Effects of Polyol Types on Underwater Curing Properties of Polyurethane
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
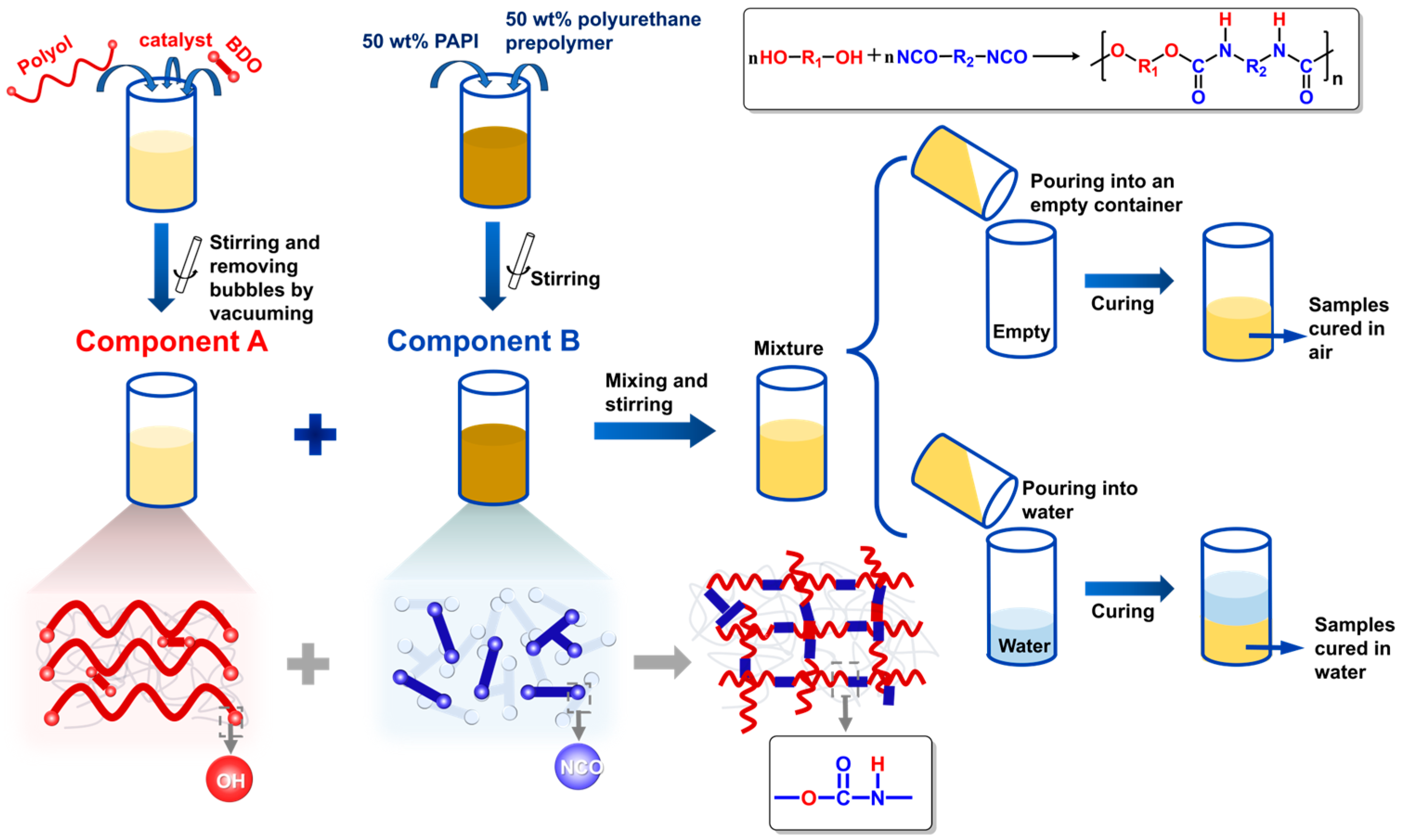
2.2. Preparation of Polyurethanes
2.3. Measurements
2.3.1. Fourier Transform Infrared (FTIR)
2.3.2. Reaction Process Temperature Monitoring
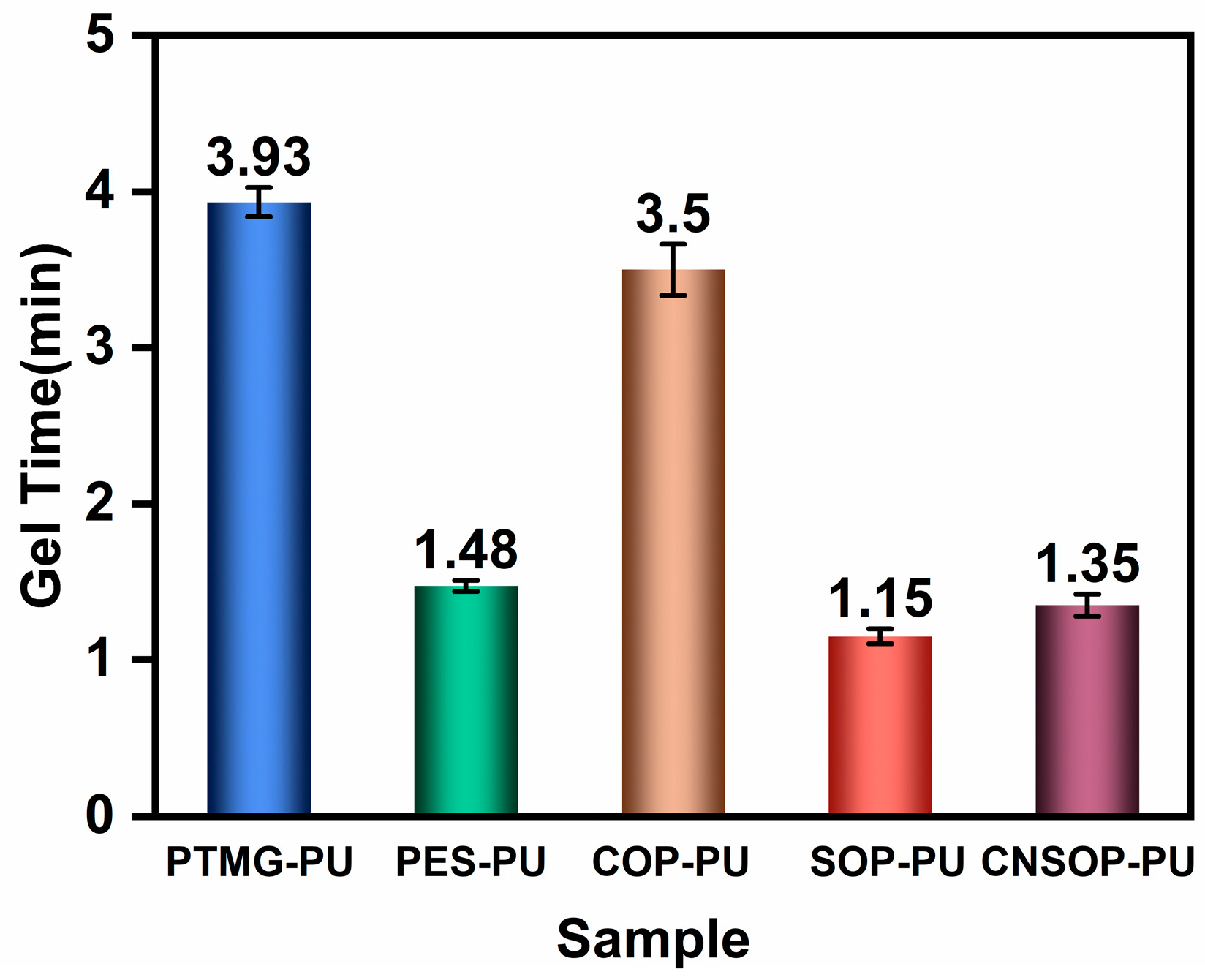
2.3.3. Gel Time
2.3.4. Spreading Diameter and Interfacial Tension Measurement
2.3.5. Volume Expansion Ratio
2.3.6. Scanning Electron Microscopy (SEM)
2.3.7. Adhesion Properties
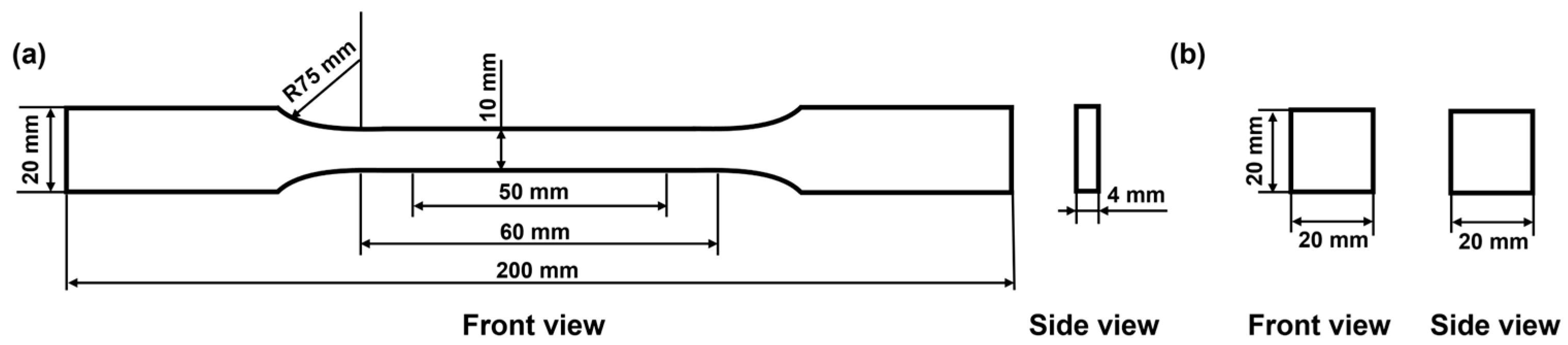
2.3.8. Mechanical Properties
3. Results
3.1. Effect of Polyol Types on Underwater Curing Reaction Characteristics of Polyurethane
3.1.1. Structural Characterization of Polyurethane
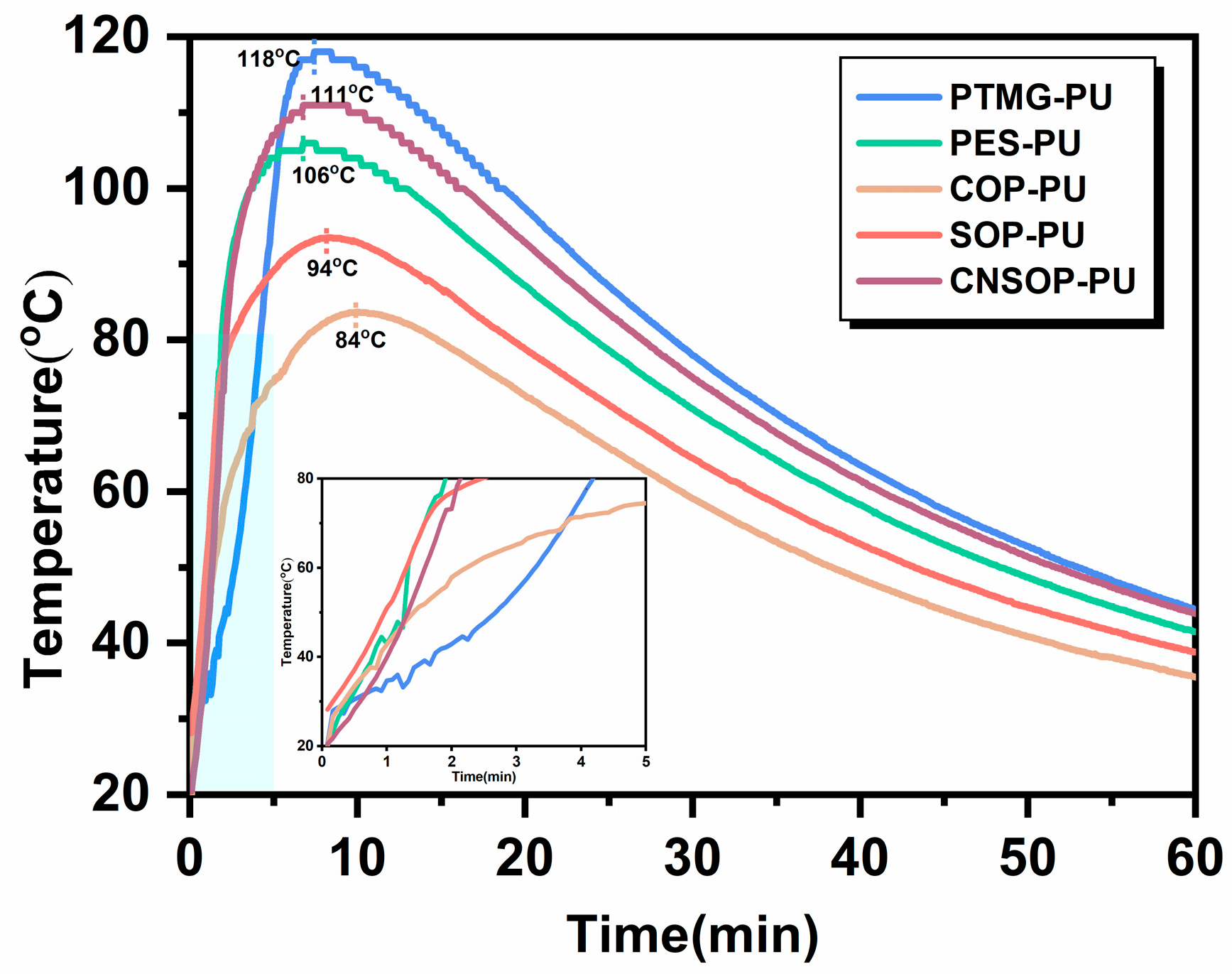
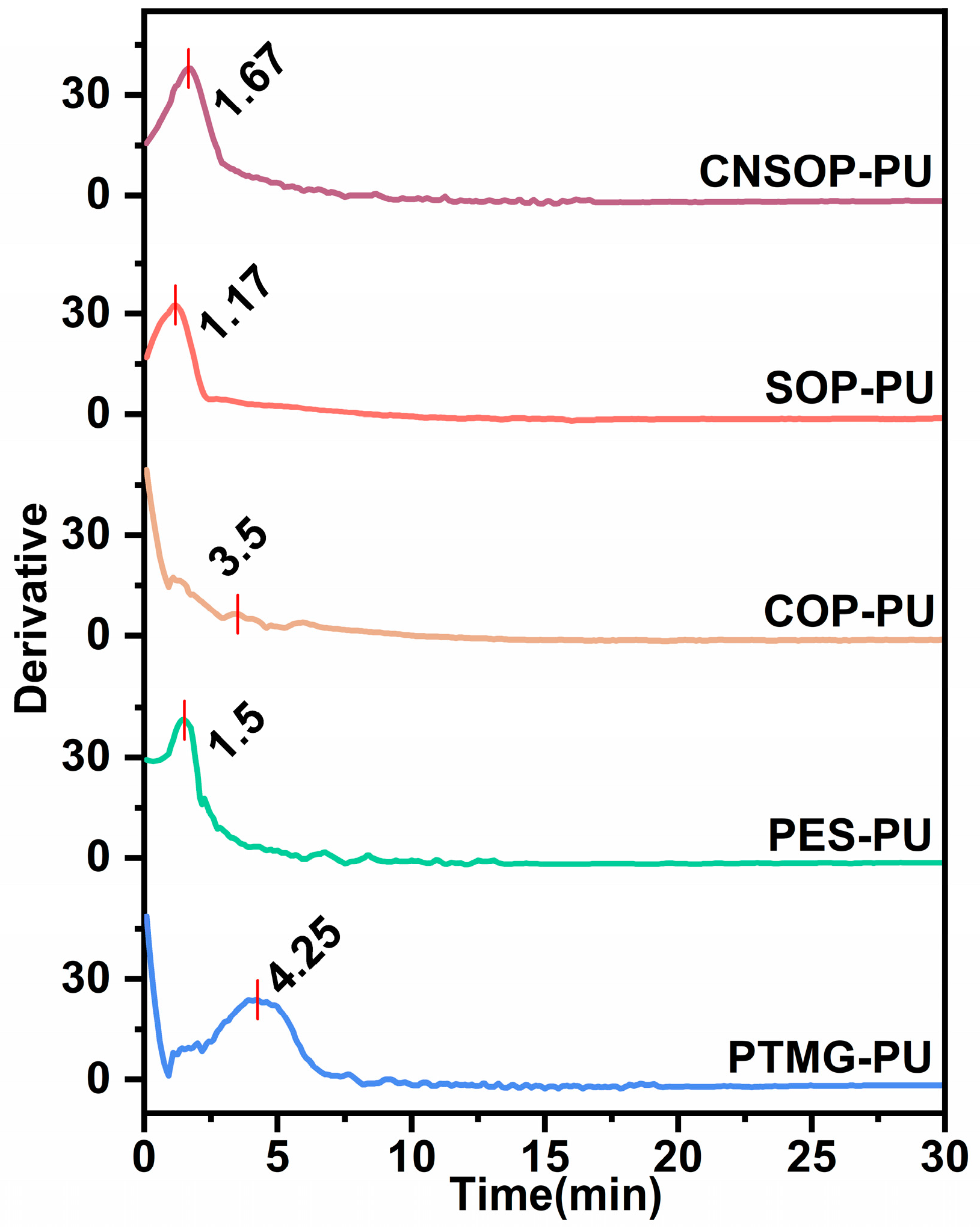
3.1.2. Study on the Exothermic Process of Curing Reactions
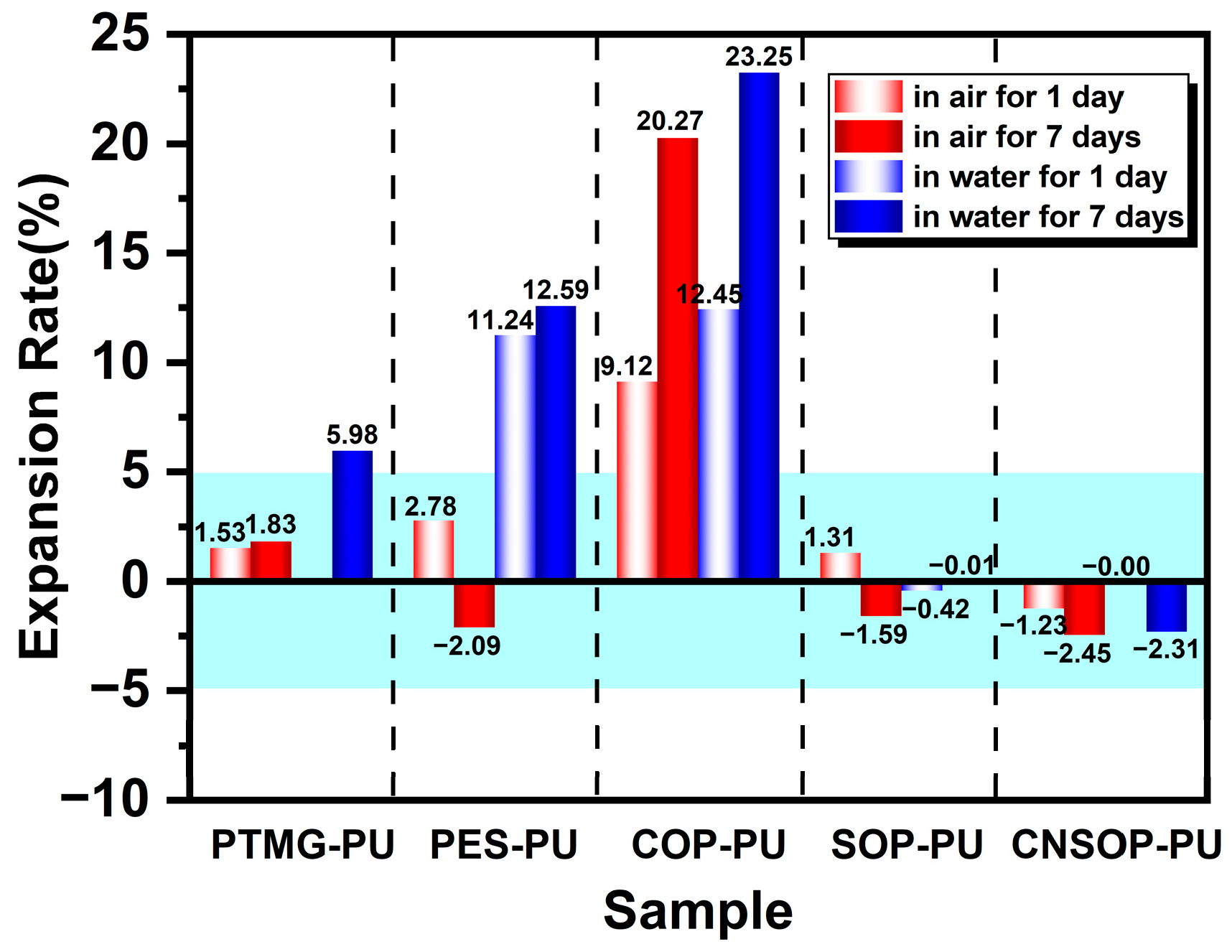
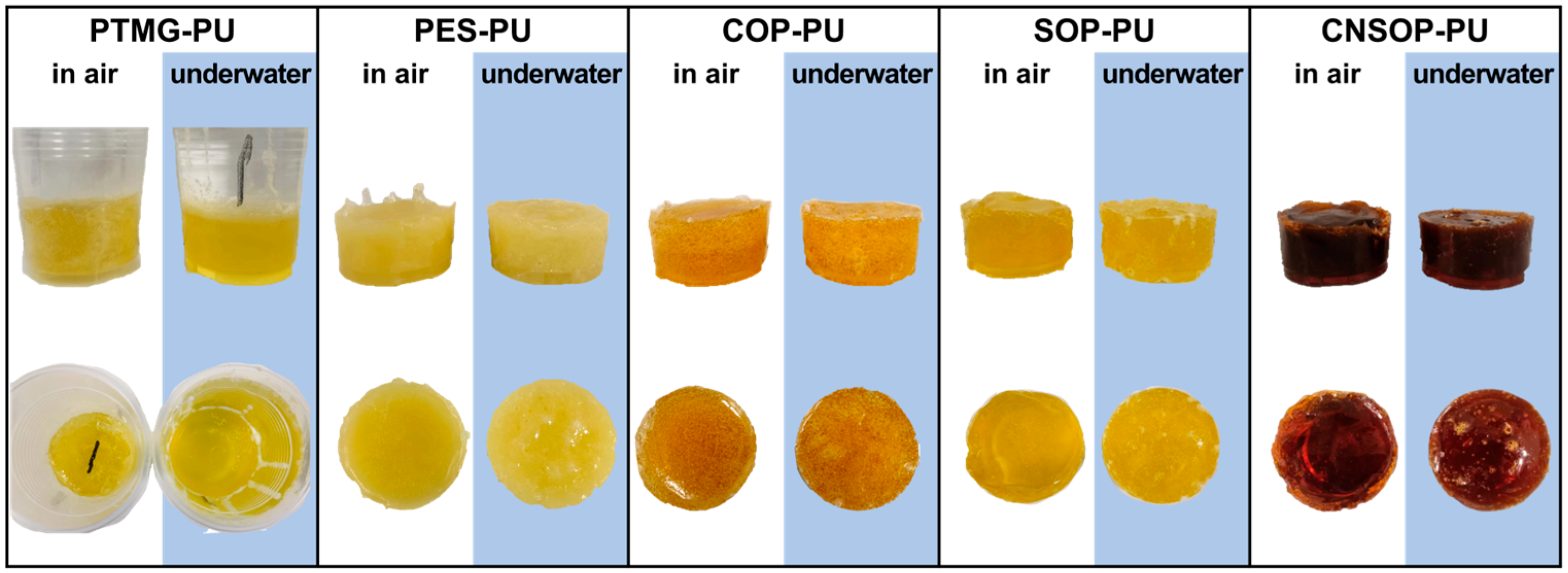
3.1.3. Macroscopic State Comparison of Underwater Curing
3.1.4. Microscopic Morphology Comparison of Underwater Curing
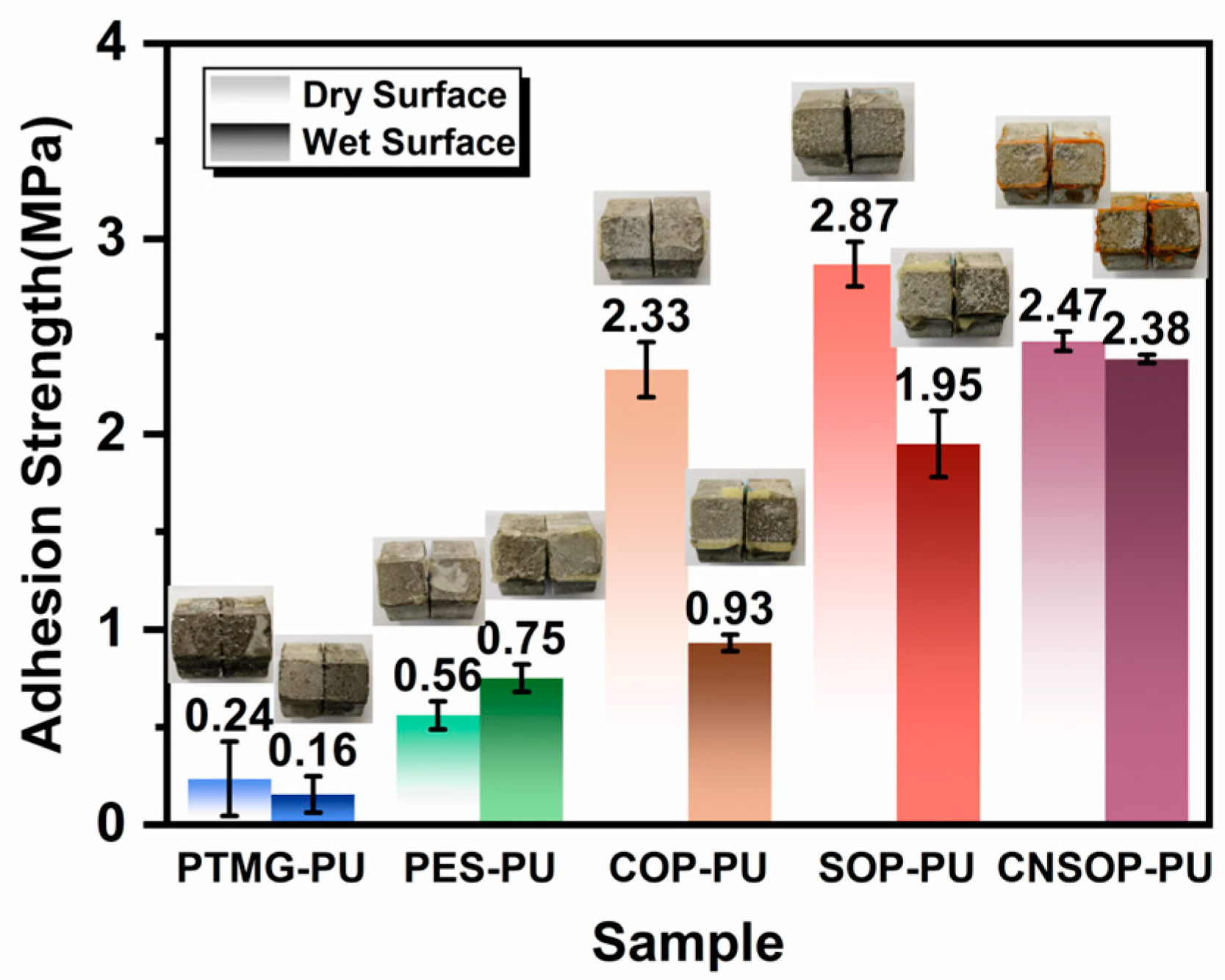
3.1.5. Effect of Different Polyol Types on Adhesion Strength on Wet Surfaces
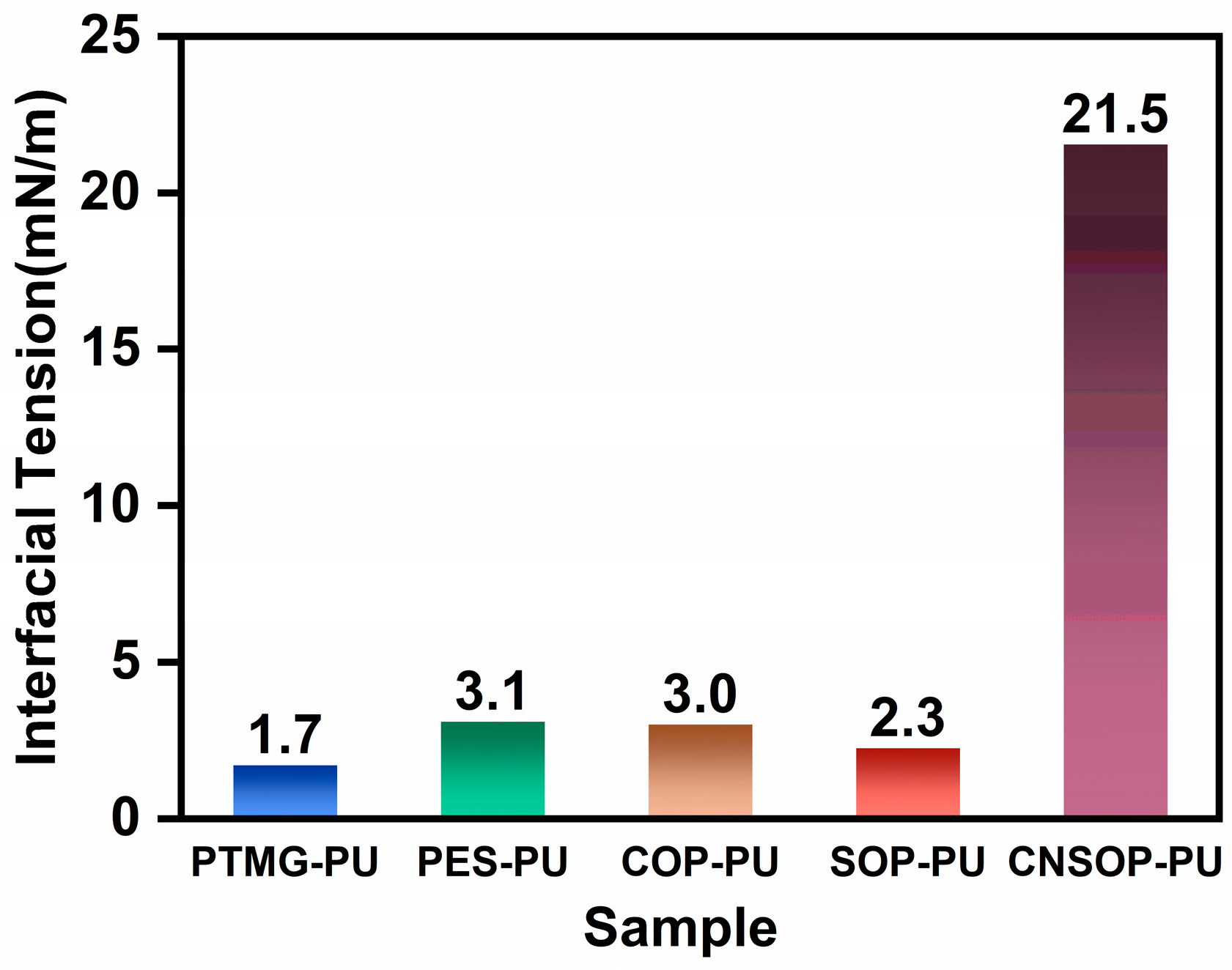
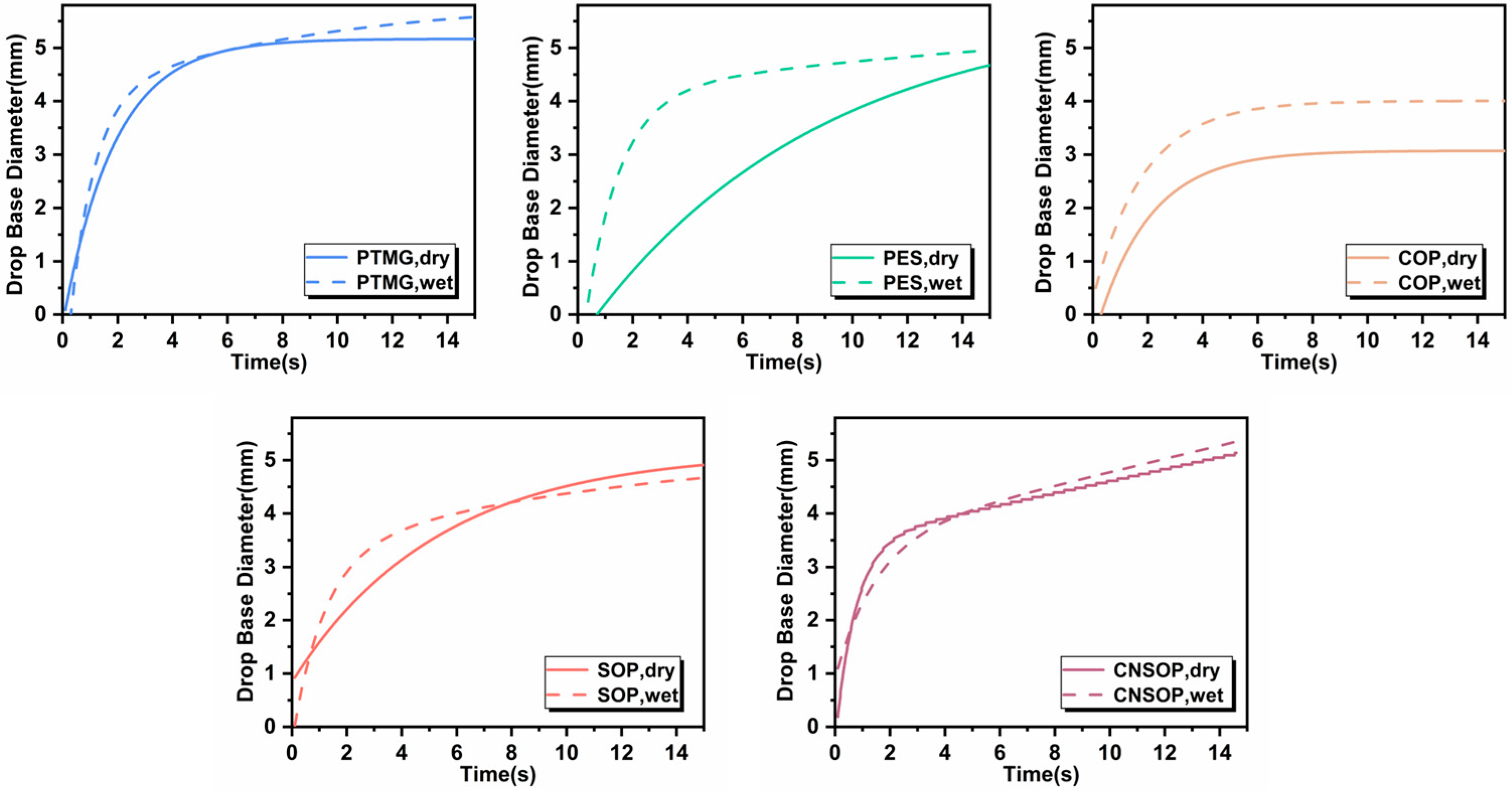
3.1.6. Hydrophobicity of Different Polyol Types
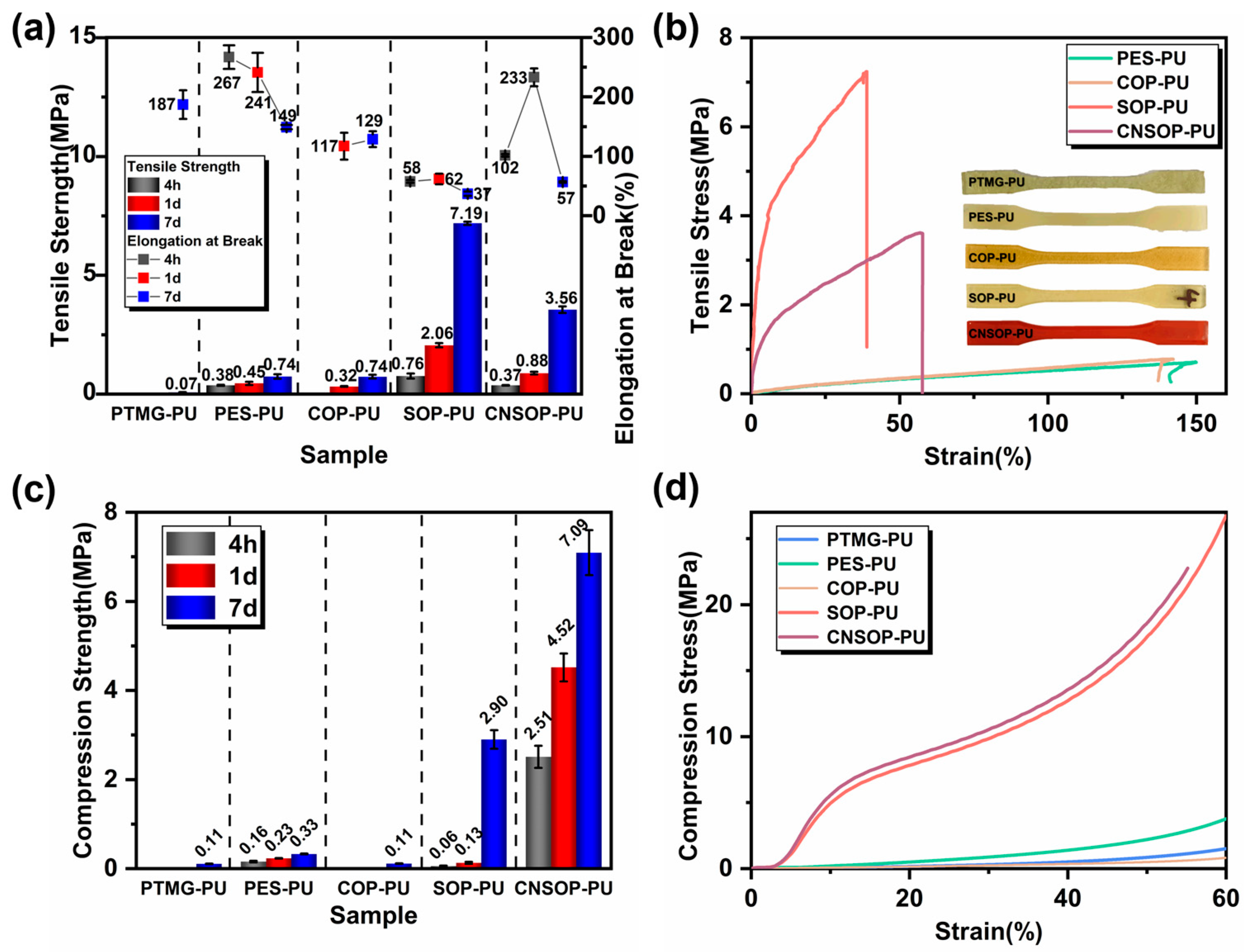
