Functionalization of Carbon Nanotubes in Polystyrene and Properties of Their Composites: A Review
Abstract
:1. Introduction
2. General Principles for the Interaction between CNTs and the Polymers
2.1. Types of CNT–Polymer Interactions
2.1.1. van der Waals and π Interactions
2.1.2. Hydrogen Bonding and Covalent Bonding
2.2. Influence of Functionalization of CNT on Dispersion and CNT–Polymer Interactions
2.3. Characterization Techniques on CNT–Polymer Interactions
2.3.1. Wetting
2.3.2. Spectroscopy
2.3.3. Atomic Force Microscopy—AFM
3. Functionalization of CNTs for CNT/PS Composites
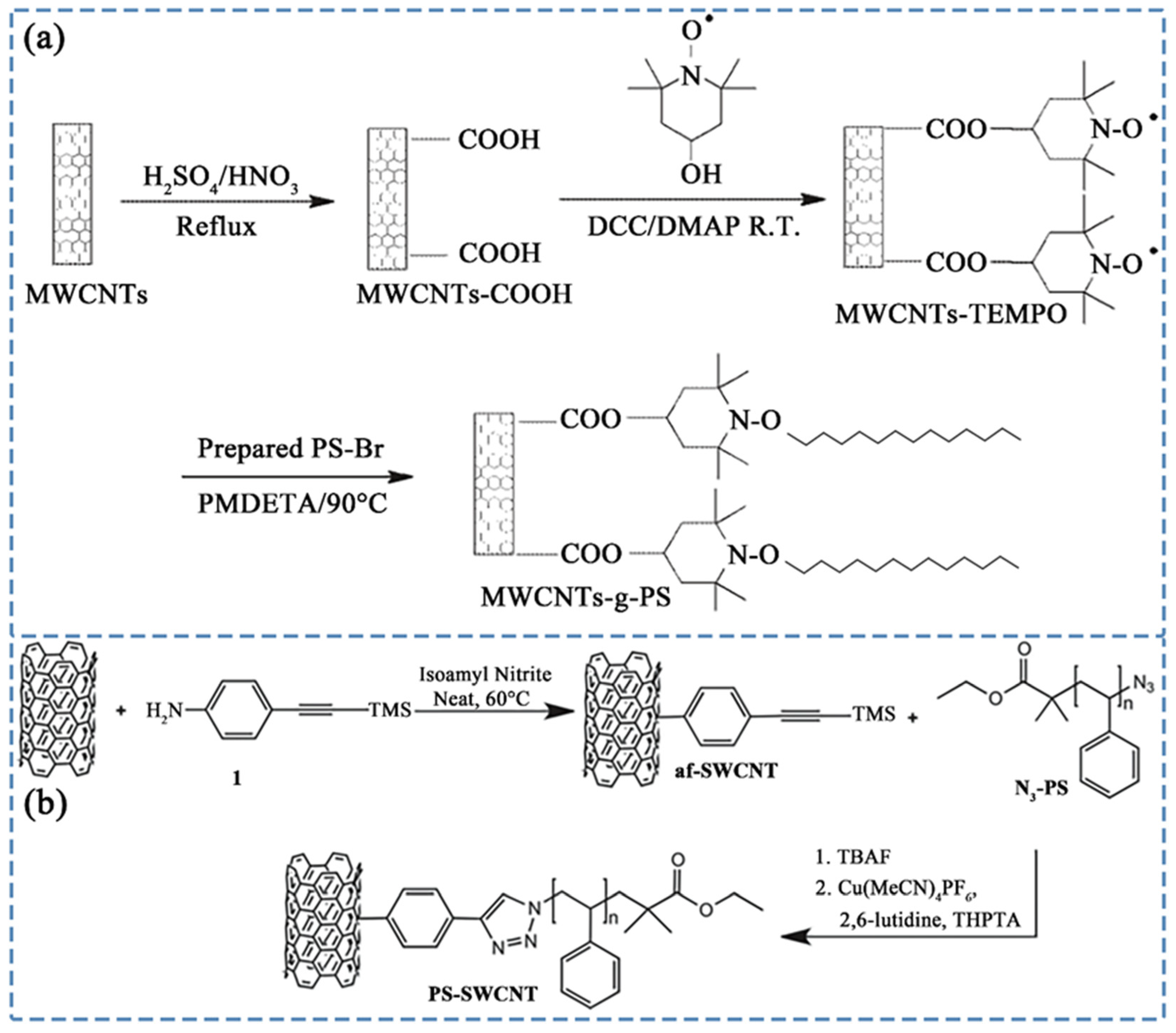
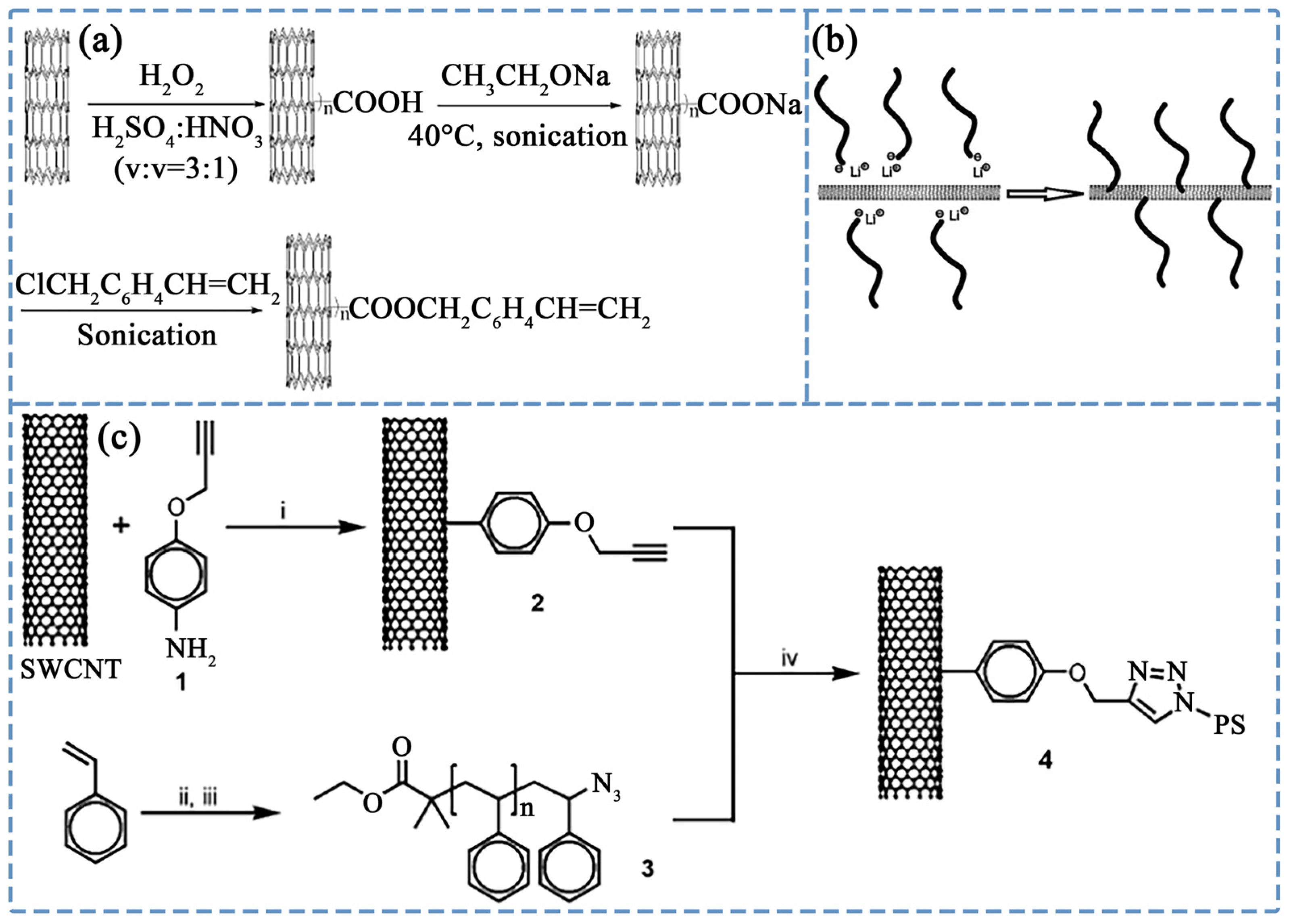
3.1. Covalent Grafting Using PS-Related Polymers
3.1.1. Atom Transfer Radical Polymerization (ATRP)
3.1.2. Other Covalent Grafting Methods
3.2. Non-Covalent Modification Using PS-Related Polymers
3.3. Other Methods
3.4. Comparisons of the Functionalization Methods
4. The Properties of CNTs/PS Composite Materials
4.1. Mechanical Properties
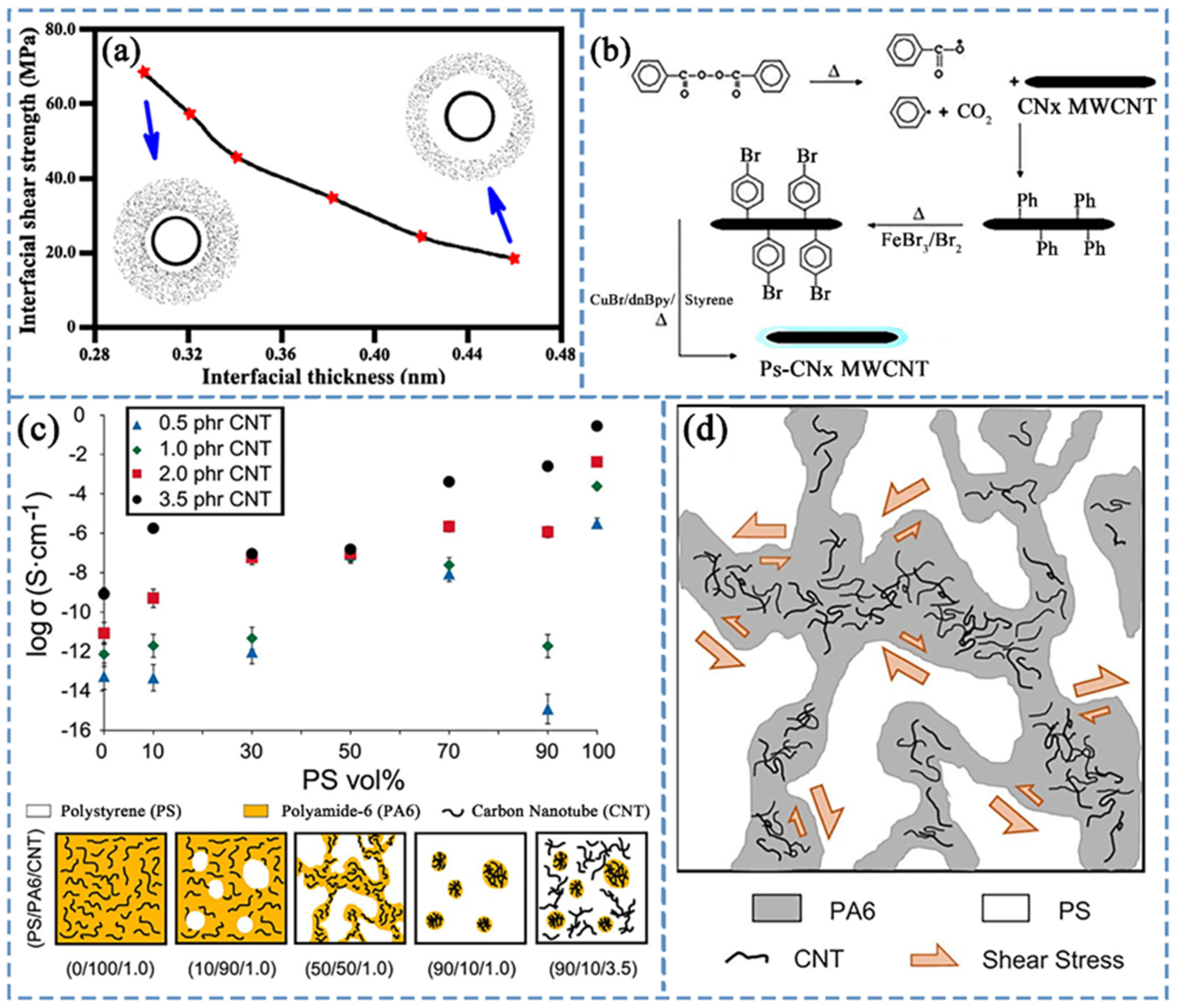
4.2. Electrical Conductivity
4.3. Electromagnetic Shielding Performance
4.4. Thermal Conductivity and Thermal Stability
4.5. Other Performance and Applications
5. Conclusions and Perspectives
5.1. Conclusions
5.2. Perspectives
- (1)
- Orientation Control for Anisotropy: Tensile and melt shear processes can control the orientation of CNTs, imparting anisotropy to CNTs/polymer nanocomposites. However, a singular orientation of CNTs can reduce the formation of conductive pathways, potentially hindering conductivity. Improving preparation methods, such as utilizing stacking or interweaving techniques to make CNT orientation more complex and unique, may unlock more intriguing material properties.
