Mechanically Tough and Conductive Hydrogels Based on Gelatin and Z–Gln–Gly Generated by Microbial Transglutaminase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Gelatin-Based Hydrogels
2.3. Characterization
2.4. Dynamic Rheology
2.5. Mechanical Property Tests
2.6. Swell Behavior Test
2.7. Cytotoxicity Assays
2.8. Conductivity and Strain-Sensing Measurements
3. Results
3.1. Preparation of Gel–TG–ZQG
3.2. Characterization of Gel–TG–ZQG
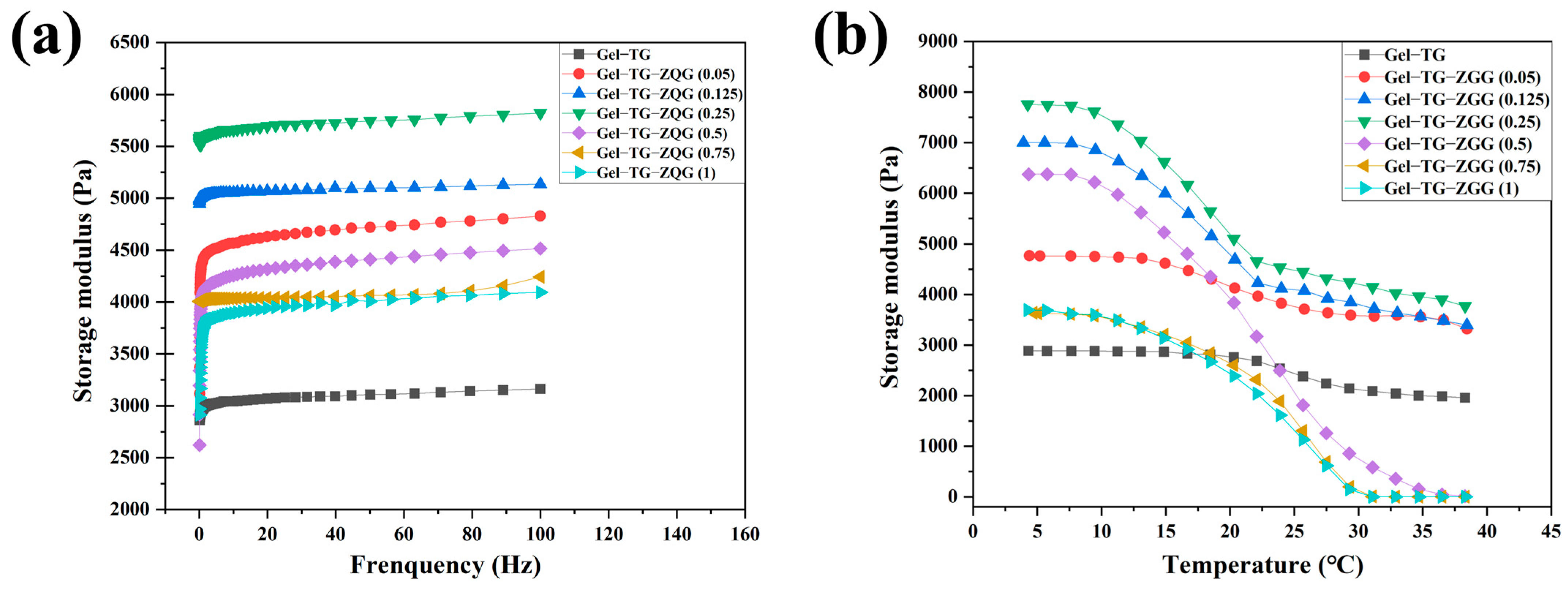
3.3. Rheological Properties of Hydrogels
3.4. Mechanical Properties of the Hydrogels
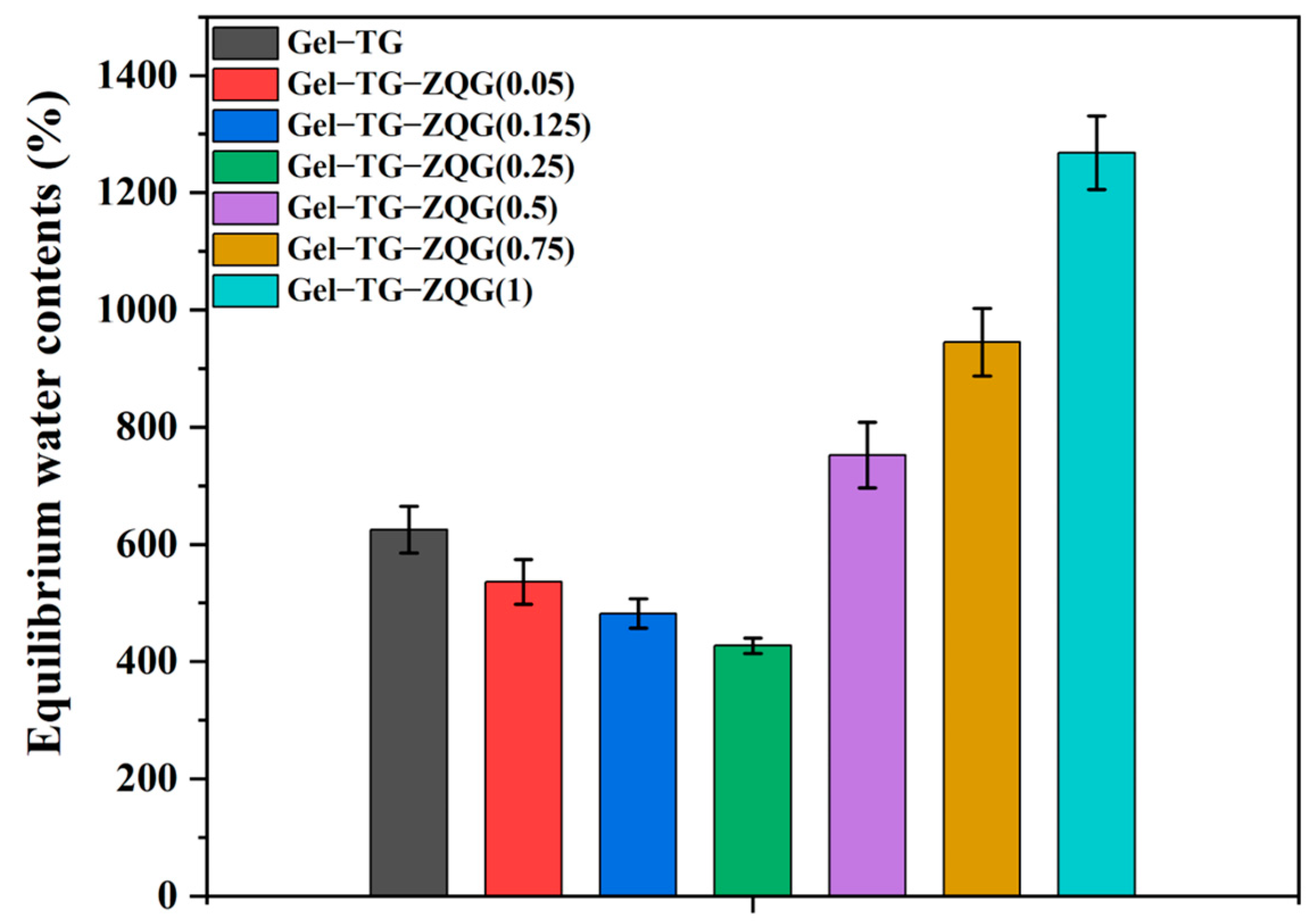
3.5. Swelling Behaviors of the Hydrogels
3.6. Cytotoxicity Levels of the Hydrogels
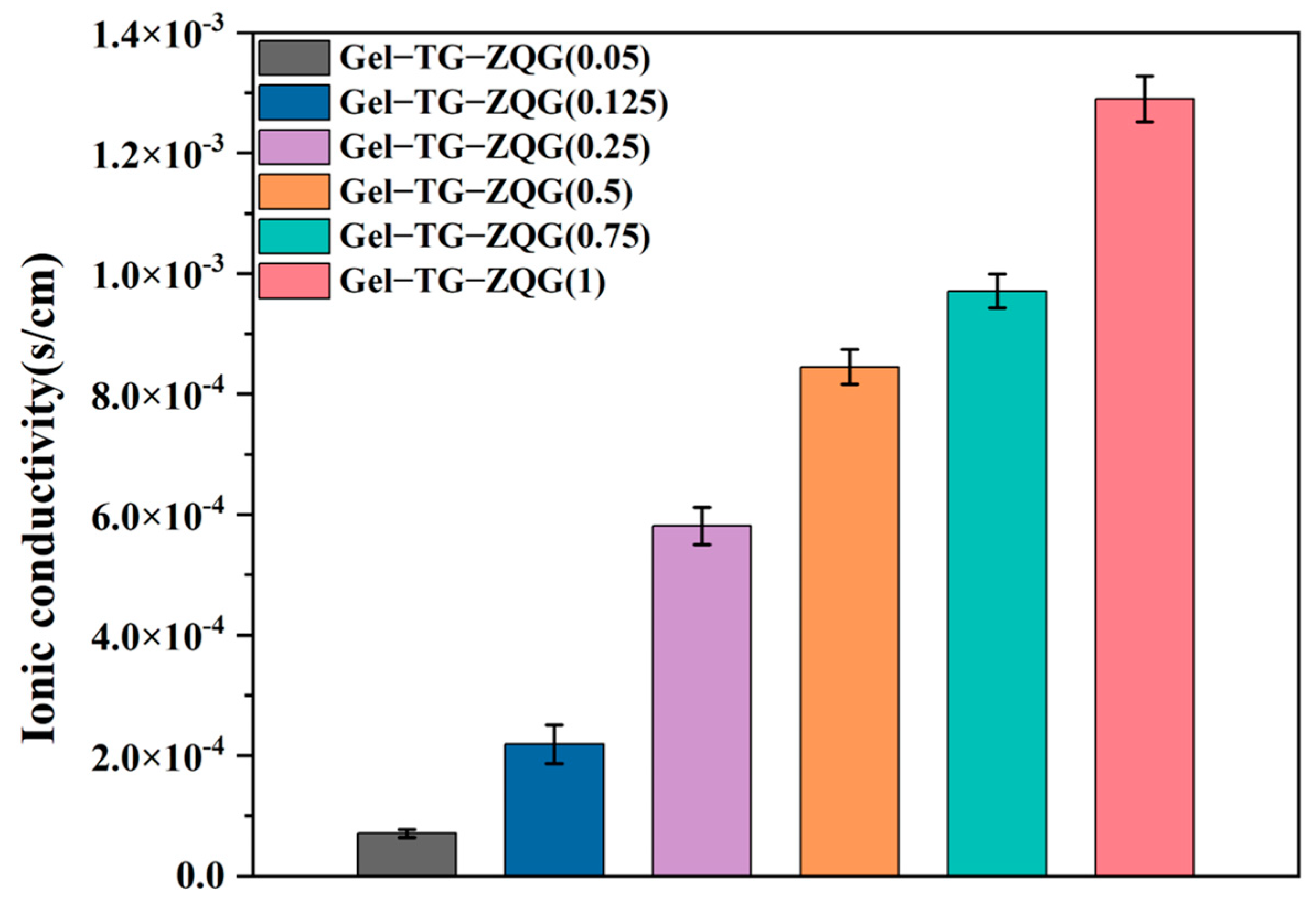
3.7. Conductivities of the Hydrogels
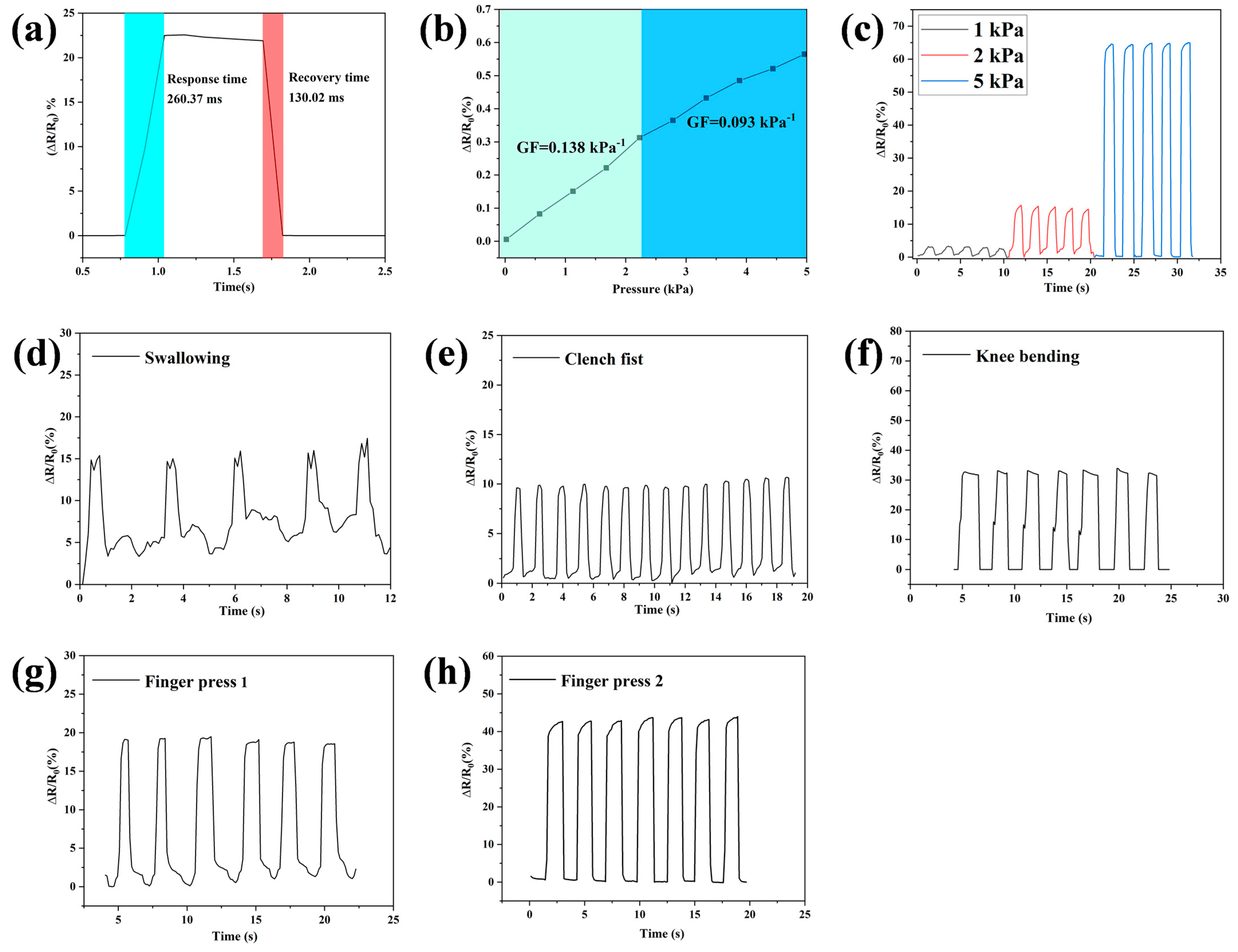
3.8. Strain Sensitivities of Hydrogel Strain Sensors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lim, J.Y.; Goh, L.; Otake, K.; Goh, S.S.; Loh, X.J.; Kitagawa, S. Biomedically-relevant metal organic framework-hydrogel composites. Biomater. Sci. 2023, 11, 2661–2677. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Ruan, L.; Dong, X.; Tian, S.; Lang, W.; Wu, M.; Chen, Y.; Lv, Q.; Lei, L. Current state of knowledge on intelligent-response biological and other macromolecular hydrogels in biomedical engineering: A review. Int. J. Biol. Macromol. 2023, 227, 472–492. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cheng, Q.; Jeng, U.; Hsu, S. A biomimetic bilayer hydrogel actuator based on thermoresponsive gelatin methacryloyl–poly (N-isopropylacrylamide) hydrogel with three-dimensional printability. ACS Appl. Mater. Interfaces 2023, 15, 5798–5810. [Google Scholar] [CrossRef]
- Pamplona, R.; González-Lana, S.; Romero, P.; Ochoa, I.; Martín-Rapún, R.; Sánchez-Somolinos, C. Tuning of Mechanical Properties in Photopolymerizable Gelatin-Based Hydrogels for In Vitro Cell Culture Systems. ACS Appl. Polym. Mater. 2023, 5, 1487–1498. [Google Scholar] [CrossRef] [PubMed]
