Nanocrystalline Cellulose-Supported Iron Oxide Composite Materials for High-Performance Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experiment Details
2.1. Materials
2.2. Fabrication of Nanocrystalline Cellulose-Supported Iron Oxide Composite
2.3. Characterization
2.4. Electrochemical Performance Measurement
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, J.; Ding, Y.; Ma, Z.; Tang, W.; Chen, X.; Lu, Y. Recent Progress on Nanostructured Transition Metal Oxides As Anode Materials for Lithium-Ion Batteries. J. Electron. Mater. 2022, 51, 3391–3417. [Google Scholar] [CrossRef]
- Cao, Y.; He, Y.; Gang, H.; Wu, B.; Yan, L.; Wei, D.; Wang, H. Stability Study of Transition Metal Oxide Electrode Materials. J. Power Sources 2023, 560, 232710. [Google Scholar] [CrossRef]
- Nandagudi, A.; Nagarajarao, S.H.; Santosh, M.S.; Basavaraja, B.M.; Malode, S.J.; Mascarenhas, R.J.; Shetti, N.P. Hydrothermal Synthesis of Transition Metal Oxides, Transition Metal Oxide/Carbonaceous Material Nanocomposites for Supercapacitor Applications. Mater. Today Sustain. 2022, 19, 100214. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, C.H.; Lee, H. Recent Research Trend in Conductive Polymer Binders for Silicon-Based Anodes of Lithium-Ion Batteries. J. Adhes. Interface 2023, 24, 9–16. [Google Scholar]
- Zhang, L.; Wu, H.B.; Lou, X.W. Iron-Oxide-Based Advanced Anode Materials for Lithium-Ion Batteries. Adv. Energy Mater. 2014, 4, 1300958. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, J. Iron Anode-based Aqueous Electrochemical Energy Storage Devices: Recent Advances and Future Perspectives. Interdiscip. Mater. 2022, 1, 116–139. [Google Scholar] [CrossRef]
- Zhang, G.; Shi, Y.; Wang, H.; Jiang, L.; Yu, X.; Jing, S.; Xing, S.; Tsiakaras, P. A Facile Route to Achieve Ultrafine Fe2O3 Nanorods Anchored on Graphene Oxide for Application in Lithium-Ion Battery. J. Power Sources 2019, 416, 118–124. [Google Scholar] [CrossRef]
