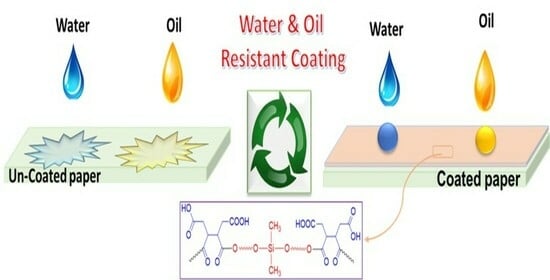
Synthesis of Water-Dispersible Poly(dimethylsiloxane) and Its Potential Application in the Paper Coating Industry as an Alternative for PFAS-Coated Paper and Single-Use Plastics
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
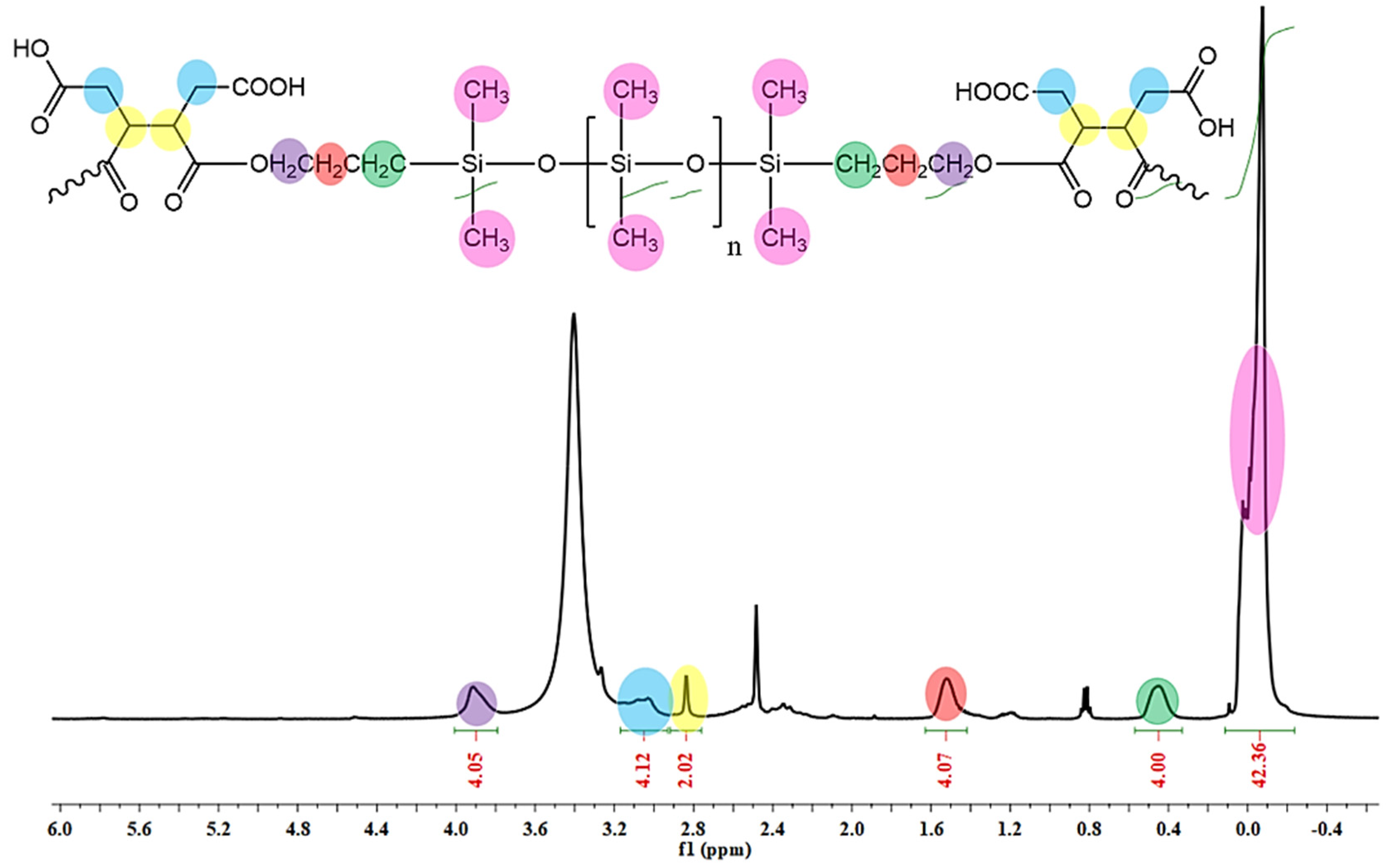
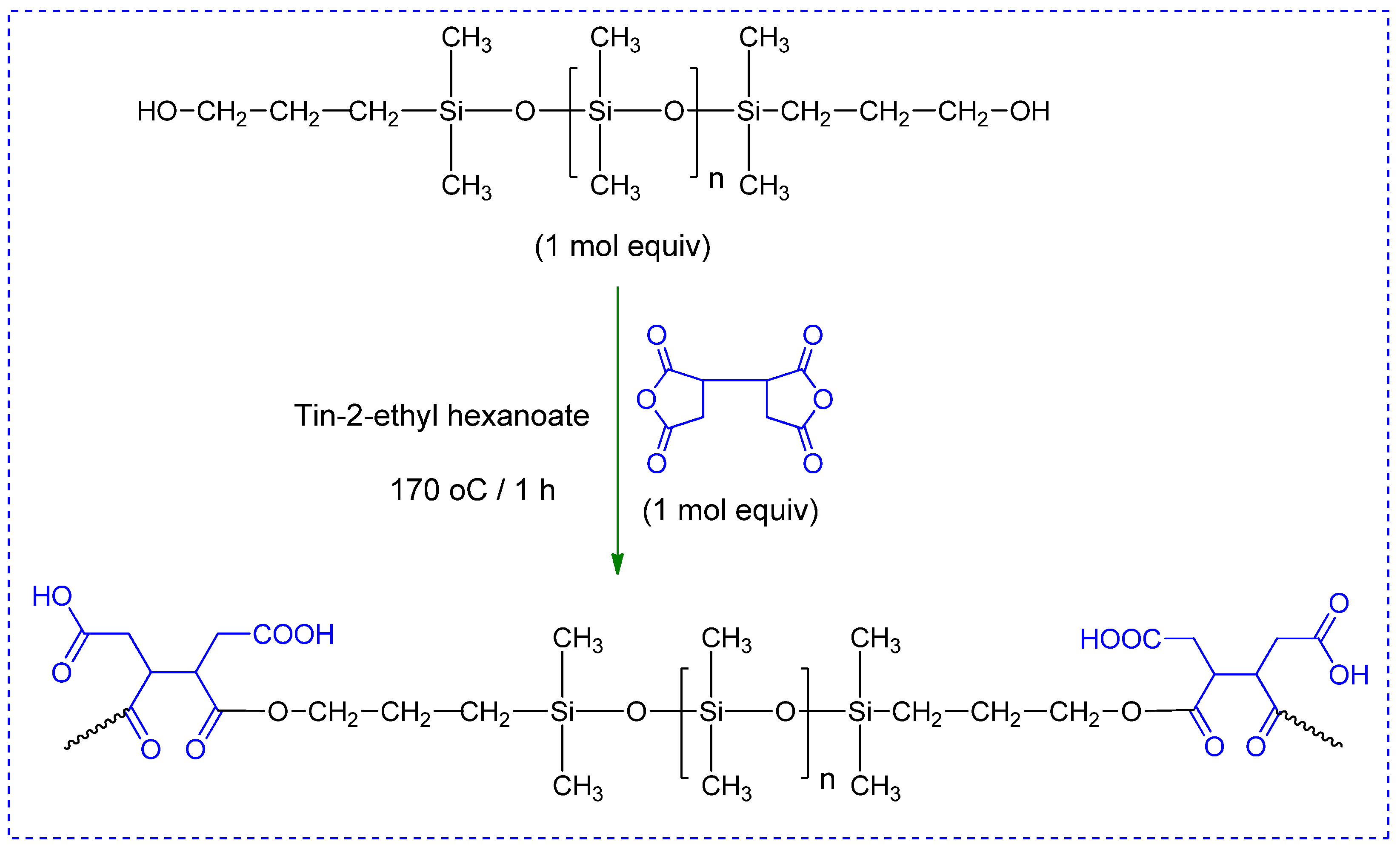
2.2.1. Synthesis of Carboxylic-Functionalized PDMS (PDMS-COOH)
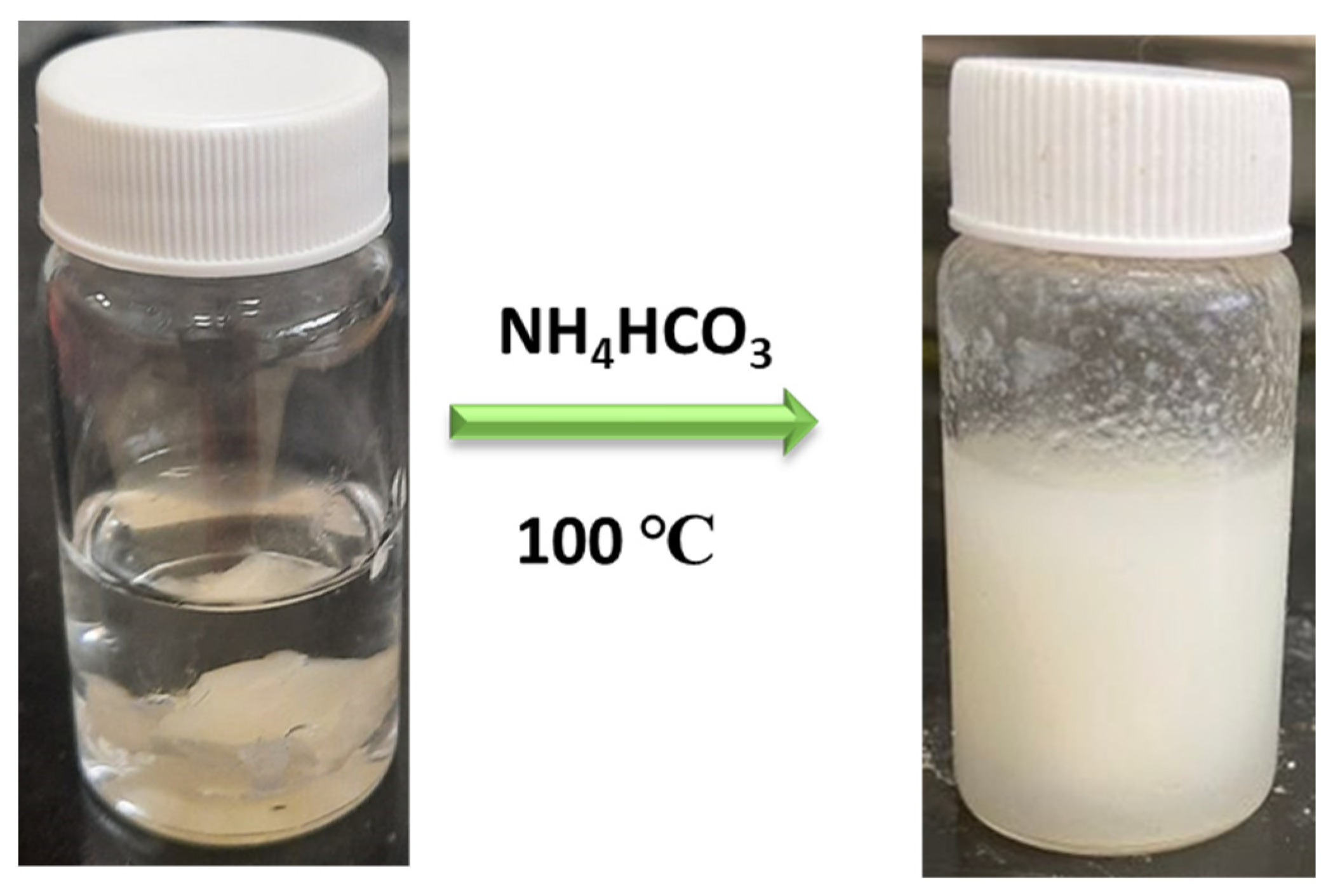
2.2.2. Emulsification of PDMS-COOH
2.2.3. Preparation of Starch Solution
2.2.4. Preparation of Starch and PDMS-COOH Mixture Solution
2.2.5. Paper Coating Procedure
3. Characterization
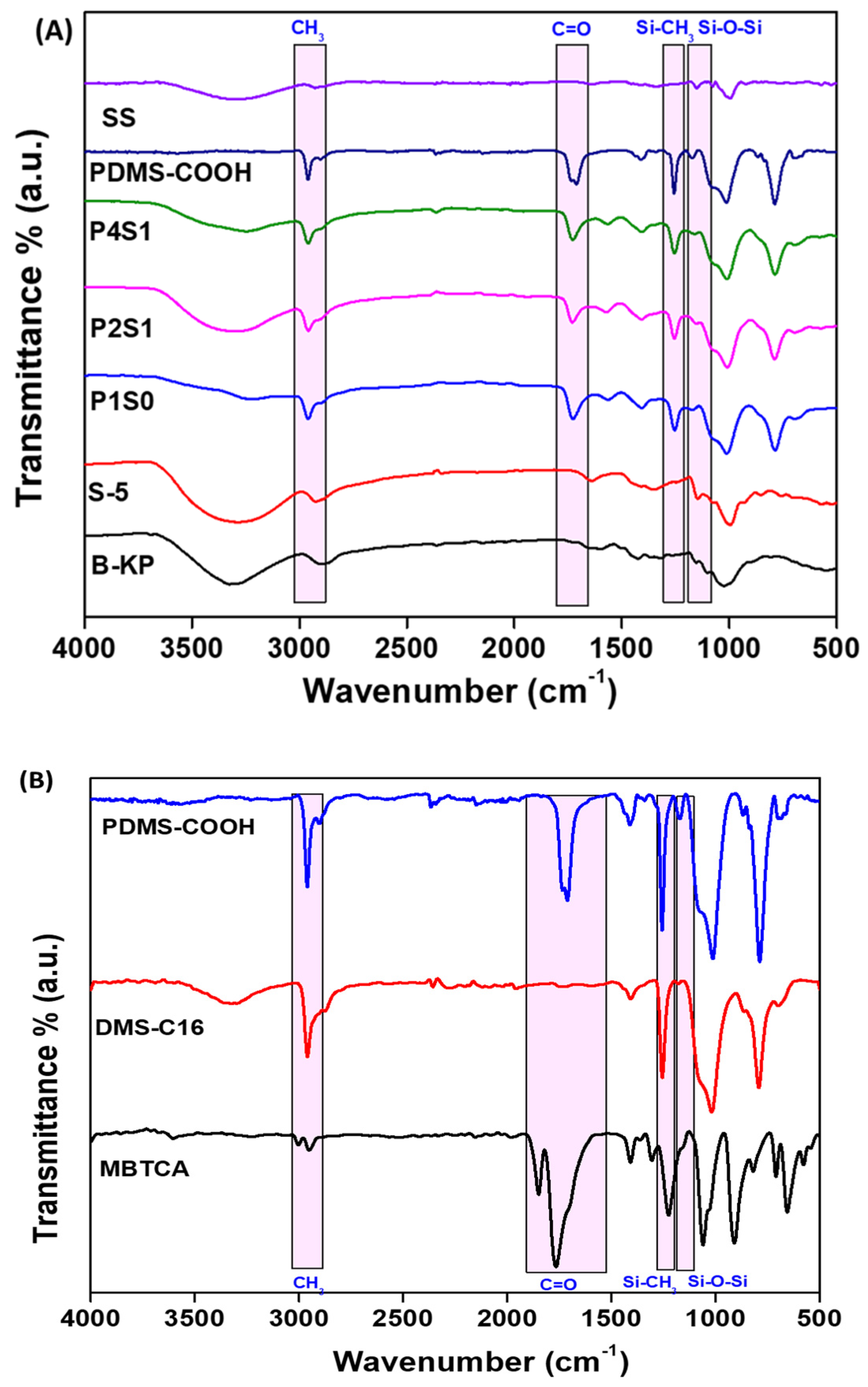
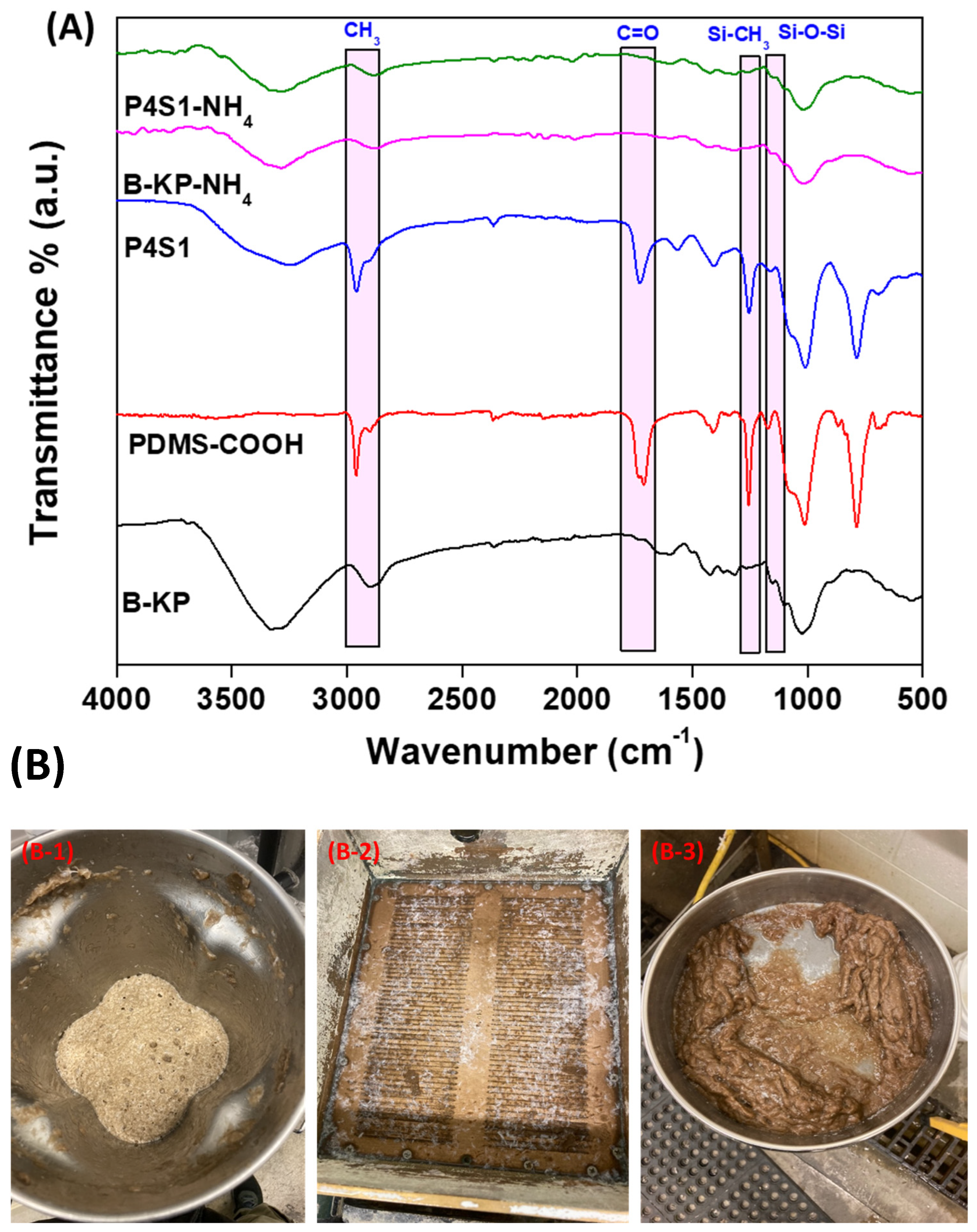
3.1. ATR-FTIR Analysis
3.2. 1H-NMR Analysis
3.3. Basis Weight and Thickness
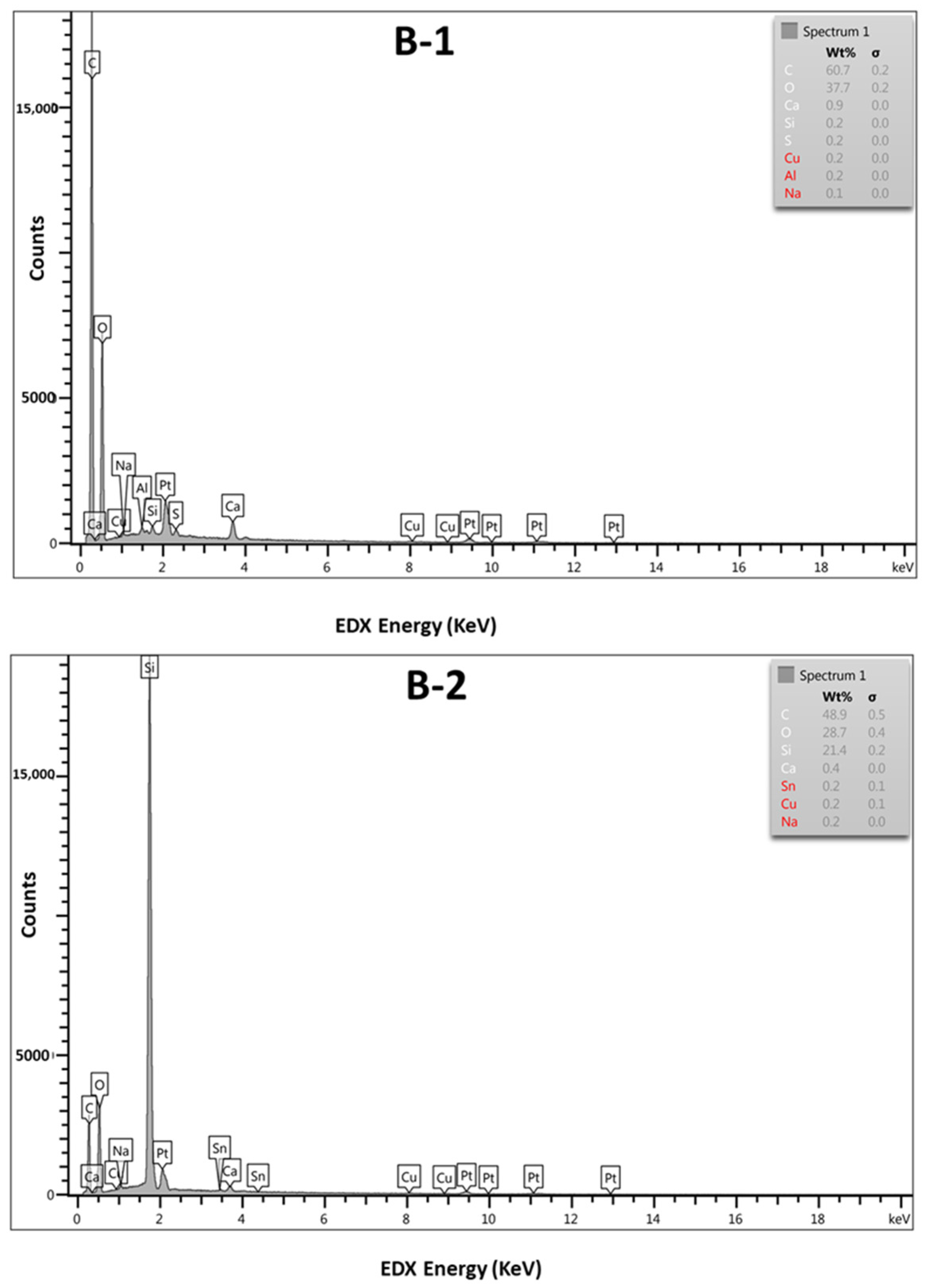
3.4. Scanning Electron Microscopy (SEM)/Energy-Dispersive X-ray Spectroscopy (EDX)
3.5. Water Resistance
3.6. Water Vapor Transmission Measurements
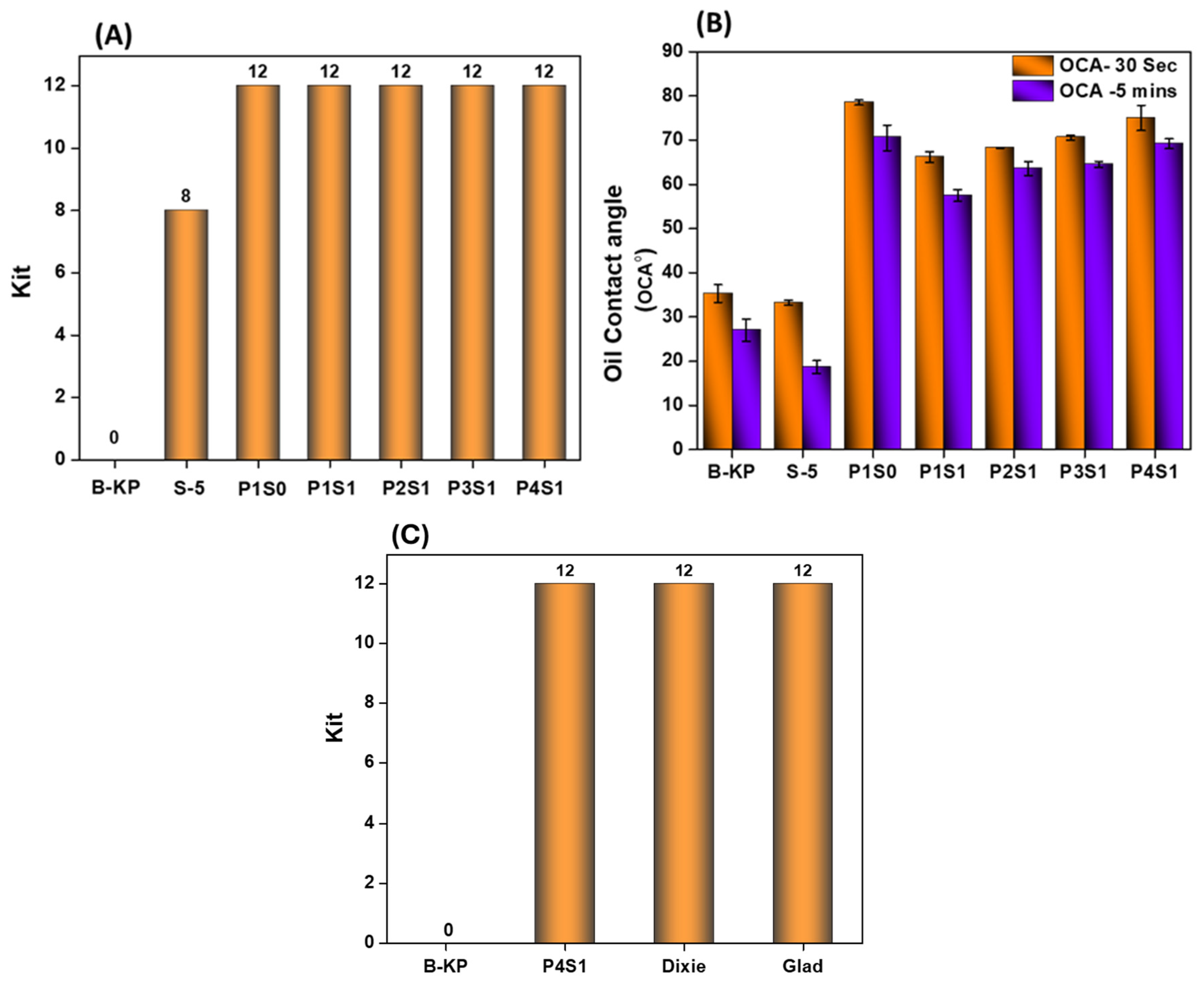
3.7. Oil Resistance Properties
3.8. Thermogravimetric Analysis (TGA)
3.9. Contact Angle Measurements
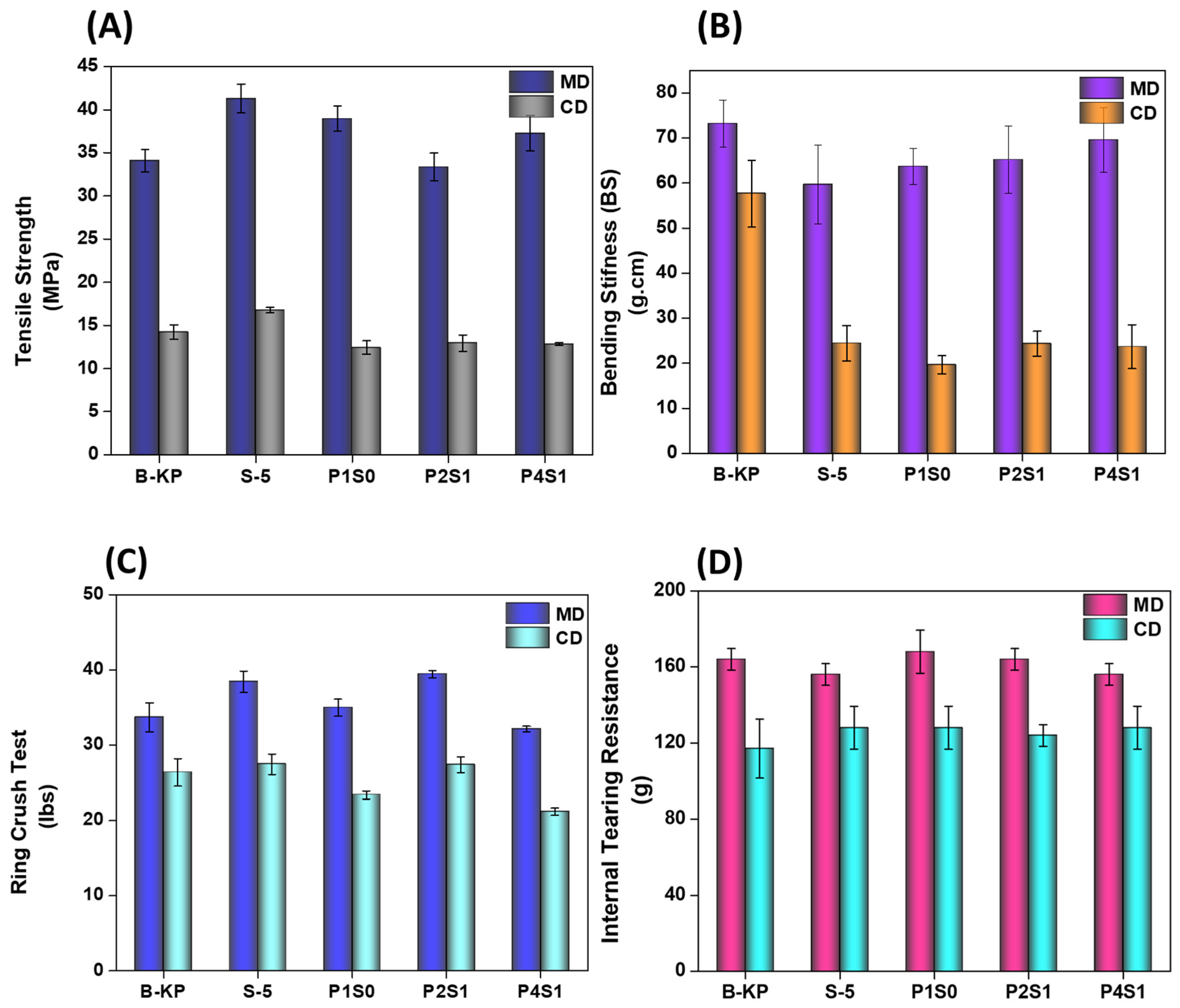
3.10. Tensile Measurements
3.11. Ring Crush Test (RCT)
3.12. Bending Stiffness (BS)
3.13. Internal Tearing Resistance (ITR)
3.14. Recyclability
4. Results and Discussion
4.1. Basis Weight and Thickness
4.2. FTIR Analysis
4.3. Scanning Electron Microscopy (SEM) Analysis
4.4. Water Resistance
4.5. Liquid Water Resistance
4.6. Water Vapor Barrier Properties
4.7. Oil Resistance
4.8. TGA Analysis
4.9. Mechanical Properties
