Photothermal Performance of Lignin-Based Nanospheres and Their Applications in Water Surface Actuators
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Lignin-Based Nanospheres
2.3. Preparation of Carbon Nanospheres Hydrophobic Coating
2.4. Characterization
2.5. Photothermal Heating
2.6. Photothermal Stability
2.7. Photothermal Driving Performance
3. Results and Discussion
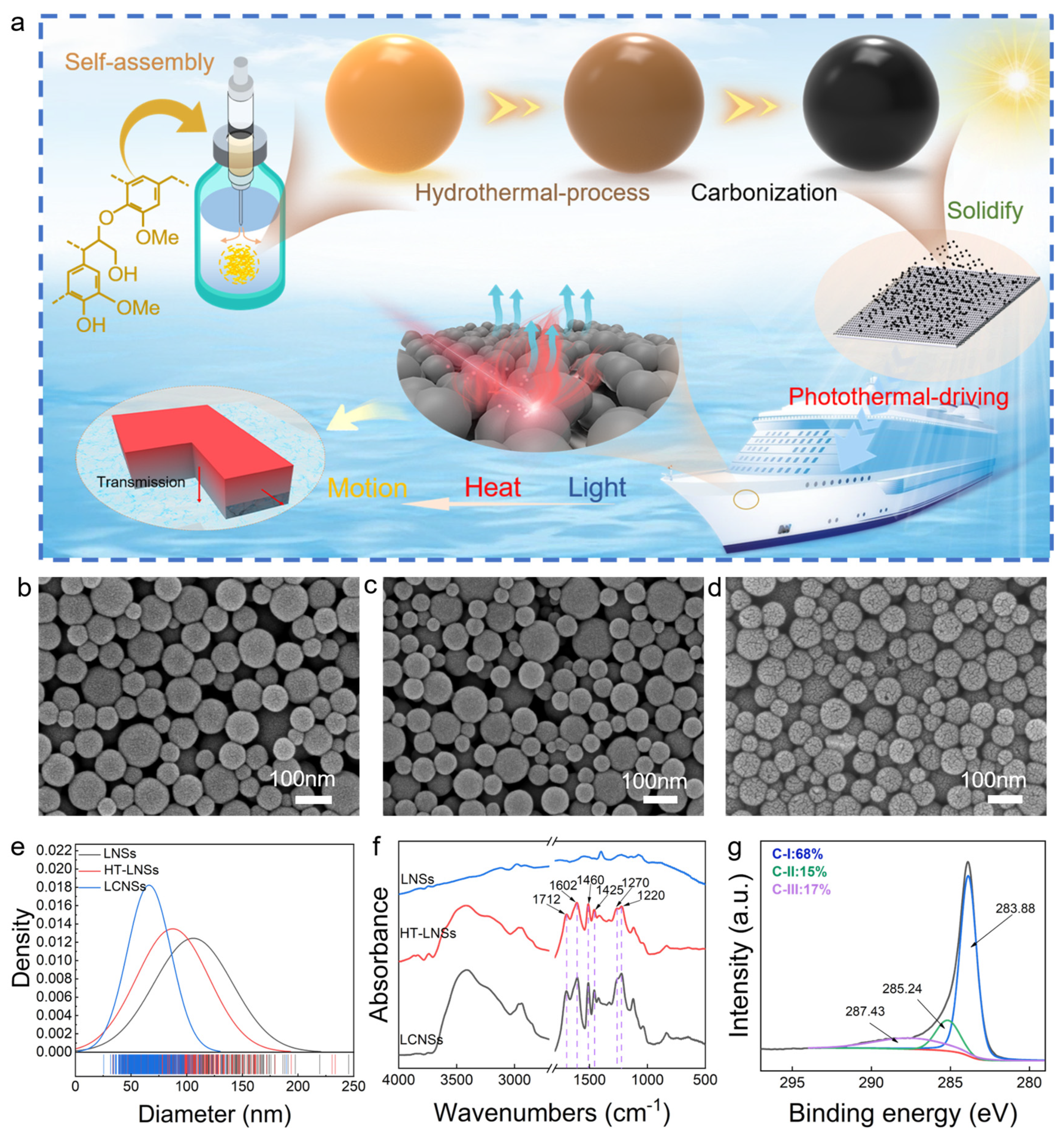
3.1. Morphology and Chemical Structural Characteristics of Lignin-Based Nanospheres
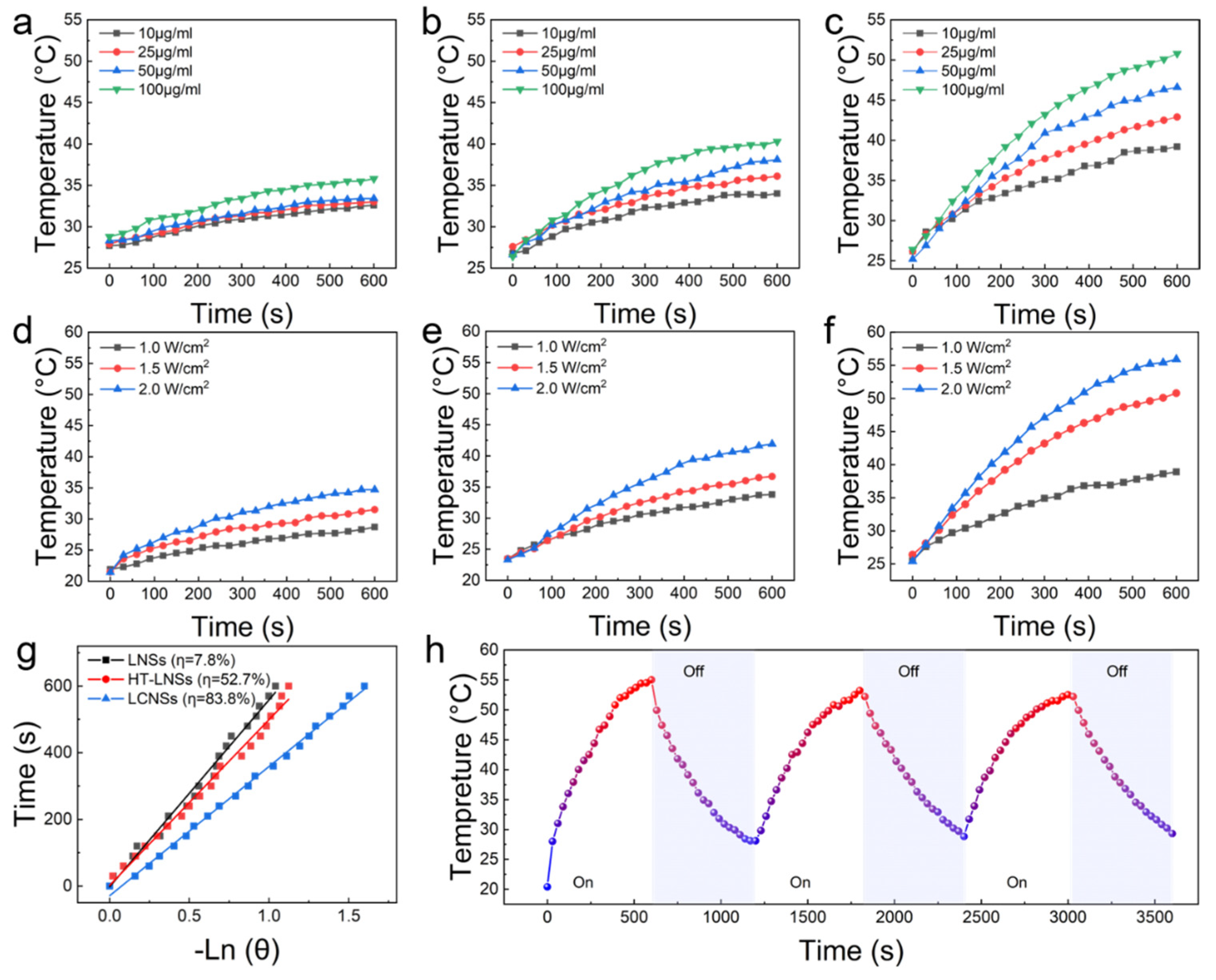
3.2. Photothermal Performance of Lignin-Based Nanospheres
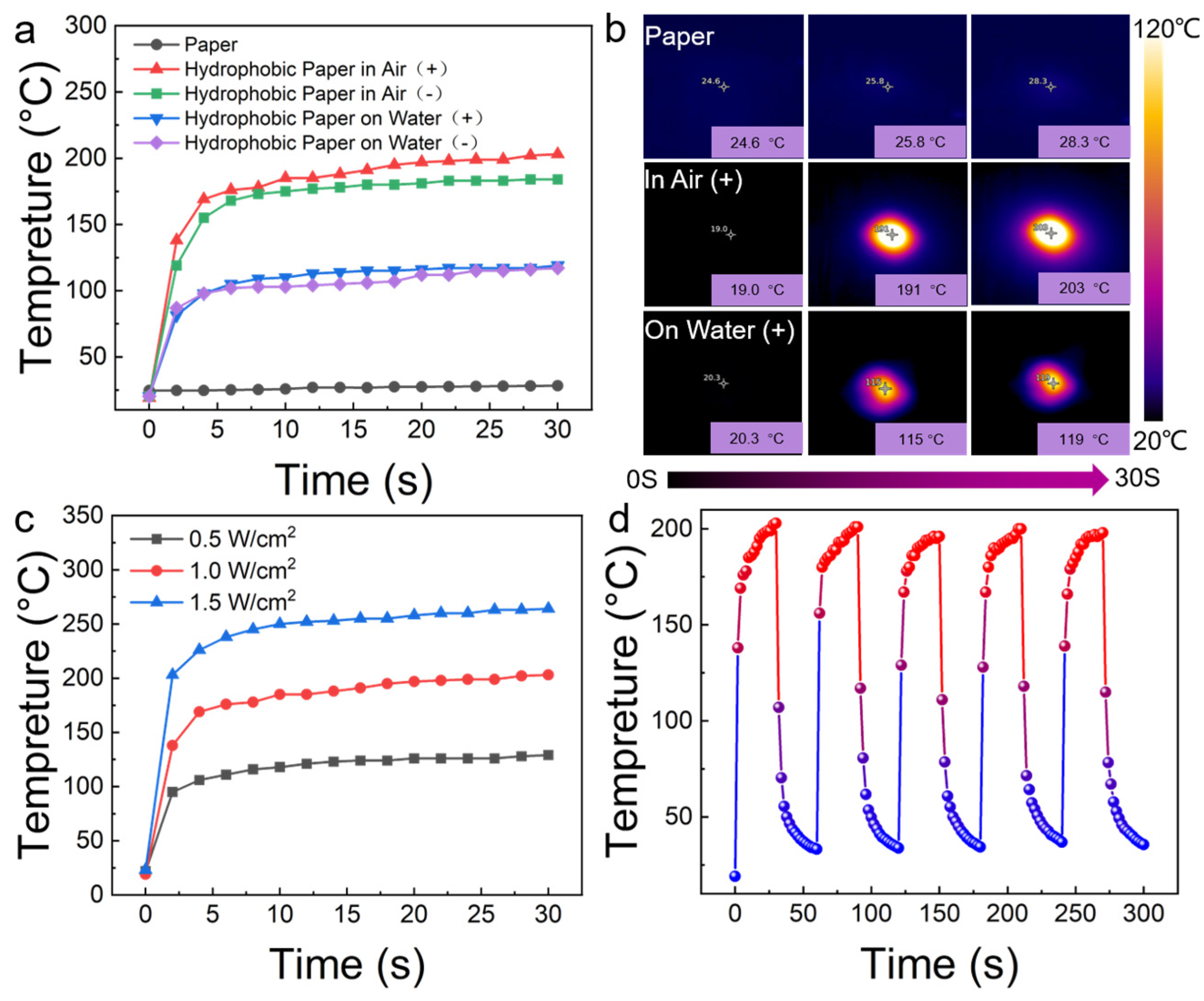
3.3. Characterization and Photothermal Performance of LCNS Hydrophobic Coating
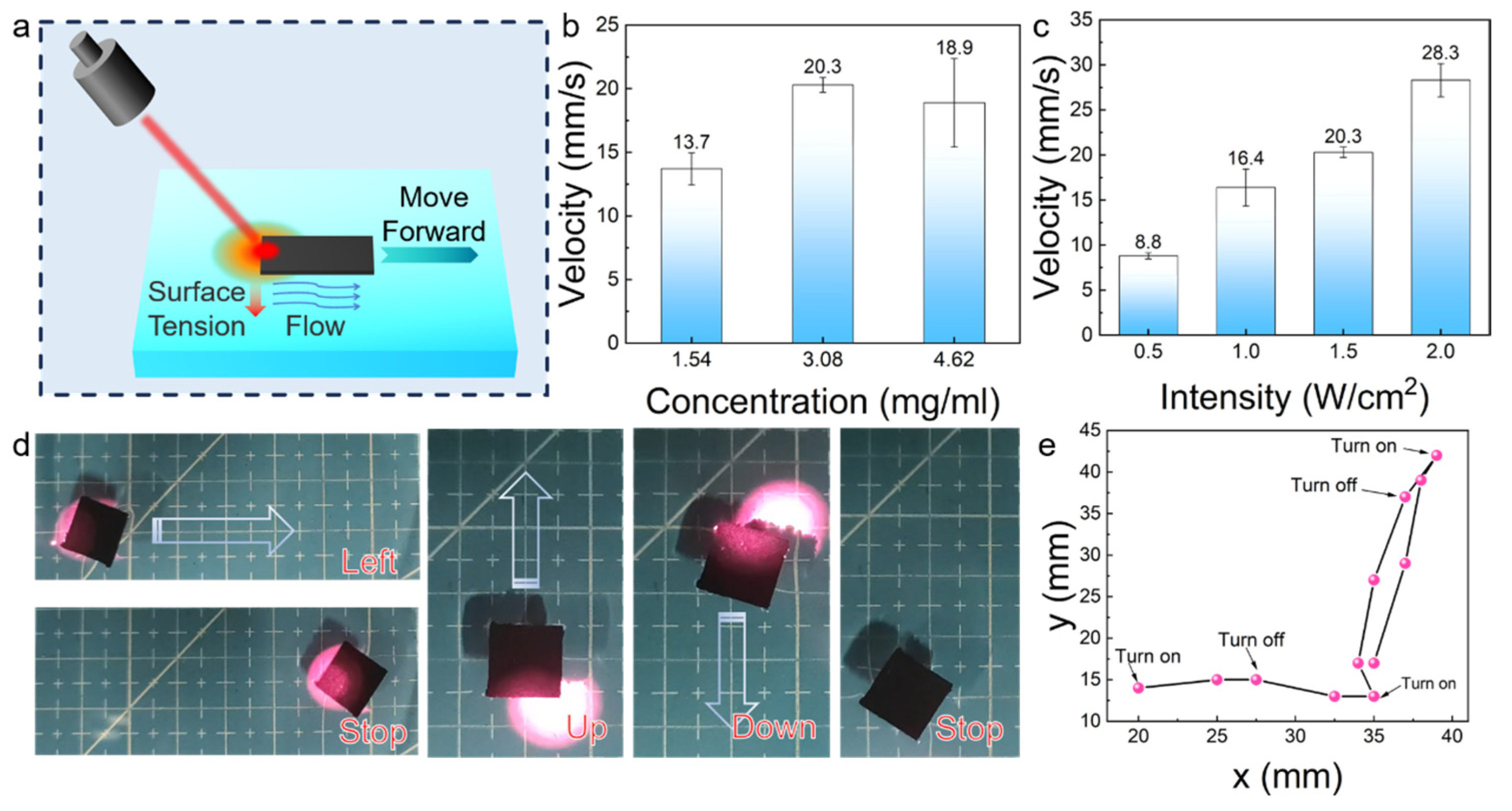
3.4. Driving Performance of the Photothermal-Driven Actuator
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Han, B.; Zhang, Y.L.; Chen, Q.D.; Sun, H.B. Carbon-Based Photothermal Actuators. Adv. Funct. Mater. 2018, 28, 1802235. [Google Scholar] [CrossRef]
- Pan, D.; Wu, D.; Li, P.J.; Ji, S.Y.; Nie, X.; Fan, S.Y.; Chen, G.Y.; Zhang, C.C.; Xin, C.; Xu, B.; et al. Transparent Light-Driven Hydrogel Actuator Based on Photothermal Marangoni Effect and Buoyancy Flow for Three-Dimensional Motion. Adv. Funct. Mater. 2021, 31, 2009386. [Google Scholar] [CrossRef]
- Yang, K.; Fu, C.; Li, C.; Ye, Y.; Ding, M.; Zhou, J.; Bai, Y.; Jiao, F.; Ma, J.; Guo, Q.; et al. Dual-responsive smart actuator based on Ti3C2Tx/polymer bilayer structure for bionic applications. Sens. Actuators A Phys. 2022, 341, 113553. [Google Scholar] [CrossRef]
