Understanding the Effects of Adding Metal Oxides to Polylactic Acid and Polylactic Acid Blends on Mechanical and Rheological Behaviour, Wettability, and Photo-Oxidation Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Melt Processing
2.3. Characterizations
2.3.1. FTIR Spectroscopy
2.3.2. Water Contact Angle (WCA)
2.3.3. Tensile Tests
2.3.4. Rheological Analysis
2.3.5. Scanning Electron Microscopy (SEM)
2.3.6. Differential Scanning Calorimetry (DSC)
2.4. Photo-Oxidation Exposure
3. Results and Discussion
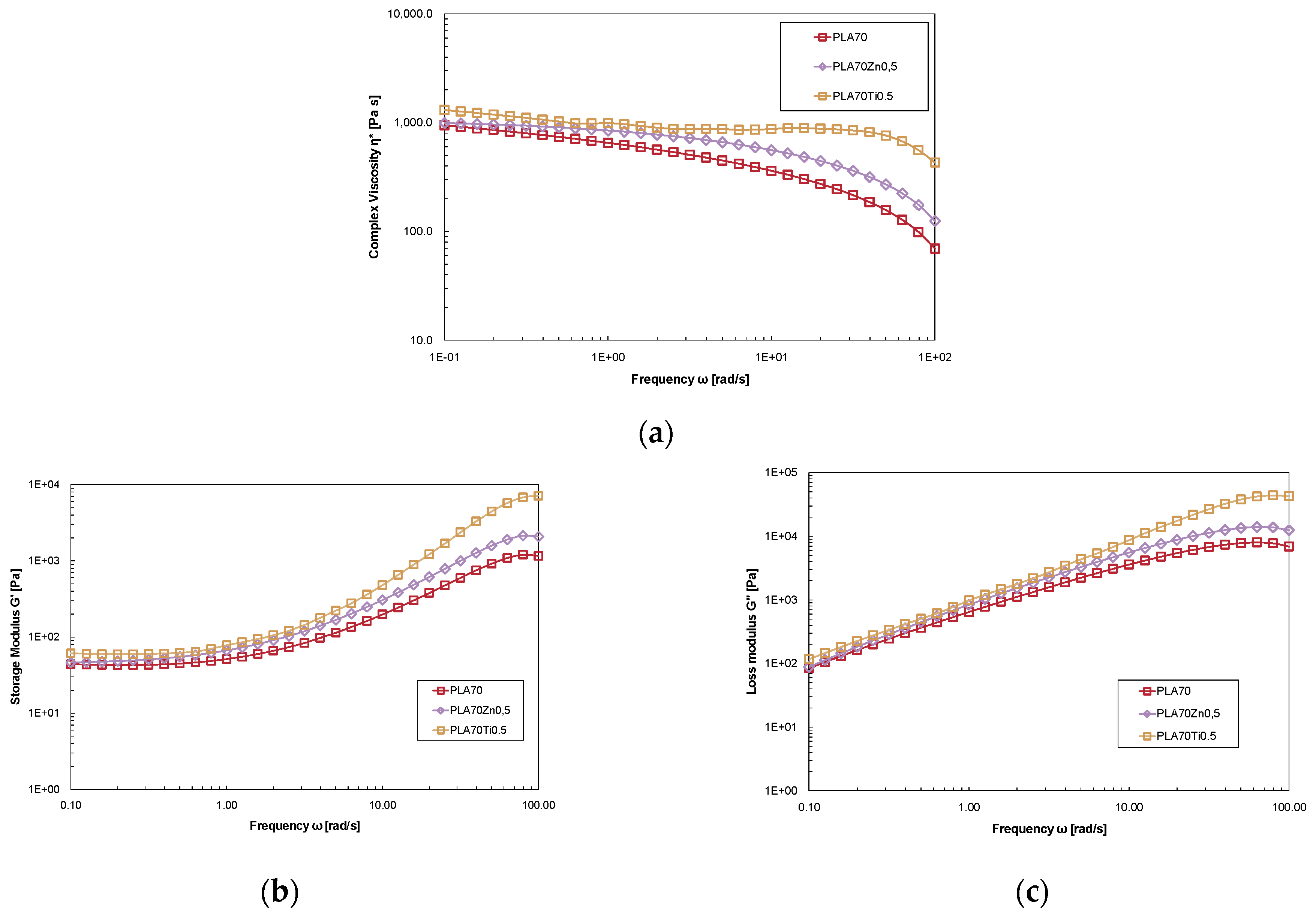
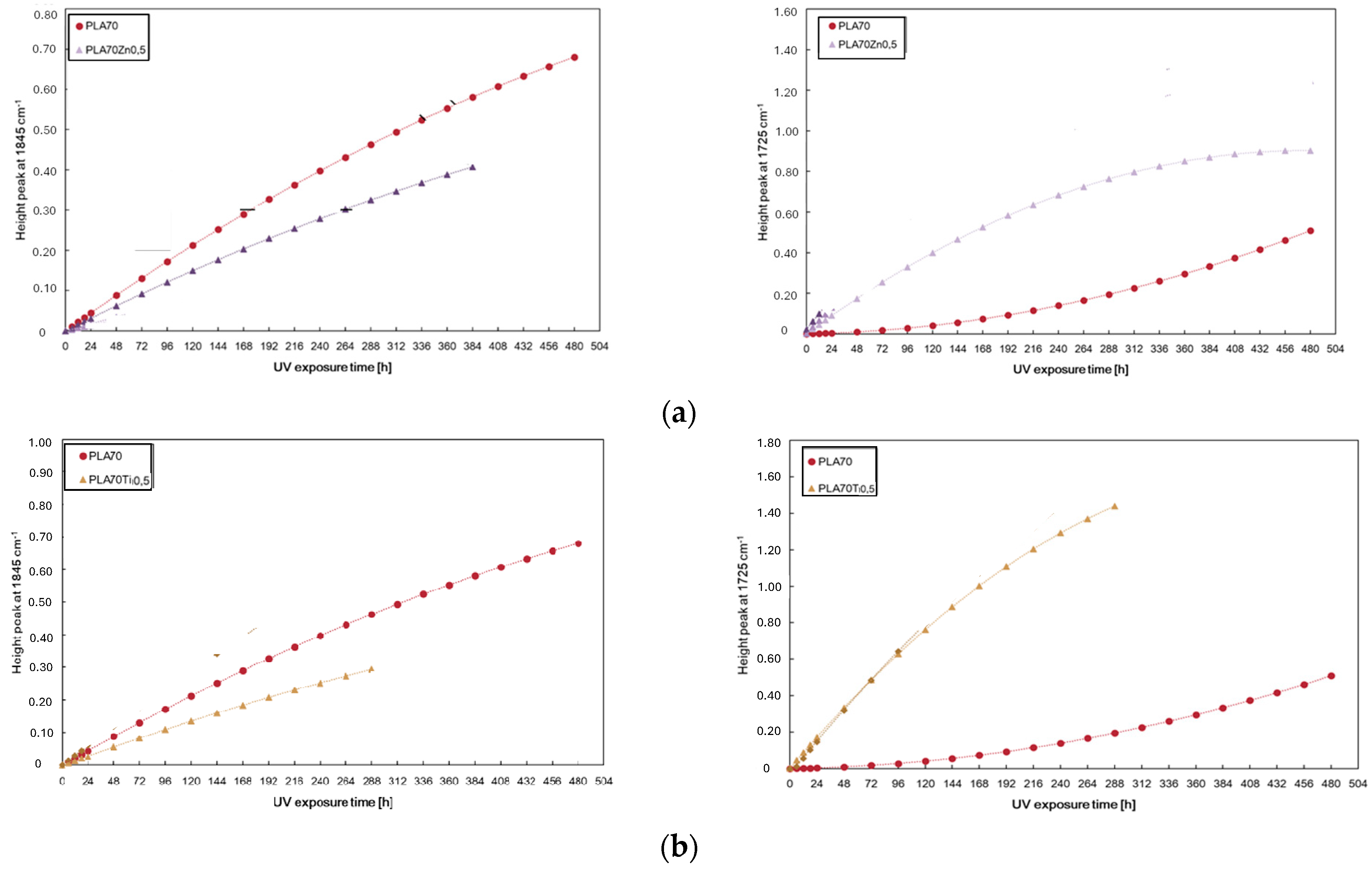
3.1. Effect of ZnO and TiO2 Adding on PLA Properties
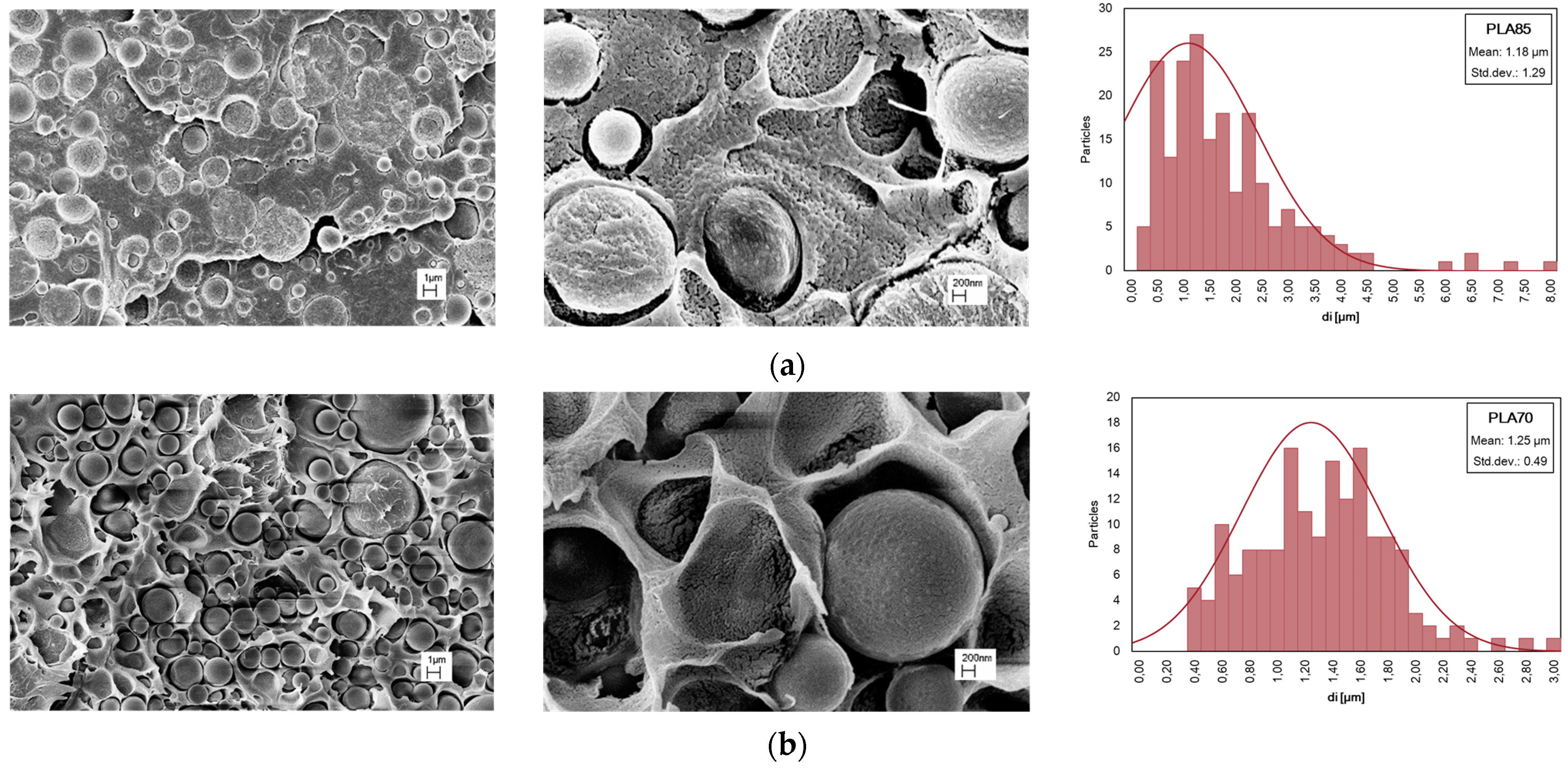
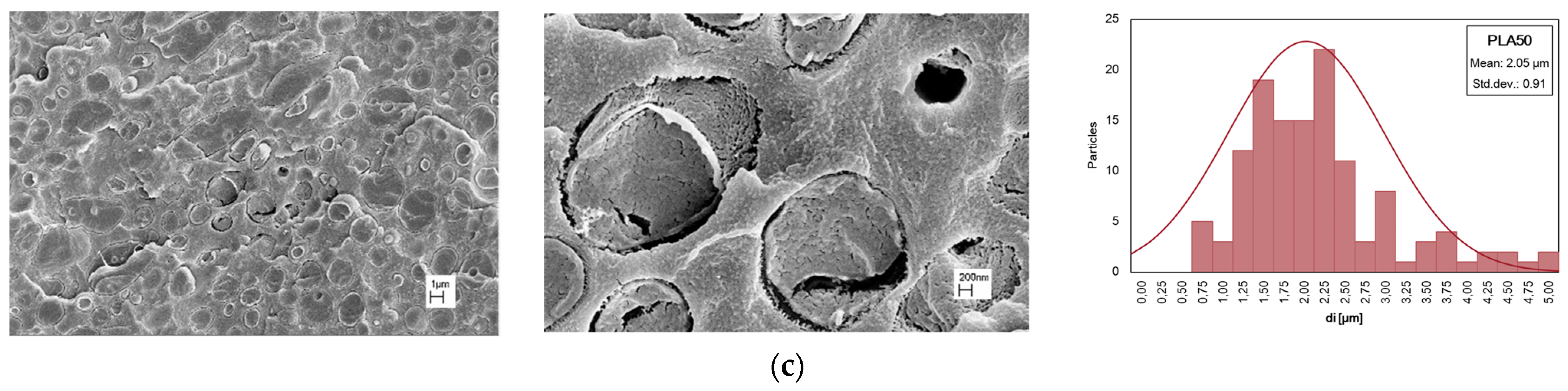
3.2. Effect of PA11 Adding on PLA Properties
3.3. Effect of ZnO and TiO2 Adding on PLA/PA11 (70/30%wt/wt) Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Siva Prasanna, S.R.V.; Balaji, K.; Pandey, S.; Rana, S. Chapter 4-Metal Oxide Based Nanomaterials and Their Polymer Nanocomposites. In Nanomaterials and Polymer Nanocomposites; Karak, N., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 123–144. ISBN 9780128146156. [Google Scholar] [CrossRef]
- Nikolaeva, A.L.; Bugrov, A.N.; Sokolova, M.P.; Ivan’kova, E.M.; Abalov, I.V.; Vlasova, E.N.; Gofman, I.V. Metal Oxide Nanoparticles: An Effective Tool to Modify the Functional Properties of Thermally Stable Polyimide Films. Polymers 2022, 14, 2580. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Singh, A.; Ushal, R.; Yusuf, M.; Kumar, S.; Kumar Ojha, A.; Khanf, W. Overview of Polymer/Metal-Oxide Nanocomposites: Synthesis, Properties, and Their Potential Applications. In Handbook of Nanofibers and Nanocomposites, 1st ed.; Yusuf, M., Haji, A., Eds.; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2024; pp. 193–220. ISBN 9781003432746. [Google Scholar]
- Zarrintaj, P.; Khalili, R.; Vahabi, H.; Reza Saeb, M.; Reza Ganjali, M.; Mozafari, M. Chapter 8–“Polyaniline/metal oxides nanocomposites”. In Fundamentals and Emerging Applications of Polyaniline; Mozafari, M., Singh Chauhan, N.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 131–141. ISBN 9780128179154. [Google Scholar] [CrossRef]