- (2)
- Dispersion Enhancement Strategies: The dispersion of CNTs is a critical issue in the preparation process of CNTs/PS or polymer composite materials. Exploring methods such as electrospinning could offer solutions to address dispersion challenges, providing a convenient approach for the preparation of CNTs–polymer composite materials.
- (3)
- Enhancing Interface Microstructure: The interface microstructure and percolation network formed between CNTs and the matrix in CNTs/PS composite materials are currently limited. Expanding and optimizing these aspects could unleash the full potential of the mechanical and electrical properties of CNTs/PS or polymer composite materials. CNTs can synergistically form unique multilevel microstructures and conductive networks with other materials, enhancing overall performance.
- (4)
- Characterization of Interface Interactions: Understanding the interface characteristics of CNTs/PS composite materials and achieving a customized interface are crucial for manufacturing high-performance CNTs composite materials. Characterizing interface interactions, especially changes in the interaction between CNTs and the polymer matrix before and after CNTs’ modification, is essential. Direct measurement methods using equipment pose challenges, but advancements in computer technology and the maturity of molecular dynamics simulation make it more convenient to study the influence of CNTs’ surface modification on interface interactions in polymer composite materials by constructing molecular dynamics models.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIBN | Azobisisobutyronitrile |
| ATRP | Atom transfer radical polymerization |
| AFM | Atomic force microscopy |
| CA | Cinnamic acid |
| CNT | Carbon nanotube |
| CPC | Conductive polymer composite |
| CPN | Electrically conductive polymer nanocomposite |
| CTAB | Cetyltrimethylammonium bromide |
| DMF | Dimethylformamide |
| DWCNT | Double-wall carbon nanotube |
| EG | Expanded graphite |
| EMI | Electromagnetic interference |
| EVA | Ethylene–vinyl acetate |
| FTIR | Fourier transform infrared spectroscopy |
| GNS | Graphene nanosheet |
| MWCNT | Multi-wall carbon nanotube |
| NaDDBS | Sodium dodecylbenzenesulfonate |
| P3HT | Polythiophene |
| PA6 | Polyamide-6 |
| PCF | Polystyrene/carbon nanotubes foam |
| PDA | Polydopamine |
| PEDOT:PSS | Poly(3,4-ethylenedioxythiophene): poly(styrene sulfonate) |
| PMMA | Poly(methyl methacrylate |
| PP | Polyphenylene |
| PPO | Polyphenylene ether |
| PS | Polystyrene |
| PyPS | Pyrene-terminated polystyrene |
| SDS | Sodium dodecyl sulfate |
| SE | Shielding effectiveness |
| St | Styrene |
| SWCNT/SWCNT | Single-wall carbon nanotubes |
| TEM | Transmission electron microscope |
| THF | Tetrahydrofuran |
| TEMPO | 2,2,6,6-tetramethylpiperidine-1-oxy |
| TMSPMA | 3-(trimethoxysilyl)propyl methacrylate |
| UVO | Ultraviolet ozone |
| Tg | Glass transition temperature |
| NaN3 | Sodium azide |
References
- Soares, B.G.; Calheiros, L.F.; Silva, A.A.; Indrusiak, T.; Barra, G.M.; Livi, S. Conducting melt blending of polystyrene and EVA copolymer with carbon nanotube assisted by phosphonium-based ionic liquid. J. Appl. Polym. Sci. 2018, 135, 45564. [Google Scholar] [CrossRef]
- Marquez, C.; Martin, C.; Linares, N.; De Vos, D. Catalytic routes towards polystyrene recycling. Mater. Horizons 2023, 10, 1625–1640. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Cheng, J.; Tang, Z.; He, Y.; Lyu, Y. Widespread occurrence of phthalates in popular take-out food containers from China and the implications for human exposure. J. Clean Prod. 2021, 290, 125851. [Google Scholar] [CrossRef]
- Yan, Y.; Cui, J.; Pötschke, P.; Voit, B. Dispersion of pristine single-walled carbon nanotubes using pyrene-capped polystyrene and its application for preparation of polystyrene matrix composites. Carbon 2010, 48, 2603–2612. [Google Scholar] [CrossRef]
- Sharma, T.; Garg, M. Effect of ZnO nanoparticles and temperature on dielectric constant/loss properties of polystyrene nanocomposite films. Eur. Phys. J. Plus 2023, 138, 611. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, H.; Quan, Y.; Ma, R.; Pentzer, E.B.; Green, M.J.; Wang, Q. Thermal stability and flammability studies of MXene–organic hybrid polystyrene nanocomposites. Polymers 2022, 14, 1213. [Google Scholar] [CrossRef]
- Mahand, S.N.; Yazdanbakhsh, A.; Tayouri, M.I.; Zarei, A.; Nouranian, S.; Ruckdäschel, H.; Khonakdar, H.A. Theoretical and experimental investigation of selective gas permeability in polystyrene/polyolefin elastomer/nanoclay nanocomposite films. Polym. Test 2023, 120, 107960. [Google Scholar] [CrossRef]
- Angulo, A.L.; Rodriguez, C.L.C.; Fechine, G.J.M. Photooxidative Behavior of Polystyrene Nanocomposites Filled with Two-Dimensional Molybdenum Disulfide. Polymers 2023, 15, 2099. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Lukyanovich, V. The structure of carbon forming in thermal decomposition of carbon monoxide on an iron catalyst. J. Phys. Chem. 1952, 26, 88–95. [Google Scholar]
- Gao, C.; Guo, M.; Liu, Y.; Zhang, D.; Gao, F.; Sun, L.; Li, J.; Chen, X.; Terrones, M.; Wang, Y. Surface modification methods and mechanisms in carbon nanotubes dispersion. Carbon 2023, 212, 118133. [Google Scholar] [CrossRef]
- Zhang, P.; Su, J.; Guo, J.; Hu, S. Influence of carbon nanotube on properties of concrete: A review. Constr. Build. Mater. 2023, 369, 130388. [Google Scholar] [CrossRef]
- Liew, K.M.; Pan, Z.; Zhang, L.-W. The recent progress of functionally graded CNT reinforced composites and structures. Sci. China Phys. Mech. Astron. 2020, 63, 234601. [Google Scholar] [CrossRef]
- Wang, R.; Sun, L.; Zhu, X.; Ge, W.; Li, H.; Li, Z.; Zhang, H.; Huang, Y.; Li, Z.; Zhang, Y.F. Carbon nanotube-based strain sensors: Structures, fabrication, and applications. Adv. Mater. Technol. 2023, 8, 2200855. [Google Scholar] [CrossRef]
- Tao, Z.; Zou, H.; Li, M.; Ren, S.; Xu, J.; Lin, J.; Yang, M.; Feng, Y.; Wang, G. Polypyrrole coated carbon nanotube aerogel composite phase change materials with enhanced thermal conductivity, high solar-/electro-thermal energy conversion and storage. J. Colloid Interface Sci. 2023, 629, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, T.; Liu, X.-L.; Bao, Z.-L.; Qian, P.-F.; Liu, K.; Shi, Y.; Ming, X.; Geng, H.-Z. Highly stable phosphotungstic acid/Au dual doped carbon nanotube transparent conductive films for transparent flexible heaters. Carbon 2023, 207, 219–229. [Google Scholar] [CrossRef]
- Li, M.; Fang, Y.; Shao, S.; Wang, X.; Chen, Z.; Li, J.; Gu, W.; Yang, W.; Xu, W.; Wang, H. Fully-Solution-Processed Enhancement-Mode Complementary Metal-Oxide-Semiconductor Carbon Nanotube Thin Film Transistors Based on BiI3-Doped Crosslinked Poly (4-Vinylphenol) Dielectrics for Ultralow-Power Flexible Electronics. Small 2023, 19, 2207311. [Google Scholar] [CrossRef]
- Gao, C.; Shi, Y.; Huang, R.; Feng, Y.; Chen, Y.; Zhu, S.; Lv, Y.; Shui, W.; Chen, Z. Creating multilayer-structured polystyrene composites for enhanced fire safety and electromagnetic shielding. Compos. Part B-Eng. 2022, 242, 110068. [Google Scholar] [CrossRef]
- Yadav, M.D.; Joshi, H.M.; Sawant, S.V.; Dasgupta, K.; Patwardhan, A.W.; Joshi, J.B. Advances in the application of carbon nanotubes as catalyst support for hydrogenation reactions. Chem. Eng. Sci. 2023, 272, 118586. [Google Scholar] [CrossRef]
- Garrido-Regife, L.; Rivero-Antúnez, P.; Morales-Flórez, V. The dispersion of carbon nanotubes in composite materials studied by computer simulation of Small Angle Scattering. Physica B 2023, 649, 414450. [Google Scholar] [CrossRef]
- Vorobei, A.M.; Ustinovich, K.B.; Chernyak, S.A.; Savilov, S.V.; Parenago, O.O.; Kiselev, M.G. Formation of Polymer-Carbon Nanotube Composites by Two-Step Supercritical Fluid Treatment. Materials 2021, 14, 7428. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhou, T.; Lei, M.; Zhang, X.; Zhou, L. Surface modification of CNTs to improve comprehensive properties of CNTs/Cu composites. Compos. Interfaces 2023, 30, 645–657. [Google Scholar] [CrossRef]
