Recycled PET/PA6 Fibers from Waste Textile with Improved Hydrophilicity by In-Situ Reaction-Induced Capacity Enhancement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
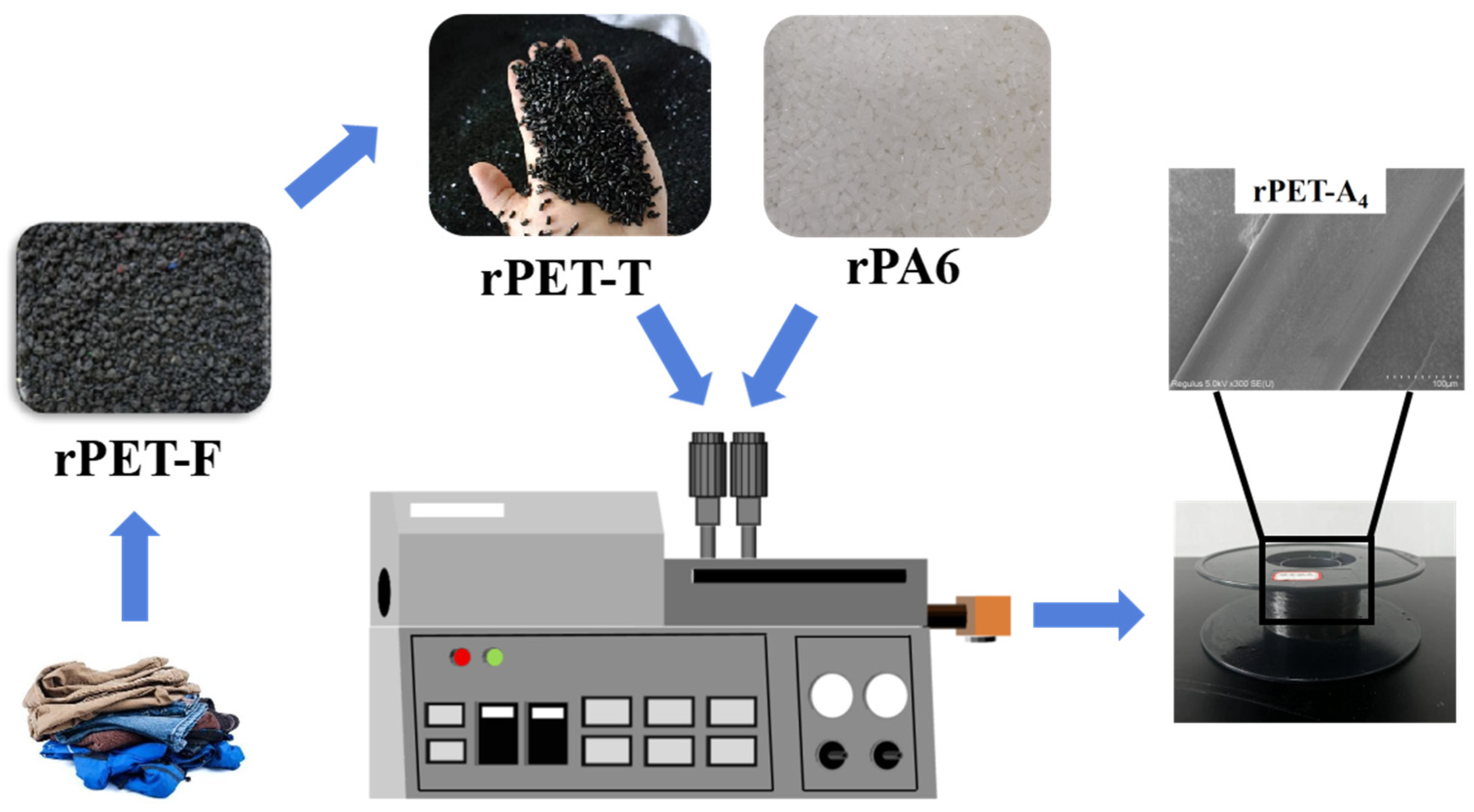
2.2. Preparation of Polymer Blends and Fibers
2.3. Characterizations
3. Results
3.1. Mechanical Properties of rPET-Ax Fibers
3.2. Hydrophilic Properties of the rPET-Ax Fibers
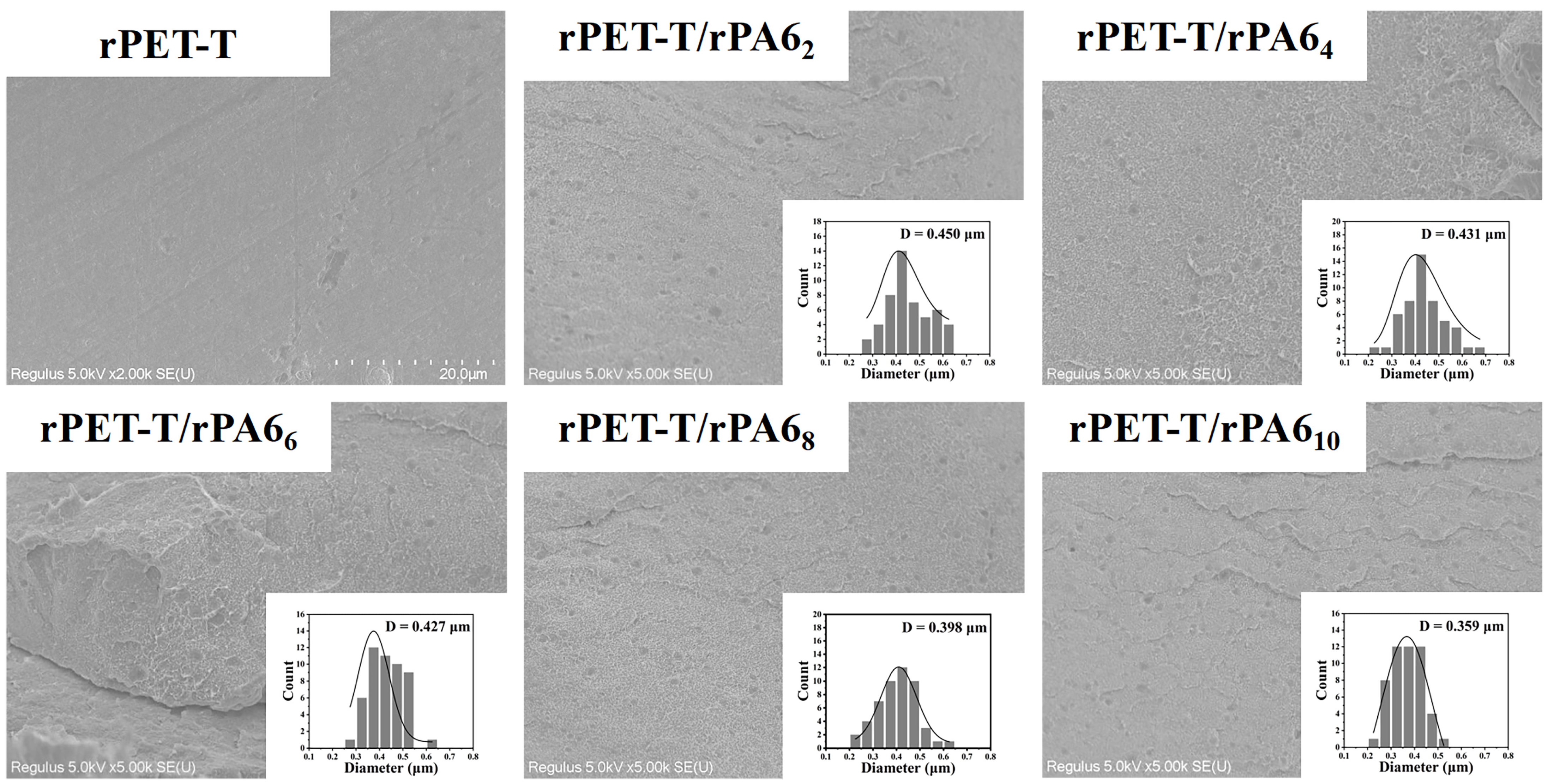
3.3. Phase Morpchology of rPET-T/rPA6 Blends
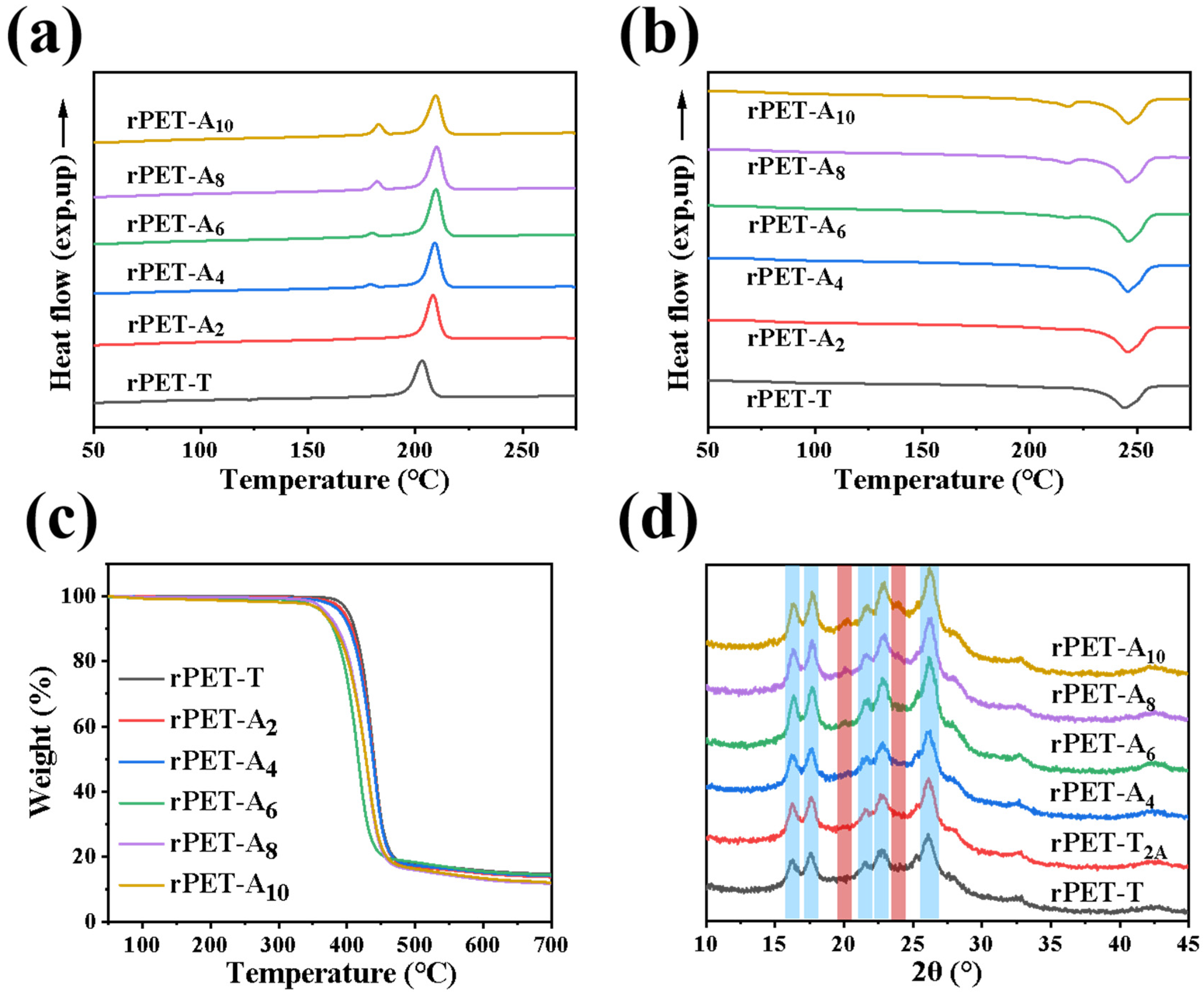
3.4. The Thermal and Crystallization Properties of rPET-Ax Fibers
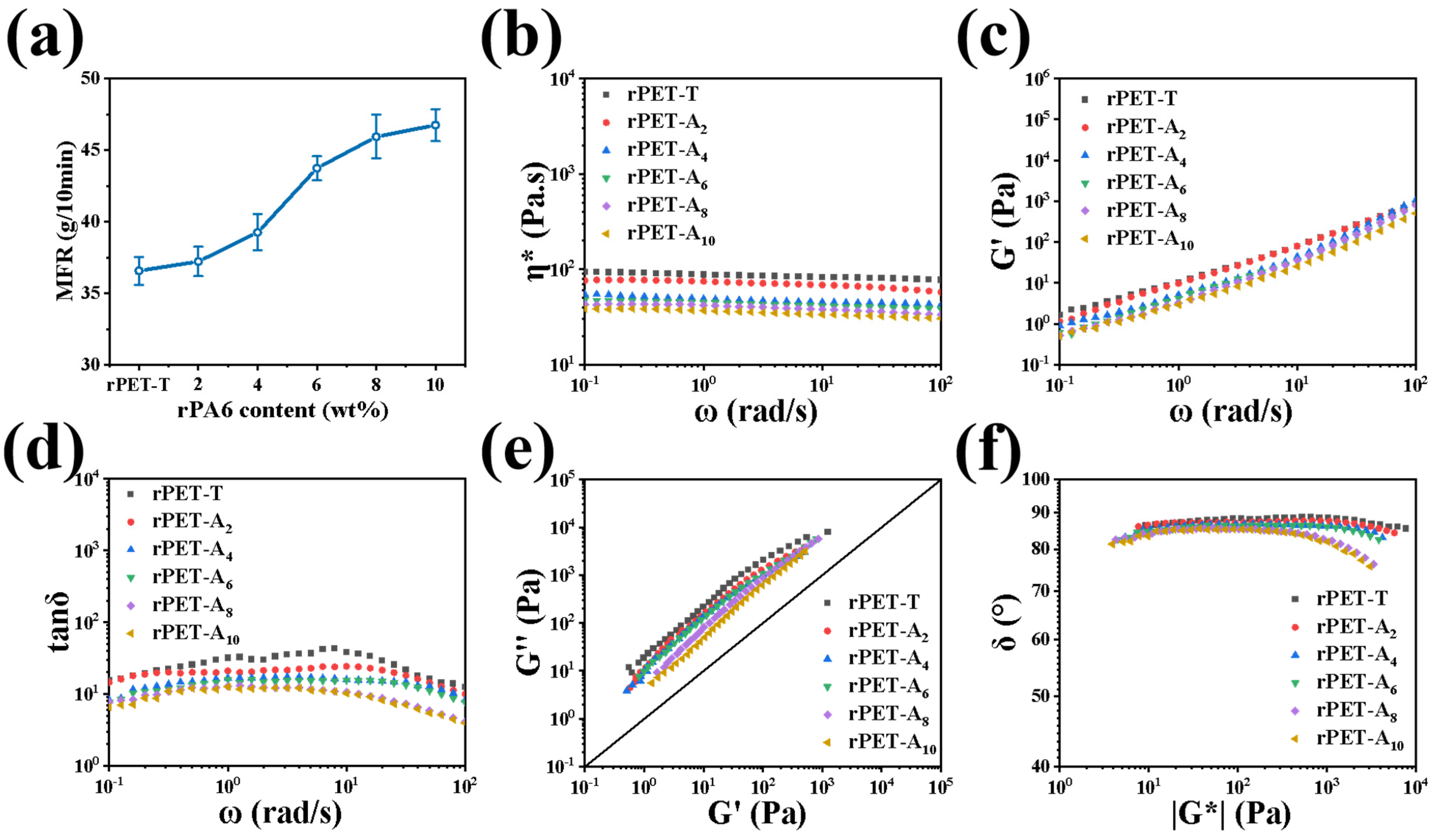
3.5. Rheological Properties of rPET-Ax Fibers Melts
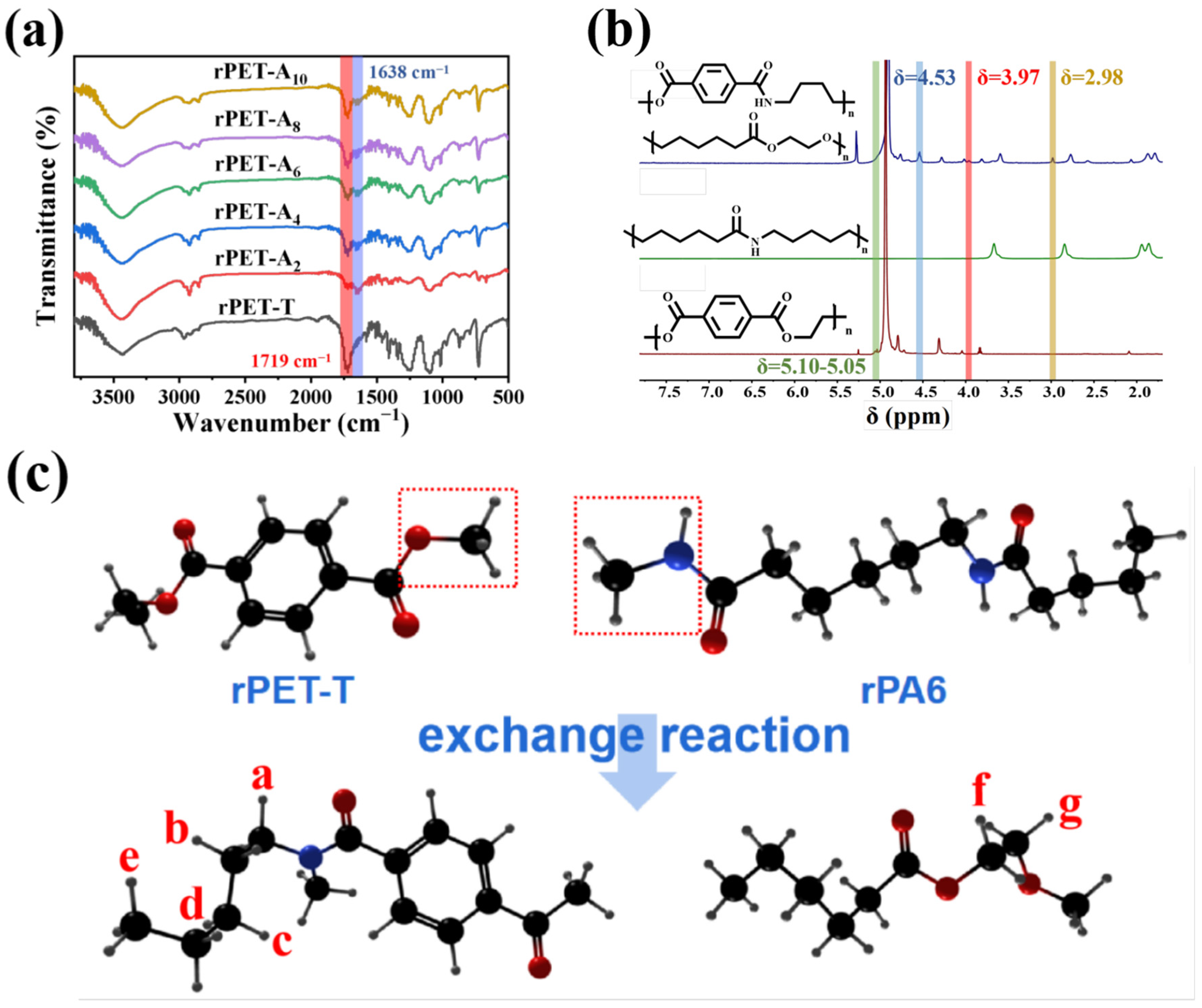
3.6. Chemical Structure of rPET−Ax Fibers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Biessey, P.; Vogel, J.; Seitz, M.; Quicker, P. Plastic Waste Utilization via Chemical Recycling: Approaches, Limitations, and the Challenges Ahead. Chem. Ing. Tech. 2023, 95, 1199–1214. [Google Scholar] [CrossRef]
- Damayanti, D.; Saputri, D.R.; Marpaung, D.S.S.; Yusupandi, F.; Sanjaya, A.; Simbolon, Y.M.; Asmarani, W.; Ulfa, M.; Wu, H.-S. Current Prospects for Plastic Waste Treatment. Polymers 2022, 14, 3133. [Google Scholar] [CrossRef] [PubMed]