- Opoku, F.; Kiarii, E.M.; Govender, P.P.; Mamo, M.A. Metal Oxide Polymer Nanocomposites in Water Treatments. In Descriptive Inorganic Chemistry Researches of Metal Compounds; Akitsu, T., Ed.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef]
- Velempini, T.; Prabakaran, E.; Pillay, K. Recent developments in the use of metal oxides for photocatalytic degradation of pharmaceutical pollutants in water—A review. Mater. Today Chem. 2021, 19, 100380. [Google Scholar] [CrossRef]
- Danish, M.S.S.; Bhattacharya, A.; Stepanova, D.; Mikhaylov, A.; Grilli, M.L.; Khosravy, M.; Senjyu, T.A. Systematic Review of Metal Oxide Applications for Energy and Environmental Sustainability. Metals 2020, 10, 1604. [Google Scholar] [CrossRef]
- Amini, M.; Ashrafi, M. Photocatalytic degradation of some organic dyes under solar light irradiation using TiO2 and ZnO nanoparticles. Nano Chem. Res. 2016, 1, 79–86. [Google Scholar] [CrossRef]
- Le, A.T.; Pung, S.; Sreekantan, S.; Matsuda, A.; Huynh, D.P. Mechanisms of removal of heavy metal ions by ZnO particles. Heliyon 2019, 5, e01440. [Google Scholar] [CrossRef] [PubMed]
- Alardhi, S.M.; Abdalsalam, A.H.; Ati, A.A.; Abdulkareem, M.H.; Ramadhan, A.A.; Taki, M.M.; Abbas, Z.Y. Fabrication of polyaniline/zinc oxide nanocomposites: Synthesis, characterization and adsorption of methylene orange. Polym. Bull. 2024, 81, 1131–1157. [Google Scholar] [CrossRef]
- Yadav, H.M.; Kim, J.-S.; Pawar, S.H. Developments in photocatalytic antibacterial activity of nano TiO2: A review. Kor. J. Chem. Eng. 2016, 33, 1989–1998. [Google Scholar] [CrossRef]
- Pachaiappan, R.; Rajendran, S.; Loke Show, P.; Manavalan, M.; Naushad, M. Metal/metal oxide nanocomposites for bactericidal effect: A review. Chemosphere 2021, 272, 128607. [Google Scholar] [CrossRef]
- Akira Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Gvozdenko, A.A.; Siddiqui, S.A.; Blinov, A.V.; Golik, A.B.; Nagdalian, A.A.; Maglakelidze, D.G.; Statsenko, E.N.; Pirogov, M.A.; Blinova, A.A.; Sizonenko, M.N.; et al. Synthesis of CuO nanoparticles stabilized with gelatin for potential use in food packaging applications. Sci. Rep. 2022, 12, 12843. [Google Scholar] [CrossRef]
