Reversibility in the Physical Properties of Agarose Gels following an Exchange in Solvent and Non-Solvent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Agarose Samples Preparation
2.2. Water Regain and Swelling Degree
2.3. Rheology
3. Results and Discussion
3.1. NMR Study
3.2. Degree of Substitution on Agarose
3.3. Degree of Swelling
3.4. Rheology of Agarose Gels
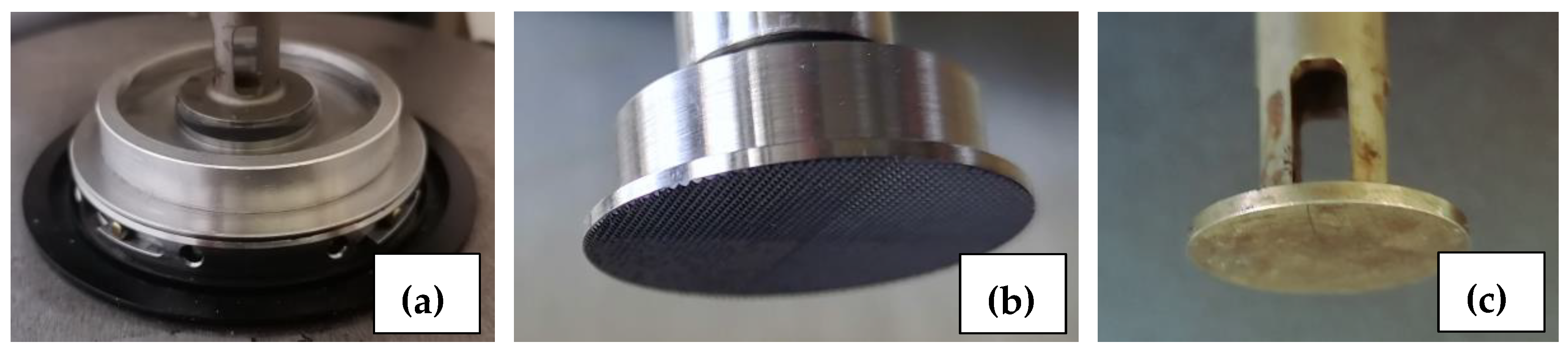
3.4.1. Selection of Experimental Conditions
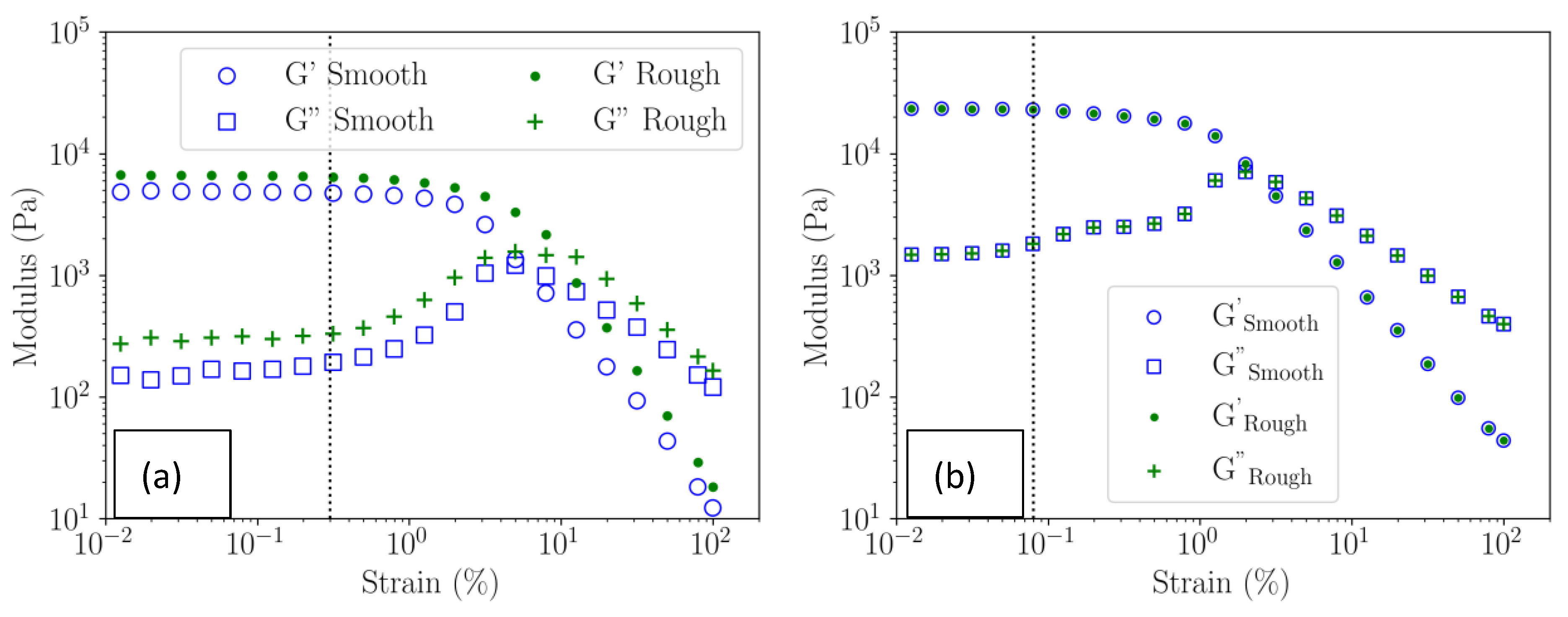
3.4.2. Viscoelastic Moduli of the Gels in the Linear Domain
3.4.3. Evidence of Ethanol–Water Reversibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mao, B. Dynamics of Agar-Based Gels in Contact with Solid Surfaces: Gelation, Adhesion, Drying and Formulation. Ph.D. Thesis, University of Bordeaux, Bordeaux, France, 2017. [Google Scholar]
- Roux, D.C.; Jeacomine, I.; Maîtrejean, G.; Caton, F.; Rinaudo, M. Characterization of Agarose Gels in Solvent and Non-Solvent Media. Polymers 2023, 15, 2162. [Google Scholar] [CrossRef]
- Kouwijzer, M.; Pérez, S. Molecular modeling of agarose helices, leading to the prediction of crystalline allomorphs. Biopolym. Orig. Res. Biomol. 1998, 46, 11–29. [Google Scholar] [CrossRef]
- Foord, S.A.; Atkins, E.D.Y. New X-ray diffraction results from agarose: Extended single helix structures and implications for gelation mechanism. Biopolym. Orig. Res. Biomol. 1989, 28, 1345–1365. [Google Scholar] [CrossRef]
- Watase, M.; Nishinari, K.; Clark, A.H.; Ross-Murphy, S.B. Differential scanning calorimetry, rheology, X-ray, and NMR of very concentrated agarose gels. Macromolecules 1989, 22, 1196–1201. [Google Scholar] [CrossRef]
- Djabourov, M.; Clark, A.H.; Rowlands, D.W.; Ross-Murphy, S.B. Small-angle X-ray scattering characterization of agarose sols and gels. Macromolecules 1989, 22, 180–188. [Google Scholar] [CrossRef]
- Schafer, S.E.; Stevens, E.S. A reexamination of the double-helix model for agarose gels using optical rotation. Biopolym. Orig. Res. Biomol. 1995, 36, 103–108. [Google Scholar] [CrossRef]
- Arnott, S.; Fulmer, A.S.W.E.; Scott, W.E.; Dea, I.C.M.; Moorhouse, R.; Rees, D.A. The agarose double helix and its function in agarose gel structure. J. Mol. Biol. 1974, 90, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Corongiu, G.; Fornili, S.L.; Clementi, E. Hydration of agarose double helix: A monte carlo simulation. Int. J. Quantum Chem. 1983, 24, 277–291. [Google Scholar] [CrossRef]
- Rochas, C.; Rinaudo, M. Mechanism of gel formation in κ-carrageenan. Biopolym. Orig. Res. Biomol. 1984, 23, 735–745. [Google Scholar] [CrossRef]
