Preparation of Activated Carbon-Reinforced Composite Beads Based on MnO2/MCM-41@Fe3O4 and Calcium Alginate for Efficient Removal of Tetracycline in Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Camellia oleifera Shell-Activated Carbon (COFAC)
2.2. Synthesis of MnO2/MCM-41@Fe3O4 Composites
2.3. Preparation of COFAC–FeMnMCM–ALG Composite Beads
2.4. Characterization and Analytical Methods
2.5. Catalytic TC Degradation Experiments
2.6. Catalyst Reuse
3. Results and Discussion
3.1. Characterization of Catalysts
3.2. Effectiveness of As-Prepared Catalysts for TC Removal
3.3. Effects of Process Parameters on TC Degradation
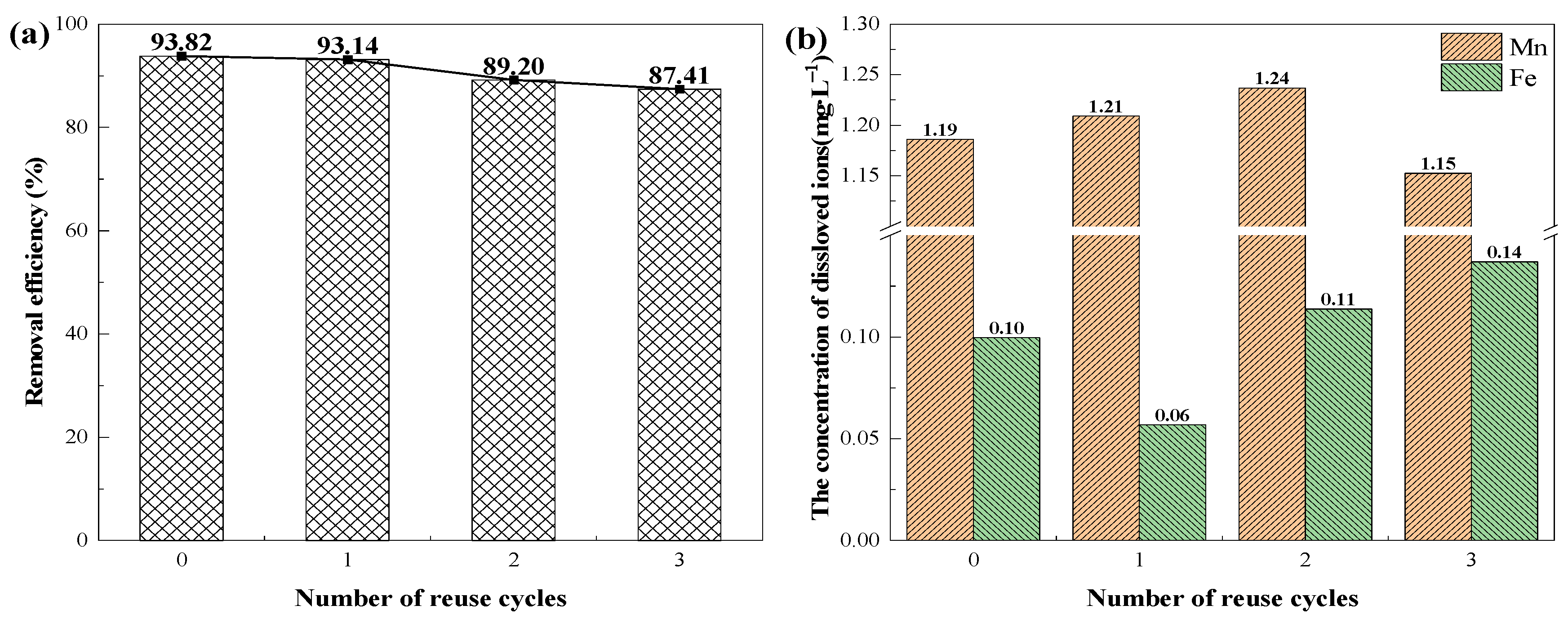
3.4. Catalyst Reuse
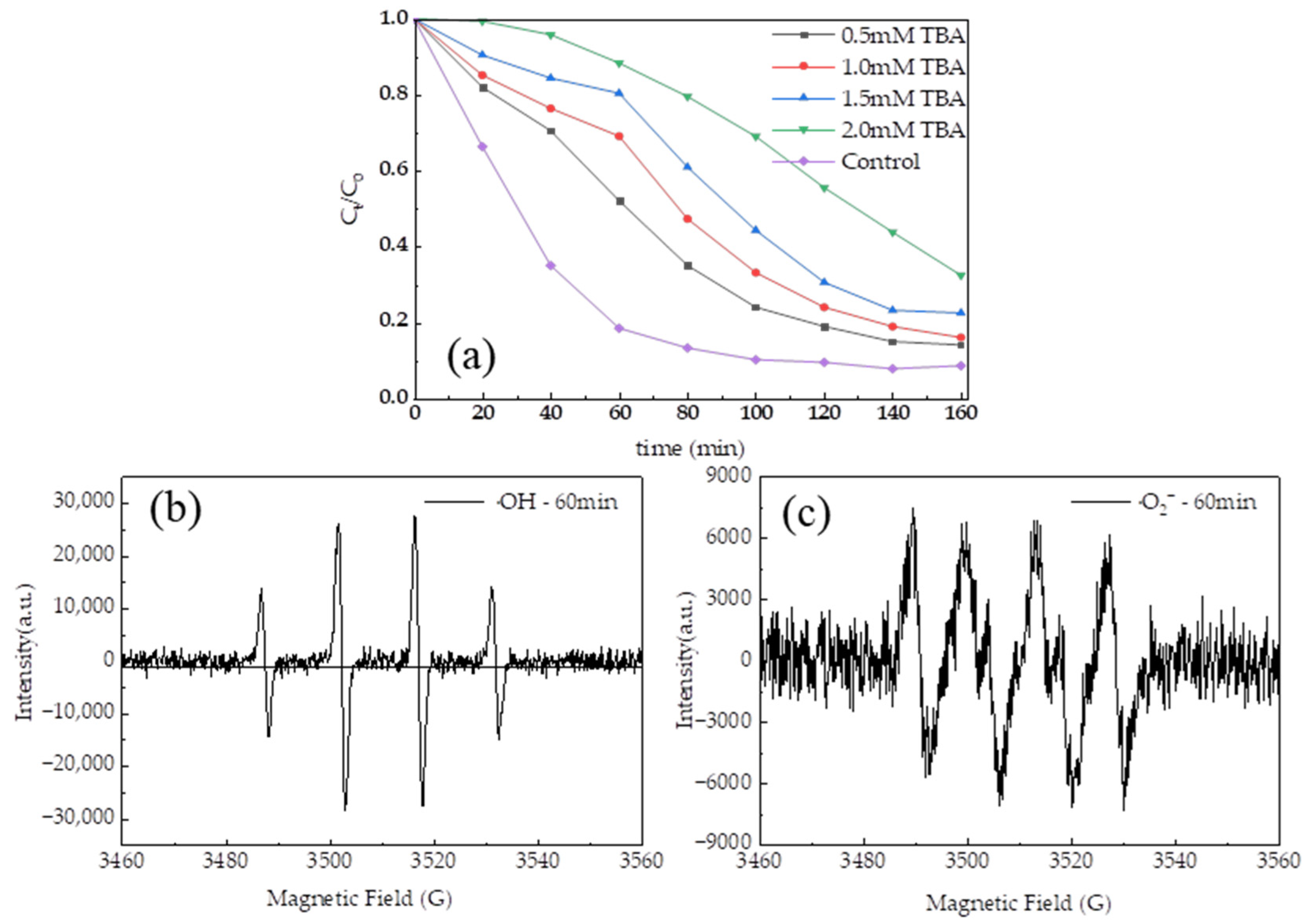
3.5. Identification of Hydroxyl Radical as the Main Reactive Species
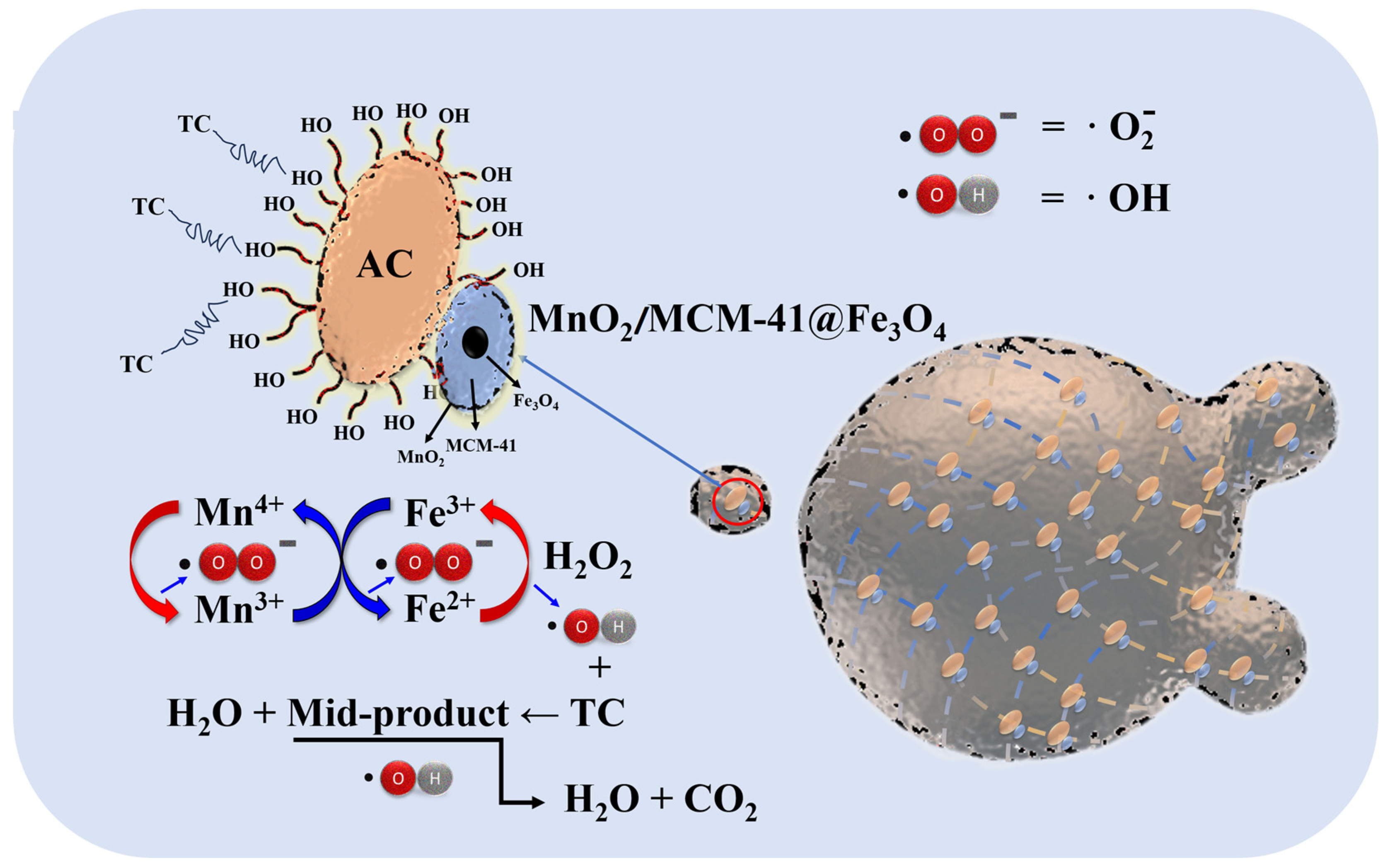
3.6. Proposed Mechanism for the Catalytic Degradation of TC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeong, J.; Song, W.; Cooper, W.J.; Jung, J.; Greaves, J. Degradation of tetracycline antibiotics: Mechanisms and kinetic studies for advanced oxidation/reduction processes. Chemosphere 2010, 78, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.T.; Santos, L. Antibiotics in the aquatic environments: A review of the European scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef] [PubMed]
- Leichtweis, J.; Vieira, Y.; Welter, N.; Silvestri, S.; Dotto, G.L.; Carissimi, E. A review of the occurrence, disposal, determination, toxicity and remediation technologies of the tetracycline antibiotic. Process Saf. Environ. Prot. 2022, 160, 25–40. [Google Scholar] [CrossRef]
- Lin, J.; Nishino, K.; Roberts, M.C.; Tolmasky, M.; Aminov, R.I.; Zhang, L. Mechanisms of antibiotic resistance. Front. Microbiol. 2015, 6, 481–511. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, E.L.; Jones, A.L. Distribution of tetracycline resistance genes and transposons among phylloplane bacteria in Michigan apple orchards. Appl. Environ. Microbiol. 1999, 65, 4898–4907. [Google Scholar] [CrossRef]
