Natural and Synthetic Polymers for Biomedical and Environmental Applications
Abstract
1. Introduction
2. Natural Polymers for Biomedical Use
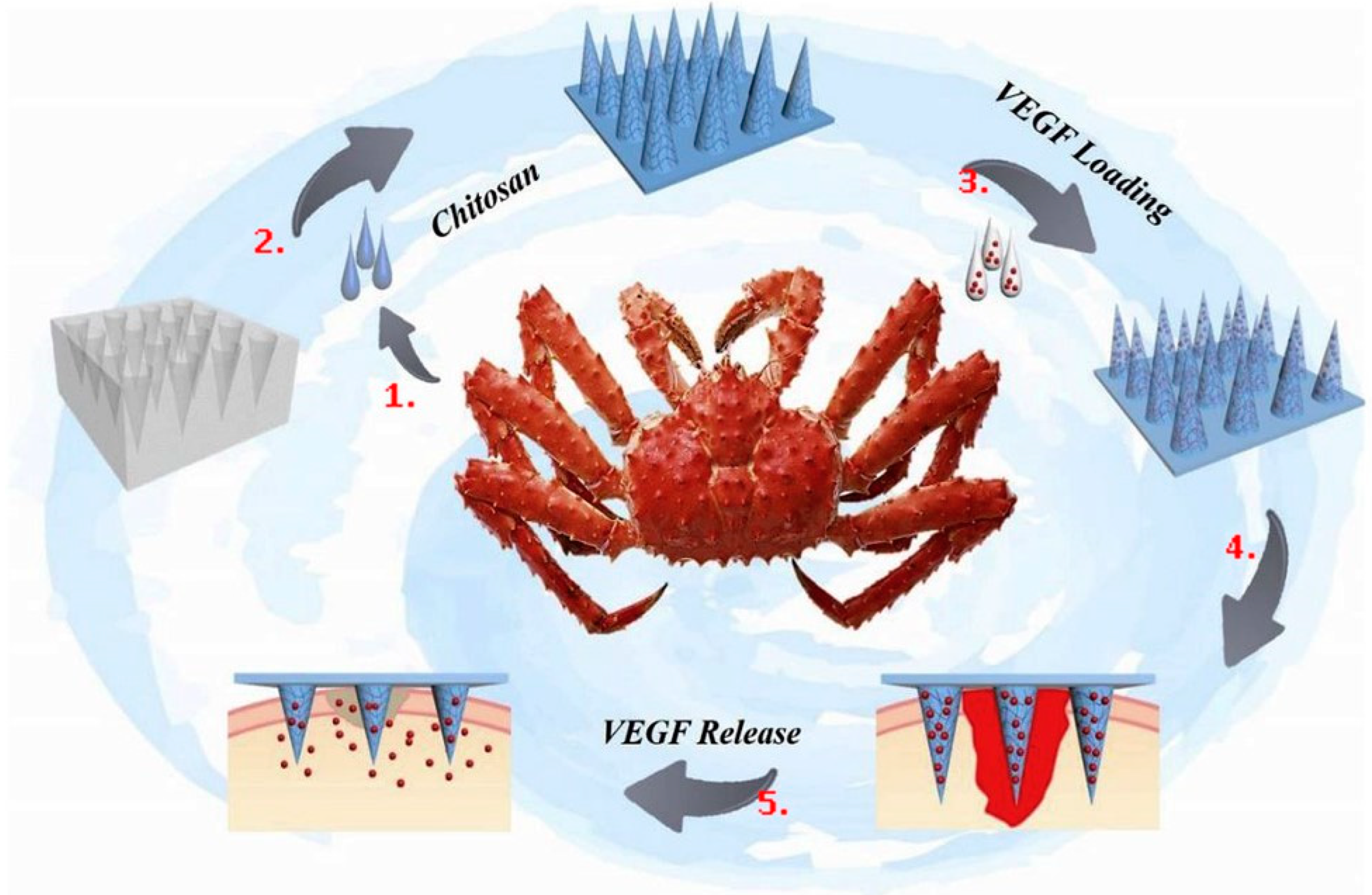
2.1. Antibacterial
2.2. Hydrogel Preparation and Application
2.3. Drug Delivery
2.4. Stem Cell Morphogenesis
2.5. Wound Healing
2.6. Skin Tissue Engineering
2.7. Bone Tissue Engineering
2.8. Cartilage Tissue Engineering
2.9. Heart Valve Tissue Engineering
2.10. Cell Encapsulation
2.11. Biofabrication
2.12. Bio-Based Monomers
3. Natural Polymers for Environmental Use
3.1. Food Packaging
3.2. Nano Fertilizers and Micronutrients
3.3. Nanocarriers of Fungicides/Bactericides/Viricides
3.4. Nanocarriers of Insecticides
3.5. Bioplastics
4. Methods of Isolation and Physicochemical Parameters of Natural Polymers
5. Synthetic Polymers for Biomedical Use
5.1. Antiviral
5.2. Antibacterial
5.3. Antifungal
5.4. Antitumor
5.5. Myocardial Tissue Engineering
5.6. Insulin Drug Carriers
5.7. Biofilm
5.8. Gene Delivery
5.9. Bioink in 3D Printing
5.10. Textiles in Medicine
6. Synthetic Polymers for Environmental Use
6.1. Agriculture
6.2. Food Packaging
6.3. Hazardous Waste Management
6.4. Phenol Degradation
6.5. Soil Stabilizers
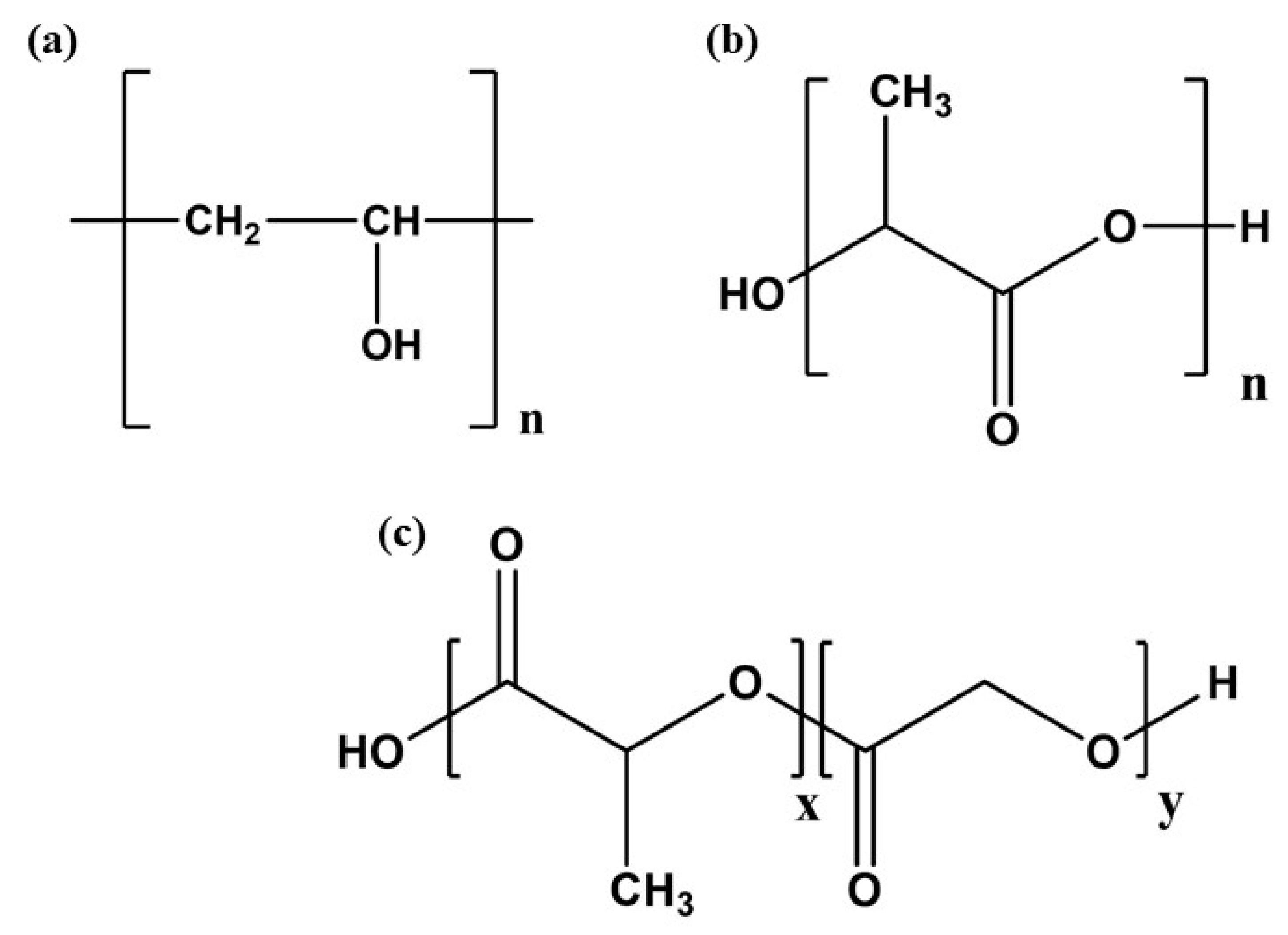
7. Methods of Synthesis and Physicochemical Parameters of Synthetic Polymers
8. Advantages and Limitations: Future Perspectives of Natural and Synthetic Polymers
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Puertas-Bartolomé, M.; Mora-Boza, A.; García-Fernández, L. Emerging Biofabrication Techniques: A Review on Natural Polymers for Biomedical Applications. Polymers 2021, 13, 1209. [Google Scholar] [CrossRef]
- Aravamudhan, A.; Ramos, D.M.; Nada, A.A.; Kumbar, S.G. Chapter 4—Natural Polymers: Polysaccharides and Their Derivatives for Biomedical Applications. In Natural and Synthetic Biomedical Polymers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 67–89. Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780123969835000041 (accessed on 15 March 2024).