- Tomczykowa, M.; Plonska-Brzezinska, M.E. Conducting polymers, hydrogels and their composites: Preparation, properties and bioapplications. Polymers 2019, 11, 350. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Cheng, Y.; Deng, Y.; Wen, F.; Lai, Y.; Li, H. Conductive hydrogel for flexible bioelectronic device: Current progress and future perspective. Adv. Funct. Mater. 2024, 34, 2308974. [Google Scholar] [CrossRef]
- Rong, Q.; Lei, W.; Liu, M. Conductive hydrogels as smart materials for flexible electronic devices. Chem. A Eur. J. 2018, 24, 16930–16943. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, M.G.; Lübben, J.F. Review on Hydrogel-Based Flexible Supercapacitors for Wearable Applications. Gels 2023, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Shymborska, Y.; Budkowski, A.; Raczkowska, J.; Donchak, V.; Melnyk, Y.; Vasiichuk, V.; Stetsyshyn, Y. Switching it Up: The Promise of Stimuli-Responsive Polymer Systems in Biomedical Science. Chem. Rec. 2024, 24, e202300217. [Google Scholar] [CrossRef]
- Li, X.; He, L.; Li, Y.; Chao, M.; Li, M.; Wan, P.; Zhang, L. Healable, degradable, and conductive MXene nanocomposite hydrogel for multifunctional epidermal sensors. ACS Nano 2021, 15, 7765–7773. [Google Scholar] [CrossRef]
- Miramon-Ortíz, D.A.; Argüelles-Monal, W.; Carvajal-Millan, E.; López-Franco, Y.L.; Goycoolea, F.M.; Lizardi-Mendoza, J. Acemannan gels and aerogels. Polymers 2019, 11, 330. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Yu, C.; Zhong, W. Facile preparation of a 3D porous aligned graphene-based wall network architecture by confined self-assembly with shape memory for artificial muscle, pressure sensor, and flexible supercapacitor. ACS Appl. Mater. Interfaces 2022, 14, 17739–17753. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.; Qin, Y.; Xia, Q.; Zhang, X.; Chen, Y. Double-Network Protein Hydrogels as Flexible Pressure Sensors for Contactless Delivery. ACS Appl. Polym. Mater. 2023, 5, 2312–2322. [Google Scholar] [CrossRef]