4.10. Recyclability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hamdani, S.S.; Li, Z.; Rabnawaz, M.; Kamdem, D.P.; Khan, B.A. Chitosan–Graft–Poly(dimethylsiloxane)/Zein Coatings for the Fabrication of Environmentally Friendly Oil- and Water-Resistant Paper. ACS Sustain. Chem. Eng. 2020, 8, 5147–5155. [Google Scholar] [CrossRef]
- Dwivedi, S.; Nag, A.; Sakamoto, S.; Funahashi, Y.; Harimoto, T.; Takada, K.; Kaneko, T. High-temperature resistant water-soluble polymers derived from exotic amino acids. RSC Adv. 2020, 10, 38069–38074. [Google Scholar] [CrossRef]
- Li, Z.; Rabnawaz, M.; Sarwar, M.G.; Khan, B.; Krishna Nair, A.; Sirinakbumrung, N.; Kamdem, D.P. A closed-loop and sustainable approach for the fabrication of plastic-free oil- and water-resistant paper products. Green Chem. 2019, 21, 5691–5700. [Google Scholar] [CrossRef]
- Ng, E.-L.; Huerta Lwanga, E.; Eldridge, S.M.; Johnston, P.; Hu, H.-W.; Geissen, V.; Chen, D. An overview of microplastic and nanoplastic pollution in agroecosystems. Sci. Total Environ. 2018, 627, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Awasthi, A.K.; Wei, F.; Tan, Q.; Li, J. Single-use plastics: Production, usage, disposal, and adverse impacts. Sci. Total Environ. 2021, 752, 141772. [Google Scholar] [CrossRef]
- Jiang, J.-Q. Occurrence of microplastics and its pollution in the environment: A review. Sustain. Prod. Consum. 2018, 13, 16–23. [Google Scholar] [CrossRef]
