Investigating How the Properties of Electrospun Poly(lactic acid) Fibres Loaded with the Essential Oil Limonene Evolve over Time under Different Storage Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospinning Process
2.3. Definition of Fibre Groups and Outline of Testing Timelines
2.4. Characterisation of the Electrospun Fibres
2.5. Antibacterial Investigations
3. Results and Discussion
3.1. Characterisation of PLA and PLA–Limonene Electrospun Fibres
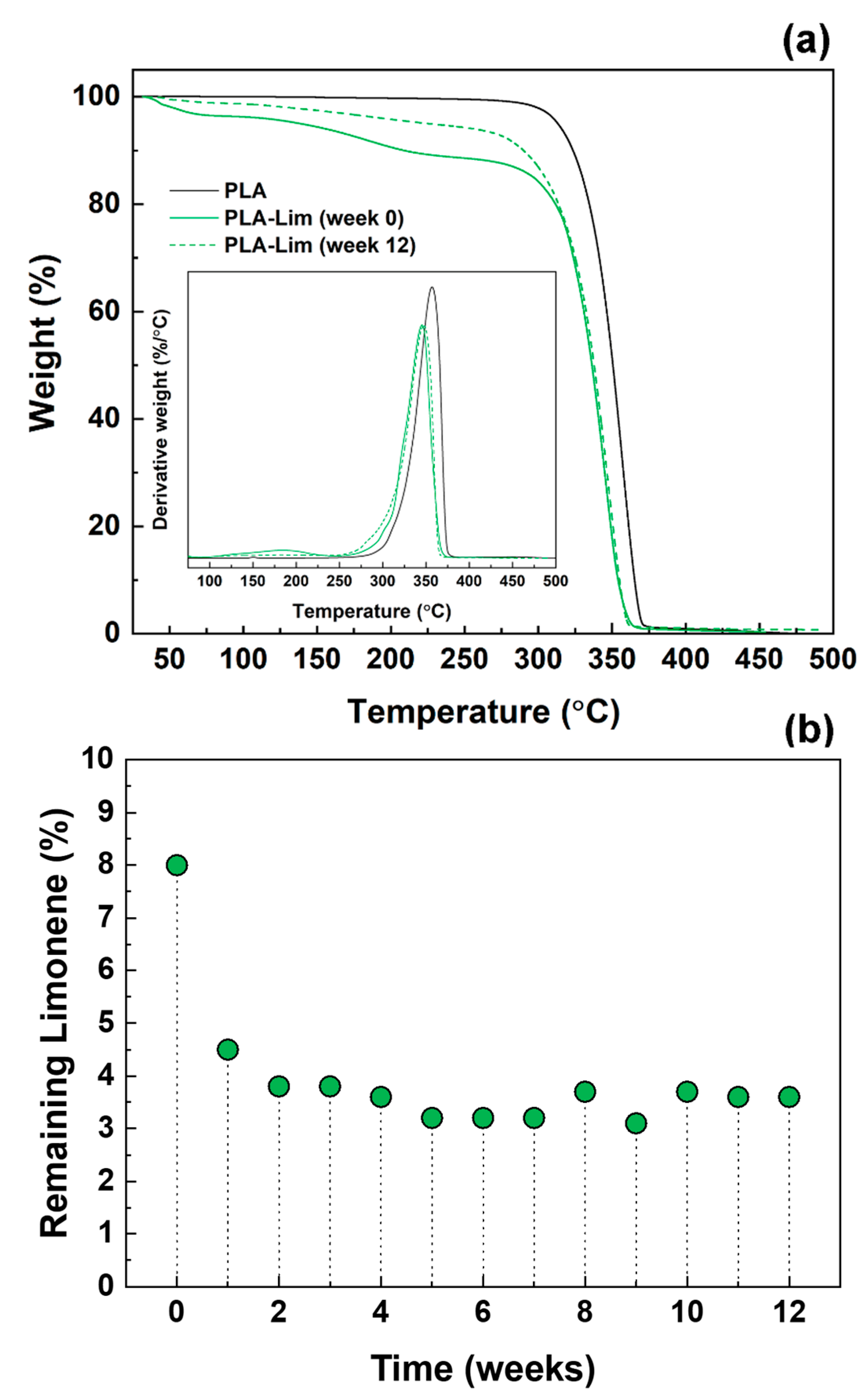
3.2. Analysis of PLA and PLA–Limonene Electrospun Fibres over Time
3.3. Antibacterial Activity of PLA and PLA–Limonene Electrospun Fibres
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liakos, I.; Rizzello, L.; Hajiali, H.; Brunetti, V.; Carzino, R.; Pompa, P.P.; Athanassiou, A.; Mele, E. Fibrous wound dressings encapsulating essential oils as natural antimicrobial agents. J. Mater. Chem. B 2015, 3, 1583–1589. [Google Scholar] [CrossRef] [PubMed]
- Rieger, K.A.; Schiffman, J.D. Electrospinning an essential oil: Cinnamaldehyde enhances the antimicrobial efficacy of chitosan/poly(ethylene oxide) nanofibres. Carbohydr. Polym. 2014, 113, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, C.; Kusmartseva, O.; Thomas, N.L.; Mele, E. Electrospinning of polylactic acid fibres containing tea tree and manuka oil. React. Funct. Polym. 2017, 117, 106–111. [Google Scholar] [CrossRef]
- Schuhladen, K.; Raghu, S.N.V.; Liverani, L.; Nescakova, Z.; Boccaccini, A.R. Production of a novel poly(ε-caprolactone)-methylcellulose electrospun wound dressing by incorporating bioactive glass and Manuka honey. J. Biomed. Mater. Res. 2020, 109, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Karami, Z.; Rezaeian, I.; Zahedi, P.; Abdollahi, M. Preparation and performance evaluations of electrospun poly(ε-caprolactone), and their hybrid (50/50) nanofibrous mats containing thymol as an herbal drug for effective wound healing. J. Appl. Polymer. Sci. 2021, 129, 756–766. [Google Scholar] [CrossRef]
- Hajiali, H.; Summa, M.; Russo, D.; Armirotti, A.; Brunetti, V.; Bertorelli, R.; Athanassiou, A.; Mele, E. Aliginate-lavender nanofibres with antibacterial and anti-inflammatory activity to effectively promote burn healing. J. Mater. Chem. B 2016, 4, 1686–1695. [Google Scholar] [CrossRef] [PubMed]
- Motealleh, B.; Zahedi, P.; Rezaeian, I.; Moghimi, M.; Abdolghaffari, A.H.; Zarandi, M.A. Morphology, drug release, antibacterial, cell proliferation, and histology studies of chamomile-loaded wound dressing mats based on electrospun nanofibrous poly(ε-caprolactone)/polystyrene blends. J. Biomed. Mat. Res. 2013, 102, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ronca, S.; Mele, E. Electrospun Nanofibres Containing Antimicrobial Plant Extracts. Nanomaterials 2017, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Milanesi, G.; Vigani, B.; Rossi, S.; Sandri, G.; Mele, E. Chitosan-coated Poly(lactic acid) nanofibres loaded with essential oils for wound healing. Polymers 2021, 13, 2582. [Google Scholar] [CrossRef]