- Trabelsi, A.B.G.; Mostafa, A.M.; Alkallas, F.H.; Elsharkawy, W.B.; Al-Ahmadi, A.N.; Ahmed, H.A.; Nafee, S.S.; Pashameah, R.A.; Mwafy, E.A. Effect of CuO Nanoparticles on the Optical, Structural, and Electrical Properties in the PMMA/PVDF Nanocomposite. Micromachines 2023, 14, 1195. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.A.; Eddine, L.S.; Souhaila, M.; Hasan, G.G.; Kir, I.; Abdullah, J.A.A. Green Synthesis of SnO2 Nanoparticles from Laurus nobilis L. Extract for Enhanced Gelatin-Based Films and CEF@SnO2 for Efficient Antibacterial Activity. Food Bioprocess. Technol. 2023. [Google Scholar] [CrossRef]
- Zagloul, H.; Dhahri, M.; Bashal, A.H.; Khaleil, M.M.; Habeeb, T.H.; Khalil, K.D. Multifunctional Ag2O/chitosan nanocomposites synthesized via sol-gel with enhanced antimicrobial, and antioxidant properties: A novel food packaging material. Int. J. Biol. Macromol. 2024, 264, 129990. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.K.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Borriello, L.; Scivicco, M.; Cacciola, N.A.; Esposito, F.; Severino, L.; Cirillo, T. Microplastics, a Global Issue: Human Exposure through Environmental and Dietary Sources. Foods 2023, 12, 3396. [Google Scholar] [CrossRef] [PubMed]
- Ravanchi, M.T.; Soleimani, M. Global warming and greenhouse effect resulted from oil, gas, and petrochemical units. In Crises in Oil, Gas and Petrochemical Industries; Rahimpour, M.R., Omidvar, B., Shirazi, N.A., Makarem, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 257–282. [Google Scholar] [CrossRef]
- Santos, A.; Barbosa-Póvoa, A.; Carvalho, A. Life cycle assessment in chemical industry—A review. Curr. Opin. Chem. Eng. 2019, 26, 139–147, ISSN 2211-3398. [Google Scholar] [CrossRef]
- Steinbüchel, A. Non-biodegradable biopolymers from renewable resources: Perspectives and impacts. Curr. Opin. Biotechnol. 2005, 16, 607–613, ISSN 0958-1669. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Ringu, T.; Ghosh, S.; Pramanik, N. A comprehensive review on recent advances in preparation, physicochemical characterization, and bioengineering applications of biopolymers. Polym. Bull. 2023, 80, 7247–7312. [Google Scholar] [CrossRef] [PubMed]
- Hanifah, A.; Mahardika, M.A.; Sumirat, R.; Nissa, R.C.; Nurhamiyah, Y. Recent Updates on Biopolymers: Precursors, Process, Properties, Challenge, and Future Perspectives. In Biomass Conversion and Sustainable Biorefinery; Lubis, M.A.R., Lee, S.H., Mardawati, E., Rahimah, S., Antov, P., Andoyo, R., Krišťák, Ľ., Nurhadi, B., Eds.; Green Energy and Technology; Springer: Singapore, 2024. [Google Scholar] [CrossRef]
