Bioinspired Thermal Conductive Cellulose Nanofibers/Boron Nitride Coating Enabled by Co-Exfoliation and Interfacial Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. TEMPO Oxidation of Cellulose Fibers
2.3. Co-Exfoliation/Dispersion of BN Nanoplate and Cellulose Nanofibers (CNFs)
2.4. Self-Polymerisation of Dopamine
2.5. Preparation of Thermal Conductive Film and Coating
2.6. Characterization
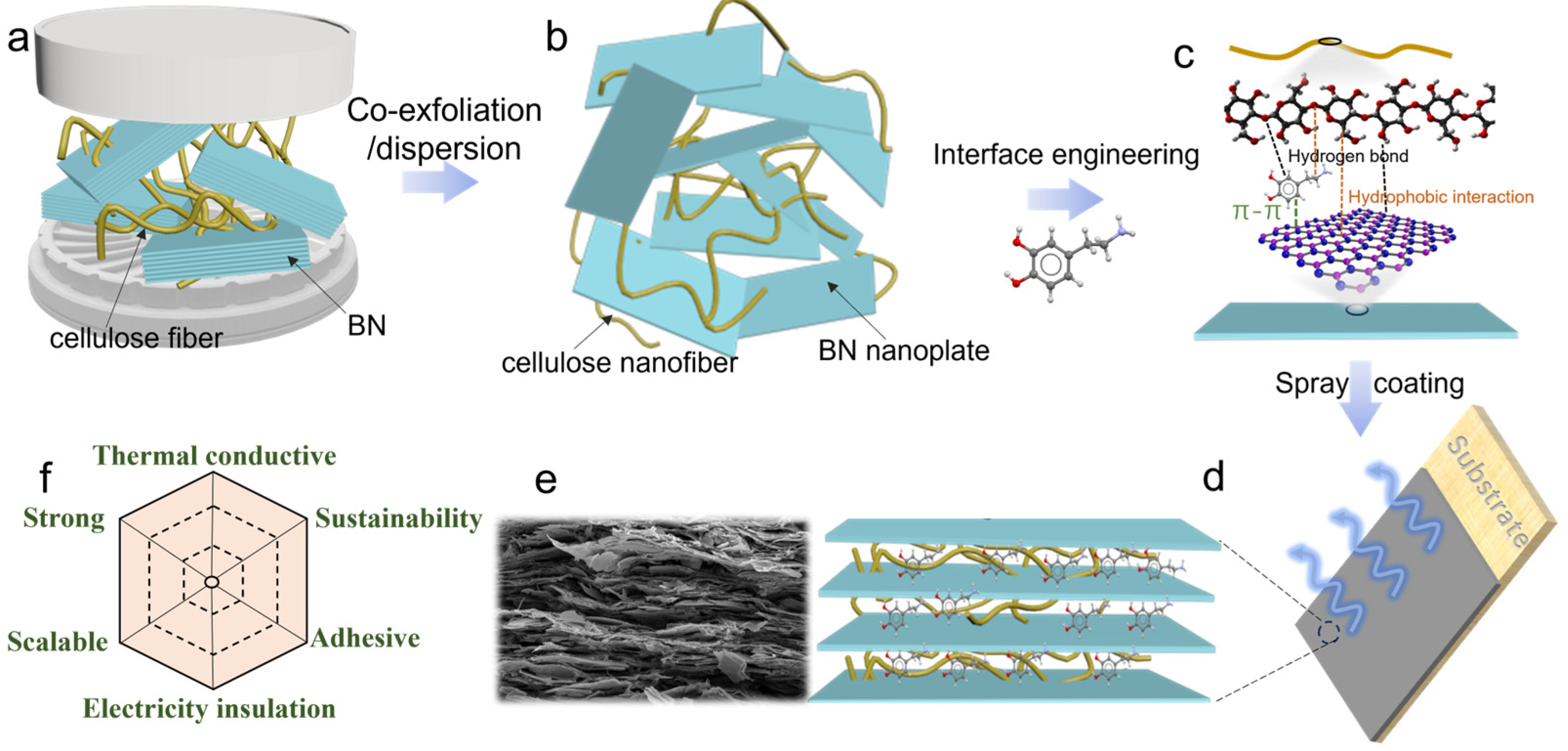
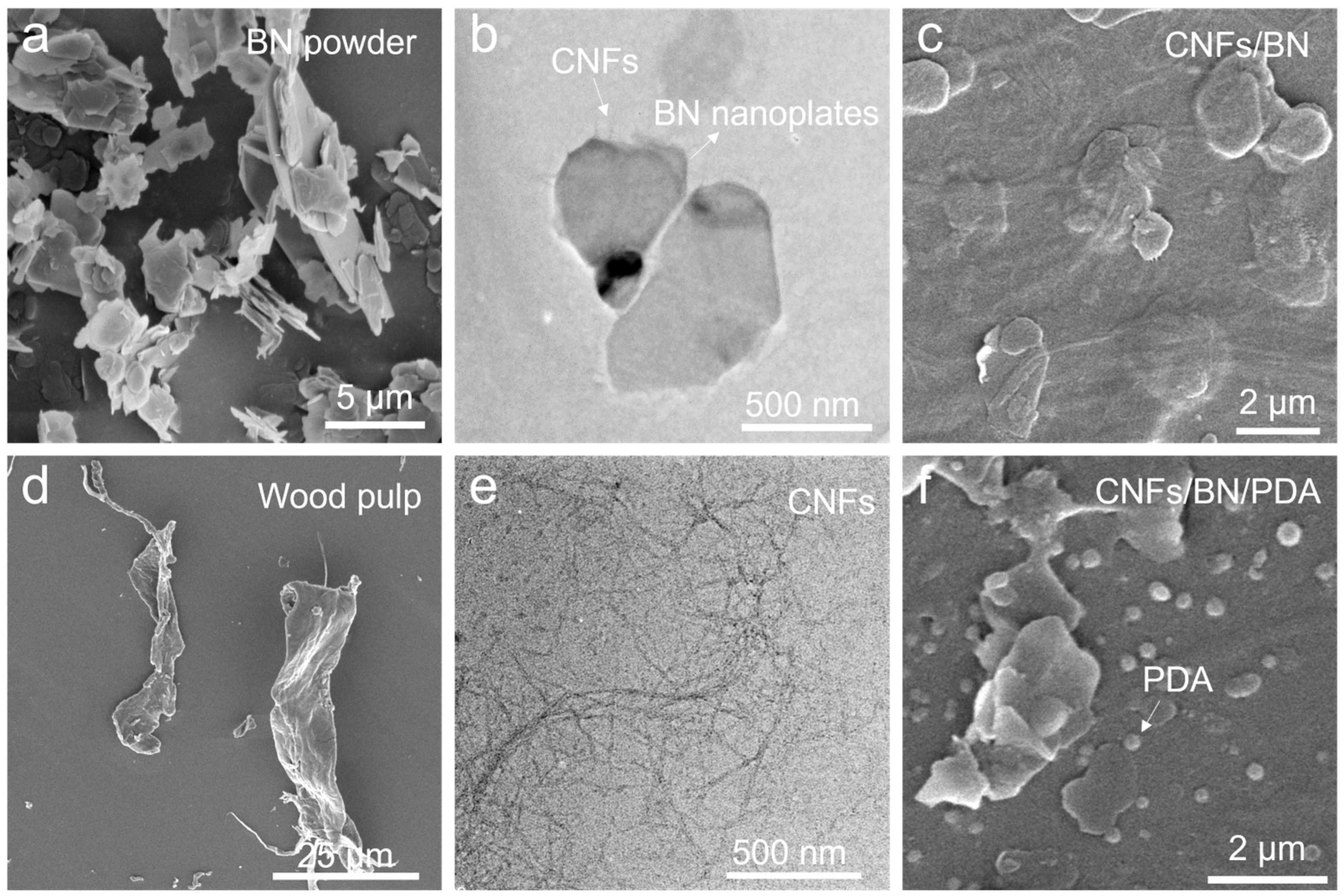
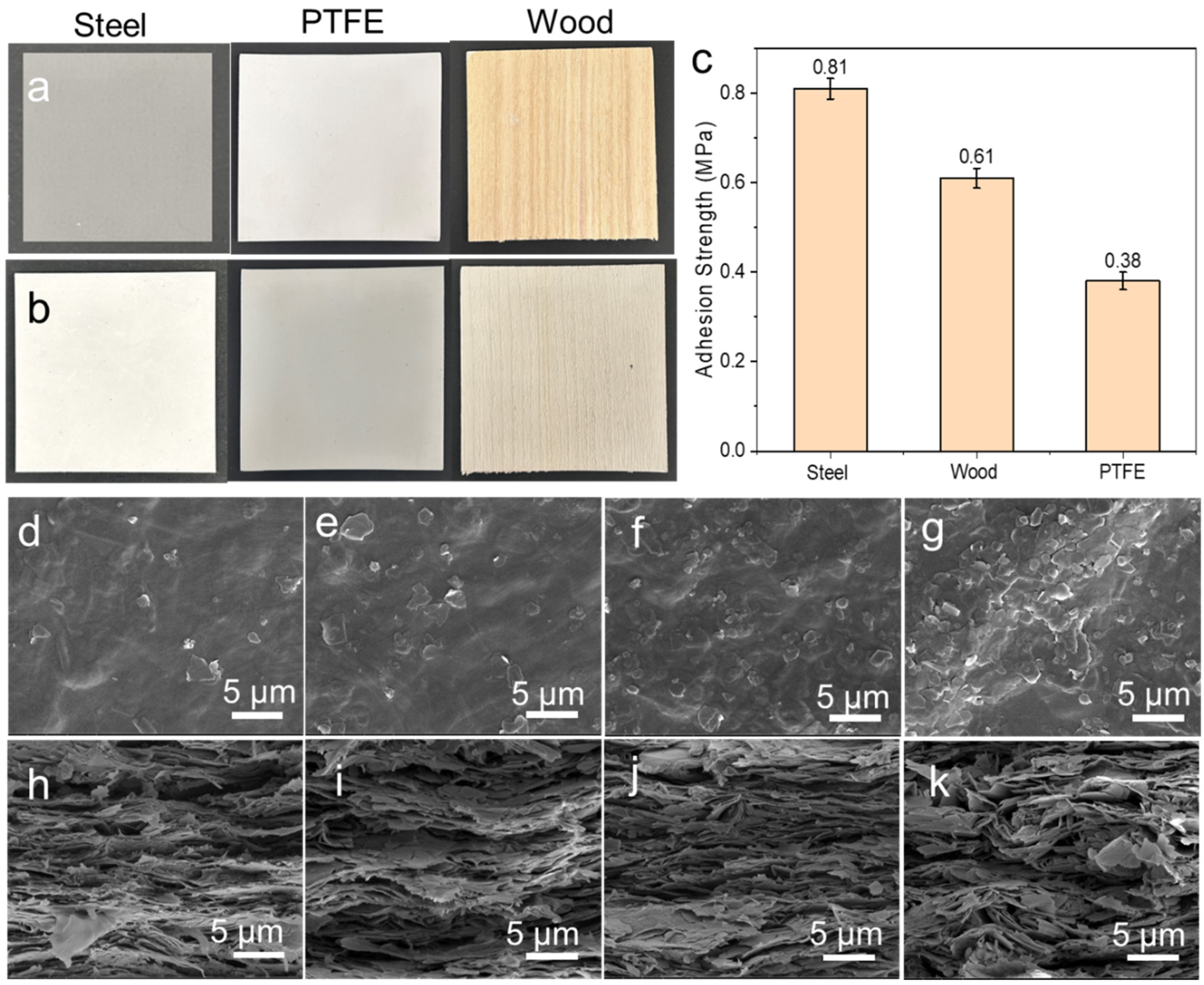
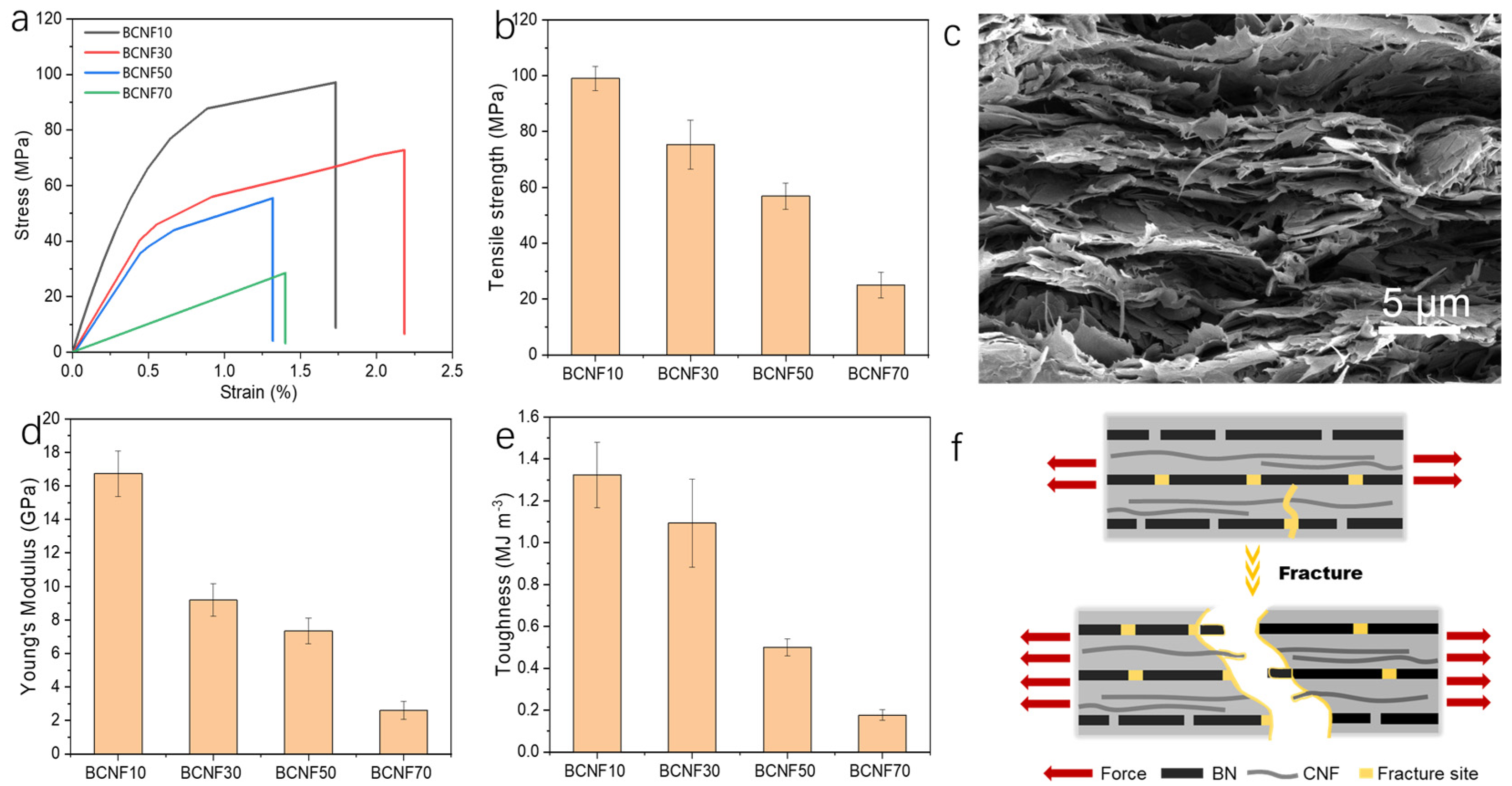
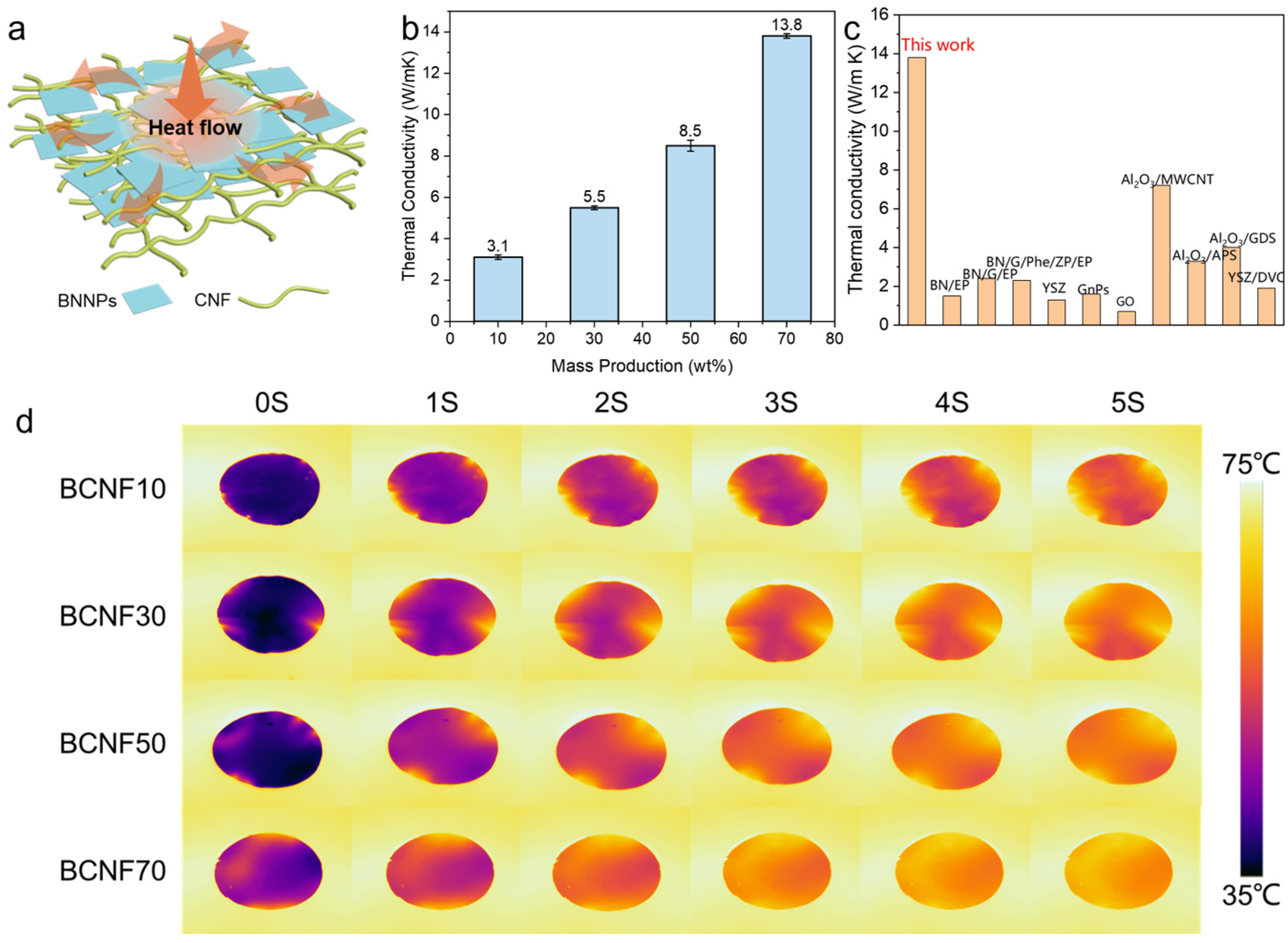
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Burger, N.; Laachachi, A.; Ferriol, M.; Lutz, M.; Toniazzo, V.; Ruch, D. Review of Thermal Conductivity in Composites: Mechanisms, Parameters and Theory. Prog. Polym. Sci. 2016, 61, 1–28. [Google Scholar] [CrossRef]
- Toberer, E.S.; Baranowski, L.L.; Dames, C. Advances in Thermal Conductivity. Annu. Rev. Mater. Res. 2012, 42, 179–209. [Google Scholar] [CrossRef]
- Han, Z.; Fina, A. Thermal Conductivity of Carbon Nanotubes and Their Polymer Nanocomposites: A Review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Fang, Z.; Huang, Y.; Xu, H.; Liu, Y.; Wu, D.; Zhuang, J.; Sun, J. Recent Advances in Preparation, Mechanisms, and Applications of Thermally Conductive Polymer Composites: A Review. J. Compos. Sci. 2020, 4, 180. [Google Scholar] [CrossRef]
