Development of Flame-Retardant Polylactic Acid Formulations for Additive Manufacturing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples
2.3. Thermal, Rheological, Mechanical and Fire Behavior
2.4. Mechanical Characterization of the Printed and Injection-Molded Samples
3. Results and Discussion
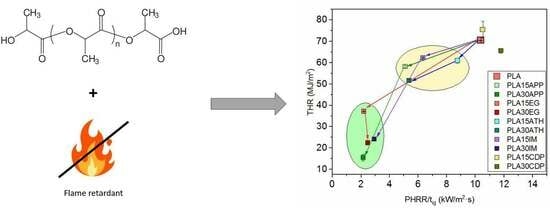
3.1. Fire Behavior
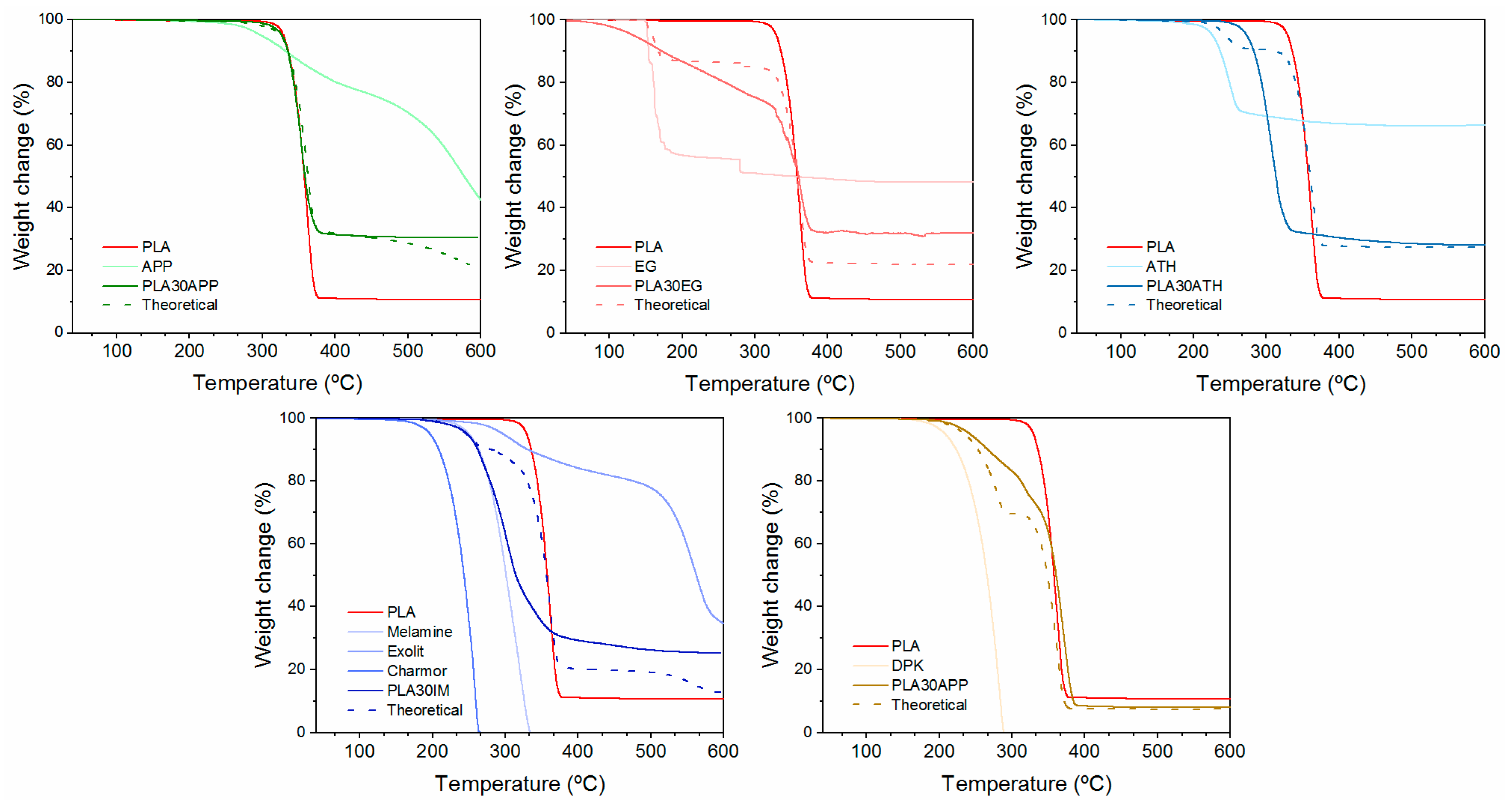
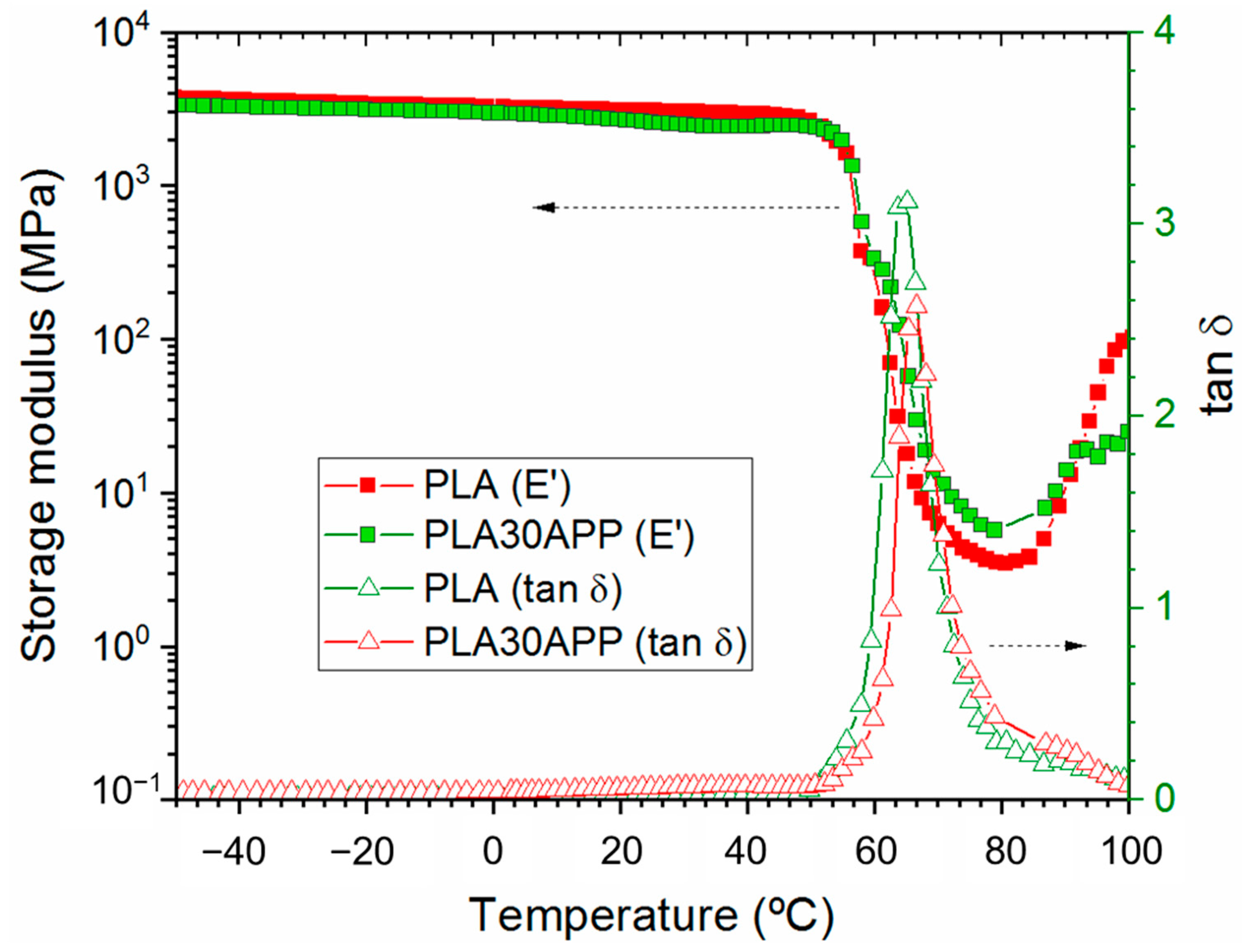
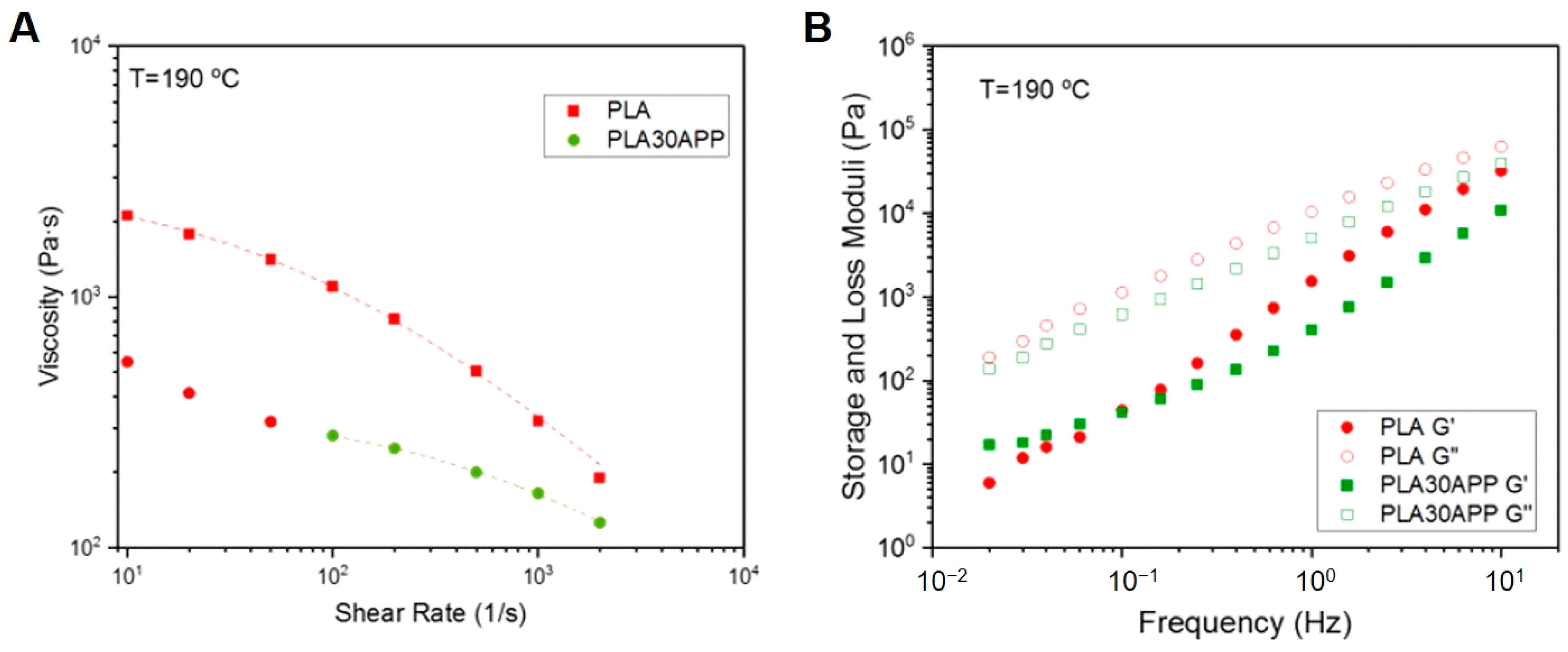
3.2. Effect of FR Addition on the Physico-Chemical, Thermal and Mechanical Properties
3.3. Printability
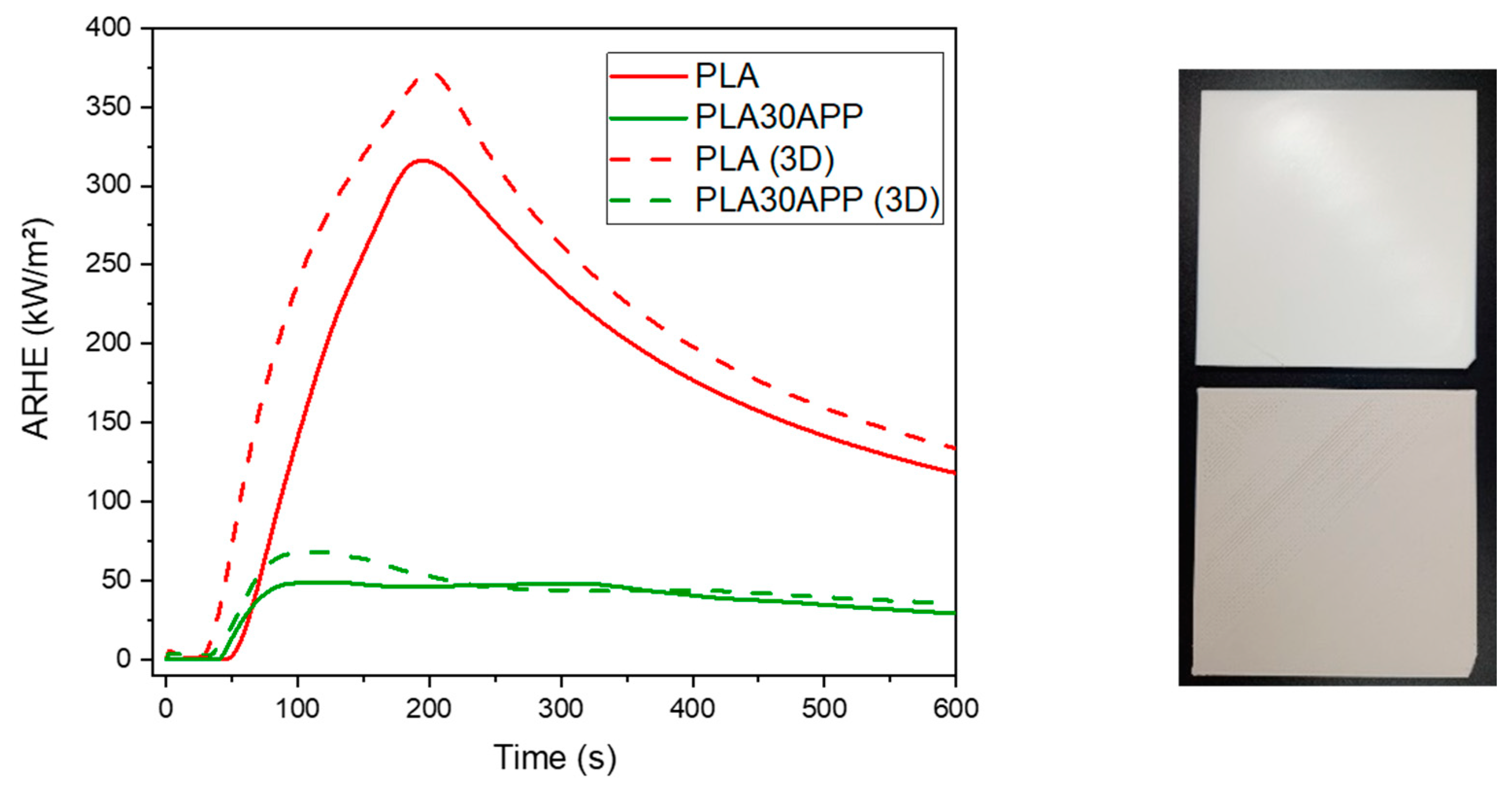
3.4. Comparison between 3D-Printed and Compression-Molded Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Applications for 3D Printing at the Heart of the Railway Industry. Available online: https://www.3dnatives.com/en/applications-for-3d-printing-at-the-heart-of-the-railway-industry-110320224/#! (accessed on 7 November 2023).