- Taib, N.A.A.B.; Rahman, M.R.; Huda, D.; Kuok, K.K.; Hamdan, S.; Bin Bakri, M.K.; Bin Julaihi, M.R.M.; Khan, A. A review on poly lactic acid (PLA) as a biodegradable polymer. Polym. Bull. 2023, 80, 1179–1213. [Google Scholar] [CrossRef]
- Park, S.B.; Lih, E.; Park, K.S.; Joung, Y.K.; Han, D.K. Biopolymer-based functional composites for medical applications. Prog. Polym. Sci. 2017, 68, 77–105. [Google Scholar] [CrossRef]
- Kulkarni, R.K.; Moore, E.G.; Hegyeli, A.F.; Leonard, F. Biodegradable poly(lactic acid) polymers. J. Biomed. Mater. Res. 1971, 5, 169–181. [Google Scholar] [CrossRef]
- NatureWorks LLC. Announces World’s First Greenhouse-GasNeutral Polymer; NatureWorks LLC.: Minneapolis, MN, USA, 2005. [Google Scholar]
- Bikiaris, N.D.; Koumentakou, I.; Samiotaki, C.; Meimaroglou, D.; Varytimidou, D.; Karatza, A.; Kalantzis, Z.; Roussou, M.; Bikiaris, R.D.; Papageorgiou, G.Z. Recent Advances in the Investigation of Poly(lactic acid) (PLA) Nanocomposites: Incorporation of Various Nanofillers and their Properties and Applications. Polymers 2023, 15, 1196. [Google Scholar] [CrossRef] [PubMed]
- Sharif, A.; Mondal, S.; Hoque, M.E. Polylactic acid (PLA)-based nanocomposites: Processing and properties. In Bio-Based Polymers and Nanocomposites; Springer: Cham, Switzerland, 2019; pp. 233–254. [Google Scholar]
- Sanyang, M.L.; Jawaid, M. (Eds.) Bio-Based Polymers and Nanocomposites: Preparation, Processing, Properties & Performance; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Stoclet, G.; Seguela, R.; Lefebvre, J.-M. Morphology, thermal behavior and mechanical properties of binary blends of compatible biosourced polymers: Polylactide/polyamide11. Polymer 2011, 52, 1417–1425. [Google Scholar] [CrossRef]
- Patel, R.; Ruehle, D.A.; Dorgan, J.R.; Halley, P.; Martin, D. Biorenewable blends of polyamide-11 and polylactide. Polym. Eng. Sci. 2013, 54, 1523–1532. [Google Scholar] [CrossRef]
- Shen, L.; Haufe, J.; Patel, M.K. Product Overview and Market Projection of Emerging Bio-Based Plastics PRO-BIP 2009. Report for European Polysaccharide Network of Excellence (EPNOE) and European Bioplastics. 2009. Volume 243. Available online: https://plasticker.de/docs/news/PROBIP2009_Final_June_2009.pdf (accessed on 15 March 2024).
