Effect of Functionalization of Texturized Polypropylene Surface by Silanization and HBII-RGD Attachment on Response of Primary Abdominal and Vaginal Fibroblasts
Abstract
:1. Introduction
2. Experimental Section
2.1. Material
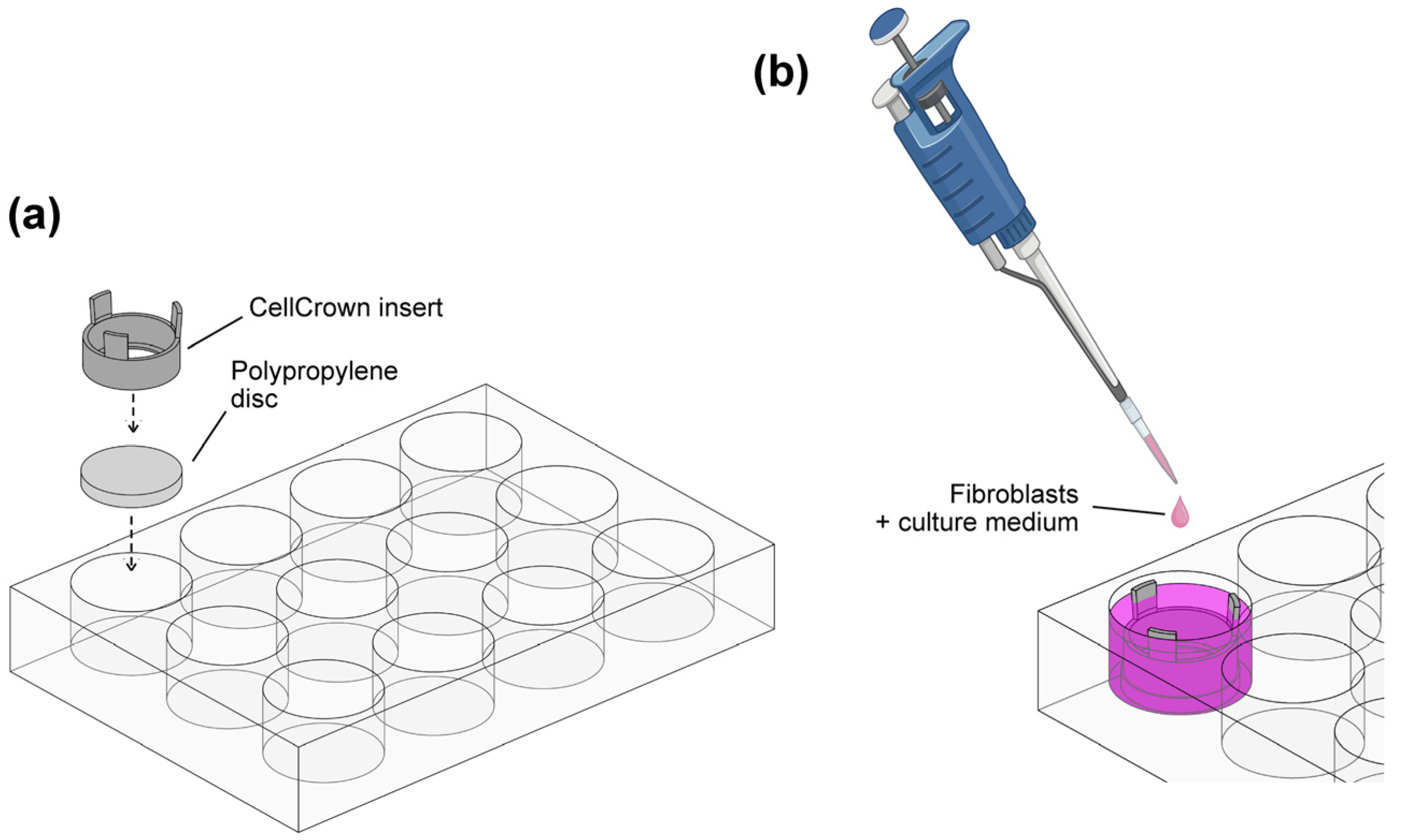
2.2. Sample Preparation
2.3. Synthesis of Recombinant HBII-RGD Fragments
2.4. PP Functionalization by Silanization and HBII-RGD Covalent Attachment
2.4.1. Oxygen Plasma
2.4.2. Silanization Process
2.5. Surface Characterization
2.5.1. Field Emission Scanning Electron Microscopy (FESEM)
2.5.2. Confocal Microscopy
2.5.3. Wettability and Contact Angle (CA)
2.5.4. X-ray Photoelectron Spectroscopy (XPS)
2.6. Cell Response
2.6.1. Isolation and Culture of Primary Human Fibroblasts
2.6.2. Fibroblast Cell Culture on Modified PP Substrates
2.6.3. Evaluation of Human Fibroblast Adhesion and Viability on PP Substrates
2.6.4. Immunofluorescence
2.6.5. Image Acquisition and Quantitative Analysis
2.7. Statistics
3. Results
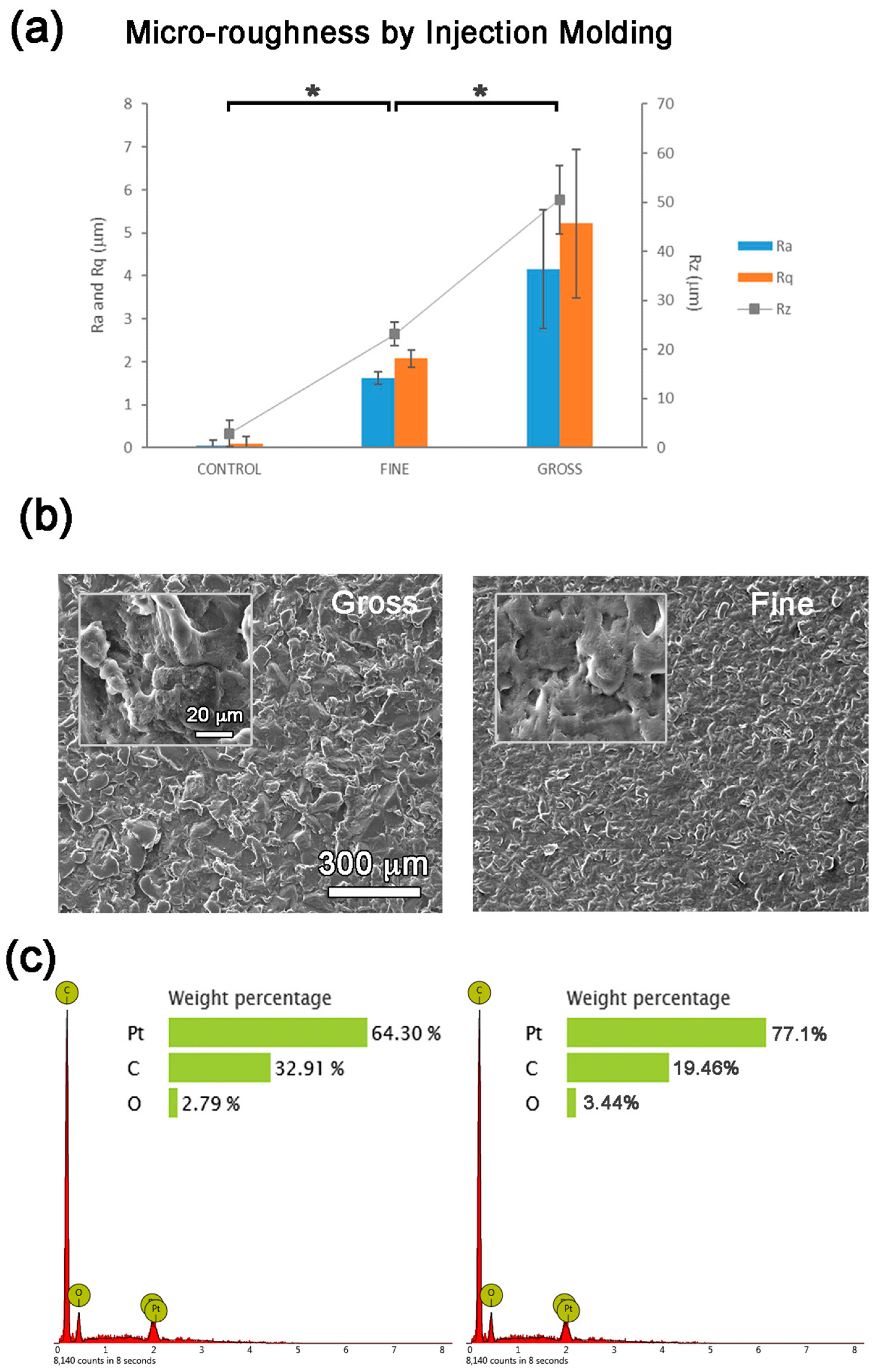
3.1. Micro-Topography
3.2. Oxygen Plasma Activation Process
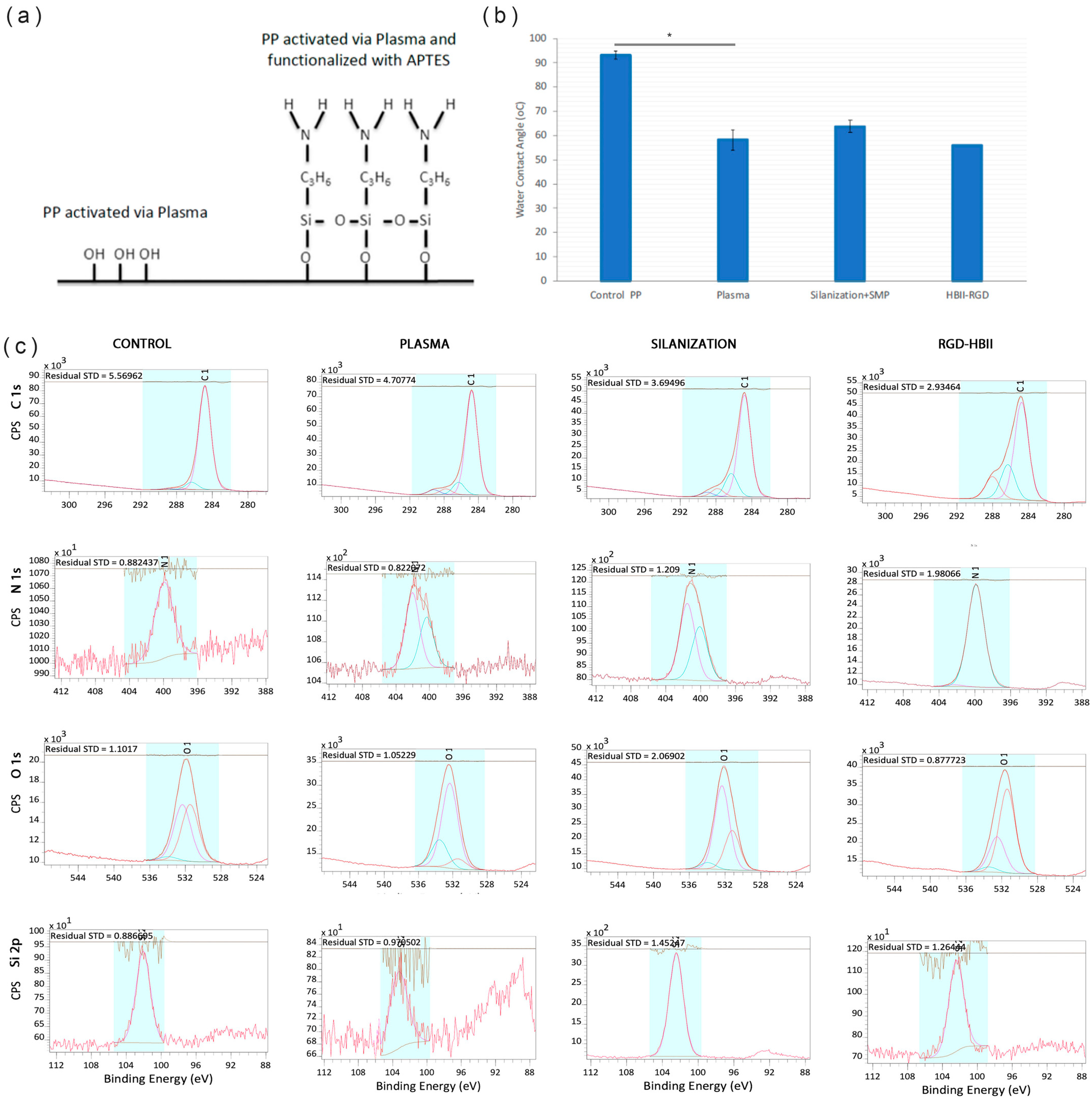
3.3. Functionalization of SMOOTH PP Surface: HBII-RGD Covalent Attachment via Silanization
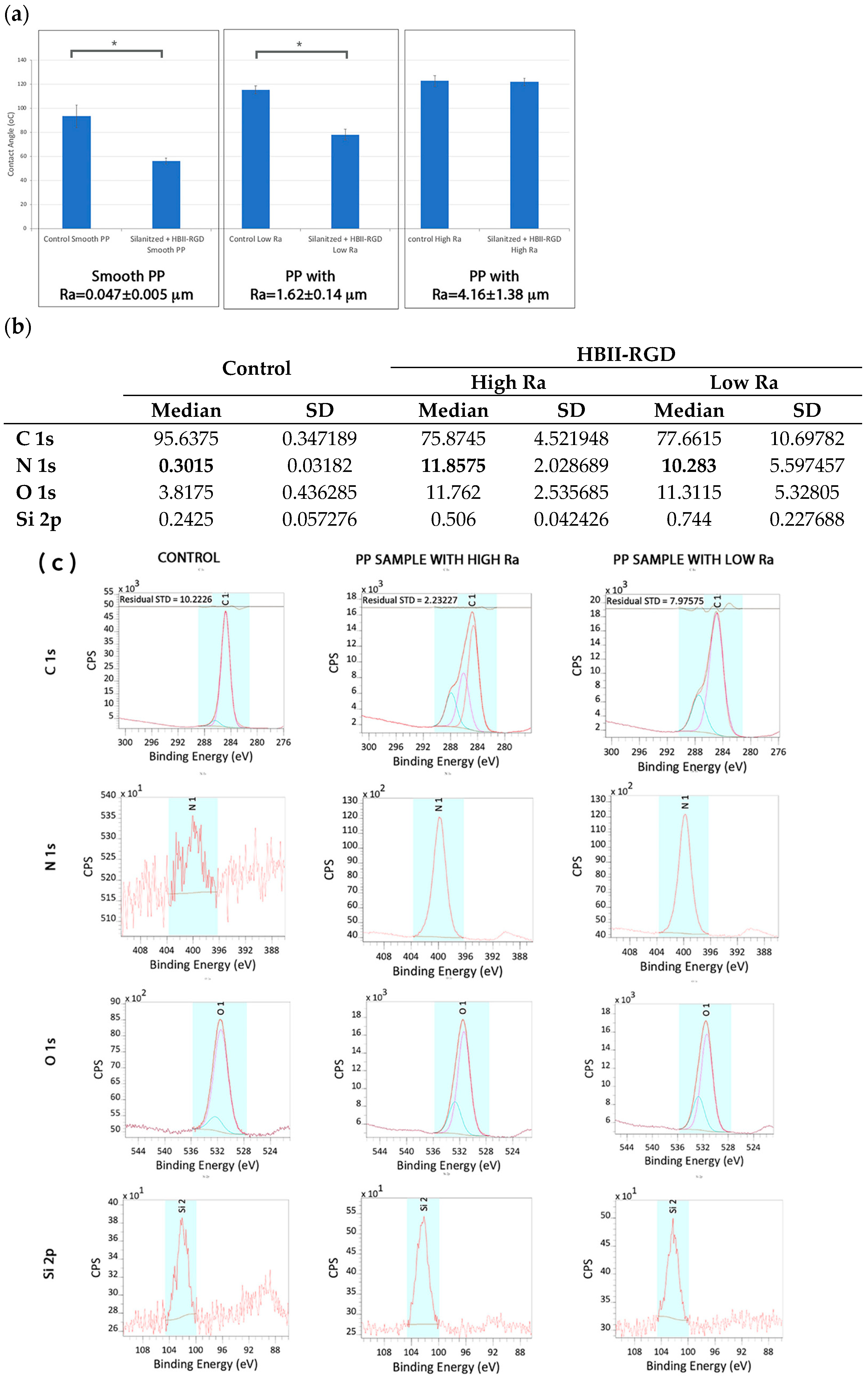
3.4. Functionalization of ROUGH PP Surface: HBII-RGD Covalent Attachment via Silanization
3.5. VW and AW Fibroblast Response to the Experimental PP Surfaces
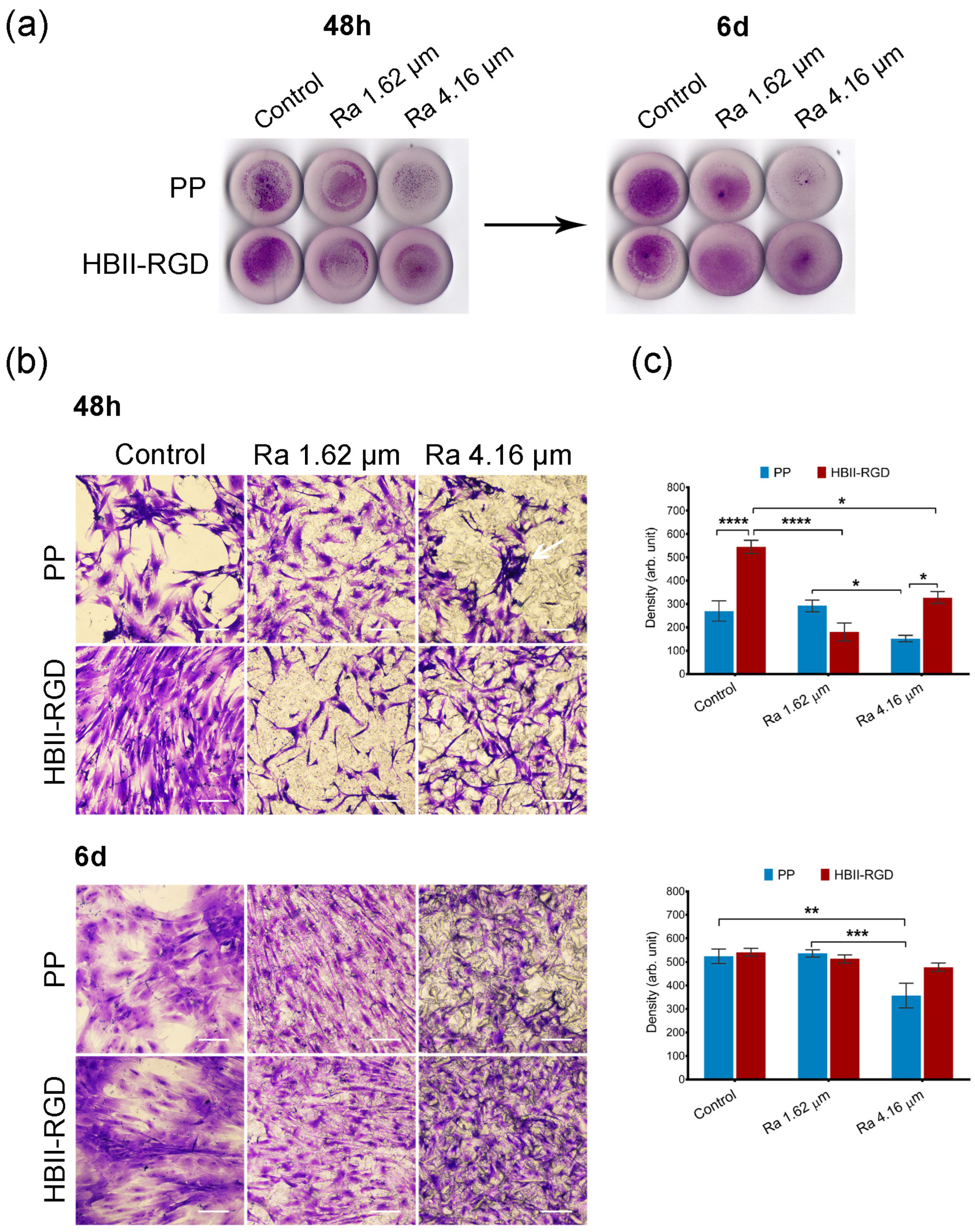
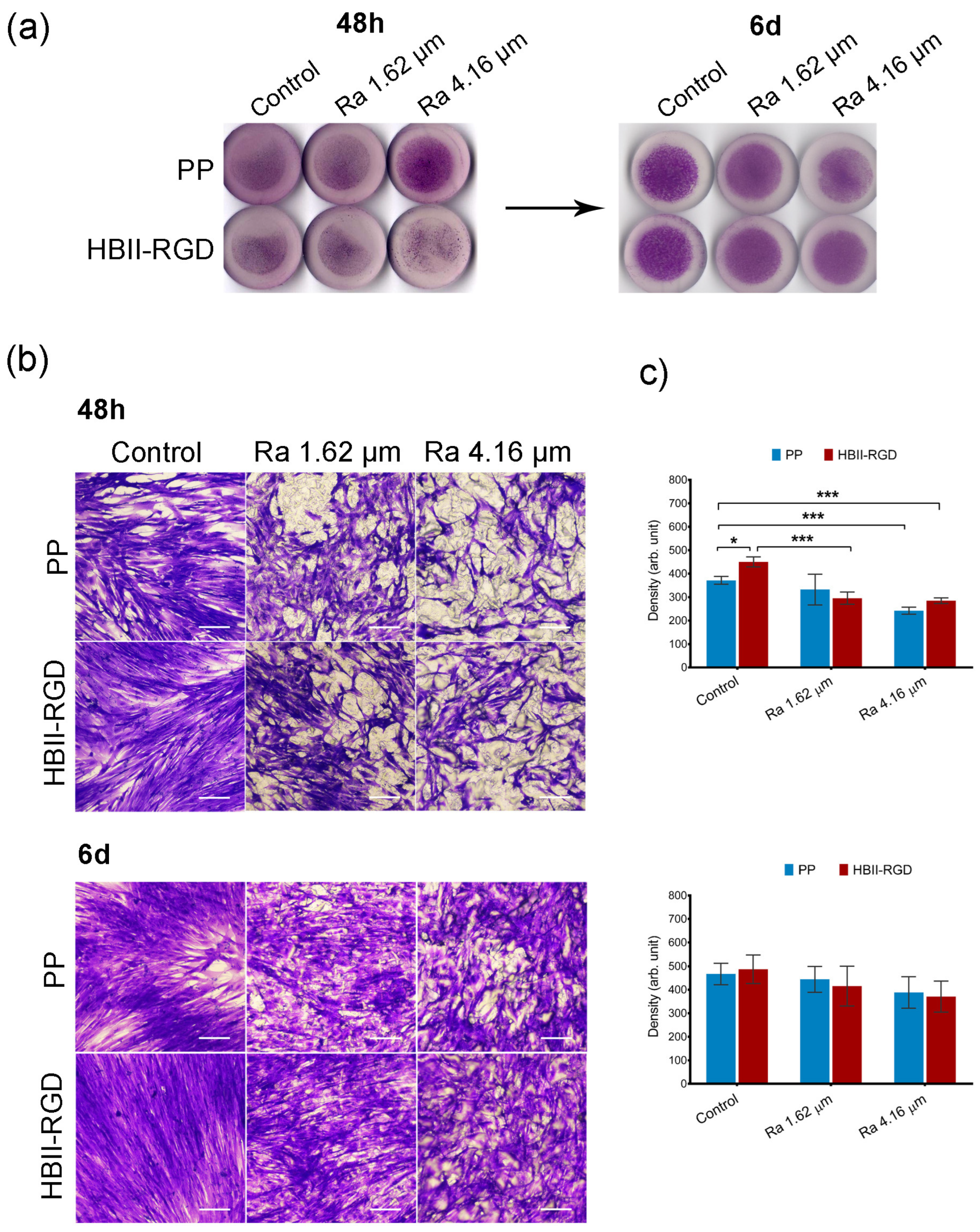
3.5.1. Fibroblast Adhesion and Viability
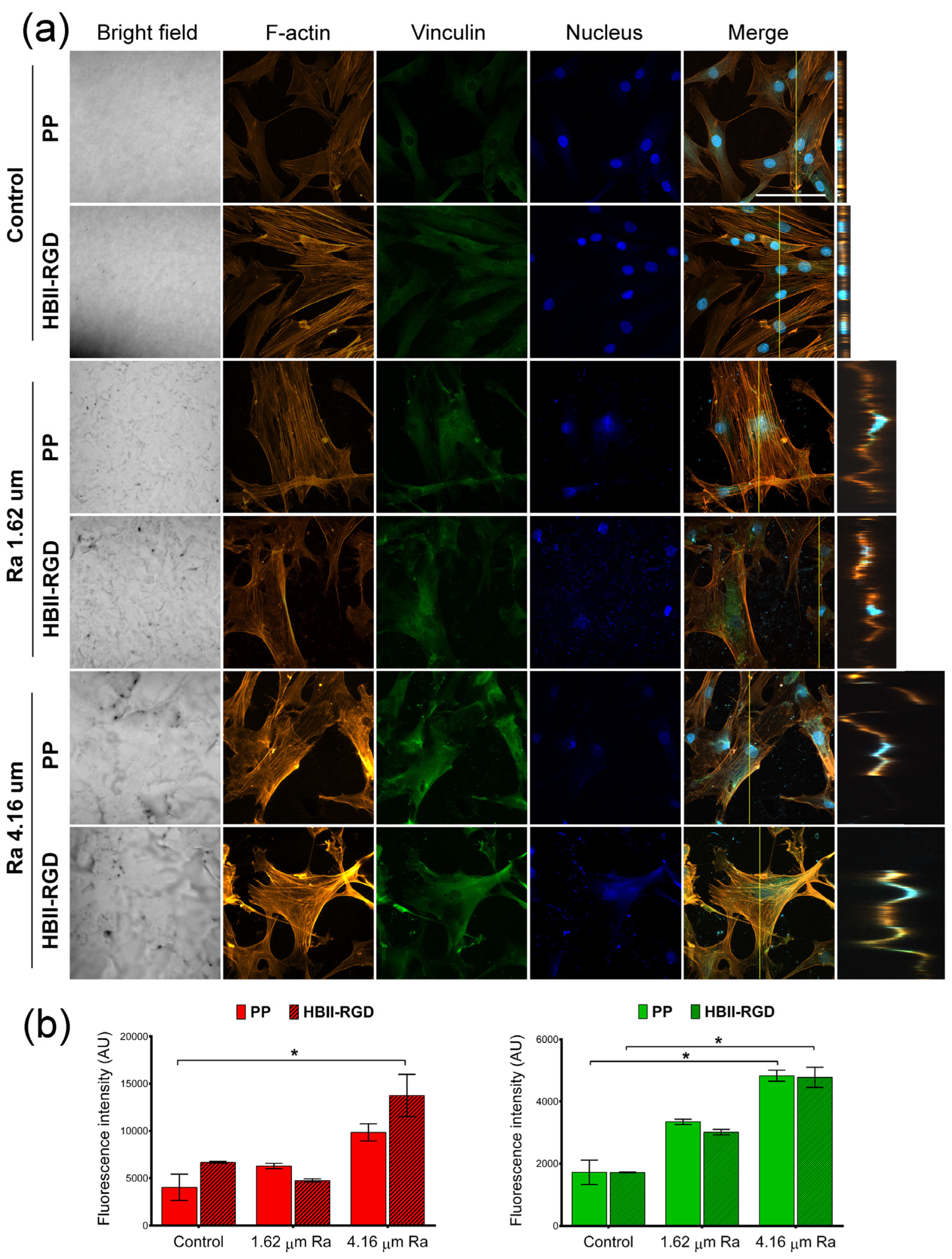
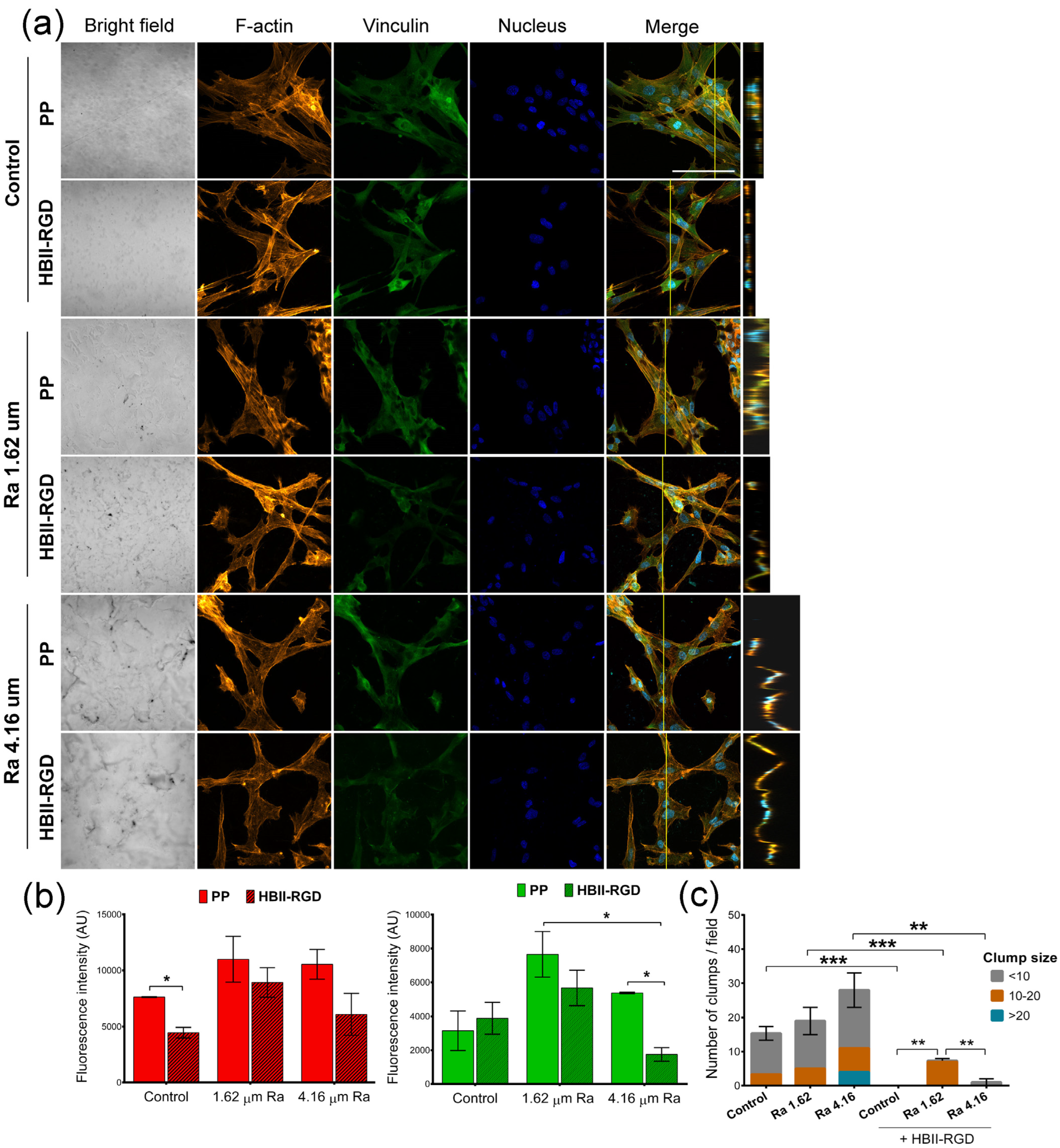
3.5.2. Fluorescent F-Actin and Vinculin Staining
3.5.3. Nuclear Morphology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kingsnorth, A.; LeBlanc, K. Hernias: Inguinal and incisional. Lancet 2003, 362, 1561–1571. [Google Scholar] [CrossRef] [PubMed]
- Barber, M.D. Pelvic organ prolapse. BMJ 2016, 354, i3853. [Google Scholar] [CrossRef] [PubMed]
- Deerenberg, E.B.; Henriksen, N.A.; Antoniou, G.A.; Antoniou, S.A.; Bramer, W.M.; Fischer, J.P.; Fortelny, R.H.; Gök, H.; Harris, H.W.; Hope, W.; et al. Updated guideline for closure of abdominal wall incisions from the European and American Hernia Societies. Br. J. Surg. 2022, 22, 1239–1250. [Google Scholar] [CrossRef]
- Brown, H.W.; Hegde, A.; Huebner, M.; Neels, H.; Barnes, H.C.; Marquini, G.V.; Mukhtarova, N.; Mbwele, B.; Tailor, V.; Kocjancic, E.; et al. International urogynecology consultation chapter 1 committee 2: Epidemiology of pelvic organ prolapse: Prevalence, incidence, natural history, and service needs. Int. Urogynecol. J. 2022, 33, 173–187. [Google Scholar] [CrossRef]