- Li, J.; Ma, Z.; Hao, S.; Di, S.; Su, L.; Qin, X.; Shao, G. In Situ Fabrication of Hierarchical Iron Oxide Spheres@N-Doped 3D Porous Graphene Aerogel for Superior Lithium Storage. Ionics 2020, 26, 2303–2314. [Google Scholar] [CrossRef]
- Han, T.; Wei, Y.; Jin, X.; Jiu, H.; Zhang, L.; Sun, Y.; Tian, J.; Shang, R.; Hang, D.; Zhao, R. Hydrothermal Self-Assembly of α-Fe2O3 Nanorings@graphene Aerogel Composites for Enhanced Li Storage Performance. J. Mater. Sci. 2019, 54, 7119–7130. [Google Scholar] [CrossRef]
- Li, L.; Zhou, G.; Weng, Z.; Shan, X.Y.; Li, F.; Cheng, H.M. Monolithic Fe2O3/Graphene Hybrid for Highly Efficient Lithium Storage and Arsenic Removal. Carbon 2014, 67, 500–507. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, H.B.; Xu, J.; Wu, X.; Yang, D.; Qu, J.; Yu, Z.Z. Highly Efficient High-Pressure Homogenization Approach for Scalable Production of High-Quality Graphene Sheets and Sandwich-Structured α-Fe2O3/Graphene Hybrids for High-Performance Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 11025–11034. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, A.Q.; Zhang, H.; Ding, G.Q.; Zhang, L. Sen A Facile Route to Achieve Fe2O3 Hollow Sphere Anchored on Carbon Nanotube for Application in Lithium-Ion Battery. Inorg. Chem. Commun. 2020, 111, 107633. [Google Scholar] [CrossRef]
- Sheng, S.; Gao, H.; Liu, X.; Wang, Z.; Zhao, Y. Rational Fabrication and Improved Lithium Ion Battery Performances of Carbon Nanofibers Incorporated with α-Fe2O3 Hollow Nanoballs. Surfaces and Interfaces 2020, 21, 100612. [Google Scholar] [CrossRef]
- Cui, X.; Zhu, Y.; Li, F.; Liu, D.; Chen, J.; Zhang, Y.; Zhang, L.L.; Ji, J. Enhanced Rate Capability of a Lithium Ion Battery Anode Based on Liquid-Solid-Solution Assembly of Fe2O3 on Crumpled Graphene. RSC Adv. 2016, 6, 9007–9012. [Google Scholar] [CrossRef]
- Liu, H.; Luo, S.H.; Yan, S.X.; Wang, Q.; Hu, D.B.; Wang, Y.L.; Feng, J.; Yi, T.F. High-Performance α-Fe 2 O 3 /C Composite Anodes for Lithium-Ion Batteries Synthesized by Hydrothermal Carbonization Glucose Method Used Pickled Iron Oxide Red as Raw Material. Compos. B Eng. 2019, 164, 576–582. [Google Scholar] [CrossRef]
