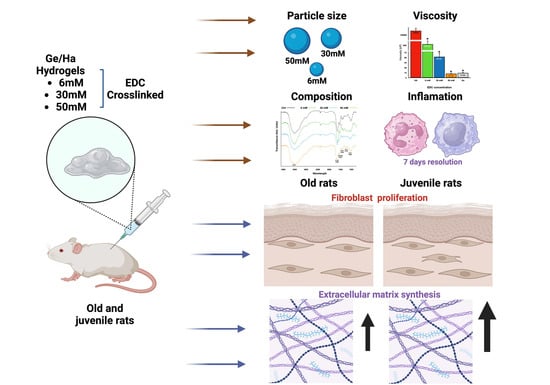
Subcutaneous Application of a Gelatin/Hyaluronic Acid Hydrogel Induces the Production of Skin Extracellular Matrix
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Ge/Ha Hydrogel Crosslinked with EDC at Different Concentrations
2.2. Determination of Particle Size
2.3. Determination of Viscosity
2.4. Infrared Spectroscopy (IR)
2.5. Subcutaneous Application of Ge/Ha Hydrogel
2.6. Histological Evaluation of Ge/HA Hydrogels
2.7. Mechanical Properties of the Skin under Uniaxial Tension
2.8. Statistic Analysis
3. Results
3.1. Ge/Ha Particle Size Analysis
3.2. Ge/Ha Viscosity Analysis
3.3. The Composition of Ge/Ha Hydrogels
3.4. Effect of the Application of Ge/Ha Hydrogels in the Dermis of Rats
3.5. Effect of Ge/Ha Hydrogels on the Dermal Extracellular Matrix
3.6. Mechanical Behavior of the Rat Skin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Witmanowski, H.; Błochowiak, K. Another Face of Dermal Fillers. Adv. Dermatol. Allergol. 2020, 37, 651–659. [Google Scholar] [CrossRef]
- Xiong, M.; Zhang, Q.; Hu, W.; Zhao, C.; Lv, W.; Yi, Y.; Wang, Y.; Tang, H.; Wu, M.; Wu, Y. The Novel Mechanisms and Applications of Exosomes in Dermatology and Cutaneous Medical Aesthetics. Pharmacol. Res. 2021, 166, 105490. [Google Scholar] [CrossRef]
- Breithaupt, A.; Fitzgerald, R. Collagen Stimulators. Facial Plast. Surg. Clin. N. Am. 2015, 23, 459–469. [Google Scholar] [CrossRef]
- Christen, M.-O. Collagen Stimulators in Body Applications: A Review Focused on Poly-L-Lactic Acid (PLLA). CCID 2022, 15, 997–1019. [Google Scholar] [CrossRef]
- Gyles, D.A.; Castro, L.D.; Silva, J.O.C.; Ribeiro-Costa, R.M. A Review of the Designs and Prominent Biomedical Advances of Natural and Synthetic Hydrogel Formulations. Eur. Polym. J. 2017, 88, 373–392. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for Biomedical Applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef]
- Day, A.J.; De La Motte, C.A. Hyaluronan Cross-Linking: A Protective Mechanism in Inflammation? Trends Immunol. 2005, 26, 637–643. [Google Scholar] [CrossRef]
- Tian, X.; Azpurua, J.; Hine, C.; Vaidya, A.; Myakishev-Rempel, M.; Ablaeva, J.; Mao, Z.; Nevo, E.; Gorbunova, V.; Seluanov, A. High-Molecular-Mass Hyaluronan Mediates the Cancer Resistance of the Naked Mole Rat. Nature 2013, 499, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Krejcova, D.; Pekarova, M.; Safrankova, B.; Kubala, L. The Effect of Different Molecular Weight Hyaluronan on Macrophage Physiology. Neuro. Endocrinol. Lett. 2009, 30 (Suppl. S1), 106–111. [Google Scholar] [PubMed]
- Ramamurthi, A.; Vesely, I. Ultraviolet Light-induced Modification of Crosslinked Hyaluronan Gels. J. Biomed. Mater. Res. 2003, 66, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Noh, I.; Kim, G.-W.; Choi, Y.-J.; Kim, M.-S.; Park, Y.; Lee, K.-B.; Kim, I.-S.; Hwang, S.-J.; Tae, G. Effects of Cross-Linking Molecular Weights in a Hyaluronic Acid–Poly(Ethylene Oxide) Hydrogel Network on Its Properties. Biomed. Mater. 2006, 1, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Prestwich, G.; Kuo, J. Chemically-Modified HA for Therapy and Regenerative Medicine. CPB 2008, 9, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan as an Immune Regulator in Human Diseases. Physiol. Rev. 2011, 91, 221–264. [Google Scholar] [CrossRef] [PubMed]
