Topographically and Chemically Enhanced Textile Polycaprolactone Scaffolds for Tendon and Ligament Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of the Surface-Modified PCL Scaffold
2.1.1. Preparation of PCL Fibers
2.1.2. Scaffold Fabrication
2.1.3. Surface Modification and Nanoparticle Loading
2.2. Cell Culture
2.2.1. Scaffold Seeding
2.2.2. Live–Dead Staining
2.3. Analytics
2.3.1. Tensile Tests
2.3.2. Scaffold Morphology
2.3.3. Sterilization
2.3.4. Confocal Laser Scanning Microscopy (CLSM)
2.3.5. Scanning Electron Microscopy (SEM)
2.3.6. 2-Photon Laser Scanning Microscopy
2.3.7. Differential Scanning Calorimetry (DSC)
2.3.8. Gel Permeation Chromatography (GPC)
2.3.9. Release and ELISA of TGF-β3
2.3.10. Statistical Analysis
3. Results
3.1. Preparation of PCL Fibers
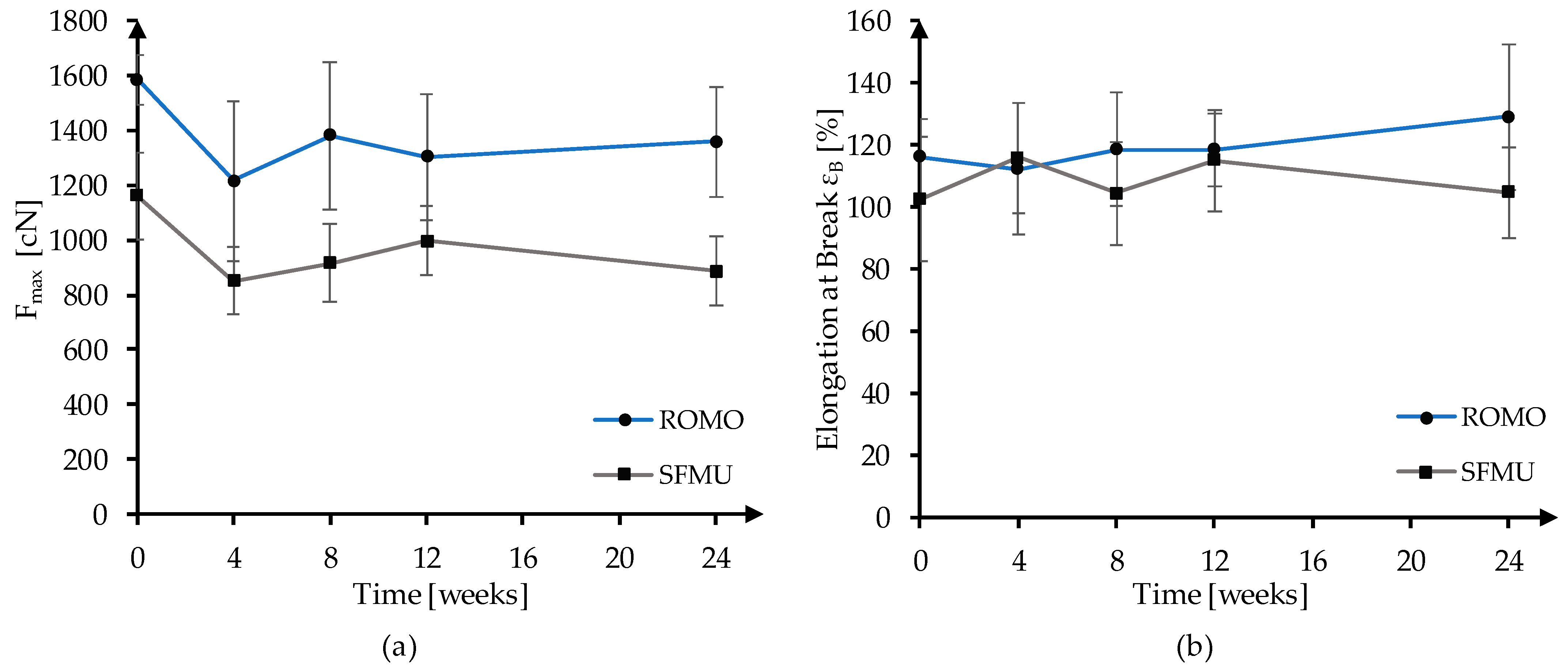
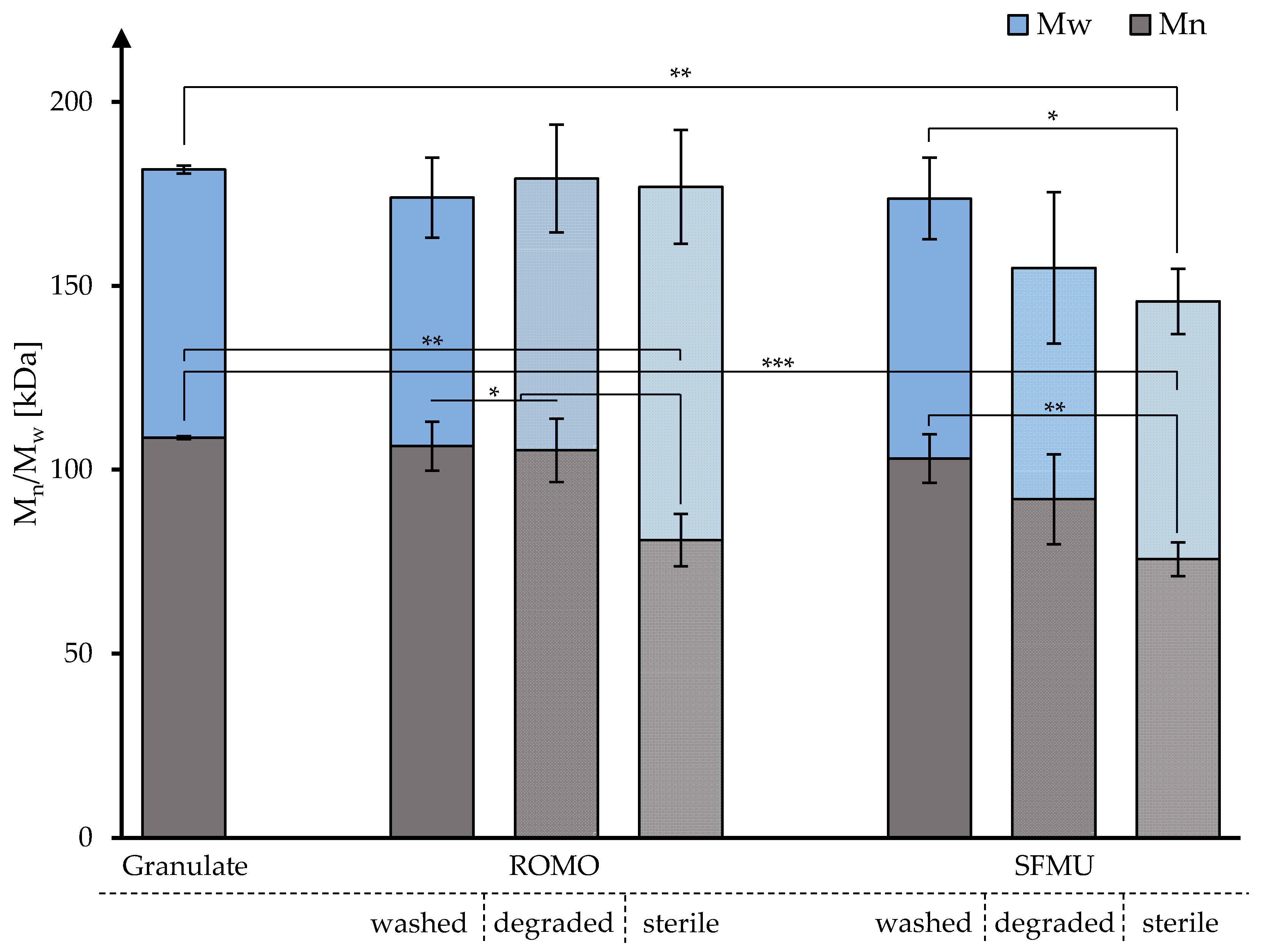
3.2. In Vitro Degradation and Molecular Weight Analysis
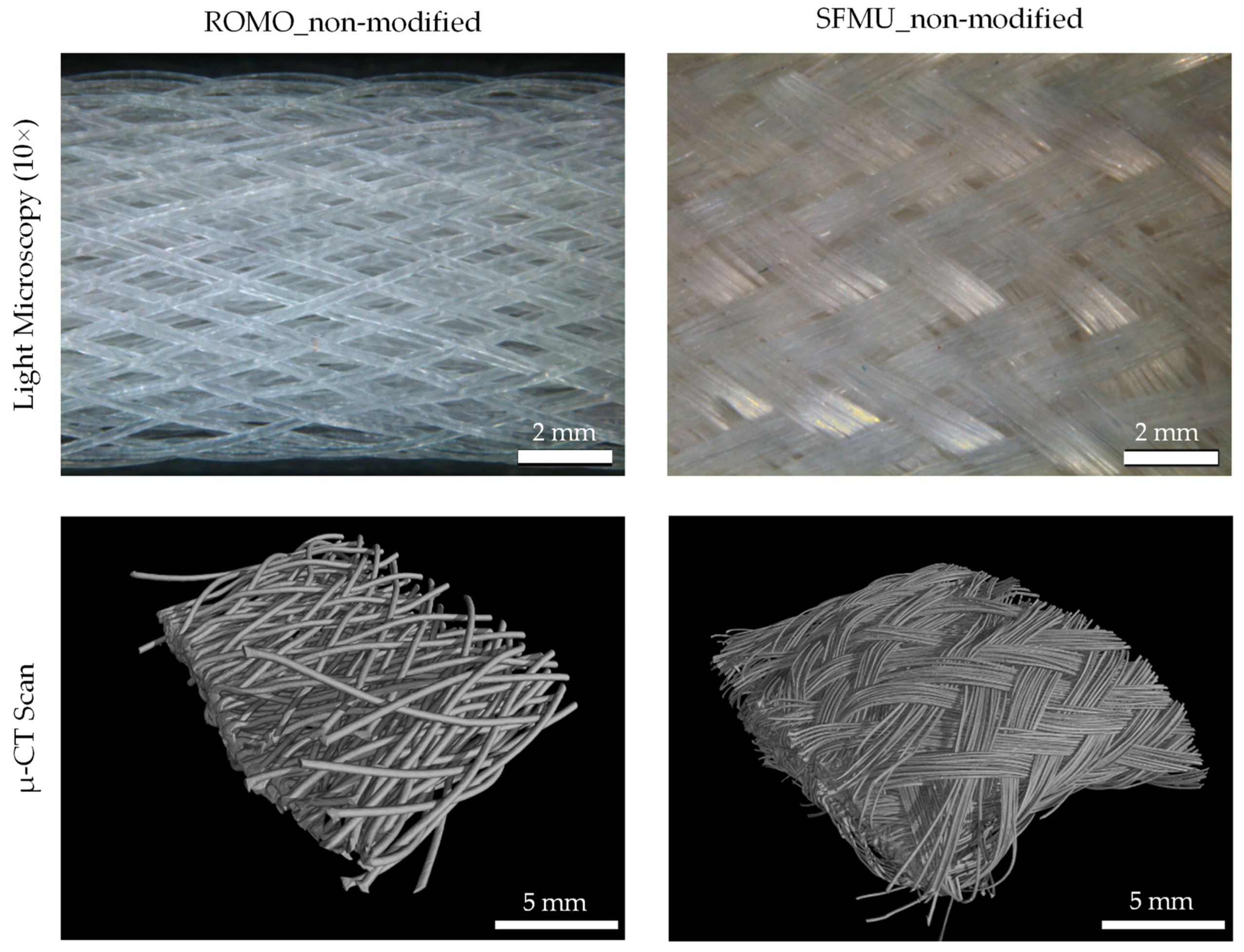
3.3. Scaffold Characterization
3.4. Live–Dead Assay
3.5. Surface Functionalization and Release of TGF-Beta
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Altman, G.H.; Horan, R.L.; Lu, H.H.; Moreau, J.; Martin, I.; Richmond, J.C.; Kaplan, D.L. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials 2002, 23, 4131–4141. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.C.; Guedes, R.M.; Marques, A.T. Development of ligament tissue biodegradable devices: A review. J. Biomech. 2009, 42, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Jedda, H.; Abessalem, S.B.; Sakli, F. The effect of cyclic loading on the mechanical properties of artificial ligaments. J. Text. Inst. 2011, 102, 332–342. [Google Scholar] [CrossRef]
- Ge, Z.; Yang, F.; Goh, J.C.H.; Ramakrishna, S.; Lee, E.H. Biomaterials and scaffolds for ligament tissue engineering. J. Biomed. Mater. Res. A 2006, 77, 639–652. [Google Scholar] [CrossRef]
- Silva, M.; Ferreira, F.N.; Alves, N.M.; Paiva, M.C. Biodegradable polymer nanocomposites for ligament/tendon tissue engineering. J. Nanobiotechnol. 2020, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Schlenker, H.-J. Tissue Engineering von Bandersatz: Einfluss Mechanischer Reize auf Humane Mesenchymale Progenitorzellen und Humane Kreuzbandzellen. Ph.D. Dissertation, Universität Ulm, Ulm, Germany, 2006. [Google Scholar]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef] [PubMed]
- Vunjak-Novakovic, G.; Altman, G.; Horan, R.; Kaplan, D.L. Tissue engineering of ligaments. Annu. Rev. Biomed. Eng. 2004, 6, 131–156. [Google Scholar] [CrossRef] [PubMed]
- Negahi Shirazi, A.; Chrzanowski, W.; Khademhosseini, A.; Dehghani, F. Anterior Cruciate Ligament: Structure, Injuries and Regenerative Treatments. Adv. Exp. Med. Biol. 2015, 881, 161–186. [Google Scholar] [CrossRef]