- Estahbanati, M.R.K.; Kong, X.Y.; Eslami, A.; Soo, H.S. Current Developments in the Chemical Upcycling of Waste Plastics Using Alternative Energy Sources. Chemsuschem 2021, 14, 4152–4166. [Google Scholar] [CrossRef] [PubMed]
- Korley, L.T.J.; Epps, T.H.; Helm, B.A.; Ryan, A.J. Toward Polymer Upcycling-Adding Value and Tackling Circularity. Science 2021, 373, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, I.; Yoshida, S.; Hiraga, K.; Miyamoto, K.; Kimura, Y.; Oda, K. Biodegradation of PET: Current Status and Application Aspects. Acs Catal. 2019, 9, 4089–4105. [Google Scholar] [CrossRef]
- Ghisellini, P.; Cialani, C.; Ulgiati, S. A Review on Circular Economy: The Expected Transition to a Balanced Interplay of Environmental and Economic Systems. J. Clean. Prod. 2016, 114, 11–32. [Google Scholar] [CrossRef]
- Aizenshtein, E.M. Polyester Fibres in the Post-Crisis Period. Fibre Chem. 2011, 42, 341–349. [Google Scholar] [CrossRef]
- Damayanti, D.; Wulandari, L.A.; Bagaskoro, A.; Rianjanu, A.; Wu, H.-S. Possibility Routes for Textile Recycling Technology. Polymers 2021, 13, 3834. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, Q.; Pan, W.; Xiang, H.; Hu, Z.; Zhu, M. Thermal Stability of Bio-Based Aliphatic-Semiaromatic Copolyester for Melt-Spun Fibers with Excellent Mechanical Properties. Macromol. Rapid Commun. 2021, 42, 498. [Google Scholar] [CrossRef]