- Deprest, J.A.; Cartwright, R.; Dietz, H.P.; Brito, L.G.O.; Koch, M.; Allen-Brady, K.; Manonai, J.; Weintraub, A.Y.; Chua, J.W.F.; Cuffolom, R.; et al. International Urogynecological Consultation (IUC): Pathophysiology of pelvic organ prolapse (POP). Int. Urogynecol. J. 2022, 33, 699–1710. [Google Scholar] [CrossRef]
- Franz, M.G. The biology of hernia formation. Surg. Clin. N. Am. 2008, 88, 1–15, vii. [Google Scholar] [CrossRef]
- Kluivers, K.B.; Lince, S.L.; Ruiz-Zapata, A.M.; Post, W.M.; Cartwright, R.; Kerkhof, M.H.; Widomska, J.; De Witte, W.; Pecanka, J.; Kiemeney, L.A.; et al. Molecular landscape of pelvic organ prolapse provides insights into disease etiology. Int. J. Mol. Sci. 2023, 24, 6087. [Google Scholar] [CrossRef]
- Huber, A.; McCabe, G.P.; Boruch, A.V.; Medberry, C.; Honerlaw, M.; Badylak, S.F. Polypropylene-containing synthetic mesh devices in soft tissue repair: A meta-analysis. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 145–154. [Google Scholar] [CrossRef]
- Köckerling, F.; Hoffmann, H.; Mayer, F.; Zarras, K.; Reinpold, W.; Fortelny, R.; Weyhe, D.; Lammers, B.; Adolf, D.; Schug-Pass, C. What are the trends in incisional hernia repair? Real-world data over 10 years from the Herniamed registry. Hernia 2021, 25, 255–265. [Google Scholar] [CrossRef]
- Maher, C.; Feiner, B.; Baessler, K.; Schmid, C. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst. Rev. 2013, 30, CD004014. [Google Scholar] [CrossRef]
- Aiolfi, C.; Cavalli, M.; Gambero, F.; Mini, E.; Lombardo, F.; Gordini, L.; Bonitta, G.; Bruni, P.G.; Bona, D.; Campanelli, G. Prophylactic mesh reinforcement for midline incisional hernia prevention: Systematic review and updated meta-analysis of randomized controlled trials. Hernia 2023, 27, 213–224. [Google Scholar] [CrossRef]
- Valverde, S.; Arbós, M.A.; Quiles, M.T.; Espín, E.; Sánchez-Garcia, J.L.; Rodrigues, V.; Pereira, J.A.; Villalobos, R.; García-Alamino, J.M.; Armengol, M.; et al. Use of a bioabsorbable mesh in midline laparotomy closure to prevent incisional hernia: Randomized controlled trial. Hernia 2022, 26, 1231–1239. [Google Scholar] [CrossRef]
- Heymann, F.; von Trotha, K.T.; Preisinger, C.; Lynen-Jansen, P.; Roeth, A.A.; Geiger, M.; Geisler, L.J.; Frank, A.K.; Conze, J.; Luedde, T.; et al. Polypropylene mesh implantation for hernia repair causes myeloid cell-driven persistent inflammation. JCI Insight 2019, 24, e123862. [Google Scholar] [CrossRef]