- Puglisi, R.; Scamporrino, A.A.; Dintcheva, N.T.; Filippone, G.; Bruno, E.; Scarfato, P.; Cerruti, P.; Carroccio, S.C. Photo- and Water-Degradation Phenomena of ZnO Bio-Blend Based on Poly(lactic acid) and Polyamide 11. Polymers 2023, 15, 1434. [Google Scholar] [CrossRef]
- Nielsen, L.; Landel, R. Mechanical Properties of Polymers and Composites; Marcel Decker: New York, NY, USA, 1994. [Google Scholar]
- Murariu, M.; Doumbia, A.; Bonnaud, L.; Dechief, A.L.; Paint, Y.; Ferreira, M.; Campagne, C.; Devaux, E.; Dubois, P. High-Performance Polylactide/ZnO Nanocomposites Designed for Films and Fibers with Special End-Use Properties. Biomacromolecules 2011, 12, 1762–1771. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, R.; Bartoli, M.; Malucelli, G. Poly(lactic acid)–Biochar Biocomposites: Effect of Processing and Filler Content on Rheological, Thermal, and Mechanical Properties. Polymers 2020, 12, 892. [Google Scholar] [CrossRef] [PubMed]
- Botta, L.; Scaffaro, R.; La Mantia, F.P.; Dintcheva, N.T. Effect of different matrices and nanofillers on the rheological behavior of polymer-clay nanocomposites. J. Polym. Sci. B Polym. Phys. 2010, 48, 344–355. [Google Scholar] [CrossRef]
- Gardette, M.; Thérias, S.; Gardette, J.-L.; Murariu, M.; Dubois, P. Photooxidation of polylactide/calcium sulphate composites. Polym. Degrad. Stab. 2011, 96, 616–623. [Google Scholar] [CrossRef]
- Pérez Amaro, L.; Cicogna, F.; Passaglia, E.; Morici, E.; Oberhauser, W.; Al-Malaika, S.; Dintcheva, N.T.; Coiai, S. Thermo-oxidative stabilization of poly(lactic acid) with antioxidant intercalated layered double hydroxides. Polym. Degrad. Stab. 2016, 133, 92–100. [Google Scholar] [CrossRef]