- Hahn, J.; Breier, A.; Brünig, H.; Heinrich, G. Long-term hydrolytic degradation study on polymer-based embroidered scaffolds for ligament tissue engineering. J. Ind. Text. 2018, 47, 1305–1320. [Google Scholar] [CrossRef]
- Laurent, C.P.; Ganghoffer, J.-F.; Babin, J.; Six, J.-L.; Wang, X.; Rahouadj, R. Morphological characterization of a novel scaffold for anterior cruciate ligament tissue engineering. J. Biomech. Eng. 2011, 133, 65001. [Google Scholar] [CrossRef]
- Liu, X.; Baldit, A.; de Brosses, E.; Velard, F.; Cauchois, G.; Chen, Y.; Wang, X.; de Isla, N.; Laurent, C. Characterization of Bone Marrow and Wharton’s Jelly Mesenchymal Stromal Cells Response on Multilayer Braided Silk and Silk/PLCL Scaffolds for Ligament Tissue Engineering. Polymers 2020, 12, 2163. [Google Scholar] [CrossRef]
- Liu, Y.; Ramanath, H.S.; Wang, D.-A. Tendon tissue engineering using scaffold enhancing strategies. Trends Biotechnol. 2008, 26, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Legnani, C.; Ventura, A.; Terzaghi, C.; Borgo, E.; Albisetti, W. Anterior cruciate ligament reconstruction with synthetic grafts. A review of literature. Int. Orthop. 2010, 34, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Han, F.; Chen, T.; Wu, Z.; Chen, S. “Swiss roll”-like bioactive hybrid scaffolds for promoting bone tissue ingrowth and tendon-bone healing after anterior cruciate ligament reconstruction. Biomater. Sci. 2020, 8, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.H.; Cooper, J.A.; Manuel, S.; Freeman, J.W.; Attawia, M.A.; Ko, F.K.; Laurencin, C.T. Anterior cruciate ligament regeneration using braided biodegradable scaffolds: In vitro optimization studies. Biomaterials 2005, 26, 4805–4816. [Google Scholar] [CrossRef] [PubMed]
- Laurencin, C.T.; Freeman, J.W. Ligament tissue engineering: An evolutionary materials science approach. Biomaterials 2005, 26, 7530–7536. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.W.; Woods, M.D.; Laurencin, C.T. Tissue engineering of the anterior cruciate ligament using a braid-twist scaffold design. J. Biomech. 2007, 40, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Ouyang, H.; Goh, J.C.-H.; Tay, T.E.; Toh, S.L. Characterization of a Novel Polymeric Scaffold for Potential Application in Tendon/Ligament Tissue Engineering. Tissue Eng. 2006, 12, 91–99. [Google Scholar] [CrossRef]
- Fuoco, T.; Mathisen, T.; Finne-Wistrand, A. Minimizing the time gap between service lifetime and complete resorption of degradable melt-spun multifilament fibers. Polym. Degrad. Stab. 2019, 163, 43–51. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Pan, Z.; Sun, H.; Wang, J.; Yu, D.; Zhu, S.; Dai, J.; Chen, Y.; Tian, N.; et al. The effects of lactate and acid on articular chondrocytes function: Implications for polymeric cartilage scaffold design. Acta Biomater. 