3.2. Effect of Polyol Types on the Mechanical Properties of Polyurethane
4. Conclusions
- To minimize the impact of side reactions between isocyanates and water in polyurethane systems for water-bearing environments, it is crucial to consider both gel time and slurry water solubility. Shorter gel times and better hydrophobicity lead to reduced side reactions, making the system more suitable for underwater applications. Among polyether, polyester, and bio-based polyols examined in this study, soybean oil- and cashew shell oil-modified polyols showed minimal side reactions, highlighting their potential for underwater construction. In contrast, polyether polyols were the least suitable, while castor oil-modified systems showed pronounced side reactions, particularly in underwater conditions.
- Soybean oil-based and cashew nut shell oil-based polyurethanes demonstrated the best overall performance among the five polyols tested; their distinct mechanical properties, due to structural differences, allow for selection based on specific project requirements. PES-PU, with acceptable wet adhesion properties, may be suitable for interface bonding in low mechanical performance applications. SOP-PU, with higher tensile strength, could be considered for deformation joint filling or leakage seam repair, while CNSOP-PU, with higher compressive strength and hydrophobicity, may be suited for underwater stress-bearing applications, such as filling wet base surfaces or repairing tunnel gaps. Further adjustments to formulation may be required to optimize performance.
- No clear correlation was observed between system temperature and the degree of side reactions. Further studies are needed to explore the effects of temperature on isocyanate-water side reactions and bubble dynamics during curing.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Souza, F.M.D.; Kahol, P.K.; Gupta, R.K. Polyurethane Chemistry: Renewable Polyols and Isocyanates; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2021; pp. 1–24. [Google Scholar]
- Somarathna, H.M.C.C.; Raman, S.N.; Mohotti, D.; Mutalib, A.A.; Badri, K.H. The use of polyurethane for structural and infrastructural engineering applications: A state-of-the-art review. Constr. Build. Mater. 2018, 190, 995–1014. [Google Scholar] [CrossRef]
- Wan, Z.; Bian, X.; Li, S.; Chen, Y.; Cui, Y. Remediation of mud pumping in ballastless high-speed railway using polyurethane chemical injection. Constr. Build. Mater. 2020, 259, 120401. [Google Scholar] [CrossRef]
- Huang, Z.; Su, Q.; Huang, J.; Dong, M.; Li, D.; Liu, T. Polyurethane grouting materials with different compositions for the treatment of mud pumping in ballastless track subgrade beds: Properties and application effect. Railw. Eng. Sci. 2022, 30, 204–220. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Wang, J.; Yuan, J.; Jiang, F.; Yu, X.; Xiao, F. Recent applications and developments of polyurethane materials in pavement engineering. Constr. Build. Mater. 2021, 304, 124639. [Google Scholar] [CrossRef]
