Strategies towards Fully Recyclable Commercial Epoxy Resins: Diels–Alder Structures in Sustainable Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
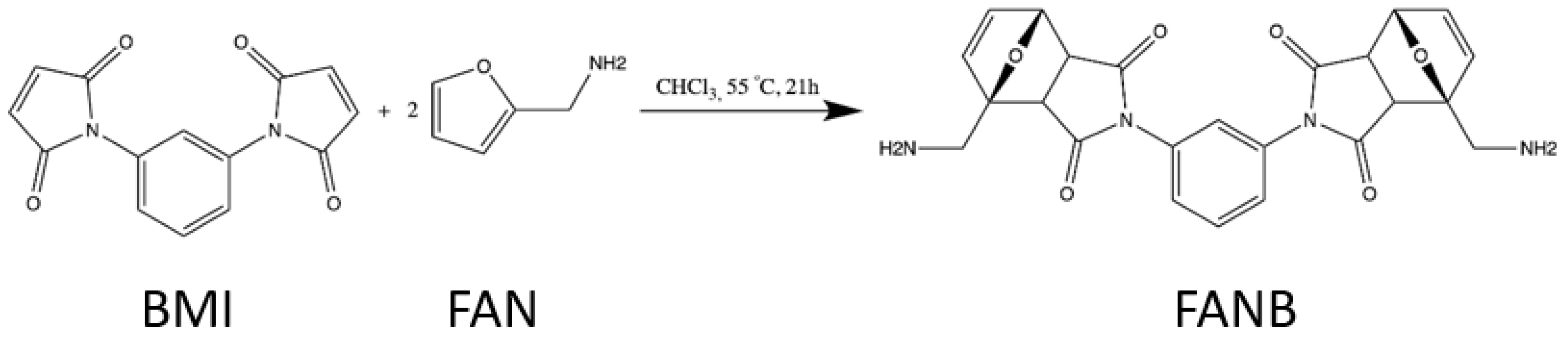
2.2. Chemical Synthesis of the Diels–Alder Adducts
2.3. Preparation of the Cured Materials
2.4. Characterization
3. Results
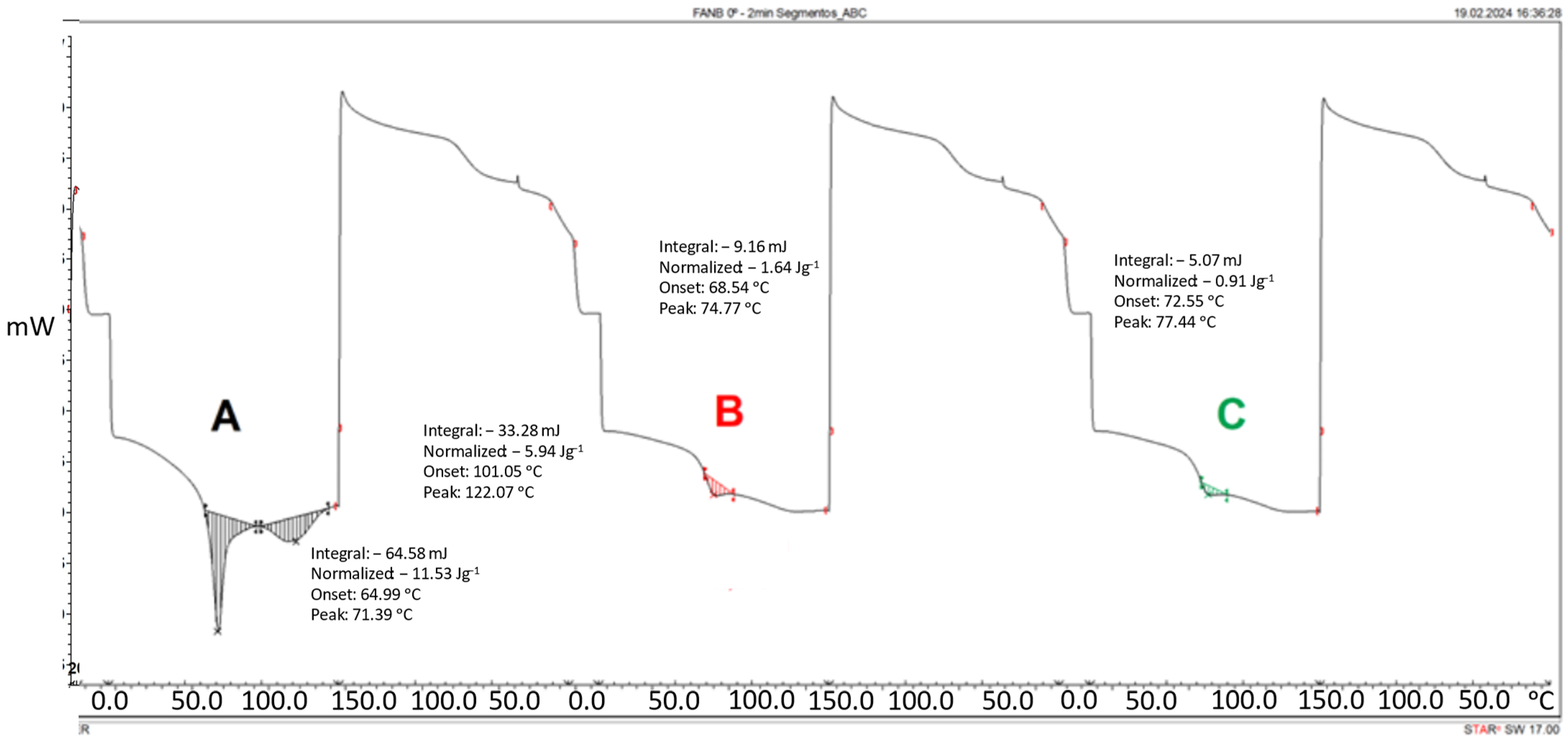
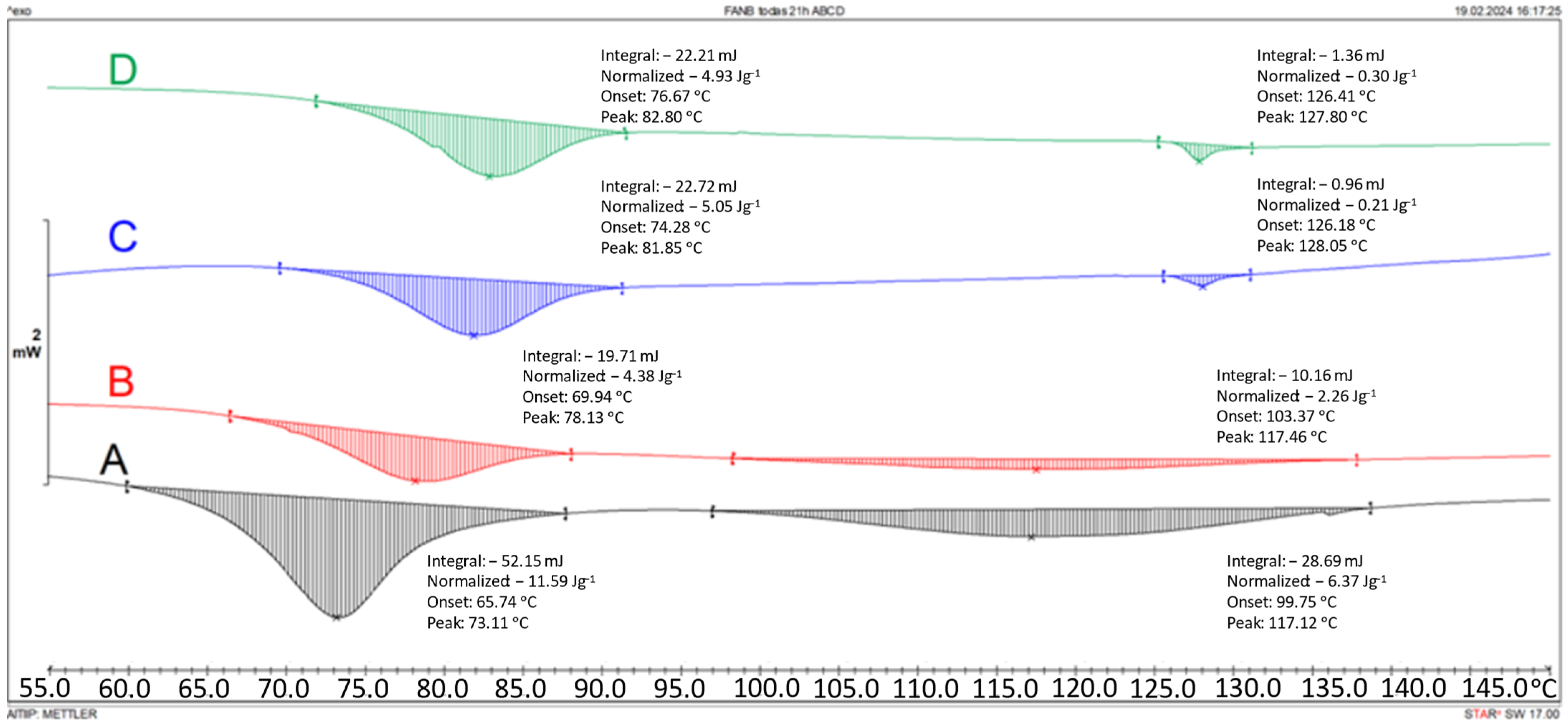
3.1. Kinetic Studies
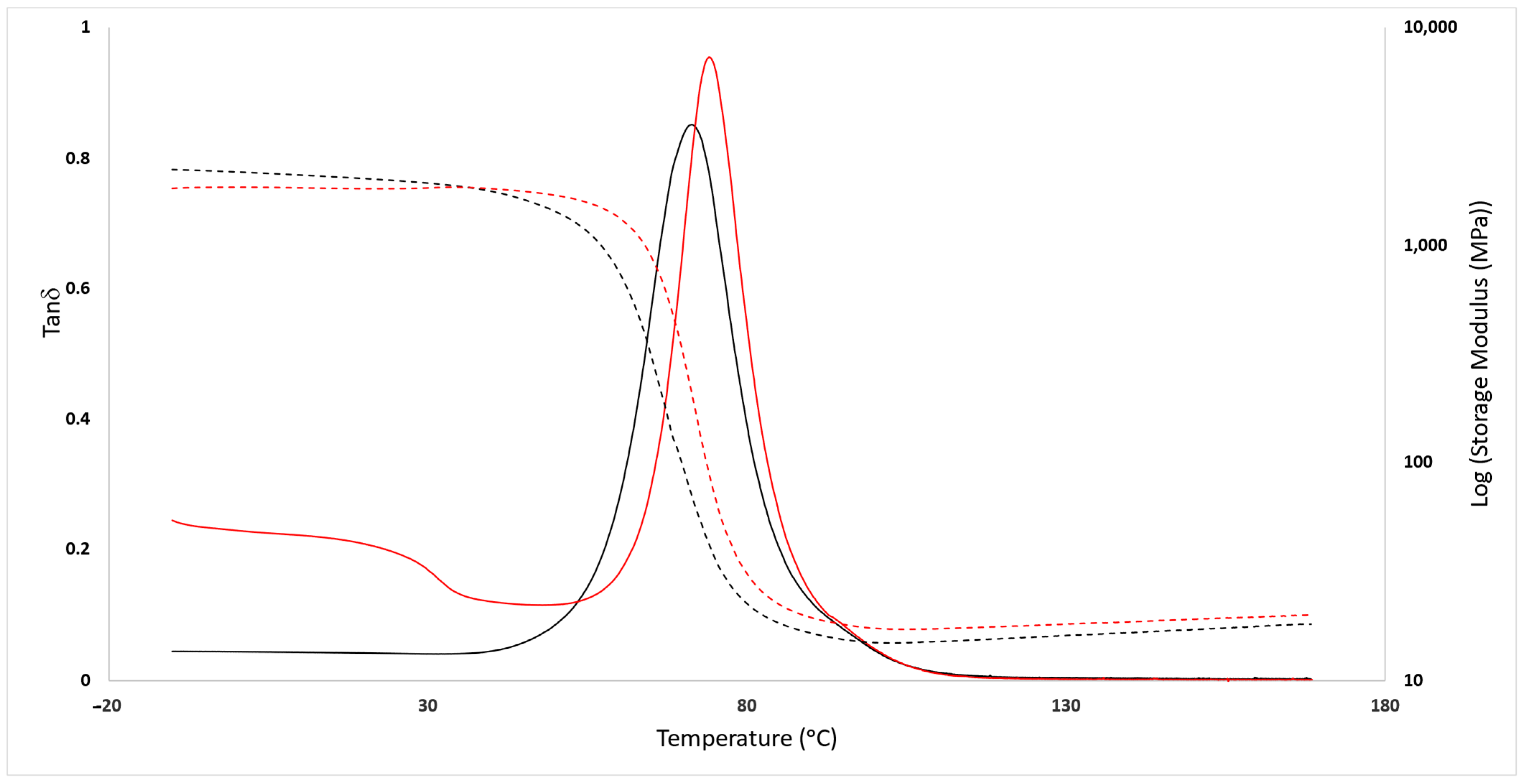
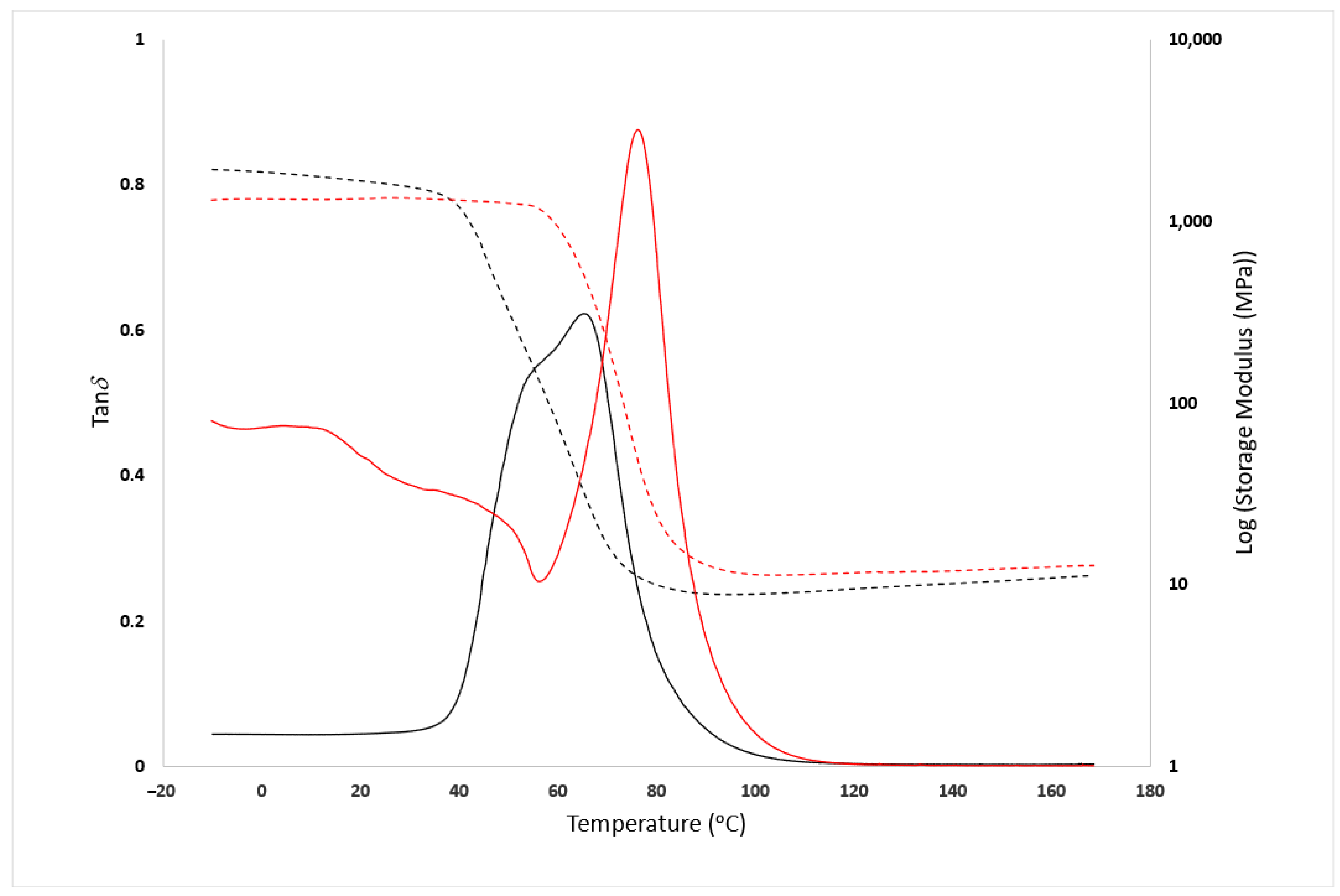
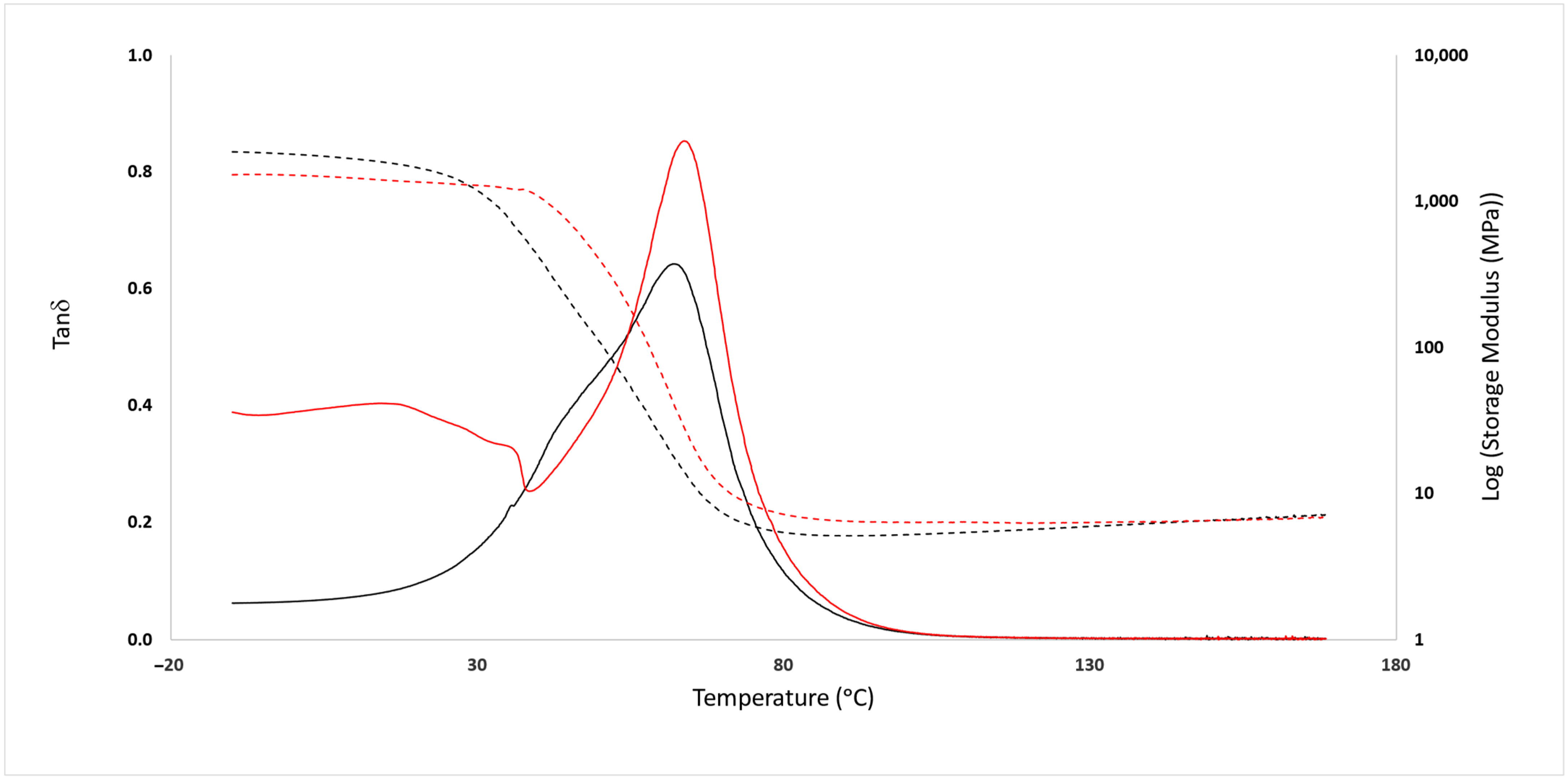
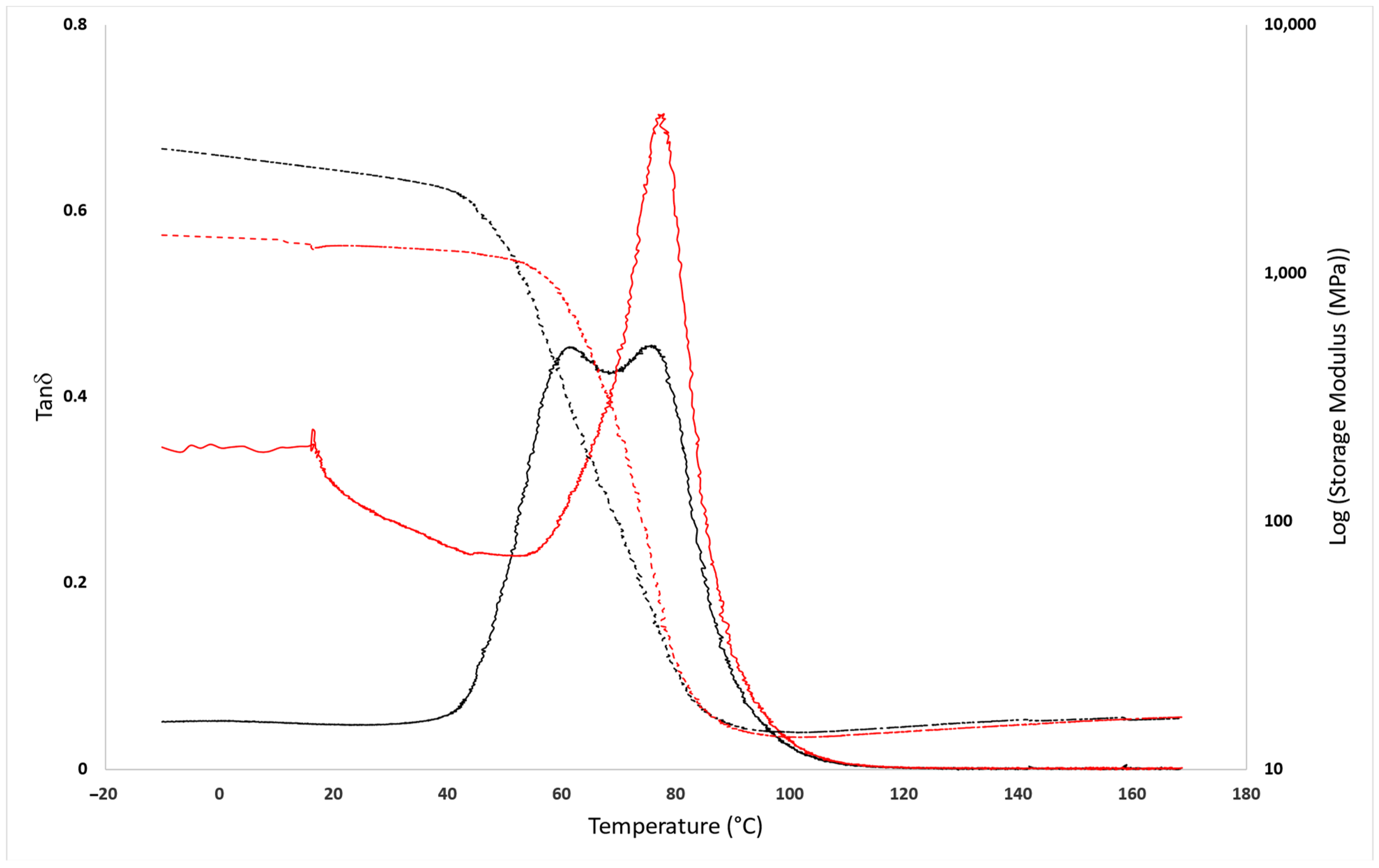
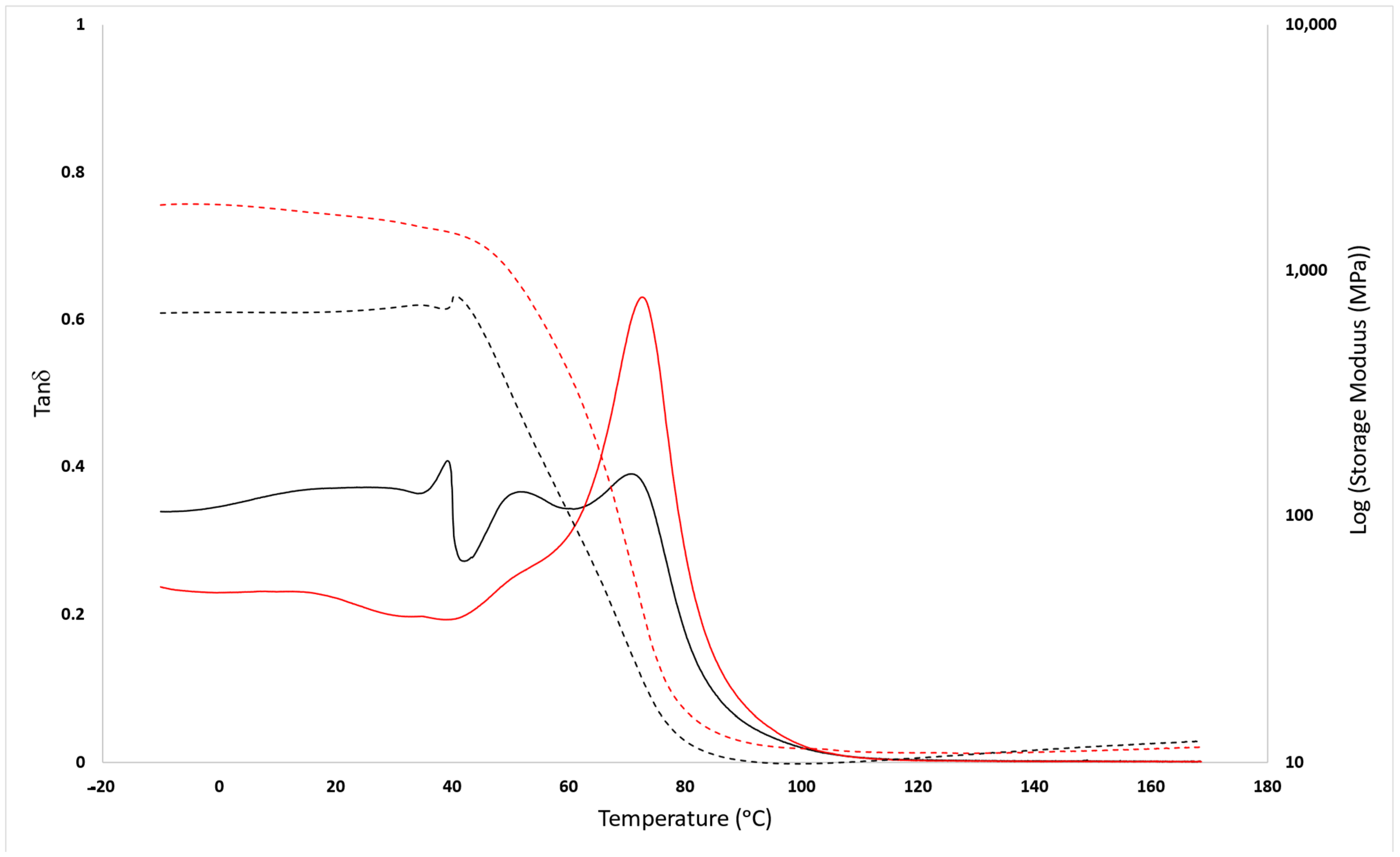
3.2. Mechanical Analysis