- Wu, H.; Luo, J.; Huang, X.; Wang, L.; Guo, Z.; Liang, J.; Zhang, S.; Xue, H.; Gao, J. Superhydrophobic, mechanically durable coatings for controllable light and magnetism driven actuators. J. Colloid Interface Sci. 2021, 603, 282–290. [Google Scholar] [CrossRef]
- Kümmel, F.; ten Hagen, B.; Wittkowski, R.; Buttinoni, I.; Eichhorn, R.; Volpe, G.; Löwen, H.; Bechinger, C. Circular Motion of Asymmetric Self-Propelling Particles. Phys. Rev. Lett. 2013, 110, 198302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chang, H.; Wu, Y.; Xiao, P.; Yi, N.; Lu, Y.; Ma, Y.; Huang, Y.; Zhao, K.; Yan, X.-Q.; et al. Macroscopic and direct light propulsion of bulk graphene material. Nat. Photonics 2015, 9, 471–476. [Google Scholar] [CrossRef]
- Jiang, W.; Niu, D.; Liu, H.; Wang, C.; Zhao, T.; Yin, L.; Shi, Y.; Chen, B.; Ding, Y.; Lu, B. Photoresponsive Soft-Robotic Platform: Biomimetic Fabrication and Remote Actuation. Adv. Funct. Mater. 2014, 24, 7598–7604. [Google Scholar] [CrossRef]
- Wang, X.; Dai, L.; Jiao, N.; Tung, S.; Liu, L. Superhydrophobic photothermal graphene composites and their functional applications in microrobots swimming at the air/water interface. Chem. Eng. J. 2021, 422, 129394. [Google Scholar] [CrossRef]
- Lu, X.; Guo, S.; Tong, X.; Xia, H.; Zhao, Y. Tunable Photocontrolled Motions Using Stored Strain Energy in Malleable Azobenzene Liquid Crystalline Polymer Actuators. Adv. Mater. 2017, 29, 1606467. [Google Scholar] [CrossRef]