- Khan, S.N.; Khan, A.U. Breaking the spell: Combating multidrug resistant ‘Superbugs’. Front. Microbiol. 2016, 7, 174662. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Chen, W.; Duan, L.; Zhu, D. Mechanisms for strong adsorption of tetracycline to carbon nanotubes: A comparative study using activated carbon and graphite as adsorbents. Environ. Sci. Technol. 2009, 43, 2322–2327. [Google Scholar] [CrossRef] [PubMed]
- Aldahash, S.A.; Siddiqui, S.; Uddin, M.K. Eco-friendly synthesis of copper nanoparticles from fiber of Trapa natans L. shells and their impregnation onto polyamide-12 for environmental applications. J. Nat. Fibers 2023, 20, 2224976. [Google Scholar] [CrossRef]
- Shan, H.; Si, Y.; Yu, J.; Ding, B. Facile access to highly flexible and mesoporous structured silica fibrous membranes for tetracyclines removal. Chem. Eng. J. 2021, 417, 129211. [Google Scholar] [CrossRef]
- Gómez-Pacheco, C.V.; Sánchez-Polo, M.; Rivera-Utrilla, J.; López-Peñalver, J. Tetracycline removal from waters by integrated technologies based on ozonation and biodegradation. Chem. Eng. J. 2011, 178, 115–121. [Google Scholar] [CrossRef]
- Choudhary, V.; Vellingiri, K.; Thayyil, M.I.; Philip, L. Removal of antibiotics from aqueous solutions by electrocatalytic degradation. Environ. Sci. Nano 2021, 8, 1133–1176. [Google Scholar] [CrossRef]
- Zhi, D.; Wang, J.; Zhou, Y.; Luo, Z.; Sun, Y.; Wan, Z.; Luo, L.; Tsang, D.C.W.; Dionysiou, D.D. Development of ozonation and reactive electrochemical membrane coupled process: Enhanced tetracycline mineralization and toxicity reduction. Chem. Eng. J. 2020, 383, 123149. [Google Scholar] [CrossRef]
- Li, X.; Cui, K.; Guo, Z.; Yang, T.; Cao, Y.; Xiang, Y.; Chen, H.; Xi, M. Heterogeneous Fenton-like degradation of tetracyclines using porous magnetic chitosan microspheres as an efficient catalyst compared with two preparation methods. Chem. Eng. J. 2020, 379, 122324. [Google Scholar] [CrossRef]
- Khedkar, C.V.; Khupse, N.D.; Thombare, B.R.; Dusane, P.R.; Lole, G.; Devan, R.S.; Deshpande, A.S.; Patil, S.I. Magnetically separable Ag-Fe3O4 catalyst for the reduction of organic dyes. Chem. Phys. Lett. 2020, 742, 137131. [Google Scholar] [CrossRef]
- Li, Y.; Lu, H.; Wang, Y.; Li, X. Deposition of Au nanoparticles on PDA-functionalized PVA beads as a recyclable catalyst for degradation of organic pollutants with NaBH4 in aqueous solution. J. Alloys Compd. 2019, 793, 115–126. [Google Scholar] [CrossRef]
