Effect of Modified Natural Rubber on the Mechanical and Thermal Properties of Poly(Lactic Acid) and Its Composites with Nanoparticles from Biowaste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of nHA from Seabass Scales
2.3. Preparation of PLA-MoNR and PLA/nHA Composites with/without MoNR
2.4. Characterization
3. Results and Discussion
3.1. Characteristics of nHA
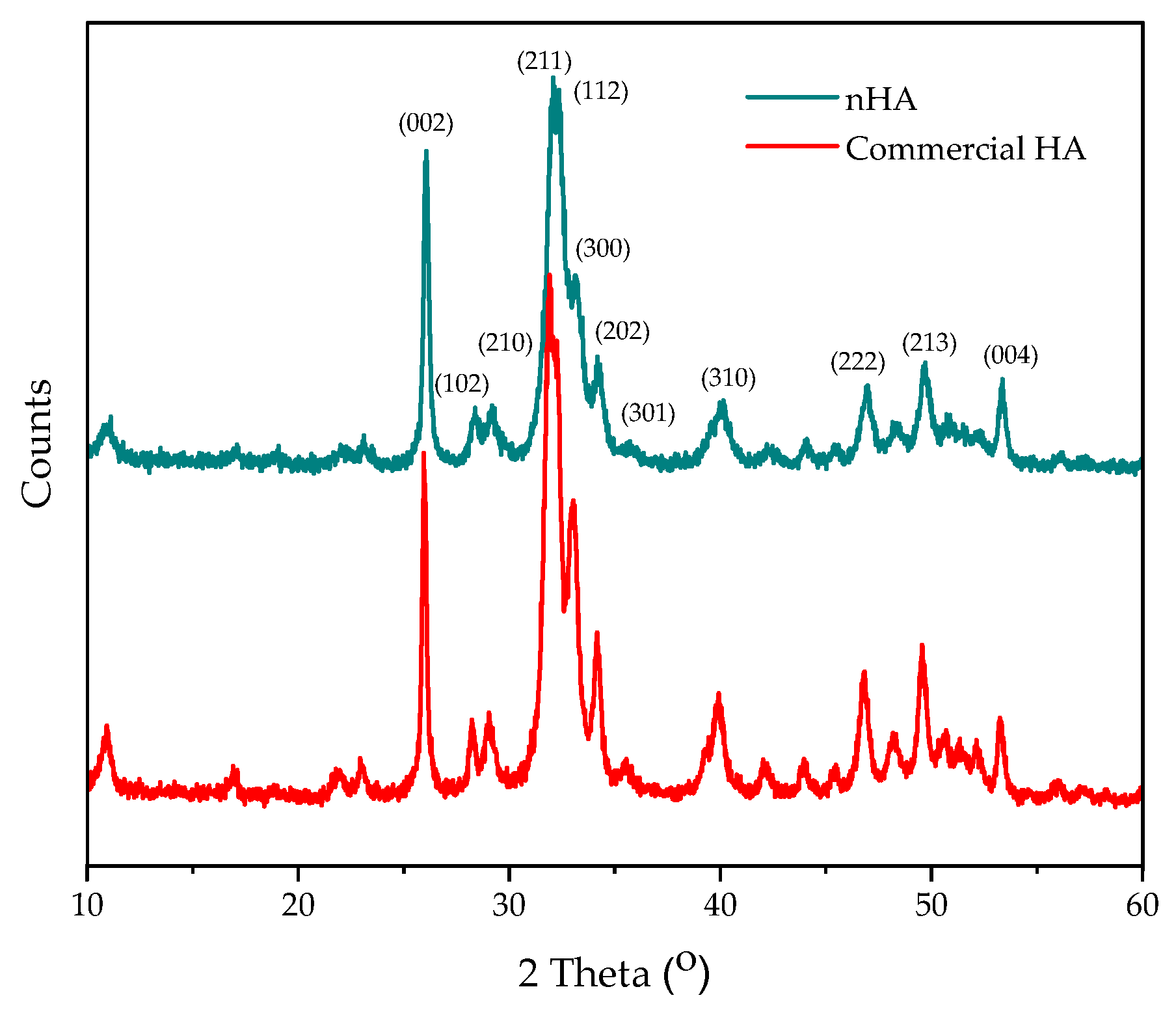
3.1.1. XRD Analysis
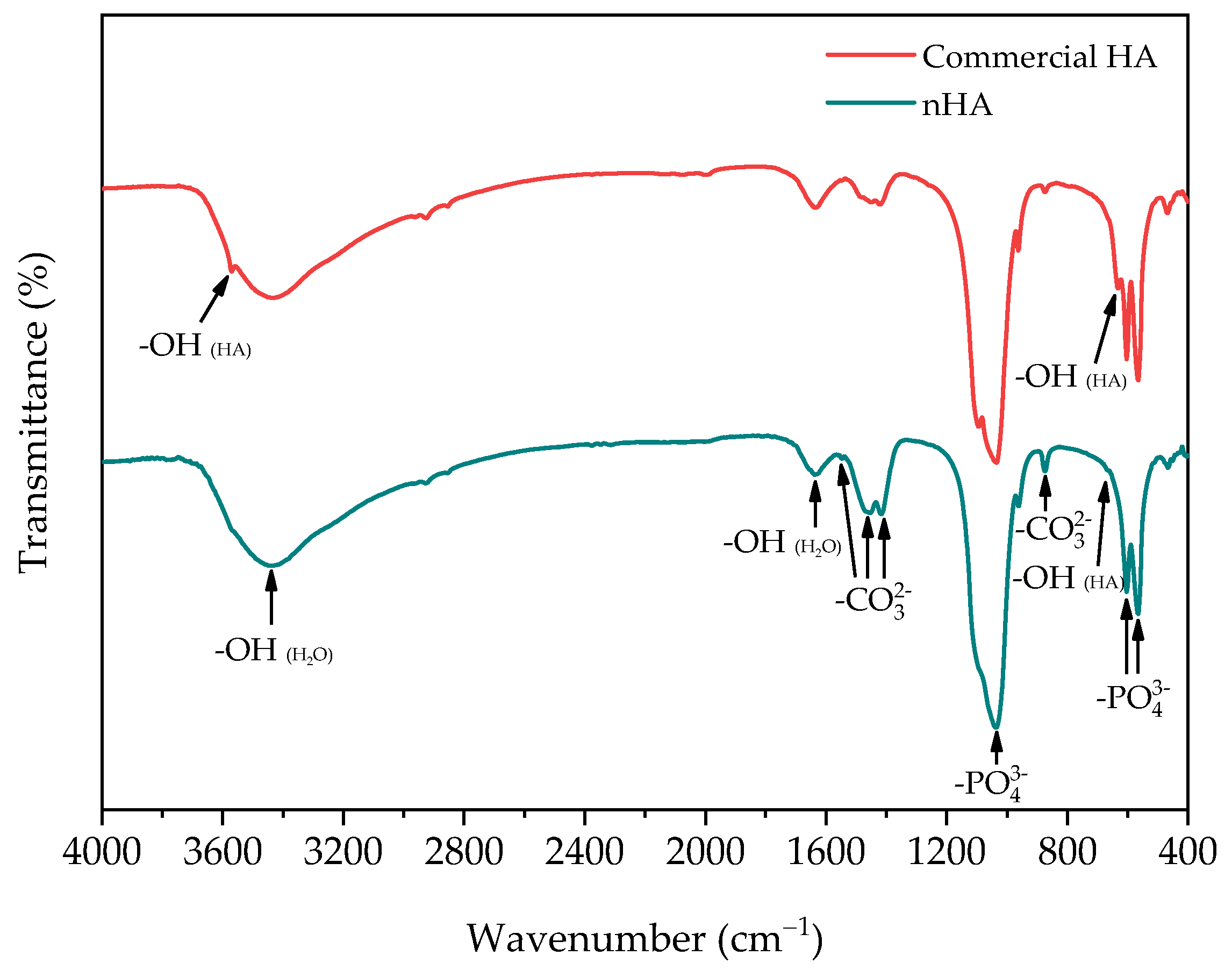
3.1.2. FTIR Analysis
3.1.3. Microstructure Analysis
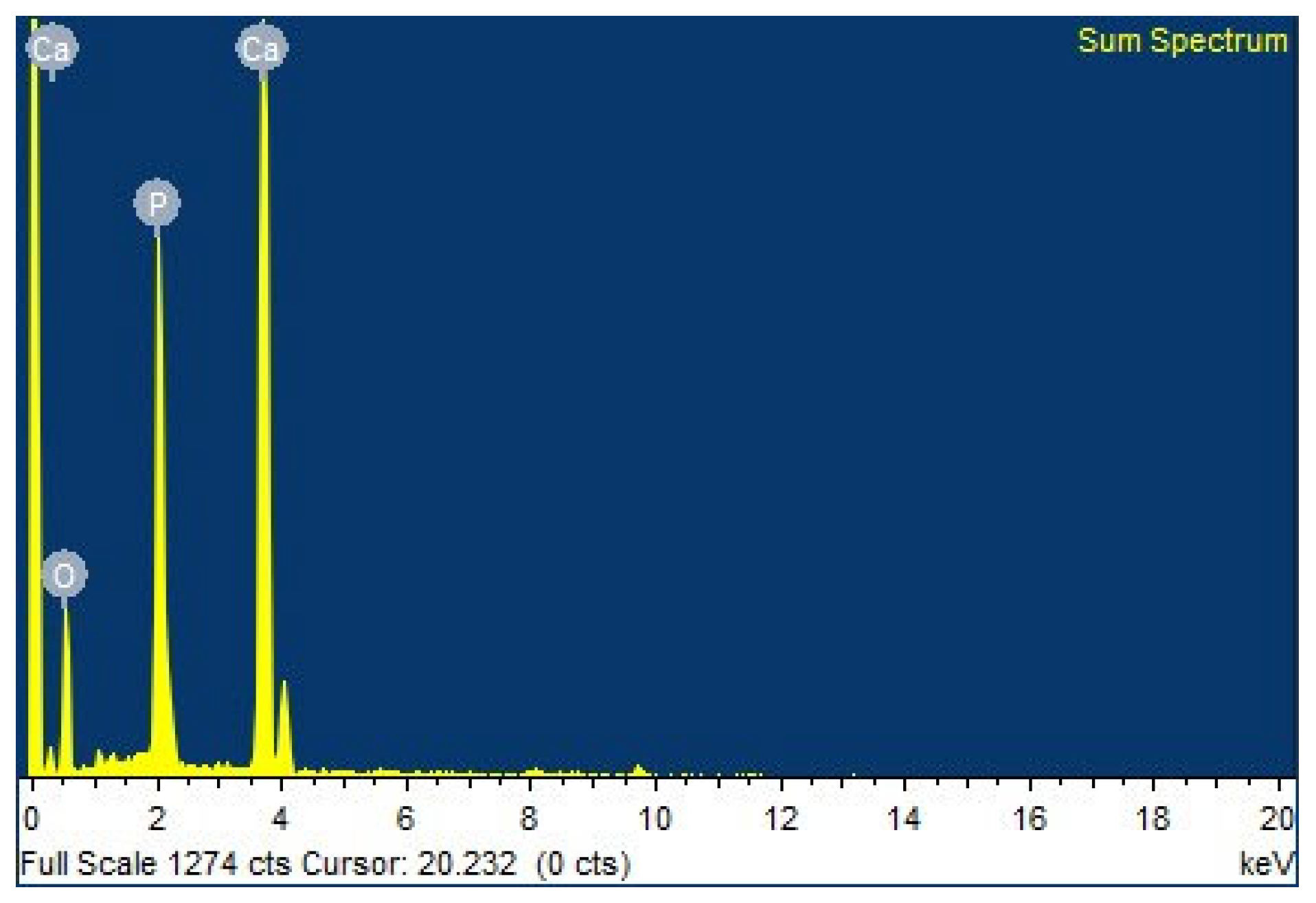
3.1.4. Chemical Elemental Composition Analysis
3.2. Characterization of Neat PLA, PLA-MoNR at Various MoNR Contents, and PLA/nHA Composites with/without MoNR
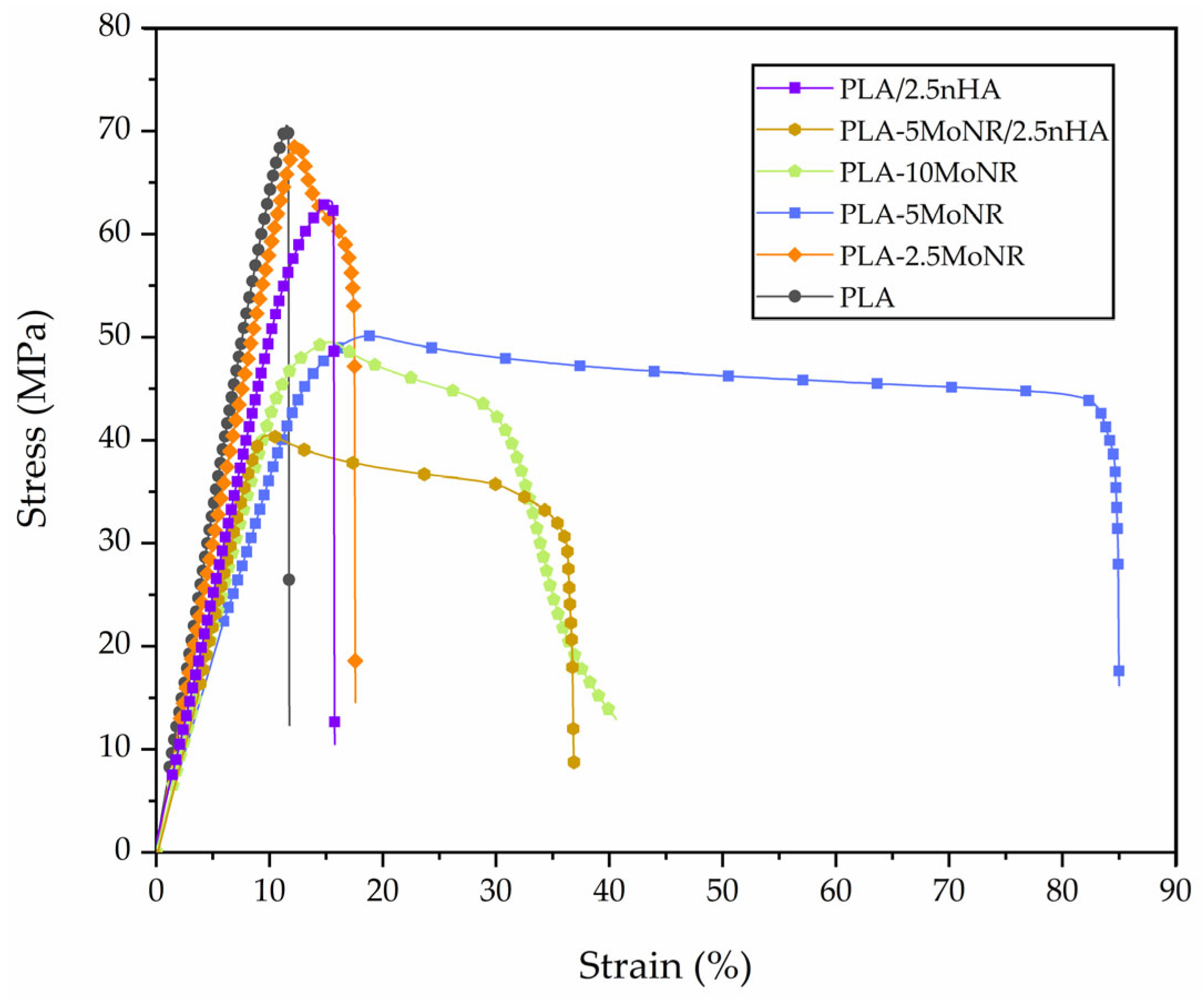
3.2.1. Mechanical Properties
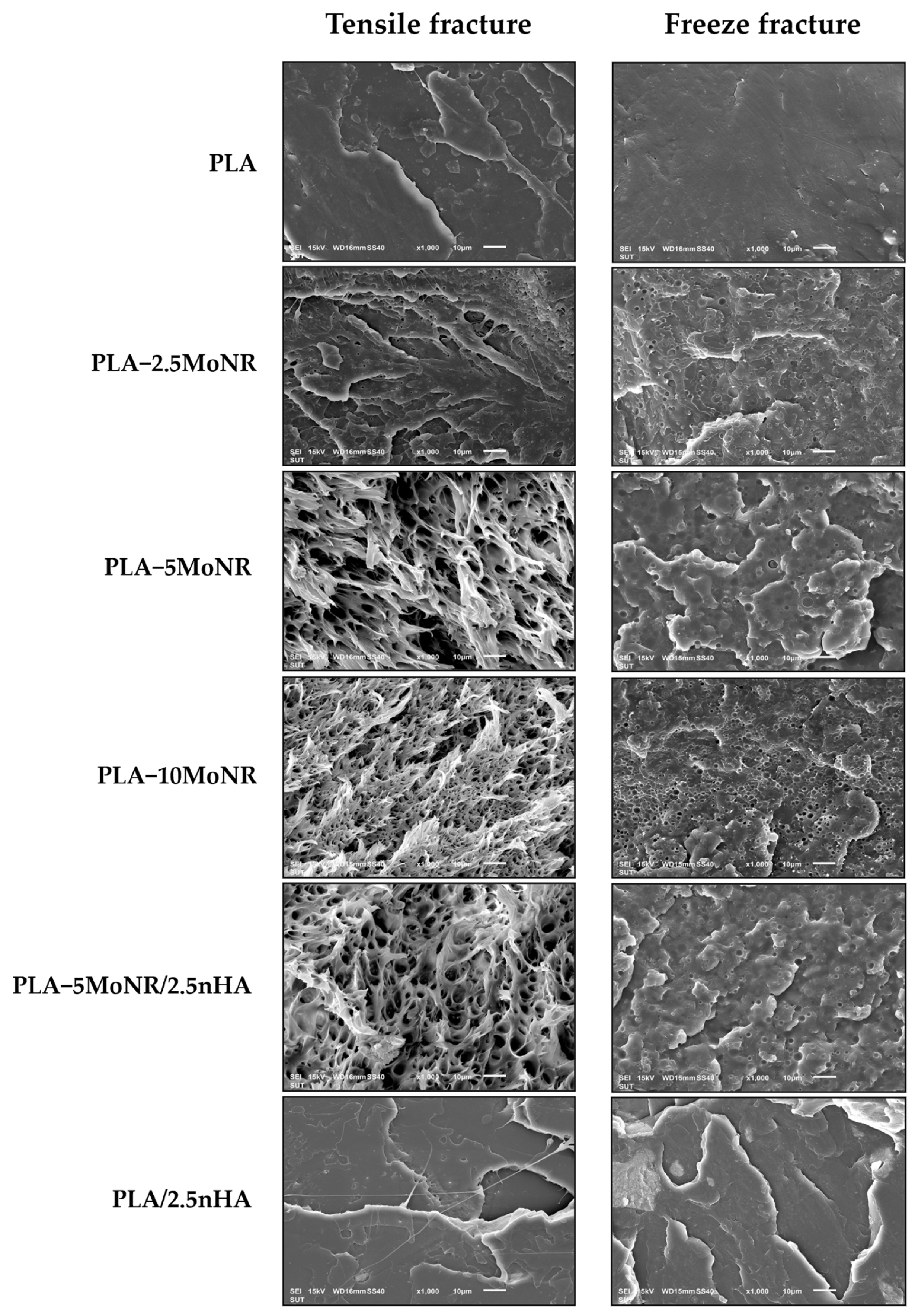
3.2.2. Morphology
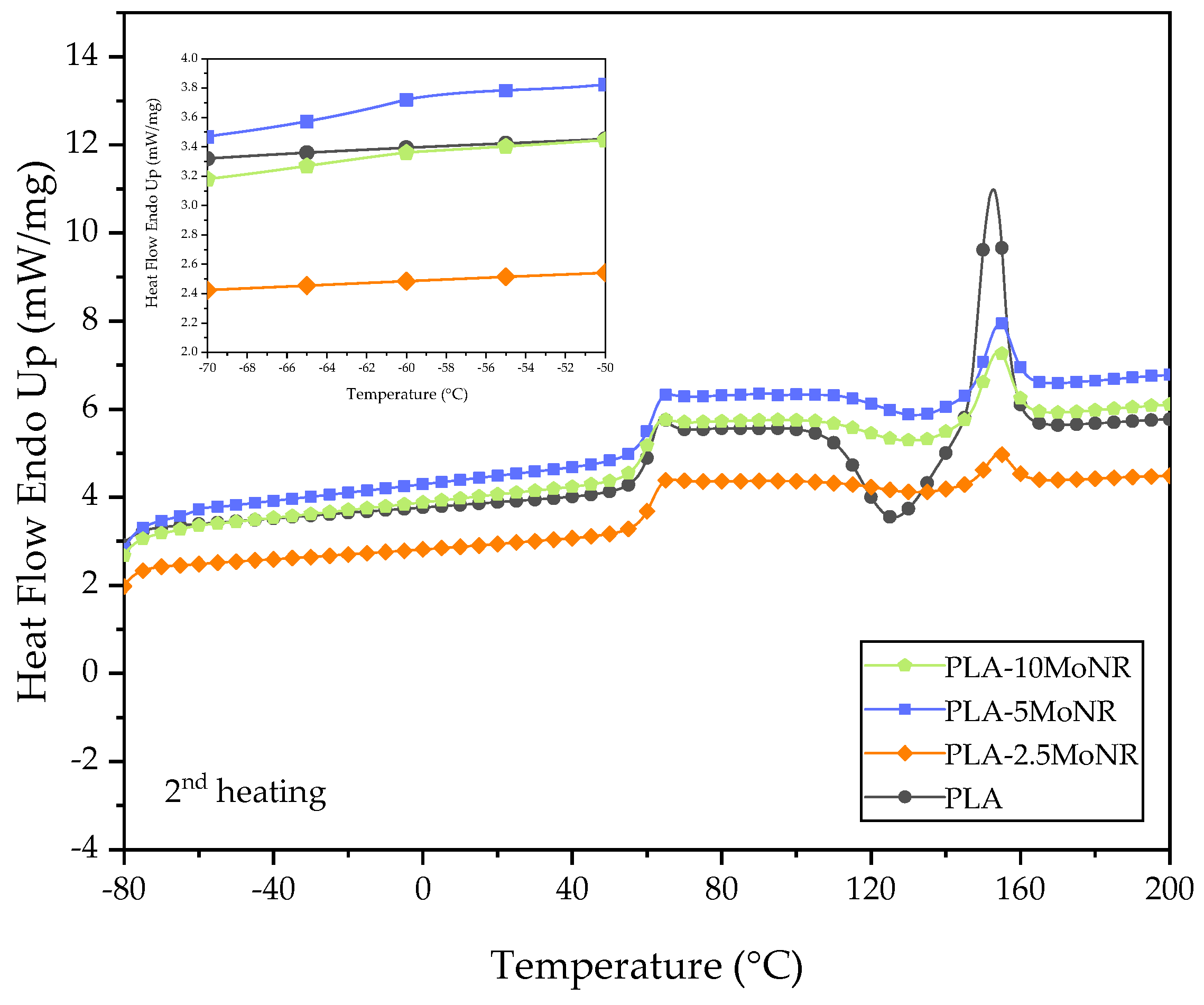
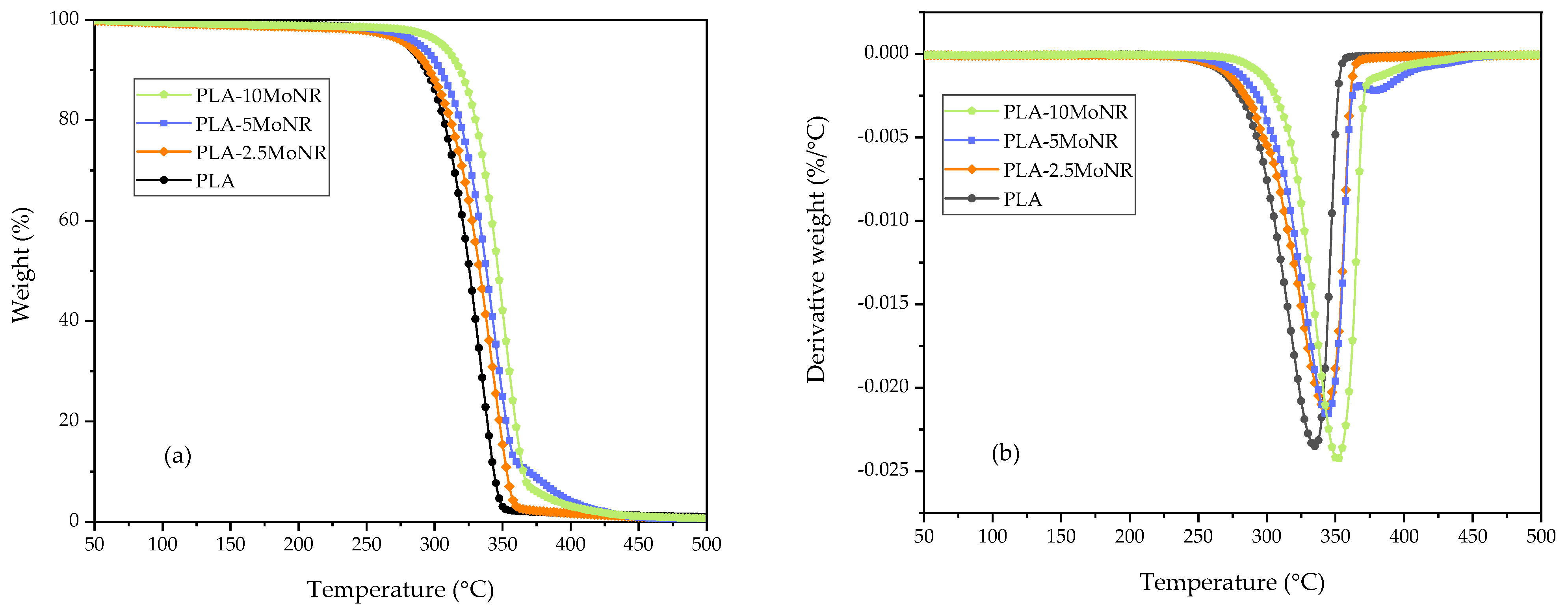
3.2.3. Thermal Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Shi, S.; Yang, F.; Cao, D.; Zhang, K.; Wang, B.; Ma, Z.; Pan, L.; Li, Y. Supertough and Transparent Poly(lactic acid) Nanostructure Blends with Minimal Stiffness Loss. ACS Omega 2020, 5, 13148–13157. [Google Scholar] [CrossRef]
- Wang, M.; Wu, Y.; Li, Y.-D.; Zeng, J.-B. Progress in Toughening Poly(Lactic Acid) with Renewable Polymers. Polym. Rev. 2017, 57, 557–593. [Google Scholar] [CrossRef]
- Edyvean, R.G.J.; Apiwatanapiwat, W.; Vaithanomsat, P.; Boondaeng, A.; Janchai, P.; Sophonthammaphat, S. The Bio-Circular Green Economy model in Thailand—A comparative review. Agric. Nat. Resour. 2023, 57, 51–64. [Google Scholar]
- Papageorgiou, G.Z. Thinking Green: Sustainable Polymers from Renewable Resources. Polymers 2018, 10, 952. [Google Scholar] [CrossRef] [PubMed]