- Knight, K.M.; King, G.E.; Palcsey, S.L.; Suda, A.; Liang, R.; Moalli, P.A. Mesh deformation: A mechanism underlying polypropylene prolapse mesh complications in vivo. Acta Biomater. 2022, 148, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Abhari, R.E.; Izett-Kay, M.L.; Morris, H.L.; Cartwright, R.; Snelling, S.J.B. Host-biomaterial interactions in mesh complications after pelvic floor reconstructive surgery. Nat. Rev. Urol. 2021, 18, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Freedman, B.R.; Mooney, D.J. Biomaterials to mimic and heal connective tissues. Adv. Mater. 2019, 31, e1806695. [Google Scholar] [CrossRef] [PubMed]
- Saha, T.; Wang, X.; Padhye, R.; Houshyar, S. A review of recent developments of polypropylene surgical mesh for hernia repair. OpenNano 2022, 7, 100046. [Google Scholar] [CrossRef]
- Vashaghian, M.; Ruiz-Zapata, A.M.; Kerkhof, M.H.; Zandieh-Doulabi, B.; Werner, A.; Roovers, J.P.; Smit, T.H. Toward a new generation of pelvic floor implants with electrospun nanofibrous matrices: A feasibility study. Neurourol. Urodyn. 2017, 36, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. The plasticity of biocompatibility. Biomaterials 2023, 296, 22077. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. Biocompatibility pathways and mechanisms for bioactive materials: The bioactivity zone. Bioact. Mater. 2022, 10, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Polak, J.M. Third-generation biomedical materials. Science 2002, 295, 1014–1017. [Google Scholar] [CrossRef]
- Rhodes, A.D.Y.; Duran-Mota, J.A.; Oliva, N. Current progress in bionanomaterials to modulate the epigenome. Biomater. Sci. 2022, 10, 5081–5091. [Google Scholar] [CrossRef] [PubMed]
- Şeker, Ş.; Elçin, A.E.; Elçin, Y.M. Advances in regenerative medicine and biomaterials. Methods Mol. Biol. 2023, 2575, 127–152. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Q.; Wang, Z.; Zhou, W.; Zhang, L.; Liu, Y.; Xu, Z.; Li, Z.; Zhu, C.; Zhang, X. Bone regeneration materials and their application over 20 years: A bibliometric study and systematic review. Front. Bioeng. Biotechnol. 2022, 10, 921092. [Google Scholar] [CrossRef]
- Mazzoni, E.; Iaquinta, M.R.; Lanzillotti, C.; Mazziotta, C.; Maritati, M.; Montesi, M.; Sprio, S.; Tampieri, A.; Tognon, M.; Martini, F. Bioactive Materials for Soft Tissue Repair. Front. Bioeng. Biotechnol. 2021, 19, 613787. [Google Scholar] [CrossRef] [PubMed]
- Aguado, B.A.; Grim, J.C.; Rosales, A.M.; Watson-Capps, J.J.; Anseth, K.S. Engineering precision biomaterials for personalized medicine. Sci. Transl. Med. 2018, 10, eaam8645. [Google Scholar] [CrossRef]
- Oliva, N.; Unterman, S.; Zhang, Y.; Conde, J.; Song, H.S.; Artzi, N. Personalizing biomaterials for precision nanomedicine considering the local tissue microenvironment. Adv. Healthc. Mater. 2015, 4, 1584–1599. [Google Scholar] [CrossRef] [PubMed]