- Chen, H.; Ginzburg, V.V.; Yang, J.; Yang, Y.; Liu, W.; Huang, Y.; Du, L.; Chen, B. Thermal Conductivity of Polymer-Based Composites: Fundamentals and Applications. Prog. Polym. Sci. 2016, 59, 41–85. [Google Scholar] [CrossRef]
- Peng, L.; Xu, Z.; Liu, Z.; Guo, Y.; Li, P.; Gao, C. Ultrahigh Thermal Conductive yet Superflexible Graphene Films. Adv. Mater. 2017, 29, 1700589. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, P.; Tang, Z.; Xu, X.; Gao, H.; Wang, G. Carbon-Based Composite Phase Change Materials for Thermal Energy Storage, Transfer, and Conversion. Adv. Sci. 2021, 8, 2001274. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, N.; Uzun, S.; Levitt, A.; Seyedin, S.; Lynch, P.A.; Qin, S.; Han, M.; Yang, W.; Liu, J.; et al. Scalable Manufacturing of Free-Standing, Strong Ti3C2Tx MXene Films with Outstanding Conductivity. Adv. Mater. 2020, 32, 2001093. [Google Scholar] [CrossRef]
- Cai, Q.; Scullion, D.; Gan, W.; Falin, A.; Zhang, S.; Watanabe, K.; Taniguchi, T.; Chen, Y.; Santos, E.J.G.; Li, L.H. High Thermal Conductivity of High-Quality Monolayer Boron Nitride and Its Thermal Expansion. Sci. Adv. 2019, 5, eaav0129. [Google Scholar] [CrossRef]
- Guerra, V.; Wan, C.; McNally, T. Thermal Conductivity of 2D Nano-Structured Boron Nitride (BN) and Its Composites with Polymers. Prog. Mater. Sci. 2019, 100, 170–186. [Google Scholar] [CrossRef]