- Locarno, S.; Gallo, S.; Arosio, P.; Biordi, C.; Dallasega, D.; Gargano, M.; Ludwig, N.; Orsini, F.; Pignoli, E.; Veronese, I. Dosimetric double network hydrogel based on Poly (vinyl-alcohol)/Phenylalanine-derivatives with enhanced mechanical properties. ACS Appl. Polym. Mater. 2023, 5, 1902–1914. [Google Scholar] [CrossRef]
- Han, K.; Bai, Q.; Wu, W.; Sun, N.; Cui, N.; Lu, T. Gelatin-based adhesive hydrogel with self-healing, hemostasis, and electrical conductivity. Int. J. Biol. Macromol. 2021, 183, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, Y.X.; Yan, J.; Quinn, D.; Dong, P.; Sawyer, S.W.; Soman, P. Fabrication of conductive gelatin methacrylate–polyaniline hydrogels. Acta Biomater. 2016, 33, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, H.J.; You, X.; Cui, K.; Wang, X. Electrically conductive tough gelatin hydrogel. Adv. Electron. Mater. 2020, 6, 2000040. [Google Scholar] [CrossRef]
- Wang, X.; Bai, Z.; Zheng, M.; Yue, O.; Hou, M.; Cui, B.; Su, R.; Wei, C.; Liu, X. Engineered gelatin-based conductive hydrogels for flexible wearable electronic devices: Fundamentals and recent advances. J. Sci. Adv. Mater. Devices 2022, 7, 100451. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, Y.; Wang, X.; Xing, L.; Shi, L.; Ran, R. Stable, strain-sensitive conductive hydrogel with antifreezing capability, remoldability, and reusability. ACS Appl. Mater. Interfaces 2018, 10, 44000–44010. [Google Scholar] [CrossRef]
- Tronci, G.; Neffe, A.T.; Pierce, B.F.; Lendlein, A. An entropy–elastic gelatin-based hydrogel system. J. Mater. Chem. 2010, 20, 8875–8884. [Google Scholar] [CrossRef]
- Zhang, H.J.; Wang, L.; Wang, X.; Han, Q.; You, X. Developing super tough gelatin-based hydrogels by incorporating linear poly (methacrylic acid) to facilitate sacrificial hydrogen bonding. Soft Matter 2020, 16, 4723–4727. [Google Scholar] [CrossRef] [PubMed]