- Guillen-Marti, J.; Diaz, R.; Quiles, M.T.; Lopez-Cano, M.; Vilallonga, R.; Huguet, P.; Ramon-y-Cajal, S.; Sanchez-Niubo, A.; Reventós, J.; Armengol, M.; et al. MMPs/TIMPs and inflammatory signalling de-regulation in human incisional hernia tissues. J. Cell. Mol. Med. 2009, 13, 4432–4443. [Google Scholar] [CrossRef]
- Henriksen, N.A.; Mortensen, J.H.; Lorentzen, L.; Ågren, M.S.; Bay-Jensen, A.C.; Jorgensen, L.N.; Karsdal, M.A. Abdominal wall hernias-A local manifestation of systemically impaired quality of the extracellular matrix. Surgery 2016, 160, 220–227. [Google Scholar] [CrossRef]
- Diaz, R.; Quiles, M.T.; Guillem-Marti, J.; Lopez-Cano, M.; Huguet, P.; Ramon-Y-Cajal, S.; Reventos, J.; Armengol, M.; Arbos, M.A. Apoptosis-like cell death induction and aberrant fibroblast properties in human incisional hernia fascia. Am. J. Pathol. 2011, 178, 2641–2653. [Google Scholar] [CrossRef]
- Quiles, M.; Sabadell, J.; Rodriguez-Contreras, A.; Manero, J.; Salicru, S.; Montero, A.; Poza, J.; Gil-Moreno, A.; Armengol, M.; Arbós, M. Differential interaction between vaginal fibroblasts and polypropylene from prolapse and healthy controls and the effect of material’s surface modifications. Continence 2022, 2, 100244. [Google Scholar] [CrossRef]
- Guler, Z.; Roovers, J.P. Role of fibroblasts and myofibroblasts on the pathogenesis and treatment of pelvic organ prolapse. Biomolecules 2022, 12, 94. [Google Scholar] [CrossRef]
- Najm, A.; Niculescu, A.G.; Gaspar, B.S.; Grumezescu, A.M.; Beuran, M. A review of abdominal meshes for hernia repair-current status and emerging solutions. Materials 2023, 16, 7124. [Google Scholar] [CrossRef]
- Saiding, Q.; Chen, Y.; Wang, J.; Pereira, C.L.; Sarmento, B.; Cui, W.; Chen, X. Abdominal wall hernia repair: From prosthetic meshes to smart materials. Mater. Today Bio 2023, 21, 100691. [Google Scholar] [CrossRef]
- Ács, J.; Szabó, A.; Fehérvári, P.; Harnos, A.; Skribek, B.; Tenke, M.; Szarvas, T.; Nyirády, P.; Ács, N.; Hegyi, P.; et al. Safety and efficacy of vaginal implants in pelvic organ prolapse surgery: A meta-analysis of 161 536 patients. Eur. Urol. Focus. 2023; in press. [Google Scholar] [CrossRef] [PubMed]
- Iturriaga, L.; Van Gordon, K.D.; Larrañaga-Jaurrieta, G.; Camarero-Espinosa, S. Strategies to introduce topographical and structural cues in 3d-printed scaffolds and implications in tissue regeneration. Adv. NanoBiomed Res. 2021, 1, 2100068. [Google Scholar] [CrossRef]
- Ermis, M.; Antmen, E.; Hasirci, V. Micro and nanofabrication methods to control cell-substrate interactions and cell behavior: A review from the tissue engineering perspective. Bioact. Mater. 2018, 3, 355–369. [Google Scholar] [CrossRef]
- Ferrari, F.; Cirisano, F.; Morán, M.C. Mammalian Cell Behavior on Hydrophobic Substrates: Influence of Surface Properties. Colloids Interfaces 2019, 3, 48. [Google Scholar] [CrossRef]