- Lin, X.; Qian, Q.; Xiao, L.; Chen, Q.; Huang, Q.; Zhang, H. Influence of Reactive Compatibilizer on the Morphology, Rheological, and Mechanical Properties of Recycled Poly(Ethylene Terephthalate)/Polyamide 6 Blends. J. Macromol. Sci. Part B-Phys. 2014, 53, 1543–1552. [Google Scholar] [CrossRef]
- Chen, H.; Guo, M.; Schiraldi, D.; Maia, J.M. Morphology optimization of poly(ethylene terephthalate)/polyamide blends compatibilized via extension-dominated twin-screw extrusion. J. Polym. Eng. 2021, 41, 218–225. [Google Scholar] [CrossRef]
- Li, C.; Xiao, Y.; Guan, G.; Liu, X.; Zhang, D. Preparation and Properties of PET/PA6 Copolymer/Montmorillonite Hybrid Nanocomposite. J. Appl. Polym. Sci. 2006, 101, 2512–2517. [Google Scholar] [CrossRef]
- Aldas, M.; Pavon, C.; Rosa-Ramírez, H.D.L.; Ferri, J.M.; Bertomeu, D.; Samper, M.D.; López-Martínez, J. The Impact of Biodegradable Plastics in the Properties of Recycled Polyethylene Terephthalate. J. Polym. Environ. 2021, 29, 2686–2700. [Google Scholar] [CrossRef]