- Zhang, Z.; Liang, J.; Zhang, X.; Yang, W.; Dong, X.; Jung, Y. Dominant Pseudocapacitive Lithium Storage in the Carbon-Coated Ferric Oxide Nanoparticles (Fe2O3@C) towards Anode Materials for Lithium-Ion Batteries. Int. J. Hydrogen Energy 2020, 45, 8186–8197. [Google Scholar] [CrossRef]
- Wang, J.; Ma, C.; Tang, J.; Yang, X.; Wang, Y.; Jia, X.; Cai, W.; Qiao, W.; Ling, L. Facile Fabrication of Fe2O3-Decorated Carbon Matrixes with a Multidimensional Structure as Anodes for Lithium-Ion Batteries. Energy Fuels 2021, 35, 816–826. [Google Scholar] [CrossRef]
- Liu, H.; Luo, S.H.; Hu, D.B.; Liu, X.; Wang, Q.; Wang, Z.Y.; Wang, Y.L.; Chang, L.J.; Liu, Y.G.; Yi, T.F.; et al. Design and Synthesis of Carbon-Coated α-Fe2O3@Fe3O4 Heterostructured as Anode Materials for Lithium Ion Batteries. Appl. Surf. Sci. 2019, 495, 143590. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Chen, Z.; Yang, K.; Zhang, Z.; Shi, Z.; Mei, T.; Qian, J.; Li, J.; Wang, X. Yolk-Double Shell Fe3O4@C@C Composite as High-Performance Anode Materials for Lithium-Ion Batteries. J. Alloys Compd. 2020, 822, 153656. [Google Scholar] [CrossRef]
- Liu, R.; Liu, H.; Yang, Q.; Ma, Y.; Dong, D.; Wang, J. Longan-Derived Biomass Carbon-Induced Cubic-Type Ferric Oxide Nanoparticles for Efficient Lithium-Ion Battery Anodes. Energy Fuels 2023, 37, 16979–16987. [Google Scholar] [CrossRef]
- Zhu, W.; Kierzek, K.; Wang, S.; Li, S.; Holze, R.; Chen, X. Improved Performance in Lithium Ion Battery of CNT-Fe3O4@graphene Induced by Three-Dimensional Structured Construction. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 126014. [Google Scholar] [CrossRef]