- Yadav, S.; Khan, A.; Hamdani, S.S.; Rabnawaz, M. Degradable Polymeric Waxes for Paper Coating Applications. ACS Appl. Polym. Mater. 2024, 6, 3263–3272. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Johnson, L.M.; Gao, L.; Shields Iv, C.W.; Smith, M.; Efimenko, K.; Cushing, K.; Genzer, J.; López, G.P. Elastomeric microparticles for acoustic mediated bioseparations. J. Nanobiotechnol. 2013, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Elliott, T.; Gillie, H.; Thomson, A. Chapter 24—European Union’s plastic strategy and an impact assessment of the proposed directive on tackling single-use plastics items. In Plastic Waste and Recycling; Letcher, T.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 601–633. [Google Scholar]
- Available online: https://usplasticspact.org/ (accessed on 30 March 2024).
- Wagner, T.P. Reducing single-use plastic shopping bags in the USA. Waste Manag. 2017, 70, 3–12. [Google Scholar] [CrossRef]
- Begley, T.H.; Hsu, W.; Noonan, G.; Diachenko, G. Migration of fluorochemical paper additives from food-contact paper into foods and food simulants. Food Addit. Contam. Part A 2008, 25, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Schaider, L.A.; Balan, S.A.; Blum, A.; Andrews, D.Q.; Strynar, M.J.; Dickinson, M.E.; Lunderberg, D.M.; Lang, J.R.; Peaslee, G.F. Fluorinated Compounds in U.S. Fast Food Packaging. Environ. Sci. Technol. Lett. 2017, 4, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Langberg, H.A.; Arp, H.P.H.; Breedveld, G.D.; Slinde, G.A.; Høiseter, Å.; Grønning, H.M.; Jartun, M.; Rundberget, T.; Jenssen, B.M.; Hale, S.E. Paper product production identified as the main source of per- and polyfluoroalkyl substances (PFAS) in a Norwegian lake: Source and historic emission tracking. Environ. Pollut. 2021, 273, 116259. [Google Scholar] [CrossRef] [PubMed]
