Comparative Analysis of the Mechanical Properties and Biocompatibility between CAD/CAM and Conventional Polymers Applied in Prosthetic Dentistry
Abstract
:1. Introduction
- Biocompatibility and Human Body Impact: Biomaterials intended for use in the oral cavity must be compatible with the surrounding tissues. This involves assessing how the material interacts with the body, whether it causes any adverse reactions, and if it integrates well with the surrounding tissues without causing inflammation or immune responses.
- Degradation in the Oral Cavity: The degradation behavior of biomaterials in the oral environment is crucial for their longevity and performance. Factors such as exposure to saliva, enzymes, pH variations, and mechanical stresses can influence the degradation process. Understanding how biomaterials degrade over time helps in predicting their lifespan and the potential risks associated with degradation by-products.
- Bacterial Colonization and Biofilm Formation: Biomaterials in the oral cavity can serve as substrates for bacterial colonization, leading to biofilm formation. Biofilms are communities of microorganisms encased within a matrix of extracellular polymeric substances. These biofilms can harbor pathogenic bacteria, contributing to various oral diseases such as dental caries, periodontal diseases, and implant-associated infections. Therefore, it is crucial to assess the propensity of biomaterials to support bacterial growth and biofilm formation.
2. Materials and Methods
3. Recent Assessment of the Mechanical Properties of Conventional Polymers Employed in Prosthodontics
- Acetals
- Polyamides
- Acrylic polymers (free from residual monomer)
- Polyolefins
- Polyesters
4. Biocompatibility of Traditional Polymers Applied in Prosthodontics
5. Mechanical Properties and Biocompatibility of Contemporary Polymers, Used in Modern Prosthodontics
5.1. PEEK
5.2. 3D-Printed Denture Base Resins (Additive Manufacturing)
5.3. Milled Denture Base Resins (Subtractive Manufacturing)
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABS | acrylonitrile butadiene styrene |
| AM | additive manufacturing |
| CAD/CAM | computer-aided design/computer-aided manufacturing |
| CD | complete dentures |
| EGDMA | Ethylene Glycol Dimethacrylate |
| ISO | International Organization of Standardization |
| PC | polycarbonate |
| PCL | poly[e-caprolactone] |
| PDMS | polydimethylsiloxane |
| PE | polyethylene |
| PEEK | polyetheretherketone |
| PEG | polyethylene glycol |
| PLA | polylactic acid |
| PMMA | polymethyl methacrylate |
| PP | polypropylene |
| PU | polyurethane |
| STL | stereolithography, standard triangle language, standard tessellation language |
| TEGDMA | Triethylene Glycol Di-methacrylate |
| 3D | three dimensional |
References
- Tregunoa, I.; Boldureva, R.; Mihaylenko, L.; Aglakelidze, V.; Tregubov, S. The Use of Thermoplastic Materials in Dentistry; Medical Press: Moscow, Russia, 2007. [Google Scholar]
- Chuchulska, B.; Yankov, S.; Hristov, I.; Aleksandrov, S. Thermoplastic Materials in the Dental Practice: A Review. Int. J. Sci. Res. 2016, 6, 1074–1076. [Google Scholar] [CrossRef]
- Dezertinskiy, A. Thermoplastics. What do we know about them. Dent. Inst. 2007, 2, 98–101. (In Russian) [Google Scholar]
- Fueki, K.; Yatabe, M.; Ohkubo, C.; Arakawa, I.; Arita, M.; Ino, S.; Kanamori, T.; Kawai, Y.; Kawara, M.; Komiyama, O.; et al. Clinical application of removable partial dentures using thermoplastic resin—Part I: Definition and indication of non-metal clasps dentures. J. Prosthodont. Res. 2014, 58, 10. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Aeran, H.; Kumar, N.; Gupta, N. Flexible Thermoplastic Denture Base Materials for Aesthetic Removable Partial Denture Framework. J. Clin. Diagn. Res. 2013, 7, 2372–2373. [Google Scholar]
- Tandon, R.; Gupta, S.; Agarwal, S.K. Denture base materials: From past to future. Ind. J. Dent. Sci. 2010, 2, 33–39. [Google Scholar]
- Yokoyama, N.; Machi, H.; Hayashi, K.; Uchida, T.; Ono, T.; Nokubi, T. Physical properties of polyamide resin (nylon group) as a polymeric material for dentures: Part 2. Surface hardness and tensile strength. J. Nippon Acad. Dent. Technol. 2004, 25, 87–92. [Google Scholar]
- Hamanaka, I.; Takahashi, Y.; Shimizu, H. Mechanical properties of injection-molded thermoplastic denture base resins. Acta Odontol. Scand. 2011, 69, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, M.; Corsalini, M.; Kazakova, R.; Vlahova, A.; Chuchulska, B.; Barile, G.; Capodiferro, S.; Kazakov, S. Comparison between Conventional PMMA and 3D Printed Resins for Denture Bases: A Narrative Review. J. Compos. Sci. 2022, 6, 87. [Google Scholar] [CrossRef]
- Hamanaka, I.M.; Iwamoto, M.; Lassila, L.; Vallittu, P.; Shimizu, H.; Takahashi, Y. Influence of water sorption on mechanical properties of injection-molded thermoplastic denture base resins. Acta Odontol. Scand. 2014, 72, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Hayran, Y.; Keskin, Y. Flexural strength of polymethyl methacrylate copolymers as a denture base resin. Dent. Mater. J. 2019, 38, 678–686. [Google Scholar] [CrossRef] [PubMed]
- ISO 20795-1:2013; Dentistry—Base Polymers—Part 1. ISO: Geneve, Switzerland, 2013. Available online: https://www.iso.org/standard/62277.html (accessed on 8 March 2024).