- Hachemaoui, M.; Mokhtar, A.; Mekki, A.; Zaoui, F.; Abdelkrim, S.; Hacini, S.; Boukoussa, B. Composites beads based on Fe3O4@MCM-41 and calcium alginate for enhanced catalytic reduction of organic dyes. Int. J. Biol. Macromol. 2020, 164, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Shaker, M.; Elhamifar, D. Core-shell structured magnetic mesoporous silica supported Schiff-base/Pd: An efficacious and reusable nanocatalyst. New J. Chem. 2020, 44, 3445–3454. [Google Scholar] [CrossRef]
- Vahidian, M.; Elhamifar, D.; Shaker, M. Core-shell structured magnetic mesoporous silica-titania: A novel, powerful and recoverable nanocatalyst. Polyhedron 2020, 178, 114326. [Google Scholar] [CrossRef]
- Patiño Ruiz, D.; Sanchez Botero, L.; Hinestroza, J.; Herrera, A. Modification of cotton fibers with magnetite and magnetic core-shell mesoporous silica nanoparticles. Phys. Status Solidi A 2018, 215, 1800266. [Google Scholar] [CrossRef]
- Ghasemi, H.; Mozaffari, S.; Mousavi, S.H.; Aghabarari, B.; Abu-Zahra, N. Decolorization of wastewater by heterogeneous Fenton reaction using MnO2-Fe3O4/CuO hybrid catalysts. J. Environ. Chem. Eng. 2021, 9, 105091. [Google Scholar] [CrossRef]
- Lei, Y.; Chen, C.-S.; Tu, Y.-J.; Huang, Y.-H.; Zhang, H. Heterogeneous degradation of organic pollutants by persulfate activated by CuO-Fe3O4: Mechanism, stability, and effects of pH and bicarbonate Ions. Environ. Sci. Technol. 2015, 49, 6838–6845. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Wang, T.; Zhao, Y.; Song, T.; Zhang, L.; Cheng, X. Construction of layered hollow Fe3O4/Fe1−xS @MoS2 composite with enhanced photo-Fenton and adsorption performance. J. Environ. Chem. Eng. 2020, 8, 103762. [Google Scholar] [CrossRef]
- Ghasemi, H.; Aghabarari, B.; Alizadeh, M.; Khanlarkhani, A.; Abu-Zahra, N. High efficiency decolorization of wastewater by Fenton catalyst: Magnetic iron-copper hybrid oxides. J. Water Process Eng. 2020, 37, 101540. [Google Scholar] [CrossRef]
