Photopolymerization of Limonene Dioxide and Vegetable Oils as Biobased 3D-Printing Stereolithographic Formulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cationic Photopolymerization
2.2. 3D Printing Application
2.3. Swelling Test
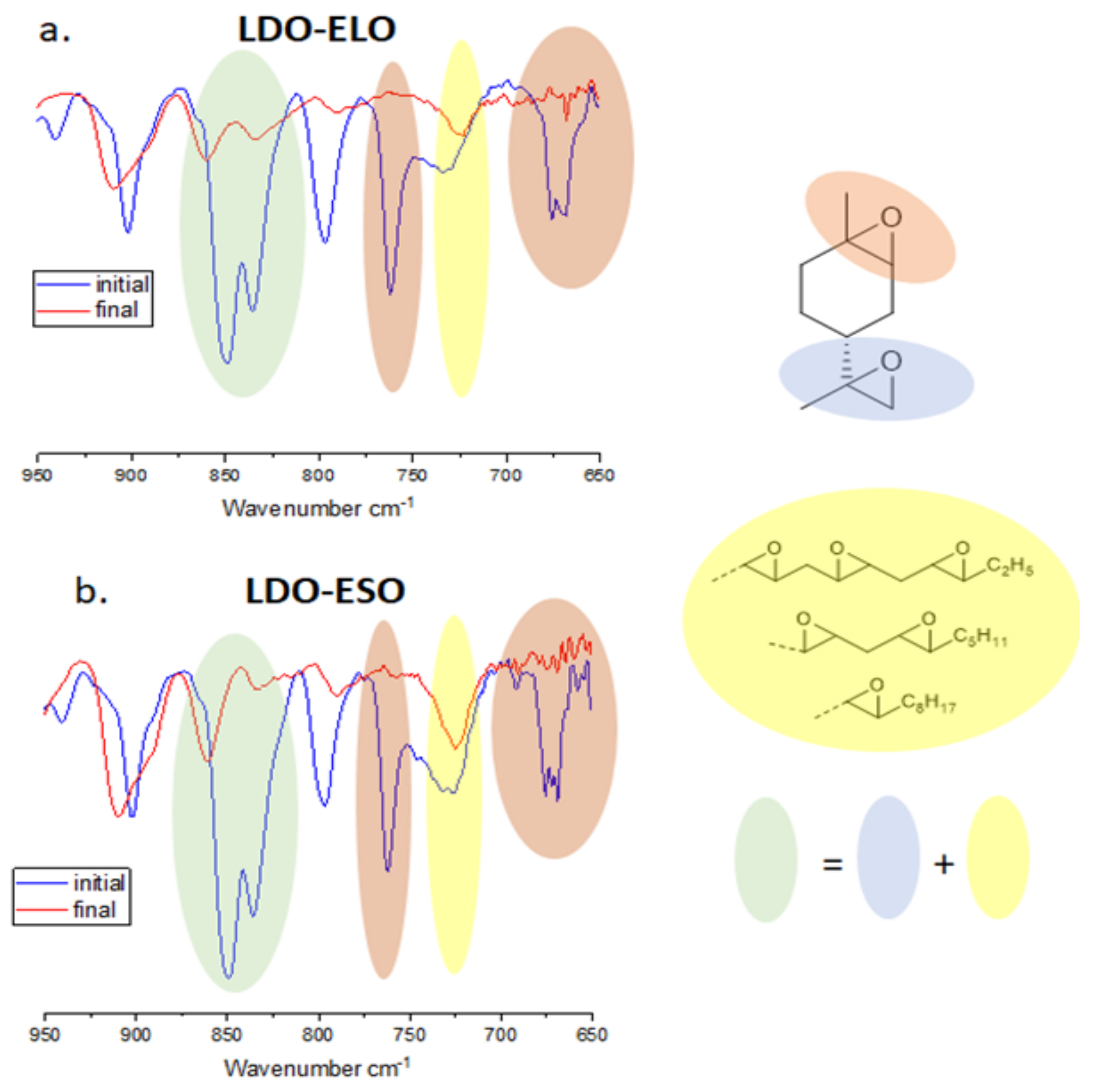
2.4. Conversion Measurement by Fourier Transform InfraRed (FTIR) Spectroscopy
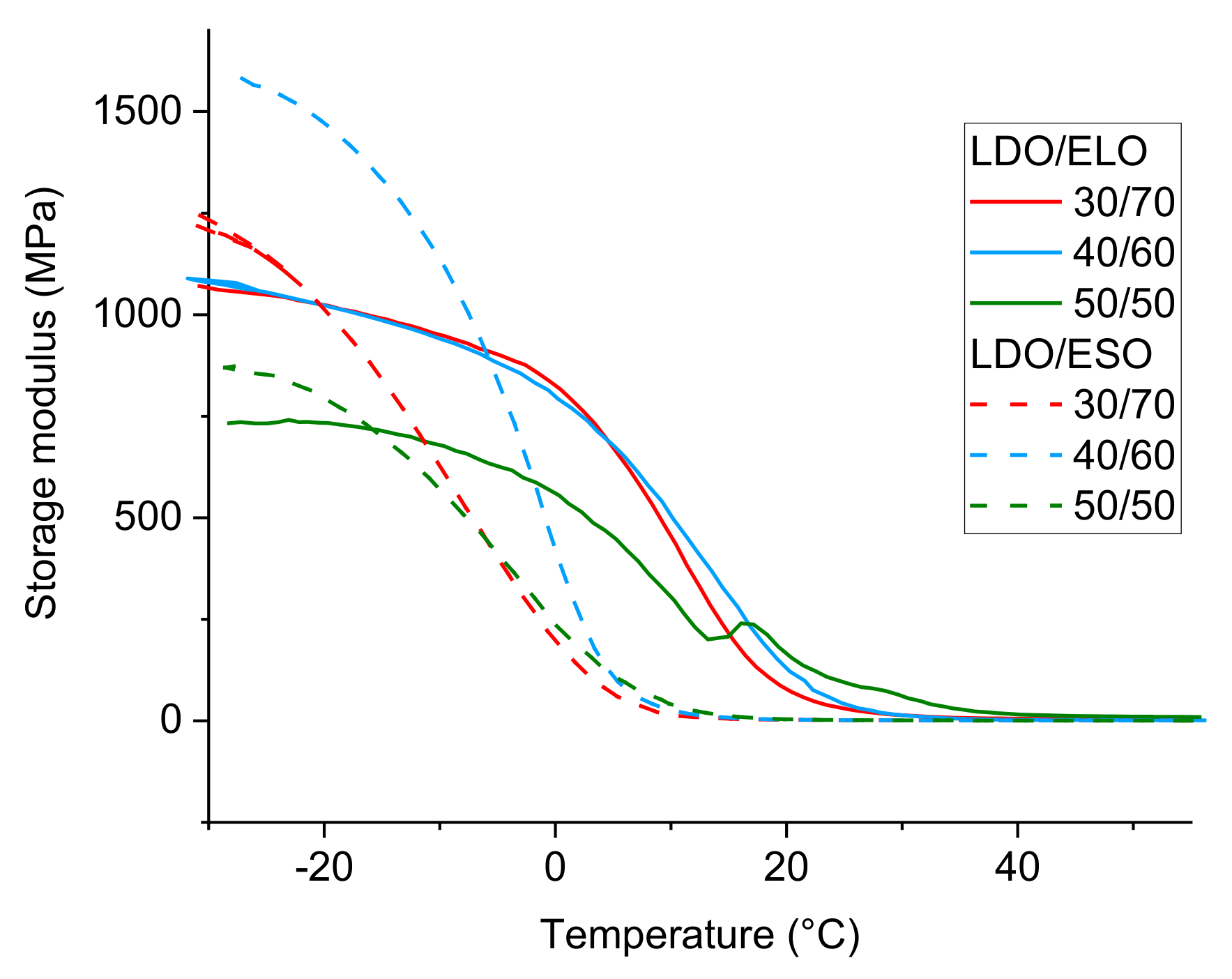
2.5. Dynamic Mechanical Temperature Measurements (DMTA)
2.6. Differential Scanning Calorimetry (DSC)
3. Results
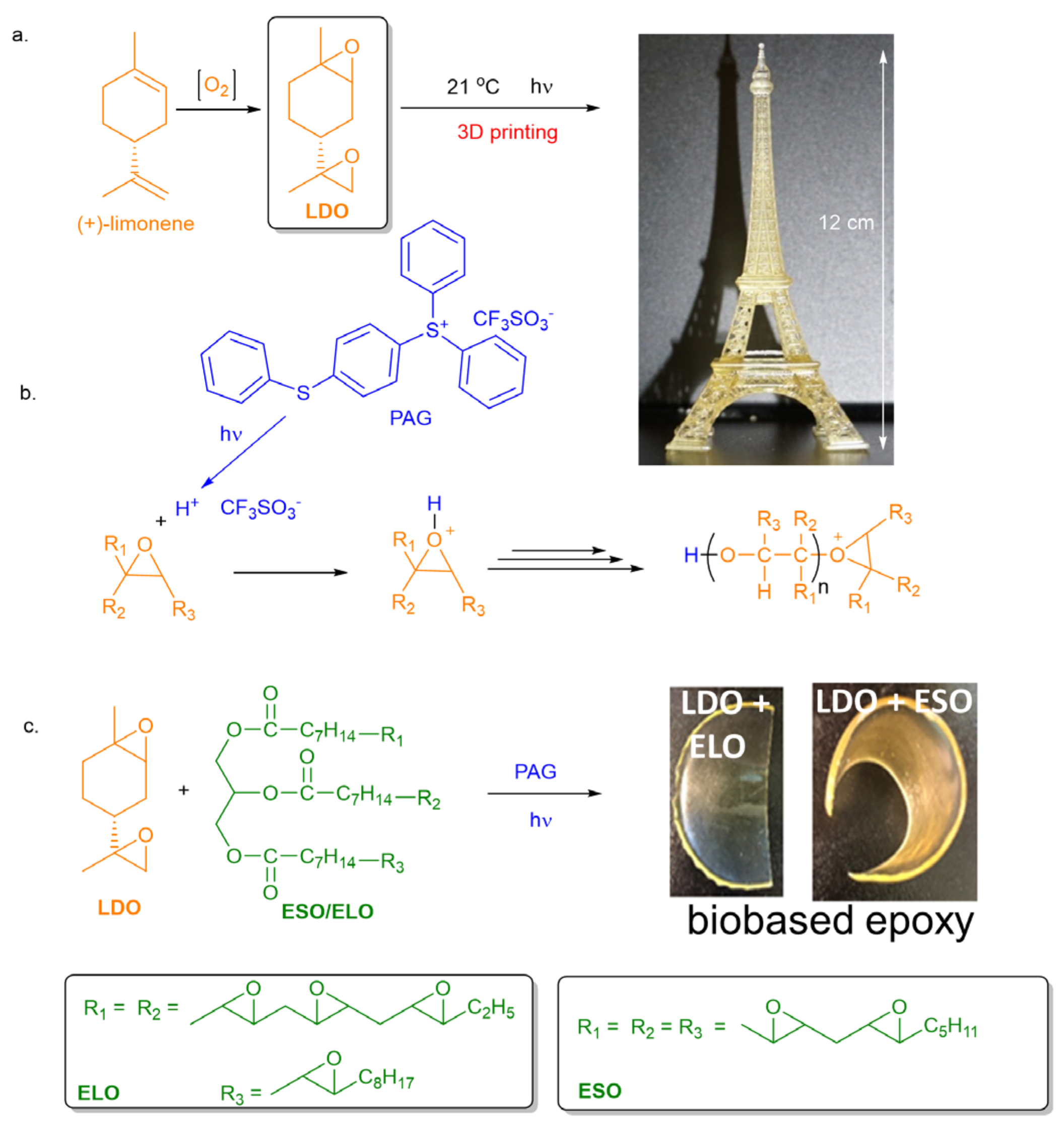
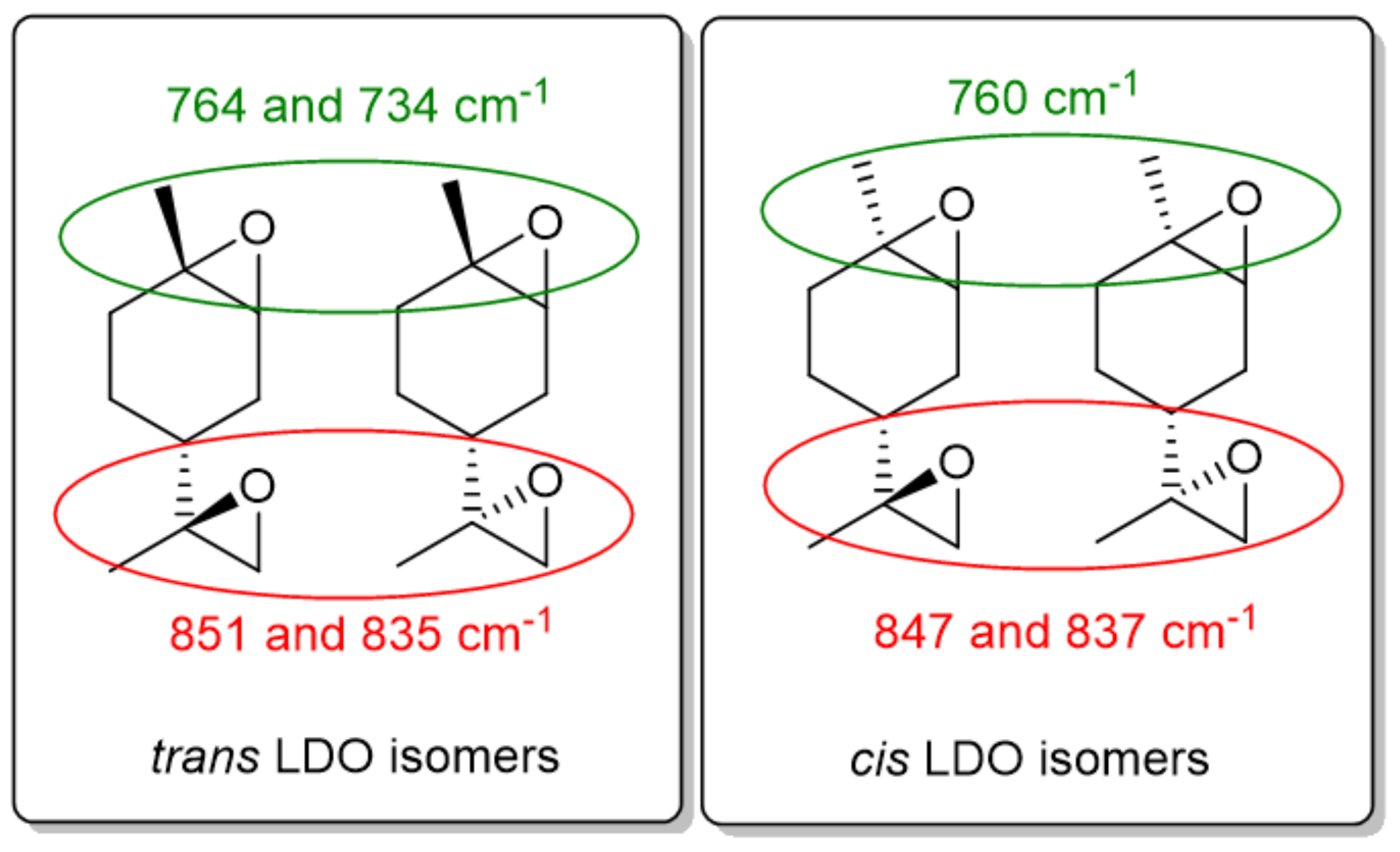
3.1. Polymerization of LDO
3.2. Copolymerization of LDO with Epoxidized Vegetable Oils
3.3. Stereolithographic (SLA) 3D Printing Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gandini, A. The Irruption of Polymers from Renewable Resources on the Scene of Macromolecular Science and Technology. Green Chem. 2011, 13, 1061–1083. [Google Scholar] [CrossRef]
- Auvergne, R.; Caillol, S.; David, G.; Boutevin, B.; Pascault, J.P. Biobased Thermosetting Epoxy: Present and Future. Chem. Rev. 2014, 114, 1082–1115. [Google Scholar] [CrossRef] [PubMed]
- Batzer, H.; Lohse, F. Epoxy Resins. Kunstst. Ger. Plast. 1976, 66, 50–53. [Google Scholar] [CrossRef]
- Raquez, J.M.; Deléglise, M.; Lacrampe, M.F.; Krawczak, P. Thermosetting (Bio)Materials Derived from Renewable Resources: A Critical Review. Prog. Polym. Sci. 2010, 35, 487–509. [Google Scholar] [CrossRef]