- Yu, X.; Wang, X.; Zhang, Z.; Peng, S.; Chen, H.; Zhao, X. High-performance fully bio-based poly(lactic acid)/polyamide11 (PLA/PA11) blends by reactive blending with multi-functionalized epoxy. Polym. Test. 2019, 78, 105980. [Google Scholar] [CrossRef]
- Rasselet, D.; Caro-Bretelle, A.-S.; Taguet, A.; Lopez-Cuesta, J.-M. Reactive Compatibilization of PLA/PA11 Blends and Their Application in Additive Manufacturing. Materials 2019, 12, 485. [Google Scholar] [CrossRef] [PubMed]
- Khankrua, R.; Wiriya-Amornchai, A.; Triamnak, N.; Suttiruengwong, S. Biopolymer blends based on poly(lactic acid) and polyamide for durable applications. Polym.-Plast. Technol. Mater. 2023, 62, 131–144. [Google Scholar] [CrossRef]
- Nuzzo, A.; Coiai, S.; Carroccio, S.C.; Dintcheva, N.T.; Gambarotti, C.; Filippone, G. Heat-resistant fully bio-based nanocomposite blends based on Poly(lactic acid). Macromol. Mater. Eng. 2014, 299, 31–40. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; Filippone, G.; La Mantia, F.P.; Acierno, D. Photo-oxidation behaviour of polyethylene/polyamide 6 blends filled with organomodified clay: Improvement of the photo-resistance through morphology modification. Polym. Degrad. Stab. 2010, 95, 527–535. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; Al-Malaika, S.; Morici, E. Novel organo-modifier for thermally-stable polymer-layered silicate nanocomposites. Polym. Degrad. Stab. 2015, 122, 88–101. [Google Scholar] [CrossRef]
- Rasouli, D.; Dintcheva, N.T.; Faezipour, M.; La Mantia, F.P.; Farahani, M.R.M.; Tajvidi, M. Effect of nano zinc oxide as UV stabilizer on the weathering performance of wood-polyethylene composite. Polym. Degrad. Stab. 2016, 133, 85–91. [Google Scholar] [CrossRef]
- Grigoriadou, I.; Paraskevopoulos, K.M.; Chrissafis, K.; Pavlidou, E.; Stamkopoulos, T.-G.; Bikiaris, D. Effect of different nanoparticles on HDPE UV stability. Polym. Degrad. Stab. 2011, 96, 151–163. [Google Scholar] [CrossRef]