- Mulla, M.Z.; Rahman, M.R.T.; Marcos, B.; Tiwari, B.; Pathania, S. Poly Lactic Acid (PLA) Nanocomposites: Effect of Inorganic Nanoparticles Reinforcement on Its Performance and Food Packaging Applications. Molecules 2021, 26, 1967. [Google Scholar] [CrossRef] [PubMed]
- Hassanajili, S.; Karami-Pour, A.; Oryan, A.; Talaei-Khozani, T. Preparation and characterization of PLA/PCL/HA composite scaffolds using indirect 3D printing for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109960. [Google Scholar] [CrossRef]
- Qin, D.; Sang, L.; Zhang, Z.; Lai, S.; Zhao, Y. Compression Performance and Deformation Behavior of 3D-Printed PLA-Based Lattice Structures. Polymers 2022, 14, 1062. [Google Scholar] [CrossRef] [PubMed]
- Eang, C.; Nim, B.; Opaprakasit, M.; Petchsuk, A.; Opaprakasit, P. Polyester-based polyurethanes derived from alcoholysis of polylactide as toughening agents for blends with shape-memory properties. RSC Adv. 2022, 12, 35328–35340. [Google Scholar] [CrossRef] [PubMed]
- Coudane, J.; Van Den Berghe, H.; Mouton, J.; Garric, X.; Nottelet, B. Poly(Lactic Acid)-Based Graft Copolymers: Syntheses Strategies and Improvement of Properties for Biomedical and Environmentally Friendly Applications: A Review. Molecules 2022, 27, 4135. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, B.; Yang, G.; Gauthier, M. Poly(Lactic Acid)-Based Biomaterials: Synthesis, Modification and Applications. Biomed. Sci. Eng. Technol. 2012, 11, 247–282. [Google Scholar]
- Das, A.; Ringu, T.; Ghosh, S.; Pramanik, N. A comprehensive review on recent advances in preparation, physicochemical characterization, and bioengineering applications of biopolymers. Polym. Bull. 2023, 80, 7247–7312. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Filho, E.A.; Luna, C.B.B.; Ferreira, E.d.S.B.; Siqueira, D.D.; Araújo, E.M. Production of PLA/NR blends compatibilized with EE-g-GMA and POE-g-GMA: An investigation of mechanical, thermal, thermomechanical properties and morphology. J. Polym. Res. 2023, 30, 132. [Google Scholar] [CrossRef]