- Madadi, S.; Charbonneau, L.; Bergeron, J.Y.; Kaliaguine, S. Aerobic Epoxidation of Limonene Using Cobalt Substituted Mesoporous SBA-16 Part 1: Optimization via Response Surface Methodology (RSM). Appl. Catal. B 2020, 260, 118049. [Google Scholar] [CrossRef]
- Ciriminna, R.; Lomeli-Rodriguez, M.; Demma Carà, P.; Lopez-Sanchez, J.A.; Pagliaro, M. Limonene: A Versatile Chemical of the Bioeconomy. Chem. Commun. 2014, 50, 15288–15296. [Google Scholar] [CrossRef] [PubMed]
- Oberleitner, N.; Ressmann, A.K.; Bica, K.; Gärtner, P.; Fraaije, M.W.; Bornscheuer, U.T.; Rudroff, F.; Mihovilovic, M.D. From Waste to Value-Direct Utilization of Limonene from Orange Peel in a Biocatalytic Cascade Reaction towards Chiral Carvolactone. Green Chem. 2017, 19, 367–371. [Google Scholar] [CrossRef]
- Soto, M.; Koschek, K. Diastereoisomeric Diversity Dictates Reactivity of Epoxy Groups in Limonene Dioxide Polymerization. Express Polym. Lett. 2018, 12, 583–589. [Google Scholar] [CrossRef]
- Schutz, L.; Kazemi, F.; Mackenzie, E.; Bergeron, J.; Gagnon, E.; Claverie, J.P. Trans-limonene Dioxide, a Promising Bio-based Epoxy Monomer. J. Polym. Sci. 2021, 59, 321–328. [Google Scholar] [CrossRef]
- Mija, A.; Louisy, E.; Lachegur, S.; Khodyrieva, V.; Martinaux, P.; Olivero, S.; Michelet, V. Limonene Dioxide as a Building Block for 100% Bio-Based Thermosets. Green Chem. 2021, 23, 9855–9859. [Google Scholar] [CrossRef]