- Schultz, B.; Snow, E.S.; Walker, S. Mechanism of D-Alanine Transfer to Teichoic Acids Shows How Bacteria Acylate Cell Envelope Polymers. Nat. Microbiol. 2023, 8, 1318–1329. [Google Scholar] [CrossRef] [PubMed]
- Caillol, S. Special Issue “Natural Polymers and Biopolymers II”. Molecules 2020, 26, 112. [Google Scholar] [CrossRef]
- Rajeswari, S. Natural Polymers: A Recent Review. World J. Pharm. Pharm. Sci. 2017, 6, 472–494. [Google Scholar] [CrossRef][Green Version]
- Silva, A.C.Q.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Natural Polymers-Based Materials: A Contribution to a Greener Future. Molecules 2022, 27, 94. [Google Scholar] [CrossRef]
- Silva, S.S.; Gomes, J.M.; Rodrigues, L.C.; Reis, R.L. Marine-Derived Polymers in Ionic Liquids: Architectures Development and Biomedical Applications. Mar. Drugs 2020, 18, 346. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, X.; Cheng, Z. Research on the Application of Synthetic Polymer Materials in Contemporary Public Art. Polymers 2022, 14, 1208. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A. Introduction to Plastics Engineering; William Andrew Publishing: Boston, MA, USA, 2018; pp. 1–16. Available online: https://www.sciencedirect.com/science/article/pii/B9780323395007000010 (accessed on 15 March 2024).
- D’Alvise, T.M.; Sunder, S.; Hasler, R.; Moser, J.; Knoll, W.; Synatschke, C.V.; Harvey, S.; Weil, T. Preparation of Ultrathin and Degradable Polymeric Films by Electropolymerization of 3-Amino-L-Tyrosine. Macromol. Rapid Commun. 2022, 44, 202200332. [Google Scholar] [CrossRef]
- Cao, S.; Ivanov, T.; de Souza Melchiors, M.; Landfester, K.; Caire da Silva, L. Controlled Membrane Transport in Polymeric Biomimetic Nanoreactors. ChemBioChem 2023, 24, 202200718. [Google Scholar] [CrossRef]
- Hunter, A.; Moghimi, S.M. Therapeutic Synthetic Polymers: A Game of Russian Roulette? Drug Discov. Today 2002, 7, 998–1001. [Google Scholar] [CrossRef]
- PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/?db=pubmed (accessed on 4 April 2024).
- Zhang, H.; Lin, X.; Cao, X.; Wang, Y.; Wang, J.; Zhao, Y. Developing Natural Polymers for Skin Wound Healing. Bioact. Mater. 2024, 33, 355–376. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhou, Y.; Zhang, J.; Liang, H.; Chen, X.; Tan, H. Natural Polymer-Based Hydrogels: From Polymer to Biomedical Applications. Pharmaceutics 2023, 15, 2514. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Xie, C.; Lu, X. Natural Polymer-Based Adhesive Hydrogel for Biomedical Applications. Biosurface Biotribology 2022, 8, 69–94. [Google Scholar] [CrossRef]
- Jao, D.; Xue, Y.; Medina, J.; Hu, X. Protein-Based Drug-Delivery Materials. Materials 2017, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Birajdar, M.S.; Joo, H.; Koh, W.-G.; Park, H. Natural Bio-Based Monomers for Biomedical Applications: A Review. Biomater. Res. 2021, 25, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic Acid: The Influence of Molecular Weight on Structural, Physical, Physico-Chemical, and Degradable Properties of Biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef]
- Teixeira-Costa, B.E.; Andrade, C.T. Natural Polymers Used in Edible Food Packaging—History, Function and Application Trends as a Sustainable Alternative to Synthetic Plastic. Polysaccharides 2022, 3, 32–58. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef]
- Bharadwaz, A.; Jayasuriya, A.C. Recent Trends in the Application of Widely Used Natural and Synthetic Polymer Nanocomposites in Bone Tissue Regeneration. Mater. Sci. Engineering. C Mater. Biol. Appl. 2020, 110, 110698. [Google Scholar] [CrossRef]
- Ciolacu, D.E.; Nicu, R.; Ciolacu, F. Natural Polymers in Heart Valve Tissue Engineering: Strategies, Advances and Challenges. Biomedicines 2022, 10, 1095. [Google Scholar] [CrossRef]
- Cardoso, L.M.d.F.; Barreto, T.; Gama, J.F.G.; Alves, L.A. Natural Biopolymers as Additional Tools for Cell Microencapsulation Applied to Cellular Therapy. Polymers 2022, 14, 2641. [Google Scholar] [CrossRef] [PubMed]
- Devi, N.; Singh, S.; Manickam, S.; Cruz-Martins, N.; Kumar, V.; Verma, R.; Kumar, D. Itaconic Acid and Its Applications for Textile, Pharma and Agro-Industrial Purposes. Sustainability 2022, 14, 13777. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable Polymers for Food Packaging: A Review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- González-López, M.E.S.; Gradilla-Hernández, M.S.; Barajas-Álvarez, P. Current Trends in Biopolymers for Food Packaging: A Review. Front. Sustain. Food Syst. 2023, 7, 1225371. [Google Scholar] [CrossRef]