- Wu, W.J.; Sun, X.L.; Chen, Q.H.; Qian, Q.R. Recycled Poly(Ethylene Terephthalate) from Waste Textiles with Improved Thermal and Rheological Properties by Chain Extension. Polymers 2022, 14, 510. [Google Scholar] [CrossRef] [PubMed]
- Welle, F.; Bayer, F.; Franz, R. Quantification of the Sorption Behavior of Polyethylene Terephthalate Polymer versus PET/PA Polymer Blends towards Organic Compounds. Packag. Technol. Sci. 2012, 25, 341–349. [Google Scholar] [CrossRef]
- Evstatiev, M.; Schultz, J.M.; Petrovich, S.; Georgiev, G.; Fakirov, S.; Friedrich, K. In situ polymer/polymer composites from poly(ethylene terephthalate), polyamide-6, and polyamide-66 blends. J. Appl. Polym. Sci. 1998, 67, 723–737. [Google Scholar]
- Huang, Y.Q.; Liu, Y.X.; Zhao, C.H. Morphology and properties of PET/PA-6/E-44 blends. J. Appl. Polym. Sci. 1998, 69, 1505–1515. [Google Scholar] [CrossRef]
- Qu, C.; Su, R.; Zhang, Q.; Du, R.N.; Fu, Q. Effect of ethylene-acrylate-(maleic anhydride) terpolymer on mechanical properties and morphology of poly(ethylene terephthalate)/polyamide-6 blends. Polym. Int. 2008, 57, 139–148. [Google Scholar] [CrossRef]
- Evstatiev, M.; Fakirov, S.; Schultz, J.M.; Friedrich, K.I. In Situ Fibrillar Reinforced PET/PA-6/PA-66 Blend(Statistical Data Included). Polym. Eng. Sci. 2001, 41, 192–204. [Google Scholar] [CrossRef]