- Mohamed Jais, I.B. Rapid remediation using polyurethane foam/resin grout in Malaysia. Geotech. Res. 2017, 4, 107–117. [Google Scholar] [CrossRef]
- Hu, C.; Shi, S. Summary of the grouting material for the void beneath cement concrete pavement slab. IOP Conf. Ser. Mater. Sci. Eng. 2018, 382, 022096. [Google Scholar] [CrossRef]
- Lat, D.C.; Ali, N.; Jais, I.B.M.; Nor Zurairahetty Mohd Yunus, R.R.; Talip, A.R.A. A review of polyurethane as a ground improvement method. Malays. J. Fundam. Appl. Sci. 2020, 16, 70–74. [Google Scholar] [CrossRef]
- Li, S.; Wang, P.; Yuan, C.; Li, J.; Ma, P.; Zhi, B.; Zhou, H.; Tian, Y. Adaptability of polyurethane/water glass grouting reinforcement to subsea tunnels. Constr. Build. Mater. 2021, 311, 125354. [Google Scholar] [CrossRef]
- Myslicki, S.; Kordy, H.; Kaufmann, M.; Créac’hcadec, R.; Vallée, T. Under water glued stud bonding fasteners for offshore structures. Int. J. Adhes. Adhes. 2020, 98, 102533. [Google Scholar] [CrossRef]
- Lat, D.C.; Jais, I.B.M.; Ali, N.; Baharom, B.; Mohd Yunus, N.Z.; Mat Yusof, D.A. Uplift and settlement prediction model of marine clay soil e integrated with polyurethane foam. Open Eng. 2019, 9, 481–489. [Google Scholar] [CrossRef]
