Phase Behavior of Polydisperse Y-Shaped Polymer Brushes under Good Solvent Conditions
Abstract
1. Introduction
2. Materials and Methods
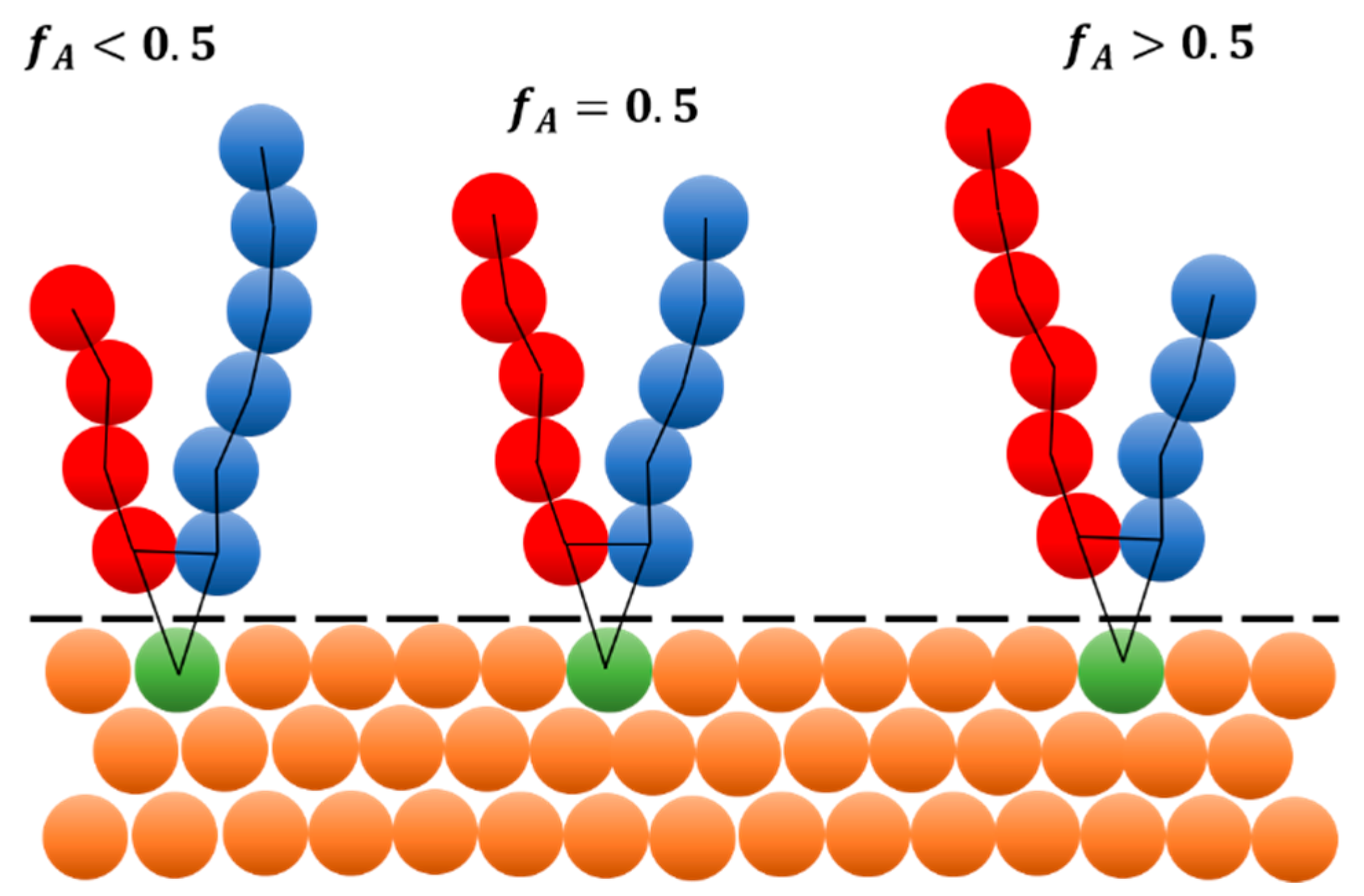
2.1. Mesoscale Model of Polydisperse Y-Shaped Brush
2.2. Mesoscale Modeling and Simulation Details
2.3. Observables
3. Results
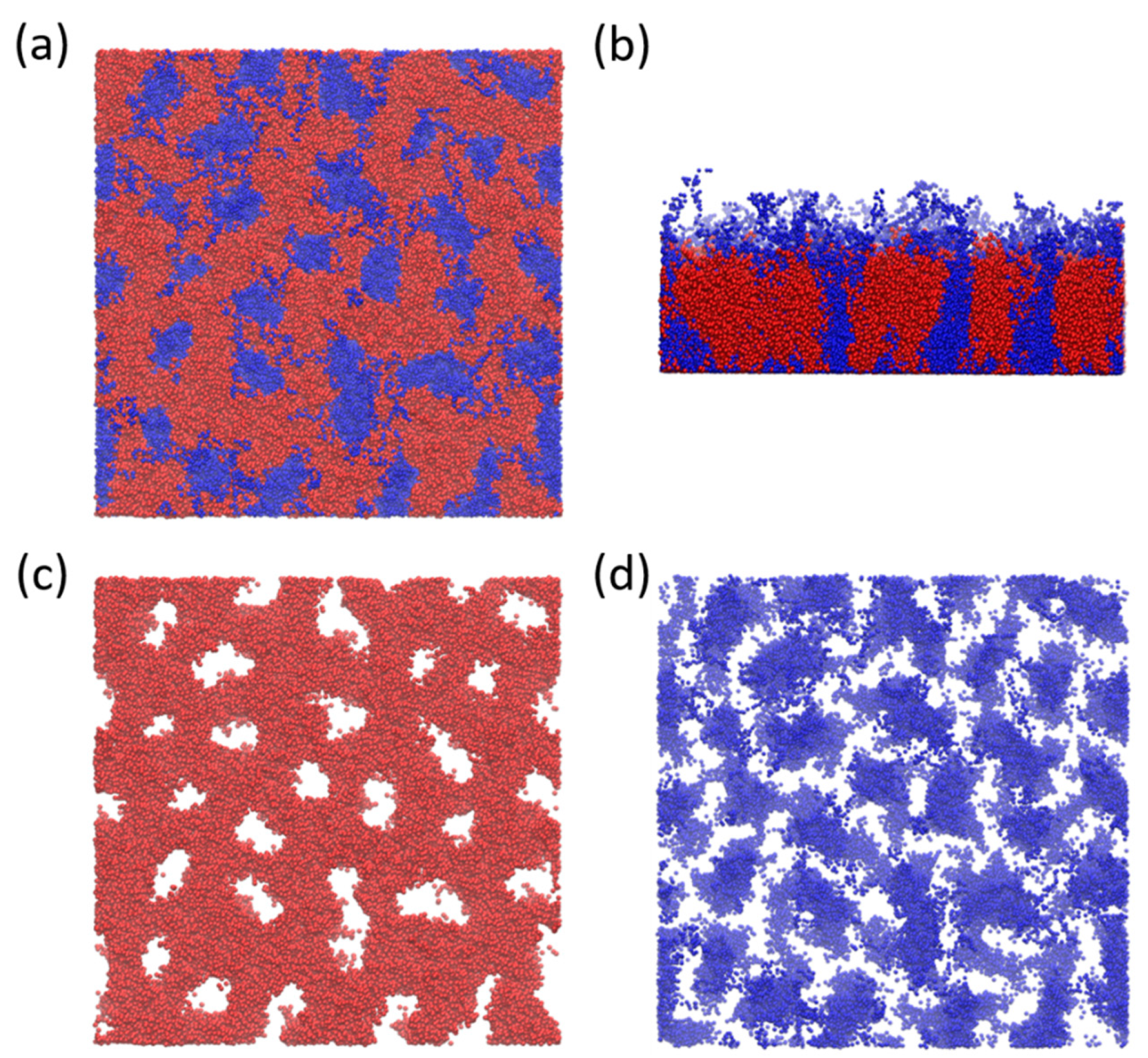
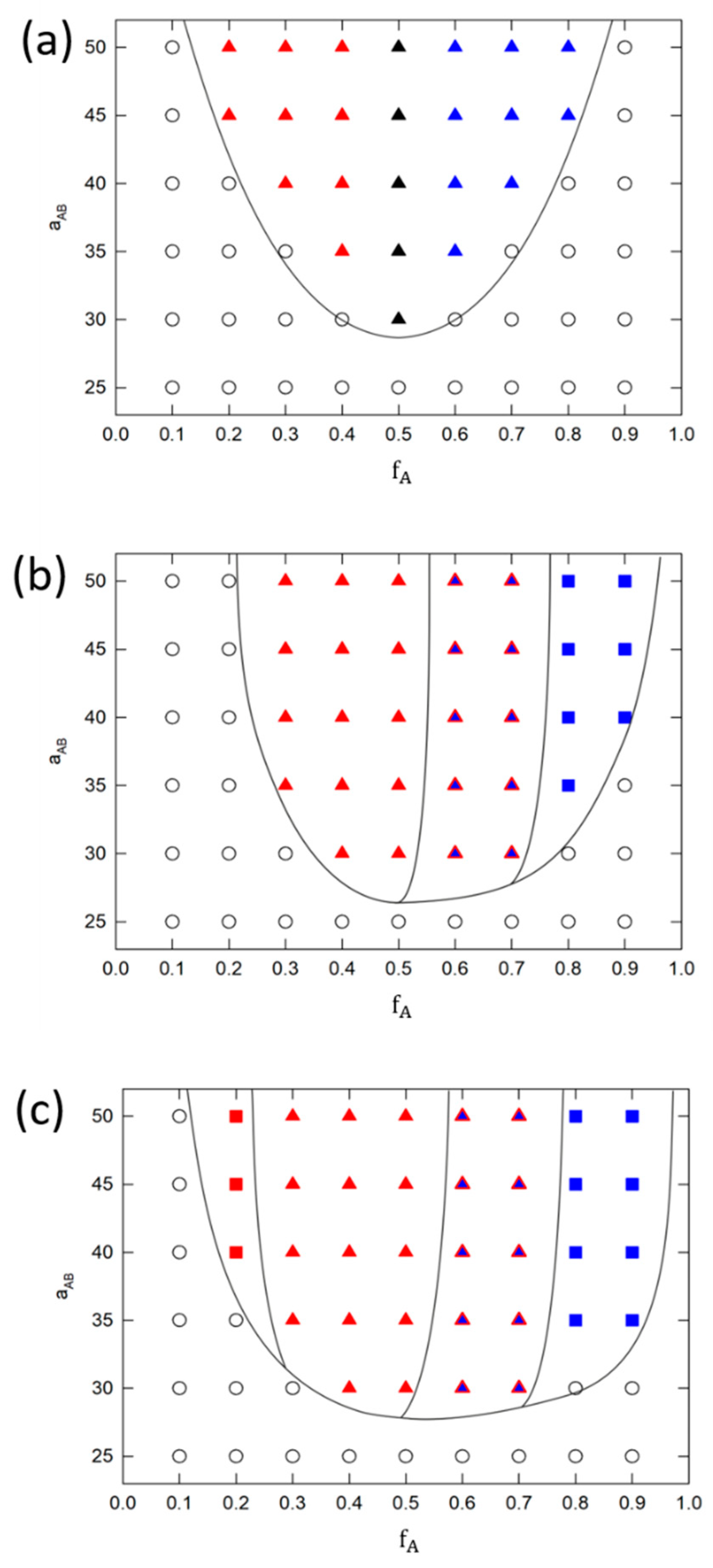
3.1. Phase Behavior of Monodisperse and Partially Polydisperse Y-Shaped Brushes
Perforated Layer
3.2. Phase Behavior of Fully Polydisperse Y-Shaped Brushes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, W.L.; Cordero, R.; Tran, H.; Ober, C.K. Polymer Brushes: Novel Surfaces for Future Materials. Macromolecules 2017, 50, 4089–4113. [Google Scholar] [CrossRef]
- Hou, W.M.; Liu, Y.Z.; Zhao, H.Y. Surface Nanostructures Based on Assemblies of Polymer Brushes. Chempluschem 2020, 85, 998–1007. [Google Scholar] [CrossRef]
- Li, M.X.; Pester, C.W. Mixed Polymer Brushes for “Smart” Surfaces. Polymers 2020, 12, 1553. [Google Scholar] [CrossRef]
- Zhao, B.; Zhu, L. Mixed Polymer Brush-Grafted Particles: A New Class of Environmentally Responsive Nanostructured Materials. Macromolecules 2009, 42, 9369–9383. [Google Scholar] [CrossRef]
- Zoppe, J.O.; Ataman, N.C.; Mocny, P.; Wang, J.; Moraes, J.; Klok, H.A. Surface-Initiated Controlled Radical Polymerization: State-of-the-Art, Opportunities, and Challenges in Surface and Interface Engineering with Polymer Brushes. Chem. Rev. 2017, 117, 4667. [Google Scholar] [CrossRef]
- Ma, S.H.; Zhang, X.Q.; Yu, B.; Zhou, F. Brushing up functional materials. NPG Asia Mater. 2019, 11, 24. [Google Scholar] [CrossRef]
- Zhulina, E.; Balazs, A.C. Designing patterned surfaces by grafting Y-shaped copolymers. Macromolecules 1996, 29, 2667–2673. [Google Scholar] [CrossRef]