- Labay, C.; Canal, J.M.; Modic, M.; Cvelbar, U.; Quiles, M.; Armengol, M.; Arbos, M.A.; Gil, F.J.; Canal, C. Antibiotic-loaded polypropylene surgical meshes with suitable biological behaviour by plasma functionalization and polymerization. Biomaterials 2015, 71, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.; Park, C.H. Contribution of surface energy and roughness to the wettability of polyamide 6 and polypropylene film in the plasma-induced process. Text. Res. J. 2016, 86, 461–471. [Google Scholar] [CrossRef]
- Wang, L.; Carrier, R.L. Biomimetic Topography: Bioinspired Cell Culture Substrates and Scaffolds. Adv. Biomimetics. 2011, 21, 454–472. [Google Scholar] [CrossRef]
- Herranz-Diez, C.; Mas-Moruno, C.; Neubauer, S.; Kessler, H.; Gil, F.J.; Pegueroles, M.; Manero, J.M.; Guillem-Marti, J. Tuning Mesenchymal Stem Cell Response onto Titanium-Niobium-Hafnium Alloy by Recombinant Fibronectin Fragments. ACS Appl. Mater. Interfaces 2016, 8, 2517–2525. [Google Scholar] [CrossRef] [PubMed]
- Guillem-Marti, J.; Gelabert, M.; Heras-Parets, A.; Pegueroles, M.; Ginebra, M.P.; Manero, J.M. RGD Mutation of the Heparin Binding II Fragment of Fibronectin for Guiding Mesenchymal Stem Cell Behavior on Titanium Surfaces. ACS Appl. Mater. Interfaces 2019, 11, 3666–3678. [Google Scholar] [CrossRef] [PubMed]
- Heras-Parets, A.; Ginebra, M.P.; Manero, J.M.; Guillem-Marti, J. Guiding fibroblast activation using an RGD-mutated heparin binding II fragment of fibronectin for gingival titanium integration. Adv. Healthc. Mater. 2023, 12, e2203307. [Google Scholar] [CrossRef] [PubMed]
- FEPA-Standard 42-1:2006; Grains of Fused Aluminium Oxide, Silicon Carbide and Other Abrasive Materials for Bonded Abrasives and for General Applications Macrogrits F 4 to F 220. Federation of European Producers of Abrasives (FEPA). FEPA Federation of European Producers of Abrasives: Katsushika City, Tokyo, Japan, 2006.
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb. Protoc. 2016, 4, 343–346. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. Res. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Ingber, D.; Wang, N.; Stamenović, D. Tensegrity, cellular biophysics, and the mechanics of living systems. Rep. Prog. Phys. 2014, 77, 046603. [Google Scholar] [CrossRef]
- Sun, Z.; Guo, S.; Fässler, R. Integrin-mediated mechanotransduction. J. Cell Biol. 2016, 215, 445–456. [Google Scholar] [CrossRef]
- Mertgen, A.S.; Trossmann, V.T.; Guex, A.G.; Maniura-Weber, K.; Scheibel, T.; Rottmar, M. Multifunctional Biomaterials: Combining Material Modification Strategies for Engineering of Cell-Contacting Surfaces. ACS Appl. Mater. Interfaces. 2020, 12, 21342. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, M.R.; Blanes, V.; Blanes, M.; Garcia, D.; Balart, R. Surface modification of low density polyethylene (LDPE) film by low pressure O2 plasma treatment. Eur. Polym. J. 2006, 42, 1558–1568. [Google Scholar] [CrossRef]