- Kim, S.; Lee, D.; Ahn, K.; Kim, J.; Kim, J. Damage-free remote SF6 plasma-treated CNTs for facile fabrication of electrochemical enzyme biosensors. Appl. Surf. Sci. 2023, 628, 157386. [Google Scholar] [CrossRef]
- Johnson, E.P.; Aquino de Carvalho, N.; Gilbertson, L.M.; Plata, D.L. Acid Treatment in Alkyl-Functionalized Carbon Nanotubes: Soft Functionalization Techniques with Lower Environmental Footprint. ACS Sustain. Chem. Eng. 2023, 11, 15523–15532. [Google Scholar] [CrossRef]
- Liu, X.; Qin, Y.; Zhao, S.; Dong, J.-Y. Nanocomposites-Turned-Nanoalloys Polypropylene/Multiwalled Carbon Nanotubes-graft-Polystyrene: Synthesis and Polymer Nanoreinforcement. Ind. Eng. Chem. Res. 2021, 60, 10167–10179. [Google Scholar] [CrossRef]
- Battery, L.-I. Preparation of Tough, Binder-Free, and Self-Supporting LiFePO. Adv. Sci 2023, 10, 2207355. [Google Scholar]
- Huang, C.-W.; Mohamed, M.G.; Zhu, C.-Y.; Kuo, S.-W. Functional supramolecular polypeptides involving π–π stacking and strong hydrogen-bonding interactions: A conformation study toward carbon nanotubes (CNTs) dispersion. Macromolecules 2016, 49, 5374–5385. [Google Scholar] [CrossRef]
- Mazzotta, G.; Dollmann, M.; Habisreutinger, S.N.; Christoforo, M.G.; Wang, Z.; Snaith, H.J.; Riede, M.K.; Nicholas, R.J. Solubilization of carbon nanotubes with ethylene-vinyl acetate for solution-processed conductive films and charge extraction layers in perovskite solar cells. ACS Appl. Mater. Interfaces 2018, 11, 1185–1191. [Google Scholar] [CrossRef]
- Matsuda, T.; Minami, D.; Khoerunnisa, F.; Sunaga, M.; Nakamura, M.; Utsumi, S.; Itoh, T.; Fujimori, T.; Hayashi, T.; Hattori, Y. Aqueous Nanosilica dispersants for carbon nanotube. Langmuir 2015, 31, 3194–3202. [Google Scholar] [CrossRef]
- He, L.; Tjong, S.C. Aqueous graphene oxide-dispersed carbon nanotubes as inks for the scalable production of all-carbon transparent conductive films. J. Mater. Chem. C 2016, 4, 7043–7051. [Google Scholar] [CrossRef]
- Ma, P.-C.; Siddiqui, N.A.; Marom, G.; Kim, J.-K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A-Appl. Sci. Manuf. 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Karousis, N.; Tagmatarchis, N.; Tasis, D. Current progress on the chemical modification of carbon nanotubes. Chem. Rev. 2010, 110, 5366–5397. [Google Scholar] [CrossRef]
- Ata, M.S.; Poon, R.; Syed, A.M.; Milne, J.; Zhitomirsky, I. New developments in non-covalent surface modification, dispersion and electrophoretic deposition of carbon nanotubes. Carbon 2018, 130, 584–598. [Google Scholar] [CrossRef]
- Kirikova, M.; Ivanov, A.; Savilov, S.; Lunin, V. Modification of multiwalled carbon nanotubes by carboxy groups and determination of the degree of functionalization. Russ. Chem. Bull. 2008, 57, 298–303. [Google Scholar] [CrossRef]
- Ivanova, T.; Maslakov, K.; Savilov, S.; Ivanov, A.; Egorov, A.; Linko, R.; Lunin, V. Carboxylated and decarboxylated nanotubes studied by X-ray photoelectron spectroscopy. Russ. Chem. Bull. 2013, 62, 640–645. [Google Scholar] [CrossRef]
- Liu, Y.; Kumar, S. Polymer/carbon nanotube nano composite fibers–a review. ACS Appl. Mater. Interfaces 2014, 6, 6069–6087. [Google Scholar] [CrossRef]
- Rahmat, M.; Hubert, P. Carbon nanotube–polymer interactions in nanocomposites: A review. Compos. Sci. Technol. 2011, 72, 72–84. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Terentjev, E.M. Dispersion of carbon nanotubes: Mixing, sonication, stabilization, and composite properties. Polymers 2012, 4, 275–295. [Google Scholar] [CrossRef]
- Hamaker, H.C. The London—Van der Waals attraction between spherical particles. Physica 1937, 4, 1058–1072. [Google Scholar] [CrossRef]
- Chazot, C.A.; Hart, A.J. Understanding and control of interactions between carbon nanotubes and polymers for manufacturing of high-performance composite materials. Compos. Sci. Technol. 2019, 183, 107795. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Z.; Tian, X.; Xiu, P.; Zhou, R. Amino acid analogues bind to carbon nanotube via π-π interactions: Comparison of molecular mechanical and quantum mechanical calculations. J. Chem. Phys. 2012, 136, 025103. [Google Scholar] [CrossRef]
- Kar, T.; Bettinger, H.F.; Scheiner, S.; Roy, A.K. Noncovalent π− π stacking and CH---π interactions of aromatics on the surface of single-wall carbon nanotubes: An MP2 study. J. Phys. Chem. C 2008, 112, 20070–20075. [Google Scholar] [CrossRef]
- Naraghi, M.; Bratzel, G.H.; Filleter, T.; An, Z.; Wei, X.; Nguyen, S.T.; Buehler, M.J.; Espinosa, H.D. Atomistic investigation of load transfer between DWNT bundles “crosslinked” by PMMA oligomers. Adv. Funct. Mater. 2013, 23, 1883–1892. [Google Scholar] [CrossRef]
- Liu, J.; Bibari, O.; Mailley, P.; Dijon, J.; Rouviere, E.; Sauter-Starace, F.; Caillat, P.; Vinet, F.; Marchand, G. Stable non-covalent functionalisation of multi-walled carbon nanotubes by pyrene–polyethylene glycol through π–π stacking. New J. Chem. 2009, 33, 1017–1024. [Google Scholar] [CrossRef]
- Mu, M.; Winey, K.I. Improved load transfer in nanotube/polymer composites with increased polymer molecular weight. J. Phys. Chem. C 2007, 111, 17923–17927. [Google Scholar] [CrossRef]
- Davijani, A.A.B.; Kumar, S. Ordered wrapping of poly (methyl methacrylate) on single wall carbon nanotubes. Polymer 2015, 70, 278–281. [Google Scholar] [CrossRef]
- Tallury, S.S.; Pasquinelli, M.A. Molecular dynamics simulations of polymers with stiff backbones interacting with single-walled carbon nanotubes. J. Phys. Chem. B 2010, 114, 9349–9355. [Google Scholar] [CrossRef]
- Coleman, J.N.; Ferreira, M. Geometric constraints in the growth of nanotube-templated polymer monolayers. Appl. Phys. Lett. 2004, 84, 798–800. [Google Scholar] [CrossRef]
- Peng, X.; Meguid, S. Molecular simulations of the influence of defects and functionalization on the shear strength of carbon nanotube-epoxy polymer interfaces. Comput. Mater. Sci. 2017, 126, 204–216. [Google Scholar] [CrossRef]
- Rahimian-Koloor, S.M.; Hashemianzadeh, S.M.; Shokrieh, M.M. Effect of CNT structural defects on the mechanical properties of CNT/Epoxy nanocomposite. Physica B 2018, 540, 16–25. [Google Scholar] [CrossRef]
- Park, O.-K.; Lee, W.; Hwang, J.Y.; You, N.-H.; Jeong, Y.; Kim, S.M.; Ku, B.-C. Mechanical and electrical properties of thermochemically cross-linked polymer carbon nanotube fibers. Compos. Part A-Appl. Sci. Manuf. 2016, 91, 222–228. [Google Scholar] [CrossRef]
- Lu, X.; Hiremath, N.; Hong, K.; Evora, M.C.; Ranson, V.H.; Naskar, A.K.; Bhat, G.S.; Kang, N.-G.; Mays, J.W. Improving mechanical properties of carbon nanotube fibers through simultaneous solid-state cycloaddition and crosslinking. Nanotechnology 2017, 28, 145603. [Google Scholar] [CrossRef]
- Frankland, S.; Caglar, A.; Brenner, D.; Griebel, M. Molecular simulation of the influence of chemical cross-links on the shear strength of carbon nanotube− polymer interfaces. J. Phys. Chem. B 2002, 106, 3046–3048. [Google Scholar] [CrossRef]
- Zheng, Q.; Xue, Q.; Yan, K.; Gao, X.; Li, Q.; Hao, L. Effect of chemisorption on the interfacial bonding characteristics of carbon nanotube–polymer composites. Polymer 2008, 49, 800–808. [Google Scholar] [CrossRef]
- Park, O.-K.; Kim, W.Y.; Kim, S.M.; You, N.-H.; Jeong, Y.; Lee, H.S.; Ku, B.-C. Effect of oxygen plasma treatment on the mechanical properties of carbon nanotube fibers. Mater. Lett. 2015, 156, 17–20. [Google Scholar] [CrossRef]
- Liu, X.; Xu, F.; Zhang, K.; Wei, B.; Gao, Z.; Qiu, Y. Characterization of enhanced interfacial bonding between epoxy and plasma functionalized carbon nanotube films. Compos. Sci. Technol. 2017, 145, 114–121. [Google Scholar] [CrossRef]
- Kim, H.J.; Choo, H.; Park, O.-K.; You, N.-H.; Jeong, Y.; Kim, H.C.; Lee, J.K.; Ku, B.-C. Mechanical properties enhanced by solid-state coupling reaction for molecular covalent bridges of carbon nanotube fibers. Mater. Lett. 2018, 211, 243–246. [Google Scholar] [CrossRef]