- Pongpilaipruet, A.; Magaraphan, R. Influence of the admicelled poly(methyl methacrylate) on the compatibility and toughness of poly(lactic acid). J. Mater. Res. 2018, 33, 662–673. [Google Scholar] [CrossRef]
- Buys, Y.; Aznan, A.; Anuar, H. Mechanical properties, morphology, and hydrolytic degradation behavior of polylactic acid/natural rubber blends. IOP Conf. Ser. Mater. Sci. Eng. 2018, 290, 012077. [Google Scholar] [CrossRef]
- Jaratrotkamjorn, R.; Khaokong, C.; Tanrattanakul, V. Toughness enhancement of poly(lactic acid) by melt blending with natural rubber. J. Appl. Polym. Sci. 2012, 124, 5027–5036. [Google Scholar] [CrossRef]
- Pongtanayut, K.; Thongpin, C.; Santawitee, O. The Effect of Rubber on Morphology, Thermal Properties and Mechanical Properties of PLA/NR and PLA/ENR Blends. Energy Procedia 2013, 34, 888–897. [Google Scholar] [CrossRef]
- Gigante, V.; Bosi, L.; Parlanti, P.; Gemmi, M.; Aliotta, L.; Lazzeri, A. Analysis of the Damage Mechanism around the Crack Tip for Two Rubber-Toughened PLA-Based Blends. Polymers 2021, 13, 4053. [Google Scholar] [CrossRef]
- Tessanan, W.; Chanthateyanonth, R.; Yamaguchi, M.; Phinyocheep, P. Improvement of mechanical and impact performance of poly(lactic acid) by renewable modified natural rubber. J. Clean. Prod. 2020, 276, 123800. [Google Scholar] [CrossRef]
- Zhang, C.; Man, C.; Pan, Y.; Wang, W.; Jiang, L.; Dan, Y. Toughening of polylactide with natural rubber grafted with poly(butyl acrylate). Polym. Int. 2011, 60, 1548–1555. [Google Scholar] [CrossRef]