- Alijotas-Reig, J.; Garcia-Gimenez, V. Delayed Immune-mediated Adverse Effects Related to Hyaluronic Acid and Acrylic Hydrogel Dermal Fillers: Clinical Findings, Long-term Follow-up and Review of the Literature. Acad. Dermatol. Venereol. 2008, 22, 150–161. [Google Scholar] [CrossRef]
- Jarquin Yanez, K.; Arenas Alatorre, J. Structural Effect of Different EDC Crosslinker Concentration in Gelatin- Hyaluronic Acid Scaffolds. J. Bioeng. Biomed. 2016, 6, 2. [Google Scholar] [CrossRef]
- Tuin, A.; Zandstra, J.; Kluijtmans, S.; Bouwstra, J.; Harmsen, M.; Van Luyn, M. Hyaluronic Acid-Recombinant Gelatin Gels as a Scaffold for Soft Tissue Regeneration. Eur. Cell. Mater. 2012, 24, 320–330. [Google Scholar] [CrossRef]
- Bard, R.L. (Ed.) Image-Guided Aesthetic Treatments; Springer: Cham, Switzerland, 2023; ISBN 978-3-031-36266-8. [Google Scholar]
- Bergström, J. Elasticity/Hyperelasticity. In Mechanics of Solid Polymers; Elsevier: Amsterdam, The Netherlands, 2015; pp. 209–307. ISBN 978-0-323-31150-2. [Google Scholar]
- Cassuto, D.; Bellia, G.; Schiraldi, C. An Overview of Soft Tissue Fillers for Cosmetic Dermatology: From Filling to Regenerative Medicine. CCID 2021, 14, 1857–1866. [Google Scholar] [CrossRef]
- Ralf Paus, L.; Berneburg, M.; Trelles, M.; Friguet, B.; Ogden, S.; Esrefoglu, M.; Kaya, G.; Goldberg, D.J.; Mordon, S.; Calderhead, R.G.; et al. How Best to Halt and/or Revert UV-induced Skin Ageing: Strategies, Facts and Fiction. Exp. Dermatol. 2008, 17, 228–229. [Google Scholar] [CrossRef]
- Makrantonaki, E.; Adjaye, J.; Herwig, R.; Brink, T.C.; Groth, D.; Hultschig, C.; Lehrach, H.; Zouboulis, C.C. Age-specific Hormonal Decline Is Accompanied by Transcriptional Changes in Human Sebocytes in Vitro. Aging Cell 2006, 5, 331–344. [Google Scholar] [CrossRef]
- Jung, T.; Höhn, A.; Catalgol, B.; Grune, T. Age-Related Differences in Oxidative Protein-Damage in Young and Senescent Fibroblasts. Arch. Biochem. Biophys. 2009, 483, 127–135. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative Stress in the Skin: Impact and Related Protection. Int. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef]
- Peres, P.S.; Terra, V.A.; Guarnier, F.A.; Cecchini, R.; Cecchini, A.L. Photoaging and Chronological Aging Profile: Understanding Oxidation of the Skin. J. Photochem. Photobiol. B Biol. 2011, 103, 93–97. [Google Scholar] [CrossRef]
- Poon, F.; Kang, S.; Chien, A.L. Mechanisms and Treatments of Photoaging. Photoderm. Photoimm. Photomed. 2015, 31, 65–74. [Google Scholar] [CrossRef]
- Papaccio, F.; D’Arino, A.; Caputo, S.; Bellei, B. Focus on the Contribution of Oxidative Stress in Skin Aging. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef]
- Wollina, U. Midfacial Rejuvenation by Hyaluronic Acid Fillers and Subcutaneous Adipose Tissue—A New Concept. Med. Hypotheses 2015, 84, 327–330. [Google Scholar] [CrossRef]
- Park, S.-N.; Lee, H.J.; Lee, K.H.; Suh, H. Biological Characterization of EDC-Crosslinked Collagen–Hyaluronic Acid Matrix in Dermal Tissue Restoration. Biomaterials 2003, 24, 1631–1641. [Google Scholar] [CrossRef]
- Weadock, K.S.; Miller, E.J.; Keuffel, E.L.; Dunn, M.G. Effect of Physical Crosslinking Methods on Collagen-Fiber Durability in Proteolytic Solutions. J. Biomed. Mater. Res. 1996, 32, 221–226. [Google Scholar] [CrossRef]
- Lee, J.M.; Edwards, H.H.L.; Pereira, C.A.; Samii, S.I. Crosslinking of Tissue-Derived Biomaterials in 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide (EDC). J. Mater. Sci. Mater. Med. 1996, 7, 531–541. [Google Scholar] [CrossRef]