- Wang, R.; Xu, C.; Sun, J.; Gao, L.; Lin, C. Flexible Free-Standing Hollow Fe3O4/Graphene Hybrid Films for Lithium-Ion Batteries. J. Mater. Chem. A Mater. 2013, 1, 1794–1800. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Li, Y.; Zhao, Y.; Tian, Y.; Kurmanbayeva, I.; Bakenov, Z. Hierarchical Sandwiched Fe3O4@C/Graphene Composite as Anode Material for Lithium-Ion Batteries. J. Electroanal. Chem. 2019, 847, 113240. [Google Scholar] [CrossRef]
- Zhong, L.; Fu, S.; Peng, X.; Zhan, H.; Sun, R. Colloidal Stability of Negatively Charged Cellulose Nanocrystalline in Aqueous Systems. Carbohydr. Polym. 2012, 90, 644–649. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Akhter, T.; Faheem, M.; Mahmood, A.; Al-Masry, W.; Nadeem, S.; Hassan, S.U.; Park, C.H. Metal-Free, Visible Light-Mediated Atom Transfer Radical Polymerization of Hydroxypropyl Cellulose-Graft-Poly(Methyl Methacrylate)s: Effect of Polymer Side Chains on Thermo-Responsive Behavior of Hydroxypropyl Cellulose. Cellulose 2023, 30, 7519–7533. [Google Scholar] [CrossRef]
- Xu, T.; Du, H.; Liu, H.; Liu, W.; Zhang, X.; Si, C.; Liu, P.; Zhang, K. Advanced Nanocellulose-Based Composites for Flexible Functional Energy Storage Devices. Adv. Mater. 2021, 33, 2101368. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hu, Y.; Shi, G.; Zhuo, H.; Ali, M.A.; Jamróz, E.; Zhang, H.; Zhong, L.; Peng, X. Advanced Flexible Materials from Nanocellulose. Adv. Funct. Mater. 2023, 33, 2214245. [Google Scholar] [CrossRef]
- Gou, J.; Liu, W.; Tang, A. A Renewable and Biodegradable Nanocellulose-Based Gel Polymer Electrolyte for Lithium-Ion Battery. J. Mater. Sci. 2020, 55, 10699–10711. [Google Scholar] [CrossRef]
- Wang, D.C.; Lei, S.N.; Zhong, S.; Xiao, X.; Guo, Q.H. Cellulose-Based Conductive Materials for Energy and Sensing Applications. Polymers 2023, 15, 4159. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, Z.; Zheng, M.; Wang, T.; Yang, J.; Yuan, F.; Lu, X.; Liu, L.; Sun, D. Amorphous Fe2O3 Nanoshells Coated on Carbonized Bacterial Cellulose Nanofibers as a Flexible Anode for High-Performance Lithium Ion Batteries. J. Power Sources 2016, 307, 649–656. [Google Scholar] [CrossRef]