- Wang, P.; Mele, E. Effect of antibacterial plant extracts on the morphology of electrospun poly(lactic acid) fibres. Materials 2018, 11, 923. [Google Scholar] [CrossRef]
- Güngör Ertuğral, T.; Akçura, S. Preparation and Characterization of Polylactic Acid Based Nanofiber Loaded with Tangerine Peel (Citrus Unshiu) Essential Oil. Celal Bayar Univ. J. Sci. 2023, 19, 283–287. [Google Scholar] [CrossRef]
- Williams, L.; Hatton, F.L.; Willcock, H.; Mele, E. Electrospinning of stimuli-responsive polymers for controlled drug delivery: pH- and temperature-driven release. Biotech. Bioeng. 2022, 119, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, D.M.; Correa, D.S.; Medeiros, E.S.; Oliveira, J.E.; Mattoso, L.H.C. Advances in functional polymer nanofibers: From spinning fabrication techniques to recent biomedical applications. ACS Appl. Mater. Interfaces 2020, 12, 45673–45701. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Shetty, S.; Yadav, K.S. Unfolding the electrospinning potential of biopolymers for preparation of nanofibers. J. Drug Deliv. Sci. Technol. 2020, 57, 101604. [Google Scholar] [CrossRef]
- Doberenz, F.; Zeng, K.; Willems, C.; Zhang, K.; Growth, T. Thermoresponsive polymers and their biomedical application in tissue engineering—A review. J. Mater. Chem. B 2020, 8, 607–628. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Camerlo, A.; Verbet-Nardin, C.; Rossi, R.M.; Popa, A.-M. Fragrance encapsulation in polymeric matrices by emulsion electrospinning. Eur. Polym. J. 2013, 49, 3806–3813. [Google Scholar] [CrossRef]
- Parin, F.N.; Ullah, A.; Yesilyurt, A.; Parin, U.; Haider, M.K.; Kharaghani, D. Development of PVA–psyllium husk meshes via emulsion electrospinning: Preparation, characterization, and antibacterial activity. Polymers 2022, 14, 1490. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Liang, X.; Lan, W.; Ahmed, S.; Liu, Y.; Qin, W. Electrospun Polyvinyl Alcohol/D-limonene fibers prepared by ultrasonic processing for antibacterial active packaging material. Molecules 2019, 24, 767. [Google Scholar] [CrossRef]
- Mahmood, K.; Kamilah, H.; Alias, A.K.; Ariffin, F.; Nafchi, A.M. Functionalization of electrospun fish gelatin mats with bioactive agents: Comparative effect on morphology, thermo-mechanical, antioxidant, antimicrobial properties, and bread shelf stability. Food Sci. Nutr. 2021, 10, 584–596. [Google Scholar] [CrossRef]
- Anandakumar, P.; Kamaraj, S.; Vanitha, M.K. D-limonene: A multifunctional compound with potent therapeutic effects. J. Food Biochem. 2021, 45, e13566. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Sun, Z.; Chen, W. Antimicrobial susceptibility and antibacterial mechanism of limonene against Listeria monocytogenes. Molecules 2020, 5, 33. [Google Scholar] [CrossRef]