- Qie, H.; Wang, Z.; Ren, J.; Lü, S.; Liu, M. A tough shape memory hydrogel strain sensor based on gelatin grafted polypyrrole. Polymer 2022, 263, 125524. [Google Scholar] [CrossRef]
- Yavin, E.; Boal, A.K.; Stemp, E.D.; Boon, E.M.; Livingston, A.L.; O’Shea, V.L.; David, S.S.; Barton, J.K. Protein–DNA charge transport: Redox activation of a DNA repair protein by guanine radical. Proc. Natl. Acad. Sci. USA 2005, 102, 3546–3551. [Google Scholar] [CrossRef] [PubMed]
- Schneebeli, S.T.; Kamenetska, M.; Cheng, Z.; Skouta, R.; Friesner, R.A.; Venkataraman, L.; Breslow, R. Single-molecule conductance through multiple π−π-stacked benzene rings determined with direct electrode-to-benzene ring connections. J. Am. Chem. Soc. 2011, 133, 2136–2139. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Chen, Z.; Dong, Y.; Lu, W.; Zhu, D. The Preparation and Properties of Composite Hydrogels Based on Gelatin and (3-Aminopropyl) Trimethoxysilane Grafted Cellulose Nanocrystals Covalently Linked with Microbial Transglutaminase. Gels 2022, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Santoso, J.W.; McCain, M.L. Characterization of gelatin hydrogels cross-linked with microbial transglutaminase as engineered skeletal muscle substrates. Bioengineering 2021, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; An, Z.; Tong, X.; Chen, Y.; Ma, M.; Shi, Y.; Wang, X. Stronger intermolecular forces or closer molecular spacing? Key impact factor research of gelator self-assembly mechanism. Langmuir 2017, 33, 14389–14395. [Google Scholar] [CrossRef] [PubMed]
- Pryazhnikov, M.I.; Mikhienkova, E.I.; Minakov, A.V. Rheological and microrheological study of microsuspension with. Top. Issues Ration. Use Nat. Resour. 2019, 2, 883. [Google Scholar]
- Cheng, K.; Zou, L.; Chang, B.; Liu, X.; Shi, H.; Li, T.; Yang, Q.; Guo, Z.; Liu, C.; Shen, C. Mechanically robust and conductive poly (acrylamide) nanocomposite hydrogel by the synergistic effect of vinyl hybrid silica nanoparticle and polypyrrole for human motion sensing. Adv. Compos. Hybrid Mater. 2022, 5, 2834–2846. [Google Scholar] [CrossRef]