- Vahabi, H.; Laoutid, F.; Mehrpouya, M.; Saeb, M.R.; Dubois, P. Flame Retardant Polymer Materials: An Update and the Future for 3D Printing Developments. Mater. Sci. Eng. R Rep. 2021, 144, 100604. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, X.; Chen, X.; He, Y.; Chang, L.; Hou, M.; Yin, J.; Hao, F.; Chen, S.; Wang, P.; et al. Additive Manufacturing of Structural Materials. Mater. Sci. Eng. R Rep. 2021, 145, 100596. [Google Scholar] [CrossRef]
- Morgan, A.B.; Gilman, J.W. An Overview of Flame Retardancy of Polymeric Materials: Application, Technology, and Future Directions. Fire Mater. 2013, 37, 259–279. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, H.; Wang, Y. Advanced Flame-Retardant Methods for Polymeric Materials. Adv. Mater. 2022, 34, 2107905. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Gilmer, E.L.; Biria, S.; Bortner, M.J. Importance of Polymer Rheology on Material Extrusion Additive Manufacturing: Correlating Process Physics to Print Properties. ACS Appl. Polym. Mater. 2021, 3, 1218–1249. [Google Scholar] [CrossRef]
- Clayton, J.; Millington-Smith, D.; Armstrong, B. The Application of Powder Rheology in Additive Manufacturing. JOM 2015, 67, 544–548. [Google Scholar] [CrossRef]
- Arjmandi, R.; Hassan, A.; Zakaria, Z. Polylactic Acid Green Nanocomposites for Automotive Applications. In Green Biocomposites. Green Energy and Technology; Springer: New York, NY, USA, 2017. [Google Scholar]
- Trivedi, A.K.; Gupta, M.K.; Singh, H. PLA Based Biocomposites for Sustainable Products: A Review. Adv. Ind. Eng. Polym. Res. 2023, 6, 382–395. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing Technologies for Poly(Lactic Acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Tümer, E.H.; Erbil, H.Y. Extrusion-Based 3D Printing Applications of PLA Composites: A Review. Coatings 2021, 11, 390. [Google Scholar] [CrossRef]
- Wang, S.; Daelemans, L.; Fiorio, R.; Gou, M.; D’hooge, D.; De Clerck, K.; Cardon, L. Improving Mechanical Properties for Extrusion-Based Additive Manufacturing of Poly(Lactic Acid) by Annealing and Blending with Poly(3-Hydroxybutyrate). Polymers 2019, 11, 1529. [Google Scholar] [CrossRef]
- Bian, Y.-H.; Yu, G.; Zhao, X.; Li, S.-X.; He, X.-L.; Tian, C.-X.; Li, Z.-Y. Exit Morphology and Mechanical Property of FDM Printed PLA: Influence of Hot Melt Extrusion Process. Adv. Manuf. 2023, 11, 56–74. [Google Scholar] [CrossRef]
- Herrera, N.; Mathew, A.P.; Oksman, K. Plasticized Polylactic Acid/Cellulose Nanocomposites Prepared Using Melt-Extrusion and Liquid Feeding: Mechanical, Thermal and Optical Properties. Compos. Sci. Technol. 2015, 106, 149–155. [Google Scholar] [CrossRef]
- Asadi, M.; Salehi, Z.; Akrami, M.; Hoseeinpour, M.; Jockenhövel, S.; Ghazanfari, S. 3D Printed PH-Responsive Tablets Containing N-Acetylglucosamine-Loaded Methylcellulose Hydrogel for Colon Drug Delivery Applications. Int. J. Pharm. 2023, 645, 123366. [Google Scholar] [CrossRef] [PubMed]
- Marano, S.; Laudadio, E.; Minnelli, C.; Stipa, P. Tailoring the Barrier Properties of PLA: A State-of-the-Art Review for Food Packaging Applications. Polymers 2022, 14, 1626. [Google Scholar] [CrossRef] [PubMed]
- Szalai, S.; Herold, B.; Kurhan, D.; Németh, A.; Sysyn, M.; Fischer, S. Optimization of 3D Printed Rapid Prototype Deep Drawing Tools for Automotive and Railway Sheet Material Testing. Infrastructures 2023, 8, 43. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, Q.; Li, J.; Tao, K.; Xue, L.; Yan, Q. Synergistic Effect between Expandable Graphite and Ammonium Polyphosphate on Flame Retarded Polylactide. Polym. Degrad. Stab. 2011, 96, 183–189. [Google Scholar] [CrossRef]
- Carretier, V.; Delcroix, J.; Pucci, M.F.; Rublon, P.; Lopez-Cuesta, J.-M. Influence of Sepiolite and Lignin as Potential Synergists on Flame Retardant Systems in Polylactide (PLA) and Polyurethane Elastomer (PUE). Materials 2020, 13, 2450. [Google Scholar] [CrossRef] [PubMed]