- Kazemi, F.; Schutz, L.; Bergeron, J.-Y.; Gagnon, E.; Yap, G.; Claverie, J.P. Lanthanide Dodecyl Sulfates, a Potent Family of Catalysts for the Preparation of Biobased Epoxy Thermosets. Chem. Commun. 2021, 57, 6784–6787. [Google Scholar] [CrossRef] [PubMed]
- Sangermano, M.; Razza, N.; Crivello, J.V. Cationic UV-Curing: Technology and Applications. Macromol. Mater. Eng. 2014, 299, 775–793. [Google Scholar] [CrossRef]
- Sangermano, M.; Roppolo, I.; Chiappone, A. New Horizons in Cationic Photopolymerization. Polymers 2018, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Kostanski, L.K.; Kim, Y.-M.; Macgregor, J.F.; Hamielec, A.E. Cationic Polymerization Using Mixed Cationic Photoinitiator Systems. Des. Monomers Polym. 2007, 10, 327–345. [Google Scholar] [CrossRef]
- Miao, S.; Wang, P.; Su, Z.; Zhang, S. Vegetable-Oil-Based Polymers as Future Polymeric Biomaterials. Acta Biomater. 2014, 10, 1692–1704. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, H.; Mohanty, A.K.; Misra, M.; Drzal, L.T. Thermo-Physical and Impact Properties of Epoxy Containing Epoxidized Linseed Oil, 1. Macromol. Mater. Eng. 2004, 289, 629–635. [Google Scholar] [CrossRef]
- Konuray, O.; Morancho, J.M.; Fernández-Francos, X.; García-Alvarez, M.; Ramis, X. Curing Kinetics of Dually-Processed Acrylate-Epoxy 3D Printing Resins. Thermochim. Acta 2021, 701, 178963. [Google Scholar] [CrossRef]