- Chuchulska, B.; Yankov, S.; Todorov, R.; Ivanova, D.; Kalachev, Y. Injection Shrinkage and Water Sorption of Some Thermoplastic Dental Materials. APESB 2019, 19, e4474. [Google Scholar] [CrossRef]
- Gu, X.; Sun, X.; Sun, Y.; Wang, J.; Liu, Y.; Yu, K.; Wang, Y.; Zhou, Y. Bioinspired Modifications of PEEK Implants for Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 8, 631616. [Google Scholar] [CrossRef]
- Nguyen, L.G.; Kopperud, H.M.; Øilo, M. Water sorption and solubility of polyamide denture base materials. Acta Biomater. Odontol. Scand. 2017, 3, 47–52. [Google Scholar] [CrossRef]
- Takabayashi, Y. Characteristics of denture thermoplastic resins for non-metal clasp dentures. Dent. Mater. J. 2010, 29, 353–361. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-1:2018; Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing within a Risk Management Process. ISO: Geneve, Switzerland, 2018. Available online: https://www.iso.org/standard/68936.html (accessed on 8 March 2024).
- Chuchulska, B.; Zlatev, S. Linear dimensional change and ultimate tensile strength of polyamide materials for denture bases. Polymers 2021, 13, 3446. [Google Scholar] [CrossRef]
- Chuchulska, B.; Tomova, Z.; Vlahova, A.; Hristov, I. Comparative Simulation Testing with Functional Loading of Two Denture Framework Materials—A Pilot Study. J. IMAB 2022, 28, 4. [Google Scholar] [CrossRef]
- Sahin, O.; Ozdemir, A.K.; Turgut, M.; Boztug, A.; Sumer, Z. Investigation of flexural strength and cytotoxicity of acrylic resin copolymers by using different polymerization methods. J. Adv. Prosthodont. 2015, 7, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Raszewski, Z.; Chojnacka, K.; Mikulewicz, M. Preparation and characterization of acrylic resins with bioactive glasses. Sci. Rep. 2022, 12, 16624. [Google Scholar] [CrossRef]
- Özer, H.; Özcan, M. Antibacterial effect of bioactive glass incorporated in acrylic resins against Streptococcus Mutans and Lactobacillus Acidophilus activity in biofilm. Braz. Dent. Sci. 2022, 25, e3317. [Google Scholar] [CrossRef]
- Hayran, Y.; Yu-Shan, H.; Cheng-Yuan, H.; Her-Hsiung, H. Surface changes and bacterial adhesion on implant abutment materials after various clinical cleaning procedures. J. Chin. Med. Assoc. 2019, 82, 643–650. [Google Scholar] [CrossRef]
- Dimitrova, M.; Chuchulska, B.; Zlatev, S.; Kazakova, R. Colour Stability of 3D-Printed and Prefabricated Denture Teeth after Immersion in Different Colouring Agents—An In Vitro Study. Polymers 2022, 14, 3125. [Google Scholar] [CrossRef]
- Tsui, C.; Kong, E.F.; Jabra-Rizk, M.A. Pathogenesis of Candida Albicans Biofilm. Pathog. Dis. 2016, 74, ftw018. [Google Scholar] [CrossRef]
- Gozhaya, L.D. Oral Mucosa Diseases Caused by Denture Materials; Abstract of a Doctoral Thesis of Medical Sciences; College of Medicine: Qatar, Doha, 2001; p. 20. [Google Scholar]
- Yankova, M.; Yordanov, B.; Dimova-Gabrovska, M.; Peev, T. Modified Approach to Ensure a Uniform Layer of Elastic Material for Relining Complete Dentures with Self-Curing Silicones. J. IMAB 2019, 25, 2781–2787. [Google Scholar] [CrossRef]
- Aslanimehr, M.; Rezvani, S.; Mahmoudi, A.; Moosavi, N. Comparison of Candida Albicans adherence to conventional acrylic denture base materials and injection molding acrylic materials. J. Dent. 2017, 18, 61. [Google Scholar]
- Sharabasy, R.; Ahmed, M.E.; Mohammed, E. Comparative study of candida albicans adherence to conventional acrylic denture base materials and injection molding acrylic materials and poly ether ether ketone. Egypt. Dent. J. 2022, 68, 3579–3585. [Google Scholar] [CrossRef]
- Chuchulska, B.; Hristov, I.; Dochev, B.; Raychev, R. Changes in the Surface Texture of Thermoplastic (Monomer-Free) Dental Materials Due to Some Minor Alterations in the Laboratory Protocol—Preliminary Study. Materials 2022, 15, 6633. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Ahmed, S.; Nandini, V.V.; Lathief, J.; Boruah, S. An In Vitro Comparison of Microbial Adhesion on Three Different Denture Base Materials and Its Relation to Surface Roughness. Cureus 2023, 15, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Durkan, R.; Ayaz, E.A.; Bagis, B. Comparative effects of denture cleansers on physical properties of polyamide and polymethyl methacrylate base polymers. Dent. Mater. J. 2013, 32, 367–375. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, W.J. Dental Materials and Their Selection, 4th ed.; Quintessence Pub. Co., Inc.: Chicago, IL, USA, 2009; pp. 78–79. Available online: http://www.quintpub.com/PDFs/book_preview/B4375.pdf (accessed on 11 July 2022).