- Tapia-Picazo, J.C.; Luna-Barcenas, J.G.; García-Chávez, A.; Gonzalez-Nuñez, R.; Bonilla-Petriciolet, A.; Alvarez-Castillo, A. Polyester Fiber Production Using Virgin and Recycled PET. Fibers Polym. 2014, 15, 547–552. [Google Scholar] [CrossRef]
- He, X.D.; Xiao, B.; Cai, G.B.; Takahashi, J. Morphological, Physicochemical, and Flexural Characterization of Carbon Fiber Paper-Reinforced Polyamide 6 for Long-Term Application in Aqueous Environments. J. Polym. Res. 2021, 28, 362. [Google Scholar] [CrossRef]
- Hale, W.; Lee, J.H.; Keskkula, H.; Paul, D.R. Effect of PBT Melt Viscosity on the Morphology and Mechanical Properties of Compatibilized and Uncompatibilized Blends with ABS. Polymer 1999, 40, 3621–3629. [Google Scholar] [CrossRef]
- Liu, Y.J.; Wei, M.H.; Liu, X.L.; Sheng, S.R. Influence of the Matrix Molecular Weight on the Morphology and Properties of Poly(Ethylene Terephthalate)/Poly(Ethylene-Octene) Blends. J. Macromol. Sci. Part B-Phys. 2022, 61, 871–881. [Google Scholar] [CrossRef]
- Mehmet, K. Polypropylene/Polyamide 6/Poss Ternary Nanocomposites: Effects of Poss Nanoparticles on the Compatibility. Polymer 2016, 105, 43–50. [Google Scholar]