2016, 42, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Gögele, C.; Hahn, J.; Elschner, C.; Breier, A.; Schröpfer, M.; Prade, I.; Meyer, M.; Schulze-Tanzil, G. Enhanced Growth of Lapine Anterior Cruciate Ligament-Derived Fibroblasts on Scaffolds Embroidered from Poly(l-lactide-co-ε-caprolactone) and Polylactic Acid Threads Functionalized by Fluorination and Hexamethylene Diisocyanate Cross-Linked Collagen Foams. Int. J. Mol. Sci. 2020, 21, 1132. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.X.F.; Hutmacher, D.W.; Schantz, J.-T.; Woodruff, M.A.; Teoh, S.H. Evaluation of polycaprolactone scaffold degradation for 6 months in vitro and in vivo. J. Biomed. Mater. Res. A 2009, 90, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Pal, J.; Kankariya, N.; Sanwaria, S.; Nandan, B.; Srivastava, R.K. Control on molecular weight reduction of poly(ε-caprolactone) during melt spinning--a way to produce high strength biodegradable fibers. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4213–4220. [Google Scholar] [CrossRef] [PubMed]
- Bauer, B.; Emonts, C.; Bonten, L.; Annan, R.; Merkord, F.; Vad, T.; Idrissi, A.; Gries, T.; Blaeser, A. Melt-Spun, Cross-Section Modified Polycaprolactone Fibers for Use in Tendon and Ligament Tissue Engineering. Fibers 2022, 10, 23. [Google Scholar] [CrossRef]
- Leroux, A.; Maurice, E.; Viateau, V.; Migonney, V. Feasibility Study of the Elaboration of a Biodegradable and Bioactive Ligament Made of Poly(ε-caprolactone)-pNaSS Grafted Fibers for the Reconstruction of Anterior Cruciate Ligament: In Vivo Experiment. IRBM 2019, 40, 38–44. [Google Scholar] [CrossRef]
- Leroux, A.; Ngoc Nguyen, T.; Rangel, A.; Cacciapuoti, I.; Duprez, D.; Castner, D.G.; Migonney, V. Long-term hydrolytic degradation study of polycaprolactone films and fibers grafted with poly(sodium styrene sulfonate): Mechanism study and cell response. Biointerphases 2020, 15, 61006. [Google Scholar] [CrossRef]
- Maurice, E.; Rangel, A.L.R.; Venkatesan, J.K.; Leroux, A.; El Hafci, H.; Pichard, D.; Manassero, M.; Godineau, T.; Vial, J.; Schmitt, G.; et al. The effect of pNaSS grafting of knitted poly(ε-caprolactone) artificial ligaments on in vitro mineralization and in vivo osseointegration. Materialia 2022, 21, 101331. [Google Scholar] [CrossRef]
- Gomes, S.; Rodrigues, G.; Martins, G.; Henriques, C.; Silva, J.C. Evaluation of nanofibrous scaffolds obtained from blends of chitosan, gelatin and polycaprolactone for skin tissue engineering. Int. J. Biol. Macromol. 2017, 102, 1174–1185. [Google Scholar] [CrossRef]
- Jung, S.-M.; Yoon, G.H.; Lee, H.C.; Shin, H.S. Chitosan nanoparticle/PCL nanofiber composite for wound dressing and drug delivery. J. Biomater. Sci. Polym. Ed. 2015, 26, 252–263. [Google Scholar] [CrossRef] [PubMed]