- Chladek, G.; Pakieła, K.; Pakieła, W.; Żmudzki, J.; Adamiak, M.; Krawczyk, C. Effect of antibacterial silver-releasing filler on the physicochemical properties of poly (methyl methacrylate) denture base material. Materials 2019, 12, 4146. [Google Scholar] [CrossRef] [PubMed]
- Bajunaid, S.O. How effective are antimicrobial agents on preventing the adhesion of candida albicans to denture base acrylic resin materials? A systematic review. Polymers 2022, 14, 908. [Google Scholar] [CrossRef]
- Totu, E.E.; Nechifor, A.C.; Nechifor, G.; Aboul-Enein, H.Y.; Cristache, C.M. Poly (Methyl Methacrylate) with TiO2 Nanoparticles Inclusion for Stereolithographic Complete Denture Manufacturing—The Future in Dental Care for Elderly Edentulous Patients? J. Dent. 2017, 59, 68–77. [Google Scholar] [CrossRef]
- Le, C.V.; Yoon, H. Advances in the Use of Conducting Polymers for Healthcare Monitoring. Int. J. Mol. Sci. 2024, 25, 1564. [Google Scholar] [CrossRef]
- Le Bars, P.; Kouadio, A.A.; Amouriq, Y.; Bodic, F.; Blery, P.; Bandiaky, O.N. Different Polymers for the Base of Removable Dentures? Part I: A Narrative Review of Mechanical and Physical Properties. Polymers 2023, 15, 3495. [Google Scholar] [CrossRef]
- Thomé, T.; Erhardt, M.C.G.; Leme, A.A.; Al Bakri, I.; Bedran-Russo, A.K.; Bertassoni, L.E. Emerging Polymers in Dentistry. In Advanced Polymers in Medicine; Puoci, F., Ed.; Springer: Cham, Switzerland, 2015; pp. 265–296. [Google Scholar]
- Peng, P.W.; Chen, M.S.; Peng, T.Y.; Huang, P.C.; Nikawa, H.; Lee, W.F. In vitro study of optimal removable partial denture clasp design made from novel high-performance polyetherketoneketone. J. Prosthodont. Res. 2024, 12, 1832. [Google Scholar] [CrossRef] [PubMed]
- Paszkiewicz, S.; Lesiak, P.; Walkowiak, K.; Irska, I.; Miądlicki, K.; Królikowski, M.; Piesowicz, E.; Figiel, P. The Mechanical, Thermal, and Biological Properties of Materials Intended for Dental Implants: A Comparison of Three Types of Poly[aryl-ether-ketones] [PEEK and PEKK]. Polymers 2023, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of polyetheretherketone [PEEK] in oral implantology and prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Panayotov, I.V.; Orti, V.; Cuisinier, F.; Yachouh, J. Polyetheretherketone [PEEK] for medical applications. J. Mater. Sci. Mater. Med. 2016, 27, 118. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, S.; Arthurs, C.; Molina, M.I.E.; Pruitt, L.; Roy, A. An overview of the tribological and mechanical properties of PEEK and CFR-PEEK for use in total joint replacements. J. Mech. Behav. Biomed. Mater. 2023, 145, 105974. [Google Scholar] [CrossRef] [PubMed]
- Schwitalla, A.D.; Spintig, T.; Kallage, I.; Müller, W.-D. Flexural behavior of PEEK materials for dental application. Dent. Mater. 2015, 31, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.S.; Bento, V.A.A.; Brunetto, J.L.; Pesqueira, A.A. Polyetheretherketone materials for removable partial denture frameworks: An integrative review. Gen. Dent. 2023, 71, 58–62. [Google Scholar] [PubMed]
- Vertucci, V.; Marrelli, B.; Ruggiero, R.; Iaquinta, M.; Marenzi, G.; Parisi, G.M.; Gasparro, R.; Pacifici, A.; Palumbo, G.; Sammartino, G.; et al. Comparative in vitro study on biomechanical behavior of zirconia and polyetheretherketone biomaterials exposed to experimental loading conditions in a prototypal simulator. Int. J. Med. Sci. 2023, 20, 639–651. [Google Scholar] [CrossRef]
- Fu, Q.; Gabriel, M.; Schmidt, F.; Müller, W.-D.; Schwitalla, A.D. The impact of different low-pressure plasma types on the physical, chemical, and biological surface properties of PEEK. Dent. Mater. 2021, 37, e15–e22. [Google Scholar] [CrossRef]
- Papathanasiou, I.; Kamposiora, P.; Papavasiliou, G.; Ferrari, M. The use of PEEK in digital prosthodontics: A narrative review. BMC Oral Health 2020, 20, 217. [Google Scholar] [CrossRef]
- Limaye, N.; Veschini, L.; Coward, T. Assessing biocompatibility & mechanical testing of 3D-printed PEEK versus milled PEEK. Heliyon 2022, 8, e12314. [Google Scholar]
- Han, X.; Gao, W.; Zhou, Z.; Yang, S.; Wang, J.; Shi, R.; Li, Y.; Jiao, J.; Qi, Y.; Zhao, J. Application of biomolecules modification strategies on PEEK and its composites for osteogenesis and antibacterial properties. Colloids Surf. B Biointerfaces 2022, 215, 112492. [Google Scholar] [CrossRef]
- Liu, Y.; Yi, N.; Davies, R.; McCutchion, P.; Ghita, O. Powder Bed Fusion Versus Material Extrusion: A Comparative Case Study on Polyether-Ether-Ketone Cranial Implants. 3D Print. Addit. Manuf. 2023, 10, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Hanemann, T.; Klein, A.; Baumgärtner, S.; Jung, J.; Wilhelm, D.; Antusch, S. Material Extrusion 3D Printing of PEEK-Based Composites. Polymers 2023, 15, 3412. [Google Scholar] [CrossRef] [PubMed]
- Mory, N.; Cascos, R.; Celemín-Viñuela, A.; Gómez-Polo, C.; Agustín-Panadero, R.; Gómez-Polo, M. Comparison of the Surface Roughness of CAD/CAM Metal-Free Materials Used for Complete-Arch Implant-Supported Prostheses: An In Vitro Study. Biomedicines 2023, 11, 3036. [Google Scholar] [CrossRef]