- Rinaudo, M.; Rochas, C. On the non-covalent gelation in polysaccharides systems. Polym. Prepr. 1986, 246. [Google Scholar]
- Morris, E.R.; Rees, D.A.; Robinson, G. Cation-specific aggregation of carrageenan helices: Domain model of polymer gel structure. J. Mol. Biol. 1980, 138, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Dea, I.C.; Rees, D.A. Affinity interactions between agarose and β-1, 4-glycans: A model for polysaccharide associations in algal cell walls. Carbohydr. Polym. 1987, 7, 183–224. [Google Scholar] [CrossRef]
- Minghou, J.; Lahaye, M.; Yaphe, W. Structure of agar from Gracilaria spp. (Rhodophyta) collected in the People’s Republic of China. Bot. Mar. 1985, 28, 521–528. [Google Scholar] [CrossRef]
- Rodríguez, M.C.; Matulewicz, M.C.; Noseda, M.D.; Ducatti, D.R.B.; Leonardi, P.I. Agar from Gracilaria gracilis (Gracilariales, Rhodophyta) of the Patagonic coast of Argentina–Content, structure and physical properties. Bioresour. Technol. 2009, 100, 1435–1441. [Google Scholar] [CrossRef]
- Rochas, C.; Lahaye, M. Average molecular weight and molecular weight distribution of agarose and agarose-type polysaccharides. Carbohydr. Polym. 1989, 10, 289–298. [Google Scholar] [CrossRef]
- Watase, M.; Nishinari, K. Rheological properties of agarose gels with different molecular weights. Rheol. Acta 1983, 22, 580–587. [Google Scholar] [CrossRef]
- Normand, V.; Lootens, D.L.; Amici, E.; Plucknett, K.P.; Aymard, P. New insight into agarose gel mechanical properties. Biomacromolecules 2000, 1, 730–738. [Google Scholar] [CrossRef]
- Xiong, J.Y.; Narayanan, J.; Liu, X.Y.; Chong, T.K.; Chen, S.B.; Chung, T.S. Topology evolution and gelation mechanism of agarose gel. J. Phys. Chem. B 2005, 109, 5638–5643. [Google Scholar] [CrossRef]
- Van Winkle, D.H.; Beheshti, A.; Rill, R.L. DNA electrophoresis in agarose gels: A simple relation describing the length dependence of mobility. Electrophoresis 2002, 23, 15–19. [Google Scholar] [CrossRef]
- Aymard, P.; Martin, D.R.; Plucknett, K.; Foster, T.J.; Clark, A.H.; Norton, I.T. Influence of thermal history on the structural and mechanical properties of agarose gels. Biopolym. Orig. Res. Biomol. 2001, 59, 131–144. [Google Scholar] [CrossRef]
- Donley, G.J.; Singh, P.K.; Shetty, A.; Rogers, S.A. Elucidating the G″ overshoot in soft materials with a yield transition via a time-resolved experimental strain decomposition. Proc. Natl. Acad. Sci. USA 2020, 117, 21945–21952. [Google Scholar] [CrossRef]
- Russ, N.; Zielbauer, B.I.; Koynov, K.; Vilgis, T.A. Influence of nongelling hydrocolloids on the gelation of agarose. Biomacromolecules 2013, 14, 4116–4124. [Google Scholar] [CrossRef]