- Gorgieva, S.; Kokol, V. Collagen- vs. Gelatine-Based Biomaterials and Their Biocompatibility: Review and Perspectives. In Biomaterials Applications for Nanomedicine; Pignatello, R., Ed.; InTech: Melbourne, FL, USA, 2011; ISBN 978-953-307-661-4. [Google Scholar]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, F.; Raza, Z.A.; Batool, S.R.; Zahid, M.; Onder, O.C.; Rafique, A.; Nazeer, M.A. Preparation, Properties, and Applications of Gelatin-Based Hydrogels (GHs) in the Environmental, Technological, and Biomedical Sectors. Int. J. Biol. Macromol. 2022, 218, 601–633. [Google Scholar] [CrossRef] [PubMed]
- Kabiri, M.; Bushnak, I.; McDermot, M.T.; Unsworth, L.D. Toward a Mechanistic Understanding of Ionic Self-Complementary Peptide Self-Assembly: Role of Water Molecules and Ions. Biomacromolecules 2013, 14, 3943–3950. [Google Scholar] [CrossRef]
- Klopfleisch, R. Macrophage Reaction against Biomaterials in the Mouse Model—Phenotypes, Functions and Markers. Acta Biomater. 2016, 43, 3–13. [Google Scholar] [CrossRef]
- Zarbock, A.; Polanowska-Grabowska, R.K.; Ley, K. Platelet-Neutrophil-Interactions: Linking Hemostasis and Inflammation. Blood Rev. 2007, 21, 99–111. [Google Scholar] [CrossRef]
- Hidalgo, A.; Chang, J.; Jang, J.-E.; Peired, A.J.; Chiang, E.Y.; Frenette, P.S. Heterotypic Interactions Enabled by Polarized Neutrophil Microdomains Mediate Thromboinflammatory Injury. Nat. Med. 2009, 15, 384–391. [Google Scholar] [CrossRef]
- Christo, S.N.; Diener, K.R.; Bachhuka, A.; Vasilev, K.; Hayball, J.D. Innate Immunity and Biomaterials at the Nexus: Friends or Foes. BioMed Res. Int. 2015, 2015, 342304. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil Recruitment and Function in Health and Inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Klopfleisch, R.; Jung, F. The Pathology of the Foreign Body Reaction against Biomaterials. J. Biomed. Mater. Res. 2017, 105, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jao, B.; McNally, A.K.; Anderson, J.M. In Vivo Quantitative and Qualitative Assessment of Foreign Body Giant Cell Formation on Biomaterials in Mice Deficient in Natural Killer Lymphocyte Subsets, Mast Cells, or the Interleukin-4 Receptorα and in Severe Combined Immunodeficient Mice. J. Biomed. Mater. Res. 2014, 102, 2017–2023. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Defife, K.; Mcnally, A.; Collier, T.; Jenney, C. Monocyte, Macrophage and Foreign Body Giant Cell Interactions with Molecularly Engineered Surfaces. J. Mater. Sci. Mater. Med. 1999, 10, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Kastellorizios, M.; Tipnis, N.; Burgess, D.J. Foreign Body Reaction to Subcutaneous Implants. In Immune Responses to Biosurfaces; Lambris, J.D., Ekdahl, K.N., Ricklin, D., Nilsson, B., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2015; Volume 865, pp. 93–108. ISBN 978-3-319-18602-3. [Google Scholar]
- Mesure, L.; De Visscher, G.; Vranken, I.; Lebacq, A.; Flameng, W. Gene Expression Study of Monocytes/Macrophages during Early Foreign Body Reaction and Identification of Potential Precursors of Myofibroblasts. PLoS ONE 2010, 5, e12949. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.; Pullen, N.A.; Oskeritzian, C.A.; Ryan, J.J.; Bowlin, G.L. Macrophage Functional Polarization (M1/M2) in Response to Varying Fiber and Pore Dimensions of Electrospun Scaffolds. Biomaterials 2013, 34, 4439–4451. [Google Scholar] [CrossRef] [PubMed]
