Catalyst Selection for Body-Temperature Curable Polyurethane Networks from Poly(δ-Decalactone) and Lysine Diisocyanate
Abstract
1. Introduction
2. Experimental Section
2.1. Material
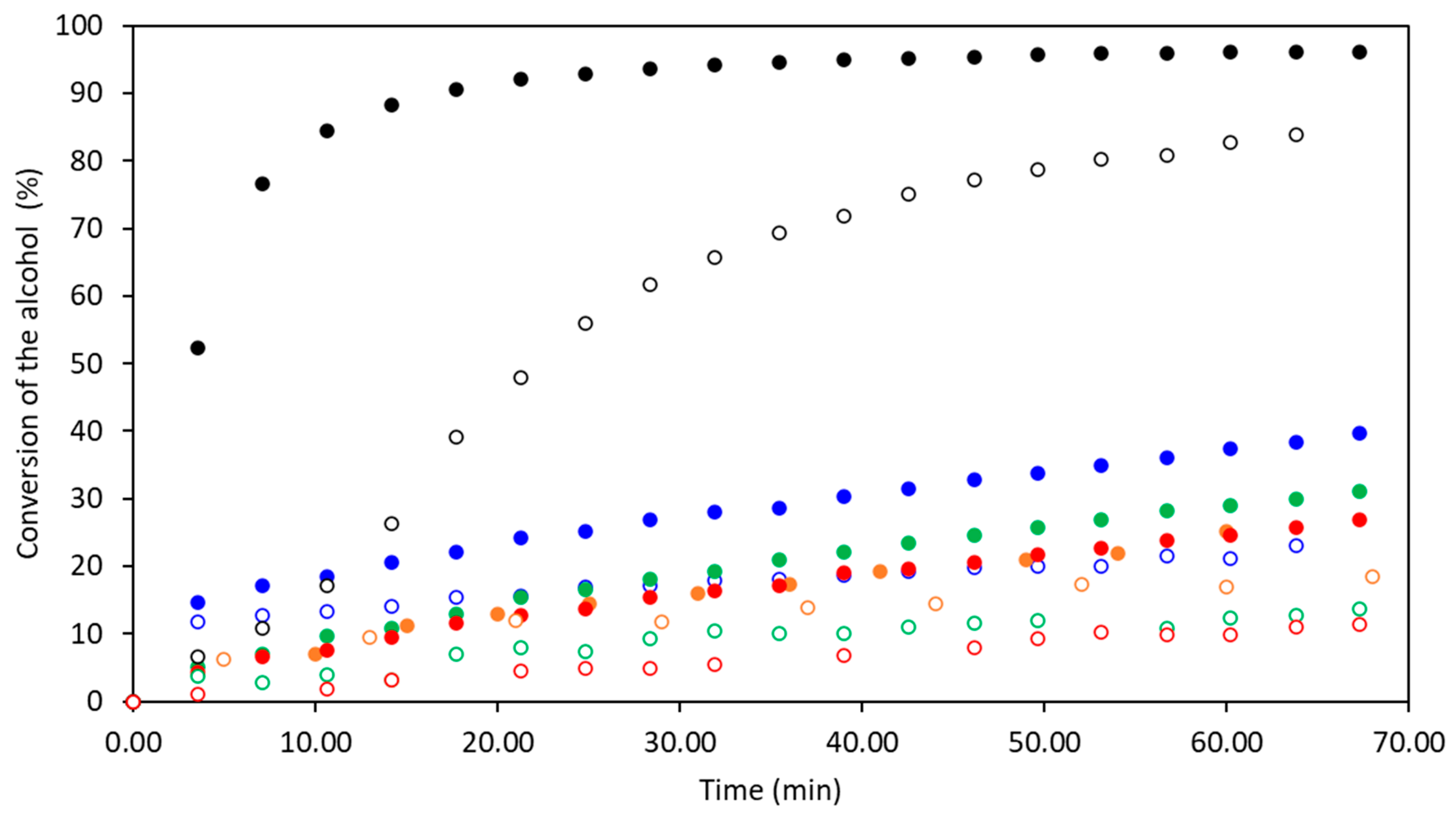
2.2. Kinetic Study
2.3. General Procedure for the Synthesis of PδDL Triol Oligomers
2.4. Functionalization of the Triol Oligomer into Isocyanate