- Yun, D.; Feng, W.; Wu, H.; Li, B.; Liu, X.; Yi, W.; Qiang, J.; Gao, S.; Yan, S. Controllable functionalization of single-wall carbon nanotubes by in situ polymerization method for organic photovoltaic devices. Synth. Met. 2008, 158, 977–983. [Google Scholar] [CrossRef]
- Wang, P.-H.; Ghoshal, S.; Gulgunje, P.; Verghese, N.; Kumar, S. Polypropylene nanocomposites with polymer coated multiwall carbon nanotubes. Polymer 2016, 100, 244–258. [Google Scholar] [CrossRef]
- Gojny, F.H.; Schulte, K. Functionalisation effect on the thermo-mechanical behaviour of multi-wall carbon nanotube/epoxy-composites. Compos. Sci. Technol. 2004, 64, 2303–2308. [Google Scholar] [CrossRef]
- Gojny, F.H.; Nastalczyk, J.; Roslaniec, Z.; Schulte, K. Surface modified multi-walled carbon nanotubes in CNT/epoxy-composites. Chem. Phys. Lett. 2003, 370, 820–824. [Google Scholar] [CrossRef]
- Ruelle, B.; Peeterbroeck, S.; Bittencourt, C.; Gorrasi, G.; Patimo, G.; Hecq, M.; Snyders, R.; De Pasquale, S.; Dubois, P. Semi-crystalline polymer/carbon nanotube nanocomposites: Effect of nanotube surface-functionalization and polymer coating on electrical and thermal properties. React. Funct. Polym. 2012, 72, 383–392. [Google Scholar] [CrossRef]
- Kim, Y.J.; Shin, T.S.; Do Choi, H.; Kwon, J.H.; Chung, Y.-C.; Yoon, H.G. Electrical conductivity of chemically modified multiwalled carbon nanotube/epoxy composites. Carbon 2005, 43, 23–30. [Google Scholar] [CrossRef]
- Han, K.-n.; Zhou, W.; Qin, R.; Wang, G.-f.; Ma, L.-H. Effects of carbon nanotubes on open-hole carbon fiber reinforced polymer composites. Mater. Today Commun. 2020, 24, 101106. [Google Scholar] [CrossRef]
- Tai, N.-H.; Yeh, M.-K.; Liu, J.-H. Enhancement of the mechanical properties of carbon nanotube/phenolic composites using a carbon nanotube network as the reinforcement. Carbon 2004, 42, 2774–2777. [Google Scholar] [CrossRef]
- Wang, H.; Hobbie, E.K. Amphiphobic carbon nanotubes as macroemulsion surfactants. Langmuir 2003, 19, 3091–3093. [Google Scholar] [CrossRef]
- Strano, M.S.; Moore, V.C.; Miller, M.K.; Allen, M.J.; Haroz, E.H.; Kittrell, C.; Hauge, R.H.; Smalley, R. The role of surfactant adsorption during ultrasonication in the dispersion of single-walled carbon nanotubes. J. Nanosci. Nanotechnol. 2003, 3, 81–86. [Google Scholar] [CrossRef]
- Vanyorek, L.; Sikora, E.; Balogh, T.; Román, K.; Marossy, K.; Pekker, P.; Szabó, T.J.; Viskolcz, B.; Fiser, B. Nanotubes as polymer composite reinforcing additive materials–A comparative study. Arab. J. Chem. 2020, 13, 3775–3782. [Google Scholar] [CrossRef]
- Su, C.; Wang, X.; Ding, L.; Yu, P. Enhancement of mechanical behavior of resin matrices and fiber reinforced polymer composites by incorporation of multi-wall carbon nanotubes. Polym. Test 2021, 96, 107077. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, P.; Du, J.; Zhao, L.; Ajayan, P.M.; Cheng, H.-M. Increasing the electrical conductivity of carbon nanotube/polymer composites by using weak nanotube–polymer interactions. Carbon 2010, 48, 3551–3558. [Google Scholar] [CrossRef]
- Najmi, L.; Hu, Z. Review on Molecular Dynamics Simulations of Effects of Carbon Nanotubes (CNTs) on Electrical and Thermal Conductivities of CNT-Modified Polymeric Composites. J. Compos. Sci. 2023, 7, 165. [Google Scholar] [CrossRef]
- Sa, V.; Kornev, K.G. Analysis of stability of nanotube dispersions using surface tension isotherms. Langmuir 2011, 27, 13451–13460. [Google Scholar] [CrossRef] [PubMed]
- Alig, I.; Pötschke, P.; Lellinger, D.; Skipa, T.; Pegel, S.; Kasaliwal, G.R.; Villmow, T. Establishment, morphology and properties of carbon nanotube networks in polymer melts. Polymer 2012, 53, 4–28. [Google Scholar] [CrossRef]
- Barber, A.H.; Cohen, S.R.; Wagner, H.D. Static and dynamic wetting measurements of single carbon nanotubes. Phys. Rev. Lett. 2004, 92, 186103. [Google Scholar] [CrossRef] [PubMed]
- Pegel, S.; Villmow, T.; Kasaliwal, G.; Pötschke, P. Polymer-carbon nanotube composites: Melt processing, properties and applications. In Synthetic All-Polymer Composites; Bhattacharyya, D., Fakirov, S., Eds.; Hanser Publishers: Munich, Germany, 2012; pp. 145–192. [Google Scholar]
- Kasaliwal, G.; Villmow, T.; Pegel, S.; Pötschke, P. Influence of material and processing parameters on carbon nanotube dispersion in polymer melts. In Polymer–Carbon Nanotube Composites; Elsevier: Amsterdam, The Netherlands, 2011; pp. 92–132. [Google Scholar]
- Tran, M.Q.; Cabral, J.T.; Shaffer, M.S.; Bismarck, A. Direct measurement of the wetting behavior of individual carbon nanotubes by polymer melts: The key to carbon nanotube− polymer composites. Nano Lett. 2008, 8, 2744–2750. [Google Scholar] [CrossRef]
- Hribova, M.; Rybnikar, F.; Vilcakova, J. Interaction of carbon nanotubes with some polymers. J. Macromol.Sci. Part B Phys. 2010, 50, 16–25. [Google Scholar] [CrossRef]
- Qian, H.; Bismarck, A.; Greenhalgh, E.S.; Shaffer, M.S. Carbon nanotube grafted silica fibres: Characterising the interface at the single fibre level. Compos. Sci. Technol. 2010, 70, 393–399. [Google Scholar] [CrossRef]
- Barber, A.H.; Cohen, S.R.; Wagner, H.D. External and internal wetting of carbon nanotubes with organic liquids. Phys. Rev. B 2005, 71, 115443. [Google Scholar] [CrossRef]
- Rahmat, M. Carbon Nanotube-Polymer Interaction in Nanocomposites; McGill University (Canada): Montréal, QC, Canada, 2011. [Google Scholar]
- López-Manchado, M.; Biagiotti, J.; Valentini, L.; Kenny, J. Dynamic mechanical and Raman spectroscopy studies on interaction between single-walled carbon nanotubes and natural rubber. J. Appl. Polym. Sci. 2004, 92, 3394–3400. [Google Scholar] [CrossRef]
- Brownlow, S.R.; Moravsky, A.P.; Kalugin, N.G.; Majumdar, B.S. Probing deformation of double-walled carbon nanotube (DWNT)/epoxy composites using FTIR and Raman techniques. Compos. Sci. Technol. 2010, 70, 1460–1468. [Google Scholar] [CrossRef]
- Young, R.J.; Deng, L.; Wafy, T.Z.; Kinloch, I.A. Interfacial and internal stress transfer in carbon nanotube based nanocomposites. J. Mater. Sci. 2016, 51, 344–352. [Google Scholar] [CrossRef]
- Wang, Z.; Ciselli, P.; Peijs, T. The extraordinary reinforcing efficiency of single-walled carbon nanotubes in oriented poly (vinyl alcohol) tapes. Nanotechnology 2007, 18, 455709. [Google Scholar] [CrossRef]
- Bernard, C.; Marsaudon, S.; Boisgard, R.; Aimé, J.-P. Competition of elastic and adhesive properties of carbon nanotubes anchored to atomic force microscopy tips. Nanotechnology 2007, 19, 035709. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, W.; Zhan, Q.; Dai, L.; Sowards, L.; Pender, M.; Naik, R.R. Direct measurements of interactions between polypeptides and carbon nanotubes. J. Phys. Chem. B 2006, 110, 12621–12625. [Google Scholar] [CrossRef]
- Barber, A.H.; Cohen, S.R.; Wagner, H.D. Measurement of carbon nanotube–polymer interfacial strength. Appl. Phys. Lett. 2003, 82, 4140–4142. [Google Scholar] [CrossRef]
- Strus, M.; Zalamea, L.; Raman, A.; Pipes, R.; Nguyen, C.; Stach, E. Peeling force spectroscopy: Exposing the adhesive nanomechanics of one-dimensional nanostructures. Nano Lett. 2008, 8, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.; Liu, W.K.; Ruoff, R.S. Load transfer mechanism in carbon nanotube ropes. Compos. Sci. Technol. 2003, 63, 1561–1569. [Google Scholar] [CrossRef]
- Cai, W.; Xiao, C.; Qian, L.; Cui, S. Detecting van der Waals forces between a single polymer repeating unit and a solid surface in high vacuum. Nano Res. 2019, 12, 57–61. [Google Scholar] [CrossRef]
- Sui, K.; Yang, C.; Gao, S.; Shan, X.; Xia, Y.; Zheng, Q. A novel route for well-defined polystyrene-grafted multiwalled carbon nanotubes via the radical coupling reaction. J. Appl. Polym. Sci. 2009, 114, 1914–1920. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, G.-J.; Wu, Y.-J. Amphiphilicity and self-assembly behaviors of polystyrene-grafted multi-walled carbon nanotubes in selective solvents. Colloid Polym. Sci. 2014, 292, 185–196. [Google Scholar] [CrossRef]