- Udomkitpanya, T.; Srikulkit, K. Properties of Poly(Lactic Acid) Blended with Natural Rubber-Graft-Poly(Acrylic Acid). Key Eng. Mater. 2020, 845, 45–50. [Google Scholar] [CrossRef]
- Ayutthaya, W.D.N.; Poompradub, S. Thermal and mechanical properties of poly(lactic acid)/natural rubber blend using epoxidized natural rubber and poly(methyl methacrylate) as co-compatibilizers. Macromol. Res. 2014, 22, 686–692. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, C.; Pan, Y.; Zhou, Y.; Jiang, L.; Dan, Y. Effect of NR on the hydrolytic degradation of PLA. Polym. Degrad. Stab. 2013, 98, 943–950. [Google Scholar] [CrossRef]
- Chumeka, W.; Tanrattanakul, V.; Pilard, J.-F.; Pasetto, P. Effect of Poly(Vinyl Acetate) on Mechanical Properties and Characteristics of Poly(Lactic Acid)/Natural Rubber Blends. J. Polym. Environ. 2012, 21, 450–460. [Google Scholar] [CrossRef]
- Inphonlek, S.; Bureewong, N.; Jarukumjorn, K.; Chumsamrong, P.; Ruksakulpiwat, C.; Ruksakulpiwat, Y. Preparation of Poly(acrylic acid-co-acrylamide)-Grafted Deproteinized Natural Rubber and Its Effect on the Properties of Natural Rubber/Silica Composites. Polymers 2022, 14, 4602. [Google Scholar] [CrossRef]
- Inphonlek, S.; Jarukumjorn, K.; Chumsamrong, P.; Ruksakulpiwat, C.; Ruksakulpiwat, Y. Preparation of Crosslinked Poly(acrylic acid-co-acrylamide)-Grafted Deproteinized Natural Rubber/Silica Composites as Coating Materials for Controlled Release of Fertilizer. Polymers 2023, 15, 1770. [Google Scholar] [CrossRef] [PubMed]
- Inphonlek, S.; Ruksakulpiwat, C.; Ruksakulpiwat, Y. The Effect of Silver Nanoparticles/Titanium Dioxide in Poly(acrylic acid-co-acrylamide)-Modified, Deproteinized, Natural Rubber Composites on Dye Removal. Polymers 2024, 16, 92. [Google Scholar] [CrossRef]