- Yang, R.-L.; Zhu, Y.-J.; Qin, D.-D.; Xiong, Z.-C. Light-Operated Dual-Mode Propulsion at the Liquid/Air Interface Using Flexible, Superhydrophobic, and Thermally Stable Photothermal Paper. ACS Appl. Mater. Interfaces 2019, 12, 1339–1347. [Google Scholar] [CrossRef]
- Yang, R.-L.; Zhu, Y.-J.; Chen, F.-F.; Qin, D.-D.; Xiong, Z.-C. Superhydrophobic Photothermal Paper Based on Ultralong Hydroxyapatite Nanowires for Controllable Light-Driven Self-Propelled Motion. ACS Sustain. Chem. Eng. 2019, 7, 13226–13235. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, F.; Liu, L.; Tong, T.; Li, R.; Zhong, H.; Chen, Z.; Qin, C.; Wang, Q.; Qi, Y.; et al. Magnetically Enhanced Marangoni Convection for Efficient Mass and Heat Transfer like a Self-Driving Liquid Conveyor Belt. Adv. Funct. Mater. 2022, 32, 2202984. [Google Scholar] [CrossRef]
- Lin, F.; Quraishy, A.N.; Tong, T.; Li, R.; Yang, G.; Mohebinia, M.; Qiu, Y.; Vishal, T.; Zhao, J.; Zhang, W.; et al. Marangoni convection-driven laser fountains on free surfaces of liquids. Mater. Today Phys. 2021, 21, 100558. [Google Scholar] [CrossRef]
- Lin, F.; Quraishy, A.N.; Li, R.; Yang, G.; Mohebinia, M.; Tong, T.; Qiu, Y.; Vishal, T.; Zhao, J.; Zhang, W.; et al. Molding, patterning and driving liquids with light. Mater. Today 2021, 51, 48–55. [Google Scholar] [CrossRef]
- Cao, W.-T.; Feng, W.; Jiang, Y.-Y.; Ma, C.; Zhou, Z.-F.; Ma, M.-G.; Chen, Y.; Chen, F. Two-dimensional MXene-reinforced robust surface superhydrophobicity with self-cleaning and photothermal-actuating binary effects. Mater. Horiz. 2019, 6, 1057–1065. [Google Scholar] [CrossRef]
