Preparation of a Lignin-Based Cationic Flocculant and Its Application in Kaolin Suspension Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
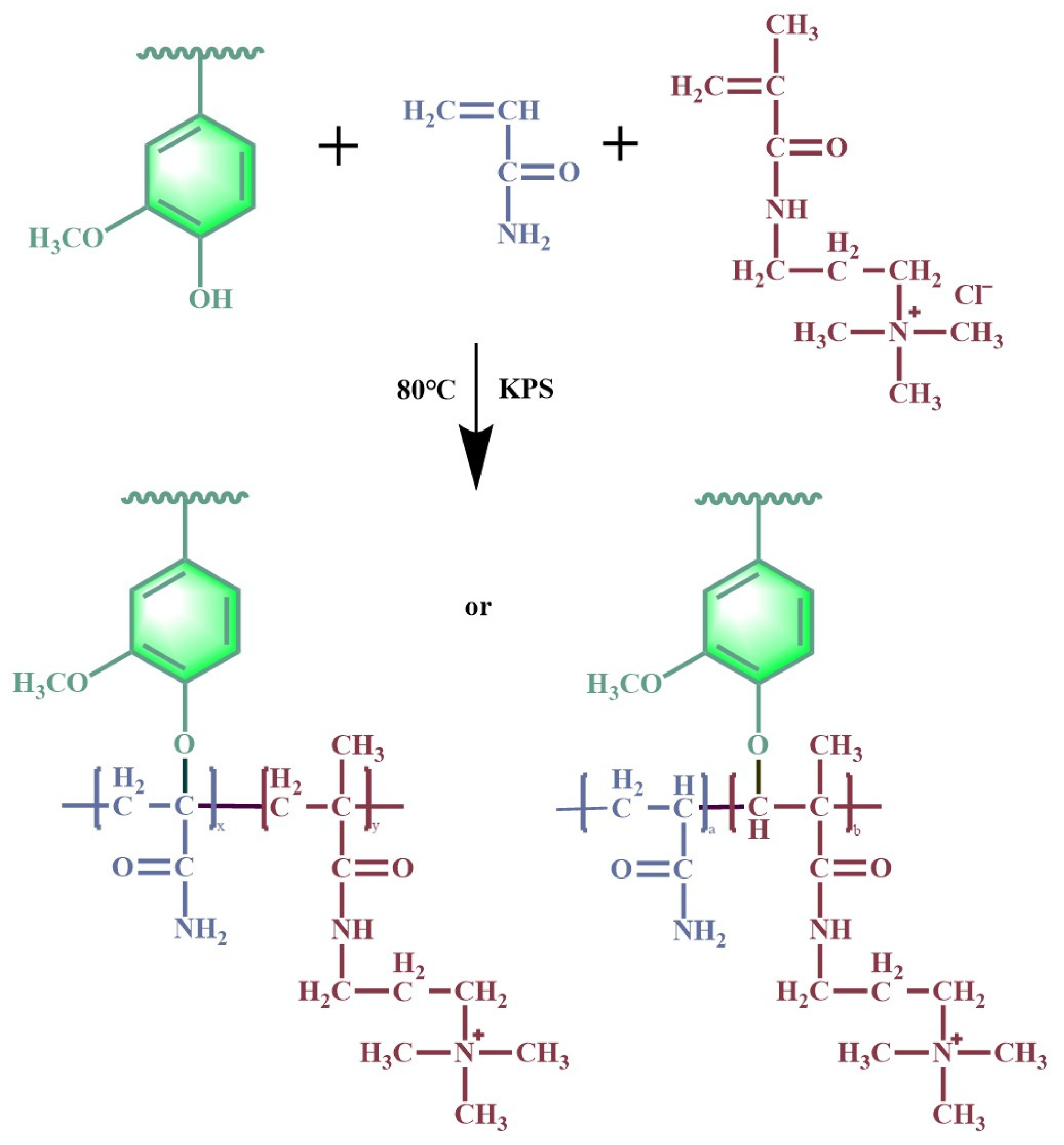
2.2. Preparation of AL-g-PAMA
2.3. Characterization of AL-g-PAMA
2.4. Grafting Efficiency of PAMA and Intrinsic Viscosity Determination
2.4.1. Determination of Grafting Efficiency
2.4.2. Determination of Intrinsic Viscosity
2.5. Flocculation-Sedimentation and Dewatering Experiments
2.5.1. Flocculation and Sedimentation Experiments
2.5.2. Dewatering Experiments
2.6. FBRM Test
3. Results and Discussion
3.1. Structural Characteristics of AL-g-PAMA
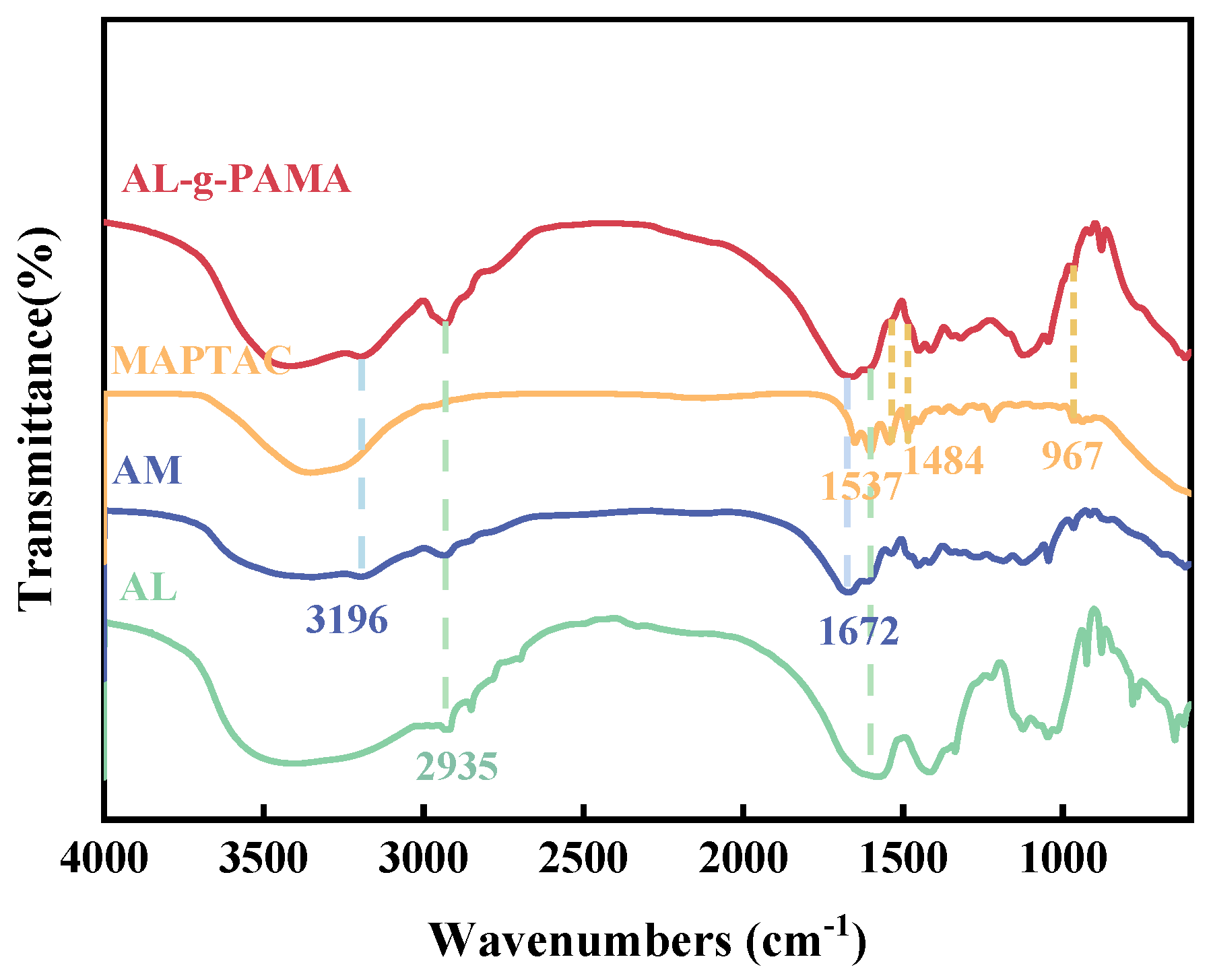
3.1.1. FTIR Analysis
3.1.2. 1H NMR Analysis
3.1.3. XRD Analysis
3.1.4. SEM Analysis
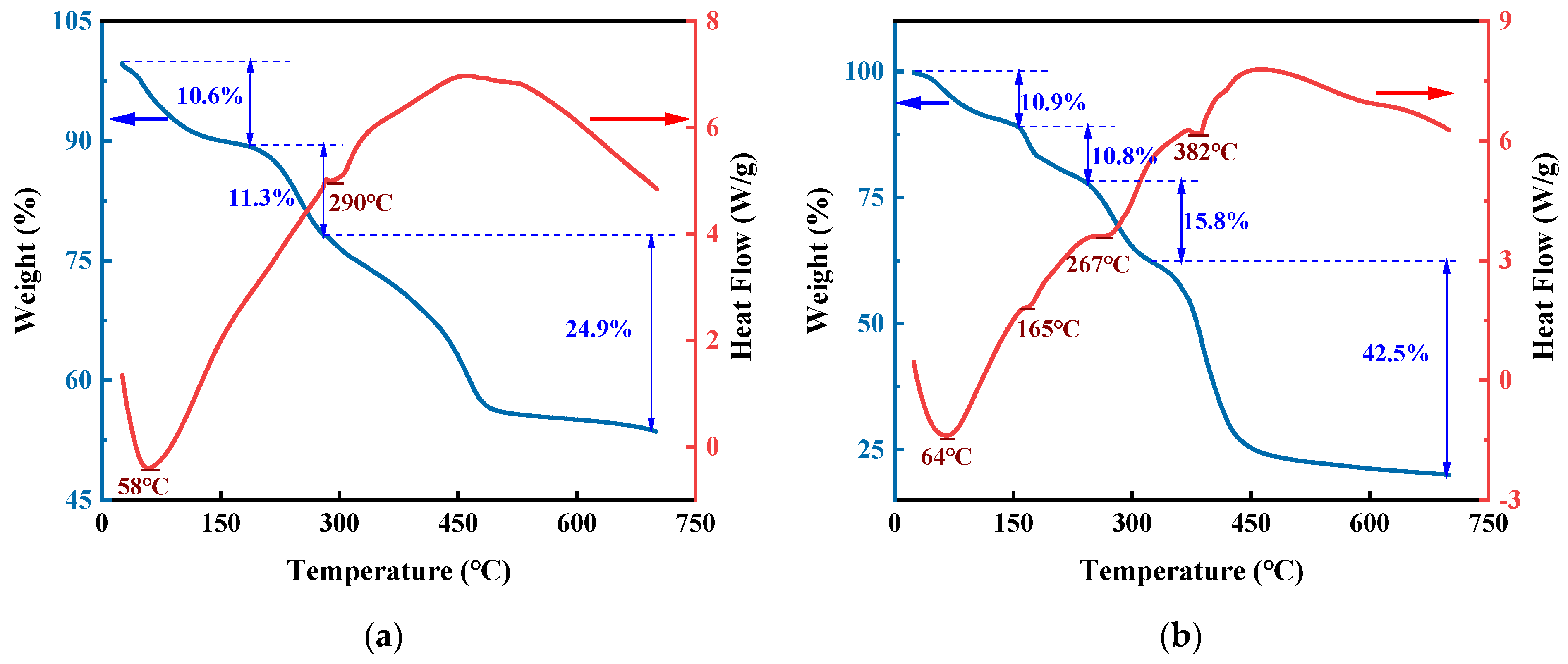
3.1.5. TG Analysis
3.2. Flocculation and Dewatering Performance
3.2.1. Effect of AL-g-PAMA Type on Flocculation Performance
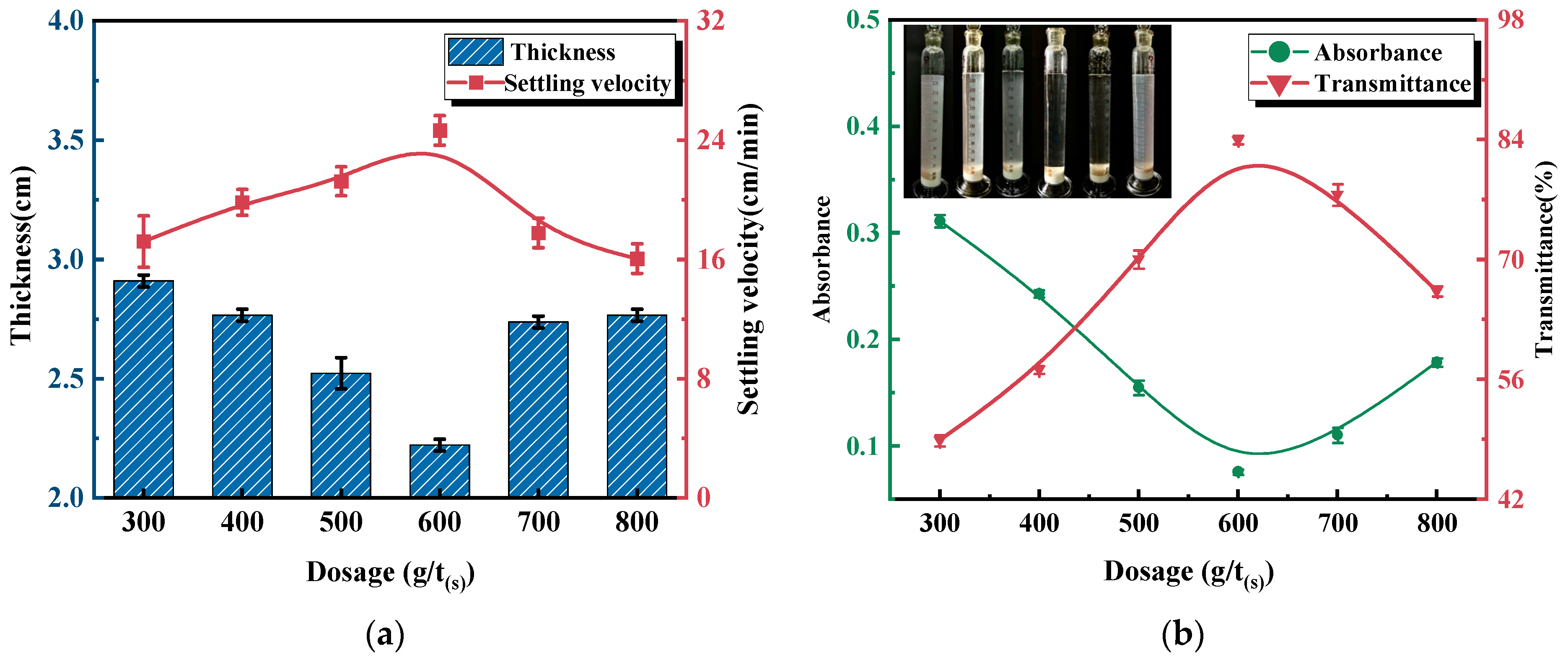
3.2.2. Effect of AL-g-PAMA #5 Dosage on Flocculation Performance
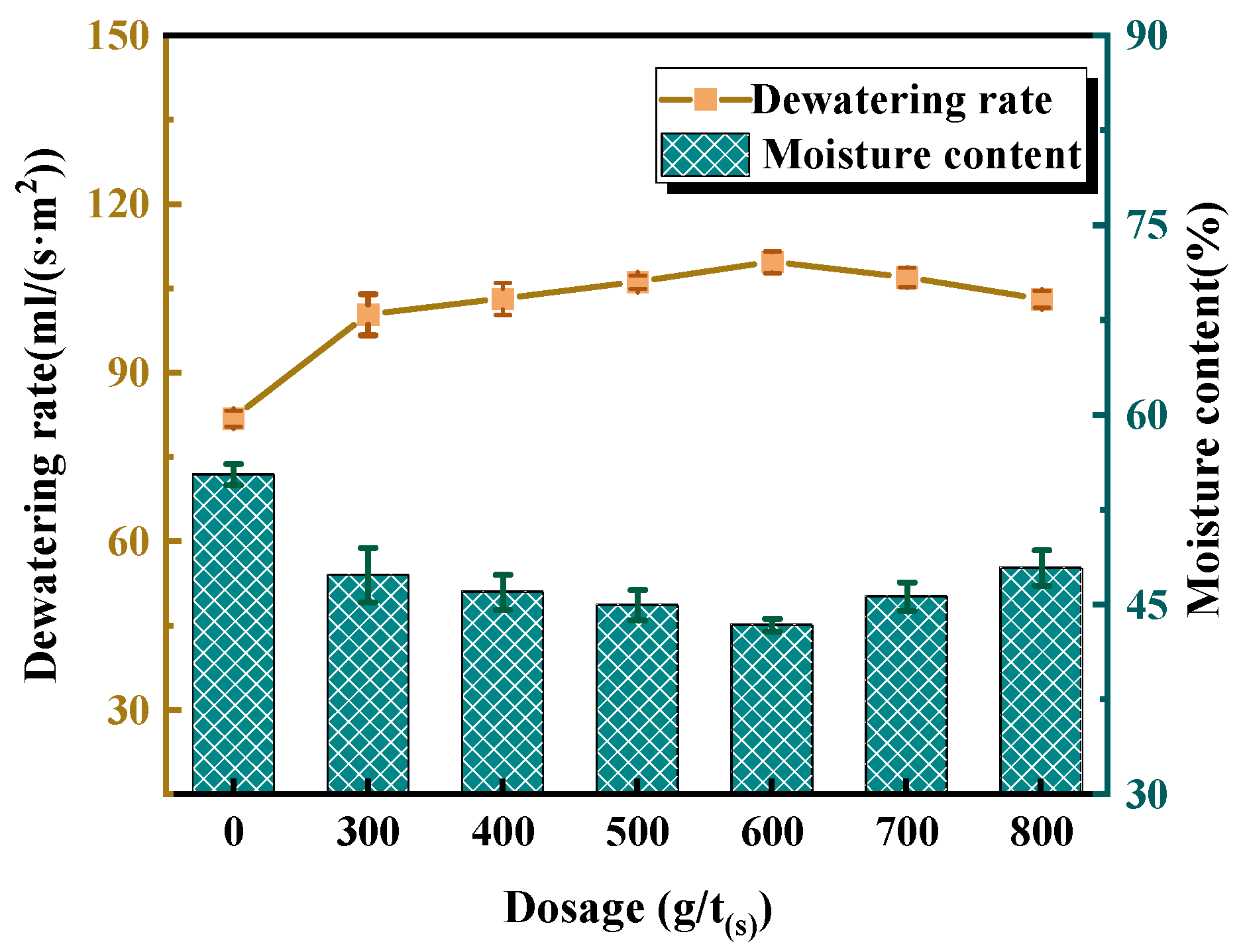
3.2.3. Effect of AL-g-PAMA #5 Dosage on Dewatering Performance
3.3. Interaction between AL-g-PAMA and Kaolin Particles
3.3.1. Zeta Potential Analysis
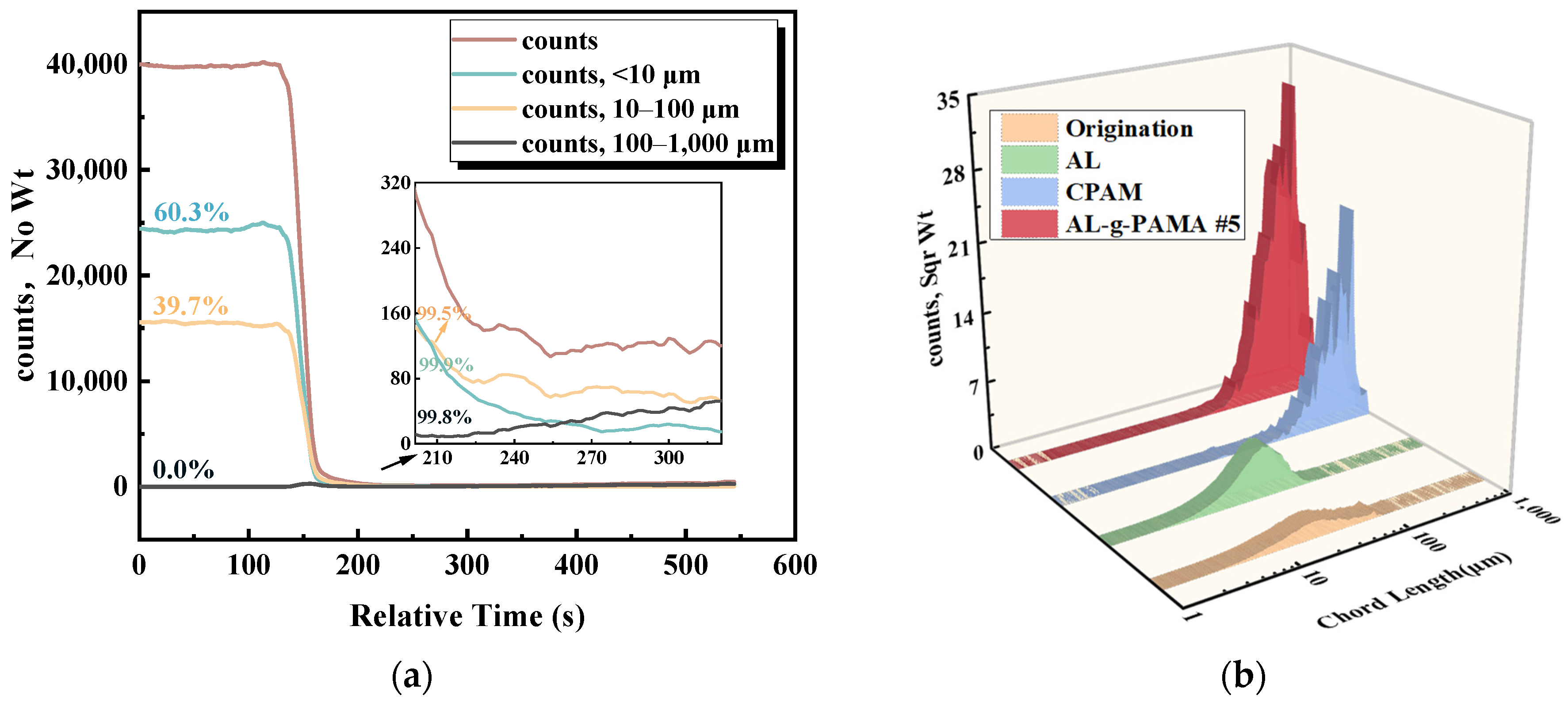
3.3.2. FBRM Results
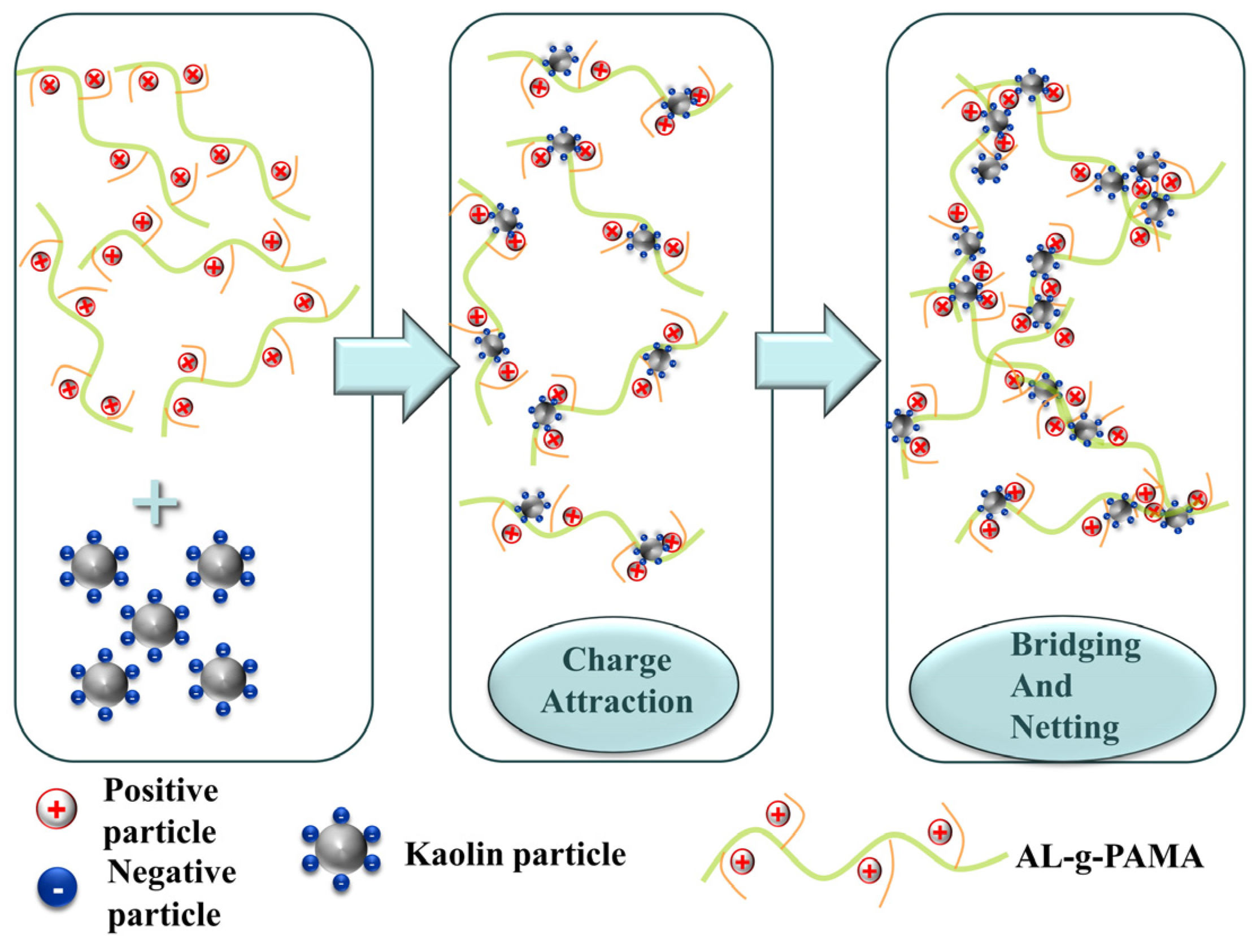
3.3.3. Flocculation Mechanism of AL-g-PAMA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shaikh, S.M.R.; Nasser, M.S.; Hussein, I.; Benamor, A.; Onaizi, S.A.; Qiblawey, H. Influence of Polyelectrolytes and Other Polymer Complexes on the Flocculation and Rheological Behaviors of Clay Minerals: A Comprehensive Review. Sep. Purif. Technol. 2017, 187, 137–161. [Google Scholar] [CrossRef]
- Ren, B.; Min, F.; Liu, L.; Chen, J.; Liu, C.; Lv, K. Adsorption of Different PAM Structural Units on Kaolinite (001) Surface: Density Functional Theory Study. Appl. Surf. Sci. 2020, 504, 144324. [Google Scholar] [CrossRef]