- Bourbigot, S.; Duquesne, S.; Fontaine, G.; Bellayer, S.; Turf, T.; Samyn, F. Characterization and Reaction to Fire of Polymer Nanocomposites with and without Conventional Flame Retardants. Mol. Cryst. Liq. Cryst. 2008, 486, 325/[1367]–339/[1381]. [Google Scholar] [CrossRef]
- Fukushima, K.; Murariu, M.; Camino, G.; Dubois, P. Effect of Expanded Graphite/Layered-Silicate Clay on Thermal, Mechanical and Fire Retardant Properties of Poly(Lactic Acid). Polym. Degrad. Stab. 2010, 95, 1063–1076. [Google Scholar] [CrossRef]
- Cheng, K.C.; Yu, C.B.; Guo, W.; Wang, S.F.; Chuang, T.H.; Lin, Y.H. Thermal Properties and Flammability of Polylactide Nanocomposites with Aluminum Trihydrate and Organoclay. Carbohydr. Polym. 2012, 87, 1119–1123. [Google Scholar] [CrossRef]
- Zhao, X.; Gao, S.; Liu, G. A THEIC-Based Polyphosphate Melamine Intumescent Flame Retardant and Its Flame Retardancy Properties for Polylactide. J. Anal. Appl. Pyrolysis 2016, 122, 24–34. [Google Scholar] [CrossRef]
- Réti, C.; Casetta, M.; Duquesne, S.; Bourbigot, S.; Delobel, R. Flammability Properties of Intumescent PLA Including Starch and Lignin. Polym. Adv. Technol. 2008, 19, 628–635. [Google Scholar] [CrossRef]
- Chollet, B.; Lopez-Cuesta, J.-M.; Laoutid, F.; Ferry, L. Lignin Nanoparticles as A Promising Way for Enhancing Lignin Flame Retardant Effect in Polylactide. Materials 2019, 12, 2132. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.H.; Li, J.; Fang, K.Y.; Zhu, Q.L.; Zhu, J.; Yan, Q.; Wang, Y.Z. Synergistic Effect between a Novel Hyperbranched Charring Agent and Ammonium Polyphosphate on the Flame Retardant and Anti-Dripping Properties of Polylactide. Polym. Degrad. Stab. 2010, 95, 763–770. [Google Scholar] [CrossRef]
- Liao, F.; Ju, Y.; Dai, X.; Cao, Y.; Li, J.; Wang, X. A Novel Efficient Polymeric Flame Retardant for Poly (Lactic Acid) (PLA): Synthesis and Its Effects on Flame Retardancy and Crystallization of PLA. Polym. Degrad. Stab. 2015, 120, 251–261. [Google Scholar] [CrossRef]
- Chen, C.; Gu, X.; Jin, X.; Sun, J.; Zhang, S. The Effect of Chitosan on the Flammability and Thermal Stability of Polylactic Acid/Ammonium Polyphosphate Biocomposites. Carbohydr. Polym. 2017, 157, 1586–1593. [Google Scholar] [CrossRef]
- Zhan, J.; Song, L.; Nie, S.; Hu, Y. Combustion Properties and Thermal Degradation Behavior of Polylactide with an Effective Intumescent Flame Retardant. Polym. Degrad. Stab. 2009, 94, 291–296. [Google Scholar] [CrossRef]
- Babu, K.; Das, O.; Shanmugam, V.; Mensah, R.A.; Försth, M.; Sas, G.; Restás, Á.; Berto, F. Fire Behavior of 3D-Printed Polymeric Composites. J. Mater. Eng. Perform. 2021, 30, 4745–4755. [Google Scholar] [CrossRef]
- Martins, R.C.; Ribeiro, S.P.d.S.; Rezende, M.J.C.; Nascimento, R.S.V.; Nascimento, M.A.C.; Batistella, M.; Lopez-Cuesta, J.-M. Flame-Retarding Properties of Injected and 3D-Printed Intumescent Bio-Based PLA Composites: The Influence of Brønsted and Lewis Acidity of Montmorillonite. Polymers 2022, 14, 1702. [Google Scholar] [CrossRef]
- Xue, Y.; Zuo, X.; Wang, L.; Zhou, Y.; Pan, Y.; Li, J.; Yin, Y.; Li, D.; Yang, R.; Rafailovich, M.H.; et al. Enhanced Flame Retardancy of Poly(Lactic Acid) with Ultra-Low Loading of Ammonium Polyphosphate. Compos. Part B Eng. 2020, 196, 108124. [Google Scholar] [CrossRef]
- Guo, Y.; Chang, C.-C.; Halada, G.; Cuiffo, M.A.; Xue, Y.; Zuo, X.; Pack, S.; Zhang, L.; He, S.; Weil, E.; et al. Engineering Flame Retardant Biodegradable Polymer Nanocomposites and Their Application in 3D Printing. Polym. Degrad. Stab. 2017, 137, 205–215. [Google Scholar] [CrossRef]
- EN 45545; Fire Protection on Railway Vehicles. European Committee for Standardization (CEN): Brussels, Belgium, 2013.