- Shan, J.; Yang, Z.; Chen, G.; Hu, Y.; Luo, Y.; Dong, X.; Zheng, W.; Zhou, W. Design and Synthesis of Free-Radical/Cationic Photosensitive Resin Applied for 3D Printer with Liquid Crystal Display (LCD) Irradiation. Polymers 2020, 12, 1346. [Google Scholar] [CrossRef]
- Fernández-Francos, X.; Konuray, O.; Ramis, X.; Serra, À.; De la Flor, S. Enhancement of 3D-Printable Materials by Dual-Curing Procedures. Materials 2020, 14, 107. [Google Scholar] [CrossRef]
- Kuang, X.; Zhao, Z.; Chen, K.; Fang, D.; Kang, G.; Qi, H.J. High-Speed 3D Printing of High-Performance Thermosetting Polymers via Two-Stage Curing. Macromol. Rapid. Commun. 2018, 39, 1700809. [Google Scholar] [CrossRef]
- Campbell, J.A.; Inglis, H.; WeiLong, E.N.; McKinley, C.; Lewis, D.A. Morphology Control in a Dual-Cure System for Potential Applications in Additive Manufacturing. Polymers 2019, 11, 420. [Google Scholar] [CrossRef] [PubMed]
- Binyamin, I.; Grossman, E.; Gorodnitsky, M.; Kam, D.; Magdassi, S.; Binyamin, I.; Gorodnitsky, M.; Kam, D.; Magdassi, S.; Grossman, E. 3D Printing Thermally Stable High-Performance Polymers Based on a Dual Curing Mechanism. Adv. Funct. Mater. 2023, 33, 2214368. [Google Scholar] [CrossRef]
- ASTM D5895-20; Standard Test Methods for Evaluating Drying or Curing during Film Formation of Organic Coatings Using Mechanical Recorders. ASTM: West Conshohocken, PA, USA, 2020.