- Fu, Y.; Lu, Y.; Lai, F.; Li, Z. Characterizing the crosslinking process of borate and hydroxypropyl guar gum by diffusing wave spectroscopy. Fuel 2022, 324, 124570. [Google Scholar] [CrossRef]
- Hu, G.; Ma, M.; Batool, Z.; Sheng, L.; Cai, Z.; Liu, Y.; Jin, Y. Gel properties of heat-induced transparent hydrogels from ovalbumin by acylation modifications. Food Chem. 2022, 369, 130912. [Google Scholar] [CrossRef] [PubMed]
- Shamekhi, M.A.; Rabiee, A.; Mirzadeh, H.; Mahdavi, H.; Mohebbi-Kalhori, D.; Eslaminejad, M.B. Fabrication and characterization of hydrothermal cross-linked chitosan porous scaffolds for cartilage tissue engineering applications. Mater. Sci. Eng. C 2017, 80, 532–542. [Google Scholar] [CrossRef]
- Rodríguez Rodríguez, R.; García Carvajal, Z.Y.; Jiménez Palomar, I.; Jiménez Avalos, J.A.; Espinosa Andrews, H. Development of gelatin/chitosan/PVA hydrogels: Thermal stability, water state, viscoelasticity, and cytotoxicity assays. J. Appl. Polym. Sci. 2019, 136, 47149. [Google Scholar] [CrossRef]
- Huo, L.; Zhang, H.; Wang, Y.; Wang, Y.; Deng, B.; Jin, L.E. Synthesis and properties of L (AS) 3-type low-molecular-mass organic gelators based on citryl aromatic amino acid cholesteryl ester. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129197. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, C.; Peng, L.; Yu, G. Conductive “smart” hybrid hydrogels with PNIPAM and nanostructured conductive polymers. Adv. Funct. Mater. 2015, 25, 1219–1225. [Google Scholar] [CrossRef]
- Xiong, J.; Wu, W.; Hu, Y.; Guo, Z.; Wang, S. An anisotropic conductive hydrogel for strain sensing and breath detection. Appl. Mater. Today 2023, 34, 101909. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Mahesh, K.; Le, N.H.; Kemp, K.C.; Timilsina, R.; Tiwari, R.N.; Kim, K.S. Reduced graphene oxide-based hydrogels for the efficient capture of dye pollutants from aqueous solutions. Carbon 2013, 56, 173–182. [Google Scholar] [CrossRef]