- Chadwick, R.C.; Khan, U.; Coleman, J.N.; Adronov, A. Polymer Grafting to Single-Walled Carbon Nanotubes: Effect of Chain Length on Solubility, Graft Density and Mechanical Properties of Macroscopic Structures. Small 2013, 9, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Homenick, C.M.; Sivasubramaniam, U.; Adronov, A. Effect of polymer chain length on the solubility of polystyrene grafted single-walled carbon nanotubes in tetrahydrofuran. Polym. Int. 2008, 57, 1007–1011. [Google Scholar] [CrossRef]
- Nayak, R.R.; Shanmugharaj, A.M.; Ryu, S.H. A Novel Route for Polystyrene Grafted Single-Walled Carbon Nanotubes and their Characterization. Macromol. Chem. Phys. 2008, 209, 1137–1144. [Google Scholar] [CrossRef]
- Lin, T.S.; Cheng, L.Y.; Hsiao, C.-C.; Yang, A.C.-M. Percolated network of entangled multi-walled carbon nanotubes dispersed in polystyrene thin films through surface grafting polymerization. Mater. Chem. Phys. 2005, 94, 438–443. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, X.; Wu, J.; Mai, Y.W. Synthesis and self-assembly of polystyrene-grafted multiwalled carbon nanotubes with a hairy-rod nanostructure. J. Polym. Sci. Pol. Chem. 2006, 44, 3869–3881. [Google Scholar] [CrossRef]
- Hua, J.; Wang, Z.; Xu, L.; Wang, X.; Zhao, J.; Li, F. Preparation polystyrene/multiwalled carbon nanotubes nanocomposites by copolymerization of styrene and styryl-functionalized multiwalled carbon nanotubes. Mater. Chem. Phys. 2013, 137, 694–698. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Chen, P.; Zhang, B.; He, J.; Hu, G.-H. Enhanced interactions between multi-walled carbon nanotubes and polystyrene induced by melt mixing. Carbon 2006, 44, 692–698. [Google Scholar] [CrossRef]
- Hu, H.; Hui, K.; Hui, K.S.; Lee, S.; Zhou, W. Facile and green method for polystyrene grafted multi-walled carbon nanotubes and their electroresponse. Colloid Surf. A-Physicochem. Eng. Asp. 2012, 396, 177–181. [Google Scholar] [CrossRef]
- Oliveira, E.Y.; Bode, R.; Escárcega-Bobadilla, M.V.; Zelada-Guillén, G.A.; Maier, G. Polymer nanocomposites from self-assembled polystyrene-grafted carbon nanotubes. New J. Chem. 2016, 40, 4625–4634. [Google Scholar] [CrossRef]
- Abbasian, M.; Ghaeminia, H.; Jaymand, M. A facile and efficient strategy for the functionalization of multiple-walled carbon nanotubes using well-defined polypropylene-grafted polystyrene. Appl. Phys. A-Mater. Sci. Process. 2018, 124, 522. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, C.; Du, Z.; Li, H.; Zou, W. Preparation of antistatic polystyrene superfine powder with polystyrene modified carbon nanotubes as antistatic agent. Compos. Sci. Technol. 2017, 138, 1–7. [Google Scholar] [CrossRef]
- Chen, X.; Tao, F.; Wang, J.; Yang, H.; Zou, J.; Chen, X.; Feng, X. Concise route to styryl-modified multi-walled carbon nanotubes for polystyrene matrix and enhanced mechanical properties and thermal stability of composite. Mater. Sci. Eng. A 2009, 499, 469–475. [Google Scholar] [CrossRef]
- Mountrichas, G.; Pispas, S.; Tagmatarchis, N. Grafting-to approach for the functionalization of carbon nanotubes with polystyrene. Mater. Sci. Eng. B 2008, 152, 40–43. [Google Scholar] [CrossRef]
- Li, H.; Cheng, F.; Duft, A.M.; Adronov, A. Functionalization of single-walled carbon nanotubes with well-defined polystyrene by “click” coupling. J. Am. Chem. Soc. 2005, 127, 14518–14524. [Google Scholar] [CrossRef] [PubMed]
- Liu, P. Modifications of carbon nanotubes with polymers. Eur. Polym. J. 2005, 41, 2693–2703. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, Y.T.; Feng, Q.P.; Xie, X.M.; Wang, X.H.; Ye, X.Y. Dispersion and noncovalent modification of multiwalled carbon nanotubes by various polystyrene-based polymers. J. Appl. Polym. Sci. 2008, 109, 3525–3532. [Google Scholar] [CrossRef]
- Rivin, D.; Suzin, Y. Calorimetric investigation of the interaction of carbon nanotubes with polystyrene. J. Polym. Sci. Part B-Polym. Phys. 2006, 44, 1821–1834. [Google Scholar] [CrossRef]
- Patole, A.S.; Patole, S.; Yoo, J.B.; Ahn, J.H.; Kim, T.H. Effective in situ synthesis and characteristics of polystyrene nanoparticle-covered multiwall carbon nanotube composite. J. Polym. Sci. Part B-Polym. Phys. 2009, 47, 1523–1529. [Google Scholar] [CrossRef]
- Zhang, Q.; Fang, F.; Zhao, X.; Li, Y.; Zhu, M.; Chen, D. Use of dynamic rheological behavior to estimate the dispersion of carbon nanotubes in carbon nanotube/polymer composites. J. Phys. Chem. B 2008, 112, 12606–12611. [Google Scholar] [CrossRef]
- Jin, H.-J.; Choi, H.J.; Yoon, S.H.; Myung, S.J.; Shim, S.E. Carbon nanotube-adsorbed polystyrene and poly (methyl methacrylate) microspheres. Chem. Mat. 2005, 17, 4034–4037. [Google Scholar] [CrossRef]
- Ham, H.T.; Choi, Y.S.; Chee, M.G.; Chung, I.J. Singlewall carbon nanotubes covered with polystyrene nanoparticles by in-situ miniemulsion polymerization. J. Polym. Sci. Pol. Chem. 2006, 44, 573–584. [Google Scholar] [CrossRef]
- London, L.; Bolton, L.; Samarakoon, D.; Sannigrahi, B.; Wang, X.; Khan, I. Effect of polymer stereoregularity on polystyrene/single-walled carbon nanotube interactions. RSC Adv. 2015, 5, 59186–59193. [Google Scholar] [CrossRef]
- Yang, M.; Koutsos, V.; Zaiser, M. Interactions between polymers and carbon nanotubes: A molecular dynamics study. J. Phys. Chem. B 2005, 109, 10009–10014. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-T.; Zhao, W.; Huang, Z.-Y.; Gao, Y.-F.; Xie, X.-M.; Wang, X.-H.; Ye, X.-Y. Noncovalent surface modification of carbon nanotubes for solubility in organic solvents. Carbon 2006, 44, 1613–1616. [Google Scholar] [CrossRef]
- Zou, J.; Liu, L.; Chen, H.; Khondaker, S.I.; McCullough, R.D.; Huo, Q.; Zhai, L. Dispersion of pristine carbon nanotubes using conjugated block copolymers. Adv. Mater. 2008, 20, 2055–2060. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, K.; Chen, Y. Mechanical performance of ozone functionalized MWCNTs/PC nanocomposites. Express Polym. Lett. 2011, 5, 516–525. [Google Scholar] [CrossRef]
- Jia, Y.; Jiang, Z.; Gong, X.; Zhang, Z. Creep of thermoplastic polyurethane reinforced with ozone functionalized carbon nanotubes. Express Polym. Lett. 2012, 6, 750–758. [Google Scholar] [CrossRef]
- Ayesh, A.S.; Ibrahim, S.S.; Aljaafari, A.A. Electrical, optical, and rheological properties of ozone-treated multiwalled carbon nanotubes–polystyrene nanocomposites. J. Reinf. Plast. Compos. 2013, 32, 359–370. [Google Scholar] [CrossRef]
- Sarvi, A.; Sundararaj, U. Rheological percolation in polystyrene composites filled with polyaniline-coated multiwall carbon nanotubes. Synth. Met. 2014, 194, 109–117. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Q.; Hong, R.; Kumar, M.R. Preparation of oleic acid modified multi-walled carbon nanotubes for polystyrene matrix and enhanced properties by solution blending. J. Mater. Sci.-Mater. Electron. 2015, 26, 8667–8675. [Google Scholar] [CrossRef]
- Amr, I.T.; Al-Amer, A.; Thomas, S.P.; Sougrat, R.; Atieh, M.A. Mechanical, rheological and thermal properties of polystyrene/1-octadecanol modified carbon nanotubes nanocomposites. Fuller. Nanotub. Carbon Nanostruct. 2015, 23, 209–217. [Google Scholar] [CrossRef]
- Sun, G.; Chen, G.; Liu, J.; Yang, J.; Xie, J.; Liu, Z.; Li, R.; Li, X. A facile gemini surfactant-improved dispersion of carbon nanotubes in polystyrene. Polymer 2009, 50, 5787–5793. [Google Scholar] [CrossRef]
- Mallakpour, S.; Nezamzadeh Ezhieh, A. Preparation of polystyrene/MWCNT-Valine composites: Investigation of optical, morphological, thermal, and electrical conductivity properties. Polym. Adv. Technol. 2018, 29, 1182–1190. [Google Scholar] [CrossRef]
- Li, Y.-x.; Gao, Y.; Yang, C.; Wang, Z.-q.; Xue, G. Facile and controllable assembly of multiwalled carbon nanotubes on polystyrene microspheres. Chin. J. Polym. Sci. 2014, 32, 711–717. [Google Scholar] [CrossRef]
- Bellayer, S.; Gilman, J.W.; Eidelman, N.; Bourbigot, S.; Flambard, X.; Fox, D.M.; De Long, H.C.; Trulove, P.C. Preparation of homogeneously dispersed multiwalled carbon nanotube/polystyrene nanocomposites via melt extrusion using trialkyl imidazolium compatibilizer. Adv. Funct. Mater. 2005, 15, 910–916. [Google Scholar] [CrossRef]