- Ortiz, R.A.; López, D.P.; Cisneros, M.D.L.G.; Valverde, J.C.R.; Crivello, J.V. A Kinetic Study of the Acceleration Effect of Substituted Benzyl Alcohols on the Cationic Photopolymerization Rate of Epoxidized Natural Oils. Polymer 2005, 46, 1535–1541. [Google Scholar] [CrossRef]
- Fernandes, F.C.; Kirwan, K.; Wilson, P.R.; Coles, S.R. Optimisation of Waste Vegetable Oil-Based Thermoset Polymers. Green Mater. 2018, 6, 38–46. [Google Scholar] [CrossRef]
- Enns, J.B.; Gillham, J.K. Time–Temperature–Transformation (TTT) Cure Diagram: Modeling the Cure Behavior of Thermosets. J. Appl. Polym. Sci. 1983, 28, 2567–2591. [Google Scholar] [CrossRef]
- Tasdelen, M.A.; Lalevée, J.; Yagci, Y. Photoinduced Free Radical Promoted Cationic Polymerization 40 Years after Its Discovery. Polym. Chem. 2020, 11, 1111–1121. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, X.; Liu, R.; Liu, X.; Liu, J. Highly Functional Bio-Based Acrylates with a Hard Core and Soft Arms: From Synthesis to Enhancement of an Acrylated Epoxidized Soybean Oil-Based UV-Curable Coating. Prog. Org. Coatings 2019, 134, 342–348. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, J.; Lu, J.; Huang, J.; Zhang, F.; Hu, Y.; Liu, C.; An, R.; Miao, H.; Chen, Y.; et al. Preparation and Properties of Plant-Oil-Based Epoxy Acrylate-like Resins for UV-Curable Coatings. Polymers 2020, 12, 2165. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Sharma, S. The Development and Comparison of Bio-Thermoset Plastics from Epoxidized Plant Oils. Ind. Crops Prod. 2012, 36, 485–499. [Google Scholar] [CrossRef]
- Tu, J.; Makarian, K.; Alvarez, N.J.; Palmese, G.R. Formulation of a Model Resin System for Benchmarking Processing-Property Relationships in High-Performance Photo 3D Printing Applications. Materials 2020, 13, 4109. [Google Scholar] [CrossRef]
- Clerget, M. Photopolymérisation du Dioxyde de Limonène et D’huile Végatle Époxydée—Application à la Stéréolithographie. M.Sc. Thesis, Université de Sherbrooke, Sherbrooke, QC, Canada, 1 May 2022. [Google Scholar]


| Oil | LDO/Oil wt% | Conversion Endocyclic Epoxide (%) | Conversion Exocyclic Epoxide (%) | Conversion Epox. Oil (%) | Drying Time (h) |
|---|---|---|---|---|---|
| none | 100/0 | 92 | 46 | 1.5 | |
| ESO | 60/40 | >95 | 90 | 73 | 14 |
| ESO | 50/50 | >95 | >95 | 56 | 19 |
| ESO | 40/60 | >95 | >95 | 61 | 22 |
| ESO | 30/70 | >95 | >95 | 63 | 24 |
| ELO | 60/40 | >95 | >95 | 73 | 12 |
| ELO | 50/50 | >95 | >95 | 76 | 17 |
| ELO | 40/60 | >95 | 88 | 58 | 21 |
| ELO | 30/70 | >95 | 93 | 47 | 23 |
| LDO/ESO wt% | Swelling in MeOH (%) | Tg by DMTA (°C) | LDO/ELO wt% | Swelling in MeOH (%) | Tg by DMTA (°C) |
|---|---|---|---|---|---|
| 100/0 | Dissolved | 100/0 | Dissolved | ||