- Kim, K.; Ryu, S.; Kim, J. Melt-Processable Aggregated Boron Nitride Particle via Polysilazane Coating for Thermal Conductive Composite. Ceram. Int. 2017, 43, 2441–2447. [Google Scholar] [CrossRef]
- Abbas, A.; Zhao, Y.; Zhou, J.; Wang, X.; Lin, T. Improving Thermal Conductivity of Cotton Fabrics Using Composite Coatings Containing Graphene, Multiwall Carbon Nanotube or Boron Nitride Fine Particles. Fibers Polym. 2013, 14, 1641–1649. [Google Scholar] [CrossRef]
- Tao, P.; Shang, W.; Song, C.; Shen, Q.; Zhang, F.; Luo, Z.; Yi, N.; Zhang, D.; Deng, T. Bioinspired Engineering of Thermal Materials. Adv. Mater. 2015, 27, 428–463. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, R.; Li, L.; Liang, C.; Yan, J.; Liang, R.; Sun, G.; Jiang, L.; Cheng, Q. Strong, Tough, and Thermally Conductive Nacre-Inspired Boron Nitride Nanosheet/Epoxy Layered Nanocomposites. Nano Res. 2023, 17, 820–828. [Google Scholar] [CrossRef]
- Han, J.; Du, G.; Gao, W.; Bai, H. An Anisotropically High Thermal Conductive Boron Nitride/Epoxy Composite Based on Nacre-Mimetic 3D Network. Adv. Funct. Mater. 2019, 29, 1900412. [Google Scholar] [CrossRef]
- Pan, G.; Yao, Y.; Zeng, X.; Sun, J.; Hu, J.; Sun, R.; Xu, J.-B.; Wong, C.-P. Learning from Natural Nacre: Constructing Layered Polymer Composites with High Thermal Conductivity. ACS Appl. Mater. Interfaces 2017, 9, 33001–33010. [Google Scholar] [CrossRef] [PubMed]