- Nguyen, H.K.; Bae, J.; Hur, J.; Park, S.J.; Park, M.S.; Kim, I.T. Tailoring of Aqueous-Based Carbon Nanotube-Nanocellulose Films as Self-Standing Flexible Anodes for Lithium-Ion Storage. Nanomaterials 2019, 9, 655. [Google Scholar] [CrossRef]
- Farooq, A.; Patoary, M.K.; Zhang, M.; Mussana, H.; Li, M.; Naeem, M.A.; Mushtaq, M.; Farooq, A.; Liu, L. Cellulose from Sources to Nanocellulose and an Overview of Synthesis and Properties of Nanocellulose/Zinc Oxide Nanocomposite Materials. Int. J. Biol. Macromol. 2020, 154, 1050–1073. [Google Scholar] [CrossRef]
- Ullah, M.W.; Manan, S.; Ul-Islam, M.; Vasilyevich, R.V.; Thomas, S.; Yang, G. Introduction to Nanocellulose. In Nanocellulose: Synthesis, Structure, Properties and Applications; World Scientific Publishers: Singapore, 2021. [Google Scholar]
- Postek, M.T.; Moon, R.J.; Rudie, A.W.; Bilodeau, M.A. Production and Applications of Cellulose Nanomaterials; Tappi Press: Atlanta, GA, USA, 2013; pp. 1–28. [Google Scholar]
- Lam, E.; Hemraz, U.D. Preparation and Surface Functionalization of Carboxylated Cellulose Nanocrystals. Nanomaterials 2021, 11, 1641. [Google Scholar] [CrossRef]
- Buqa, H.; Holzapfel, M.; Krumeich, F.; Veit, C.; Novák, P. Study of Styrene Butadiene Rubber and Sodium Methyl Cellulose as Binder for Negative Electrodes in Lithium-Ion Batteries. J. Power Sources 2006, 161, 617–622. [Google Scholar] [CrossRef]
- Wan, Y.; Yang, Z.; Xiong, G.; Luo, H. A General Strategy of Decorating 3D Carbon Nanofiber Aerogels Derived from Bacterial Cellulose with Nano-Fe3O4 for High-Performance Flexible and Binder-Free Lithium-Ion Battery Anodes. J. Mater. Chem. A Mater. 2015, 3, 15386–15393. [Google Scholar] [CrossRef]