- Socher, R.; Krause, B.; Boldt, R.; Hermasch, S.; Wursche, R.; Pötschke, P. Melt mixed nano composites of PA12 with MWNTs: Influence of MWNT and matrix properties on macrodispersion and electrical properties. Compos. Sci. Technol. 2011, 71, 306–314. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Guo, B.-H.; Guo, Z.-X.; Yu, J. Electrical conductivity of carbon nanotube-filled miscible poly (phenylene oxide)/polystyrene blends prepared by melt compounding. Compos. Part B-Eng. 2019, 176, 107213. [Google Scholar] [CrossRef]
- Zhou, T.n.; Hou, Z.c.; Wang, K.; Zhang, Q.; Fu, Q. Polystyrene-wrapping multi-walled carbon nanotubes obtained via simple physical modification of melt mixing. Polym. Adv. Technol. 2011, 22, 1359–1365. [Google Scholar] [CrossRef]
- Sachdev, V.K.; Bhattacharya, S.; Patel, K.; Sharma, S.K.; Mehra, N.C.; Tandon, R.P. Electrical and EMI shielding characterization of multiwalled carbon nanotube/polystyrene composites. J. Appl. Polym. Sci. 2014, 131, 40201. [Google Scholar] [CrossRef]
- Arash, B.; Wang, Q.; Varadan, V. Mechanical properties of carbon nanotube/polymer composites. Sci Rep 2014, 4, 6479. [Google Scholar] [CrossRef]
- Li, A.; Wang, J.; He, W.; Wei, Z.; Wang, X.; He, Q. Enhancing mechanical property and thermal conductivity of fluororubber by the synergistic effect of CNT and BN. Diam. Relat. Mat. 2023, 134, 109790. [Google Scholar] [CrossRef]
- Chen, J.; Yan, L.; Song, W.; Xu, D. Interfacial characteristics of carbon nanotube-polymer composites: A review. Compos. Part A-Appl. Sci. Manuf. 2018, 114, 149–169. [Google Scholar] [CrossRef]
- Wernik, J.; Cornwell-Mott, B.; Meguid, S. Determination of the interfacial properties of carbon nanotube reinforced polymer composites using atomistic-based continuum model. Int. J. Solids Struct. 2012, 49, 1852–1863. [Google Scholar] [CrossRef]
- Xiong, Q.; Meguid, S. Atomistic investigation of the interfacial mechanical characteristics of carbon nanotube reinforced epoxy composite. Eur. Polym. J. 2015, 69, 1–15. [Google Scholar] [CrossRef]
- Rafiee, R.; Ghorbanhosseini, A. Investigating interaction between CNT and polymer using cohesive zone model. Polym. Compos. 2018, 39, 3903–3911. [Google Scholar] [CrossRef]
- Hsiao, C.C.; Lin, T.S.; Cheng, L.Y.; Ma, C.C.M.; Yang, A.C.M. The nanomechanical properties of polystyrene thin films embedded with surface-grafted multiwalled carbon nanotubes. Macromolecules 2005, 38, 4811–4818. [Google Scholar] [CrossRef]
- Nayak, R.R.; Lee, K.Y.; Shanmugharaj, A.; Ryu, S.H. Synthesis and characterization of styrene grafted carbon nanotube and its polystyrene nanocomposite. Eur. Polym. J. 2007, 43, 4916–4923. [Google Scholar] [CrossRef]
- Yuan, J.M.; Fan, Z.F.; Chen, X.H.; Chen, X.H.; Wu, Z.J.; He, L.P. Preparation of polystyrene–multiwalled carbon nanotube composites with individual-dispersed nanotubes and strong interfacial adhesion. Polymer 2009, 50, 3285–3291. [Google Scholar] [CrossRef]
- Putz, K.; Krishnamoorti, R.; Green, P.F. The role of interfacial interactions in the dynamic mechanical response of functionalized SWNT–PS nanocomposites. Polymer 2007, 48, 3540–3545. [Google Scholar] [CrossRef]
- Fragneaud, B.; Masenelli-Varlot, K.; Gonzalez-Montiel, A.; Terrones, M.; Cavaillé, J.-Y. Efficient coating of N-doped carbon nanotubes with polystyrene using atomic transfer radical polymerization. Chem. Phys. Lett. 2006, 419, 567–573. [Google Scholar] [CrossRef]
- Fragneaud, B.; Masenelli-Varlot, K.; Gonzalez-Montiel, A.; Terrones, M.; Cavaille, J.-Y. Mechanical behavior of polystyrene grafted carbon nanotubes/polystyrene nanocomposites. Compos. Sci. Technol. 2008, 68, 3265–3271. [Google Scholar] [CrossRef]
- Amr, I.T.; Al-Amer, A.; Al-Harthi, M.; Girei, S.A.; Sougrat, R.; Atieh, M.A. Effect of acid treated carbon nanotubes on mechanical, rheological and thermal properties of polystyrene nanocomposites. Compos. Part B-Eng. 2011, 42, 1554–1561. [Google Scholar] [CrossRef]
- Jia, Y.; Jiang, Z.; Peng, J.; Gong, X.; Zhang, Z. Resistance to time-dependent deformation of polystyrene/carbon nanotube composites under cyclic tension. Compos. Part A-Appl. Sci. Manuf. 2012, 43, 1561–1568. [Google Scholar] [CrossRef]
- Hoseini, A.H.A.; Arjmand, M.; Sundararaj, U.; Trifkovic, M. Tunable electrical conductivity of polystyrene/polyamide-6/carbon nanotube blend nanocomposites via control of morphology and nanofiller localization. Eur. Polym. J. 2017, 95, 418–429. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Zhang, B.-Y.; Guo, B.-H.; Yu, J.; Guo, Z.-X. Improved electrical conductivity of polymer/carbon black composites by simultaneous dispersion and interaction-induced network assembly. Compos. Sci. Technol. 2019, 179, 106–114. [Google Scholar] [CrossRef]
- Granados-Martínez, F.G.; Domratcheva-Lvova, L.; Flores-Ramírez, N.; García-González, L.; Zamora-Peredo, L.; Mondragón-Sánchez, M.d.L. Composite films from polystyrene with hydroxyl end groups and carbon nanotubes. Mater. Res. 2017, 19, 133–138. [Google Scholar] [CrossRef]
- Pokharel, P.; Xiao, D.; Erogbogbo, F.; Keles, O. A hierarchical approach for creating electrically conductive network structure in polyurethane nanocomposites using a hybrid of graphene nanoplatelets, carbon black and multi-walled carbon nanotubes. Compos. Part B-Eng. 2019, 161, 169–182. [Google Scholar] [CrossRef]
- Fei, G.; Gong, Q.; Li, D.; Lavorgna, M.; Xia, H. Relationship between electrical conductivity and spatial arrangements of carbon nanotubes in polystyrene nanocomposites: The effect of thermal annealing and plasticization on electrical conductivity. Compos. Sci. Technol. 2017, 146, 99–109. [Google Scholar] [CrossRef]
- Hoseini, A.H.A.; Arjmand, M.; Sundararaj, U.; Trifkovic, M. Significance of interfacial interaction and agglomerates on electrical properties of polymer-carbon nanotube nanocomposites. Mater. Des. 2017, 125, 126–134. [Google Scholar] [CrossRef]
- Yan, K.; Xue, Q.; Zheng, Q.; Hao, L. The interface effect of the effective electrical conductivity of carbon nanotube composites. Nanotechnology 2007, 18, 255705. [Google Scholar] [CrossRef]
- Haggenmueller, R.; Gommans, H.H.; Rinzler, A.G.; Fischer, J.E.; Winey, K.I. Aligned single-wall carbon nanotubes in composites by melt processing methods. Chem. Phys. Lett. 2000, 330, 219–225. [Google Scholar] [CrossRef]
- Mitchell, C.A.; Bahr, J.L.; Arepalli, S.; Tour, J.M.; Krishnamoorti, R. Dispersion of functionalized carbon nanotubes in polystyrene. Macromolecules 2002, 35, 8825–8830. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, M.; Li, H.-L.; Guo, X.-Y. SWNTs–polystyrene composites preparations and electrical properties research. Mater. Chem. Phys. 2006, 100, 77–81. [Google Scholar] [CrossRef]
- Chang, T.E.; Kisliuk, A.; Rhodes, S.; Brittain, W.; Sokolov, A. Conductivity and mechanical properties of well-dispersed single-wall carbon nanotube/polystyrene composite. Polymer 2006, 47, 7740–7746. [Google Scholar] [CrossRef]
- Zhang, B.; Fu, R.W.; Zhang, M.Q.; Dong, X.M.; Lan, P.L.; Qiu, J.S. Preparation and characterization of gas-sensitive composites from multi-walled carbon nanotubes/polystyrene. Sens. Actuator B-Chem. 2005, 109, 323–328. [Google Scholar] [CrossRef]
- Faraguna, F.; Poetschke, P.; Pionteck, J. Preparation of polystyrene nanocomposites with functionalized carbon nanotubes by melt and solution mixing: Investigation of dispersion, melt rheology, electrical and thermal properties. Polymer 2017, 132, 325–341. [Google Scholar] [CrossRef]
- Zhou, S.; Hrymak, A.N.; Kamal, M.R. Electrical and morphological properties of microinjection molded polystyrene/multiwalled carbon nanotubes nanocomposites. Polym. Eng. Sci. 2016, 56, 1182–1190. [Google Scholar] [CrossRef]
- Wu, T.-M.; Chen, E.-C. Preparation and characterization of conductive carbon nanotube–polystyrene nanocomposites using latex technology. Compos. Sci. Technol. 2008, 68, 2254–2259. [Google Scholar] [CrossRef]