- Wegst, U.G.K.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired Structural Materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Liao, T.; Li, W.; Qiao, Y.; Ostrikov, K. (Ken) Beyond Seashells: Bioinspired 2D Photonic and Photoelectronic Devices. Adv. Funct. Mater. 2019, 29, 1901460. [Google Scholar] [CrossRef]
- Ling, S.; Chen, W.; Fan, Y.; Zheng, K.; Jin, K.; Yu, H.; Buehler, M.J.; Kaplan, D.L. Biopolymer Nanofibrils: Structure, Modeling, Preparation, and Applications. Prog. Polym. Sci. 2018, 85, 1–56. [Google Scholar] [CrossRef]
- Iioka, M.; Kawanabe, W.; Tsujimura, S.; Kobayashi, T.; Shohji, I. An Evaluation of the Wear Resistance of Electroplated Nickel Coatings Composited with 2,2,6,6-Tetramethylpiperidine 1-Oxyl-Oxidized Cellulose Nanofibers. Polymers 2024, 16, 224. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Lazzara, G.; Milioto, S. Supramolecular Systems Based on Chitosan and Chemically Functionalized Nanocelluloses as Protective and Reinforcing Fillers of Paper Structure. Carbohydr. Polym. Technol. Appl. 2023, 6, 100380. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xue, Z.; Zhao, J.; Ao, C.; Jia, X.; Xia, T.; Wang, Q.; Deng, X.; Zhang, W.; Lu, C. Mass Production of High Thermal Conductive Boron Nitride/Nanofibrillated Cellulose Composite Membranes. Chem. Eng. J. 2020, 383, 123101. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Ruppel, S.S.; Liang, J. Tunable Properties of Polydopamine Nanoparticles and Coated Surfaces. Langmuir 2022, 38, 5020–5029. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Du, X.; Hou, W.; Liu, X.; Zhu, C.; Gao, B.; Sun, L.; Li, Q.; Liao, J.; Levkin, P.A.; et al. UV-Triggered Polydopamine Secondary Modification: Fast Deposition and Removal of Metal Nanoparticles. Adv. Funct. Mater. 2019, 29, 1901875. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-Oxidized Cellulose Nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- Yang, K.-P.; Chen, H.; Han, Z.-M.; Yin, C.-H.; Liu, H.-C.; Li, D.-H.; Yang, H.-B.; Sun, W.-B.; Guan, Q.-F.; Yu, S.-H. Bioinspired Multifunctional High-Performance Electromagnetic Shielding Coatings Resistant to Extreme Space Environments. Innov. Mater. 2023, 1, 100010–100025. [Google Scholar] [CrossRef]
- Xiong, R.; Kim, H.S.; Zhang, L.; Korolovych, V.F.; Zhang, S.; Yingling, Y.G.; Tsukruk, V.V. Wrapping Nanocellulose Nets around Graphene Oxide Sheets. Angew. Chem. Int. Ed. 2018, 57, 8508–8513. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, H.; Shen, F.; Wan, J.; Lacey, S.; Fang, Z.; Dai, H.; Hu, L. Nanocellulose as Green Dispersant for Two-Dimensional Energy Materials. Nano Energy 2015, 13, 346–354. [Google Scholar] [CrossRef]