- Ponnusamy, P.G.; Mani, S. Material and Environmental Properties of Natural Polymers and Their Composites for Packaging Applications—A Review. Polymers 2022, 14, 4033. [Google Scholar] [CrossRef] [PubMed]
- Wypij, M.; Trzcińska-Wencel, J.; Golińska, P.; Avila-Quezada, G.D.; Ingle, A.P.; Rai, M. The Strategic Applications of Natural Polymer Nanocomposites in Food Packaging and Agriculture: Chances, Challenges, and Consumers’ Perception. Front. Chem. 2023, 10, 1106230. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, S.; Wang, J.; Chen, Q. A Review on Polymer and Lipid-Based Nanocarriers and Its Application to Nano-Pharmaceutical and Food-Based Systems. Front. Nutr. 2021, 8, 783831. [Google Scholar] [CrossRef] [PubMed]
- Acquavia, M.A.; Pascale, R.; Martelli, G.; Bondoni, M.; Bianco, G. Natural Polymeric Materials: A Solution to Plastic Pollution from the Agro-Food Sector. Polymers 2021, 13, 158. [Google Scholar] [CrossRef]
- Ngwuluka, N.C.; Ochekpe, N.A.; Aruoma, O.I. Naturapolyceutics: The Science of Utilizing Natural Polymers for Drug Delivery. Polymers 2014, 6, 1312–1332. [Google Scholar] [CrossRef]
- Akbari, A.; Bigham, A.; Rahimkhoei, V.; Sharifi, S.; Jabbari, E. Antiviral Polymers: A Review. Polymers 2022, 14, 1634. [Google Scholar] [CrossRef]
- Kamatar, A.; Gunay, G.; Acar, H. Natural and Synthetic Biomaterials for Engineering Multicellular Tumor Spheroids. Polymers 2020, 12, 2506. [Google Scholar] [CrossRef]
- Li, Y.; Meng, Q.; Chen, S.; Ling, P.; Kuss, M.A.; Duan, B.; Wu, S. Advances, Challenges, and Prospects for Surgical Suture Materials. Acta Biomater. 2023, 168, 78–112. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef] [PubMed]
- McMahan, S.; Taylor, A.; Copeland, K.M.; Pan, Z.; Liao, J.; Hong, Y. Current Advances in Biodegradable Synthetic Polymer Based Cardiac Patches. J. Biomed. Mater. Res. Part A 2020, 108, 972–983. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, X. Synthetic Polymers for Organ 3D Printing. Polymers 2020, 12, 1765. [Google Scholar] [CrossRef] [PubMed]
- Kalirajan, C.; Dukle, A.; Nathanael, A.J.; Oh, T.-H.; Manivasagam, G. A Critical Review on Polymeric Biomaterials for Biomedical Applications. Polymers 2021, 13, 3015. [Google Scholar] [CrossRef] [PubMed]
- Thadepalli, S. Review of Multifarious Applications of Polymers in Medical and Health Care Textiles. Mater. Today Proc. 2021, 55, 330–336. [Google Scholar] [CrossRef]
- Vallejos, S.; Trigo-López, M.; Arnaiz, A.; Miguel, Á.; Muñoz, A.; Mendía, A.; García, J.M. From Classical to Advanced Use of Polymers in Food and Beverage Applications. Polymers 2022, 14, 4954. [Google Scholar] [CrossRef] [PubMed]
- Kadhem, A.J.; Gentile, G.J.; Fidalgo de Cortalezzi, M.M. Molecularly Imprinted Polymers (MIPs) in Sensors for Environmental and Biomedical Applications: A Review. Molecules 2021, 26, 6233. [Google Scholar] [CrossRef]
- Zhao, X.; Lv, L.; Pan, B.; Zhang, W.; Zhang, S.; Zhang, Q. Polymer-Supported Nanocomposites for Environmental Application: A Review. Chem. Eng. J. 2011, 170, 381–394. [Google Scholar] [CrossRef]
- Shoukat, H.; Buksh, K.; Noreen, S.; Pervaiz, F.; Maqbool, I. Hydrogels as Potential Drug-Delivery Systems: Network Design and Applications. Ther. Deliv. 2021, 12, 375–396. [Google Scholar] [CrossRef] [PubMed]
- Ravula, T.; Hardin, N.Z.; Ramamoorthy, A. Polymer Nanodiscs: Advantages and Limitations. Chem. Phys. Lipids 2019, 219, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Zhang, X.; Chen, C.; Shao, C.; Zhao, Y.; Wang, Y. Antibacterial and Angiogenic Chitosan Microneedle Array Patch for Promoting Wound Healing. Bioact. Mater. 2020, 5, 253–259. [Google Scholar] [CrossRef] [PubMed]
- García, M.A.; Hensler, G.; Al-Gousous, J.; Pielenhofer, J.; Wagner, M.; Lennernäs, H.; Langguth, P. Novel Food Drug Interaction Mechanism Involving Acyclovir, Chitosan and Endogenous Mucus. Drug Metab. Pharmacokinet. 2023, 49, 100491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Z.; Zhang, H.; Chen, C.; Zhang, D.; Zhao, Y. Protein-Based Hybrid Responsive Microparticles for Wound Healing. ACS Appl. Mater. Interfaces 2021, 13, 18413–18422. [Google Scholar] [CrossRef]