- Vidič, M.; Bartenjev, I. An Adverse Reaction after Hyaluronic Acid Filler Application: A Case Report. Acta Dermatovenerol. Alp. Pannonica Adriat. 2018, 27, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Snozzi, P.; Van Loghem, J.A.J. Complication Management Following Rejuvenation Procedures with Hyaluronic Acid Fillers—An Algorithm-Based Approach. Plast. Reconstr. Surg.-Glob. Open 2018, 6, e2061. [Google Scholar] [CrossRef] [PubMed]
- Pavicic, T.; Funt, D. Dermal Fillers in Aesthetics: An Overview of Adverse Events and Treatment Approaches. CCID 2013, 295, 295–316. [Google Scholar] [CrossRef] [PubMed]
- Signorini, M.; Liew, S.; Sundaram, H.; De Boulle, K.L.; Goodman, G.J.; Monheit, G.; Wu, Y.; Trindade De Almeida, A.R.; Swift, A.; Vieira Braz, A. Global Aesthetics Consensus: Avoidance and Management of Complications from Hyaluronic Acid Fillers—Evidence- and Opinion-Based Review and Consensus Recommendations. Plast. Reconstr. Surg. 2016, 137, 961e–971e. [Google Scholar] [CrossRef] [PubMed]
- Röck, K.; Fischer, K.; Fischer, J.W. Hyaluronan Used for Intradermal Injections Is Incorporated into the Pericellular Matrix and Promotes Proliferation in Human Skin Fibroblasts in Vitro. Dermatology 2010, 221, 219–228. [Google Scholar] [CrossRef]
- Xu, Q.; Torres, J.E.; Hakim, M.; Babiak, P.M.; Pal, P.; Battistoni, C.M.; Nguyen, M.; Panitch, A.; Solorio, L.; Liu, J.C. Collagen- and Hyaluronic Acid-Based Hydrogels and Their Biomedical Applications. Mater. Sci. Eng. R Rep. 2021, 146, 100641. [Google Scholar] [CrossRef]
- Knudson, C.B. Hyaluronan and CD44: Strategic Players for Cell–Matrix Interactions during Chondrogenesis and Matrix Assembly. Birth Defects Res. Pt. C 2003, 69, 174–196. [Google Scholar] [CrossRef]
- Lebbink, R.J.; De Ruiter, T.; Adelmeijer, J.; Brenkman, A.B.; Van Helvoort, J.M.; Koch, M.; Farndale, R.W.; Lisman, T.; Sonnenberg, A.; Lenting, P.J.; et al. Collagens Are Functional, High Affinity Ligands for the Inhibitory Immune Receptor LAIR-1. J. Exp. Med. 2006, 203, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Goldring, S.R. Osteoarthritis. J. Cell. Physiol. 2007, 213, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Humphries, J.D.; Byron, A.; Humphries, M.J. Integrin Ligands at a Glance. J. Cell Sci. 2006, 119, 3901–3903. [Google Scholar] [CrossRef] [PubMed]
- Sheikholeslam, M.; Wright, M.E.E.; Jeschke, M.G.; Amini-Nik, S. Biomaterials for Skin Substitutes. Adv. Healthc. Mater. 2018, 7, 1700897. [Google Scholar] [CrossRef]
- Ghosh, P.; Guidolin, D. Potential Mechanism of Action of Intra-Articular Hyaluronan Therapy in Osteoarthritis: Are the Effects Molecular Weight Dependent? Semin. Arthritis Rheum. 2002, 32, 10–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Garza, L.A.; Kang, S.; Varani, J.; Orringer, J.S.; Fisher, G.J.; Voorhees, J.J. In Vivo Stimulation of De Novo Collagen Production Caused by Cross-Linked Hyaluronic Acid Dermal Filler Injections in Photodamaged Human Skin. Arch. Dermatol. 2007, 143, 155–163. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Biggs, L.C.; Kim, C.S.; Miroshnikova, Y.A.; Wickström, S.A. Mechanical Forces in the Skin: Roles in Tissue Architecture, Stability, and Function. J. Investig. Dermatol. 2020, 140, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Leavitt, T.; Bayer, L.R.; Orgill, D.P. Effect of Negative Pressure Wound Therapy on Wound Healing. Curr. Probl. Surg. 2014, 51, 301–331. [Google Scholar] [CrossRef]