- Motamedi, P.; Bagheri, R. Modification of Nanostructure and Improvement of Mechanical Properties of Polypropylene/Polyamide 6/Layered Silicate Ternary Nanocomposites through Variation of Processing Route. Compos. Part B-Eng. 2016, 85, 207–215. [Google Scholar] [CrossRef]
- Aparna, S.; Doddipatla, P. Influence of PP Content on Mechanical Properties, Water Absorption, and Morphology in PA6/PP Blend. J. Appl. Polym. Sci. 2019, 136, 47690. [Google Scholar]
- Wang, X.C.; Zheng, Q.; Yang, G. Crystalline Morphology and Crystallization Characteristics of In-situ Blends of Anionic Polyamide 6 with Noncrystallizable Semiaromatic Polyamide Copolymer. Chem. Res. Chin. Univ. 2007, 23, 360–365. [Google Scholar] [CrossRef]
- Mukherjee, M.; Das, C.K.; Kharitonov, A.P.; Banik, K.; Mennig, G.; Chung, T.N. Properties of syndiotactic polystyrene composites with surface modified short Kevlar fiber. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2006, 441, 206–214. [Google Scholar] [CrossRef]
- Su, K.H.; Lin, J.H.; Lin, C.C. Influence of Reprocessing on the Mechanical Properties and Structure of Polyamide 6. J. Mater. Process. Technol. 2007, 192–193, 532–538. [Google Scholar] [CrossRef]
- Hussein, I.A. Influence of Composition Distribution and Branch Content on the Miscibility of M-LLDPE and Hdpe Blends: Rheological Investigation. Macromolecules 2003, 36, 2024–2031. [Google Scholar] [CrossRef]