- Shao, J.; Wang, B.; Li, J.; Jansen, J.A.; Walboomers, X.F.; Yang, F. Antibacterial Effect and Wound Healing Ability of Silver Nanoparticles Incorporation into Chitosan-Based Nanofibrous Membranes. Mater. Sci. Eng. C 2019, 98, 1053–1063. [Google Scholar] [CrossRef]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Stickdorn, J.; Czysch, C.; Medina-Montano, C.; Stein, L.; Xu, L.; Scherger, M.; Schild, H.; Grabbe, S.; Nuhn, L. Peptide-Decorated Degradable Polycarbonate Nanogels for Eliciting Antigen-Specific Immune Responses. Int. J. Mol. Sci. 2023, 24, 15417. [Google Scholar] [CrossRef] [PubMed]
- Nordin, N.N.; Aziz, N.K.; Naharudin, I.; Anuar, N.K. Effects of Drug-Free Pectin Hydrogel Films on Thermal Burn Wounds in Streptozotocin-Induced Diabetic Rats. Polymers 2022, 14, 2873. [Google Scholar] [CrossRef]
- Kamenova, K.; Prancheva, A.; Stoyanova, S.; Radeva, L.; Tibi, I.P.-E.; Yoncheva, K.; Ravutsov, M.A.; Marinova, M.K.; Simeonov, S.P.; Mitova, S.; et al. Functional Hydrogels for Delivery of the Proteolytic Enzyme Serratiopeptidase. Gels 2024, 10, 156. [Google Scholar] [CrossRef]
- Wang, R.; Yu, Y.; Gai, M.; Mateos‐Maroto, A.; Morsbach, S.; Xia, X.; He, M.; Fan, J.; Peng, X.; Landfester, K.; et al. Liposomal Enzyme Nanoreactors Based on Nanoconfinement for Efficient Anti-Tumor Therapy. Angew. Chem. Int. Ed. 2023, 62, 202308761. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Brocchini, S. Synthetic Approaches to Uniform Polymers. Adv. Drug Deliv. Rev. 2006, 58, 1671–1687. [Google Scholar] [CrossRef] [PubMed]
- Alleva, N.; Eigen, K.; Ng, D.Y.; Weil, T. A Versatile and Efficient Method to Isolate DNA–Polymer Conjugates. ACS Macro Lett. 2023, 12, 1257–1263. [Google Scholar] [CrossRef]
- Tong, X.; Pan, W.; Su, T.; Zhang, M.; Dong, W.; Qi, X. Recent Advances in Natural Polymer-Based Drug Delivery Systems. React. Funct. Polym. 2020, 148, 104501. [Google Scholar] [CrossRef]
- Giliopoulos, D.; Zamboulis, A.; Giannakoudakis, D.; Bikiaris, D.; Triantafyllidis, K. Polymer/Metal Organic Framework (MOF) Nanocomposites for Biomedical Applications. Molecules 2020, 25, 185. [Google Scholar] [CrossRef] [PubMed]
- Krauhausen, I.; Coen, C.; Spolaor, S.; Gkoupidenis, P.; van de Burgt, Y. Brain-Inspired Organic Electronics: Merging Neuromorphic Computing and Bioelectronics Using Conductive Polymers. Adv. Funct. Mater. 2023, 34, 202307729. [Google Scholar] [CrossRef]
- Zhang, N.; Kohn, D.H. Using Polymeric Materials to Control Stem Cell Behavior for Tissue Regeneration. Birth Defects Res. C Embryo Today Rev. 2012, 96, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.; Bellion, M.; Kissmann, A.-K.; Herberger, T.; Synatschke, C.V.; Bozdogan, A.; Andersson, J.; Rodriguez, A.; Ständker, L.; Wiese, S.; et al. Aptamers as Novel Binding Molecules on an Antimicrobial Peptide-Armored Composite Hydrogel Wound Dressing for Specific Removal and Efficient Eradication of Pseudomonas aeruginosa. Int. J. Mol. Sci. 2023, 24, 4800. [Google Scholar] [CrossRef]
- Ter Horst, B.; Moiemen, N.S.; Grover, L.M. Natural Polymers. In Biomaterials for Skin Repair and Regeneration; Woodhead Publishing: Cambridge, UK, 2019; pp. 151–192. [Google Scholar] [CrossRef]
- Belluati, A.; Harley, I.; Lieberwirth, I.; Bruns, N. An Outer Membrane-Inspired Polymer Coating Protects and Endows Escherichia Coli with Novel Functionalities. Small 2023, 19, 202303384. [Google Scholar] [CrossRef]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural Polymers for the Microencapsulation of Cells. J. R. Soc. Interface 2014, 11, 20140817. [Google Scholar] [CrossRef]
- Papadopoulos, L.; Malletzidou, L.; Patsiaoura, D.; Magaziotis, A.; Psochia, E.; Terzopoulou, Z.; Chrissafis, K.; Markessini, C.; Papadopoulou, E.; Bikiaris, D.N. Synthesis and Characterization of Unsaturated Succinic Acid Biobased Polyester Resins. Appl. Sci. 2021, 11, 896. [Google Scholar] [CrossRef]
- Kim, H.J.; Koo, J.M.; Kim, S.H.; Hwang, S.Y.; Im, S.S. Synthesis of Super Absorbent Polymer Using Citric Acid as a Bio-Based Monomer. Polym. Degrad. Stab. 2017, 144, 128–136. [Google Scholar] [CrossRef]
- Sabbah, M.; Di Pierro, P.; Ruffo, F.; Schiraldi, C.; Alfano, A.; Cammarota, M.; Porta, R. Glutamic Acid as Repeating Building Block for Bio-Based Films. Polymers 2020, 12, 1613. [Google Scholar] [CrossRef] [PubMed]
- Scelsi, E.; Angelini, A.; Pastore, C. Deep Eutectic Solvents for the Valorisation of Lignocellulosic Biomasses towards Fine Chemicals. Biomass 2021, 1, 29–59. [Google Scholar] [CrossRef]