- Rasilainen, T.; Kirveslahti, A.; Nevalainen, P.; Pakkanen, T. Controlling the movement of water droplets with micro- and hierarchical micro/nanostructures. Surf. Rev. Lett. 2011, 18, 209–222. [Google Scholar] [CrossRef]
- Rasilainen, T.; Kirveslahti, A.; Nevalainen, P.; Suvanto, M.; Pakkanen, T. Modification of polypropylene surfaces with micropits and hierarchical micropits/nanodepressions. Surf. Sci. 2010, 604, 2036–2042. [Google Scholar] [CrossRef]
- Rasilainen, T.; Suvanto, M.; Pakanen, T. Anisotropically microstructured and micro/nanostructured polypropylene surfaces. Surf. Sci. 2009, 603, 2240–2247. [Google Scholar] [CrossRef]
- Majhy, B.; Priyadarshini, P.; Sen, A.K. Effect of surface energy and roughness on cell adhesion and growth—Facile surface modification for enhanced cell culture. RSC Adv. 2021, 11, 15467–15476. [Google Scholar] [CrossRef] [PubMed]
- Koufaki, N.; Ranella, A.; Aifantis, K.E.; Barberoglou, M.; Psycharakis, S.; Fotakis, C.; Stratakis, E. Controlling cell adhesion via replication of laser micro/nano-textured surfaces on polymers. Biofabrication 2011, 3, 045004. [Google Scholar] [CrossRef]
- Janiszewska, N.; Orzechowska, B.; Awsiuk, K.; Rysz, J.; Tymetska, S.; Raczkowska, J. Cell-specific response of NSIP- and IPF-derived fibroblasts to the modification of the elasticity, biological properties, and 3D architecture of the substrate. Int. J. Mol. Sci. 2022, 23, 14714. [Google Scholar] [CrossRef]
- Talbott, H.; Mascharak, S.; Griffin, M.; Wan, D.; Longaker, M. Wound healing, fibroblast heterogeneity, and fibrosis. Cell Stem Cell 2022, 29, 1161–1180. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, R.; Holmes, K. Actin Structure and Function. Annu. Rev. Biophys. 2011, 40, 169–186. [Google Scholar] [CrossRef]
- Humphries, J.; Wang, P.; Streuli, C.; Geiger, B.; Humphries, M.; Ballestrem, C. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J. Cell Biol. 2007, 179, 1043–1057. [Google Scholar] [CrossRef]
- Carthew, J.; Abdelmaksoud, H.; Hodgson-Garms, M.; Aslanoglou, S.; Ghavamian, S.; Elnathan, R.; Spatz, J.P.; Brugger, J.; Thissen, H.; Voelker, N.H.; et al. Precision surface microtopography regulates cell fate via changes to actomyosin contractility and nuclear architecture. Adv. Sci. 2021, 8, 2003186. [Google Scholar] [CrossRef]
- Lee, R.; Campanello, L.; Hourwitz, M.; Alvarez, P.; Omidvar, A.; Fourkas, J.; Losert, W. Quantifying topography-guided actin dynamics across scales using optical flow. Mol. Biol. Cell. 2020, 31, 1753–1764. [Google Scholar] [CrossRef]