- Glenn, G.; Shogren, R.; Jin, X.; Orts, W.; Hart-Cooper, W.; Olson, L. Per- and polyfluoroalkyl substances and their alternatives in paper food packaging. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2596–2625. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, A.B.; Strynar, M.J.; Libelo, E.L. Polyfluorinated Compounds: Past, Present, and Future. Environ. Sci. Technol. 2011, 45, 7954–7961. [Google Scholar] [CrossRef] [PubMed]
- Hamdani, S.S.; Li, Z.; Rolland, E.; Mohiuddin, M.; Rabnawaz, M. Barrier and mechanical properties of biodegradable paper bilayer-coated with plasticized starch and zein. J. Appl. Polym. Sci. 2023, 140, e53440. [Google Scholar] [CrossRef]
- Reis, A.B.; Yoshida, C.M.P.; Reis, A.P.C.; Franco, T.T. Application of chitosan emulsion as a coating on Kraft paper. Polym. Int. 2011, 60, 963–969. [Google Scholar] [CrossRef]
- Wang, W.; Qin, C.; Li, W.; Ge, J.; Feng, C. Improving moisture barrier properties of paper sheets by cellulose stearoyl ester-based coatings. Carbohydr. Polym. 2020, 235, 115924. [Google Scholar] [CrossRef] [PubMed]
- Rodionova, G.; Lenes, M.; Eriksen, Ø.; Gregersen, Ø. Surface chemical modification of microfibrillated cellulose: Improvement of barrier properties for packaging applications. Cellulose 2011, 18, 127–134. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, H.; Qian, L. Enhanced water vapour barrier and grease resistance of paper bilayer-coated with chitosan and beeswax. Carbohydr. Polym. 2014, 101, 401–406. [Google Scholar] [CrossRef]