- Li, Y.; Sun, J.; Sun, S.-P. Mn2+-mediated homogeneous Fenton-like reaction of Fe(III)-NTA complex for efficient degradation of organic contaminants under neutral conditions. J. Hazard. Mater. 2016, 313, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Nouri, L.; Hemidouche, S.; Boudjemaa, A.; Kaouah, F.; Sadaoui, Z.; Bachari, K. Elaboration and characterization of photobiocomposite beads, based on titanium (IV) oxide and sodium alginate biopolymer, for basic blue 41 adsorption/photocatalytic degradation. Int. J. Biol. Macromol. 2020, 151, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Benhouria, A.; Islam, M.A.; Zaghouane-Boudiaf, H.; Boutahala, M.; Hameed, B.H. Calcium alginate-bentonite-activated carbon composite beads as highly effective adsorbent for methylene blue. Chem. Eng. J. 2015, 270, 621–630. [Google Scholar] [CrossRef]
- Marrakchi, F.; Fazeli Zafar, F.; Wei, M.; Wang, S. Cross-linked FeCl3-activated seaweed carbon/MCM-41/alginate hydrogel composite for effective biosorption of bisphenol A plasticizer and basic dye from aqueous solution. Bioresour. Technol. 2021, 331, 125046. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zhao, H.; Lin, X.; Yang, J.; Shi, R. Preparation of activated carbon from Camellia oleifera shell and its application to adsorption of hexavalent chromium from aqueous solution: Kinetics, equilibrium, and thermodynamics. Desalin. Water Treat. 2020, 198, 170–179. [Google Scholar] [CrossRef]
- Sharifi, N.; Nasiri, A.; Martínez, S.S.; Amiri, H. Synthesis of Fe3O4@activated carbon to treat metronidazole effluents by adsorption and heterogeneous Fenton with effluent bioassay. J. Photochem. Photobiol. A Chem. 2022, 427, 113845. [Google Scholar] [CrossRef]
- Wei, Y.; Han, B.; Hu, X.; Lin, Y.; Wang, X.; Deng, X. Synthesis of Fe3O4 Nanoparticles and their Magnetic Properties. Procedia Eng. 2012, 27, 632–637. [Google Scholar] [CrossRef]
- Khorshidi, A.; Shariati, S. Sulfuric acid functionalized MCM-41 coated on magnetite nanoparticles as a recyclable core–shell solid acid catalyst for three-component condensation of indoles, aldehydes and thiols. RSC Adv. 2014, 4, 41469–41475. [Google Scholar] [CrossRef]