- Wang, X.; Gonzalez-Rodriguez, D.; Vourch, T.; Silberzan, P.; Barakat, A. Contractility-induced self-organization of smooth muscle cells: From multilayer cell sheets to dynamic three-dimensional clusters. Commun. Biol. 2023, 6, 262. [Google Scholar] [CrossRef]
- Azatov, M.; Sun, X.; Suberi, A.; Fourkas, J.; Upadhyaya, A. Topography on a subcellular scale modulates cellular adhesions and actin stress fiber dynamics in tumor associated fibroblasts. Phys. Biol. 2017, 14, 065003. [Google Scholar] [CrossRef]
- Bao, M.; Xie, J.; Piruska, A.; Huck, W. 3D microniches reveal the importance of cell size and shape. Nat. Commun. 2017, 8, 1962. [Google Scholar] [CrossRef]
- Leclech, C.; Villard, C. Cellular and Subcellular Contact Guidance on Microfabricated Substrates. Front. Bioeng. Biotechnol. 2020, 8, 551505. [Google Scholar] [CrossRef]
- Diedrich, C.; Roovers, J.P.; Smit, T.; Guler, Z. Fully absorbable poly-4-hydroxybutyrate implants exhibit more favorable cell-matrix interactions than polypropylene. Mater. Sci. Eng. C 2021, 120, 111702. [Google Scholar] [CrossRef]
- Malakpour-Permlid, A.; Buzzi, I.; Hegardt, C.; Johansson, F.; Oredsson, S. Identification of extracellular matrix proteins secreted by human dermal fibroblasts cultured in 3D electrospun scaffolds. Sci. Rep. 2021, 11, 6655. [Google Scholar] [CrossRef]
- Chakraborty, S.; Gourain, V.; Benz, M.; Scheiger, J.; Levkin, P.; Popova, A. Droplet microarrays for cell culture: Effect of surface properties and nanoliter culture volume on global transcriptomic landscape. Mater. Today Bio. 2021, 11, 100112. [Google Scholar] [CrossRef]

| Control | Plasma | Silanization | HBII-RGD | |||||
|---|---|---|---|---|---|---|---|---|
| Median | SD | Median | SD | Median | SD | Median | SD | |
| C 1s | 93.65 | 0.12 | 87.42 | 0.19 | 71.35 | 1.41 | 73.51 | 0.47 |
| N 1s | 0.45 | 0.21 | 0.88 | 0.13 | 4.66 | 0.33 | 13.07 | 0.13 |
| O 1s | 5.02 | 0.43 | 11.52 | 0.11 | 19.74 | 0.88 | 12.78 | 0.27 |
| Si 2p | 0.50 | 0.02 | 0.19 | 0.05 | 4.25 | 0.20 | 0.63 | 0.07 |
| Control | Plasma | Silanization | HBII-RGD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median | SD | Median | SD | Median | SD | Median | SD | ||
| C 1s | C-C, C-H | 90.94 | 0.00 | 81.70 | 0.12 | 73.80 | 0.04 | 62.99 | 0.39 |
| C-O | 6.46 | 0.04 | 9.88 | 0.01 | 17.28 | 0.00 | 22.49 | 0.17 | |
| C=O | 1.75 | 0.04 | 4.55 | 0.00 | 5.88 | 0.08 | 14.52 | 0.22 | |
| N-C=O, O-C=O | 0.71 | 0.12 | 3.87 | 0.01 | 3.05 | 0.00 | NP | NP | |
| N 1s | (NH4)/(NR4) | 0.00 | 0.00 | 57.53 | 3.43 | 55.96 | 3.81 | 1.41 | 0.31 |
| N-(C=O)- | 100.00 | 0.00 | 42.47 | 3.43 | 44.04 | 3.81 | 98.59 | 0.31 | |