- Hussein, H.S.; Shaarawy, H.H.; Hussien, N.H.; Hawash, S.I. Preparation of Nano-Fertilizer Blend from Banana Peels. Bull. Natl. Res. Cent. 2019, 43, 26. [Google Scholar] [CrossRef]
- Wang, J.; Emmerich, L.; Wu, J.; Vana, P.; Zhang, K. Hydroplastic Polymers as Eco-Friendly Hydrosetting Plastics. Nat. Sustain. 2021, 4, 877–883. [Google Scholar] [CrossRef]
- Bashir, K.; Aggarwal, M. Physicochemical, Structural and Functional Properties of Native and Irradiated Starch: A Review. J. Food Sci. Technol. 2019, 56, 513–523. [Google Scholar] [CrossRef]
- Gamage, A.; Thiviya, P.; Mani, S.; Ponnusamy, P.G.; Manamperi, A.; Evon, P.; Merah, O.; Madhujith, T. Environmental Properties and Applications of Biodegradable Starch-Based Nanocomposites. Polymers 2022, 14, 4578. [Google Scholar] [CrossRef]
- Jacob, J.; Gopi, S. Isolation and Physicochemical Characterization of Biopolymers. In Biopolymers and Their Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 45–79. [Google Scholar] [CrossRef]
- Biswas, D.; Das, S.; Mohanto, S.; Mantry, S. Extraction, Modification, and Characterization of Natural Polymers used in Transdermal Drug Delivery System: An updated review. Asian J. Pharm. Clin. Res. 2020, 13, 10–20. [Google Scholar] [CrossRef]
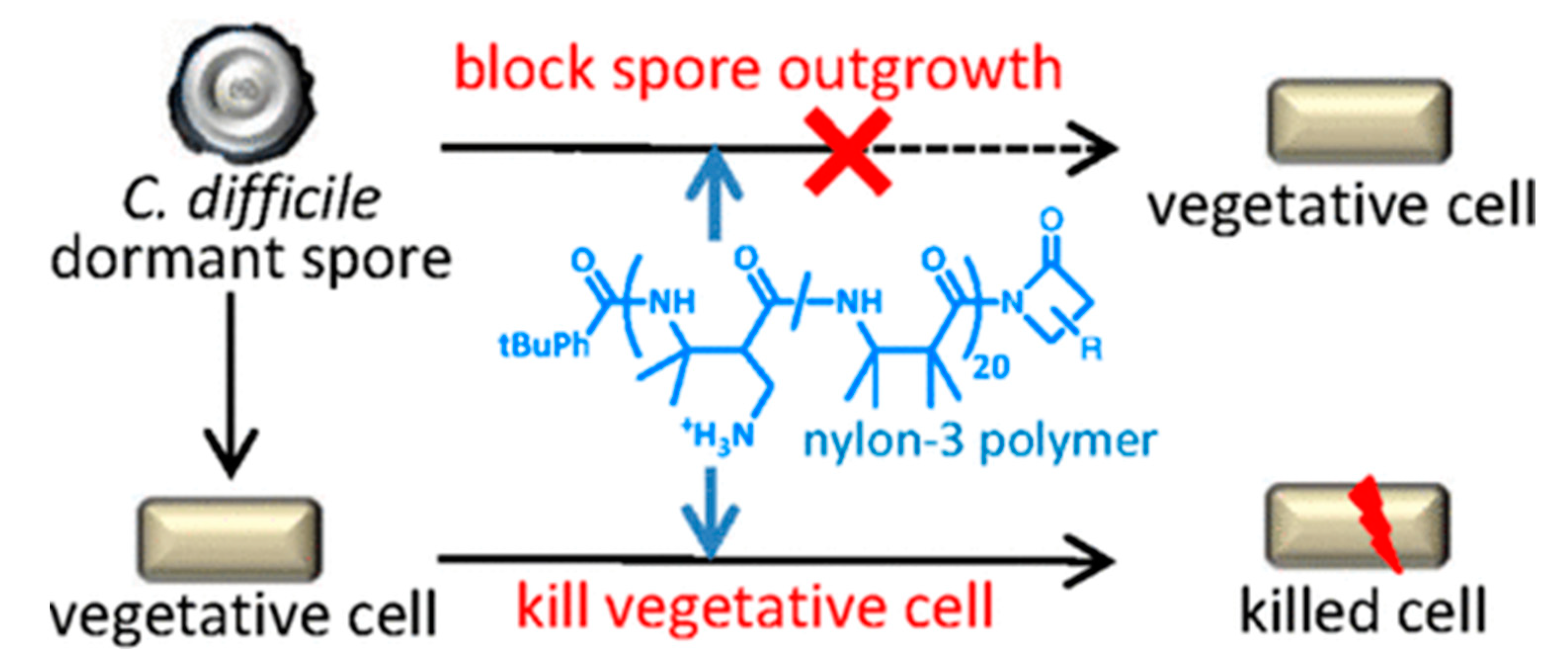
- Liu, R.; Suárez, J.M.; Weisblum, B.; Gellman, S.H.; McBride, S.M. Synthetic Polymers Active against Clostridium Difficile Vegetative Cell Growth and Spore Outgrowth. J. Am. Chem. Soc. 2014, 136, 14498–14504. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, X.; Yu, L.; Chen, X.; Zhang, J.; Zhang, S.; Wu, S. Electrospun Polyasparthydrazide Nanofibrous Hydrogel Loading with In-Situ Synthesized Silver Nanoparticles for Full-Thickness Skin Wound Healing Application. Mater. Des. 2024, 239, 112818. [Google Scholar] [CrossRef]
- Mejía, S.P.; Marques, R.d.C.; Landfester, K.; Orozco, J.; Mailänder, V. Effect of Protein Corona on the Specificity and Efficacy of Nanobioconjugates to Treat Intracellular Infections. Macromol. Biosci. 2024, 24, e2300197. [Google Scholar] [CrossRef] [PubMed]
- Petrov, P.D.; Yoncheva, K.; Gancheva, V.; Konstantinov, S.; Trzebicka, B. Multifunctional Block Copolymer Nanocarriers for Co-Delivery of Silver Nanoparticles and Curcumin: Synthesis and Enhanced Efficacy against Tumor Cells. Eur. Polym. J. 2016, 81, 24–33. [Google Scholar] [CrossRef]
- Wang, J.; Luo, Y.; Wu, H.; Cao, S.; Abdelmohsen, L.K.; Shao, J.; van Hest, J.C. Inherently Fluorescent Peanut-Shaped Polymersomes for Active Cargo Transportation. Pharmaceutics 2023, 15, 1986. [Google Scholar] [CrossRef]
- Kim, P.-H.; Cho, J.-Y. Myocardial Tissue Engineering Using Electrospun Nanofiber Composites. BMB Rep. 2016, 49, 26–36. [Google Scholar] [CrossRef] [PubMed]
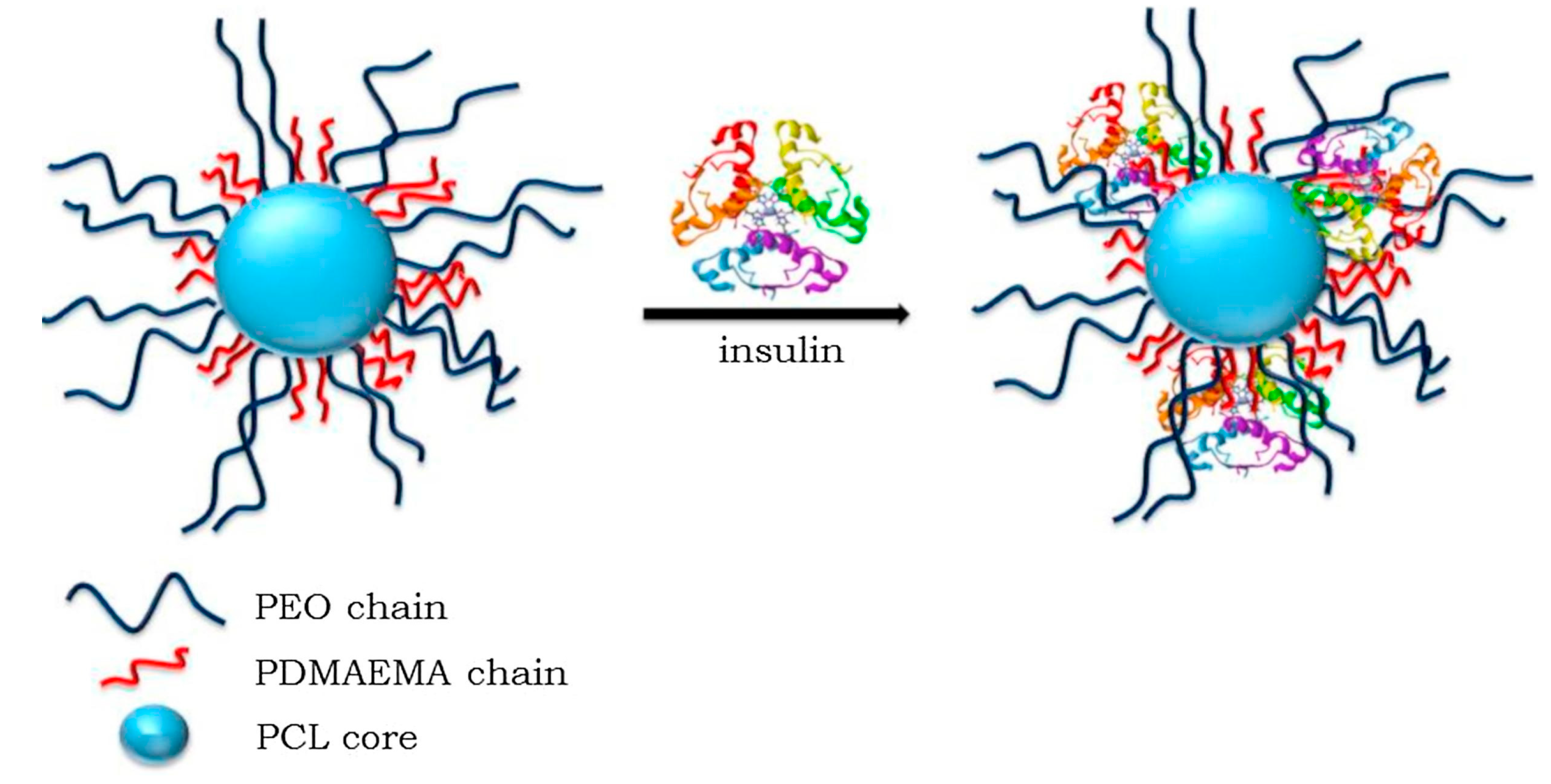
- Kamenova, K.; Haladjova, E.; Grancharov, G.; Kyulavska, M.; Tzankova, V.; Aluani, D.; Yoncheva, K.; Pispas, S.; Petrov, P. Co-Assembly of Block Copolymers as a Tool for Developing Novel Micellar Carriers of Insulin for Controlled Drug Delivery. Eur. Polym. J. 2018, 104, 1–9. [Google Scholar] [CrossRef]
- Borisova, D.; Haladjova, E.; Kyulavska, M.; Petrov, P.; Pispas, S.; Stoitsova, S.; Paunova-Krasteva, T. Application of Cationic Polymer Micelles for the Dispersal of Bacterial Biofilms. Eng. Life Sci. 2018, 18, 943–948. [Google Scholar] [CrossRef]
- Haladjova, E.; Toncheva-Moncheva, N.; Apostolova, M.D.; Trzebicka, B.; Dworak, A.; Petrov, P.; Dimitrov, I.; Rangelov, S.; Tsvetanov, C.B. Polymeric Nanoparticle Engineering: From Temperature-Responsive Polymer Mesoglobules to Gene Delivery Systems. Biomacromolecules 2014, 15, 4377–4395. [Google Scholar] [CrossRef]
- Kaygisiz, K.; Rauch-Wirth, L.; Iscen, A.; Hartenfels, J.; Kremer, K.; Münch, J.; Synatschke, C.V.; Weil, T. Peptide Amphiphiles as Biodegradable Adjuvants for Efficient Retroviral Gene Delivery. Adv. Healthc. Mater. 2023, 13, e2301364. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Wu, F.; Mincheva, R.; Hakkarainen, M.; Raquez, J.-M.; Mielewski, D.F.; Narayan, R.; Netravali, A.N.; Misra, M. Sustainable Polymers. Nat. Rev. Methods Primers 2022, 2, 1–27. [Google Scholar] [CrossRef]
- Chang, C.; Ginn, B.; Livingston, N.K.; Yao, Z.; Slavin, B.; King, M.W.; Chung, S.; Mao, H.-Q. 1.4.6—Medical Fibers and Biotextiles. In Biomaterials Science; Academic Press: Cambridge, MA, USA, 2020; pp. 575–600. Available online: https://www.sciencedirect.com/science/article/pii/B9780128161371000386 (accessed on 15 March 2024).