- Ha, N.M.; Huong, T.T.; The Son, N. Synthesis of the MnO2-Fe3O4 catalyst support on amorphous silica: A new Fenton’s reagent in the degradation of the reactive blue-19 in aqueous solution. J. Environ. Sci. Health Part A 2023, 58, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Gong, T.; Yang, X.; Guo, Y. Interpenetrating network gels with tunable physical properties: Glucono-δ-lactone induced gelation of mixed Alg/gellan sol systems. Int. J. Biol. Macromol. 2020, 151, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhao, Y.; Li, Y.; Xu, J.; Fu, X.; Gao, X.; Mao, X.; Li, Z. UV-shielding alginate films crosslinked with Fe3+ containing EDTA. Carbohydr. Polym. 2020, 239, 115480. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yuan, H.; Chen, S.; Zheng, C.; Wu, X.; Li, Z.; Liang, C.; Dai, P.; Wang, Q.; Ma, X.; et al. Cost-effective, high-yield production of biotemplated catalytic tubular micromotors as self-propelled microcleaners for water treatment. ACS Appl. Mater. Interfaces 2021, 13, 31226–31235. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Zhang, J.; Li, X.; Hao, B.; Xu, F.; Liu, K. Self-assembled morphology-controlled hierarchical Fe3O4 @LDH for Cr(VI) removal. J. Environ. Chem. Eng. 2023, 11, 110129. [Google Scholar] [CrossRef]
- Liang, L.; Li, X.; Lin, Z.; Tian, C.; Guo, Y. The removal of Cd by sulfidated nanoscale zero-valent iron: The structural, chemical bonding evolution and the reaction kinetics. Chem. Eng. J. 2020, 382, 122933. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Macedo, J.D.S.; Da Costa Júnior, N.B.; Almeida, L.E.; Vieira, E.F.D.S.; Cestari, A.R.; Gimenez, I.D.F.; Villarreal Carreño, N.L.; Barreto, L.S. Kinetic and calorimetric study of the adsorption of dyes on mesoporous activated carbon prepared from coconut coir dust. J. Colloid Interface Sci. 2006, 298, 515–522. [Google Scholar] [CrossRef]
- Qin, Y.; Chai, B.; Wang, C.; Yan, J.; Fan, G.; Song, G. Removal of tetracycline onto KOH-activated biochar derived from rape straw: Affecting factors, mechanisms and reusability inspection. Colloids Surf. Physicochem. Eng. Aspects 2022, 640, 128466. [Google Scholar] [CrossRef]
- Ahmad, T.; Manzar, M.S.; Georgin, J.; Franco, D.S.P.; Khan, S.; Meili, L.; Ullah, N. Development of a new hyper crosslinked resin based on polyamine-isocyanurate for the efficient removal of endocrine disruptor bisphenol-A from water. J. Water Process Eng. 2023, 53, 103623. [Google Scholar] [CrossRef]