- Minko, S.; Müller, M.; Usov, D.; Scholl, A.; Froeck, C.; Stamm, M. Lateral versus perpendicular segregation in mixed polymer brushes: Art. Phys. Rev. Lett. 2002, 88, 035502. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.H.; Jiang, R.; Li, B.H.; Jin, Q.H.; Ding, D.T.; Shi, A.C. Self-assembly of grafted Y-shaped triblock copolymers in solutions. J. Chem. Phys. 2008, 129, 154903. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Müller, M. Microphase Separation of Mixed Polymer Brushes: Dependence of the Morphology on Grafting Density, Composition, Chain-Length Asymmetry, Solvent Quality, and Selectivity. J. Phys. Chem. B 2009, 113, 11384–11402. [Google Scholar] [CrossRef] [PubMed]
- Dodd, P.M.; Jayaraman, A. Monte carlo simulations of polydisperse polymers grafted on spherical surfaces. J. Polym. Sci. Pol. Phys. 2012, 50, 694–705. [Google Scholar] [CrossRef]
- Jiao, G.S.; Li, Y.; Qian, H.J.; Lu, Z.Y. Computer simulation study of polydispersity effect on the phase behavior of short diblock copolymers. Polymer 2016, 96, 6–12. [Google Scholar] [CrossRef]
- Qi, S.H.; Klushin, L.I.; Skvortsov, A.M.; Schmid, F. Polydisperse Polymer Brushes: Internal Structure, Critical Behavior, and Interaction with Flow. Macromolecules 2016, 49, 9665–9683. [Google Scholar] [CrossRef]
- Klushin, L.I.; Skvortsov, A.M.; Qi, S.; Schmid, F. Polydisperse Brush with the Linear Density Profile. Polym. Sci. Ser. C+ 2018, 60, 84–94. [Google Scholar] [CrossRef]
- Zimm, B.H. Apparatus and Methods for Measurement and Interpretation of the Angular Variation of Light Scattering—Preliminary Results on Polystyrene Solutions. J. Chem. Phys. 1948, 16, 1099–1116. [Google Scholar] [CrossRef]
- Patil, R.R.; Turgman-Cohen, S.; Srogl, J.; Kiserow, D.; Genzer, J. On-Demand Degrafting and the Study of Molecular Weight and Grafting Density of Poly(methyl methacrylate) Brushes on Flat Silica Substrates. Langmuir 2015, 31, 2372–2381. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.M.; Liu, H.; Lu, Z.Y.; Sun, Z.Y.; An, L.J. The structures of thin layer formed by microphase separation of grafted Y-shaped block copolymers in solutions. J. Chem. Phys. 2013, 138, 2372–2381. [Google Scholar] [CrossRef]