3.3. Structural Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Nurazzi, N.M.; Asyraf, M.R.M.; Khalina, A.; Abdullah, N.; Aisyah, H.A.; Rafiqah, S.A.; Sabaruddin, F.A.; Kamarudin, S.H.; Norrrahim, M.N.F.; Ilyas, R.A.; et al. A Review on Natural Fiber Reinforced Polymer Composite for Bullet Proof and Ballistic Applications. Polymers 2021, 13, 646. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M. An Overview of PEEK Biomaterials. In PEEK Biomaterials Handbook; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1–7. [Google Scholar] [CrossRef]
- Xu, Z.; Croft, Z.L.; Guo, D.; Cao, K.; Liu, G. Recent Development of Polyimides: Synthesis, Processing, and Application in Gas Separation. J. Polym. Sci. 2021, 59, 943–962. [Google Scholar] [CrossRef]
- Kiskan, B. Adapting Benzoxazine Chemistry for Unconventional Applications. React. Funct. Polym. 2018, 129, 76–88. [Google Scholar] [CrossRef]
- Martins, G.; Batista, C.; Domingues, J.; Santos, C.; Antunes, F.; Mateus, A. Thermoplastic Composites Reinforced with Wool and Wood: Application in the Automotive Industry. Mater. Proc. 2022, 8, 152. [Google Scholar] [CrossRef]
- Fitri, M.; Mahzan, S.; Anggara, F. The Mechanical Properties Requirement for Polymer Composite Automotive Parts—A Review. Int. J. Adv. Technol. Mech. Mechatron. Mater. 2021, 1, 125–133. [Google Scholar] [CrossRef]
- Dinu, R.; Lafont, U.; Damiano, O.; Mija, A. High Glass Transition Materials from Sustainable Epoxy Resins with Potential Applications in the Aerospace and Space Sectors. ACS Appl. Polym. Mater. 2022, 4, 3636–3646. [Google Scholar] [CrossRef]
- Fernandes, A.P.; Prado, K.S.; Castanho, M.N.; Paiva, J.M.F. Preparation and Characterization of Polymeric Composites Assembled from Fiberglass Fabric Waste from the Wind Blades Manufacturing Process. Fibers Polym. 2022, 23, 3606–3614. [Google Scholar] [CrossRef]
- Chakraborty, B.C. Naval Applications of Structural Adhesives. In Structural Adhesives; Wiley: Hoboken, NJ, USA, 2023; pp. 397–444. [Google Scholar]
- Morici, E.; Dintcheva, N.T. Recycling of Thermoset Materials and Thermoset-Based Composites: Challenge and Opportunity. Polymers 2022, 14, 4153. [Google Scholar] [CrossRef] [PubMed]
- Polymers Market Size to Hit around USD 1207.11 Billion by 2032. Available online: https://www.precedenceresearch.com/polymers-market (accessed on 26 September 2023).