- Zhang, H. Research progress and application of polyurethane material in hydraulic engineering. J. Water Resour. Archit. Eng. 2022, 20, 8–18. [Google Scholar]
- Guo, C.; Chu, X.; Wang, F. The feasibility of non-water reaction polymer grouting technology application in seepage prevention for tailings reservoirs. Water Supply 2017, 18, 203–213. [Google Scholar] [CrossRef]
- Chen, Y.; Li, A.; Yang, D.; Liu, T.; Li, X.; Tang, J.; Jiang, C. Study on the interaction between low-viscosity high-permeability pregrouting sealing material and coal and its application. Adv. Polym. Technol. 2020, 2020, 1217285. [Google Scholar] [CrossRef]
- Shkapenko, G.; Gmitter, G.T.; Gruber, E.E. Mechanism of the water-isocyanate reaction. Ind. Eng. Chem. 1960, 52, 605–608. [Google Scholar] [CrossRef]
- Hannay, R.C. Method for Setting or Resetting Poles in the Ground with Foamed Polyurethane Resin. U.S. Patent 3,968,657, 1976. [Google Scholar]
- Botrie, A.; Goodman, N. Polyurethane Foam in Foundation Footings for Load-Bearing Structures. U.S. Patent 2,016,015,316,2A1, 2015. [Google Scholar]
- Stamenkovic, J.; Cakic, S.; Nikolic, G. Study of the catalytic selectivity of an aqueous two-component polyurethane system by ftir spectroscopy. Hem. Ind. 2003, 57, 559–562. [Google Scholar] [CrossRef]
- Li, X.; Wang, F.; Cai, X.; Meng, S.; Hu, X.; Tang, L. A porous IPN-structured polyurethane/epoxy grouting material with low viscosity, high strength and low volume shrinkage. Constr. Build. Mater. 2024, 449, 138312. [Google Scholar] [CrossRef]