- Yin, Y.H.; Jiang, R.; Wang, Z.; Li, B.H.; Shi, A.C. Influence of Grafting Point Distribution on the Surface Structures of Y-Shaped Polymer Brushes in Solution. Langmuir 2016, 32, 7467–7475. [Google Scholar] [CrossRef]
- Miliou, K.; Gergidis, L.N.; Vlahos, C. Mixed brushes consisting of oppositely charged Y-shaped polymers in salt free, monovalent, and divalent salt solutions. J. Polym. Sci. 2020, 58, 1757–1770. [Google Scholar] [CrossRef]
- Fridrich, P.; Posel, Z. Self-Assembly of Y-Shaped Polymer Brushes with Low Poly-Dispersity. Mater. Proc. 2022, 9, 26. [Google Scholar]
- Julthongpiput, D.; Lin, Y.H.; Teng, J.; Zubarev, E.R.; Tsukruk, V.V. Y-shaped polymer brushes: Nanoscale switchable surfaces. Langmuir 2003, 19, 7832–7836. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.P.; Ai, P.; Sa, Z.P.; Zhao, Y.L.; Li, M.; Wang, D.; Sha, K. Y-Shaped Diblock Copolymer with Epoxy-Based Block of Poly(glycidyl methacrylate): Synthesis, Characterization, and Its Morphology Study. J. Polym. Sci. Pol. Chem. 2009, 47, 5509–5526. [Google Scholar] [CrossRef]
- Bao, C.H.; Tang, S.D.; Horton, J.M.; Jiang, X.M.; Tang, P.; Qiu, F.; Zhu, L.; Zhao, B. Effect of Overall Grafting Density on Microphase Separation of Mixed Homopolymer Brushes Synthesized from Y-Initiator-Functionalized Silica Particles. Macromolecules 2012, 45, 8027–8036. [Google Scholar] [CrossRef]
- Bao, C.H.; Tang, S.D.; Wright, R.A.E.; Tang, P.; Qiu, F.; Zhu, L.; Zhao, B. Effect of Molecular Weight on Lateral Microphase Separation of Mixed Homopolymer Brushes Grafted on Silica Particles. Macromolecules 2014, 47, 6824–6835. [Google Scholar] [CrossRef]
- Tonhauser, C.; Golriz, A.A.; Moers, C.; Klein, R.; Butt, H.J.; Frey, H. Stimuli-Responsive Y-Shaped Polymer Brushes Based on Junction-Point-Reactive Block Copolymers. Adv. Mater. 2012, 24, 5559–5563. [Google Scholar] [CrossRef]