- Hamdani, S.S.; Li, Z.; Sirinakbumrung, N.; Rabnawaz, M. Zein and PVOH-Based Bilayer Approach for Plastic-Free, Repulpable and Biodegradable Oil- and Water-Resistant Paper as a Replacement for Single-Use Plastics. Ind. Eng. Chem. Res. 2020, 59, 17856–17866. [Google Scholar] [CrossRef]
- Almeida, C.B.d.; Corradini, E.; Forato, L.A.; Fujihara, R.; Lopes Filho, J.F. Microstructure and thermal and functional properties of biodegradable films produced using zein. Polímeros 2018, 28, 30–37. [Google Scholar] [CrossRef]
- Zou, Y.; Hsieh, J.S.; Mehnert, E.; Kokoszka, J. The study of PET recyclable polymers as paper coatings. Prog. Org. Coat. 2007, 60, 127–131. [Google Scholar] [CrossRef]
- Peng, L.; Fu, D.; Qi, H.; Lan, C.Q.; Yu, H.; Ge, C. Micro- and nano-plastics in marine environment: Source, distribution and threats—A review. Sci. Total Environ. 2020, 698, 134254. [Google Scholar] [CrossRef] [PubMed]
- Gunaalan, K.; Fabbri, E.; Capolupo, M. The hidden threat of plastic leachates: A critical review on their impacts on aquatic organisms. Water Res. 2020, 184, 116170. [Google Scholar] [CrossRef] [PubMed]
- Curtzwiler, G.W.; Silva, P.; Hall, A.; Ivey, A.; Vorst, K. Significance of Perfluoroalkyl Substances (PFAS) in Food Packaging. Integr. Environ. Assess. Manag. 2021, 17, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Surma, M.; Wiczkowski, W.; Zieliński, H.; Cieślik, E. Determination of Selected Perfluorinated Acids (PFCAs) and Perfluorinated Sulfonates (PFASs) in Food Contact Materials Using LC-MS/MS. Packag. Technol. Sci. 2015, 28, 789–799. [Google Scholar] [CrossRef]