- Su, X.; Li, H.; Lai, X.; Yang, Z.; Chen, Z.; Wu, W.; Zeng, X. Vacuum-assisted layer-by-layer superhydrophobic carbon nanotube films with electrothermal and photothermal effects for deicing and controllable manipulation. J. Mater. Chem. A 2018, 6, 16910–16919. [Google Scholar] [CrossRef]
- Xiong, F.; Han, Y.; Wang, S.; Li, G.; Qin, T.; Chen, Y.; Chu, F. Preparation and formation mechanism of size-controlled lignin nanospheres by self-assembly. Ind. Crops Prod. 2017, 100, 146–152. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, C.; Xiao, D.; Wang, P.; Luo, X.; Liu, W.; Liu, S.; Li, J.; Li, S.; Chen, Z. Melanin-Inspired Design: Preparing Sustainable Photothermal Materials from Lignin for Energy Generation. ACS Appl. Mater. Interfaces 2021, 13, 7600–7607. [Google Scholar] [CrossRef] [PubMed]
- Vélez-Cordero, J.R.; Hernández-Cordero, J. Heat generation and conduction in PDMS-carbon nanoparticle membranes irradiated with optical fibers. Int. J. Therm. Sci. 2015, 96, 12–22. [Google Scholar] [CrossRef]
- Xiong, F.; Han, Y.; Li, G.; Qin, T.; Wang, S.; Chu, F. Synthesis and characterization of renewable woody nanoparticles fluorescently labeled by pyrene. Ind. Crops Prod. 2016, 83, 663–669. [Google Scholar] [CrossRef]
- Cao, Y.; Dou, J.-H.; Zhao, N.-J.; Zhang, S.; Zheng, Y.-Q.; Zhang, J.-P.; Wang, J.-Y.; Pei, J.; Wang, Y. Highly Efficient NIR-II Photothermal Conversion Based on an Organic Conjugated Polymer. Chem. Mater. 2016, 29, 718–725. [Google Scholar] [CrossRef]