- Vajihinejad, V.; Guillermo, R.; Soares, J.B.P. Dewatering Oil Sands Mature Fine Tailings (MFTs) with Poly(acrylamide-co-diallyldimethylammonium chloride): Effect of Average Molecular Weight and Copolymer Composition. Ind. Eng. Chem. Res. 2017, 56, 1256–1266. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, L. Synergistic Mechanism of CTAB and Nonionic Polyacrylamide on Pore Structure of Kaolinite Filter cake. Sep. Purif. Technol. 2023, 324, 124575. [Google Scholar] [CrossRef]
- Doi, A.; Nguyen, T.A.H.; Nguyen, N.N.; Nguyen, C.V.; Raji, F.; Nguyen, A.V. Enhancing Shear Strength And Handleability Of Dewatered Clay-Rich Coal Tailings For Dry-Stacking. J. Environ. Manag. 2023, 344, 118488. [Google Scholar] [CrossRef] [PubMed]
- Ban, W.; Yang, Q.; Huang, W.; Li, X.; Wang, Z.; Chen, S.; Xiang, L.; Yan, B. Mussel-Inspired Catechol-Grafted Quaternized Chitosan Flocculant for Efficiently Treating Suspended Particles and Refractory Soluble Organic Pollutants. Ind. Eng. Chem. Res. 2023, 62, 1099–1111. [Google Scholar] [CrossRef]
- Yusoff, M.S.; Aziz, H.A.; Zamri, M.F.M.A.; Suja, F.; Abdullah, A.Z.; Basri, N.E.A. Floc Behavior and Removal Mechanisms of Cross-Linked Durio Zibethinus Seed Starch as a Natural Flocculant for Landfill Leachate Coagulation-Flocculation Treatment. Waste Manag. 2018, 74, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Sabaghi, S.; Fatehi, P. Phenomenological Changes in Lignin Following Polymerization and Its Effects on Flocculating Clay Particles. Biomacromolecules 2019, 20, 3940–3951. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Wang, Z.; Zhang, Y.; Peng, P.; She, D. Lignin-Based Controlled Release Fertilizers: A Review. Int. J. Biol. Macromol. 2022, 222, 1801–1817. [Google Scholar] [CrossRef]
- Wang, B.; Wang, S.-F.; Lam, S.S.; Sonne, C.; Yuan, T.-Q.; Song, G.-Y.; Sun, R.-C. A Review on Production of Lignin-Based Flocculants: Sustainable Feedstock and Low Carbon Footprint Applications. Renew. Sustain. Energy Rev. 2020, 134, 110384. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, X.; Liu, M.; Wen, J.; Yuan, T.-Q. Uncovering the Structure of Lignin from Moso Bamboo with Different Tissues and Growing Ages for Efficient Ambient-Pressure Lignin Depolymerization. ACS Sustain. Chem. Eng. 2023, 11, 13778–13786. [Google Scholar] [CrossRef]