2.5. Synthesis of the Elastomeric Joint
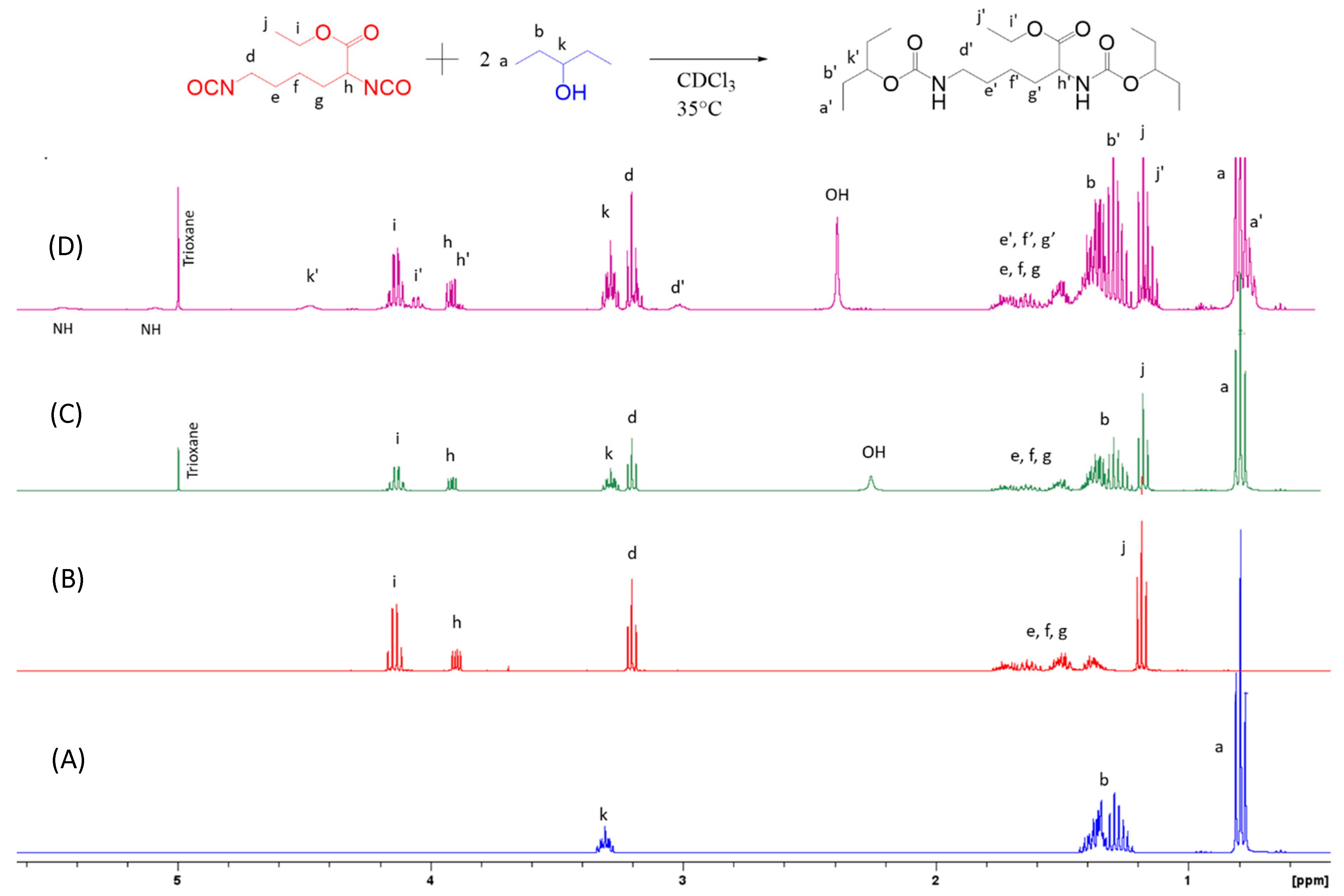
2.6. Nuclear Magnetic Resonance (NMR)
2.7. Size Exclusion Chromatography (SEC)