- de Cassan, D.; Sydow, S.; Schmidt, N.; Behrens, P.; Roger, Y.; Hoffmann, A.; Hoheisel, A.L.; Glasmacher, B.; Hänsch, R.; Menzel, H. Attachment of nanoparticulate drug-release systems on poly(ε-caprolactone) nanofibers via a graftpolymer as interlayer. Colloids Surf. B Biointerfaces 2018, 163, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Willbold, E.; Wellmann, M.; Welke, B.; Angrisani, N.; Gniesmer, S.; Kampmann, A.; Hoffmann, A.; de Cassan, D.; Menzel, H.; Hoheisel, A.L.; et al. Possibilities and limitations of electrospun chitosan-coated polycaprolactone grafts for rotator cuff tear repair. J. Tissue Eng. Regen. Med. 2020, 14, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Roger, Y.; Sydow, S.; Burmeister, L.; Menzel, H.; Hoffmann, A. Sustained release of TGF-β3 from polysaccharide nanoparticles induces chondrogenic differentiation of human mesenchymal stromal cells. Colloids Surf. B Biointerfaces 2020, 189, 110843. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, H.; Li, T.; He, T.; Temple, J.; King, M.W.; Spagnoli, A. Tissue engineering a tendon-bone junction with biodegradable braided scaffolds. Biomater. Res. 2019, 23, 11. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Lee, B.-K.; Na, M.H.; Kim, D.S. Melt-spun shaped fibers with enhanced surface effects: Fiber fabrication, characterization and application to woven scaffolds. Acta Biomater. 2013, 9, 7719–7726. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.-Y.; Wang, X.-C.; Peng, X.-F.; Turng, L.-S. Shish-kebab-structured poly(ε-caprolactone) nanofibers hierarchically decorated with chitosan-poly(ε-caprolactone) copolymers for bone tissue engineering. ACS Appl. Mater. Interfaces 2015, 7, 6955–6965. [Google Scholar] [CrossRef]
- Strand, B.L.; Mørch, Y.A.; Espevik, T.; Skjåk-Braek, G. Visualization of alginate-poly-L-lysine-alginate microcapsules by confocal laser scanning microscopy. Biotechnol. Bioeng. 2003, 82, 386–394. [Google Scholar] [CrossRef]
- DIN EN ISO 139; Textiles—Standard Atmospheres for Conditioning and Testing (ISO 139:2005 + Amd.1:2011); German version EN ISO 139:2005 + A1:2011. Beuth Verlag GmbH: Berlin, Germany, 2011.
- DIN EN 13392; Textiles—Monofilaments—Determination of Linear Density; German version EN 13392:2001. Beuth Verlag GmbH: Berlin, Germany, 2001.
- DIN EN 13895; Textiles—Monofilaments—Determination of Tensile Properties; German version EN 13895:2003. Beuth Verlag GmbH: Berlin, Germany, 2003.
- DIN EN ISO 2062; Textiles—Yarns from Packages—Determination of Single-End Breaking Force and Elongation at Break Using Constant Rate of Extension (CRE) Tester (ISO 2062:2009); German version EN ISO 2062:2009. Beuth Verlag GmbH: Berlin, Germany, 2010.
- DIN EN ISO 11137-1; Sterilization of Health Care Products—Radiation—Part 1: Requirements for Development, Validation and Routine Control of a Sterilization Process for Medical Devices (ISO 11137-1:2006, including Amd.1:2013 + Amd.2:2018); German version EN ISO 11137-1:2015 + A2:2019. Beuth Verlag GmbH: Berlin, Germany, 2020.