- Mattos, B.D.; Tardy, B.L.; Greca, L.G.; Kämäräinen, T.; Xiang, W.; Cusola, O.; Magalhães, W.L.E.; Rojas, O.J. Nanofibrillar Networks Enable Universal Assembly of Superstructured Particle Constructs. Sci. Adv. 2020, 6, eaaz7328. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-C.; Wu, Q.; Song, K.; Lee, S.; Qing, Y.; Wu, Y. Cellulose Nanoparticles: Structure–Morphology–Rheology Relationships. ACS Sustain. Chem. Eng. 2015, 3, 821–832. [Google Scholar] [CrossRef]
- Chen, K.; Peng, L.; Fang, Z.; Lin, X.; Sun, C.; Qiu, X. Dispersing Boron Nitride Nanosheets with Carboxymethylated Cellulose Nanofibrils for Strong and Thermally Conductive Nanocomposite Films with Improved Water-Resistance. Carbohydr. Polym. 2023, 321, 121250. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Yi, Y.; Chen, B.; Deng, P.; Zhou, Z.; Lu, C. Rheology-Guided Assembly of a Highly Aligned MXene/Cellulose Nanofiber Composite Film for High-Performance Electromagnetic Interference Shielding and Infrared Stealth. ACS Appl. Mater. Interfaces 2022, 14, 36060–36070. [Google Scholar] [CrossRef]
- Wu, K.; Fang, J.; Ma, J.; Huang, R.; Chai, S.; Chen, F.; Fu, Q. Achieving a Collapsible, Strong, and Highly Thermally Conductive Film Based on Oriented Functionalized Boron Nitride Nanosheets and Cellulose Nanofiber. ACS Appl. Mater. Interfaces 2017, 9, 30035–30045. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, Q.; Lin, L.; Jiang, L. Synergistic Toughening of Bioinspired Poly(Vinyl Alcohol)–Clay–Nanofibrillar Cellulose Artificial Nacre. ACS Nano 2014, 8, 2739–2745. [Google Scholar] [CrossRef]