- Injorhor, P.; Trongsatitkul, T.; Wittayakun, J.; Ruksakulpiwat, C.; Ruksakulpiwat, Y. Biodegradable Polylactic Acid-Polyhydroxyalkanoate-Based Nanocomposites with Bio-Hydroxyapatite: Preparation and Characterization. Polymers 2023, 15, 1261. [Google Scholar] [CrossRef] [PubMed]
- Injorhor, P.; Trongsatitkul, T.; Wittayakun, J.; Ruksakulpiwat, C.; Ruksakulpiwat, Y. Nano-Hydroxyapatite from White Seabass Scales as a Bio-Filler in Polylactic Acid Biocomposite: Preparation and Characterization. Polymers 2022, 14, 4158. [Google Scholar] [CrossRef]
- ASTM D638-14; Standard Test Method for Tensile Properties of Plastics. American Society for Testing and Materials: West Conshohocken, PA, USA, 2014.
- ASTM D256-10; Standard Test Methods for Determining the Izod Pendulum Impact Resistance of Plastics. American Society for Testing and Materials: West Conshohocken, PA, USA, 2010.
- El Boujaady, H.; Mourabet, M.; Abdelhadi, E.R.; Bennani-Ziatni, M.; El Hamri, R.; Abderrahim, T. Adsorption of a textile dye on synthesized calcium deficient hydroxyapatite (CDHAp): Kinetic and thermodynamic studies. J. Mater. Environ. Sci. 2016, 7, 4049–4063. [Google Scholar]
- Amanda, P.; Putri, A.; Masruchin, N.; Kusumaningrum, W.B.; Ningrum, R.S.; Ismadi, I. Autoclave-assisted deacetylation: A rapid method to recycling cigarette butts to cellulose. J. Sains Mater. Indones. 2021, 22, 32–39. [Google Scholar] [CrossRef]
- Kongsri, S.; Janpradit, K.; Buapa, K.; Techawongstien, S.; Chanthai, S. Nanocrystalline hydroxyapatite from fish scale waste: Preparation, characterization and application for selenium adsorption in aqueous solution. J. Chem. Eng. 2013, 215–216, 522–532. [Google Scholar] [CrossRef]
- Pluduma, L.; Gross, K.; Rey, C.; Ubelis, A.; Berzina, A. Production and Characterization of Oxyhydroxyapatites. Key Eng. Mater. 2018, 762, 48–53. [Google Scholar] [CrossRef]