- Sanchez-Sobrado, O.; Visniakov, N.; Bureika, G.; Losada, R.; Rodriguez, E. Effect of the Chemical Surrounding Environment on the Physical and Mechanical Properties of Aged Thermoplastic Polymers. Heliyon 2024, 10, e241462024. [Google Scholar] [CrossRef] [PubMed]
- Wiessner, A.; Wassmann, T.; Wiessner, J.M.; Schubert, A.; Wiechens, B.; Hampe, T.; Burgers, R. In Vivo Biofilm Formation on Novel PEEK, Titanium, and Zirconia Implant Abutment Materials. Int. J. Mol. Sci. 2023, 24, 1779. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.P.; Dable, R.; Raj, A.P.N.; Mutneja, P.; Srivastava, S.B.; Haque, M. Comparison of Mechanical Properties of PEEK and PMMA: An In Vitro Study. J. Contemp. Dent. Pract. 2021, 22, 179–183. [Google Scholar]
- Muhsin, S.A.; Hatton, P.V.; Johnson, A.; Sereno, N.; Wood, D.J. Determination of Polyetheretherketone (PEEK) Mechanical Properties as a Denture Material. Saudi Dent. J. 2019, 31, 382–391. [Google Scholar] [CrossRef]
- Tushar, T.; Rani, P.; Ananya; Kumar, S.; Prakash, J.; B, J.M. Evaluation of Impact Strength and Flexural Strength of Polyether Ether Ketone vs. Computer-Aided Design/Computer-Aided Manufacturing Polymethyl Methacrylate Denture Base Materials: An In-Vitro Study. Cureus 2023, 15, e479292023. [Google Scholar]
- Wu, S.; Qian, C.; Jiao, T.; Sun, J. Comparison of the Retention and Fit of Polyether Ether Ketone Clasps during Fatigue Circulation Tests. Heliyon 2023, 9, e199592023. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Müller, W.-D.; Rumjahn, A.; Schmidt, F.; Schwitalla, A.D. Mechanical Properties of Fused Filament Fabricated PEEK for Biomedical Applications Depending on Additive Manufacturing Parameters. J. Mech. Behav. Biomed. Mater. 2021, 115, 104250. [Google Scholar] [CrossRef] [PubMed]
- Johann, K.S.; Wolf, A.; Bonten, C. Mechanical Properties of 3D-Printed Liquid Crystalline Polymers with Low and High Melting Temperatures. Materials 2023, 17, 152. [Google Scholar] [CrossRef]
- Priester, M.; Müller, W.-D.; Beuer, F.; Schmidt, F.; Schwitalla, A.D. Performance of PEEK Based Telescopic Crowns, a Comparative Study. Dent. Mater. 2021, 37, 1667–1675. [Google Scholar] [CrossRef]
- Georgiev, Z.; Alexandrov, S.; Tomova, Z. Experimental Setting for Testing the Bond Strength Between Metal Alloy and Peek. J. IMAB Annu. Proceeding 2021, 27, 3881–3884. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Feng, S.-W.; Chiang, K.-Y.; Li, Y.-C.; Peng, T.-Y.; Nikawa, H. Effects of Various Functional Monomers’ Reaction on the Surface Characteristics and Bonding Performance of Polyetheretherketone. J. Prosthodont. Res. 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Soares Machado, P.; Cadore Rodrigues, A.C.; Chaves, E.T.; Susin, A.H.; Valandro, L.F.; Pereira, G.K.R.; Rippe, M.P. Surface Treatments and Adhesives Used to Increase the Bond Strength Between Polyetheretherketone and Resin-Based Dental Materials: A Scoping Review. J. Adhes. Dent. 2022, 24, 233–245. [Google Scholar] [PubMed]
- Turkkal, F.; Culhaoglu, A.K.; Sahin, V. Composite-Veneering of Polyether-Ether-Ketone (PEEK): Evaluating the Effects of Different Surface Modification Methods on Surface Roughness, Wettability, and Bond Strength. Lasers Med. Sci. 2023, 38, 95. [Google Scholar] [CrossRef]
- Kimura, H.; Morita, K.; Nishio, F.; Abekura, H.; Tsuga, K. Clinical Report of Six-Month Follow-Up after Cementing PEEK Crown on Molars. Sci. Rep. 2022, 12, 19070. [Google Scholar] [CrossRef]
- Attia, M.A.; Blunt, L.; Bills, P.; Tawfik, A.; Radawn, M. Micro-CT Analysis of Marginal and Internal Fit of Milled and Pressed Polyetheretherketone Single Crowns. J. Prosthet. Dent. 2023, 129, 906.e1–906.e10. [Google Scholar] [CrossRef] [PubMed]
- Nagi, N.; Fouda, A.M.; Bourauel, C. Comparative Evaluation of Internal Fit and Marginal Gap of Endocrowns Using Lithium Disilicate and Polyether Ether Ketone Materials—An In Vitro Study. BMC Oral Health 2023, 23, 207. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.-Y.; Ma, T.-L.; Lee, I.-T.; Wu, S.-H.; Mine, Y.; Lin, C.-C. Enhancing Dental Cement Bond Strength with Autofocus-Laser-Cutter-Generated Grooves on Polyetheretherketone Surfaces. Polymers 2023, 15, 3670. [Google Scholar] [CrossRef]
- Kuttuva Balasubramanian Sivaprakash Babu, A.D.; Singaravel Chidambara Nathan, A.; Balasubramanium, M.K. Effect of Laser Surface Treatment on Shear Bond Strength Between Polyetheretherketone and Heat-Activated Polymethylmethacrylate Resin. J. Prosthodont. Off. J. Am. Coll. Prosthodont. 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Atsu, S.; Erol, U. Marginal Fit and Fracture Resistance of Polyetheretherketone, Zirconia, and Titanium Implant-Supported Prosthesis Frameworks for a Partially Edentulous Arch after Thermomechanical Aging. J. Prosthet. Dent. 2023, 131, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Emam, M.; Arafa, A.M. Stress distribution and fracture resistance of green reprocessed polyetheretherketone (PEEK) single implant crown restorations compared to unreprocessed PEEK and Zirconia: An in-vitro study. BMC Oral Health 2023, 23, 275. [Google Scholar] [CrossRef] [PubMed]