- Li, M.; Fang, H.; Zhang, C.; Du, M.; Wang, F. Study on the new polyurethane material suitable for foaming in water. Constr. Build. Mater. 2022, 354, 129163. [Google Scholar] [CrossRef]
- Prisacariu, C.; Agherghinei, I. Reactions in solid state within polyurethanes. Kinetics and postcure reaction mechanism in casting polyurethane elastomers. J. Macromol. Sci. Part A 2000, 37, 785–806. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, W.; Tan, J.; Yang, D.; Zhang, Q. New method to evaluate antiwashout performance of grout for preventing water-inrush disasters. Int. J. Geomech. 2020, 20, 06019021. [Google Scholar] [CrossRef]
- Hao, M.; Li, X.; Zhong, Y.; Zhang, B.; Wang, F. Experimental study of polyurethane grout diffusion in a water-bearing fracture. J. Mater. Civ. Eng. 2021, 33, 04020485. [Google Scholar] [CrossRef]
- GB/T 7193-2008; Test Methods for Unsaturated Polyester Resins. Standards Press of China: Beijing, China, 2008.
- GB/T 16777-2008; Test Methods for Building Waterproofing Coatings. Standards Press of China: Beijing, China, 2008.
- JC/T 1041-2007; Epoxy Grouting Resin for Concrete Crack. Standards Press of China: Beijing, China, 2007.
- GB/T 2567-2008; Test Methods for Properties of Resin Casting Body. Standards Press of China: Beijing, China, 2008.