- Li, Y.; Lin, D.; Li, Y.; Jiang, P.; Fang, X.; Yu, B. Nonradical-dominated peroxymonosulfate activation through bimetallic Fe/Mn-loaded hydroxyl-rich biochar for efficient degradation of tetracycline. Nano Res. 2023, 16, 155–165. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, J. Degradation of sulfamethazine using Fe3O4-Mn3O4/reduced graphene oxide hybrid as Fenton-like catalyst. J. Hazard. Mater. 2017, 324, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Zhang, Q.; Zhang, C.; Wang, R.; Deng, R.; Luo, H.; Li, T.; Li, J.; Chen, S.; Liu, C. Mn doped magnetic biochar as persulfate activator for the degradation of tetracycline. Chem. Eng. J. 2020, 391, 123532. [Google Scholar] [CrossRef]
- Soumia, A.; Adel, M.; Amina, S.; Bouhadjar, B.; Amal, D.; Farouk, Z.; Abdelkader, B.; Mohamed, S. Fe3O4-alginate nanocomposite hydrogel beads material: One-pot preparation, release kinetics and antibacterial activity. Int. J. Biol. Macromol. 2020, 145, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, J.; Sun, Y.; Yu, F.; Ma, J. Ionically cross-linked sodium alginate/ĸ-carrageenan double-network gel beads with low-swelling, enhanced mechanical properties, and excellent adsorption performance. Chem. Eng. J. 2019, 372, 1091–1103. [Google Scholar] [CrossRef]
- Aichour, A.; Zaghouane-Boudiaf, H. Single and competitive adsorption studies of two cationic dyes from aqueous mediums onto cellulose-based modified citrus peels/calcium alginate composite. Int. J. Biol. Macromol. 2020, 154, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Fernandes, R.; de Moura, M.R.; Glenn, G.M.; Aouada, F.A. Thermal, microstructural, and spectroscopic analysis of Ca2+ alginate/clay nanocomposite hydrogel beads. J. Mol. Liq. 2018, 265, 327–336. [Google Scholar] [CrossRef]
- Wasilewska, M.; Deryło-Marczewska, A. Adsorption of non-steroidal anti-inflammatory drugs on alginate-carbon composites—Equilibrium and kinetics. Materials 2022, 15, 6049. [Google Scholar] [CrossRef]