- de Cassan, D.; Hoheisel, A.L.; Glasmacher, B.; Menzel, H. Impact of sterilization by electron beam, gamma radiation and X-rays on electrospun poly-(ε-caprolactone) fiber mats. J. Mater. Sci. Mater. Med. 2019, 30, 42. [Google Scholar] [CrossRef]
- Noyes, F.; Grood, E. The strength of the anterior cruciate ligament in humans and Rhesus. J. Bone Jt. Surg. Am. 1976, 58, 1074–1082. [Google Scholar] [CrossRef]
- Woo, S.L.; Hollis, J.M.; Adams, D.J.; Lyon, R.M.; Takai, S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. Am. J. Sports Med. 1991, 19, 217–225. [Google Scholar] [CrossRef]
- Sundermann, J.; Sydow, S.; Burmeister, L.; Hoffmann, A.; Menzel, H.; Bunjes, H. Spatially and Temporally Controllable BMP-2 and TGF-β3 Double Release from Polycaprolactone Fiber Scaffolds via Chitosan-Based Polyelectrolyte Coatings. ACS Biomater. Sci. Eng. 2022, 10, 89–98. [Google Scholar] [CrossRef]
- Berten-Schunk, L.; Roger, Y.; Bunjes, H.; Hoffmann, A. Release of TGF-β3 from Surface-Modified PCL Fiber Mats Triggers a Dose-Dependent Chondrogenic Differentiation of Human Mesenchymal Stromal Cells. Pharmaceutics 2023, 15, 1303. [Google Scholar] [CrossRef]
- Barry, F.; Boynton, R.E.; Liu, B.; Murphy, J.M. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: Differentiation-dependent gene expression of matrix components. Exp. Cell Res. 2001, 268, 189–200. [Google Scholar] [CrossRef]
- Mueller, M.B.; Fischer, M.; Zellner, J.; Berner, A.; Dienstknecht, T.; Prantl, L.; Kujat, R.; Nerlich, M.; Tuan, R.S.; Angele, P. Hypertrophy in mesenchymal stem cell chondrogenesis: Effect of TGF-beta isoforms and chondrogenic conditioning. Cells Tissues Organs 2010, 192, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Emonts, C.; Wienen, D.; Bauer, B.; Idrissi, A.; Gries, T. 3D-Braided Poly-ε-Caprolactone-Based Scaffolds for Ligament Tissue Engineering. JFB 2022, 13, 230. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.; Schulze-Tanzil, G.; Schröpfer, M.; Meyer, M.; Gögele, C.; Hoyer, M.; Spickenheuer, A.; Heinrich, G.; Breier, A. Viscoelastic Behavior of Embroidered Scaffolds for ACL Tissue Engineering Made of PLA and P(LA-CL) After In Vitro Degradation. Int. J. Mol. Sci. 2019, 20, 4655. [Google Scholar] [CrossRef]
- Mengsteab, P.Y.; Freeman, J.; Barajaa, M.A.; Nair, L.S.; Laurencin, C.T. Ligament Regenerative Engineering: Braiding Scalable and Tunable Bioengineered Ligaments Using a Bench-Top Braiding Machine. Regen. Eng. Transl. Med. 2021, 7, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.A.; Lu, H.H.; Ko, F.K.; Freeman, J.W.; Laurencin, C.T. Fiber-based tissue-engineered scaffold for ligament replacement: Design considerations and in vitro evaluation. Biomaterials 2005, 26, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C.P.; Durville, D.; Mainard, D.; Ganghoffer, J.-F.; Rahouadj, R. A multilayer braided scaffold for Anterior Cruciate Ligament: Mechanical modeling at the fiber scale. J. Mech. Behav. Biomed. Mater. 2012, 12, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Madhavarapu, S.; Rao, R.; Libring, S.; Fleisher, E.; Yankannah, Y.; Freeman, J.W. Design and characterization of three-dimensional twist-braid scaffolds for anterior cruciate ligament regeneration. Technology 2017, 05, 98–106. [Google Scholar] [CrossRef]
- Chandrashekar, N.; Slauterbeck, J.; Hashemi, J. Sex-based differences in the anthropometric characteristics of the anterior cruciate ligament and its relation to intercondylar notch geometry: A cadaveric study. Am. J. Sports Med. 2005, 33, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Laschke, M.W.; Harder, Y.; Amon, M.; Martin, I.; Farhadi, J.; Ring, A.; Torio-Padron, N.; Schramm, R.; Rücker, M.; Junker, D.; et