- Han, W.; Xiao, Y.; Yin, J.; Gong, Y.; Tuo, X.; Cao, J. Fe3O4@carbon Nanofibers Synthesized from Cellulose Acetate and Application in Lithium-Ion Battery. Langmuir 2020, 36, 11237–11244. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Luo, Q.; Zhou, G.; Xu, X. Recent Advances on Cellulose Nanocrystals and Their Derivatives. Polymers 2021, 13, 3247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lang, J.; Liu, L.; Liu, L.; Li, H.; Gu, Y.; Yan, X.; Ding, X. Effect of Carboxylic Acid Groups on the Supercapacitive Performance of Functional Carbon Frameworks Derived from Bacterial Cellulose. Chin. Chem. Lett. 2017, 28, 2212–2218. [Google Scholar] [CrossRef]
- Shen, F.; Zhu, H.; Luo, W.; Wan, J.; Zhou, L.; Dai, J.; Zhao, B.; Han, X.; Fu, K.; Hu, L. Chemically Crushed Wood Cellulose Fiber towards High-Performance Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 23291–23296. [Google Scholar] [CrossRef]
- Lu, H.; Behm, M.; Leijonmarck, S.; Lindbergh, G.; Cornell, A. Flexible Paper Electrodes for Li-Ion Batteries Using Low Amount of TEMPO-Oxidized Cellulose Nanofibrils as Binder. ACS Appl. Mater. Interfaces 2016, 8, 18097–18106. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Mattinen, U.; Guccini, V.; Liu, H.; Salazar-Alvarez, G.; Lindström, R.W.; Lindbergh, G.; Cornell, A. Feasibility of Chemically Modified Cellulose Nanofiber Membranes as Lithium-Ion Battery Separators. ACS Appl. Mater. Interfaces 2020, 12, 41211–41222. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, B.; Yu, H.; Chen, G.Z. Synergistic Effects of 1-Octyl-3-Methylimidazolium Hexafluorophosphate and Cellulose Nanocrystals on Improving Polyacrylate Waterborne Anti-Corrosion Coatings. Polymers 2023, 15, 810. [Google Scholar] [CrossRef]
- Marcuello, C.; Foulon, L.; Chabbert, B.; Molinari, M.; Aguié-Béghin, V. Langmuir-Blodgett Procedure to Precisely Control the Coverage of Functionalized AFM Cantilevers for SMFS Measurements: Application with Cellulose Nanocrystals. Langmuir 2018, 34, 9376–9386. [Google Scholar] [CrossRef]
- Fan, J.; Xu, M.; Xu, Y.T.; Hamad, W.Y.; Meng, Z.; MacLachlan, M.J. A Visible Multi-Response Electrochemical Sensor Based on Cellulose Nanocrystals. Chem. Eng. J. 2023, 457, 141175. [Google Scholar] [CrossRef]