- Wang, H.; Xiong, F.; Tan, Y.; Yang, J.; Qing, Y.; Chu, F.; Wu, Y. Preparation and Formation Mechanism of Covalent–Noncovalent Forces Stabilizing Lignin Nanospheres and Their Application in Superhydrophobic and Carbon Materials. ACS Sustain. Chem. Eng. 2021, 9, 3811–3820. [Google Scholar] [CrossRef]
- He, Z.-W.; Yang, J.; Lü, Q.-F.; Lin, Q. Effect of Structure on the Electrochemical Performance of Nitrogen- and Oxygen-Containing Carbon Micro/Nanospheres Prepared from Lignin-Based Composites. ACS Sustain. Chem. Eng. 2013, 1, 334–340. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, X.; Xiong, F.; Ding, J.; Qing, Y.; Wu, Y. Preparation of lignin-based porous carbon as an efficient absorbent for the removal of methylene blue. Ind. Crops Prod. 2021, 171, 113980. [Google Scholar] [CrossRef]
- Wang, H.; Xiong, F.; Yang, J.; Ma, B.; Qing, Y.; Chu, F.; Wu, Y. Preparation of size-controlled all-lignin based carbon nanospheres and their electrochemical performance in supercapacitor. Ind. Crops Prod. 2022, 179, 114689. [Google Scholar] [CrossRef]
- Guo, N.; Li, M.; Sun, X.; Wang, F.; Yang, R. Enzymatic hydrolysis lignin derived hierarchical porous carbon for supercapacitors in ionic liquids with high power and energy densities. Green Chem. 2017, 19, 2595–2602. [Google Scholar] [CrossRef]
- Fu, F.; Yang, D.; Zhang, W.; Wang, H.; Qiu, X. Green self-assembly synthesis of porous lignin-derived carbon quasi-nanosheets for high-performance supercapacitors. Chem. Eng. J. 2020, 392, 123721. [Google Scholar] [CrossRef]
- Li, J.; Liu, W.; Qiu, X.; Zhao, X.; Chen, Z.; Yan, M.; Fang, Z.; Li, Z.; Tu, Z.; Huang, J. Lignin: A sustainable photothermal block for smart elastomers. Green Chem. 2022, 24, 823–836. [Google Scholar] [CrossRef]