- Yu, J.; Lu, K.; Sourty, E.; Grossiord, N.; Koning, C.E.; Loos, J. Characterization of conductive multiwall carbon nanotube/polystyrene composites prepared by latex technology. Carbon 2007, 45, 2897–2903. [Google Scholar] [CrossRef]
- Song, J.P.; Choi, S.H.; Chung, D.-W.; Lee, S.J. Latex-based polystyrene nanocomposites with non-covalently modified carbon nanotubes. Polymers 2021, 13, 1168. [Google Scholar] [CrossRef]
- Pang, H.; Chen, C.; Zhang, Y.-C.; Ren, P.-G.; Yan, D.-X.; Li, Z.-M. The effect of electric field, annealing temperature and filler loading on the percolation threshold of polystyrene containing carbon nanotubes and graphene nanosheets. Carbon 2011, 49, 1980–1988. [Google Scholar] [CrossRef]
- Patole, A.S.; Patole, S.P.; Jung, S.-Y.; Yoo, J.-B.; An, J.-H.; Kim, T.-H. Self assembled graphene/carbon nanotube/polystyrene hybrid nanocomposite by in situ microemulsion polymerization. Eur. Polym. J. 2012, 48, 252–259. [Google Scholar] [CrossRef]
- Li, J.; Xu, Y.; Wei, H.; Huo, T.; Wang, E. Electrochemiluminescence sensor based on partial sulfonation of polystyrene with carbon nanotubes. Anal. Chem. 2007, 79, 5439–5443. [Google Scholar] [CrossRef]
- Shah, A.H.; Rizvi, T. Improvement in electrical and thermal behavior of polystyrene/multiwalled carbon nanotubes nanocomposites. Measurement 2013, 46, 1541–1550. [Google Scholar] [CrossRef]
- Kausar, A.; Ahmad, S.; Salman, S.M. Effectiveness of polystyrene/carbon nanotube composite in electromagnetic interference shielding materials: A review. Polym.-Plast. Technol. Eng. 2017, 56, 1027–1042. [Google Scholar] [CrossRef]
- Kazakova, M.A.; Moseenkov, S.I.; Golubtsov, G.V.; Korovin, E.Y.; Ishchenko, A.V.; Selyutin, A.G.; Zavorin, A.V.; Zhuravlev, V.A.; Suslyaev, V.I.; Kuznetsov, V.L. Structural and electromagnetic properties of Fe2Co-multi-walled carbon nanotubes-polystyrene based composite. J. Alloys Compd. 2020, 844, 156107. [Google Scholar] [CrossRef]
- Rohini, R.; Bose, S. Electromagnetic interference shielding materials derived from gelation of multiwall carbon nanotubes in polystyrene/poly (methyl methacrylate) blends. ACS Appl. Mater. Interfaces 2014, 6, 11302–11310. [Google Scholar] [CrossRef]
- Pang, H.; Xu, L.; Yan, D.-X.; Li, Z.-M. Conductive polymer composites with segregated structures. Prog. Polym. Sci. 2014, 39, 1908–1933. [Google Scholar] [CrossRef]
- Yan, D.X.; Pang, H.; Li, B.; Vajtai, R.; Xu, L.; Ren, P.G.; Wang, J.H.; Li, Z.M. Structured reduced graphene oxide/polymer composites for ultra-efficient electromagnetic interference shielding. Adv. Funct. Mater. 2015, 25, 559–566. [Google Scholar] [CrossRef]
- Keshmiri, N.; Hoseini, A.H.A.; Najmi, P.; Liu, J.; Milani, A.S.; Arjmand, M. Highly conductive polystyrene/carbon nanotube/PEDOT: PSS nanocomposite with segregated structure for electromagnetic interference shielding. Carbon 2023, 212, 118104. [Google Scholar] [CrossRef]
- Xie, Y.; Li, Z.; Tang, J.; Li, P.; Chen, W.; Liu, P.; Li, L.; Zheng, Z. Microwave-assisted foaming and sintering to prepare lightweight high-strength polystyrene/carbon nanotube composite foams with an ultralow percolation threshold. J. Mater. Chem. C 2021, 9, 9702–9711. [Google Scholar] [CrossRef]
- Nakai, Y.; Tsukada, R.; Ichimura, R.; Miyata, Y.; Saito, T.; Hata, K.; Maniwa, Y. Observation of the intrinsic magnetic susceptibility of highly purified single-wall carbon nanotubes. Phys. Rev. B 2015, 92, 041402. [Google Scholar] [CrossRef]
- Sen, P.; Suresh, K.; Kumar, R.V.; Kumar, M.; Pugazhenthi, G. A simple solvent blending coupled sonication technique for synthesis of polystyrene (PS)/multi-walled carbon nanotube (MWCNT) nanocomposites: Effect of modified MWCNT content. J. Sci. 2016, 1, 311–323. [Google Scholar] [CrossRef]
- Wang, K.; Li, N.; Ren, K.; Zhang, Q.; Fu, Q. Exploring interfacial enhancement in polystyrene/multiwalled carbon nanotube monofilament induced by stretching. Compos. Part A-Appl. Sci. Manuf. 2014, 61, 84–90. [Google Scholar] [CrossRef]
- Yu, B.; Fu, S.; Wu, Z.; Bai, H.; Ning, N.; Fu, Q. Molecular dynamics simulations of orientation induced interfacial enhancement between single walled carbon nanotube and aromatic polymers chains. Compos. Part A-Appl. Sci. Manuf. 2015, 73, 155–165. [Google Scholar] [CrossRef]
- Makarova, T.; Geydt, P.; Zakharchuk, I.; Lahderanta, E.; Komlev, A.; Zyrianova, A.; Kanygin, M.; Sedelnikova, O.; Suslyaev, V.; Bulusheva, L. Correlation between manufacturing processes and anisotropic magnetic and electromagnetic properties of carbon nanotube/polystyrene composites. Compos. Part B-Eng. 2016, 91, 505–512. [Google Scholar] [CrossRef]
- Makarova, T.L.; Zakharchuk, I.; Geydt, P.; Lahderanta, E.; Komlev, A.; Zyrianova, A.; Lyubchyk, A.; Kanygin, M.; Sedelnikova, O.; Kurenya, A. Assessing carbon nanotube arrangement in polystyrene matrix by magnetic susceptibility measurements. Carbon 2016, 96, 1077–1083. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Arjmand, M.; Sundararaj, U.; Park, S. The electrical conductivity and electromagnetic interference shielding of injection molded multi-walled carbon nanotube/polystyrene composites. Carbon 2012, 50, 1455–1464. [Google Scholar] [CrossRef]
- Arjmand, M.; Apperley, T.; Okoniewski, M.; Sundararaj, U. Comparative study of electromagnetic interference shielding properties of injection molded versus compression molded multi-walled carbon nanotube/polystyrene composites. Carbon 2012, 50, 5126–5134. [Google Scholar] [CrossRef]
- Qavamnia, S.S.; Nasouri, K. Facile fabrication of carbon nanotubes/polystyrene composite nanofibers for high-performance electromagnetic interference shielding. Fiber. Polym. 2016, 17, 1977–1984. [Google Scholar] [CrossRef]
- Yang, Y.; Gupta, M.C.; Dudley, K.L.; Lawrence, R.W. Novel carbon nanotube− polystyrene foam composites for electromagnetic interference shielding. Nano Lett. 2005, 5, 2131–2134. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Wang, Y.; Wang, K.; Feng, A. The effect of modified AlN on the thermal conductivity, mechanical and thermal properties of AlN/polystyrene composites. RSC Adv. 2016, 6, 102542–102548. [Google Scholar] [CrossRef]
- Hatui, G.; Bhattacharya, P.; Sahoo, S.; Dhibar, S.; Das, C.K. Combined effect of expanded graphite and multiwall carbon nanotubes on the thermo mechanical, morphological as well as electrical conductivity of in situ bulk polymerized polystyrene composites. Compos. Part A-Appl. Sci. Manuf. 2014, 56, 181–191. [Google Scholar] [CrossRef]
- Chaudhary, A.K.; Vijayakumar, R. Synthesis of polystyrene/starch/CNT composite and study on its biodegradability. J. Polym. Res. 2020, 27, 187. [Google Scholar] [CrossRef]
- Gong, P.; Buahom, P.; Tran, M.-P.; Saniei, M.; Park, C.B.; Pötschke, P. Heat transfer in microcellular polystyrene/multi-walled carbon nanotube nanocomposite foams. Carbon 2015, 93, 819–829. [Google Scholar] [CrossRef]
- Zhong, H.; Lukes, J.R. Interfacial thermal resistance between carbon nanotubes: Molecular dynamics simulations and analytical thermal modeling. Phys. Rev. B 2006, 74, 125403. [Google Scholar] [CrossRef]
- Huxtable, S.T.; Cahill, D.G.; Shenogin, S.; Xue, L.; Ozisik, R.; Barone, P.; Usrey, M.; Strano, M.S.; Siddons, G.; Shim, M. Interfacial heat flow in carbon nanotube suspensions. Nat. Mater. 2003, 2, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Gojny, F.H.; Wichmann, M.H.; Fiedler, B.; Kinloch, I.A.; Bauhofer, W.; Windle, A.H.; Schulte, K. Evaluation and identification of electrical and thermal conduction mechanisms in carbon nanotube/epoxy composites. Polymer 2006, 47, 2036–2045. [Google Scholar] [CrossRef]
- Yang, S.Y.; Ma, C.C.M.; Teng, C.C.; Huang, Y.W.; Liao, S.H.; Huang, Y.L.; Tien, H.W.; Lee, T.M.; Chiou, K.C. Effect of functionalized carbon nanotubes on the thermal conductivity of epoxy composites. Carbon 2010, 48, 592–603. [Google Scholar] [CrossRef]
- Baudot, C.; Tan, C.M. Covalent functionalization of carbon nanotubes and their use in dielectric epoxy composites to improve heat dissipation. Carbon 2011, 49, 2362–2369. [Google Scholar] [CrossRef]