- Wang, B.; Cao, X.-F.; Yu, S.-X.; Sun, Z.-H.; Shen, X.-J.; Wen, J.-L.; Yuan, T.-Q. New Sustainable Mannich-Functionalized Lignin Flocculants for Ultra-Efficiently Tailoring Wastewater Purification. J. Clean. Prod. 2023, 387, 135801. [Google Scholar] [CrossRef]
- Feng, Q.; Gao, B.; Yue, Q.; Guo, K. Flocculation Performance of Papermaking Sludge-Based Flocculants in Different DYE WASTEWater Treatment: Comparison with Commercial Lignin and Coagulants. Chemosphere 2021, 262, 128416. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chen, T.-Y.; Wang, H.-M.; Li, H.-Y.; Liu, C.-F.; Wen, J.-L. Amination of Biorefinery Technical Lignins Using Mannich Reaction Synergy with Subcritical Ethanol Depolymerization. Int. J. Biol. Macromol. 2018, 107, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.D.; Ebikade, E.; Vlachos, D.G.; Lobo, R.F. Lignin-Based Water-Soluble Polymers Exhibiting Biodegradability and Activity as Flocculating Agents. ACS Sustain. Chem. Eng. 2022, 10, 11117–11129. [Google Scholar] [CrossRef]
- Kollman, M.; Jiang, X.; Thompson, S.J.; Mante, O.; Dayton, D.C.; Chang, H.-m.; Jameel, H. Improved Understanding of Technical Lignin Functionalization through Comprehensive Structural Characterization of Fractionated Pine Kraft Lignins Modified by the Mannich Reaction. Green Chem. 2021, 23, 7122–7136. [Google Scholar] [CrossRef]
- Wang, B.; Wen, J.-L.; Sun, S.-L.; Wang, H.-M.; Wang, S.-F.; Liu, Q.-Y.; Charlton, A.; Sun, R.-C. Chemosynthesis and Structural Characterization of a Novel Lignin-Based Bio-Sorbent and Its Strong Adsorption for Pb (II). Ind. Crop. Prop. 2017, 108, 72–80. [Google Scholar] [CrossRef]
- Moore, C.; Gao, W.; Fatehi, P. Cationic Lignin Polymers as Flocculants for Municipal Wastewater. Water Environ. J. 2023, 37, 95–102. [Google Scholar] [CrossRef]