- Biegel, L.B.; Hurtt, M.E.; Frame, S.R.; O’Connor, J.C.; Cook, J.C. Mechanisms of Extrahepatic Tumor Induction by Peroxisome Proliferators in Male CD Rats. Toxicol. Sci. 2001, 60, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Barry, V.; Winquist, A.; Steenland, K. Perfluorooctanoic Acid (PFOA) Exposures and Incident Cancers among Adults Living Near a Chemical Plant. Environ. Health Perspect. 2013, 121, 1313–1318. [Google Scholar] [CrossRef]
- Fei, C.; McLaughlin Joseph, K.; Tarone Robert, E.; Olsen, J. Perfluorinated Chemicals and Fetal Growth: A Study within the Danish National Birth Cohort. Environ. Health Perspect. 2007, 115, 1677–1682. [Google Scholar] [CrossRef]
- Stein, C.R.; Savitz, D.A.; Dougan, M. Serum Levels of Perfluorooctanoic Acid and Perfluorooctane Sulfonate and Pregnancy Outcome. Am. J. Epidemiol. 2009, 170, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Espinosa, M.-J.; Mondal, D.; Armstrong, B.; Bloom Michael, S.; Fletcher, T. Thyroid Function and Perfluoroalkyl Acids in Children Living Near a Chemical Plant. Environ. Health Perspect. 2012, 120, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Vested, A.; Ramlau-Hansen Cecilia, H.; Olsen Sjurdur, F.; Bonde Jens, P.; Kristensen Susanne, L.; Halldorsson Thorhallur, I.; Becher, G.; Haug Line, S.; Ernst Emil, H.; Toft, G. Associations of in Utero Exposure to Perfluorinated Alkyl Acids with Human Semen Quality and Reproductive Hormones in Adult Men. Environ. Health Perspect. 