- Chen, J.; Wei, W.; Qian, Q.R.; Xiao, L.R.; Liu, X.P.; Xu, J.; Huang, B.Q.; Chen, Q.H. The Structure and Properties of Long-Chain Branching Poly(Trimethylene Terephthalate). Rheol. Acta 2014, 53, 67–74. [Google Scholar] [CrossRef]
- Tian, J.H.; Yu, W.; Zhou, C.X. The Preparation and Rheology Characterization of Long Chain Branching Polypropylene (Vol 47, Pg 7962, 2006). Polymer 2007, 48, 2186. [Google Scholar] [CrossRef]
- Vega, J.F.; Santamaria, A.; Munoz-Escalona, A.; Lafuente, P. Small-amplitude oscillatory shear flow measurements as a tool to detect very low amounts of long chain branching in polyethylenes. Macromolecules 1998, 31, 3639–3647. [Google Scholar] [CrossRef]
- Tapia, J.J.B.; Tenorio-Lopez, J.A.; Martinez-Estrada, A.; Guerrero-Sanchez, C. Application of Raft-Synthesized Reactive Tri-Block Copolymers for the Recycling of Post-Consumer R-PET by Melt Processing. Mater. Chem. Phys. 2019, 229, 474–481. [Google Scholar] [CrossRef]
- Trinkle, S.; Walter, P.; Friedrich, C. Van Gurp-Palmen Plot II—Classification of long chain branched polymers by their topology. Rheol. Acta 2002, 41, 103–113. [Google Scholar] [CrossRef]



| Sample | rPET-T(wt%) | rPA6(wt%) | Drafting Condition |