- Grethe, T. Biodegradable Synthetic Polymers in Textiles– What Lies beyond PLA and Medical Applications? A Review. Tekstilec 2021, 64, 33–47. [Google Scholar] [CrossRef]
- Sikder, A.; Pearce, A.K.; Parkinson, S.J.; Napier, R.; O’Reilly, R.K. Recent Trends in Advanced Polymer Materials in Agriculture Related Applications. ACS Appl. Polym. Mater. 2021, 3, 1203–1217. [Google Scholar] [CrossRef]
- Austin, H.P.; Allen, M.D.; Donohoe, B.S.; Rorrer, N.A.; Kearns, F.L.; Silveira, R.L.; Pollard, B.C.; Dominick, G.; Duman, R.; El Omari, K.; et al. Characterization and Engineering of a Plastic-Degrading Aromatic Polyesterase. Proc. Natl. Acad. Sci. USA 2018, 115, E4350–E4357. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Fu, S.; Yang, H.; He, P.; Sun, Z.; Duan, X.; Jia, D.; Colombo, P.; Zhou, Y. Exploiting Bifunctional 3D-Printed Geopolymers for Efficient Cesium Removal and Immobilization: An Approach for Hazardous Waste Management. J. Clean. Prod. 2024, 437, 140599. [Google Scholar] [CrossRef]
- Satchanska, G.; Topalova, Y.; Dimkov, R.; Groudeva, V.; Petrov, P.; Tsvetanov, C.; Selenska-Pobell, S.; Golovinsky, E. Phenol Degradation by Environmental Bacteria Entrapped in Cryogels. Biotechnol. Biotechnol. Equip. 2015, 29, 514–521. [Google Scholar] [CrossRef]
- Schöpfer, L.; Schnepf, U.; Marhan, S.; Brümmer, F.; Kandeler, E.; Pagel, H. Hydrolyzable Microplastics in Soil—Low Biodegradation but Formation of a Specific Microbial Habitat? Biol. Fertil. Soils 2022, 58, 471–486. [Google Scholar] [CrossRef]
- Sander, M.; Kohler, H.-P.E.; McNeill, K. Assessing the Environmental Transformation of Nanoplastic through 13C-Labelled Polymers. Nat. Nanotechnol. 2019, 14, 301–303. [Google Scholar] [CrossRef]
- Boaventura, N.F.; Sousa, T.F.d.P.; Casagrande, M.D.T. The Application of an Eco-Friendly Synthetic Polymer as a Sandy Soil Stabilizer. Polymers 2023, 15, 4626. [Google Scholar] [CrossRef]
- Nelson, T.F.; Baumgartner, R.; Jaggi, M.; Bernasconi, S.M.; Battagliarin, G.; Sinkel, C.; Künkel, A.; Kohler, H.-P.E.; McNeill, K.; Sander, M. Biodegradation of Poly (Butylene Succinate) in Soil Laboratory Incubations Assessed by Stable Carbon Isotope Labelling. Nat. Commun. 2022, 13, 5691. [Google Scholar] [CrossRef]
- Eubeler, J.P.; Zok, S.; Bernhard, M.; Knepper, T.P. Environmental Biodegradation of Synthetic Polymers I. Test Methodologies and Procedures. TrAC Trends Anal. Chem. 2009, 28, 1057–1072. [Google Scholar] [CrossRef]
- Choubey, R.; Sonker, N.; Bajpai, J.; Jain, P.; Singh, A. Synthesis of Polymer Nanomaterials, Mechanisms, and Their Structural Control. In Advances in Polymeric Nanomaterials for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 41–63. [Google Scholar] [CrossRef]
- McDonald, S.M.; Yang, Q.; Hsu, Y.-H.; Nikam, S.P.; Hu, Z.; Wang, Z.; Asheghali, D.; Yen, T.; Dobrynin, A.V.; Rogers, J.A.; et al. Resorbable Barrier Polymers for Flexible Bioelectronics. Nat. Commun. 2023, 14, 7299. [Google Scholar] [CrossRef] [PubMed]
- Feist, J.D.; Lee, D.C.; Xia, Y. A Versatile Approach for the Synthesis of Degradable Polymers via Controlled Ring-Opening Metathesis Copolymerization. Nat. Chem. 2022, 14, 53–58. [Google Scholar] [CrossRef] [PubMed]
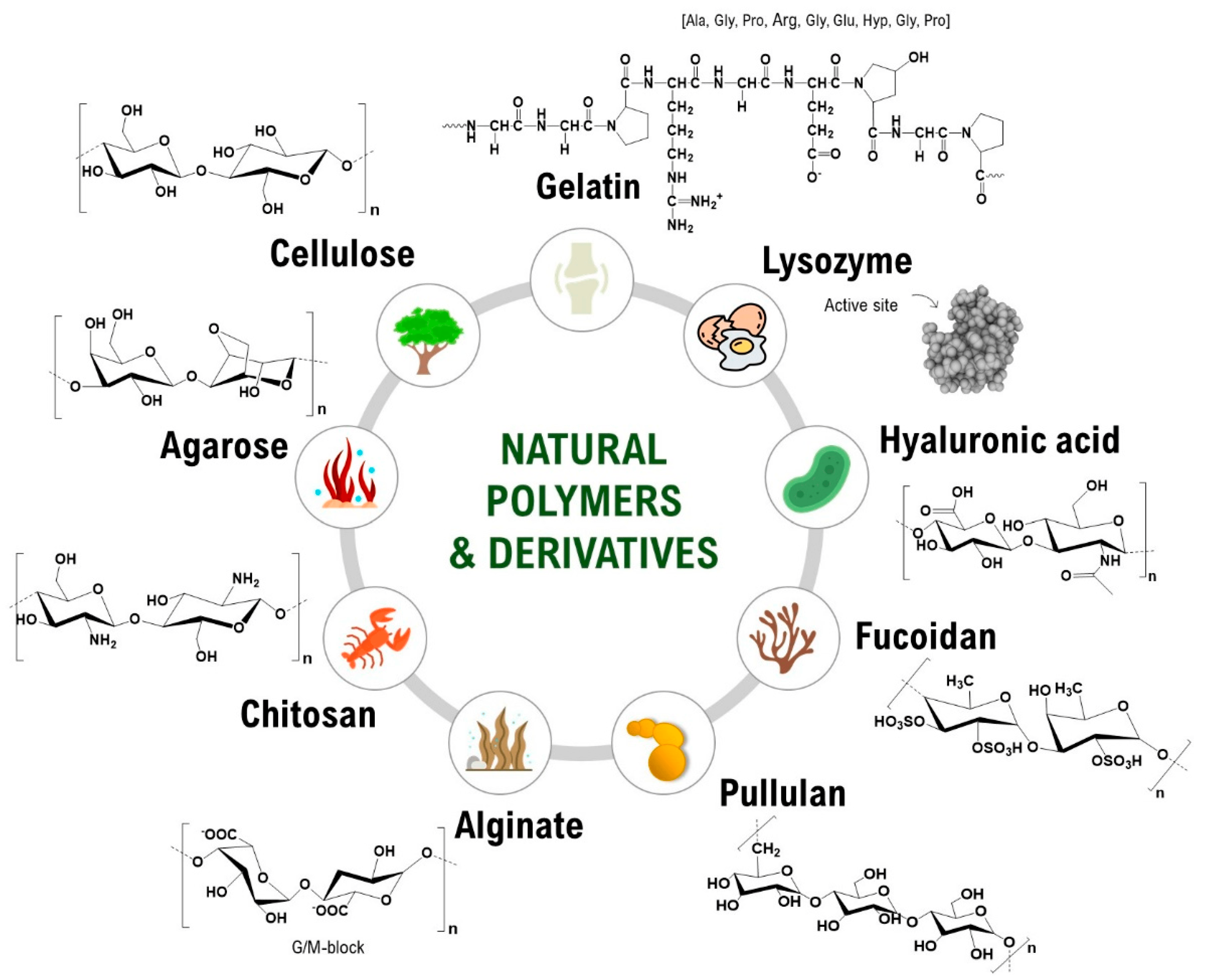



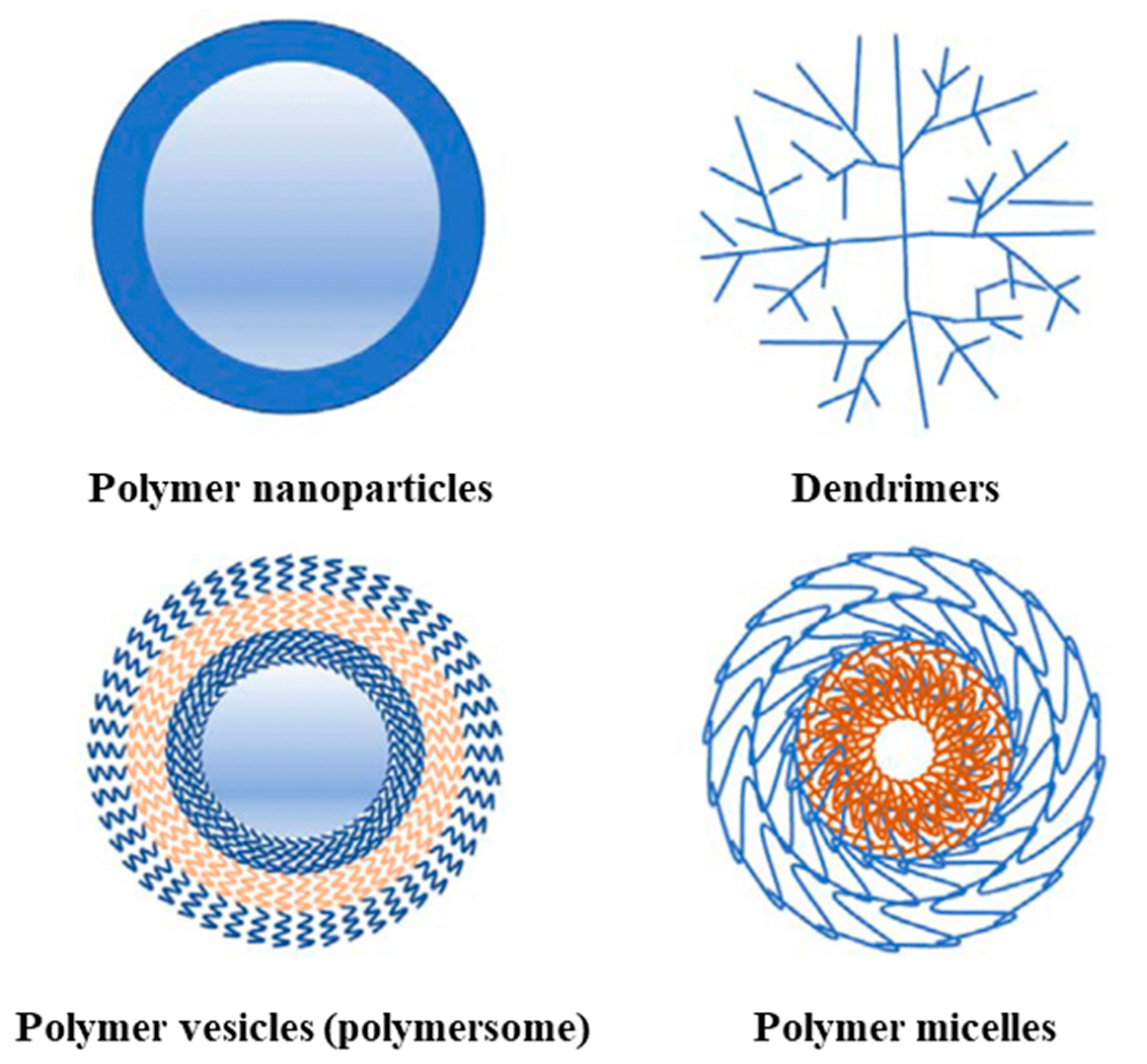
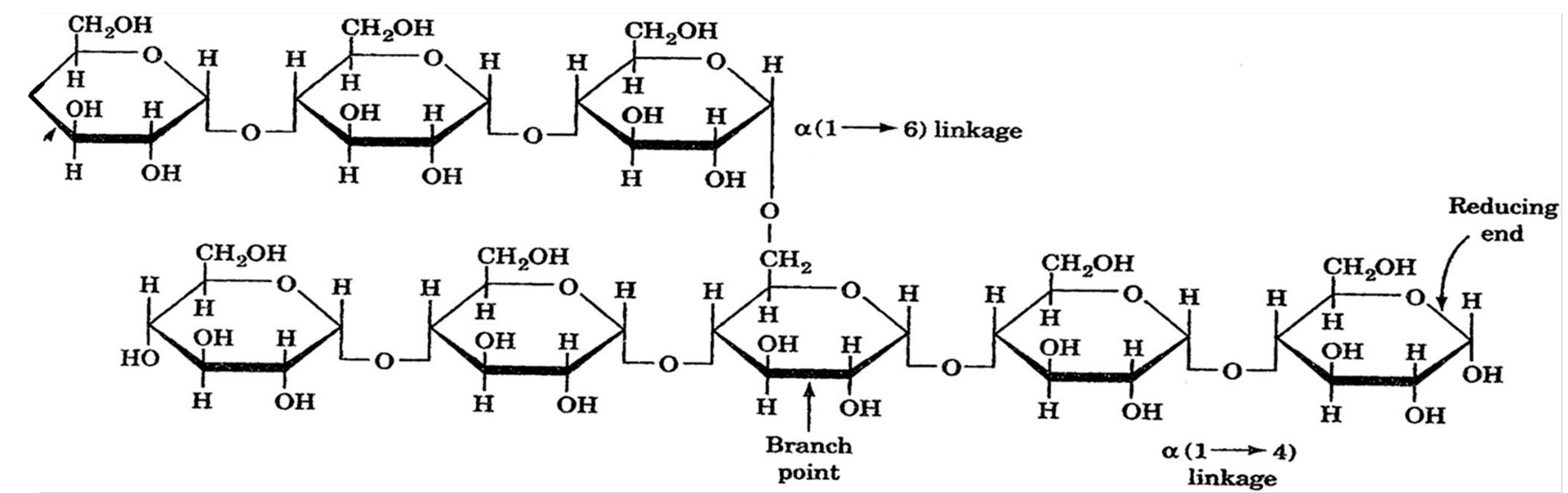


| Natural Polymers | Synthetic Polymers |
|---|---|
| In use for millions of years | First produced 125 years ago |
| Similar or nonidentical repeating units | Identical repeating unit |
| Properties are naturally controlled | Properties are engineered |
| Usually biodegradable | Some are biodegradable |
| The backbone structure is carbon, oxygen, and nitrogen | The backbone is mostly carbon |
| Environmentally friendly | Some are friendly, and some are toxic to the environment |