- Munoz, J.E.; Rossi, D.C.P.; Jabes, D.L.; Barbosa, D.A.; Cunha, F.F.M.; Nunes, L.R.; Arruda, D.C.; Taborda, C.P. In vitro and in vivo inhibitory activity of limonene against different isolates of Candida spp. J. Fungi 2020, 6, 183. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Sun, Z.; Hui, P.; Chen, W.; Jiang, X. Composite film with antibacterial gold nanoparticles and silk fibroin for treating multidrug-resistant E. coli-infected wounds. ACS Biomater. Sci. Eng. 2020, 7, 1827–1835. [Google Scholar] [CrossRef]
- Madkour, A.E.; Tew, G.N. Towards self-sterilizing medical devices: Controlling infection. Polym. Int. 2008, 57, 6–10. [Google Scholar] [CrossRef]
- Valente, T.A.M.; Silva, D.M.; Gomes, P.S.; Fernandes, M.H.; Santos, J.D.; Sencadas, V. Effect of sterilization methods on electrospun poly (lactic acid) (PLA) fiber alignment for biomedical applications. ACS Appl. Mater. Interf. 2016, 8, 3241–3249. [Google Scholar] [CrossRef]
- Mele, E. Electrospinning of essential oils. Polymers 2020, 12, 908. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, P.; Ye, K.; He, Y.; Chen, S.; Yuan, S.; Chen, F.; Yang, W. Preparation, characterization, in vitro release, and antibacterial activity of oregano essential oil chitosan nanoparticles. Foods 2020, 11, 3756. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Zandi, M.; Rezaei, M.; Farahmandghavi, F. Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: Preparation, characterization and in vitro release study. Carbohydr. Polym 2013, 95, 50–56. [Google Scholar] [CrossRef]
- Barbosa, R.F.D.S.; Yudice, E.D.C.; Mitra, S.K.; Rosa, D.D.S. Characterization of Rosewood and Cinnamon Cassia essential oil polymeric capsules: Stability, loading efficiency, release rate and antimicrobial properties. Food Control. 2021, 121, 107605. [Google Scholar] [CrossRef]
- Dong, Y.; Marshall, J.; Haroosh, H.J.; Mohammadzadehmoghadam, S.; Liu, D.; Qi, X.; Lau, K.-T. Polylactic acid (PLA)/halloysite nanotube (HNT) composite mats: Influence of HNT content and modification. Compos. Part A Appl. Sci. Manufact. 2015, 76, 28–36. [Google Scholar] [CrossRef]
- Wongkanya, R.; Teeranachaideekul, V.; Makarasen, A.; Chuysinuan, P.; Yingyuad, P.; Nooeaid, P.; Techasakul, S.; Chuenchom, L.; Dechtrirat, D. Electrospun poly(lactic acid) nanofiber mats for controlled transdermal delivery of essential oil from Zingiber cassumunar Roxb. Mater. Res. Express 2020, 7, 55305. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Lopez, J.; Ferrandiz, S.; Peltzer, M.A. Characterization of PLA-limonene blends for food packaging applications. Polym. Test. 2013, 32, 760–768. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Lopez, J.; Hernandez, A.; Rayon, E. Ternary PLA-PHB-Limonene blends intended for biodegradable food packaging applications. Eu. Polym. J. 2014, 50, 255–270. [Google Scholar] [CrossRef]
- Fortunati, E.; Luzi, F.; Puglia, D.; Dominici, F.; Santulli, C.; Kenny, J.M.; Torre, L. Investigation of thermo-mechanical, chemical and degradative properties of PLA-limonene films reinforced with cellulose nanocrystals extracted from Phormium tenax leaves. Eu. Polym. J. 2014, 56, 77–91. [Google Scholar] [CrossRef]