- Mourad, K.E.; Altonbary, G.Y.; Emera, R.M.K.; Hegazy, S.A.F. Polyetheretherketone Computer-Aided Design and Computer-Aided Manufacturing Framework for All-on-Four Mandibular Full-Arch Prosthesis: 3 Years’ Retrospective Study of Peri-Implant Soft Tissue Changes and Ridge Base Relationship. J. Prosthodont. 2023, 32, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Silva Júnior, E.V.; Basting, R.T.; Turssi, C.P.; França, F.M. Precision of Polyether Ether Ketone (PEEK) or Cobalt-Chrome Implant Bar Fit to Implants after Mechanical Cycling. Acta Odontol. Latinoam. 2023, 36, 71–77. [Google Scholar] [CrossRef]
- Delrieu, J.; Joniot, S.; Vergé, T.; Destruhaut, F.; Nasr, K.; Canceill, T. The Use of PEEK as an Occlusal Splint in a Patient with Histaminosis: A Case Report. Spec. Care Dent. 2022, 42, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Jiang, Z.; Ge, Y.; Yin, H.; Yang, Q.; Li, R.; Chen, Z.; Zhang, H. Mechanical Properties, Biosafety, and Shearing Bonding Strength of Glass Fiber-Reinforced PEEK Composites Used as Post-Core Materials. J. Mech. Behav. Biomed. Mater. 2023, 145, 106047. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Li, M.; Zhang, Y.; Yin, M.; Che, W.; Bi, Z.; Yang, Y.; Ouyang, J. Polyetheretherketone (PEEK) as a Potential Material for the Repair of Maxillofacial Defect Compared with E-poly[tetrafluoroethylene] (e-PTFE) and Silicone. ACS Biomater. Sci. Eng. 2023, 9, 4328–4340. [Google Scholar] [CrossRef] [PubMed]
- Ghazy, E.S.; Altonbary, G.Y.; Gebreel, A.; El-Daker, M.A.; Hegazy, S.A. Evaluation and Comparison of Retention and Patient Satisfaction with Milled Polyetheretherketone versus Metal Maxillary Obturators. J. Prosthet. Dent. 2023; in press. [Google Scholar]
- Moncayo-Matute, F.P.; Vázquez-Silva, E.; Peña-Tapia, P.G.; Torres-Jara, P.B.; Moya-Loaiza, D.P.; Viloria-Ávila, T.J. Finite Element Analysis of Patient-Specific 3D-Printed Cranial Implant Manufactured with PMMA and PEEK: A Mechanical Comparative Study. Polymers 2023, 15, 3620. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, F.; Golbang, A.; Jindal, S.; Dixon, D.; McIlhagger, A.; Harkin-Jones, E.; Crawford, D.; Mancuso, E. 3D Printed PEEK/HA Composites for Bone Tissue Engineering Applications: Effect of Material Formulation on Mechanical Performance and Bioactive Potential. J. Mech. Behav. Biomed. Mater. 2021, 121, 104601. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Tang, T. Current Strategies to Improve the Bioactivity of PEEK. Int. J. Mol. Sci. 2014, 15, 5426–5445. [Google Scholar] [CrossRef] [PubMed]
- AlOtaibi, N.; Naudi, K.; Conway, D.; Ayoub, A. The Current State of PEEK Implant Osseointegration and Future Perspectives: A Systematic Review. Eur. Cell. Mater. 2020, 40, 1–20. [Google Scholar] [CrossRef]
- Gao, W.; Han, X.; Sun, D.; Li, Y.; Liu, X.; Yang, S.; Zhou, Z.; Qi, Y.; Jiao, J.; Zhao, J. Antibacterial Properties of Antimicrobial Peptide HHC36 Modified Polyetheretherketone. Front. Microbiol. 2023, 14, 1103956. [Google Scholar] [CrossRef]
- Ferroni, L.; D’Amora, U.; Leo, S.; Tremoli, E.; Raucci, M.G.; Ronca, A.; Ambrosio, L.; Zavan, B. PEEK and Hyaluronan-Based 3D Printed Structures: Promising Combination to Improve Bone Regeneration. Molecules 2022, 27, 8749. [Google Scholar] [CrossRef]
- Kumar, S.R.; Hu, C.-C.; Vi, T.T.T.; Chen, D.W.; Lue, S.J. Antimicrobial Peptide Conjugated on Graphene Oxide-Containing Sulfonated Polyetheretherketone Substrate for Effective Antibacterial Activities against Staphylococcus aureus. Antibiotics 2023, 12, 1407. [Google Scholar] [CrossRef]
- Çayır Bozoğlu, Ü.; Kiremitçi, A.; Yurtsever, M.Ç.; Gümüşderelioğlu, M. Peek Dental Implants Coated with Boron-Doped Nano-Hydroxyapatites: Investigation of In-Vitro Osteogenic Activity. J. Trace Elem. Med. Biol. 2022, 73, 127026. [Google Scholar] [CrossRef] [PubMed]
- Rodzeń, K.; McIvor, M.J.; Sharma, P.K.; Acheson, J.G.; McIlhagger, A.; Mokhtari, M.; McFerran, A.; Ward, J.; Meenan, B.J.; Boyd, A.R. The Surface Characterisation of Fused Filament Fabricated (FFF) 3D Printed PEEK/Hydroxyapatite Composites. Polymers 2021, 13, 3117. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Chowdhary, R. PEEK Materials as an Alternative to Titanium in Dental Implants: A Systematic Review. Clin. Implant Dent. Relat. Res. 2019, 21, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Lei, X.; Zhu, H. Modification of Polyetheretherketone (PEEK) Physical Features to Improve Osteointegration. J. Zhejiang Univ. Sci. B 2022, 23, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, D.; Kassem, Y.M.; Omar, S.S.; Shalaby, Y. Nano-Topographical Surface Engineering for Enhancing Bioactivity of PEEK Implants (In Vitro-Histomorphometric Study). Clin. Oral Investig. 2023, 27, 6789–6799. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.A. Conditioning of PEEK Implant Abutment Surfaces Using Photodynamic Therapy, Nd:YAG Laser, and Conventional Methods to Evaluate Shear Bond Strength. Photobiomodul. Photomed. Laser Surg. 2024, 42, 90–95. [Google Scholar] [CrossRef]