- Kumar, A.; Sharma, K.; Dixit, A.R. A Review of the Mechanical and Thermal Properties of Graphene and Its Hybrid Polymer Nanocomposites for Structural Applications. J. Mater. Sci. 2019, 54, 5992–6026. [Google Scholar] [CrossRef]
- Dowbysz, A.; Samsonowicz, M.; Kukfisz, B. Recent Advances in Bio-Based Additive Flame Retardants for Thermosetting Resins. Int. J. Environ. Res. Public. Health 2022, 19, 4828. [Google Scholar] [CrossRef]
- Mora, P.; Nunwong, C.; Sriromreun, P.; Kaewsriprom, P.; Srisorrachatr, U.; Rimdusit, S.; Jubsilp, C. High Performance Composites Based on Highly Filled Glass Fiber-Reinforced Polybenzoxazine for Post Application. Polymers 2022, 14, 4321. [Google Scholar] [CrossRef] [PubMed]
- Mishnaevsky, L. Sustainable End-of-Life Management of Wind Turbine Blades: Overview of Current and Coming Solutions. Materials 2021, 14, 1124. [Google Scholar] [CrossRef] [PubMed]
- Chatziparaskeva, G.; Papamichael, I.; Voukkali, I.; Loizia, P.; Sourkouni, G.; Argirusis, C.; Zorpas, A.A. End-of-Life of Composite Materials in the Framework of the Circular Economy. Microplastics 2022, 1, 377–392. [Google Scholar] [CrossRef]
- Qureshi, J. A Review of Recycling Methods for Fibre Reinforced Polymer Composites. Sustainability 2022, 14, 16855. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Du, S.; Zhu, J.; Ma, S. Upcycling of Thermosetting Polymers into High-Value Materials. Mater. Horiz. 2023, 10, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; He, L.; Liu, J.; Liu, Y.; Rudd, C.; Liu, X. Recovery of Carbon Fibre from Waste Prepreg via Microwave Pyrolysis. Polymers 2021, 13, 1231. [Google Scholar] [CrossRef] [PubMed]
- Krauklis, A.E.; Karl, C.W.; Gagani, A.I.; Jørgensen, J.K. Composite Material Recycling Technology—State-of-the-Art and Sustainable Development for the 2020s. J. Compos. Sci. 2021, 5, 28. [Google Scholar] [CrossRef]
- Dolz, M.; Mateljak, I.; Méndez-Sánchez, D.; Sánchez-Moreno, I.; Gomez de Santos, P.; Viña-Gonzalez, J.; Alcalde, M. Colorimetric High-Throughput Screening Assay to Engineer Fungal Peroxygenases for the Degradation of Thermoset Composite Epoxy Resins. Front. Catal. 2022, 2, 883263. [Google Scholar] [CrossRef]
- Shen, M.; Cao, H.; Robertson, M.L. Hydrolysis and Solvolysis as Benign Routes for the End-of-Life Management of Thermoset Polymer Waste. Annu. Rev. Chem. Biomol. Eng. 2020, 11, 183–201. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, C.; André, A.; Juntikka, M.; Tränkle, T.; Sott, R. Chemical Recycling of End-of-Life Wind Turbine Blades by Solvolysis/HTL. IOP Conf. Ser. Mater. Sci. Eng. 2020, 942, 012013. [Google Scholar] [CrossRef]
- Samanta, S.; Kim, S.; Saito, T.; Sokolov, A.P. Polymers with Dynamic Bonds: Adaptive Functional Materials for a Sustainable Future. J. Phys. Chem. B 2021, 125, 9389–9401. [Google Scholar] [CrossRef] [PubMed]