- Huang, J.; He, T.T.; He, X.M.; Xu, J.Q.; Zuo, B.; Wang, X.P. Fabrication of V-Shaped Brushes Consisting of Two Highly Incompatible Arms of PEG and Fluorinated PMMA and Their Protein-Resistance Performance. J. Polym. Sci. Pol. Chem. 2016, 54, 2599–2610. [Google Scholar] [CrossRef]
- Li, J.J.; Zhou, Y.N.; Luo, Z.H. Mussel-inspired V-shaped copolymer coating for intelligent oil/water separation. Chem. Eng. J. 2017, 322, 693–701. [Google Scholar] [CrossRef]
- Wei, W.; Kim, T.Y.; Balamurugan, A.; Sun, J.; Chen, R.; Ghosh, A.; Rodolakis, F.; McChesney, J.L.; Lakkham, A.; Evans, P.G.; et al. Phase Behavior of Mixed Polymer Brushes Grown from Ultrathin Coatings. ACS Macro Lett. 2019, 8, 1086–1090. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Hou, W.M.; Zhao, H.Y. Synthesis of Y-Shaped Polymer Brushes on Silica Particles and Hierarchical Surface Structures Fabricated by the Coassembly Approach. Macromolecules 2020, 53, 5001–5014. [Google Scholar] [CrossRef]
- Pivkin, I.V.; Karniadakis, G.E. A new method to impose no-slip boundary conditions in dissipative particle dynamics. J. Comput. Phys. 2005, 207, 114–128. [Google Scholar] [CrossRef]
- Español, P.; Warren, P.B. Perspective: Dissipative particle dynamics. J. Chem. Phys. 2017, 146, 150901. [Google Scholar] [CrossRef]
- Skvor, J.; Posel, Z. Simulation Aspects of Lamellar Morphology: Incommensurability Effect. Macromol. Theor. Simul. 2015, 24, 141–151. [Google Scholar] [CrossRef]
- Karatrantos, A.; Composto, R.J.; Winey, K.I.; Kröger, M.; Clarke, N. Modeling of Entangled Polymer Diffusion in Melts and Nanocomposites: A Review. Polymers 2019, 11, 876. [Google Scholar] [CrossRef] [PubMed]
- Posel, Z.; Posocco, P.; Fermeglia, M.; Lísal, M.; Pricl, S. Modeling hierarchically structured nanoparticle/diblock copolymer systems. Soft Matter 2013, 9, 2936–2946. [Google Scholar] [CrossRef]