- Sepúlveda, F.A.; Rivera, F.; Loyo, C.; Canales, D.; Moreno-Serna, V.; Benavente, R.; Rivas, L.M.; Ulloa, M.T.; Gil-Castell, O.; Ribes-Greus, A.; et al. Poly (lactic acid)/D-limonene/ZnO bio-nanocomposites with antimicrobial properties. J. Appl. Polymer Sci. 2020, 139, 51542. [Google Scholar] [CrossRef]
- Beikzadeh, S.; Akbarinejad, A.; Swift, S.; Perera, J.; Kilmartin, P.A.; Travas-Sejdic, J. Cellulose acetate electrospun nanofibers encapsulating Lemon Myrtle essential oil as active agent with potent and sustainable antimicrobial activity. React. Funct. Polym. 2020, 157, 104769. [Google Scholar] [CrossRef]
- Goksen, G.; Fabra, M.J.; Perez-Cataluna, A.; Ekiz, H.I.; Sanchez, G.; Lopez-Rubio, A. Biodegradable active food packaging structures based on hybrid cross-linked electrospun polyvinyl alcohol fibers containing essential oils and their application in the preservation of chicken breast fillets. Food Packag. Shelf Life 2021, 27, 100613. [Google Scholar] [CrossRef]
- Soltanzadeh, M.; Peighambardoust, S.H.; Ghanbarzadeh, B.; Mohammadi, M.; Lorenzo, J.M. Chitosan nanoparticles encapsulating lemongrass (Cymbopogon commutatus) essential oil: Physicochemical, structural, antimicrobial and in-vitro release properties. Int. J. Biolog. Macromol. 2021, 192, 1084–1097. [Google Scholar] [CrossRef]
- Shetta, A.; Kegere, J.; Mamdouh, W. Comparative study of encapsulated peppermint and green tea essential oils in chitosan nanoparticles: Encapsulation, thermal stability, in-vitro release, antioxidant and antibacterial activities. Int. J. Biolog. Macromol. 2019, 126, 731–742. [Google Scholar] [CrossRef]
- Keawchaoon, L.; Yoksan, R. Preparation, characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids Surf. B Biointerfaces 2011, 84, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Pirani, S.; Abushammala, H.M.N.; Hashaikeh, R. Preparation and characterization of electrospun PLA/nanocrystalline cellulose-based composites. J. Appl. Polym. Sci. 2013, 130, 3345–3354. [Google Scholar] [CrossRef]
- Echeverria, C.; Limon, I.; Munoz-Bonilla, A.; Fernandez-Garcia, M.; Lopez, D. Development of highly crystalline polylactic acid with-crystalline phase from the induced alignment of electrospun fibers. Polymers 2021, 13, 2860. [Google Scholar] [CrossRef]
- Suner, S.C.; Yildirim, Y.; Yurt, F.; Ozel, D.; Oral, A.; Ozturk, I. Antibiotic loaded electrospun poly (lactic acid) nanofiber mats for drug delivery system. J. Drug Deliv. Sci. Technol. 2022, 71, 103263. [Google Scholar] [CrossRef]
- Leones, A.; Salaris, V.; Mujica-Garcia, A.; Arrieta, M.P.; Lopez, D.; Lieblich, M.; Kenny, J.M.; Peponi, L. PLA electrospun fibers reinforced with organic and inorganic nanoparticles: A comparative study. Molecules 2021, 26, 4925. [Google Scholar] [CrossRef] [PubMed]
- Bruster, B.; Adjoua, Y.-O.; Dieden, R.; Grysan, P.; Federico, C.E.; Berthe, V.; Addiego, F. Plasticization of polylactide with myrcene and limonene as bio-based plasticizers: Conventional vs. reactive extrusion. Polymers 2019, 11, 1363. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Jeyakumar, E.; Lawrence, R. Strategic approach of multifaceted antibacterial mechanism of limonene traced in Escherichia coli. Sci. Rep. 2021, 11, 13816. [Google Scholar] [CrossRef]