- Post, W.; Susa, A.; Blaauw, R.; Molenveld, K.; Knoop, R.J.I. A Review on the Potential and Limitations of Recyclable Thermosets for Structural Applications. Polym. Rev. 2020, 60, 359–388. [Google Scholar] [CrossRef]
- Lazăr, S.; Dobrotă, D.; Breaz, R.-E.; Racz, S.-G. Eco-Design of Polymer Matrix Composite Parts: A Review. Polymers 2023, 15, 3634. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, C.; Genua, A.; Mija, A. Fully Bio-Based Reprocessable Thermosetting Resins Based on Epoxidized Vegetable Oils Cured with Itaconic Acid. Ind. Crops Prod. 2022, 185, 115116. [Google Scholar] [CrossRef]
- Genua, A.; Montes, S.; Azcune, I.; Rekondo, A.; Malburet, S.; Daydé-Cazals, B.; Graillot, A. Build-To-Specification Vanillin and Phloroglucinol Derived Biobased Epoxy-Amine Vitrimers. Polymers 2020, 12, 2645. [Google Scholar] [CrossRef] [PubMed]
- Gamardella, F.; Guerrero, F.; De la Flor, S.; Ramis, X.; Serra, A. A New Class of Vitrimers Based on Aliphatic Poly(Thiourethane) Networks with Shape Memory and Permanent Shape Reconfiguration. Eur. Polym. J. 2020, 122, 109361. [Google Scholar] [CrossRef]
- Krishnakumar, B.; Sanka, R.V.S.P.; Binder, W.H.; Parthasarthy, V.; Rana, S.; Karak, N. Vitrimers: Associative Dynamic Covalent Adaptive Networks in Thermoset Polymers. Chem. Eng. J. 2020, 385, 123820. [Google Scholar] [CrossRef]
- Chakma, P.; Morley, C.N.; Sparks, J.L.; Konkolewicz, D. Exploring How Vitrimer-like Properties Can Be Achieved from Dissociative Exchange in Anilinium Salts. Macromolecules 2020, 53, 1233–1244. [Google Scholar] [CrossRef]
- Rusayyis, M.A.B.; Torkelson, J.M. Reprocessable Covalent Adaptable Networks with Excellent Elevated-Temperature Creep Resistance: Facilitation by Dynamic, Dissociative Bis(Hindered Amino) Disulfide Bonds. Polym. Chem. 2021, 12, 2760–2771. [Google Scholar] [CrossRef]
- Manarin, E.; Da Via, F.; Rigatelli, B.; Turri, S.; Griffini, G. Bio-Based Vitrimers from 2,5-Furandicarboxylic Acid as Repairable, Reusable, and Recyclable Epoxy Systems. ACS Appl. Polym. Mater. 2023, 5, 828–838. [Google Scholar] [CrossRef]
- Van Aart, J.; Den Doelder, J.; Heuts, J.P.A. Reprocessing polyurethane foam using dynamic covalent chemistry in extrusion. J. Appl. Polym. Sci. 2023, 141, e54941. [Google Scholar] [CrossRef]
- Xu, X.; Ma, S.; Wang, S.; Wang, B.; Feng, H.; Li, P.; Liu, Y.; Yu, Z.; Zhu, J. Fast-Reprocessing, Postadjustable, Self-Healing Covalent Adaptable Networks with Schiff Base and Diels–Alder Adduct. Macromol. Rapid Commun. 2022, 43, 2100777. [Google Scholar] [CrossRef] [PubMed]