- Chayanun, S.; Chanamuangkon, T.; Boonsuth, B.; Boccaccini, A.R.; Lohwongwatana, B. Enhancing PEEK Surface Bioactivity: Investigating the Effects of Combining Sulfonation with Sub-Millimeter Laser Machining. Mater. Today Bio 2023, 22, 100754. [Google Scholar] [CrossRef]
- Schwitalla, A.; Müller, W.-D. PEEK Dental Implants: A Review of the Literature. J. Oral Implantol. 2013, 39, 743–749. [Google Scholar] [CrossRef]
- Bruno, L.; Canullo, L.; Mayer, Y.; Schoenbaum, T.; Giuzio, F.; Maletta, C. Static and Fatigue Mechanical Performance of Abutments Materials for Dental Restorations. Materials 2023, 16, 3713. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Pinho, S.S.; Braz, M.P.; Silva, F.S.; Henriques, B. Carbon Fiber-Reinforced PEEK in Implant Dentistry: A Scoping Review on the Finite Element Method. Comput. Methods Biomech. Biomed. Engin. 2021, 24, 1355–1367. [Google Scholar] [CrossRef]
- Hung, C.C.U.; Costa, R.C.; Pereira, G.; Abdo, V.L.; Noronha, M.S.; Retamal-Valdes, B.; Bertolini, M.; Feres, M.; Shibli, J.A.; Barão, V.A.R.; et al. Oral Microbial Colonization on Titanium and Polyetheretherketone Dental Implant Healing Abutments: An In Vitro and In Vivo Study. J. Prosthet. Dent. 2023, in press. [Google Scholar] [CrossRef]
- Ortega-Martínez, J.; Delgado, L.M.; Ortiz-Hernández, M.; Punset, M.; Cano-Batalla, J.; Cayon, M.R.; Cabratosa-Termes, J. In Vitro Assessment of PEEK and Titanium Implant Abutments: Screw Loosening and Microleakage Evaluations under Dynamic Mechanical Testing. J. Prosthet. Dent. 2022, 127, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Saravi, B.; Flohr, A.; Patzelt, S.B.; Spies, B.C.; Hazard, D.; Kohal, R.J. Fatigue and Fracture Resistance Testing of Polyether Ether Ketone (PEEK) Implant Abutments in an Ex Vivo Chewing Simulator Model. Materials 2022, 15, 6927. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, M.; Vlahova, A.; Kalachev, Y.; Zlatev, S.; Kazakova, R.; Capodiferro, S. Recent Advances in 3D Printing of Polymers for Application in Prosthodontics. Polymers 2023, 15, 4525. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.R.; Lamprou, D.A. Polymer coatings for biomedical applications: A review. Trans. Ins. Met. 2014, 92, 9–19. [Google Scholar] [CrossRef]
- Nathanael, A.J.; Oh, T.H. Biopolymer Coatings for Biomedical Applications. Polymers 2020, 12, 3061. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Upadhyay, A.; Khayambashi, P.; Farooq, I.; Sabri, H.; Tarar, M.; Lee, K.T.; Harb, I.; Zhou, S.; Wang, Y.; et al. Dental 3D-Printing: Transferring Art from the Laboratories to the Clinics. Polymers 2021, 13, 157. [Google Scholar] [CrossRef] [PubMed]
- Abduo, J.; Lyons, K.; Bennamoun, M. Trends in Computer-Aided Manufacturing in Prosthodontics: A Review of the Available Streams. Int. J. Dent. 2014, 2014, 783948. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, J.-J.; Yang, T.-S.; Lee, W.-F.; Chen, H.; Chang, H.-M. Mechanical Properties and Biocompatibility of Urethane Acrylate-Based 3D-Printed Denture Base Resin. Polymers 2021, 13, 822. [Google Scholar] [CrossRef]
- Yankova, M.; Peev, T.; Yordanov, B.; Dimova-Gabrovska, M.; Dimitrova, D. Study of the Knowledge and Use of Resilient Denture Lining Materials in Clinical Practice. J. IMAB 2021, 27, 3668–3675. [Google Scholar] [CrossRef]
- Ujfalusi, Z.; Pentek, A.; Told, R.; Schiffer, A.; Nyitrai, M.; Maroti, P. Detailed Thermal Characterization of Acrylonitrile Butadiene Styrene and Polylactic Acid Based Carbon Composites Used in Additive Manufacturing. Polymers 2020, 12, 2960. [Google Scholar] [CrossRef]
- Heo, H.; Jin, Y.; Yang, D.; Wier, C.; Minard, A.; Dahotre, N.B.; Neogi, A. Manufacturing and Characterization of Hybrid Bulk Voxelated Biomaterials Printed by Digital Anatomy 3D Printing. Polymers 2021, 13, 123. [Google Scholar] [CrossRef] [PubMed]
- Clark Ligon, S.; Liska, R.; Stampfl, J.; Gurr, M.; Mulhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed]
- Perea-Lowery, L.; Minja, I.K.; Lassila, L.; Ramakrishnaiah, R.; Vallittu, P.K. Assessment of CAD-CAM polymers for digitally fabricated complete dentures. J. Prosthet Dent. 2021, 125, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Stansbury, J.W.; Idacavage, M.J. 3D printing with polymers: Challenges among expanding options and opportunities. Dent. Mater. 2016, 32, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Tomova, Z.; Zhekov, Y.; Alexandrov, G.; Vlahova, A.; Vasileva, E. Application of CAD/CAM technologies and materials for prosthetic restoration of severely damaged teeth-clinical cases. Aust Dent J. 2023, 68, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Official Webpage of Hira Dental Lab. Available online: https://www.hiradentallab.com/fully-guided-surgical-stent/ (accessed on 11 November 2023).