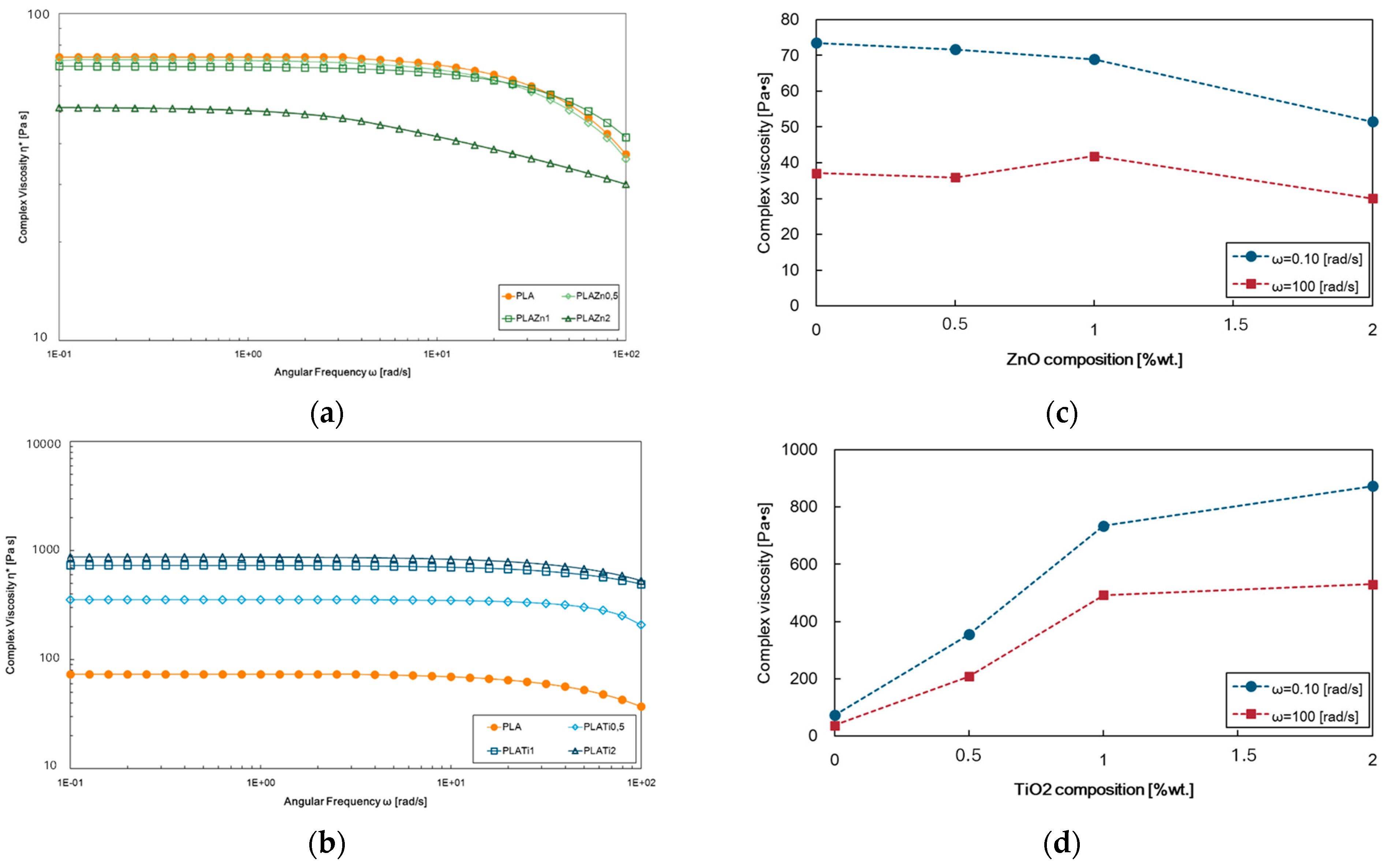

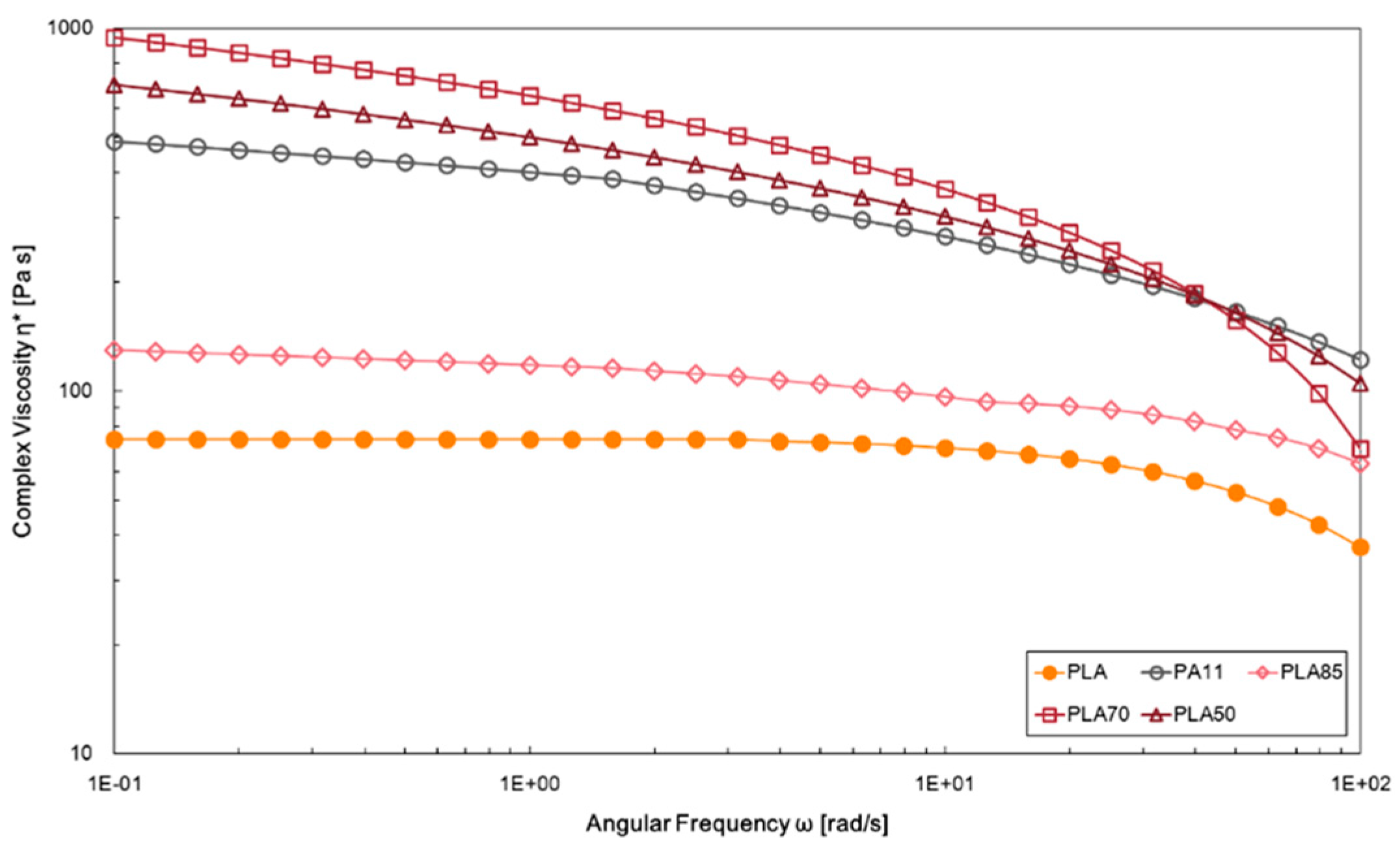
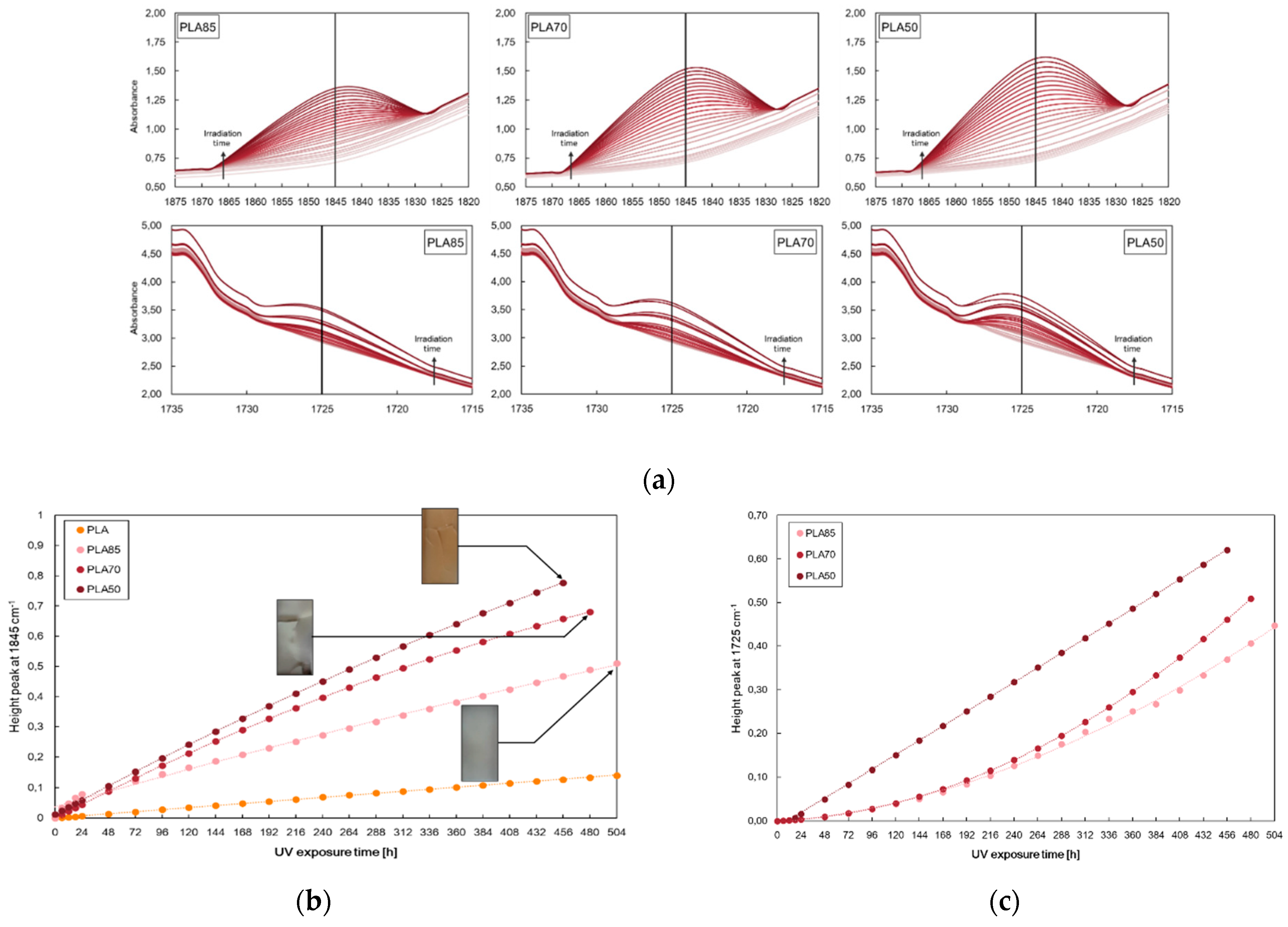
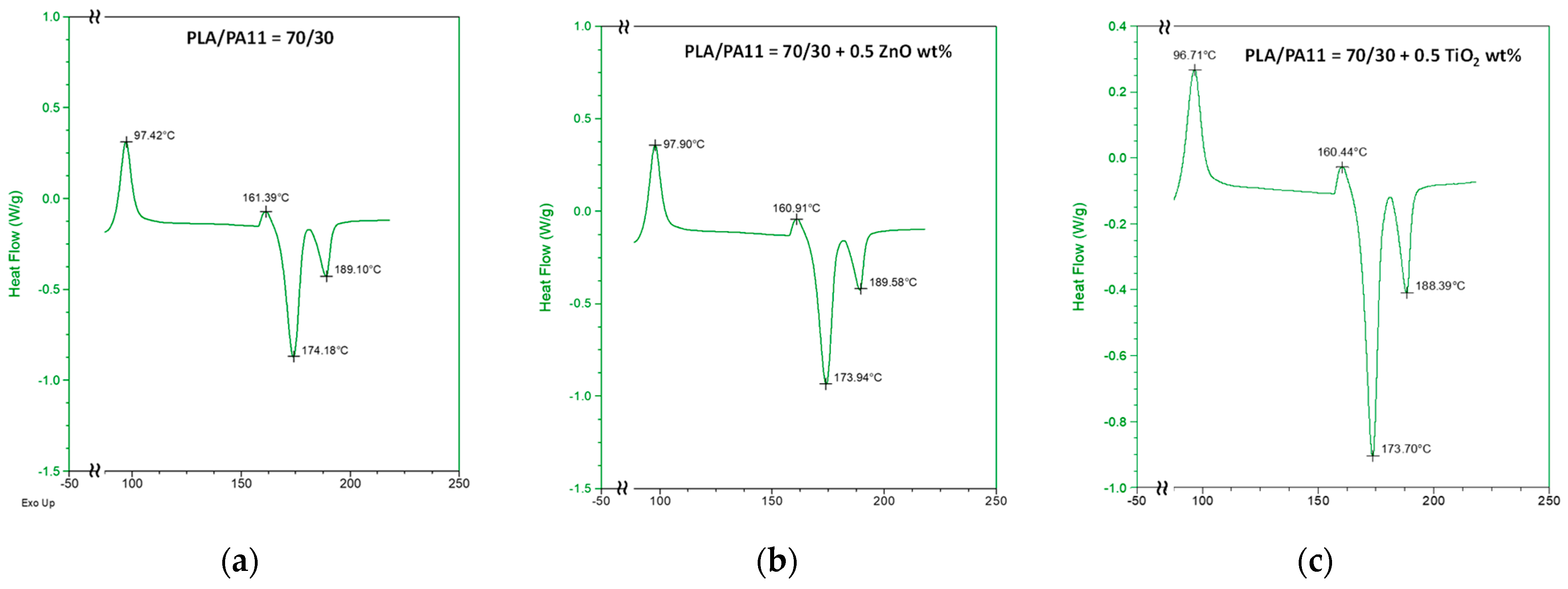
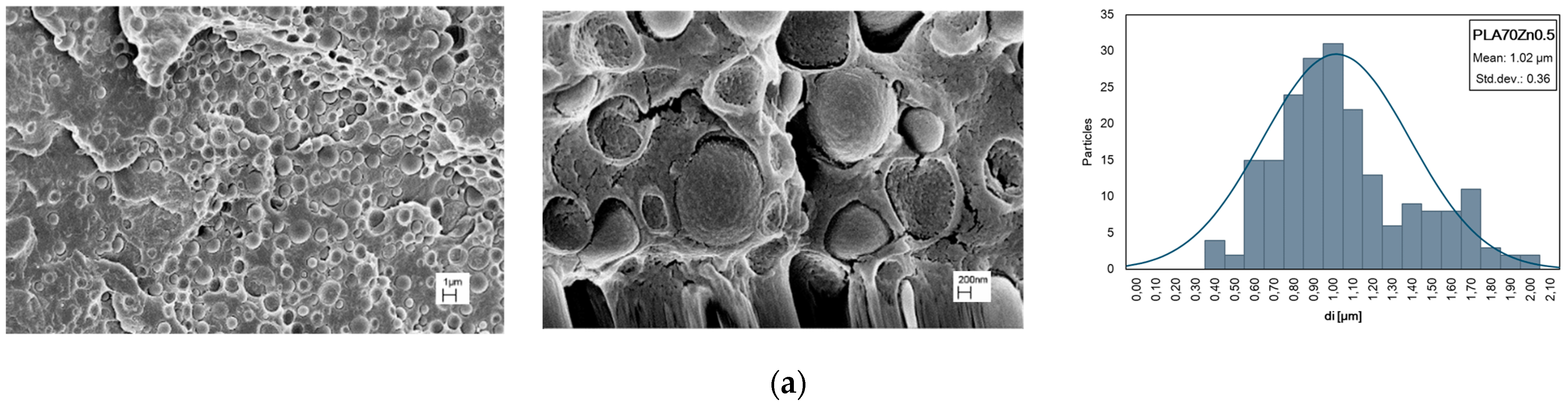
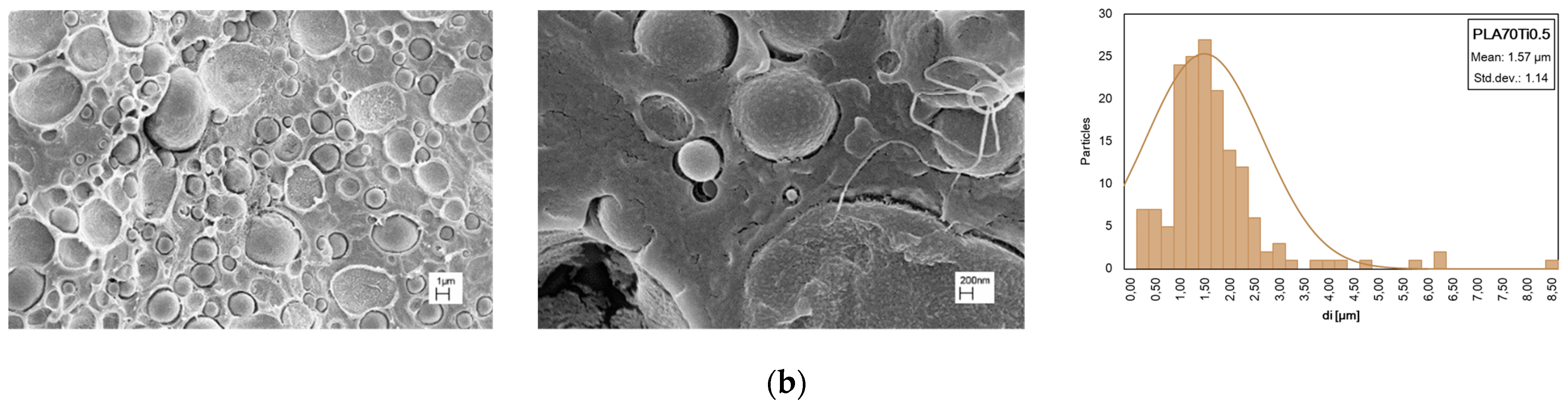
| Samples | E, MPa | TS, MPa | EB, % | WCA, deg | Δ, % |
|---|---|---|---|---|---|
| PLA | 980 ± 65 | 34.9 ± 2.5 | 6.2 ± 0.5 | 60.10 ± 1.0 | 9.5 |
| PLA + ZnO at 0.5%wt | 542 ± 52 | 33.3 ± 3.1 | 3.5 ± 0.4 | 65.15 ± 1.2 | 6.1 |
| PLA + ZnO at 1%wt | 424 ± 45 | 29.8 ± 3.0 | 2.9 ± 0.3 | 70.19 ± 1.3 | 5.8 |
| PLA + ZnO at 2%wt | 199 ± 22 | 22.9 ± 3.1 | 3.6 ± 0.3 | 78.61 ± 1.2 | 4.3 |
| PLA + TiO2 at 0.5%wt | 741 ± 68 | 35.9 ± 3.4 | 7.9 ± 0.5 | 62.64 ± 1.1 | 6.7 |
| PLA + TiO2 at 1%wt | 750 ± 69 | 29.1 ± 3.2 | 8.7 ± 0.5 | 65.18 ± 1.2 | 5.2 |
| PLA + TiO2 at 2%wt | 744 ± 65 | 22.5 ± 2.7 | 9.3 ± 0.6 | 53.90 ± 1.0 | 4.8 |
| Samples | E, MPa | TS, MPa | EB, % | WCA, deg |
|---|---|---|---|---|
| PLA | 980 ± 65 | 34.9 ± 2.5 | 6.2 ± 0.5 | 60.10 ± 1.0 |
| PA11 | 1250 ± 110 | 19.7 ± 1.5 | 22.8 ± 2.5 | 85.3 ± 2.3 |
| PLA/PA11 (85/15%wt/wt) | 632 ± 65 | 12.7 ± 1.2 | 2.1 ± 0.3 | 62.4 ± 1.2 |
| PLA/PA11 (70/30%wt/wt) | 579 ± 54 | 14.8 ± 1.5 | 5.0 ± 0.7 | 64.7 ± 1.0 |
| PLA/PA11 (50/50%wt/wt) | 828 ± 77 | 21.5 ± 2.1 | 8.2 ± 0.8 | 64.7 ± 1.1 |
| Samples | Σni | ||
|---|---|---|---|
| (a) | |||
| PLA/PA11 (85/15%wt/wt) | 201 | 1.28 | 3.46 |
| PLA/PA11 (70/30%wt/wt) | 166 | 1.35 | 1.41 |
| PLA/PA11 (50/50%wt/wt) | 129 | 2.05 | 1.65 |
| (b) | |||
| PLA/PA11 (70/30) + ZnO at 0.5%wt | 204 | 1.02 | 1.36 |
| PLA/PA11 (70/30) + TiO2 at 0.5%wt | 132 | 1.57 | 3.50 |
| Samples | E, MPa | TS, MPa | EB, % | WCA, deg |