- Uetani, K.; Okada, T.; Oyama, H.T. Crystallite Size Effect on Thermal Conductive Properties of Nonwoven Nanocellulose Sheets. Biomacromolecules 2015, 16, 2220–2227. [Google Scholar] [CrossRef]
- Nie, S.; Hao, N.; Zhang, K.; Xing, C.; Wang, S. Cellulose Nanofibrils-Based Thermally Conductive Composites for Flexible Electronics: A Mini Review. Cellulose 2020, 27, 4173–4187. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, M.; Cui, Y.; Bao, D.; Peng, J.; Gao, Y.; Lin, D.; Geng, H.; Zhu, Y.; Wang, H. A Novel Polymer Composite Coating with High Thermal Conductivity and Unique Anti-Corrosion Performance. Chem. Eng. J. 2022, 439, 135660. [Google Scholar] [CrossRef]
- Chi, W.; Sampath, S.; Wang, H. Ambient and High-Temperature Thermal Conductivity of Thermal Sprayed Coatings. J. Therm. Spray Technol. 2006, 15, 773–778. [Google Scholar] [CrossRef]
- Ligati, S.; Ohayon-Lavi, A.; Keyes, J.; Ziskind, G.; Regev, O. Enhancing Thermal Conductivity in Graphene-Loaded Paint: Effects of Phase Change, Rheology and Filler Size. Int. J. Therm. Sci. 2020, 153, 106381. [Google Scholar] [CrossRef]
- Bakshi, S.R.; Balani, K.; Agarwal, A. Thermal Conductivity of Plasma-Sprayed Aluminum Oxide—Multiwalled Carbon Nanotube Composites. J. Am. Ceram. Soc. 2008, 91, 942–947. [Google Scholar] [CrossRef]