- Hasan, A.; Fatehi, P. Cationic Kraft Lignin-Acrylamide as a Flocculant for Clay Suspensions: 1. Molecular Weight Effect. Sep. Purif. Technol. 2018, 207, 213–221. [Google Scholar] [CrossRef]
- Hasan, A.; Fatehi, P. Cationic Kraft Lignin-Acrylamide Copolymer as a Flocculant for Clay Suspensions: (2) Charge Density Effect. Sep. Purif. Technol. 2019, 210, 963–972. [Google Scholar] [CrossRef]
- Chen, N.; Liu, W.; Huang, J.; Qiu, X. Preparation of Octopus-Like Lignin-Grafted Cationic Polyacrylamide Flocculant and Its Application For Water Flocculation. Int. J. Biol. Macromol. 2020, 146, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.; Mishra, S.; Sen, G. Microwave Based Synthesis of Polymethyl Methacrylate Grafted Sodium Alginate: Its Application as Flocculant. Carbohydr. Polym. 2013, 91, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Tang, Q.; Chen, W.; Rong, X.; Zhang, K.; Ma, D.; Wei, Z. Insight into the Purification of Algael Water by a Novel Flocculant with Enhanced Branched Nanochitosan Structure. J. Environ. Manag. 2023, 331, 117283. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Ma, X.; Dong, X.; Fan, Y.; Chen, R. The Synergistic Effects of Al3+ and Chitosan on the Solid-Liquid Separation of Coal Wastewater and Their Mechanism of Action. Polymers 2022, 14, 3970. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhang, C.; Hu, Y.; Su, P.; Quan, B.; Li, X.; Zhang, Z. Nano Aluminum-Based Hybrid Flocculant: Synthesis, Characterization, Application in Mine Drainage, Flocculation Mechanism. J. Clean. Prod. 2023, 399, 136582. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, H.; Wei, X.; Chen, Y.; Yu, B.; Wang, Y.; Liu, H. Preparation of a Cationic Block Copolymer Composite Coagulant TP (AM-DAC-PPFS) and Its Coagulation Performance for Treating Oilfield Sewage. Process. Saf. Environ. 2023, 171, 341–352. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, J.; Sun, Q.; An, Y.; Zhao, R.; Zheng, H.; Li, H. Efficient Removal of Both Positively and Negatively Charged Colloidal Contaminants Using Amphoteric Starch-Based Flocculants Synthesized By Low-Pressure Uv Initiation. Sep. Purif. Technol. 2022, 282, 120120. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, D.; Liu, W.; Huang, J.; Yang, D.; Qiu, X.; Li, S. Preparation of Carboxymethylated Lignin-Based Multifunctional Flocculant and Its Application for Copper-Containing Wastewater. Eur. Polym. J. 2022, 164, 110967. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, H.; Sun, Y.; Ren, J.; Zheng, X.; Sun, Q.; Jiang, S.; Ding, W. Synthesis of Novel Chitosan-Based Flocculants With Amphiphilic Structure And Its Application In Sludge Dewatering: Role of Hydrophobic Groups. J. Clean. Prod. 2020, 249, 119350. [Google Scholar] [CrossRef]
- Guo, K.; Gao, B.; Yue, Q.; Xu, X.; Li, R.; Shen, X. Characterization and Performance of a Novel Lignin-Based Flocculant for the Treatment of Dye Wastewater. Int. Biodeter. Biodegr. 2018, 133, 99–107. [Google Scholar] [CrossRef]
- Li, X.; Zheng, H.; Gao, B.; Sun, Y.; Liu, B.; Zhao, C. UV-Initiated Template Copolymerization of AM and MAPTAC: Microblock Structure, Copolymerization mechanism, and Flocculation Performance. Chemosphere 2017, 167, 71–81. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, S.; Sun, Y.; Xu, Y. Synthesis and Evaluation of Cationic Flocculant P(Dac-Paptac-Am) for Flocculation of Coal Chemical Wastewater. J. Environ. Sci. 2021, 99, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-h.; Zeng, T.; Wu, M.; Zhang, H.-b.; Hu, X.-q. Effects of Esterification on the Structural, Physicochemical, and Flocculation Properties of Dextran. Carbohydr. Polym. 2017, 174, 1129–1137. [Google Scholar] [CrossRef]
- Sun, Y.; Ren, M.; Zhu, C.; Xu, Y.; Zheng, H.; Xiao, X.; Wu, H.; Xia, T.; You, Z. UV-Initiated Graft Copolymerization of Cationic Chitosan-Based Flocculants for Treatment of Zinc Phosphate-Contaminated Wastewater. Ind. Eng. Chem. Res. 2016, 55, 10025–10035. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, Z.; Chen, J.; Yang, G.; Chen, H.; Lucia, L.A. Synthesis and Characterization of Alkali Lignin-based Hydrogels from Ionic Liquids. Bioresources 2017, 12, 5395–5406. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, C.; Sun, W.; Xu, Y.; Xiao, X.; Zheng, H.; Wu, H.; Liu, C. Plasma-Initiated Polymerization of Chitosan-Based CS-g-P(AM-DMDAAC) Flocculant for the Enhanced Flocculation of Low-Algal-Turbidity Water. Carbohydr. Polym. 2017, 164, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xue, F.; Ding, E. Preparation of Polyacrylamide Grafted Collagen Extracted from Leather Wastes and their Application in Kaolin Flocculation. J. Appl. Polym. Sci. 2015, 132, 41556. [Google Scholar] [CrossRef]
- Guo, D.; Wu, S.; Lyu, G.; Guo, H. Effect of Molecular Weight on the Pyrolysis Characteristics of Alkali Lignin. Fuel 2017, 193, 45–53. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Yang, M.; Huang, Z.; Hu, H.; Huang, A.; Feng, Z. Acylation of Lignin with Different Acylating Agents by Mechanical Activation-Assisted Solid Phase Synthesis: Preparation and Properties. Polymers 2018, 10, 907. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, H.; Guan, Q.; Teng, H.; Zhao, C.; Zhao, C. Fabricating a Flocculant with Controllable Cationic Microblock Structure: Characterization and Sludge Conditioning Behavior Evaluation. Ind. Eng. Chem. Res. 2016, 55, 2892–2902. [Google Scholar] [CrossRef]
- Su, G.; Jiang, Y.-J.; Ju, H.-B.; Wang, Y.-K.; Yu, S.-X.; Luo, Y.-Y.; Geng, T. Fabrication and Application of Cationic Polyacrylamide. Tenside Surfactants Deterg. 2020, 57, 168–174. [Google Scholar] [CrossRef]
- Lv, S.; Sun, T.; Zhou, Q.; Liu, J.; Ding, H. Synthesis of Starch-g-p(DMDAAC) Using HRP Initiation and the Correlation of Its Structure and Sludge Dewaterability. Carbohydr. Polym. 2014, 103, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Shi, J.; Ding, H.; Zhu, G.; Fu, K.; Fu, X. Synthesis of Cationic Polyacrylamide by Low-Pressure UV Initiation for Turbidity Water Flocculation. Chem. Eng. J. 2017, 312, 20–29. [Google Scholar] [CrossRef]