|---|---|---|---|
| rPET−A2 | 98 | 2 | Hot draft temperature: 100 °C Draft multiple: 3 |
| rPET−A4 | 96 | 4 | |
| rPET−A6 | 94 | 6 | |
| rPET−A8 | 92 | 8 | |
| rPET−A10 | 90 | 10 | |
| rPET-T/rPA62 | 98 | 2 | Without drafting |
| rPET-T/rPA64 | 96 | 4 | |
| rPET-T/rPA66 | 94 | 6 | |
| rPET-T/rPA68 | 92 | 8 | |
| rPET-T/rPA610 | 90 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, L.-B.; Chen, R.; Lian, Y.-X.; Wu, W.-J.; Zhang, J.-H.; Fu, C.-X.; Sun, X.-L.; Xiao, L.-R. Recycled PET/PA6 Fibers from Waste Textile with Improved Hydrophilicity by In-Situ Reaction-Induced Capacity Enhancement. Polymers 2024, 16, 1052. https://doi.org/10.3390/polym16081052
Luo L-B, Chen R, Lian Y-X, Wu W-J, Zhang J-H, Fu C-X, Sun X-L, Xiao L-R. Recycled PET/PA6 Fibers from Waste Textile with Improved Hydrophilicity by In-Situ Reaction-Induced Capacity Enhancement. Polymers. 2024; 16(8):1052. https://doi.org/10.3390/polym16081052
Chicago/Turabian StyleLuo, Li-Bin, Rong Chen, Yu-Xin Lian, Wen-Jun Wu, Jia-Hong Zhang, Chong-Xian Fu, Xiao-Li Sun, and Li-Ren Xiao. 2024. "Recycled PET/PA6 Fibers from Waste Textile with Improved Hydrophilicity by In-Situ Reaction-Induced Capacity Enhancement" Polymers 16, no. 8: 1052. https://doi.org/10.3390/polym16081052