- Quiles-Díaz, S.; Seyler, H.; Ellis, G.J.; Shuttleworth, P.S.; Flores, A.; Gómez-Fatou, M.A.; Salavagione, H.J. Designing New Sustainable Polyurethane Adhesives: Influence of the Nature and Content of Diels–Alder Adducts on Their Thermoreversible Behavior. Polymers 2022, 14, 3402. [Google Scholar] [CrossRef] [PubMed]
- Memon, H.; Wei, Y.; Zhang, L.; Jiang, H.Q.; Liu, W. An Imine-Containing Epoxy Vitrimer with Versatile Recyclability and Its Application in Fully Recyclable Carbon Fiber Reinforced Composites. Compos. Sci. Technol. 2020, 199, 108314. [Google Scholar] [CrossRef]
- Nie, Y.; Li, M.; Li, S.; Lin, M.; Yao, N.; Deng, T.; Feng, X.; Yang, X.; Ding, H.; Xu, L. Design, Fabrication and Characterization of Dynamic Covalent Polymer Networks Containing Reversible Diels-Alder Adducts Derived from Vegetable Oil and Furfural. Polym. Test. 2023, 127, 108196. [Google Scholar] [CrossRef]
- Rulev, A.Y. Aza-Michael Reaction: A Decade Later—Is the Research Over? Eur. J. Org. Chem. 2023, 26, e202300451. [Google Scholar] [CrossRef]
- Strachota, B.; Hodan, J.; Dybal, J.; Matejka, L. Self-Healing Epoxy and Reversible Diels-Alder Based Interpenetrating Networks. Macromol. Mater. Eng. 2021, 306, 2000474. [Google Scholar] [CrossRef]
- Colledani, M.; Turri, S.; Diani, M.; Mathes, V. Introduction, Context, and Motivations of a Circular Economy for Composite Materials. In Systemic Circular Economy Solutions for Fiber Reinforced Composites; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–15. [Google Scholar] [CrossRef]
- ISO 3001; Plastics. Epoxy Compounds. Determination of Epoxy Equivalent. ISO: Geneva, Switzerland, 1999.
- ISO 1877-2; Products and Systems for the Protection and Repair of Concrete Structures. Test Methods. Reactive Functions Related to Epoxy Resins. Part 2: Determination of Amine Functions Using the Total Basicity Number. ISO: Geneva, Switzerland, 2000.
- Nikolic, G.; Zlatkovic, S.; Cakic, M.; Cakic, S.; Lacnjevac, C.; Rajic, Z. Fast Fourier transform IR characterization of epoxy GY systems crosslinked with aliphatic and cycloaliphatic EH polyamine adducts. Sensors 2010, 10, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Maity, P.; Kasisomayajula, S.V.; Parameswaran, V.; Basu, S.; Gupta, N. Improvement in surface degradation properties of polymer composites due to pre-processed nanometric alumina fillers. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 63–72. [Google Scholar] [CrossRef]
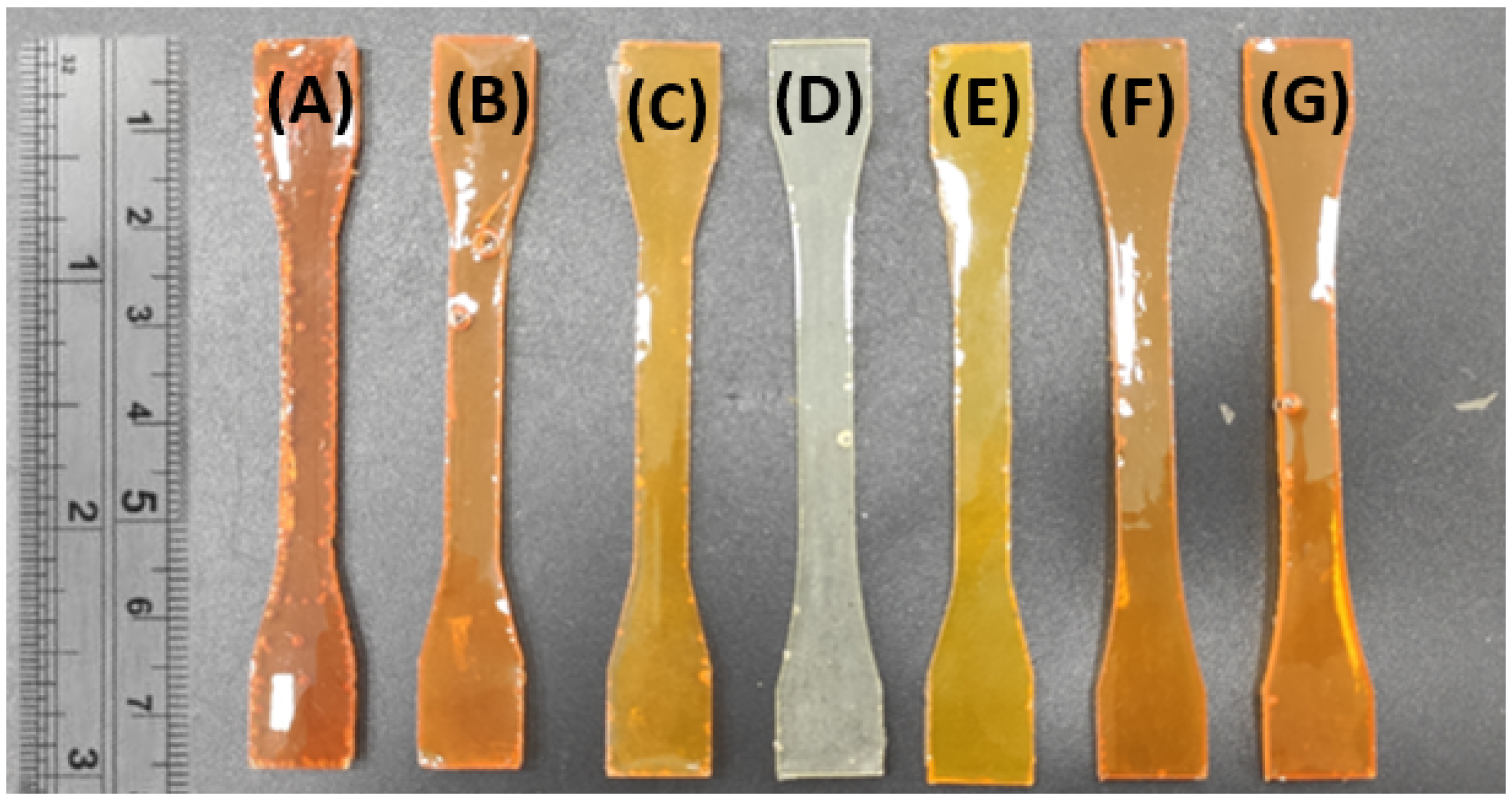
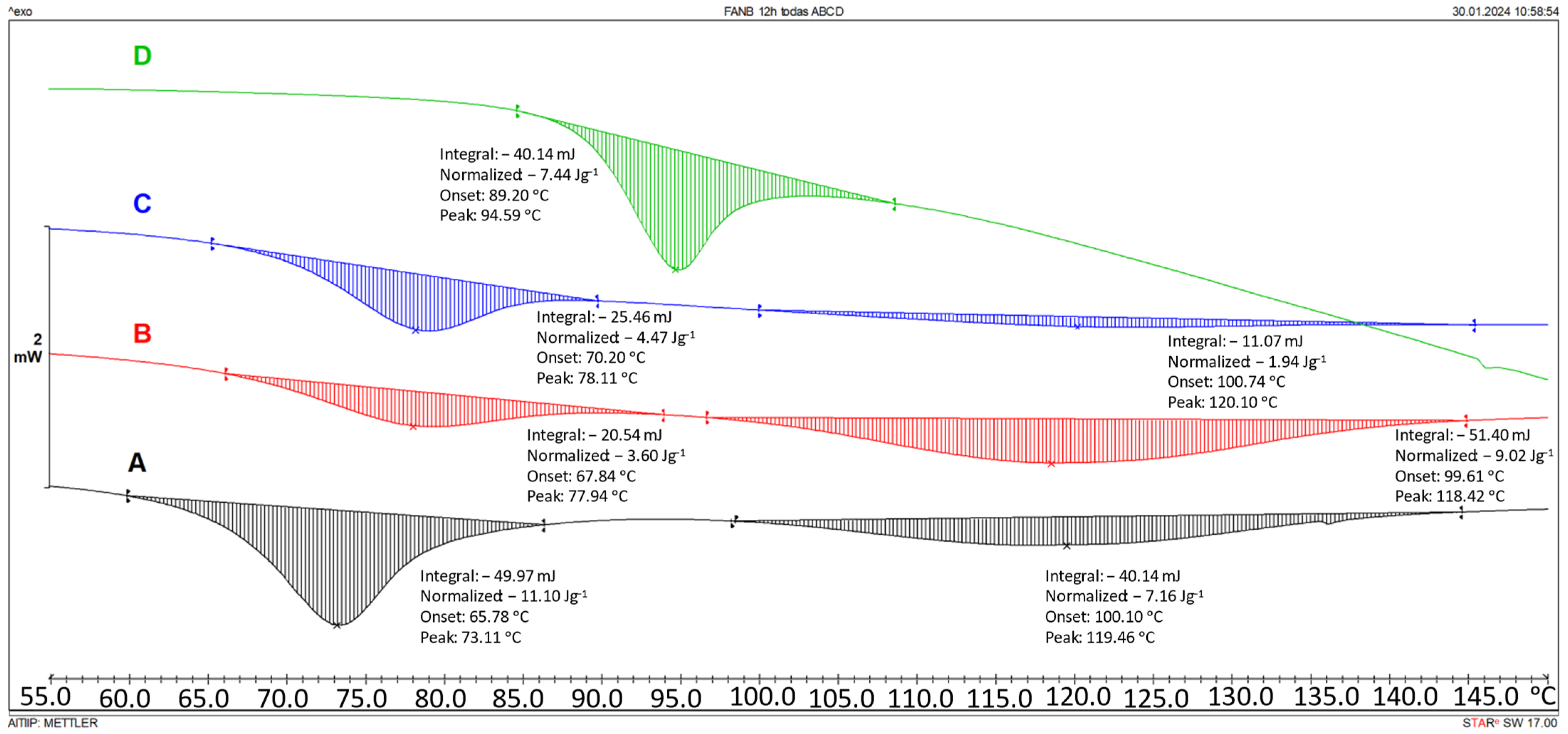

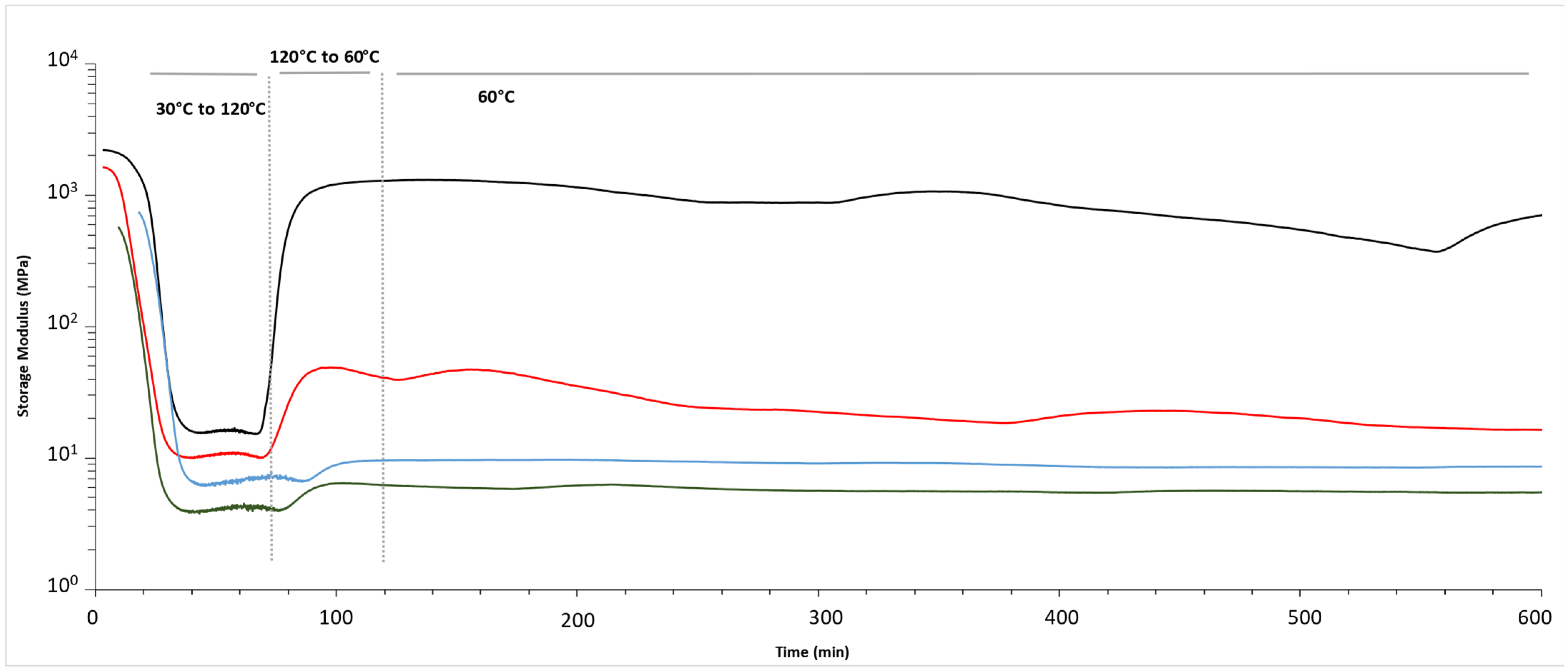
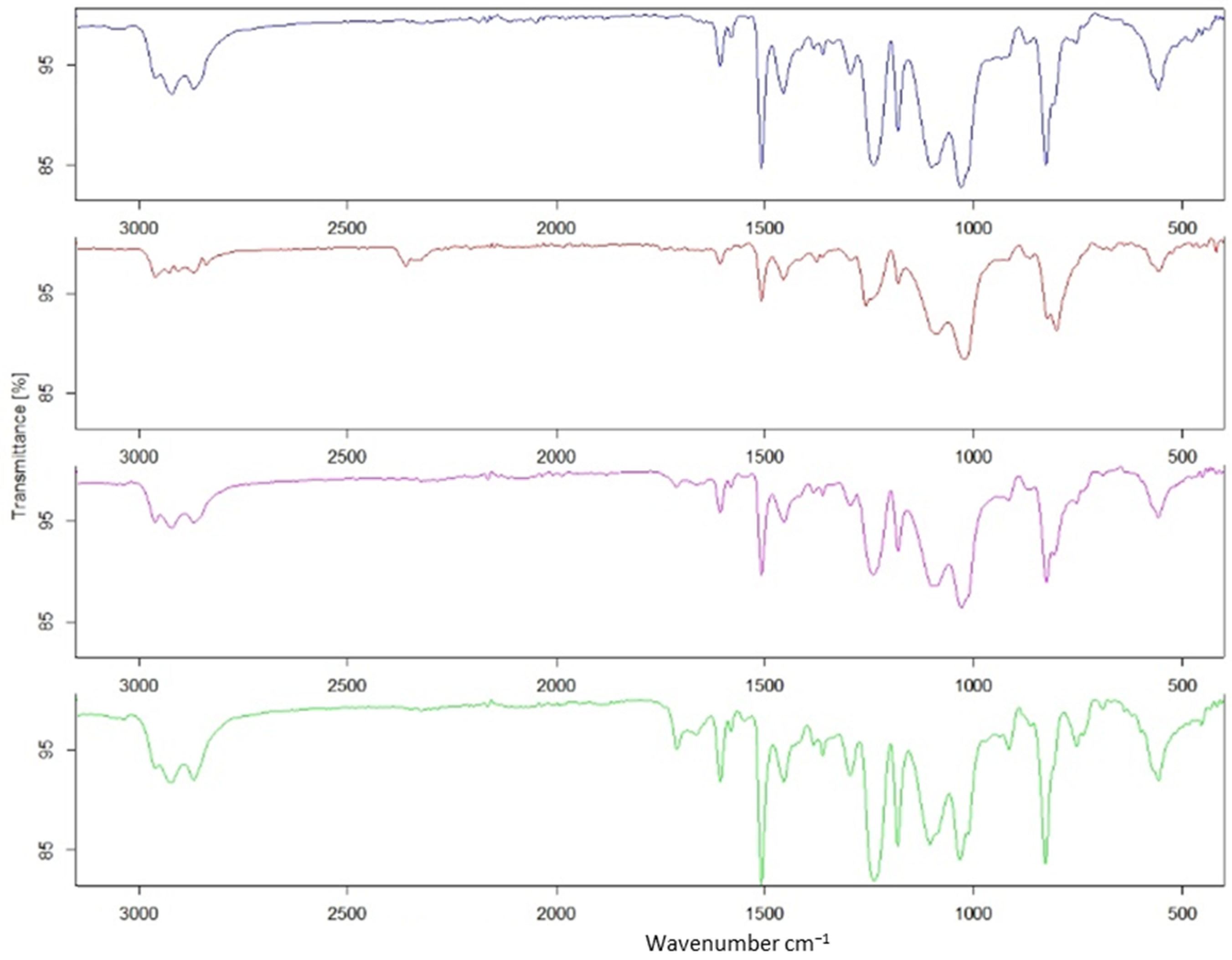

| Epoxy | Hardener | FANB vs. Epoxy | |||||
|---|---|---|---|---|---|---|---|
| g | % (wt) | g | % (wt) | g | % (wt) | ||
| Stoichiometric | Commercial | 15.50 | 77.50 | 4.50 | 22.50 | - | - |
| 5% FANB | 15.50 | 76.02 | 4.13 | 20.26 | 0.76 | 3.73 | |
| 10% FANB | 15.51 | 74.57 | 3.75 | 18.03 | 1.54 | 7.40 | |