- Srinivasan, M.; Gjengedal, H.; Cattani-Lorente, M.; Moussa, M.; Durual, S.; Schimmel, M.; Müller, F. CAD/CAM milled complete removable dental prostheses: An in vitro evaluation of biocompatibility, mechanical properties, and surface roughness. Dent. Mater. J. 2018, 37, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Theus, A.S.; Ning, L.; Hwang, B.; Gil, C.; Chen, S.; Wombwell, A.; Mehta, R.; Serpooshan, V. Bioprintability: Physiomechanical and Biological Requirements of Materials for 3D Bioprinting Processes. Polymers 2020, 12, 2262. [Google Scholar] [CrossRef] [PubMed]
- Nold, J.; Wesemann, C.; Rieg, L.; Binder, L.; Witkowski, S.; Spies, B.C.; Kohal, R.J. Does Printing Orientation Matter? In-Vitro Fracture Strength of Temporary Fixed Dental Prostheses after a 1-Year Simulation in the Artificial Mouth. Materials 2021, 14, 259. [Google Scholar] [CrossRef]
- Dimitrova, M.; Vlahova, A.; Raychev, R.; Chuchulska, B.; Kazakova, R. A 3D-simulation study of the deformation, tension, and stress of 3D-printed and conventional denture base materials after immersion in artificial saliva. Folia Medica 2024, 66, 104–113. [Google Scholar] [CrossRef]
- Osman, R.B.; Alharbi, N.; Wismeijer, D. Build angle: Does it influence the accuracy of 3D-printed dental restorations using digital light-processing technology? Int. J. Prosthodont. 2017, 30, 182–188. [Google Scholar] [CrossRef]
- Ausiello, P.; Gloria, A.; Maietta, S.; Watts, D.C.; Martorelli, M. Stress Distributions for Hybrid Composite Endodontic Post Designs with and without a Ferrule: FEA Study. Polymers 2020, 12, 1836. [Google Scholar] [CrossRef]
- de Andrade, G.-S.; Tribst, J.-P.-M.; Dal Piva, A.-M.O.; Bottino, M.-A.; Borges, A.-L.-S.; Valandro, L.-F.; Özcan, M. A Study on Stress Distribution to Cement Layer and Root Dentin for Post and Cores Made of CAD/CAM Materials with Different Elasticity Modulus in the Absence of Ferrule. J. Clin. Exp. Dent. 2019, 11, e1–e8. [Google Scholar] [CrossRef]
- Ruschel, G.H.; Gomes, É.A.; Silva-Sousa, Y.T.; Pinelli, R.G.P.; Sousa-Neto, M.D.; Pereira, G.K.R.; Spazzin, A.O. Mechanical Properties and Superficial Characterization of a Milled CAD-CAM Glass Fiber Post. J. Mech. Behav. Biomed. Mater. 2018, 82, 187–192. [Google Scholar] [CrossRef]
- Bilgin, M.S.; Erdem, A.; Dilber, E.; Ersoy, İ. Comparison of Fracture Resistance between Cast, CAD/CAM Milling, and Direct Metal Laser Sintering Metal Post Systems. J. Prosthodont. Res. 2016, 60, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Lalama, M.; Rocha, M.G.; O’Neill, E.; Zoidis, P. Polyetheretherketone [PEEK] Post and Core Restorations: A 3D Accuracy Analysis between Heat-Pressed and CAD-CAM Fabrication Methods. J. Prosthodont. 2022, 31, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, K.N.; Duque, T.M.; Maia, H.P.; Gonçalves, T. Fracture Resistance and Failure Mode of Custom-Made Post-and-Cores of Polyetheretherketone and Nano-Ceramic Composite. Oper. Dent. 2020, 45, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Al-Dwairi, Z.N.; Tahboub, K.Y.; Baba, N.Z.; Goodacre, C.J. A comparison of the flexural and impact strengths and flexural modulus of CAD/CAM and conventional heat-cured polymethyl methacrylate (PMMA). J. Prosthodont. 2020, 29, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Iemsaengchairat, R.; Aksornmuang, J. Fracture Resistance of Thin Wall Endodontically Treated Teeth without Ferrules Restored with Various Techniques. J. Esthet. Restor. Dent. 2022, 34, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Sadowsky, S.J. Comments Regarding: Batista VES, Bitencourt SB, Bastos NA, Pellizzer EP, Goiato MC, Dos Santos DM. Influence of the Ferrule Effect on the Failure of Fiber-Reinforced Composite Post-and-Core Restorations: A Systematic Review and Meta-Analysis. J. Prosthet. Dent. 2020, 123, 239–245. [Google Scholar]