al. Angiogenesis in tissue engineering: Breathing life into constructed tissue substitutes. Tissue Eng. 2006, 12, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Kyosev, Y. (Ed.) Advances in Braiding Technology: Specialized Techniques and Applications; Woodhead Publishing: Amsterdam, The Netherlands; Cambridge, UK, 2016; ISBN 978-0-08-100926-0. [Google Scholar]
- Dong, Z.; Cui, H.; Wang, Y.; Wang, C.; Li, Y.; Wang, C. Biocompatible AIE material from natural resources: Chitosan and its multifunctional applications. Carbohydr. Polym. 2020, 227, 115338. [Google Scholar] [CrossRef]
- Meier, N.; Menzel, H. Multifunctional use of casein as release system and blocking layer for implant coatings. In Proceedings of the SPhERe Proceedings: 4th International Symposium on Pharmaceutical Engineering Research, Braunschweig, Germany, 15–17 September 2021. [Google Scholar] [CrossRef]
- Wang, S.; Hashemi, S.; Stratton, S.; Arinzeh, T.L. The Effect of Physical Cues of Biomaterial Scaffolds on Stem Cell Behavior. Adv. Healthc. Mater. 2021, 10, e2001244. [Google Scholar] [CrossRef]
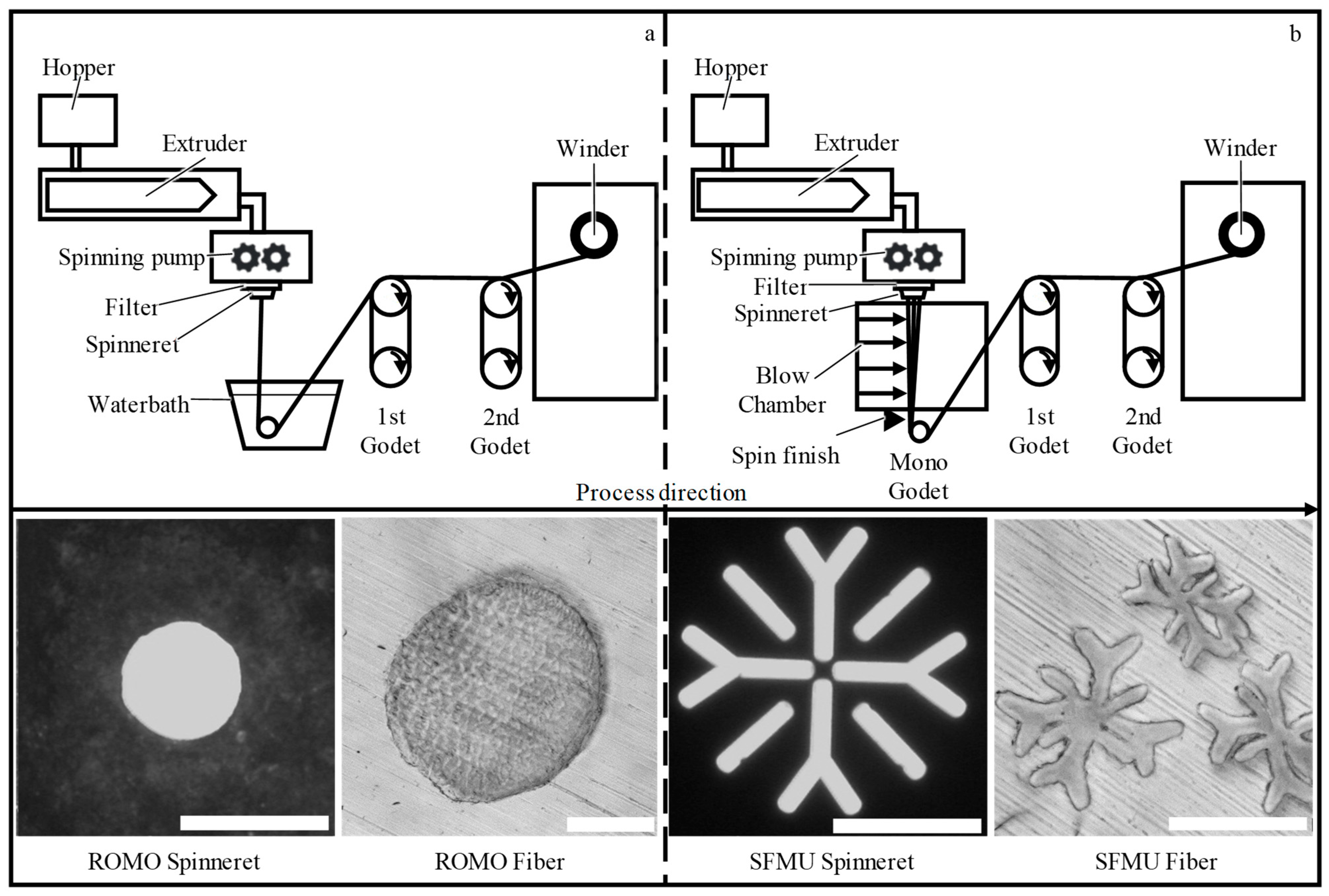

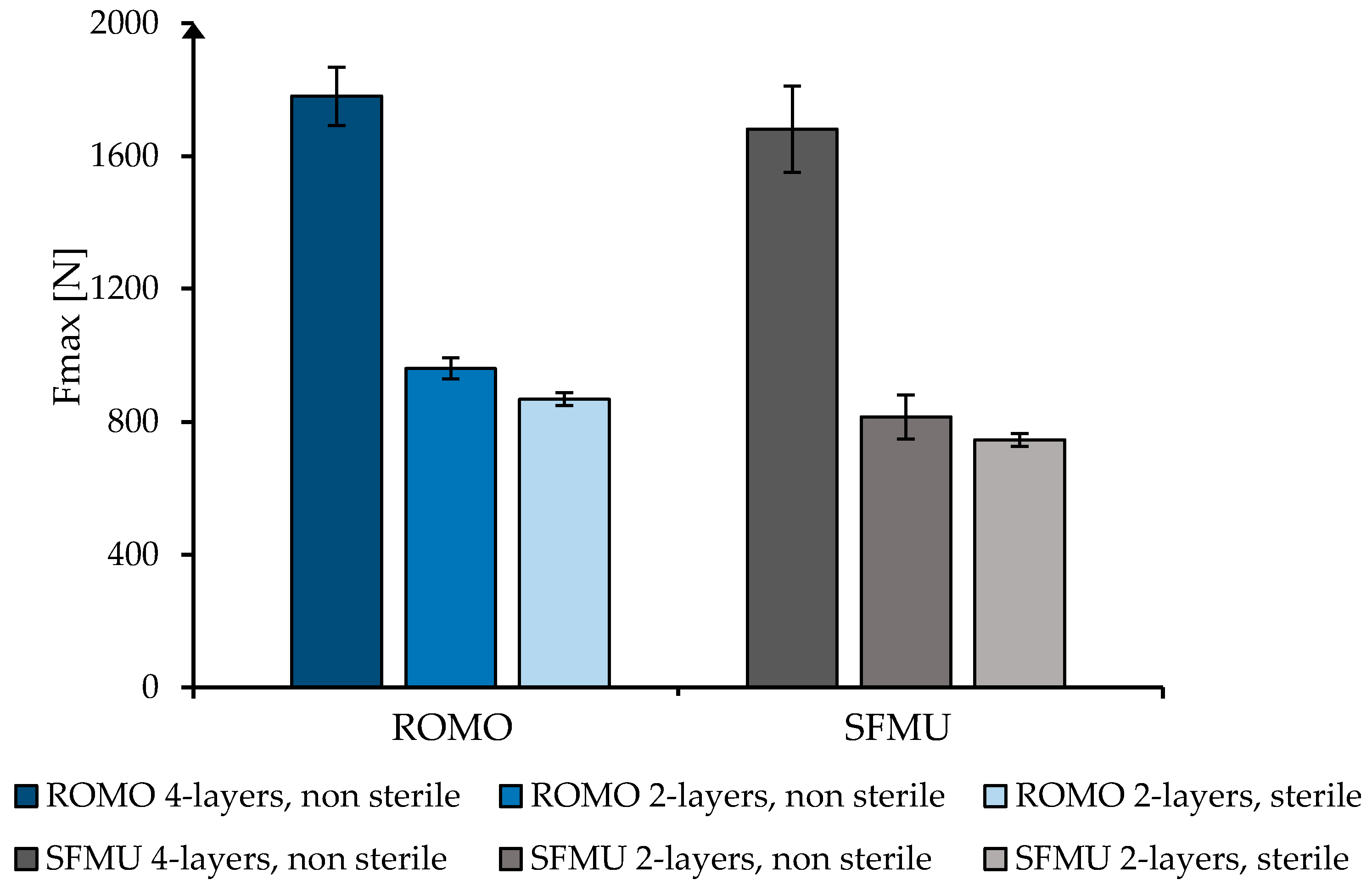

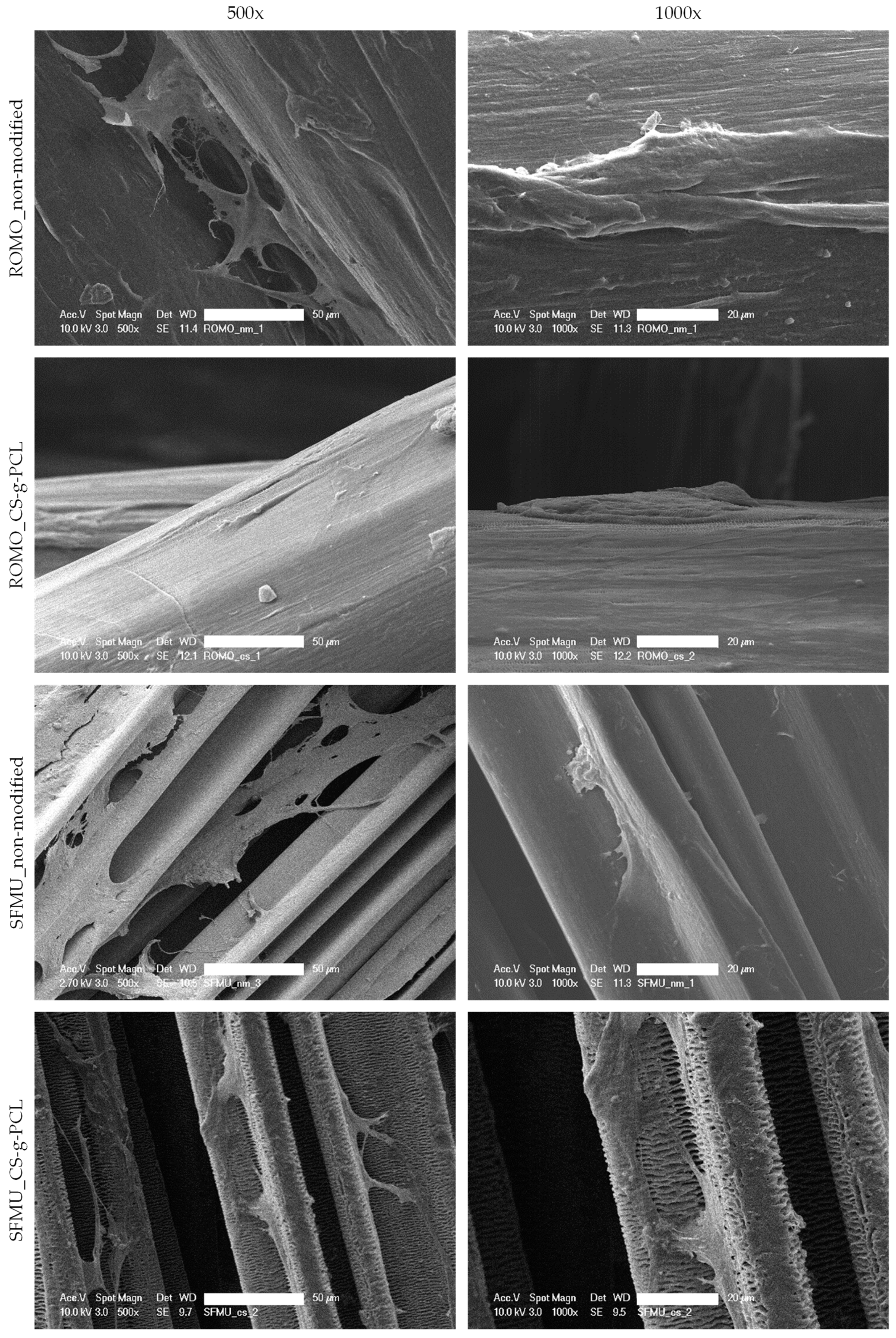
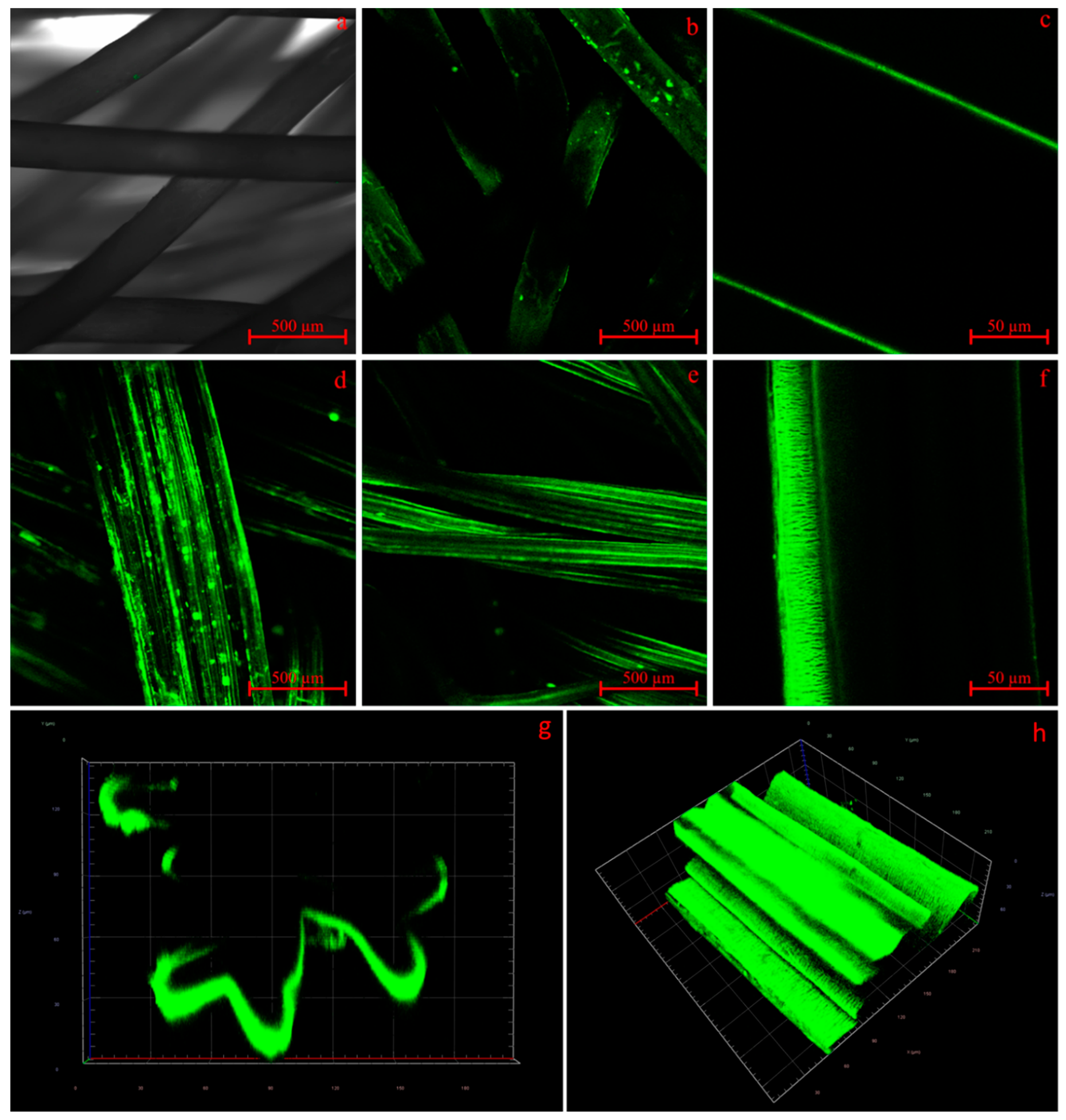

| Fiber | Cross-Section | Yarn Structure | Draw Ratio | Yarn Count [dtex] | Fmax [cN] |
|---|---|---|---|---|---|
| ROMO | Round | Monofilament | 5.58 | 458.6 ± 5.1 | 1644 ± 100 |
| SFMU | Snowflake | Multifilament | 5.58 | 436.7 ± 7.5 | 1274 ± 168 |
| Granulate | Melt-Spun Fiber | Degraded Fiber (after 24 Weeks) | Sterilized Sample | |||||
|---|---|---|---|---|---|---|---|---|
| Fiber | Mn t4 [kDa] | Mw t4 [kDa] | Mn t4 [kDa] | Mw t4 [kDa] | Mn t4 [kDa] | Mw t4 [kDa] | Mn t4 [kDa] | Mw t4 [kDa] |
| ROMO | 109 (0.35) | 184 (0.16) | 106 (6.24) | 174 (6.24) | 105 (8.18) | 179 (8.18) | 81 (8.75) | 177 (8.74) |