- Guerzoni, S.; Deplaine, H.; Bennagi, J.; Amorós, P.; Pradas, M.; Edlund, U.; Gallego Ferrer, G. Combination of silica nanoparticles with hydroxyapatite reinforces poly (L-lactide acid) scaffolds without loss of bioactivity. J. Bioact. Compat. Polym. 2013, 29, 15–31. [Google Scholar] [CrossRef]
- Coelho, C.C.; Grenho, L.; Gomes, P.S.; Quadros, P.A.; Fernandes, M.H. Nano-hydroxyapatite in oral care cosmetics: Characterization and cytotoxicity assessment. Sci. Rep. 2019, 9, 11050. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, D.; Avram, D.; Angelescu, N.; Catangiu, A.; Anghelina, F.; Despa, V. Comparative Study of Bioceramic Powders Synthesis Based on Calcium and Phosphates. Sci. Bull. Valahia Univ. Mater. Mech. 2018, 16, 13–16. [Google Scholar] [CrossRef]
- Ahmed, Y.; El-Sheikh, S.; Zaki, Z. Changes in hydroxyapatite powder properties via heat treatment. Bull. Mater. Sci. 2015, 38, 1807–1819. [Google Scholar] [CrossRef]
- Kumar, G.; Sivakumar, T.; Sekar, K.; Raji, G.; Girija, E. Fish Scale Derived Nanocrystalline Hydroxyapatite: A Potential Candidate for Orthopedic Applications. J. Bionanosci. 2016, 10, 140–144. [Google Scholar] [CrossRef]
- Granito, R.N.; Muniz Renno, A.C.; Yamamura, H.; de Almeida, M.C.; Menin Ruiz, P.L.; Ribeiro, D.A. Hydroxyapatite from Fish for Bone Tissue Engineering: A Promising Approach. Int. J. Mol. Cell Med. 2018, 7, 80–90. [Google Scholar] [PubMed]
- Zhao, X.; Hu, H.; Wang, X.; Yu, X.; Zhou, W.; Peng, S. Super tough poly(lactic acid) blends: A comprehensive review. RSC Adv. 2020, 10, 13316–13368. [Google Scholar] [CrossRef] [PubMed]
- Tessanan, W.; Phinyocheep, P. Toughening modification of poly(lactic acid) using modified natural rubber. Iran. Polym. J. 2022, 31, 455–469. [Google Scholar] [CrossRef]