- Khoshnoodi, J.; Pedchenko, V.; Hudson, B.G. Mammalian Collagen IV. Microsc. Res. Tech. 2008, 71, 357–370. [Google Scholar] [CrossRef]
- Pöschl, E.; Schlötzer-Schrehardt, U.; Brachvogel, B.; Saito, K.; Ninomiya, Y.; Mayer, U. Collagen IV Is Essential for Basement Membrane Stability but Dispensable for Initiation of Its Assembly during Early Development. Development 2004, 131, 1619–1628. [Google Scholar] [CrossRef]
- Ferguson, M.W.J.; O’Kane, S. Scar–Free Healing: From Embryonic Mechanisms to Adult Therapeutic Intervention. Phil. Trans. R. Soc. Lond. B 2004, 359, 839–850. [Google Scholar] [CrossRef]
- Gosline, J.; Lillie, M.; Carrington, E.; Guerette, P.; Ortlepp, C.; Savage, K. Elastic Proteins: Biological Roles and Mechanical Properties. Phil. Trans. R. Soc. Lond. B 2002, 357, 121–132. [Google Scholar] [CrossRef] [PubMed]







| Experiments | Time | Conditions | Injections | Biopsies |
|---|---|---|---|---|
| Short term | 1, 2, 4, 7, and 14 days | Control, 6, 30, and 50 mM | 50 μL of each condition were inoculated in one rat. Each rat received 4 inoculations (Figure 1A) Three rats per time. | 4 biopsies (one for each condition) in one rat. 3 rats per time. 15 rats in total with 60 biopsies. |
| Long term | 1 and 3 months | Control, 6, 30, and 50 mM | 20 inoculations of 50 μL in 3 rats per condition, distributed in the dorsal skin (Figure 1B) | A biopsy from a place treated with 50 μL of each condition in one rat. The rest of the skin was used for mechanical tests. 3 rats per condition (12 biopsies total in each experimental time). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarquín-Yáñez, K.; Herrera-Enríquez, M.Á.; Benítez-Barrera, D.I.; Sánchez-Arévalo, F.M.; Benítez-Martínez, J.A.; Piñón-Zárate, G.; Hernández-Téllez, B.; Sandoval, D.M.A.; Castell-Rodríguez, A.E. Subcutaneous Application of a Gelatin/Hyaluronic Acid Hydrogel Induces the Production of Skin Extracellular Matrix. Polymers 2024, 16, 573. https://doi.org/10.3390/polym16050573
Jarquín-Yáñez K, Herrera-Enríquez MÁ, Benítez-Barrera DI, Sánchez-Arévalo FM, Benítez-Martínez JA, Piñón-Zárate G, Hernández-Téllez B, Sandoval DMA, Castell-Rodríguez AE. Subcutaneous Application of a Gelatin/Hyaluronic Acid Hydrogel Induces the Production of Skin Extracellular Matrix. Polymers. 2024; 16(5):573. https://doi.org/10.3390/polym16050573
Chicago/Turabian StyleJarquín-Yáñez, Katia, Miguel Ángel Herrera-Enríquez, Diego Ivan Benítez-Barrera, Francisco M. Sánchez-Arévalo, Jorge Alejandro Benítez-Martínez, Gabriela Piñón-Zárate, Beatriz Hernández-Téllez, Diana M. Aguilar Sandoval, and Andrés E. Castell-Rodríguez. 2024. "Subcutaneous Application of a Gelatin/Hyaluronic Acid Hydrogel Induces the Production of Skin Extracellular Matrix" Polymers 16, no. 5: 573. https://doi.org/10.3390/polym16050573
APA StyleJarquín-Yáñez, K., Herrera-Enríquez, M. Á., Benítez-Barrera, D. I., Sánchez-Arévalo, F. M., Benítez-Martínez, J. A., Piñón-Zárate, G., Hernández-Téllez, B., Sandoval, D. M. A., & Castell-Rodríguez, A. E. (2024). Subcutaneous Application of a Gelatin/Hyaluronic Acid Hydrogel Induces the Production of Skin Extracellular Matrix. Polymers, 16(5), 573. https://doi.org/10.3390/polym16050573