- Garcia, P.P.; Wambier, L.M.; de Geus, J.L.; da Cunha, L.F.; Correr, G.M.; Gonzaga, C.C. Do Anterior and Posterior Teeth Treated with Post-and-Core Restorations Have Similar Failure Rates? A Systematic Review and Meta-Analysis. J. Prosthet. Dent. 2019, 121, 887–894.e4. [Google Scholar] [CrossRef] [PubMed]
- Mosharraf, R.; Fathi, A.; Botshekan, S.S. Comparative Evaluation of the Effect of Core Type and Antirotational Post on Stress Distribution in an Endodontically Treated Maxillary First Molar: FEA. Int. J. Dent. 2022, 2022, 4336980. [Google Scholar] [CrossRef] [PubMed]
- Al-Qarni, F.D. Customized Post and Cores Fabricated with CAD/CAM Technology: A Literature Review. Int. J. Gen. Med. 2022, 15, 4771–4779. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, M.; Vlahova, A.; Kalachev, Y.; Kazakova, R.; Capodiferro, S. Future Prospects and Challenges in Additive Manufacturing for Complete Dentures: A Narrative Review. Oral 2024, 4, 23–35. [Google Scholar] [CrossRef]
- Vichi, A.; Balestra, D.; Scotti, N.; Louca, C.; Paolone, G. Translucency of CAD/CAM and 3D Printable Composite Materials for Permanent Dental Restorations. Polymers 2023, 15, 1443. [Google Scholar] [CrossRef] [PubMed]
- Ferrini, F.; Paolone, G.; Di Domenico, G.L.; Pagani, N.; Gherlone, E.F. SEM Evaluation of the Marginal Accuracy of Zirconia, Lithium Disilicate, and Composite Single Crowns Created by CAD/CAM Method: Comparative Analysis of Different Materials. Materials 2023, 16, 2413. [Google Scholar] [CrossRef] [PubMed]
- Anadioti, E.; Aquilino, S.A.; Gratton, D.G.; Holloway, J.A.; Denry, I.; Thomas, G.W.; Qian, F. 3D and 2D Marginal Fit of Pressed and CAD/CAM Lithium Disilicate Crowns Made from Digital and Conventional Impressions. J. Prosthodont. 2014, 23, 610–617. [Google Scholar] [CrossRef]
- Mostafa, N.Z.; Ruse, N.D.; Ford, N.L.; Carvalho, R.M.; Wyatt, C.C.L. Marginal Fit of Lithium Disilicate Crowns Fabricated Using Conventional and Digital Methodology: A Three-Dimensional Analysis. J. Prosthodont. 2018, 27, 145–152. [Google Scholar] [CrossRef]
- Hamza, T.A.; Ezzat, H.A.; El-Hossary, M.M.K.; Katamish, H.A.E.M.; Shokry, T.E.; Rosenstiel, S.F. Accuracy of Ceramic Restorations Made with Two CAD/CAM Systems. J. Prosthet. Dent. 2013, 109, 83–87. [Google Scholar] [CrossRef]
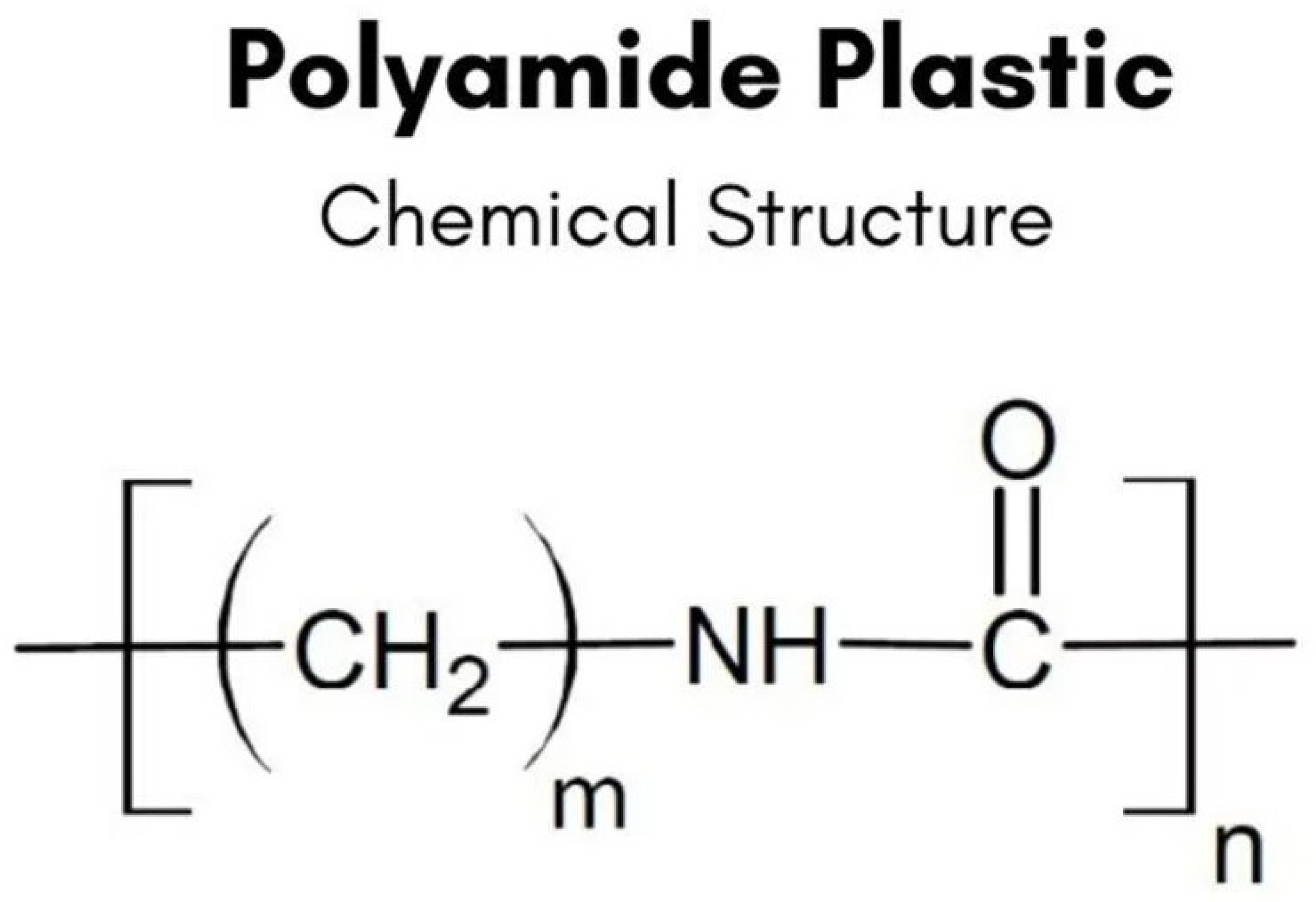



| Polymer Type | Flexural Strength (MPa) | Elastic Modulus (GPa) | Toughness (MPa) | Hardness (Shore D) |
|---|---|---|---|---|
| Conventional PMMA | 65–90 | 2–3 | 3–10 | 75–95 |
| CAD/CAM (Milled) Polymers | 80–120 | 2–5 | 5–15 | 70–85 |
| Composite (Laboratory) Resins | 80–100 | 2–4 | 5–12 | 65–80 |
| Thermoplastic Polyurethane | 25–45 | 0.5–1.5 | 15–25 | 70–90 |
| Polyetheretherketone (PEEK) | 90–120 | 3–4 | 5–15 | 80–90 |
| Polylactic Acid (PLA) | 50–70 | 3–4 | 5–15 | 65–75 |
| Acrylonitrile Butadiene Styrene (ABS) | 30–50 | 2–3 | 5–12 | 75–85 |
| Stereolithography (SLA) Resins | 60–80 | 2–4 | 5–12 | 70–85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuchulska, B.; Dimitrova, M.; Vlahova, A.; Hristov, I.; Tomova, Z.; Kazakova, R. Comparative Analysis of the Mechanical Properties and Biocompatibility between CAD/CAM and Conventional Polymers Applied in Prosthetic Dentistry. Polymers 2024, 16, 877. https://doi.org/10.3390/polym16070877
Chuchulska B, Dimitrova M, Vlahova A, Hristov I, Tomova Z, Kazakova R. Comparative Analysis of the Mechanical Properties and Biocompatibility between CAD/CAM and Conventional Polymers Applied in Prosthetic Dentistry. Polymers. 2024; 16(7):877. https://doi.org/10.3390/polym16070877
Chicago/Turabian StyleChuchulska, Bozhana, Mariya Dimitrova, Angelina Vlahova, Ilian Hristov, Zlatina Tomova, and Rada Kazakova. 2024. "Comparative Analysis of the Mechanical Properties and Biocompatibility between CAD/CAM and Conventional Polymers Applied in Prosthetic Dentistry" Polymers 16, no. 7: 877. https://doi.org/10.3390/polym16070877