- Dias, O.A.T.; Konar, S.; Leão, A.L.; Yang, W.; Tjong, J.; Sain, M. Current State of Applications of Nanocellulose in Flexible Energy and Electronic Devices. Front. Chem. 2020, 8, 420. [Google Scholar] [CrossRef]
- Rhim, Y.R.; Zhang, D.; Fairbrother, D.H.; Wepasnick, K.A.; Livi, K.J.; Bodnar, R.J.; Nagle, D.C. Changes in Electrical and Microstructural Properties of Microcrystalline Cellulose as Function of Carbonization Temperature. Carbon 2010, 48, 1012–1024. [Google Scholar] [CrossRef]
- Vo, T.N.; Nguyen, T.A.; Nguyen, D.M.K.; Nguyen, M.T.; Nguyen, T.L.T.; Le, M.L.P.; Nguyen, V.H.; Tran, V.M.; Nguyen, D.Q.; Kim, I.T.; et al. Synthesis of ZnFe2O4 Spinel Nanoparticles at Varying PH Values and Application in Anode Material for Lithium-Ion Battery. Ceram. Int. 2023, 49, 38824–38834. [Google Scholar] [CrossRef]
- Jia, X.; Lian, D.; Shi, B.; Dai, R.; Li, C.; Wu, X. Facile Synthesis of α-Fe2O3@graphene Oxide Nanocomposites for Enhanced Gas-Sensing Performance to Ethanol. J. Mater. Sci. Mater. Electron. 2017, 28, 12070–12079. [Google Scholar] [CrossRef]
- Wang, R.; Xu, C.; Du, M.; Sun, J.; Gao, L.; Zhang, P.; Yao, H.; Lin, C. Solvothermal-Induced Self-Assembly of Fe2O3/GS Aerogels for High Li-Storage and Excellent Stability. Small 2014, 10, 2260–2269. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.Y.; Liu, Z.; Nam, K.W.; Borkiewicz, O.J.; Cheng, J.; Hua, X.; Dunstan, M.T.; Yu, X.; Wiaderek, K.M.; Du, L.S.; et al. Origin of Additional Capacities in Metal Oxide Lithium-Ion Battery Electrodes. Nat. Mater. 2013, 12, 1130–1136. [Google Scholar] [CrossRef]
- Luo, J.; Liu, J.; Zeng, Z.; Ng, C.F.; Ma, L.; Zhang, H.; Lin, J.; Shen, Z.; Fan, H.J. Three-Dimensional Graphene Foam Supported Fe3O4 Lithium Battery Anodes with Long Cycle Life and High Rate Capability. Nano Lett. 2013, 13, 6136–6143. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Yuan, L.; Shi, C.; Wu, C.; Qiao, H.; Wang, K. Porous Fe2O3 Nanorod-Decorated Hollow Carbon Nanofibers for High-Rate Lithium Storage. Adv. Compos. Hybrid. Mater. 2022, 5, 370–382. [Google Scholar] [CrossRef]