- Huang, J.; Lisowski, M.S.; Runt, J.; Hall, E.S.; Kean, R.T.; Buehler, N.; Lin, J.S. Crystallization and Microstructure of Poly(L-Lactide-Co-Meso-Lactide) Copolymers. Macromolecules 1998, 31, 2593–2599. [Google Scholar] [CrossRef]
- ISO 5660-1; Reaction-to-Fire Tests—Heat Release, Smoke Production and Mass Loss Rate—Part 1: Heat Release Rate (Cone Calorimeter Method) and Smoke Production Rate (Dynamic Measurement). International Organization for Standardization (ISO): Geneva, Switzerland, 2015.
- ISO 178; Plastics—Determination of Flexural Properties. International Organization for Standardization (ISO): Geneva, Switzerland, 2019.
- ISO 527-1; Plastics—Determination of Tensile Properties—Part 1: General Principles. International Organization for Standardization (ISO): Geneva, Switzerland, 2012.
- Regazzi, A.; Pucci, M.-F.; Dumazert, L.; Gallard, B.; Buonomo, S.; Ravel, R.; Lopez-Cuesta, J.M. Controlling the Distribution of Fire Retardants in Poly(Lactic Acid) by Fused Filament Fabrication in Order to Improve Its Fire Behaviour. Polym. Degrad. Stab. 2019, 163, 143–150. [Google Scholar] [CrossRef]
- Batistella, M.; Roux, J.-C.; Masarra, N.-A.; le Saout, G.; Xenopoulos, C.; Lopez-Cuesta, J.M. Incorporation of Fly Ash in Flame-Retardant Systems of Biopolyesters. Polymers 2023, 15, 2771. [Google Scholar] [CrossRef] [PubMed]
- Madyaratri, E.-W.; Ridho, M.; Aristri, M.; Lubis, M.; Iswanto, A.; Nawawi, D.; Antov, P.; Kristak, L.; Majlingová, A.; Fatriasari, W. Recent Advances in the Development of Fire-Resistant Biocomposites—A Review. Polymers 2022, 14, 362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chu, F.; Xu, Z.; Zhou, Y.; Qiu, Y.; Qian, L.; Hu, Y.; Wang, B.; Hu, W. The Improvement of Fire Safety Performance of Flexible Polyurethane Foam by Highly-Efficient P-N-S Elemental Hybrid Synergistic Flame Retardant. J. Colloid Interface Sci. 2022, 606, 768–783. [Google Scholar] [CrossRef] [PubMed]
- Camino, G.; Lomakin, S. 10—Intumescent Materials. In Fire Retardant Materials; Woodhead Publishing: Cambridge, UK, 2001; pp. 318–336. [Google Scholar]
- Shen, J.; Liang, J.; Lin, X.; Lin, H.; Yu, J.; Wang, S. The Flame-Retardant Mechanisms and Preparation of Polymer Composites and Their Potential Application in Construction Engineering. Polymers 2022, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, S.G.; Lee, J.S.; Ma, B.C. Understanding the Flame Retardant Mechanism of Intumescent Flame Retardant on Improving the Fire Safety of Rigid Polyurethane Foam. Polymers 2022, 14, 4904. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, S.; Stec, A.A.; Hull, T.R. The Effect of Gas Phase Flame Retardants on Fire Effluent Toxicity. Polym. Degrad. Stab. 2014, 106, 36–46. [Google Scholar] [CrossRef]
- Horrocks, A.R.; Price, D. Fire Retardant Materials; Woodhead Publishing Ltd.: Cambridge, UK, 2001; ISBN 9781855734197. [Google Scholar]
- Sánchez, A.; Villanueva, S. Effect of Aryl Phosphates on Toxicity of Combustion Gases of Flame-retardant Polycarbonate/Acrylonitrile Butadiene Styrene Blends According to EN 45545 Railway Standard. Fire Mater. 2022, 46, 1197–1207. [Google Scholar] [CrossRef]
- Troitzsch, J.H. Fire Performance Durability of Flame Retardants in Polymers and Coatings. Adv. Ind. Eng. Polym. Res. 2023, in press. [Google Scholar] [CrossRef]
- Kopinke, F.D.; Remmler, M.; Mackenzie, K.; Möder, M.; Wachsen, O. Thermal Decomposition of Biodegradable Polyesters—II. Poly(Lactic Acid). Polym. Degrad. Stab. 1996, 53, 329–342. [Google Scholar] [CrossRef]