| O 1s | O-C, O-H | 48.10 | 0.07 | 70.61 | 1.68 | 63.40 | 1.66 | 28.56 | 0.09 |
| C=O | 3.16 | 0.06 | 21.65 | 0.68 | 4.56 | 0.58 | 2.35 | 2.50 | |
| O-C=O, N-C=O | 48.74 | 0.01 | 7.74 | 1.01 | 32.04 | 2.23 | 69.10 | 2.41 | |
| Si 2p | Si-O | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 |
| Control-Smooth Ti | Ra Low | Ra High | ||||||
|---|---|---|---|---|---|---|---|---|
| Position | Median | SD | Median | SD | Median | SD | ||
| C 1s | C-C, C-H | 284.79 | 95.31 | 0.08 | 73.64 | 4.41 | 68.30 | 21.20 |
| C-O | 286.31 | 4.69 | 0.08 | 18.25 | 0.33 | 19.78 | 12.90 | |
| C=O | 287.80 | ND | ND | 17.24 | 8.49 | 17.78 | 0.00 | |
| N-C=O, O-C=O | 289.19 | ND | ND | ND | ND | 6.04 | 0.00 | |
| N 1s | (NH4)/(NR4) | 402.05 | ND | ND | ND | ND | ND | ND |
| N-(C=O)- | 400.34 | ND | ND | 100 | 0 | 100 | 0 | |
| O 1S | O-C, O-H | 532.34 | 13.76 | 2.71 | 75.47 | 1.53 | 73.30 | 1.04 |
| O-C=O, N-C=O | 531.36 | 86.24 | 2.71 | 24.53 | 1.53 | 26.70 | 1.04 | |
| Si 1s | Si-O | 103.32 | 100 | 0 | 100 | 0 | 100 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quiles, M.T.; Rodríguez-Contreras, A.; Guillem-Marti, J.; Punset, M.; Sánchez-Soto, M.; López-Cano, M.; Sabadell, J.; Velasco, J.; Armengol, M.; Manero, J.M.; et al. Effect of Functionalization of Texturized Polypropylene Surface by Silanization and HBII-RGD Attachment on Response of Primary Abdominal and Vaginal Fibroblasts. Polymers 2024, 16, 667. https://doi.org/10.3390/polym16050667
Quiles MT, Rodríguez-Contreras A, Guillem-Marti J, Punset M, Sánchez-Soto M, López-Cano M, Sabadell J, Velasco J, Armengol M, Manero JM, et al. Effect of Functionalization of Texturized Polypropylene Surface by Silanization and HBII-RGD Attachment on Response of Primary Abdominal and Vaginal Fibroblasts. Polymers. 2024; 16(5):667. https://doi.org/10.3390/polym16050667
Chicago/Turabian StyleQuiles, Maria Teresa, Alejandra Rodríguez-Contreras, Jordi Guillem-Marti, Miquel Punset, Miguel Sánchez-Soto, Manuel López-Cano, Jordi Sabadell, Janice Velasco, Manuel Armengol, Jose Maria Manero, and et al. 2024. "Effect of Functionalization of Texturized Polypropylene Surface by Silanization and HBII-RGD Attachment on Response of Primary Abdominal and Vaginal Fibroblasts" Polymers 16, no. 5: 667. https://doi.org/10.3390/polym16050667
APA StyleQuiles, M. T., Rodríguez-Contreras, A., Guillem-Marti, J., Punset, M., Sánchez-Soto, M., López-Cano, M., Sabadell, J., Velasco, J., Armengol, M., Manero, J. M., & Arbós, M. A. (2024). Effect of Functionalization of Texturized Polypropylene Surface by Silanization and HBII-RGD Attachment on Response of Primary Abdominal and Vaginal Fibroblasts. Polymers, 16(5), 667. https://doi.org/10.3390/polym16050667