- Weng, Y.; Guan, S.; Wang, L.; Lu, H.; Meng, X.; Waterhouse, G.I.N.; Zhou, S. Defective Porous Carbon Polyhedra Decorated with Copper Nanoparticles for Enhanced NIR-Driven Photothermal Cancer Therapy. Small 2019, 16, 1905184. [Google Scholar] [CrossRef]
- Jaque, D.; Maestro, L.M.; del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.; Rodríguez, E.M.; Solé, J.G. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef]
- Weng, Y.; Guan, S.; Wang, L.; Qu, X.; Zhou, S. Hollow carbon nanospheres derived from biomass by-product okara for imaging-guided photothermal therapy of cancers. J. Mater. Chem. B 2019, 7, 1920–1925. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Jin, X.; Wu, C.; Brozovic, A.; Zhang, W. Carbon spheres with high photothermal conversion efficiency for photothermal therapy of tumor. Diam. Relat. Mater. 2022, 126, 109048. [Google Scholar] [CrossRef]
- Zhou, L.; Jing, Y.; Liu, Y.; Liu, Z.; Gao, D.; Chen, H.; Song, W.; Wang, T.; Fang, X.; Qin, W.; et al. Mesoporous Carbon Nanospheres as a Multifunctional Carrier for Cancer Theranostics. Theranostics 2018, 8, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tseng, Y.-T.; Suo, G.; Chen, L.; Yu, J.; Chiu, W.-J.; Huang, C.-C.; Lin, C.-H. Photothermal Therapeutic Response of Cancer Cells to Aptamer–Gold Nanoparticle-Hybridized Graphene Oxide under NIR Illumination. ACS Appl. Mater. Interfaces 2015, 7, 5097–5106. [Google Scholar] [CrossRef]