- Hyon, S.-H.; Jamshidi, K.; Ikada, Y. Effects of Residual Monomer on the Degradation of DL-Lactide Polymer. Polym. Int. 1998, 46, 196–202. [Google Scholar] [CrossRef]
- Liu, F. Fire Retardant Coating Composition. Patent US6084008A, 15 July 1999. [Google Scholar]
- Xia, Y.; Chai, W.; Liu, Y.; Su, X.; Liao, C.; Gao, M.; Li, Y.; Zheng, Z. Facile Fabrication of Starch-Based, Synergistic Intumescent and Halogen-Free Flame Retardant Strategy with Expandable Graphite in Enhancing the Fire Safety of Polypropylene. Ind. Crops Prod. 2022, 184, 115002. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, S.; Liu, B.; Wang, Z.; Xie, H. Synergistic Effect of Combining Amino Trimethylphosphonate Calcium and Expandable Graphite on Flame Retardant and Thermal Stability of Rigid Polyurethane Foam. Int. J. Polym. Anal. Charact. 2022, 27, 302–315. [Google Scholar] [CrossRef]
- Murariu, M.; Dechief, A.L.; Bonnaud, L.; Paint, Y.; Gallos, A.; Fontaine, G.; Bourbigot, S.; Dubois, P. The Production and Properties of Polylactide Composites Filled with Expanded Graphite. Polym. Degrad. Stab. 2010, 95, 889–900. [Google Scholar] [CrossRef]
- Carrasco, F.; Pagès, P.; Gámez-Pérez, J.; Santana, O.O.; Maspoch, M.L. Processing of Poly(Lactic Acid): Characterization of Chemical Structure, Thermal Stability and Mechanical Properties. Polym. Degrad. Stab. 2010, 95, 116–125. [Google Scholar] [CrossRef]
- Liu, X.; Khor, S.; Petinakis, E.; Yu, L.; Simon, G.; Dean, K.; Bateman, S. Effects of Hydrophilic Fillers on the Thermal Degradation of Poly(Lactic Acid). Thermochim. Acta 2010, 509, 147–151. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Quian, S.; Liu, Z.; Weng, Y.; Chang, Y. Depolymerization and Re/Upcycling of Biodegradable PLA Plastics. ACS Omega 2024, 9, 13509–13521. [Google Scholar] [CrossRef]
- Ma, B.; Wang, X.; He, Y.; Dong, Z.; Zhang, X.; Chen, X.; Liu, T. Effect of Poly(Lactic Acid) Crystallization on Its Mechanical and Heat Resistance Performances. Polymer 2021, 212, 123280. [Google Scholar] [CrossRef]
- Song, P.; Shen, Y.; Du, B.; Peng, M.; Shen, L.; Fang, Z. Effects of Reactive Compatibilization on the Morphological, Thermal, Mechanical, and Rheological Properties of Intumescent Flame-Retardant Polypropylene. ACS Appl. Mater. Interfaces 2009, 1, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Arockiam, A.J.; Subramanian, K.; Padmanabhan, R.G.; Selvaraj, R.; Bagal, D.K.; Rajesh, S. A Review on PLA with Different Fillers Used as a Filament in 3D Printing. Mater. Today Proc. 2022, 50, 2057–2064. [Google Scholar] [CrossRef]
- Singamneni, S.; Smith, D.; LeGuen, M.-J.; Truong, D. Extrusion 3D Printing of Polybutyrate-Adipate-Terephthalate-Polymer Composites in the Pellet Form. Polymers 2018, 10, 922. [Google Scholar] [CrossRef] [PubMed]




| Sample | Flame Retardant Type | Composition (wt%) |
|---|---|---|
| PLA15APP | APP | 15 |
| PLA30APP | APP | 30 |
| PLA7.5EG | EG | 7.5 |
| PLA15EG | EG | 15 |
| PLA30EG | EG | 30 |
| PLA15ATH | ATH | 15 |
| PLA30ATH | ATH | 30 |
| PLA15IM | MEL/APP/PA | 15 (3.75/7.5/3.75) |
| PLA30IM | MEL/APP/PA | 30 (7.5/15/7.5) |
| PLA15CDP | CDP | 15 |
| PLA30CDP | CDP | 30 |
| Sample | tignition (s) | HRRpeak (kW/m2) | THR (MJ/m2) | MARHE (kW/m2) | CO/CO2 (×103) Ratio | SEA (m2/kg) | Residue (%) |
|---|---|---|---|---|---|---|---|