- Posel, Z.; Posocco, P. Tuning the Properties of Nanogel Surfaces by Grafting Charged Alkylamine Brushes. Nanomaterials 2019, 9, 1514. [Google Scholar] [CrossRef] [PubMed]
- Beránek, P.; Posocco, P.; Posel, Z. Phase Behavior of Gradient Copolymer Melts with Different Gradient Strengths Revealed by Mesoscale Simulations. Polymers 2020, 12, 2462. [Google Scholar] [CrossRef] [PubMed]
- Posel, Z.; Svoboda, M.; Colina, C.M.; Lísal, M. Flow and aggregation of rod-like proteins in slit and cylindrical pores coated with polymer brushes: An insight from dissipative particle dynamics. Soft Matter 2017, 13, 1634–1645. [Google Scholar] [CrossRef] [PubMed]
- Groot, R.D.; Madden, T.J. Dynamic simulation of diblock copolymer microphase separation. J. Chem. Phys. 1998, 108, 8713–8724. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular-Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Plimpton, S.J. Accelerating dissipative particle dynamics simulations for soft matter systems. Comp. Mater. Sci. 2015, 100, 173–180. [Google Scholar] [CrossRef]
- Rehse, S.; Mecke, K.; Magerle, R. Characterization of the dynamics of block copolymer microdomains with local morphological measures. Phys. Rev. E 2008, 77, 051805. [Google Scholar] [CrossRef] [PubMed]
- Raczkowska, J.; Bernasik, A.; Budkowski, A.; Cyganik, P.; Rysz, J.; Raptis, I.; Czuba, P. Pattern guided structure formation in polymer films of asymmetric blends. Surf. Sci. 2006, 600, 1004–1011. [Google Scholar] [CrossRef]
- Schubert, E.; Sander, J.; Ester, M.; Kriegel, H.P.; Xu, X.W. DBSCAN Revisited, Revisited: Why and How You Should (Still) Use DBSCAN. ACM Trans. Database Syst. 2017, 42, 3. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Gavrilov, A.A.; Kudryavtsev, Y.V.; Chertovich, A.V. Phase diagrams of block copolymer melts by dissipative particle dynamics simulations. J. Chem. Phys. 2013, 139, 224901. [Google Scholar] [CrossRef] [PubMed]