| SFMU | 103 (6.4) | 174 (6.39) | 92 (13.28) | 155 (13.27) | 76 (6.07) | 146 (6.07) | ||
| Scaffold | Pix/cm | Braiding Angle α [°] | Porosity P [%] | Width [mm] |
|---|---|---|---|---|
| ROMO_4-layer | 3.84 ± 0.37 | 21.12 ± 0.97 | 83.73 ± 0.00 | 6.58 ± 0.66 |
| ROMO_2-layer_unst | 4.50 ± 0.51 | 19.61 ± 2.07 | 86.31 ± 0.02 | 6.19 ± 0.10 |
| ROMO_2-layer_st | ||||
| SFMU_4-layer | 4.53 ± 0.50 | 24.19 ± 1.52 | 73.70 ± 0.00 | 8.86 ± 0.79 |
| SFMU_2-layer_unst | 4.52 ± 0.44 | 21.37 ± 2.55 | 87.00 ± 0.05 | 6.35 ± 0.83 |
| SFMU_2-layer_st |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauer, B.; Emonts, C.; Pitts, J.; Buhl, E.M.; Eschweiler, J.; Hänsch, R.; Betsch, M.; Gries, T.; Menzel, H. Topographically and Chemically Enhanced Textile Polycaprolactone Scaffolds for Tendon and Ligament Tissue Engineering. Polymers 2024, 16, 488. https://doi.org/10.3390/polym16040488
Bauer B, Emonts C, Pitts J, Buhl EM, Eschweiler J, Hänsch R, Betsch M, Gries T, Menzel H. Topographically and Chemically Enhanced Textile Polycaprolactone Scaffolds for Tendon and Ligament Tissue Engineering. Polymers. 2024; 16(4):488. https://doi.org/10.3390/polym16040488
Chicago/Turabian StyleBauer, Benedict, Caroline Emonts, Johannes Pitts, Eva Miriam Buhl, Jörg Eschweiler, Robert Hänsch, Marcel Betsch, Thomas Gries, and Henning Menzel. 2024. "Topographically and Chemically Enhanced Textile Polycaprolactone Scaffolds for Tendon and Ligament Tissue Engineering" Polymers 16, no. 4: 488. https://doi.org/10.3390/polym16040488
APA StyleBauer, B., Emonts, C., Pitts, J., Buhl, E. M., Eschweiler, J., Hänsch, R., Betsch, M., Gries, T., & Menzel, H. (2024). Topographically and Chemically Enhanced Textile Polycaprolactone Scaffolds for Tendon and Ligament Tissue Engineering. Polymers, 16(4), 488. https://doi.org/10.3390/polym16040488