- Wang, D.; Sun, Q.; Hokkanen, M.J.; Zhang, C.; Lin, F.-Y.; Liu, Q.; Zhu, S.-P.; Zhou, T.; Chang, Q.; He, B.; et al. Design of robust superhydrophobic surfaces. Nature 2020, 582, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.L.; Xiong, F.Q.; Wang, H.; Qing, Y.; Chu, F.X.; Wu, Y.Q. Tailorable and scalable production of eco-friendly lignin micro-nanospheres and their application in functional superhydrophobic coating. Chem. Eng. J. 2023, 457, 141309. [Google Scholar] [CrossRef]
- Nine, M.J.; Tung, T.T.; Alotaibi, F.; Tran, D.N.H.; Losic, D. Facile Adhesion-Tuning of Superhydrophobic Surfaces between “Lotus” and “Petal” Effect and Their Influence on Icing and Deicing Properties. ACS Appl. Mater. Interfaces 2017, 9, 8393–8402. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, P.; Yu, P.; Leydecker, T.; Bayer, I.S.; Losic, D.; Neogi, A.; Wang, Z. Efficient photothermal deicing employing superhydrophobic plasmonic MXene composites. Adv. Compos. Hybrid Mater. 2022, 5, 3035–3044. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.Q.; Liu, Y.; Han, B.; Wang, H.; Han, D.D.; Wang, J.N.; Zhang, Y.L.; Sun, H.B. Direct Laser Writing of Superhydrophobic PDMS Elastomers for Controllable Manipulation via Marangoni Effect. Adv. Funct. Mater. 2017, 27, 1702946. [Google Scholar] [CrossRef]
- Lu, S.; Yao, Z.; Hao, P.; Fu, C. Drag reduction in ultrahydrophobic channels with micro-nano structured surfaces. Sci. China Phys. Mech. Astron. 2010, 53, 1298–1305. [Google Scholar] [CrossRef]