- Liu, L.; Yang, X.; Lv, C.; Zhu, A.; Zhu, X.; Guo, S.; Chen, C.; Yang, D. Seaweed-Derived Route to Fe2O3 Hollow Nanoparticles/N-Doped Graphene Aerogels with High Lithium Ion Storage Performance. ACS Appl. Mater. Interfaces 2016, 8, 7047–7053. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Jin, Y.; Wang, D.; Xu, Z.; Li, L.; Zou, X.; Zhang, M.; Wang, Z.; Yang, H. Fe2O3/Carbon Derived from Peanut Shell Hybrid as an Advanced Anode for High Performance Lithium Ion Batteries. J. Energy Storage 2023, 68, 107731. [Google Scholar] [CrossRef]


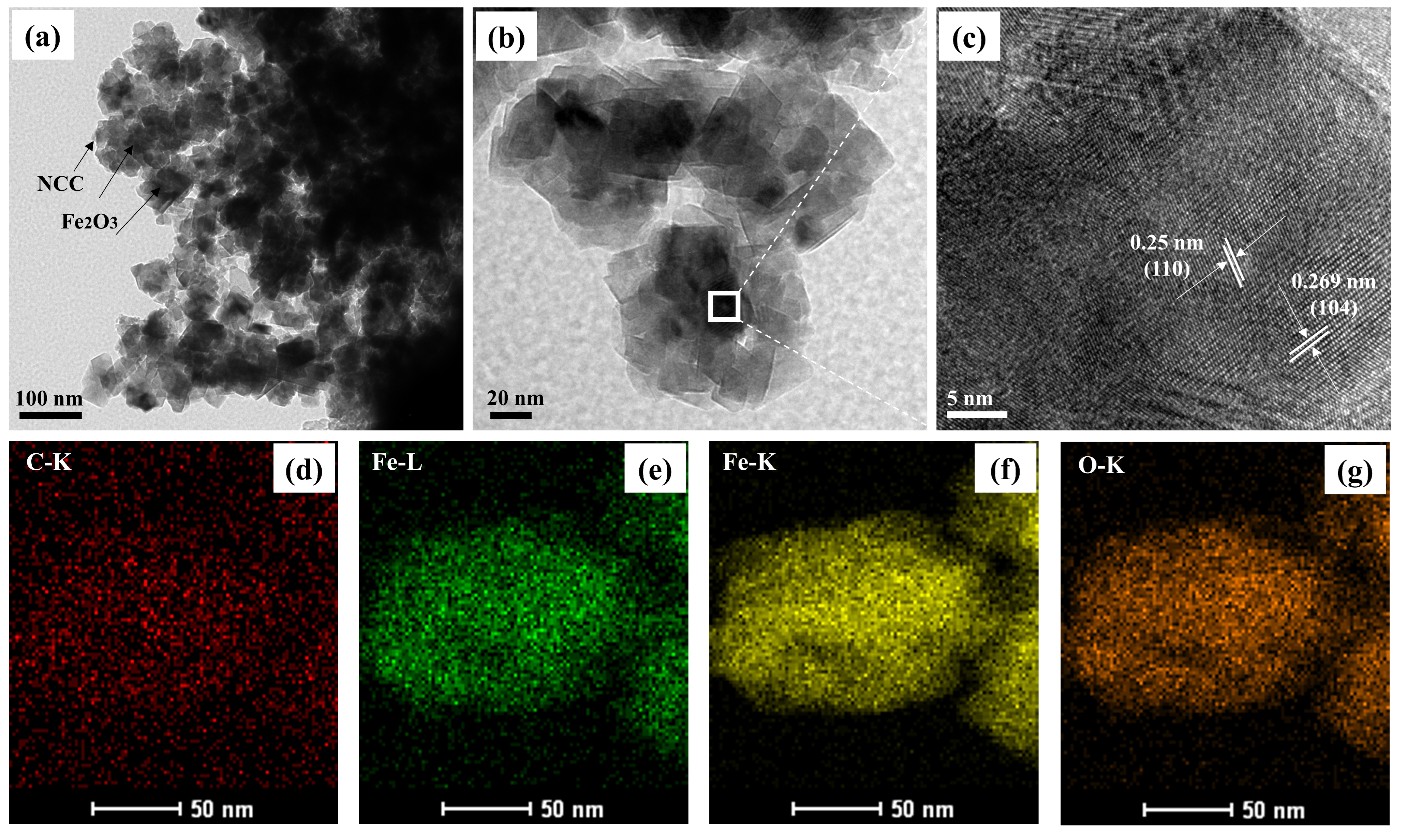
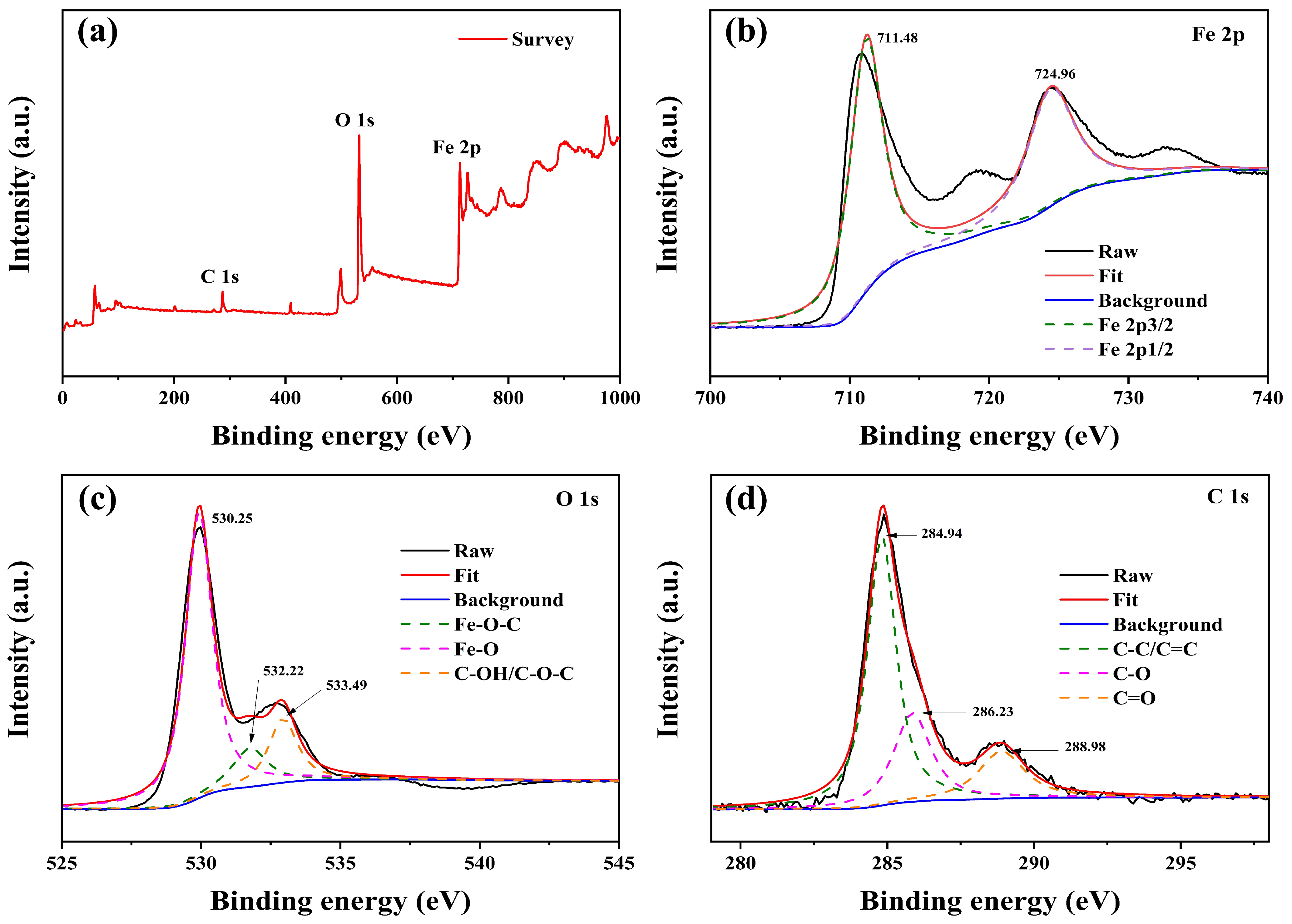
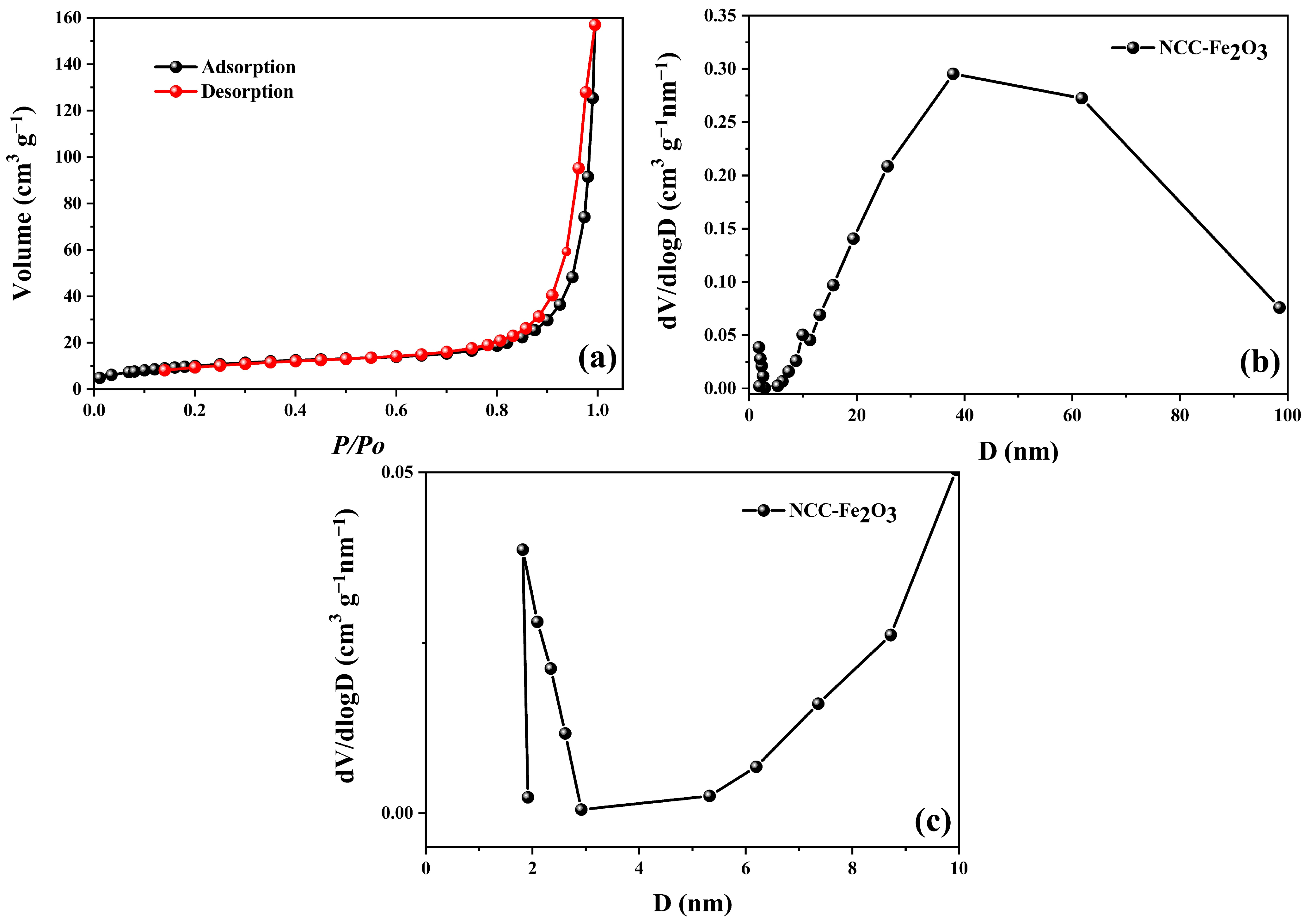


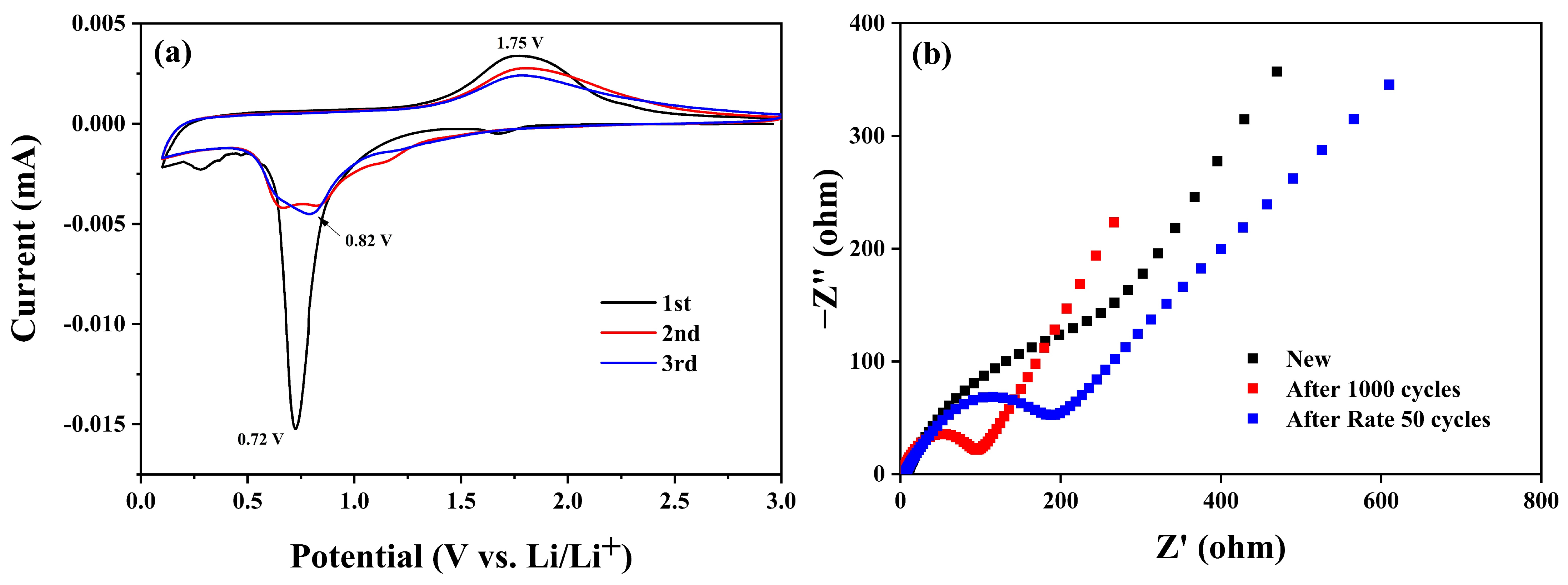
| Sample | C (atom %) | Fe (atom %) | O (atom %) | Weight Percentage (Fe/C) |
|---|---|---|---|---|
| NCC–Fe2O3 | 60.25 | 14.98 | 24.77 | 0.39 |
| Fe2O3 | 35.13 | 27.57 | 37.30 | 3.65 |
| Composite | Initial Capacity | Initial Coulombic Efficiency | Cycle Number | Remaining Capacity | Coulombic Efficiency |
|---|---|---|---|---|---|
| NCC–Fe2O3 | 1044.35 mAh g−1 | 69.00% | 1000 | 576.70 mAh g−1 | 99.77% |
| Fe2O3 | 852.35 mAh g−1 | 30.48% | 1000 | 171.82 mAh g−1 | 99.72% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, Q.N.; Park, C.H.; Le, T.H. Nanocrystalline Cellulose-Supported Iron Oxide Composite Materials for High-Performance Lithium-Ion Batteries. Polymers 2024, 16, 691. https://doi.org/10.3390/polym16050691
Tran QN, Park CH, Le TH. Nanocrystalline Cellulose-Supported Iron Oxide Composite Materials for High-Performance Lithium-Ion Batteries. Polymers. 2024; 16(5):691. https://doi.org/10.3390/polym16050691
Chicago/Turabian StyleTran, Quang Nhat, Chan Ho Park, and Thi Hoa Le. 2024. "Nanocrystalline Cellulose-Supported Iron Oxide Composite Materials for High-Performance Lithium-Ion Batteries" Polymers 16, no. 5: 691. https://doi.org/10.3390/polym16050691