- Wen, J.; Jiang, S.; Wang, J.; Li, X.; Bi, Z.; Jin, Y.; Fan, L.; Wang, L.; Wu, Y.; Gan, F. Polyimide solution with reversible sol-gel transition by construction of dynamic π–π stacking. Polymer 2023, 278, 126006. [Google Scholar] [CrossRef]
- Sarbon, N.M.; Badii, F.; Howell, N.K. The effect of chicken skin gelatin and whey protein interactions on rheological and thermal properties. Food Hydrocoll. 2015, 45, 83–92. [Google Scholar] [CrossRef]
- Simha, N.K.; Carlson, C.S.; Lewis, J.L. Evaluation of fracture toughness of cartilage by micropenetration. J. Mater. Sci. Mater. Med. 2004, 15, 631–639. [Google Scholar] [CrossRef]
- Han, L.; Yan, L.; Wang, K.; Fang, L.; Zhang, H.; Tang, Y.; Ding, Y.; Weng, L.; Xu, J.; Weng, J. Tough, self-healable and tissue-adhesive hydrogel with tunable multifunctionality. NPG Asia Mater. 2017, 9, e372. [Google Scholar] [CrossRef]
- Jayakumar, A.; Jose, V.K.; Lee, J.M. Hydrogels for medical and environmental applications. Small Methods 2020, 4, 1900735. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, S.; Lu, W.; Chen, N.; Zhu, D.; Li, Y. Preparation and characterization of enzymatically cross-linked gelatin/cellulose nanocrystal composite hydrogels. RSC Adv. 2021, 11, 10794–10803. [Google Scholar] [CrossRef] [PubMed]
- Bao, R.; Tan, B.; Liang, S.; Zhang, N.; Wang, W.; Liu, W. A π–π conjugation-containing soft and conductive injectable polymer hydrogel highly efficiently rebuilds cardiac function after myocardial infarction. Biomaterials 2017, 122, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Gu, S.; Jia, F.; Gao, G. A skin-matchable, recyclable and biofriendly strain sensor based on a hydrolyzed keratin-containing hydrogel. J. Mater. Chem. A 2020, 8, 24175–24183. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Q.; Song, S.; Duan, L.; Gao, G. Bioinspired dynamic cross-linking hydrogel sensors with skin-like strain and pressure sensing behaviors. Chem. Mat. 2019, 31, 9522–9531. [Google Scholar] [CrossRef]