- Tertyshnaya, Y.; Karpova, S.; Moskovskiy, M.; Dorokhov, A. Electrospun Polylactide/Natural Rubber Fibers: Effect Natural Rubber Content on Fiber Morphology and Properties. Polymers 2021, 13, 2232. [Google Scholar] [CrossRef] [PubMed]
- Bijarimi, M.; Ahmad, S.; Rasid, R. Mechanical, thermal and morphological properties of poly(lactic acid)/natural rubber nanocomposites. J. Reinf. Plast. Compos. 2013, 32, 1656–1667. [Google Scholar] [CrossRef]
- Juntuek, P.; Ruksakulpiwat, C.; Chumsamrong, P.; Ruksakulpiwat, Y. Effect of glycidyl methacrylate-grafted natural rubber on physical properties of polylactic acid and natural rubber blends. J. Appl. Polym. Sci. 2012, 125, 745–754. [Google Scholar] [CrossRef]




| Designation | PLA (phr 1) | MoNR (phr 1) | nHA (phr 1) |
|---|---|---|---|
| PLA | 100 | - | - |
| PLA-2.5MoNR | 100 | 2.5 | - |
| PLA-5MoNR | 100 | 5 | - |
| PLA-10MoNR | 100 | 10 | - |
| PLA-5MoNR/2.5nHA | 100 | 5 | 2.5 |
| PLA/2.5nHA | 100 | - | 2.5 |
| Elements | Atomic % |
|---|---|
| O | 63.95 |
| P | 13.24 |
| Ca | 22.81 |
| Designation | Young’s Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) | Impact Strength (kJ/m2) |
|---|---|---|---|---|
| PLA | 599 ± 70 | 72.5 ± 4.9 | 14.8 ± 3.9 | 2.7 ± 0.1 |
| PLA-2.5MoNR | 504 ± 85 | 68.7 ± 12.0 | 18.0 ± 1.0 | 2.7 ± 0.2 |
| PLA-5MoNR | 455 ± 94 | 48.4 ± 4.8 | 75.5 ± 13.0 | 3.1 ± 0.4 |
| PLA-10MoNR | 454 ± 57 | 50.0 ± 2.5 | 39.1 ± 3.3 | 6.0 ± 0.5 |
| PLA-5MoNR/2.5nHA | 428 ± 43 | 46.6 ± 7.8 | 33.4 ± 4.1 | 3.3 ± 0.7 |
| PLA/2.5nHA | 527 ± 124 | 66.4 ± 4.9 | 16.1 ± 1.0 | 2.6 ± 0.2 |
| Designation | First Heating | Second Heating | ||||
|---|---|---|---|---|---|---|
| ∆Hcc (Jg−1) | ∆Hm (Jg−1) | Xc (%) | ∆Hcc (Jg−1) | ∆Hm (Jg−1) | Xc (%) | |
| PLA | 18.74 | 22.83 | 4.40 | 16.11 | 16.18 | 0.08 |