- Feng, Z.; Fan, Y.; Dong, X.; Ma, X.; Chen, R. Permeability Estimation in Filter Cake Based on X-Ray Microtomography and Lattice Boltzmann method. Sep. Purif. Technol. 2021, 275, 119114. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, J.; Yu, W.; Zhang, S.; Liang, S.; Song, J.; Xu, Q.; Ye, N.; He, S.; Yang, C.; et al. Synergetic Conditioning of Sewage Sludge via Fe2+/Persulfate and Skeleton Builder: Effect on Sludge Characteristics and Dewaterability. Chem. Eng. J. 2015, 270, 572–581. [Google Scholar] [CrossRef]
- Wang, H.-F.; Hu, H.; Wang, H.-J.; Zeng, R.J. Combined use of Inorganic Coagulants and Cationic Polyacrylamide for Enhancing Dewaterability of Sewage Sludge. J. Clean. Prod. 2019, 211, 387–395. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, H.; Wang, Y.; Zheng, X.; Wang, M.; Ren, J.; Zhao, C. Synthesis of a Cationic Polyacrylamide by a Photocatalytic Surface-Initiated Method and Evaluation of its Flocculation and Dewatering performance: Nano-TiO2 as a Photo Initiator. Rsc. Adv. 2018, 8, 28329–28340. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Peng, C.; Zhan, Z.; Ma, F.; Zhang, J. A Novel Treatment for Amelioration of Sludge Dewaterability Using Green Starch-grafted Flocculant and Realized Mechanism. Sep. Purif. Technol. 2022, 301, 122060. [Google Scholar] [CrossRef]
- Moore, C.; Gao, W.; Fatehi, P. Cationic Lignin Polymers as Flocculant for Municipal Wastewater. Polymers 2021, 13, 3871. [Google Scholar] [CrossRef]
- Sabaghi, S.; Fatehi, P. Polarity of Cationic Lignin Polymers: Physicochemical Behavior in Aqueous Solutions and Suspensions. Chemsuschem 2020, 13, 4722–4734. [Google Scholar] [CrossRef]
- Sun, X.; Ma, X.; Fan, Y.; Dong, X.; Chang, M.; Feng, Z.; Peng, D. Insight into Filter Cake Characteristics of Fine Coal Tailings Assisted by CPAM and α-HH during Pressure Filtration. Sep. Purif. Technol. 2023, 326, 124822. [Google Scholar] [CrossRef]