- Sun, X.; Yao, F.; Wang, C.; Qin, Z.; Zhang, H.; Yu, Q.; Zhang, H.; Dong, X.; Wei, Y.; Li, J. Ionically conductive hydrogel with fast self-recovery and low residual strain as strain and pressure sensors. Macromol. Rapid Commun. 2020, 41, 2000185. [Google Scholar] [CrossRef] [PubMed]
- Hang, C.; Zhao, X.; Xi, S.; Shang, Y.; Yuan, K.; Yang, F.; Wang, Q.; Wang, J.; Zhang, D.W.; Lu, H. Highly stretchable and self-healing strain sensors for motion detection in wireless human-machine interface. Nano Energy 2020, 76, 105064. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, K.; Yu, Y.; Yue, X.; Dai, K.; Zheng, G.; Liu, C.; Shen, C. Highly stretchable, transparent, and bio-friendly strain sensor based on self-recovery ionic-covalent hydrogels for human motion monitoring. Macromol. Mater. Eng. 2019, 304, 1900227. [Google Scholar] [CrossRef]
- Lu, Y.; Qu, X.; Zhao, W.; Ren, Y.; Si, W.; Wang, W.; Wang, Q.; Huang, W.; Dong, X. Highly stretchable, elastic, and sensitive MXene-based hydrogel for flexible strain and pressure sensors. Research 2020, 2020, 2038560. [Google Scholar] [CrossRef]
- Roy, A.; Manna, K.; Ray, P.G.; Dhara, S.; Pal, S. β-Cyclodextrin-based ultrahigh stretchable, flexible, electro-and pressure-responsive, adhesive, transparent hydrogel as motion sensor. ACS Appl. Mater. Interfaces 2022, 14, 17065–17080. [Google Scholar] [CrossRef] [PubMed]





| Samples | Gelatin | ZQG | Molar Ratio of ZQG to the Primary Amines of Gelatin | ||
|---|---|---|---|---|---|
| Mass (g) | Primary Amines (μmol) | Mass (mg) | Mole (μmol) | ||
| Gel−TG | 0.79 | 498.03 | 0 | 0 | 0 |
| Gel–TG–ZQG (0.05) | 0.79 | 498.03 | 8.40 | 24.90 | 0.05 |
| Gel–TG–ZQG (0.125) | 0.79 | 498.03 | 21.00 | 62.25 | 0.125 |
| Gel–TG–ZQG (0.25) | 0.79 | 498.03 | 42.00 | 124.51 | 0.25 |
| Gel–TG–ZQG (0.5) | 0.79 | 498.03 | 84.00 | 249.01 | 0.5 |
| Gel–TG–ZQG (0.75) | 0.79 | 498.03 | 126.00 | 373.52 | 0.75 |