- Jakubinek, M.B.; White, M.A.; Mu, M.; Winey, K.I. Temperature dependence of thermal conductivity enhancement in single-walled carbon nanotube/polystyrene composites. Appl. Phys. Lett. 2010, 96, 083105. [Google Scholar] [CrossRef]
- Han, W.; Song, W.; Shen, Y.; Ge, C.; Zhang, R.; Zhang, X. Multiwalled carbon nanotubes encapsulated polystyrene: A facile one-step synthesis, electrical and thermal properties. J. Mater. Sci. 2019, 54, 6227–6237. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, L.; Chen, Y. Fabrication of carbon nanotubes/polystyrene nanocomposites via Pickering emulsion polymerization. Fuller. Nanotub. Carbon Nanostruct. 2021, 29, 840–843. [Google Scholar] [CrossRef]
- Wu, C.; Huang, X.; Wu, X.; Yu, J.; Xie, L.; Jiang, P. TiO2-nanorod decorated carbon nanotubes for high-permittivity and low-dielectric-loss polystyrene composites. Compos. Sci. Technol. 2012, 72, 521–527. [Google Scholar] [CrossRef]
- Gul, G.; Faller, R.; Ileri-Ercan, N. Coarse-grained modeling of polystyrene-modified CNTs and their interactions with lipid bilayers. Biophys. J. 2023, 122, 1748–1761. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.; Du, J.; Yang, K.; Ren, T.; Wan, D.; Pu, H. Superhydrophobic and superoleophilic polystyrene/carbon nanotubes foam for oil/water separation. J. Environ. Chem. Eng. 2021, 9, 106038. [Google Scholar] [CrossRef]
- Calaresu, I.; Hernandez, J.; Rauti, R.; Rodilla, B.L.; Arché-Núñez, A.; Perez, L.; Camarero, J.; Miranda, R.; González, M.T.; Rodríguez, I. Polystyrene nanopillars with inbuilt carbon nanotubes enable synaptic modulation and stimulation in interfaced neuronal networks. Adv. Mater. Interfaces 2021, 8, 2002121. [Google Scholar] [CrossRef]
- Li, Y.; Pionteck, J.; Pötschke, P.; Voit, B. Thermal annealing to influence the vapor sensing behavior of co-continuous poly (lactic acid)/polystyrene/multiwalled carbon nanotube composites. Mater. Des. 2020, 187, 108383. [Google Scholar] [CrossRef]
- Ismail, A.; Goh, P.; Sanip, S.; Aziz, M. Transport and separation properties of carbon nanotube-mixed matrix membrane. Sep. Purif. Technol. 2009, 70, 12–26. [Google Scholar] [CrossRef]
- Wu, B.; Li, X.; An, D.; Zhao, S.; Wang, Y. Electro-casting aligned MWCNTs/polystyrene composite membranes for enhanced gas separation performance. J. Membr. Sci. 2014, 462, 62–68. [Google Scholar] [CrossRef]
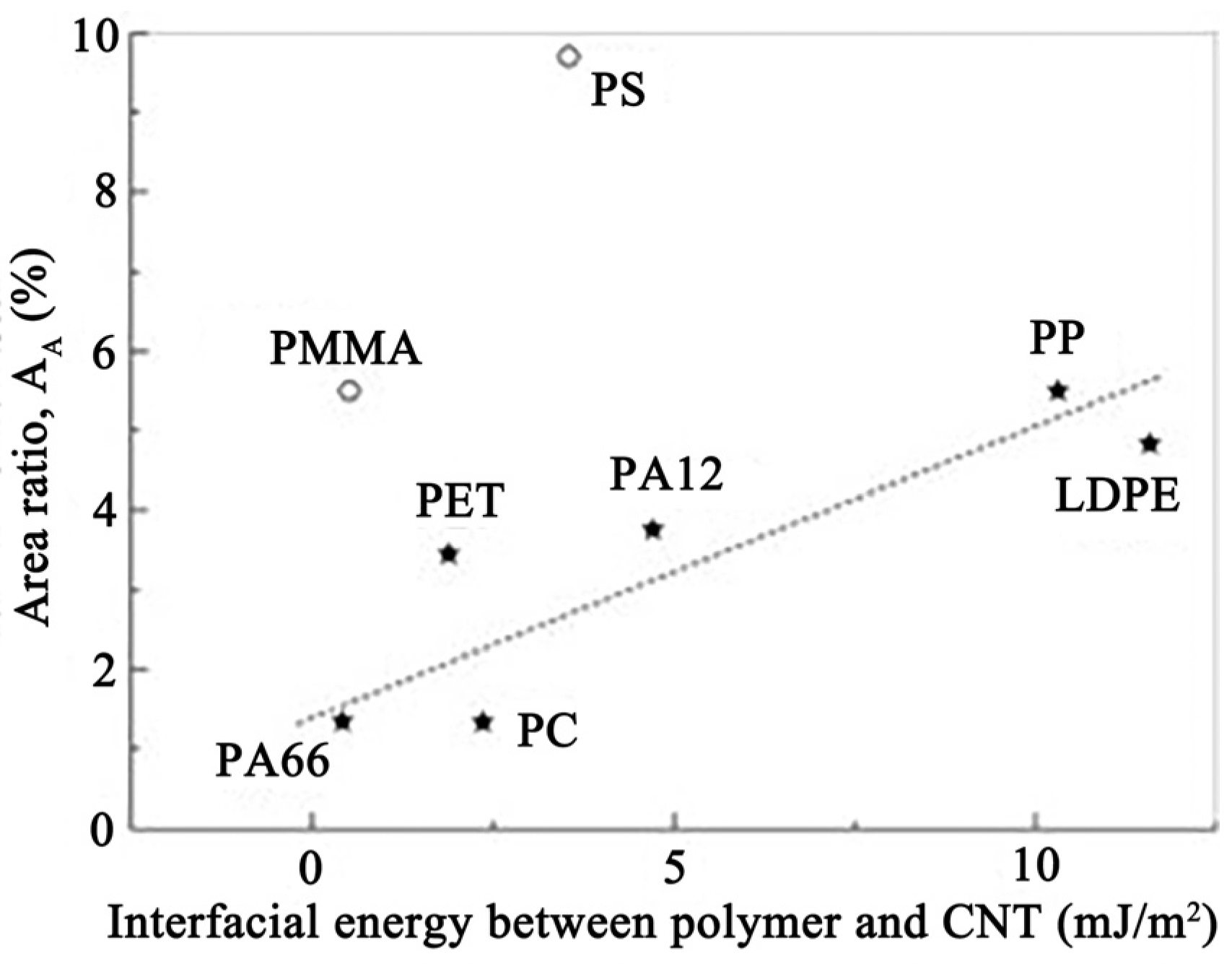




| Type | Interaction Energy (kcal/mol) |
|---|---|
| Van der Waals | ~0.1–1 |
| π–π stacking [41] | ~10 |
| CH–π/NH–π [42] | ~1–10 |
| Hydrogen bond | ~2–30 |
| Methods | Characterizations | Mechanisms | Advantages | Disadvantages |
|---|---|---|---|---|
| Covalent grafting of PS-related polymers | PS-related polymers are directly or indirectly connected to CNTs through chemical bonds. | Chemical reaction, partial change in carbon atoms from sp2 hybridization to sp3. | Strong bonding between the surface modification agent; high compatibility with the PS matrix. | The preparation process is complex and costly, involves a significant amount of chemical reagents, and results in the destruction of the inherent characteristics of CNTs. |
| Non-covalent modification of PS-related polymers | PS-related polymers encapsulate/wrap around the surface of CNTs through physical interactions. | Van der Waals forces, electrostatic interactions, π–π stacking, hydrophobic interactions, physical adsorption, steric hindrance. | Intact sp2 carbon atomic structure and the properties of CNTs are maintained; high compatibility with PS. | The interaction between CNTs and polymers is relatively weak. |
| Other methods | The modification process does not involve PS or PS-related polymers. | Chemical reaction, partial change in carbon atoms from sp2 hybridization to sp3, van der Waals forces, amide–π, π–π, π–cation interactions, physical adsorption, steric hindrance. | The method is highly selective and has a wide range of applicability, most of them are simple and easy to operate. | Compared to PS-related polymers, the compatibility with PS for modifying CNTs is lower. |
| Method | Types of CNTs | Dispersion Improvement | Property Improvement | Ref. |
|---|---|---|---|---|
| A | MWCNTs | Disperses well in CH2Cl2, Tetrahydrofuran (THF) and toluene | - | [92] |
| A | MWCNTs | Disperses well in THF and benzene | - | [93] |
| A | SWCNTs | - | Increased mechanical properties | [94] |
| A | SWCNTs | Disperses well in THF | - | [95] |
| A | SWCNTs | Disperses well in THF | Increased Tg and conductivity | [96] |
| A | MWCNTs | Disperses well in solution | Increased tensile strength | [97] |
| A | MWCNTs | Disperses well in solution | Improved Tg and thermal stability | [98] |
| A | MWCNTs | Disperses well in PS | Improved thermal stability | [99] |
| A | MWCNTs | Disperses well in toluene and xylene | Increased Tg and conductivity | [101] |
| A | MWCNTs | - | Increased elastic modulus | [102] |
| A | MWCNTs | Dispersed well in polymers | Good compatibility | [103] |
| A | MWCNTs | Disperses well in PS | Surface resistance and percolation threshold are reduced, and compatibility is good. | [104] |
| B | MWCNTs | Improved dispersion | Improved impact and tensile strength, as well as thermal stability, good compatibility | [105] |
| B | SWCNTs | Dispersed well in THF, CHCl3, and CH2Cl2 | - | [107] |
| C | SWCNTs | Disperses well in PS solution | Low current percolation threshold (0.095 wt.%) | [4] |
| C | MWCNTs | Evenly dispersed and stable in solution | - | [109] |
| C | MWCNTs | Disperses well in PS | - | [110] |
| C | MWCNTs | Disperses well in organic solvents | - | [111] |
| C | SWCNTs | Disperses well in PS solution | - | [112] |
| D | MWCNTs | Improved dispersion | Improved conductivity (percolation threshold is 0.05–0.08 wt.%) and electromagnetic shielding performance | [1] |
| D | MWCNTs | Disperses well in PS | Enhanced photoelectric and rheological properties | [121] |
| D | MWCNTs | Disperses well in PS | Low current and rheological thresholds | [122] |
| D | MWCNTs | Disperses well in PS | Improved electrical conductivity and thermal stability | [123] |
| D | MWCNTs | Disperses well in PS | Increased conductivity, tensile strength, and modulus | [125] |
| D | MWCNTs | Disperses well in PS | Improved conductivity (current percolation threshold 0.5–1 wt.%) and thermal stability | [126] |
| D | CNTs | Improved dispersion | Increased conductivity (current percolation threshold is 1.44 wt.%) | [130] |
| D | MWCNTs | Disperses well in PS | Increased tensile strength and elongation at break | [131] |
| D | MWCNTs | Improved dispersion | Good electrical conductivity (current percolation threshold is 0.05 wt.%) | [132] |
| Types of CNTs | Method | CNTs Content | Percolation Threshold | Frequency (GHz) | EMI SE | Ref. |
|---|---|---|---|---|---|---|
| MWCNTs | Build a separation structure | 2.0 wt.% | 0.009 vol% | 8.2–12.4 | 55.7 dB/mm | [173] |
| CNTs | Foaming and sintering | 0.046 vol% | 0.0014 vol% | 12.4 | 211.5 dB cm3 g−1 | [174] |
| MWCNTs | Injection molding Compression molding | 5.0 wt.% | - | 8.2–12.4 | 8.05–11.46 dB 17.2 dB | [181] |
| MWCNTs | Electrospinning | 7.5 wt.% | 0.45 vol% | 8.2–12.4 | 32 dB | [183] |
| MWCNTs | Foaming | 7.0 wt.% | - | 8.2–12.4 | ≈20 dB | [184] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Wang, G.; Wu, Y.; Jiang, N.; Niu, K. Functionalization of Carbon Nanotubes in Polystyrene and Properties of Their Composites: A Review. Polymers 2024, 16, 770. https://doi.org/10.3390/polym16060770
Li H, Wang G, Wu Y, Jiang N, Niu K. Functionalization of Carbon Nanotubes in Polystyrene and Properties of Their Composites: A Review. Polymers. 2024; 16(6):770. https://doi.org/10.3390/polym16060770
Chicago/Turabian StyleLi, Hongfu, Guangfei Wang, Ying Wu, Naisheng Jiang, and Kangmin Niu. 2024. "Functionalization of Carbon Nanotubes in Polystyrene and Properties of Their Composites: A Review" Polymers 16, no. 6: 770. https://doi.org/10.3390/polym16060770