| 60/40 | 38.5 | - | 60/40 | 11.5 | 40 |
| 50/50 | 11.1 | 21 | 50/50 | 4.1 | 32 |
| 40/60 | 8.4 | 22 | 40/60 | 2.4 | 31 |
| 30/70 | 5.7 | 17 | 30/70 | 1.3 | 26 |
| LDO/AESO/TMTPA wt% | % Conversion Epoxide (760 cm−1) | % Conversion Acrylate (1630 cm−1) | Tg by DMTA (°C) |
|---|---|---|---|
| 53/38/9 | 32 | 59 | - |
| 31/56/13 | 45 | 60 | −8 |
| 18/66/16 | 66 | 62 | −8 |
| 18/66/161 | 75 | 95 | −12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clerget, M.; Gagnon, E.; Claverie, J.P. Photopolymerization of Limonene Dioxide and Vegetable Oils as Biobased 3D-Printing Stereolithographic Formulation. Polymers 2024, 16, 965. https://doi.org/10.3390/polym16070965
Clerget M, Gagnon E, Claverie JP. Photopolymerization of Limonene Dioxide and Vegetable Oils as Biobased 3D-Printing Stereolithographic Formulation. Polymers. 2024; 16(7):965. https://doi.org/10.3390/polym16070965
Chicago/Turabian StyleClerget, Mégane, Eric Gagnon, and Jerome P. Claverie. 2024. "Photopolymerization of Limonene Dioxide and Vegetable Oils as Biobased 3D-Printing Stereolithographic Formulation" Polymers 16, no. 7: 965. https://doi.org/10.3390/polym16070965
APA StyleClerget, M., Gagnon, E., & Claverie, J. P. (2024). Photopolymerization of Limonene Dioxide and Vegetable Oils as Biobased 3D-Printing Stereolithographic Formulation. Polymers, 16(7), 965. https://doi.org/10.3390/polym16070965