|---|---|---|---|---|
| PLA/PA11 (70/30%wt/wt) | 579 ± 54 | 14.8 ± 1.5 | 5.0 ± 0.7 | 64.7 ± 1.0 |
| PLA/PA11 + ZnO at 0.5%wt | 662 ± 63 | 14.8 ± 1.4 | 4.4 ± 0.4 | 53.5 ± 1.2 |
| PLA/PA11+ TiO2 at 0.5%wt | 589 ± 51 | 9.2 ± 0.9 | 3.9 ± 0.3 | 66.7 ± 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morici, E.; Pecoraro, G.; Carroccio, S.C.; Bruno, E.; Scarfato, P.; Filippone, G.; Dintcheva, N.T. Understanding the Effects of Adding Metal Oxides to Polylactic Acid and Polylactic Acid Blends on Mechanical and Rheological Behaviour, Wettability, and Photo-Oxidation Resistance. Polymers 2024, 16, 922. https://doi.org/10.3390/polym16070922
Morici E, Pecoraro G, Carroccio SC, Bruno E, Scarfato P, Filippone G, Dintcheva NT. Understanding the Effects of Adding Metal Oxides to Polylactic Acid and Polylactic Acid Blends on Mechanical and Rheological Behaviour, Wettability, and Photo-Oxidation Resistance. Polymers. 2024; 16(7):922. https://doi.org/10.3390/polym16070922
Chicago/Turabian StyleMorici, Elisabetta, Giuseppe Pecoraro, Sabrina Carola Carroccio, Elena Bruno, Paola Scarfato, Giovanni Filippone, and Nadka Tz. Dintcheva. 2024. "Understanding the Effects of Adding Metal Oxides to Polylactic Acid and Polylactic Acid Blends on Mechanical and Rheological Behaviour, Wettability, and Photo-Oxidation Resistance" Polymers 16, no. 7: 922. https://doi.org/10.3390/polym16070922
APA StyleMorici, E., Pecoraro, G., Carroccio, S. C., Bruno, E., Scarfato, P., Filippone, G., & Dintcheva, N. T. (2024). Understanding the Effects of Adding Metal Oxides to Polylactic Acid and Polylactic Acid Blends on Mechanical and Rheological Behaviour, Wettability, and Photo-Oxidation Resistance. Polymers, 16(7), 922. https://doi.org/10.3390/polym16070922