- Posel, Z.; Rousseau, B.; Lisal, M. Scaling behaviour of different polymer models in dissipative particle dynamics of unentangled melts. Mol. Simulat. 2014, 40, 1274–1289. [Google Scholar] [CrossRef]
- Posel, Z.; Posocco, P.; Lisal, M.; Fermeglia, M.; Pricl, S. Highly grafted polystyrene/polyvinylpyridine polymer gold nanoparticles in a good solvent: Effects of chain length and composition. Soft Matter 2016, 12, 3600–3611. [Google Scholar] [CrossRef]
- Guskova, O.A.; Seidel, C. Mesoscopic Simulations of Morphological Transitions of Stimuli-Responsive Diblock Copolymer Brushes. Macromolecules 2011, 44, 671–682. [Google Scholar] [CrossRef]
- Karatrantos, A.; Clarke, N.; Kroger, M. Modeling of Polymer Structure and Conformations in Polymer Nanocomposites from Atomistic to Mesoscale: A Review. Polym. Rev. 2016, 56, 385–428. [Google Scholar] [CrossRef]


| Label | ||
|---|---|---|
| 1.00 | 1.00 | S1 * |
| 1.00 | 1.10 | S2 * |
| 1.00 | 1.50 | S3 |
| 1.00 | 2.00 | S4 |
| 1.10 | 1.10 | S5 |
| 1.10 | 1.50 | S6 |
| 1.10 | 2.00 | S7 |
| 1.50 | 1.50 | S8 |
| 1.50 | 2.00 | S9 |
| 2.00 | 2.00 | S10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fridrich, P.; Posel, Z. Phase Behavior of Polydisperse Y-Shaped Polymer Brushes under Good Solvent Conditions. Polymers 2024, 16, 721. https://doi.org/10.3390/polym16050721
Fridrich P, Posel Z. Phase Behavior of Polydisperse Y-Shaped Polymer Brushes under Good Solvent Conditions. Polymers. 2024; 16(5):721. https://doi.org/10.3390/polym16050721
Chicago/Turabian StyleFridrich, Petr, and Zbyšek Posel. 2024. "Phase Behavior of Polydisperse Y-Shaped Polymer Brushes under Good Solvent Conditions" Polymers 16, no. 5: 721. https://doi.org/10.3390/polym16050721
APA StyleFridrich, P., & Posel, Z. (2024). Phase Behavior of Polydisperse Y-Shaped Polymer Brushes under Good Solvent Conditions. Polymers, 16(5), 721. https://doi.org/10.3390/polym16050721