2013, 121, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Rabnawaz, M. Fabrication of Food-Safe Water-Resistant Paper Coatings Using a Melamine Primer and Polysiloxane Outer Layer. ACS Omega 2018, 3, 11909–11916. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Rabnawaz, M. Oil- and Water-Resistant Coatings for Porous Cellulosic Substrates. ACS Appl. Polym. Mater. 2019, 1, 103–111. [Google Scholar] [CrossRef]
- Kansal, D.; Hamdani, S.S.; Ping, R.; Sirinakbumrung, N.; Rabnawaz, M. Food-Safe Chitosan–Zein Dual-Layer Coating for Water- and Oil-Repellent Paper Substrates. ACS Sustain. Chem. Eng. 2020, 8, 6887–6897. [Google Scholar] [CrossRef]
- Kansal, D.; Hamdani, S.S.; Ping, R.; Rabnawaz, M. Starch and Zein Biopolymers as a Sustainable Replacement for PFAS, Silicone Oil, and Plastic-Coated Paper. Ind. Eng. Chem. Res. 2020, 59, 12075–12084. [Google Scholar] [CrossRef]
- Kumar, V.; Khan, A.; Rabnawaz, M. A plant oil-based eco-friendly approach for paper coatings and their packaging applications. Prog. Org. Coat. 2023, 176, 107386. [Google Scholar] [CrossRef]
- Hamdani, S.S.; Li, Z.; Ruoqi, P.; Rollend, E.; Rabnawaz, M. Oxygen and water vapor barrier properties of polyvinyl alcohol and zein bilayer-coated paper. J. Appl. Polym. Sci. 2022, 139, 51707. [Google Scholar] [CrossRef]
- Rastogi, V.K.; Samyn, P. Bio-Based Coatings for Paper Applications. Coatings 2015, 5, 887–930. [Google Scholar] [CrossRef]
- Standard Test Method for Mass per Unit Area of Paper and Paperboard of Aramid Papers (Basis Weight). Available online: https://www.astm.org/d0646-13.html (accessed on 30 March 2024).