| PLA-2.5MoNR | 6.22 | 14.09 | 8.64 | 2.20 | 2.28 | 0.09 |
| PLA-5MoNR | 15.84 | 16.82 | 1.11 | 4.34 | 4.35 | 0.01 |
| PLA-10MoNR | 14.73 | 19.77 | 5.96 | 4.61 | 4.76 | 0.18 |
| PLA-5MoNR/2.5nHA | 13.41 | 14.73 | 1.53 | 3.24 | 3.47 | 0.27 |
| PLA/2.5nHA | 15.55 | 18.84 | 3.61 | 9.43 | 9.45 | 0.02 |
| Designation | Tg PLA (°C) | Tcc (°C) | Tm (°C) |
|---|---|---|---|
| PLA | 61.33 | 126.33 | 152.67 |
| PLA-2.5MoNR | 61.33 | 133.00 | 154.33 |
| PLA-5MoNR | 61.33 | 133.00 | 154.33 |
| PLA-10MoNR | 60.33 | 133.00 | 154.33 |
| PLA-5MoNR/2.5nHA | 60.67 | 131.67 | 153.67 |
| PLA/2.5nHA | 62.33 | 131.67 | 156.00 |
| Designation | (°C) | (°C) |
|---|---|---|
| PLA | 302.82 | 334.83 |
| PLA-2.5MoNR | 311.23 | 342.67 |
| PLA-5MoNR | 314.64 | 343.83 |
| PLA-10MoNR | 326.71 | 351.50 |
| PLA-5MoNR/2.5nHA | 304.92 | 340.00 |
| PLA/2.5nHA | 306.48 | 341.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Injorhor, P.; Inphonlek, S.; Ruksakulpiwat, Y.; Ruksakulpiwat, C. Effect of Modified Natural Rubber on the Mechanical and Thermal Properties of Poly(Lactic Acid) and Its Composites with Nanoparticles from Biowaste. Polymers 2024, 16, 812. https://doi.org/10.3390/polym16060812
Injorhor P, Inphonlek S, Ruksakulpiwat Y, Ruksakulpiwat C. Effect of Modified Natural Rubber on the Mechanical and Thermal Properties of Poly(Lactic Acid) and Its Composites with Nanoparticles from Biowaste. Polymers. 2024; 16(6):812. https://doi.org/10.3390/polym16060812
Chicago/Turabian StyleInjorhor, Preeyaporn, Supharat Inphonlek, Yupaporn Ruksakulpiwat, and Chaiwat Ruksakulpiwat. 2024. "Effect of Modified Natural Rubber on the Mechanical and Thermal Properties of Poly(Lactic Acid) and Its Composites with Nanoparticles from Biowaste" Polymers 16, no. 6: 812. https://doi.org/10.3390/polym16060812