| PLA | 55.5 ± 1.5 | 575.7 ± 28.3 | 70.5 ± 0.1 | 310.8 ± 5.3 | 9.2 ± 0.8 | 3.3 ± 2.7 | 10.5 ± 0.9 |
| PLA15APP | 44.5 ± 0.5 | 226.7 ± 3.4 | 58.2 ± 0.5 | 174.5 ± 0.3 | 32.3 ± 1.5 | 12.6 ± 1.2 | 23.7 ± 0.3 |
| PLA30APP | 48.0 ± 0.1 | 102.8 ± 0.1 | 15.5 ± 1.5 | 49.3 ± 0.5 | 157.7 ± 42.2 | 56.6 ± 4.4 | 53.7 ± 0.2 |
| PLA7.5EG | 51.5 ± 0.2 | 306.9 ± 3.1 | 74.2 ± 1.2 | 165.0 ± 2.5 | 24.6 ± 0.8 | 6.2 ± 0.6 | 17.0 ± 1.5 |
| PLA15EG | 59.0 ± 1.0 | 129.3 ± 1.2 | 37.2 ± 0.1 | 73.5 ± 0.3 | 59.8 ± 2.5 | 2.5 ± 2.5 | 44.4 ± 0.1 |
| PLA30EG | 45.5 ± 0.5 | 112.3 ± 5.5 | 22.4 ± 0.3 | 61.1 ± 0.3 | 114.8 ± 1.9 | 3.7 ± 2.6 | 45.5 ± 0.7 |
| PLA15ATH | 53.0 ± 0.1 | 463.9 ± 10.0 | 61.1 ± 1.2 | 261.9 ± 4.0 | 18.3 ± 0.9 | 4.7 ± 0.2 | 19.9 ± 1.9 |
| PLA30ATH | 56.0 ± 3.1 | 300.8 ± 0.5 | 51.6 ± 0.6 | 190.7 ± 4.0 | 21.9 ± 1.1 | 1.8 ± 0.9 | 15.1 ± 0.6 |
| PLA15IM | 55.0 ± 0.2 | 348.9 ± 1.9 | 62.3 ± 0.2 | 226.7 ± 3.3 | 33.0 ± 4.6 | 19 ± 0.3 | 15.1 ± 0.6 |
| PLA30IM | 54.5 ± 0.5 | 159.1 ± 2.2 | 24.2 ± 0.5 | 91.3 ± 2.5 | 67.4 ± 7.7 | 5.8 ± 1.7 | 48.4 ± 1.4 |
| PLA15CDP | 52.0 ± 1.2 | 546.1 ± 22.1 | 75.5 ± 3.8 | 306.3 ± 5.6 | 76.5 ± 2.2 | 254.1 ± 23.5 | 9.0 ± 0.4 |
| PLA30CDP | 49.0 ± 1.1 | 577.0 ± 5.3 | 65.6 ± 1.2 | 288.6 ± 5.8 | 182.1 ± 1.5 | 485.8 ± 5.9 | 6.4 ± 0.6 |
| Sample | Ti (°C) | Tmax (°C) | Residual Chart (%) |
|---|---|---|---|
| PLA | 330 | 360 | 11 |
| PLA30APP | 327 | 350 | 30 |
| PLA30EG | 133 | 175/360 | 32 |
| PLA30ATH | 273 | 305 | 28 |
| PLA30IM | 248 | 300 | 25 |
| PLA30CDP | 242 | 318 | 8.2 |
| Sample | Mn (g/mol) | Mw (g/mol) | PI |
|---|---|---|---|
| PLA | 93,900 | 150,300 | 1.6 |
| PLA30APP | 48,300 | 70,200 | 1.4 |
| PLA15EG | 5600 | 9000 | 1.6 |
| PLA30EG | 3700 | 4800 | 1.3 |
| PLA30IM | 8300 | 17,000 | 2.0 |
| Sample | 1st Scan | Cooling | 2nd Scan | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Tg (°C) | Tcc (°C) | Tm (°C) | Xc (%) | Tcc (°C) | Tg (°C) | Tcc (°C) | Tm (°C) | Xc (%) | |
| PLA | 60 | 108 | 173 | 5 | 95 | 60 | 108 | 172 | 6 |
| PLA30APP | 57 | 102 | 176 | 12 | 95 | 57 | 105 | 173 | 5 |
| Sample | Young’s Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|
| PLA (3D) | 3664 ± 207 | 45.7 ± 1 | 3.5 ± 0.7 |
| PLA30APP (3D) | 5284 ± 1527 | 30.5 ± 2.4 | 2.0 ± 1.2 |
| PLA | 4584 ± 83 | 55.8 ± 1.7 | 5.7 ± 0.3 |
| PLA30APP | 5333 ± 63 | 49.3 ± 3 | 2.1 ± 0.7 |
| Sample | Flexural Modulus (MPa) | Flexural Strength (MPa) |
|---|---|---|
| PLA (3D) | 2458 ± 98 | 55.83 ± 1.7 |
| PLA30APP (3D) | 3835 ± 54 | 49.26 ± 3.0 |
| PLA | 4399 ± 70 | 95.80 ± 3.6 |
| PLA30APP | 5209 ± 60 | 61.80 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguirresarobe, R.; Calafel, I.; Villanueva, S.; Sanchez, A.; Agirre, A.; Sukia, I.; Esnaola, A.; Saralegi, A. Development of Flame-Retardant Polylactic Acid Formulations for Additive Manufacturing. Polymers 2024, 16, 1030. https://doi.org/10.3390/polym16081030
Aguirresarobe R, Calafel I, Villanueva S, Sanchez A, Agirre A, Sukia I, Esnaola A, Saralegi A. Development of Flame-Retardant Polylactic Acid Formulations for Additive Manufacturing. Polymers. 2024; 16(8):1030. https://doi.org/10.3390/polym16081030
Chicago/Turabian StyleAguirresarobe, Robert, Itxaso Calafel, Sara Villanueva, Alberto Sanchez, Amaia Agirre, Itxaro Sukia, Aritz Esnaola, and Ainara Saralegi. 2024. "Development of Flame-Retardant Polylactic Acid Formulations for Additive Manufacturing" Polymers 16, no. 8: 1030. https://doi.org/10.3390/polym16081030