- Fang, K.; Wang, B.; Zhang, Y.; Li, H. Optimized Production and Characterization of Cation-Independent Bioflocculant Produced by Klebsiella sp. 59L. Environ. Sci. Pollut. R. 2021, 28, 7981–7993. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, H. Enhanced Dewaterability of Sewage Sludge by Grafted Cationic Lignin-Based Flocculants. Sci. Total Environ. 2023, 903, 166958. [Google Scholar] [CrossRef] [PubMed]






| Flocculant | Weight of AL (g) | Weight of AM (g) | Volume of MAPTAC (mL) | Weight of KPS (g) | Volume of the Solution (mL) | Intrinsic Viscosity (dL/g) | Grafting Efficiency of PAMA (%) |
|---|---|---|---|---|---|---|---|
| AL-g-PAMA #1 | 8.7 | 21.8 | 12.6 | 2.0 | 100 | 3.4 | 77.1 |
| AL-g-PAMA #2 | 8.7 | 21.8 | 12.6 | 3.0 | 100 | 4.7 | 79.8 |
| AL-g-PAMA #3 | 11.2 | 36.5 | 8.1 | 4.0 | 100 | 5.3 | 67.5 |
| AL-g-PAMA #4 | 8.7 | 21.8 | 12.6 | 5.0 | 100 | 6.6 | 73.8 |
| AL-g-PAMA #5 | 8.7 | 21.8 | 12.6 | 4.0 | 100 | 6.4 | 87.7 |
| CPAM | - | - | - | - | - | 7.6 | - |
| Flocculant Type | Transmittance (%) | Settling Velocity (cm/min) | Thickness of the Compressed Layer (cm) | Filter Cake Moisture Content (%) |
|---|---|---|---|---|
| AL-g-PAMA #5 | 84.0 ± 0.5 | 24.1 ± 1.0 | 2.2 | 43.4 ± 0.5 |
| CPAM | 84.2 ± 1.1 | 31.0 ± 1.7 | 2.8 | 47.7 ± 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Yao, S.; Dong, X.; Fan, Y.; Ma, X.; Zhu, B.; Chang, M. Preparation of a Lignin-Based Cationic Flocculant and Its Application in Kaolin Suspension Treatment. Polymers 2024, 16, 1131. https://doi.org/10.3390/polym16081131
Li Y, Yao S, Dong X, Fan Y, Ma X, Zhu B, Chang M. Preparation of a Lignin-Based Cationic Flocculant and Its Application in Kaolin Suspension Treatment. Polymers. 2024; 16(8):1131. https://doi.org/10.3390/polym16081131
Chicago/Turabian StyleLi, Yan, Suling Yao, Xianshu Dong, Yuping Fan, Xiaomin Ma, Benkang Zhu, and Ming Chang. 2024. "Preparation of a Lignin-Based Cationic Flocculant and Its Application in Kaolin Suspension Treatment" Polymers 16, no. 8: 1131. https://doi.org/10.3390/polym16081131