| 15% FANB | 15.50 | 73.15 | 3.37 | 15.90 | 2.32 | 10.95 | |
| Amine excess | 5% FANB | 15.51 | 74.64 | 4.49 | 21.61 | 0.78 | 3.75 |
| 10% FANB | 15.50 | 71.96 | 4.49 | 20.84 | 1.55 | 7.20 | |
| 15% FANB | 15.51 | 69.52 | 4.48 | 20.08 | 2.32 | 10.40 | |
| 99% Conversion at 60 °C (min) | 99% Conversion at 80 °C (min) | 99% Conversion at 100 °C (min) | ||
|---|---|---|---|---|
| Stoichiometric | Commercial | 389 | 123 | 43 |
| 5% FANB | 441 | 128 | 42 | |
| 10% FANB | 5850 | 394 | 62 | |
| 15% FANB | 14,600 | 1009 | 93 | |
| Amine excess | 5% FANB | 329 | 107 | 42 |
| 10% FANB | 262 | 82 | 35 | |
| 15% FANB | 272 | 77 | 27 | |
| Stoichiometric | Amine Excess | |||||
|---|---|---|---|---|---|---|
| Commercial | 5% FANB | 10% FANB | 15% FANB | 5% FANB | 10% FANB | 15% FANB |
| 86.7 | 66.4 | 54.9 | 50.0 | 72.2 | 80.4 | 82.6 |
| Tanδ Peaks | 1st Measurement | 2nd Measurement | |||
|---|---|---|---|---|---|
| Diels–Alder (°C) | Tg (°C) | Diels–Alder (°C) | Tg (°C) | ||
| Stoichiometric | Commercial | - | 71.8 | - | 74.1 |
| 5% FANB | 57.4 | 65.7 | - | 76.2 | |
| 10% FANB | 47.4 | 61.9 | - | 63.8 | |
| 15% FANB | 45.0 | 59.2 | - | 53.2 | |
| Amine excess | 5% FANB | 61.4 | 75.8 | - | 78.0 |
| 10% FANB | 50.4 | 67.0 | - | 70.5 | |
| 15% FANB | 51.8 | 70.9 | - | 72.8 | |
| Storage Modulus at 30 °C (MPa) | Storage Modulus at 60 °C (MPa) | Storage Modulus at 120 °C (MPa) | Tensile Modulus at 60 °C (MPa) * | ||
|---|---|---|---|---|---|
| Stoichiometric | Commercial | 2210 | 1341 | 16 | 1310 |
| 5% FANB | 1635 | 135 | 11 | 49 | |
| 10% FANB | 732 | 13 | 7 | 10 | |
| 15% FANB | 568 | 11 | 4 | 6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidal, J.; Hornero, C.; De la Flor, S.; Vilanova, A.; Dieste, J.A.; Castell, P. Strategies towards Fully Recyclable Commercial Epoxy Resins: Diels–Alder Structures in Sustainable Composites. Polymers 2024, 16, 1024. https://doi.org/10.3390/polym16081024
Vidal J, Hornero C, De la Flor S, Vilanova A, Dieste JA, Castell P. Strategies towards Fully Recyclable Commercial Epoxy Resins: Diels–Alder Structures in Sustainable Composites. Polymers. 2024; 16(8):1024. https://doi.org/10.3390/polym16081024
Chicago/Turabian StyleVidal, Julio, Carlos Hornero, Silvia De la Flor, Anna Vilanova, Jose Antonio Dieste, and Pere Castell. 2024. "Strategies towards Fully Recyclable Commercial Epoxy Resins: Diels–Alder Structures in Sustainable Composites" Polymers 16, no. 8: 1024. https://doi.org/10.3390/polym16081024
APA StyleVidal, J., Hornero, C., De la Flor, S., Vilanova, A., Dieste, J. A., & Castell, P. (2024). Strategies towards Fully Recyclable Commercial Epoxy Resins: Diels–Alder Structures in Sustainable Composites. Polymers, 16(8), 1024. https://doi.org/10.3390/polym16081024