- Naseem, K.; Begum, R.; Wu, W.; Irfan, A.; Al-Sehemi, A.G.; Farooqi, Z.H. Catalytic reduction of toxic dyes in the presence of silver nanoparticles impregnated core-shell composite microgels. J. Clean. Prod. 2019, 211, 855–864. [Google Scholar] [CrossRef]
- Baye, A.F.; Appiah-Ntiamoah, R.; Kim, H. Synergism of transition metal (Co, Ni, Fe, Mn) nanoparticles and “active support” Fe3O4@C for catalytic reduction of 4-nitrophenol. Sci. Total Environ. 2020, 712, 135492. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.-H.; Li, Z.; Jiang, W.-T.; Jean, J.-S. Adsorption and intercalation of tetracycline by swelling clay minerals. Appl. Clay Sci. 2009, 46, 27–36. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, J. Degradation of sulfamethazine antibiotics using Fe3O4–Mn3O4 nanocomposite as a Fenton-like catalyst. J. Chem. Technol. Biotechnol. 2017, 92, 874–883. [Google Scholar] [CrossRef]
- Gao, P.; Song, Y.; Hao, M.; Zhu, A.; Yang, H.; Yang, S. An effective and magnetic Fe2O3-ZrO2 catalyst for phenol degradation under neutral pH in the heterogeneous Fenton-like reaction. Sep. Purif. Technol. 2018, 201, 238–243. [Google Scholar] [CrossRef]
- Panjwani, M.K.; Wang, Q.; Ma, Y.; Lin, Y.; Xiao, F.; Yang, S. High degradation efficiency of sulfamethazine with the dual-reaction-center Fe-Mn-SiO2 Fenton-like nanocatalyst in a wide pH range. Environ. Sci. Nano 2021, 8, 2204–2213. [Google Scholar] [CrossRef]
- He, J.; Pei, B.; Deng, C.; Huang, D.; Lv, B.; Peng, C.; Luo, G.; Lin, J. A new catalyst CHCP-Fe2O3 for enhanced removal of tetracycline through the Fenton-like process: Economical synthesis, catalytic performance, and practicability. J. Water Process Eng. 2023, 51, 103481. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, D.; Li, W.; Chang, J.; Lin, Q.; Xu, D.; Ma, H. Degradation of methylene blue in a heterogeneous Fenton reaction catalyzed by chitosan crosslinked ferrous complex. J. Taiwan Inst. Chem. Eng. 2016, 67, 355–361. [Google Scholar] [CrossRef]
- Yang, X.; He, J.; Yang, Q.; Jiao, R.; Liao, G.; Wang, D. Cu(I)-doped Fe3O4 nanoparticles/porous C composite for enhanced H2O2 oxidation of carbamazepine. J. Colloid Interface Sci. 2019, 551, 16–25. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Zhao, Y.; Sun, Z.; Ma, J.; He, X. Novel application of MnO2-H2O2 system for highly efficient arsenic adsorption and oxidation. Sustainability 2023, 15, 9080. [Google Scholar] [CrossRef]