| Gel–TG–ZQG (1) | 0.79 | 498.03 | 168.00 | 498.03 | 1.00 |
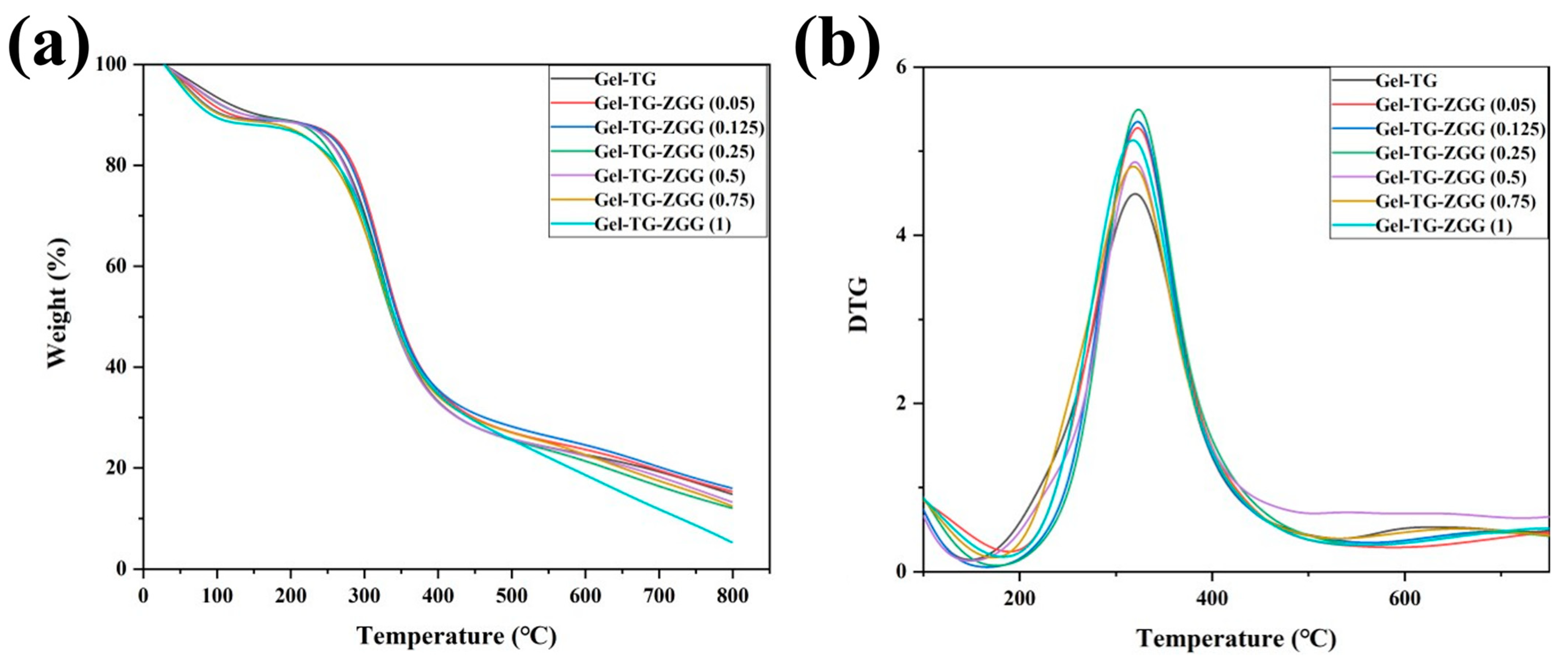
| Samples | T50 (°C) | Td (°C) | Tm (°C) |
|---|---|---|---|
| Gel−TG | 341.65 | 173.06 | 318.02 |
| Gel–TG–ZQG (0.05) | 341.08 | 175.75 | 319.98 |
| Gel–TG–ZQG (0.125) | 345.09 | 183.14 | 322.98 |
| Gel–TG–ZQG (0.25) | 347.06 | 189.66 | 323.65 |
| Gel–TG–ZQG (0.5) | 338.58 | 166.54 | 322.32 |
| Gel–TG–ZQG (0.75) | 339.55 | 149.31 | 319.75 |
| Gel–TG–ZQG (1) | 337.18 | 148.95 | 317.67 |
| Samples | Response Time | Recovery Time | References |
|---|---|---|---|
| G-PPy/PAAm-TA | 400 ms | 400 ms | [22] |
| HK/PVA/NaCl | 170 ms | 191 ms | [45] |
| PAAm/Gelatin | 200 ms | 600 ms | [47] |
| PAAm | 150 ms | 400 ms | [48] |
| PAM/SA | 800 ms | / | [49] |
| PVA/PVP/Ti3AlC2 | 233 ms | 233 ms | [50] |
| AAm/HMAm/β-CD | 210 ms | 130 ms | [51] |
| Gel–TG–ZQG (0.25) | 260.37 ms | 130.02 ms | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Zhang, R.; Zhao, S.; Li, B.; Wang, S.; Lu, W.; Zhu, D. Mechanically Tough and Conductive Hydrogels Based on Gelatin and Z–Gln–Gly Generated by Microbial Transglutaminase. Polymers 2024, 16, 999. https://doi.org/10.3390/polym16070999
Chen Z, Zhang R, Zhao S, Li B, Wang S, Lu W, Zhu D. Mechanically Tough and Conductive Hydrogels Based on Gelatin and Z–Gln–Gly Generated by Microbial Transglutaminase. Polymers. 2024; 16(7):999. https://doi.org/10.3390/polym16070999
Chicago/Turabian StyleChen, Zhiwei, Ruxin Zhang, Shouwei Zhao, Bing Li, Shuo Wang, Wenhui Lu, and Deyi Zhu. 2024. "Mechanically Tough and Conductive Hydrogels Based on Gelatin and Z–Gln–Gly Generated by Microbial Transglutaminase" Polymers 16, no. 7: 999. https://doi.org/10.3390/polym16070999
APA StyleChen, Z., Zhang, R., Zhao, S., Li, B., Wang, S., Lu, W., & Zhu, D. (2024). Mechanically Tough and Conductive Hydrogels Based on Gelatin and Z–Gln–Gly Generated by Microbial Transglutaminase. Polymers, 16(7), 999. https://doi.org/10.3390/polym16070999