- Espina, L.; Gelaw, T.K.; Lamo-Castellvi, S.D.; Pagan, R.; Garcia-Gonzalo, D. Mechanism of bacterial inactivation by (+)-limonene and its potential use in food preservation combined processes. PLoS ONE 2013, 8, e56769. [Google Scholar] [CrossRef]
- Han, Y.; Chen, W.; Sun, Z. Antimicrobial activity and mechanism of limonene against Staphylococcus aureus. J. Food Saf. 2021, 41, e12918. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Dey, A.; Pandit, S.; Joshi, S.; Upadhye, V.J.; Ray, R.R. Citrus essential eils: A treasure trove of antibioflm agent. Appl. Biochem. Biotech. 2022, 194, 4625–4638. [Google Scholar] [CrossRef]
- Mancuso, M.; Catalfamo, M.; Lagana, P.; Rappazzo, A.C.; Raymo, V.; Zampino, D.; Zaccone, R. Screening of antimicrobial activity of citrus essential oils against pathogenic bacteria and Candida strains. Flavour Fragr. J. 2019, 34, 187–200. [Google Scholar] [CrossRef]
- Ambrosio, C.M.S.; Ikeda, N.Y.; Miano, A.C.; Saldana, E.; Moreno, A.M.; Stashenko, E.; Contreras-Castillo, C.J.; Da Gloria, E.M. Unraveling the selective antibacterial activity and chemical composition of citrus essential oils. Sci. Rep. 2019, 9, 17719. [Google Scholar] [CrossRef] [PubMed]




| Fibre Batch Details | Reference Name | Timepoints Tested (Weeks) |
|---|---|---|
| PLA nanofibres without any additives, stored at ~20 °C | PLA | Various between 0 and 12 |
| PLA nanofibres loaded with 10 v/v% limonene, stored at ~20 °C | PLA–Lim | Various between 0 and 12 |
| PLA nanofibres loaded with 10 v/v% limonene, stored in sealed conditions (in either Petri dishes or DSC pans). | Sealed PLA–Lim | Various between 0 and 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, L.; Hatton, F.L.; Righetti, M.C.; Mele, E. Investigating How the Properties of Electrospun Poly(lactic acid) Fibres Loaded with the Essential Oil Limonene Evolve over Time under Different Storage Conditions. Polymers 2024, 16, 1005. https://doi.org/10.3390/polym16071005
Williams L, Hatton FL, Righetti MC, Mele E. Investigating How the Properties of Electrospun Poly(lactic acid) Fibres Loaded with the Essential Oil Limonene Evolve over Time under Different Storage Conditions. Polymers. 2024; 16(7):1005. https://doi.org/10.3390/polym16071005
Chicago/Turabian StyleWilliams, Leah, Fiona L. Hatton, Maria Cristina Righetti, and Elisa Mele. 2024. "Investigating How the Properties of Electrospun Poly(lactic acid) Fibres Loaded with the Essential Oil Limonene Evolve over Time under Different Storage Conditions" Polymers 16, no. 7: 1005. https://doi.org/10.3390/polym16071005
APA StyleWilliams, L., Hatton, F. L., Righetti, M. C., & Mele, E. (2024). Investigating How the Properties of Electrospun Poly(lactic acid) Fibres Loaded with the Essential Oil Limonene Evolve over Time under Different Storage Conditions. Polymers, 16(7), 1005. https://doi.org/10.3390/polym16071005