- Fang, Z.d.; Zhang, K.; Liu, J.; Fan, J.y.; Zhao, Z.w. Fenton-like oxidation of azo dye in aqueous solution using magnetic Fe3O4-MnO2 nanocomposites as catalysts. Water Sci. Eng. 2017, 10, 326–333. [Google Scholar] [CrossRef]
- Tang, X.; Huang, J.; Liu, K.; Feng, Q.; Li, Z.; Ao, M. Synthesis of magnetically separable MnO2/Fe3O4/silica nanofiber composite with enhanced Fenton-like catalytic activity for degradation of Acid Red 73. Surf. Coat. Technol. 2018, 354, 18–27. [Google Scholar] [CrossRef]
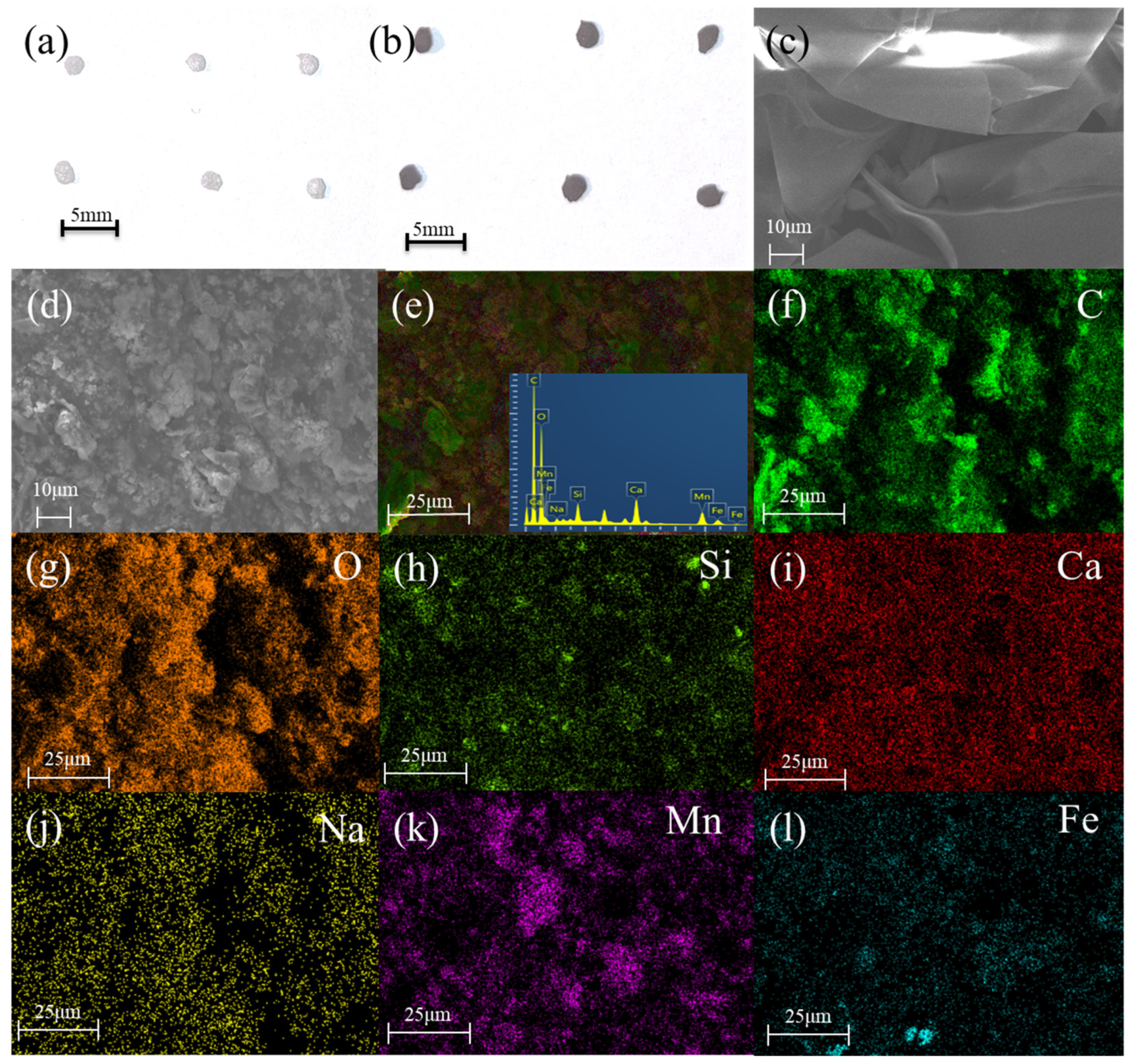
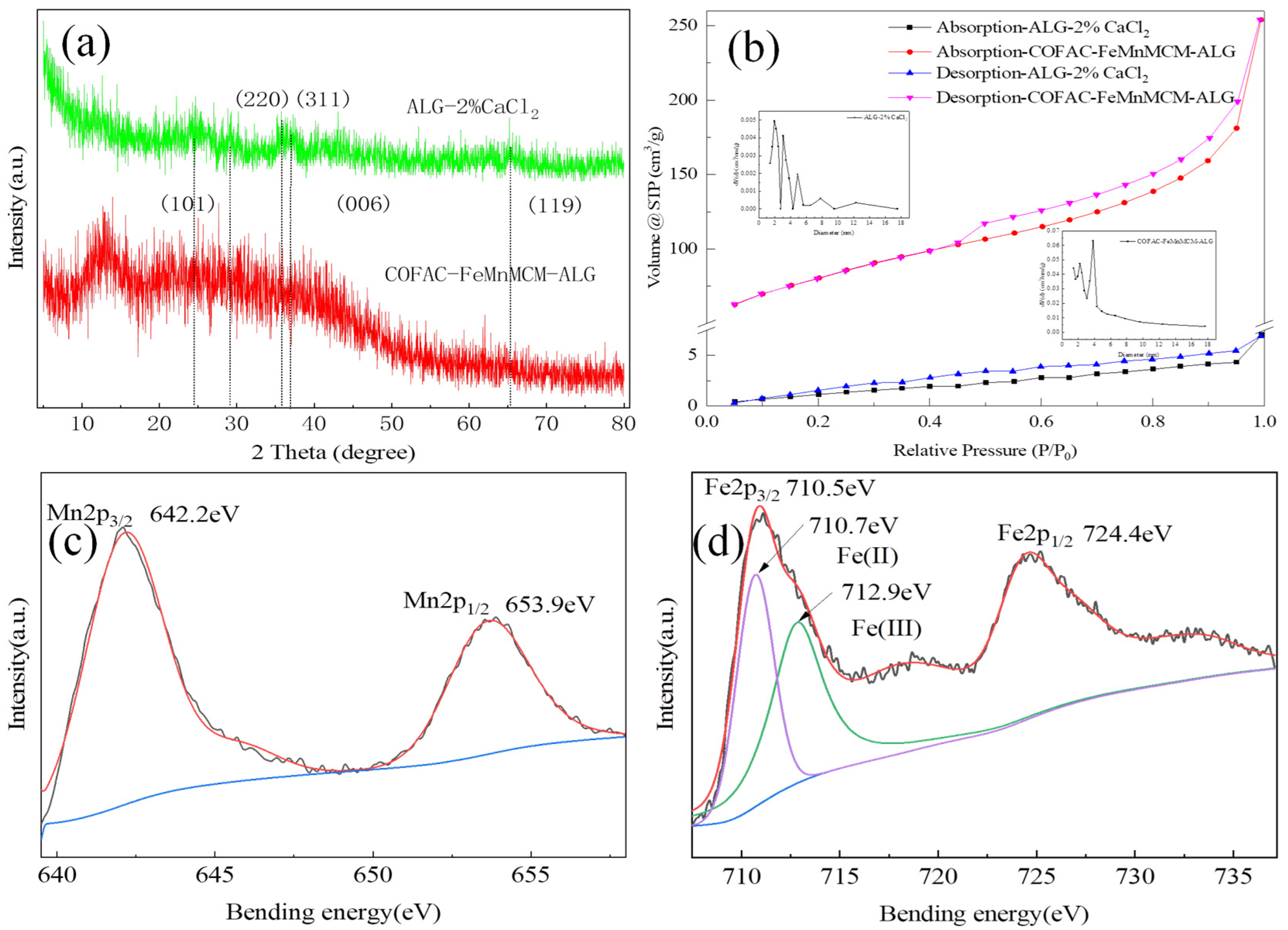




| Samples | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| ALG-2%CaCl2 | 6.458 | 0.01093 | 1.938 |
| COFAC | 1585.602 | 1.055 | 3.930 |
| MnO2/MCM-41@Fe3O4 | 81.338 | 0.6256 | 17.522 |
| COFAC–FeMnMCM–ALG | 280.154 | 0.3941 | 3.826 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Z.; Shi, R.; Zhang, X.; Ni, Y.; Zhang, H. Preparation of Activated Carbon-Reinforced Composite Beads Based on MnO2/MCM-41@Fe3O4 and Calcium Alginate for Efficient Removal of Tetracycline in Aqueous Solutions. Polymers 2024, 16, 1115. https://doi.org/10.3390/polym16081115
Zheng Z, Shi R, Zhang X, Ni Y, Zhang H. Preparation of Activated Carbon-Reinforced Composite Beads Based on MnO2/MCM-41@Fe3O4 and Calcium Alginate for Efficient Removal of Tetracycline in Aqueous Solutions. Polymers. 2024; 16(8):1115. https://doi.org/10.3390/polym16081115
Chicago/Turabian StyleZheng, Zhigong, Ronghui Shi, Xiaoping Zhang, Yonghao Ni, and Hui Zhang. 2024. "Preparation of Activated Carbon-Reinforced Composite Beads Based on MnO2/MCM-41@Fe3O4 and Calcium Alginate for Efficient Removal of Tetracycline in Aqueous Solutions" Polymers 16, no. 8: 1115. https://doi.org/10.3390/polym16081115
APA StyleZheng, Z., Shi, R., Zhang, X., Ni, Y., & Zhang, H. (2024). Preparation of Activated Carbon-Reinforced Composite Beads Based on MnO2/MCM-41@Fe3O4 and Calcium Alginate for Efficient Removal of Tetracycline in Aqueous Solutions. Polymers, 16(8), 1115. https://doi.org/10.3390/polym16081115