- Song, X.; Huang, X.; Luo, J.; Long, B.; Zhang, W.; Wang, L.; Gao, J.; Xue, H. Flexible, superhydrophobic and multifunctional carbon nanofiber hybrid membranes for high performance light driven actuators. Nanoscale 2021, 13, 12017–12027. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, Y.; Ji, F.; Zhu, J.; Ma, P.; Su, H.; Chen, P.; Feng, X.; Du, W.; Liu, B.-F. Patterning candle soot for light-driven actuator via Marangoni effect. Sens. Actuators B Chem. 2021, 347, 130613. [Google Scholar] [CrossRef]

| Intensity of the Irradiation [W/cm2] | Contact Angle [°] | Velocity of the Actuator [mm/s] | Reference |
|---|---|---|---|
| 1.0 | —— | 2.4 | Song et al. [41] |
| 0.9 | 153.6 | 4.6 | Wang et al. [42] |
| 1.0 | 163.1 | 7.9 | Cao et al. [15] |
| 1.5 | 157.4 | 8.9 | Wu et al. [4] |
| 3.4 | 161.8 | 7.5 | Wang et al. [8] |
| 1.5 | 151 | 20.3 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, M.; Wang, H.; Ma, B.; Xiong, F. Photothermal Performance of Lignin-Based Nanospheres and Their Applications in Water Surface Actuators. Polymers 2024, 16, 927. https://doi.org/10.3390/polym16070927
Wen M, Wang H, Ma B, Xiong F. Photothermal Performance of Lignin-Based Nanospheres and Their Applications in Water Surface Actuators. Polymers. 2024; 16(7):927. https://doi.org/10.3390/polym16070927
Chicago/Turabian StyleWen, Mingshan, Hang Wang, Bole Ma, and Fuquan Xiong. 2024. "Photothermal Performance of Lignin-Based Nanospheres and Their Applications in Water Surface Actuators" Polymers 16, no. 7: 927. https://doi.org/10.3390/polym16070927
APA StyleWen, M., Wang, H., Ma, B., & Xiong, F. (2024). Photothermal Performance of Lignin-Based Nanospheres and Their Applications in Water Surface Actuators. Polymers, 16(7), 927. https://doi.org/10.3390/polym16070927