- Tappi T441 Standard Protocol. Available online: https://www.tappi.org/content/tag/sarg/t441.pdf (accessed on 30 March 2024).
- Grease Resistance Test for Paper and Paperboard, Test Method T 559 cm-22. Available online: https://imisrise.tappi.org/TAPPI/Products/01/T/0104T559.aspx (accessed on 30 March 2024).
- TAPPI T494 Paper and Paperboard Tensile Testing. Available online: https://www.admet.com/testing-applications/testing-standards/tappi-t494-paper-paperboard-tensile-testing/ (accessed on 30 March 2024).
- Ring Crush of Paperboard. Available online: https://www.tappi.org/content/sarg/t822.pdf (accessed on 30 March 2024).
- Bending Resistance (Stiffness) of Paper and Paper Board. Available online: https://www.tappi.org/content/tag/sarg/t489.pdf (accessed on 30 March 2024).
- Internal Tearing Resistance of Paper (Elmendorf-Type Method). Available online: https://imisrise.tappi.org/TAPPI/Products/01/T/0104T414.aspx (accessed on 30 March 2024).
- Fibre Box Association. Voluntary Standard for Repulping and Recycling Corrugated Fiberboard Treated to Improve Its Performance in the Presence of Water and Water Vapor. 2010. Available online: https://www.yumpu.com/en/document/view/49737625/voluntary-standard-for-repulping-and-recycling-corrugated (accessed on 30 March 2024).
- Halsall, T.G.; Hirst, E.L.; Jones, J.K.N. Structure of Starch and Cellulose. Nature 1947, 159, 97. [Google Scholar] [CrossRef]
- Reiznautt, Q.B.; Garcia, I.T.S.; Samios, D. Oligoesters and polyesters produced by the curing of sunflower oil epoxidized bio-diesel with cis-cyclohexane dicarboxylic anhydride: Synthesis and characterization. Mater. Sci. Eng. C 2009, 29, 2302–2311. [Google Scholar] [CrossRef]


| Abbreviated Name | PDMS-COOH % | Starch % |
|---|---|---|
| B-KP a | - | - |
| S-5 b | - | - |
| P1S0 c | 100 | 0 |
| P1S1 d | 50 | 50 |
| P2S1 e | 66.6 | 33.3 |
| P3S1 f | 75 | 25 |
| P4S1 g | 80 | 20 |
| Sample No. | Material Thickness (µm) | Basis Weight (g/m2) | Coating Loading (g/m2) | Coating (Load by wt%) |
|---|---|---|---|---|
| B-KP | 181.7 ± 3.4 | 137.5 ± 0.5 | 0 | 0 |
| S-5 | 201.0 ± 5.6 | 145.2 ± 0.6 | 7.7 ± 0.7 | 5.36 ± 0.43 |
| P1S0 | 232.2 ± 10.4 | 173.5 ± 2.5 | 36.0 ± 2.5 | 26.99 ± 2.90 |
| P1S1 | 261.5 ± 15.1 | 193.9 ± 1.7 | 56.4 ± 1.7 | 41.43 ± 0.51 |
| P2S1 | 232.3 ± 5.9 | 185.5 ± 0.6 | 48.0 ± 0.5 | 34.82 ± 0.43 |
| P3S1 | 278.1 ± 6.9 | 183.5 ± 1.1 | 46.0 ± 1.1 | 32.72 ± 0.36 |
| P4S1 | 239.2 ± 8.8 | 186.6 ± 2.2 | 49.0 ± 2.3 | 35.41 ± 1.59 |
| Content | Unit | P1S0 |
|---|---|---|
| Moisture | (wt%) | 6.12 |
| Sample charged | (g) | 25.00 |
| Screen reject | (g) | 1.83 |
| Screen accepts | (g) | 18.79 |
| Yield of sample | (wt%) | 91.10 |
| Pass/Fail | Pass |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamdani, S.S.; Elkholy, H.M.; Alford, A.; Jackson, K.; Naveed, M.; Wyman, I.; Wang, Y.; Li, K.; Haider, S.W.; Rabnawaz, M. Synthesis of Water-Dispersible Poly(dimethylsiloxane) and Its Potential Application in the Paper Coating Industry as an Alternative for PFAS-Coated Paper and Single-Use Plastics. Polymers 2024, 16, 1006. https://doi.org/10.3390/polym16071006
Hamdani SS, Elkholy HM, Alford A, Jackson K, Naveed M, Wyman I, Wang Y, Li K, Haider SW, Rabnawaz M. Synthesis of Water-Dispersible Poly(dimethylsiloxane) and Its Potential Application in the Paper Coating Industry as an Alternative for PFAS-Coated Paper and Single-Use Plastics. Polymers. 2024; 16(7):1006. https://doi.org/10.3390/polym16071006
Chicago/Turabian StyleHamdani, Syeda Shamila, Hazem M. Elkholy, Alexandra Alford, Kang Jackson, Muhammad Naveed, Ian Wyman, Yun Wang, Kecheng Li, Syed W. Haider, and Muhammad Rabnawaz. 2024. "Synthesis of Water-Dispersible Poly(dimethylsiloxane) and Its Potential Application in the Paper Coating Industry as an Alternative for PFAS-Coated Paper and Single-Use Plastics" Polymers 16, no. 7: 1006. https://doi.org/10.3390/polym16071006
APA StyleHamdani, S. S., Elkholy, H. M., Alford, A., Jackson, K., Naveed, M., Wyman, I., Wang, Y., Li, K., Haider, S. W., & Rabnawaz, M. (2024). Synthesis of Water-Dispersible Poly(dimethylsiloxane) and Its Potential Application in the Paper Coating Industry as an Alternative for PFAS-Coated Paper and Single-Use Plastics. Polymers, 16(7), 1006. https://doi.org/10.3390/polym16071006