- Xu, Q.; Lin, J.; Jiang, G. Synthesis, characterization and properties of soybean oil-based polyurethane. Polymers 2022, 14, 2201. [Google Scholar] [CrossRef] [PubMed]
- Kathalewar, M.; Sabnis, A.; D’Melo, D. Polyurethane coatings prepared from CNSL based polyols: Synthesis, characterization and properties. Prog. Org. Coat. 2014, 77, 616–626. [Google Scholar] [CrossRef]
- Gama, N.; Ferreira, A.; Barros-Timmons, A. Cure and performance of castor oil polyurethane adhesive. Int. J. Adhes. Adhes. 2019, 95, 102413. [Google Scholar] [CrossRef]
- Wang, Z.; Du, M.; Fang, H.; Zhao, P.; Yao, X.; Chen, S. The development of high-strength, anticorrosive, and strongly adhesive polyaspartate polyurea coating suitable for pipeline spray repairs. J. Appl. Polym. Sci. 2024, 141, e55668. [Google Scholar] [CrossRef]
- Huang, Y.J.; Liang, C.M. Volume shrinkage characteristics in the cure of low-shrink unsaturated polyester resins. Polymer 1996, 37, 401–412. [Google Scholar] [CrossRef]
- Shahid, M.Z.; Usman, M.R.; Akram, M.S.; Khawaja, S.Y.; Afzal, W. Initial interfacial tension for various organic–water systems and study of the effect of solute concentration and temperature. J. Chem. Eng. Data 2017, 62, 1198–1203. [Google Scholar] [CrossRef]



| Sample | Polyol Type | Polyol Hydroxyl Value (mg KOH/g) | Polyol (wt%) | 1,4-Butanediol (wt%) | Catalyst (wt%) | Isocyanate Component (wt%) |
|---|---|---|---|---|---|---|