- Shakhova, I.; Mironov, E.; Azarmi, F.; Safonov, A. Thermo-Electrical Properties of the Alumina Coatings Deposited by Different Thermal Spraying Technologies. Ceram. Int. 2017, 43, 15392–15401. [Google Scholar] [CrossRef]
- Curry, N.; VanEvery, K.; Snyder, T.; Markocsan, N. Thermal Conductivity Analysis and Lifetime Testing of Suspension Plasma-Sprayed Thermal Barrier Coatings. Coatings 2014, 4, 630–650. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, X.; Xia, X.; Chen, Y.; Lin, D.; Zhou, Y.; Xiong, R. Bioinspired Thermal Conductive Cellulose Nanofibers/Boron Nitride Coating Enabled by Co-Exfoliation and Interfacial Engineering. Polymers 2024, 16, 805. https://doi.org/10.3390/polym16060805
Wan X, Xia X, Chen Y, Lin D, Zhou Y, Xiong R. Bioinspired Thermal Conductive Cellulose Nanofibers/Boron Nitride Coating Enabled by Co-Exfoliation and Interfacial Engineering. Polymers. 2024; 16(6):805. https://doi.org/10.3390/polym16060805
Chicago/Turabian StyleWan, Xinyuan, Xiaojian Xia, Yunxiang Chen, Deyuan Lin, Yi Zhou, and Rui Xiong. 2024. "Bioinspired Thermal Conductive Cellulose Nanofibers/Boron Nitride Coating Enabled by Co-Exfoliation and Interfacial Engineering" Polymers 16, no. 6: 805. https://doi.org/10.3390/polym16060805
APA StyleWan, X., Xia, X., Chen, Y., Lin, D., Zhou, Y., & Xiong, R. (2024). Bioinspired Thermal Conductive Cellulose Nanofibers/Boron Nitride Coating Enabled by Co-Exfoliation and Interfacial Engineering. Polymers, 16(6), 805. https://doi.org/10.3390/polym16060805