| Limited recyclability | Most of them are capable of being recycled multiple times |
| Bio-Based Monomers | Source | Characteristics |
|---|---|---|
| Itaconic acid | Aspergillus itaconicus | Antimicrobial activity, non-toxic, biocompatible, biodegradable, chemical reactivity, surfactant forming ability, hydrophilic activity, wound-healing activity, coating forming ability, water uptake ability, drug carrier ability, and hydrogel-forming ability |
| Succinic acid | Actinobacillus succinogenes, Anaerobiospirillum, and Mannheimia succiniciproducens | Biocompatible, biodegradable, non-toxic, chemical reactivity, food additives ability, food flavoring ability, surfactant/detergent extender/foaming ability, drug carrier ability, pH control ability, antimicrobial activity, and corrosion prevention ability |
| Citric acid | Citrus fruits and Aspergillus niger | Biocompatible, biodegradable, non-toxic, excellent chelating property, anti-odor property, chemical reactivity, pH control ability, food additives ability, food flavoring/preservative ability, and drug carrier ability |
| Glutamic acid | Bacillus subtilis and Bacillus licheniformis | Biodegradable, biocompatible, non-toxic, excellent chelating property, heavy metal removal ability, cosmetic property, drug carrier ability, hydrophilic activity, anionic property, thickener property, aging inhibitor ability, and use as an additive |
| Synthetic Polymer | Method of Synthesis | Application |
|---|---|---|
| Polylactide | Ring-opening polymerization of lactide; Polycondensation of lactic acid | Cartilage regeneration; repairing cartilage; bone tissue engineering; controlled drug release formulations that function as carriers; continuous drug-releasing systems |
| Polyurethane | Polyaddition reaction between diols and diisocyanates | Fabrication of polymeric capsules of drugs; myocardial tissue engineering; textiles in medicine; bioink in 3D-printing; phenol degradation |
| Poly(lactic-co-glycolic acid) | Ring-opening copolymerization; Polycondensation of lactic acid and glycolic acid | Myocardial tissue engineering; bioink in 3D-printing; drug-releasing systems |
| Poly (methyl methacrylate) | Free-radical polymerization of methyl methacrylate | Prosthetic dental applications; soft contact lenses; bone tissue regeneration; drug delivery and controlled drug-delivery systems |
| Silicone rubber | Crosslinking of poly(dimethyl siloxanes) | Medical implants; electrical insulation; waterproof coating |
| Poly(vinyl alcohol) | Hydrolysis of polyvinyl acetate | Used in drug production; textiles |
| Poly(vinyl pyrrolidone) | Free radical polymerization of vinylpyrrolidone | Cartilage regeneration |
| Polymer | Advantages | Disadvantages | Perspectives |
|---|---|---|---|
| Collagen * | Good for cell adhesion, proliferation, differentiation, ECM secretion; excellent biocompatibility; biodegradability; low toxicity; rough surface morphology; low immunogenicity; weak antigenicity | Low mechanical strength; difficult disinfection; deformation of collagen-based scaffolds restricts their use in load-bearing tissues; poor stability in aqueous environments; potential for antigenicity through telopeptides | Drug delivery; wound healing; tissue engineering |
| Gelatin * | Infiltration, adhesion, spreading, and proliferation of cells on resulting scaffolds; good stability at high temperatures in a broad pH range; biodegradability; osteoconductivity; non-immunogenic; low antigenicity | Low bioactivity in higher-order gelatin structures in scaffolds; low stability in physiological conditions | Antibacterial; wound healing; tissue engineering |