| PTMG-PU | polyether polyol | 173 | 60.08 | 3.17 | 2 | 34.75 |
| PES-PU | polyester polyol | 167 | 60.83 | 3.20 | 2 | 33.97 |
| COP-PU | castor oil-modified polyol | 195 | 57.95 | 3.05 | 2 | 37.00 |
| SOP-PU | soybean oil-modified polyol | 180 | 59.36 | 3.13 | 2 | 35.52 |
| CNSOP-PU | cashew nut shell oil-modified polyol | 175 | 59.72 | 3.15 | 2 | 35.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zhang, Y.; Liu, Y.; Cui, Y.; Zhao, M.; Peng, S.; Wang, H.; Song, Z.; Zhang, Q.; Shi, D.; et al. Effects of Polyol Types on Underwater Curing Properties of Polyurethane. Polymers 2025, 17, 5. https://doi.org/10.3390/polym17010005
Zhang C, Zhang Y, Liu Y, Cui Y, Zhao M, Peng S, Wang H, Song Z, Zhang Q, Shi D, et al. Effects of Polyol Types on Underwater Curing Properties of Polyurethane. Polymers. 2025; 17(1):5. https://doi.org/10.3390/polym17010005
Chicago/Turabian StyleZhang, Cheng, Yixuan Zhang, Yao Liu, Yiming Cui, Ming Zhao, Shuai Peng, Hecong Wang, Zuobao Song, Qunchao Zhang, Dean Shi, and et al. 2025. "Effects of Polyol Types on Underwater Curing Properties of Polyurethane" Polymers 17, no. 1: 5. https://doi.org/10.3390/polym17010005
APA StyleZhang, C., Zhang, Y., Liu, Y., Cui, Y., Zhao, M., Peng, S., Wang, H., Song, Z., Zhang, Q., Shi, D., & Zhu, Y. (2025). Effects of Polyol Types on Underwater Curing Properties of Polyurethane. Polymers, 17(1), 5. https://doi.org/10.3390/polym17010005