- Watase, M.; Arakawa, K. Rheological properties of hydrogels of agar-agar. III. Stress relaxation of agarose gels. Bull. Chem. Soc. Jpn. 1968, 41, 1830–1834. [Google Scholar] [CrossRef]
- Tanaka, T. Collapse of gels and the critical endpoint. Phys. Rev. Lett. 1978, 40, 820. [Google Scholar] [CrossRef]
- Tanaka, T. Phase transition of gels. In Polyelectrolyte Gels; Properties, Preparation and Applications; Harland, R.A., Prud’homme, R.K., Eds.; ACS Symposium Series No. 480; American Chemical Society: Washington, DC, USA, 1991; pp. 1–21. [Google Scholar]
- Rinaudo, M.; Landry, S. On the volume change on non covalent gels in solvent-non solvent mixtures. Polym. Bull. 1987, 17, 563–565. [Google Scholar] [CrossRef]
- Divoux, T.; Mao, B.; Snabre, P. Syneresis and delayed detachment in agar plates. Soft Matter 2015, 11, 3677–3685. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Divoux, T.; Snabre, P. Normal force controlled rheology applied to agar gelation. J. Rheol. 2016, 60, 473–489. [Google Scholar] [CrossRef]
- Ewoldt, R.; Johnston, M.; Caretta, L. Experimental challenges of shear rheology: How to avoid bad data. In Complex Fluids in Biological Systems; Springer: New York, NY, USA, 2015; Chapter 6; pp. 207–241. [Google Scholar]
- Yamamoto, I.; Saito, S.; Makino, T.; Yamaguchi, M.; Takamasu, T. The anisotropic properties of magnetically ordered gel. Sci. Technol. Adv. Mater. 2006, 7, 322–326. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roux, D.C.D.; Caton, F.; Jeacomine, I.; Maîtrejean, G.; Rinaudo, M. Reversibility in the Physical Properties of Agarose Gels following an Exchange in Solvent and Non-Solvent. Polymers 2024, 16, 811. https://doi.org/10.3390/polym16060811
Roux DCD, Caton F, Jeacomine I, Maîtrejean G, Rinaudo M. Reversibility in the Physical Properties of Agarose Gels following an Exchange in Solvent and Non-Solvent. Polymers. 2024; 16(6):811. https://doi.org/10.3390/polym16060811
Chicago/Turabian StyleRoux, Denis C. D., François Caton, Isabelle Jeacomine, Guillaume Maîtrejean, and Marguerite Rinaudo. 2024. "Reversibility in the Physical Properties of Agarose Gels following an Exchange in Solvent and Non-Solvent" Polymers 16, no. 6: 811. https://doi.org/10.3390/polym16060811
APA StyleRoux, D. C. D., Caton, F., Jeacomine, I., Maîtrejean, G., & Rinaudo, M. (2024). Reversibility in the Physical Properties of Agarose Gels following an Exchange in Solvent and Non-Solvent. Polymers, 16(6), 811. https://doi.org/10.3390/polym16060811