| Starch * | Biocompatible; thermoplastic; non-cytotoxic; guides various developmental stages of cells; hydrophilicity; substrate for cell adhesion; good biodegradation period | Very high water uptake; low mechanical strength; unstable for long-term application; chemical modifications can lead to toxic byproducts and reduce the degradation rate | Bioplastics |
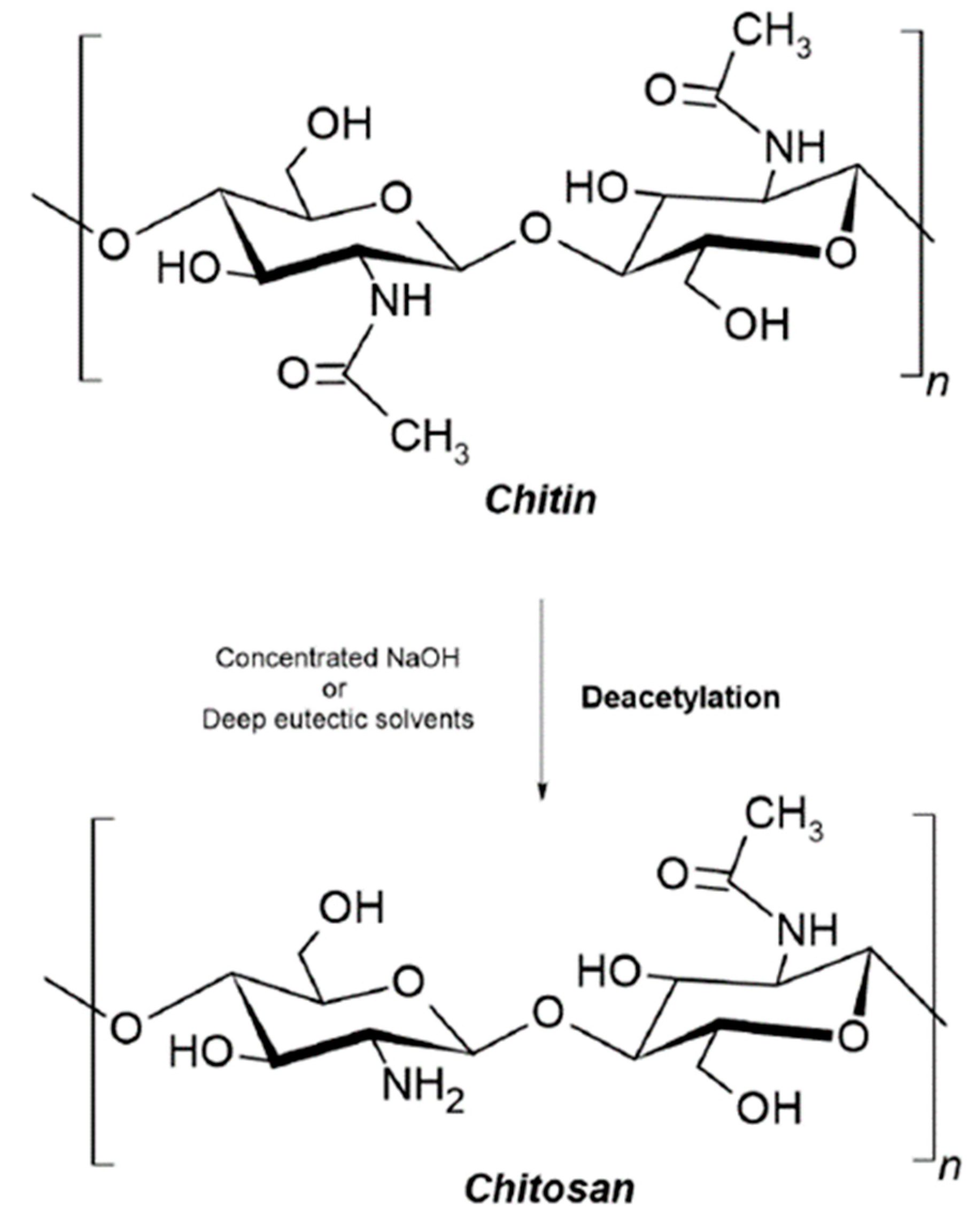
| Chitin/chitosan * | Accelerates tissue repair; prevents the formation of scar tissue; promotes cell adhesion; non-toxic and non-allergenic; bioactivity; anti-inflammatory; osteoconductivity; hemostatic potential; scaffolds could be used for a more extended period; chitosan-based scaffolds can immobilize growth factors | Poor mechanical strength and stability; high viscosity and low solubility at neutral pH; rapid in vivo degradation rate | Textiles in medicine; nano carriers used for nano fertilizers and micronutrients; antibacterial; drug delivery; wound healing; tissue engineering |
| Cellulose * | Stable for tissue engineering applications; good mechanical strength; hydrophilicity; biocompatibility; cytocompatibility; bioactivity | In the human organism, it behaves as a nondegradable or very slowly degradable material | Heart valve tissue engineering; nano carriers used for nano fertilizers and micronutrients; bioplastics; textiles in medicine |
| Polylactic acid (PLA) ** | Biocompatible; cytocompatibility; thermal stability; excellent mechanical strength; good degradation rate; non-toxic degradation products | PLA-based materials lack ideal surface chemistry for cell adhesion and proliferation; brittleness; poor thermal stability; hydrophobicity | Bioplastics; bioink in 3D-printing; drug-releasing systems |
| Polylactic-co-glycolic acid (PLGA) ** | Excellent cell adhesion and proliferation; good mechanical properties; wide range of degradation rates | Poor osteoconductivity; may develop biocompatibility problems | Myocardial tissue engineering; bioink in 3D-printing; drug-releasing systems |
| Polyglycolic acid (PGA) ** | Biocompatible; high tensile modulus; high melting point; undergoes bulk degradation; hydrophilicity | High sensitivity to hydrolysis; challenging to obtain porous PGA scaffolds without toxic solvents | Bioink in 3D-printing |
| Poly(ethylene glycol) (PEG) ** | Bioadhesive; mucoadhesive; hinders protein adsorption; hydrophilic; can be modified to different moieties to pass different requirements of a skin substitute like cell adhesion, short-term degradation, and minimum inflammation; non-immunogenic | Lacks cell-interactive character due to its bio-inert nature; nonreactive, creates insoluble networks | Antifungal |
| Polyvinyl alcohol (PVA) ** | Biocompatible, nontoxic, and noncarcinogenic, it displays a reduced protein-binding tendency, relatively higher elasticity, and water content. It is a highly hydrated water-soluble synthetic polymer with relatively similar tensile strength to human articular cartilages and good lubrication | Lack of cell-adhesive property; less ingrowth of bone cells | Used in drug production; textiles |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satchanska, G.; Davidova, S.; Petrov, P.D. Natural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers 2024, 16, 1159. https://doi.org/10.3390/polym16081159
Satchanska G, Davidova S, Petrov PD. Natural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers. 2024; 16(8):1159. https://doi.org/10.3390/polym16081159
Chicago/Turabian StyleSatchanska, Galina, Slavena Davidova, and Petar D. Petrov. 2024. "Natural and Synthetic Polymers for Biomedical and Environmental Applications" Polymers 16, no. 8: 1159. https://doi.org/10.3390/polym16081159
APA StyleSatchanska, G., Davidova, S., & Petrov, P. D. (2024). Natural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers, 16(8), 1159. https://doi.org/10.3390/polym16081159