2.8. PδDL Triol HEW Determination
2.9. PδDL Triisocyanate IEW Determination
2.10. Viscosity and Gelation Time Determination
2.11. Swelling Index (SI) and Gel Content (GC)
2.12. Thermogravimetric Analyses (TGA)
2.13. Differential Scanning Calorimetry (DSC)
2.14. Fourier Transform Infrared Spectroscopy (FTIR)
2.15. Shore A Hardness
2.16. Tensile Test
3. Results and Discussion
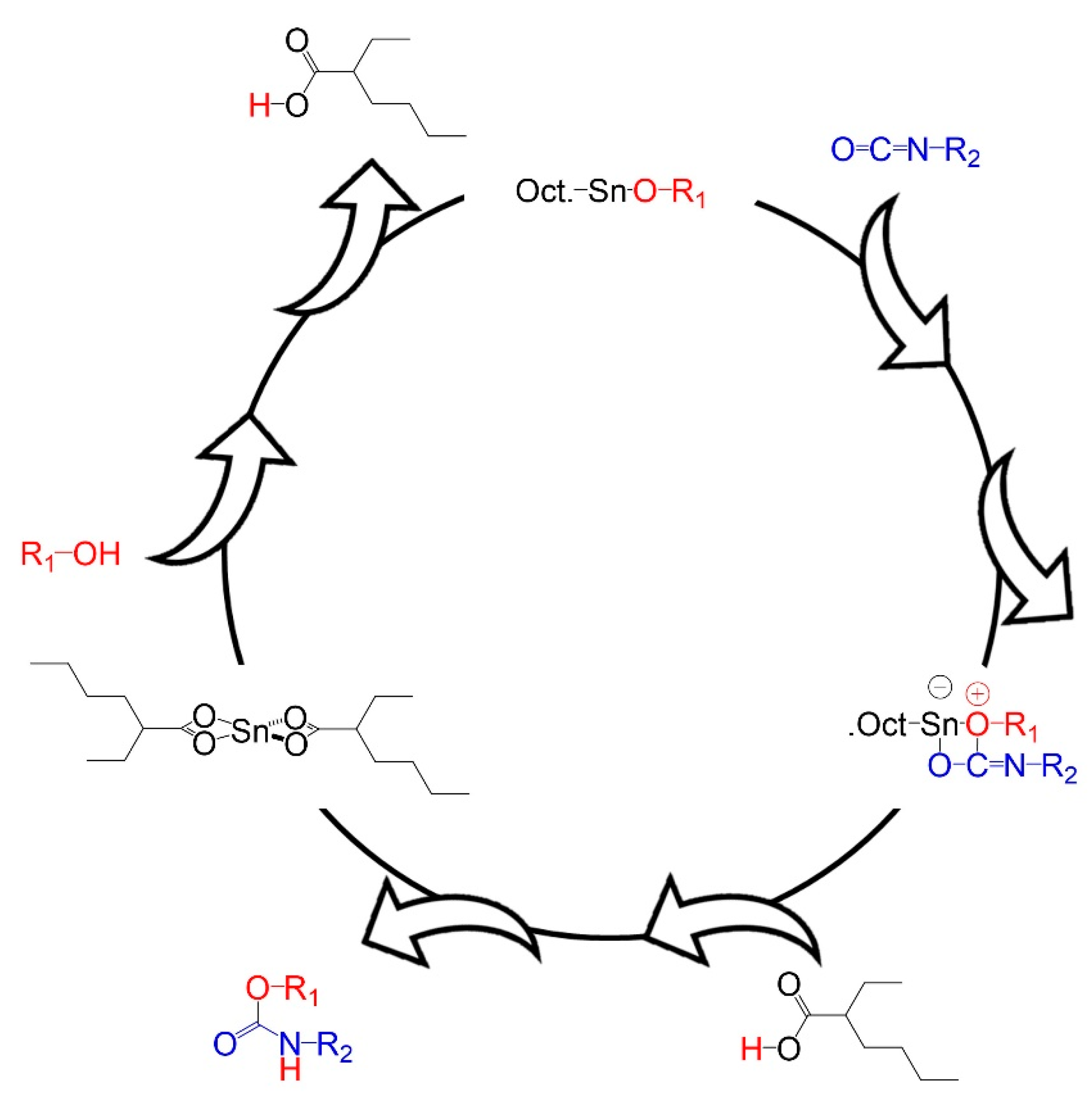
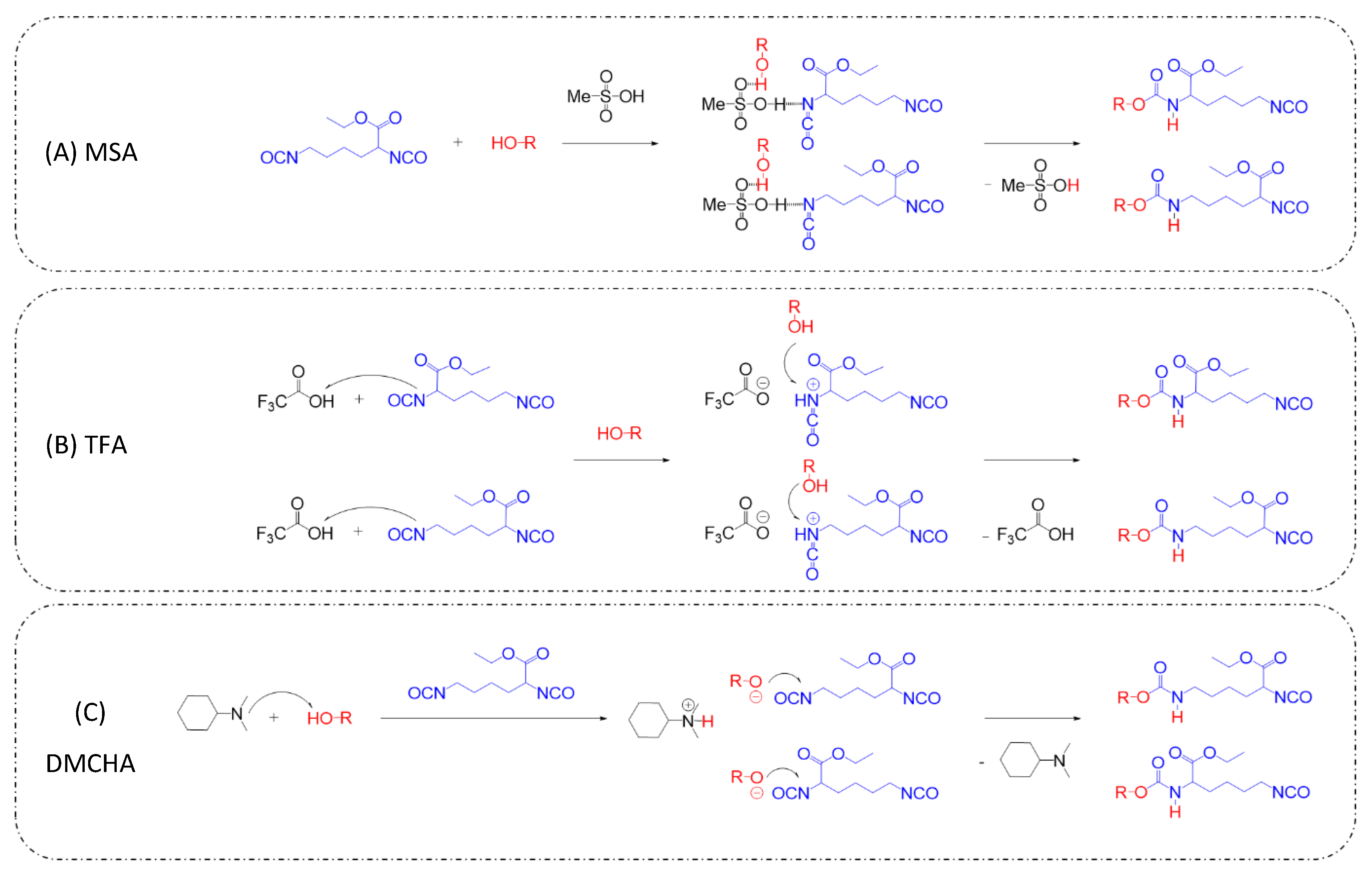
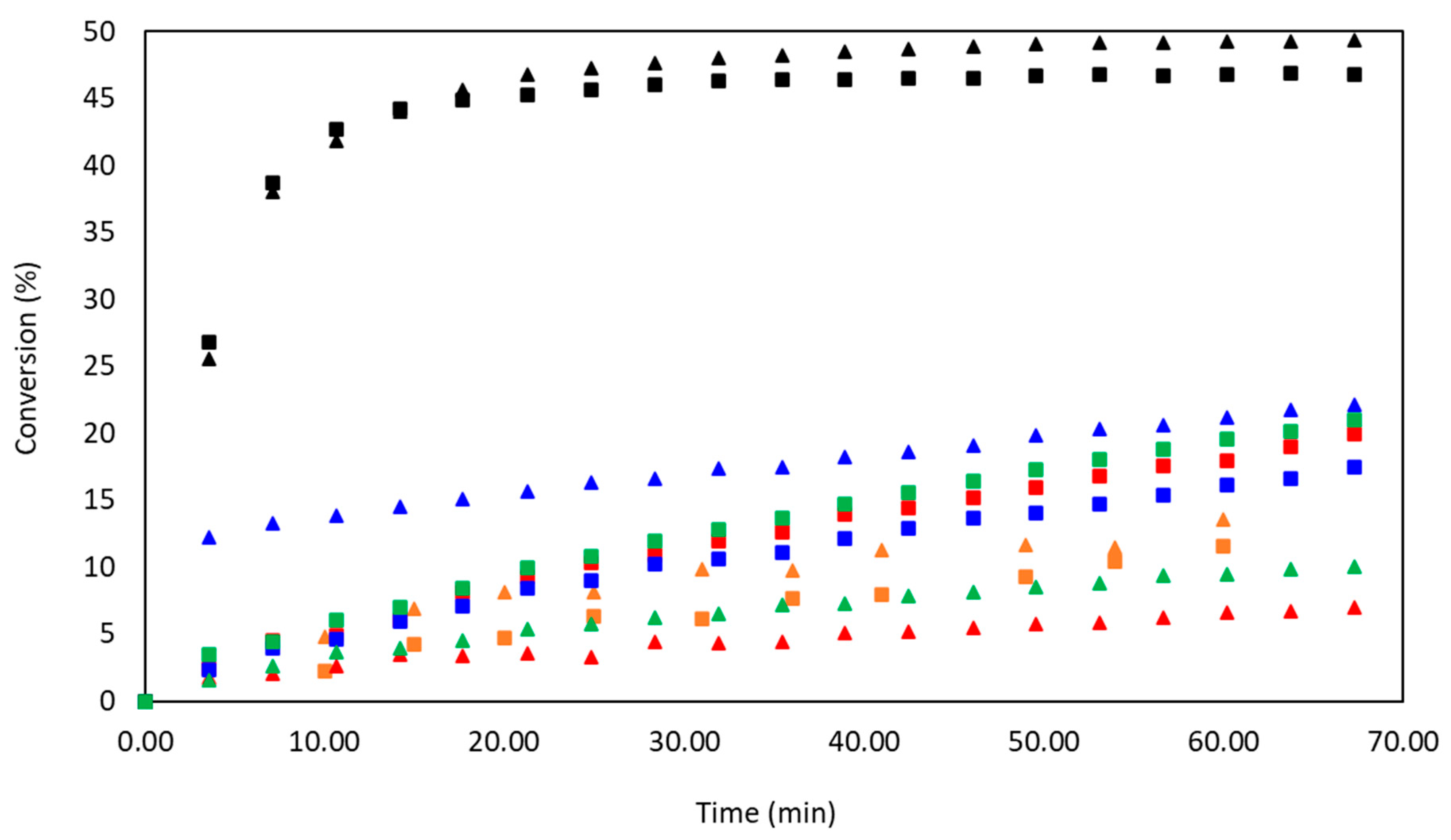
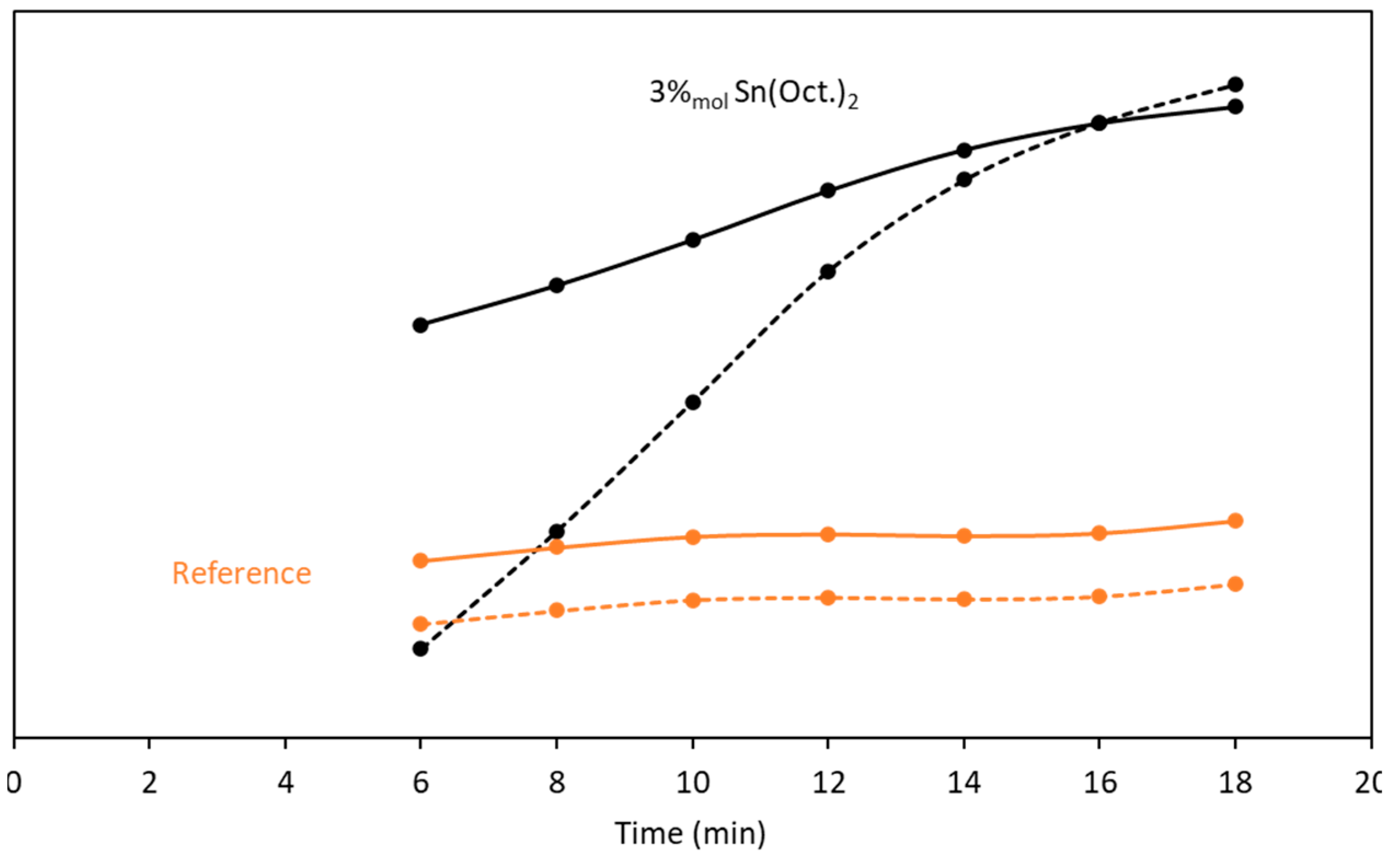
3.1. Kinetic Study
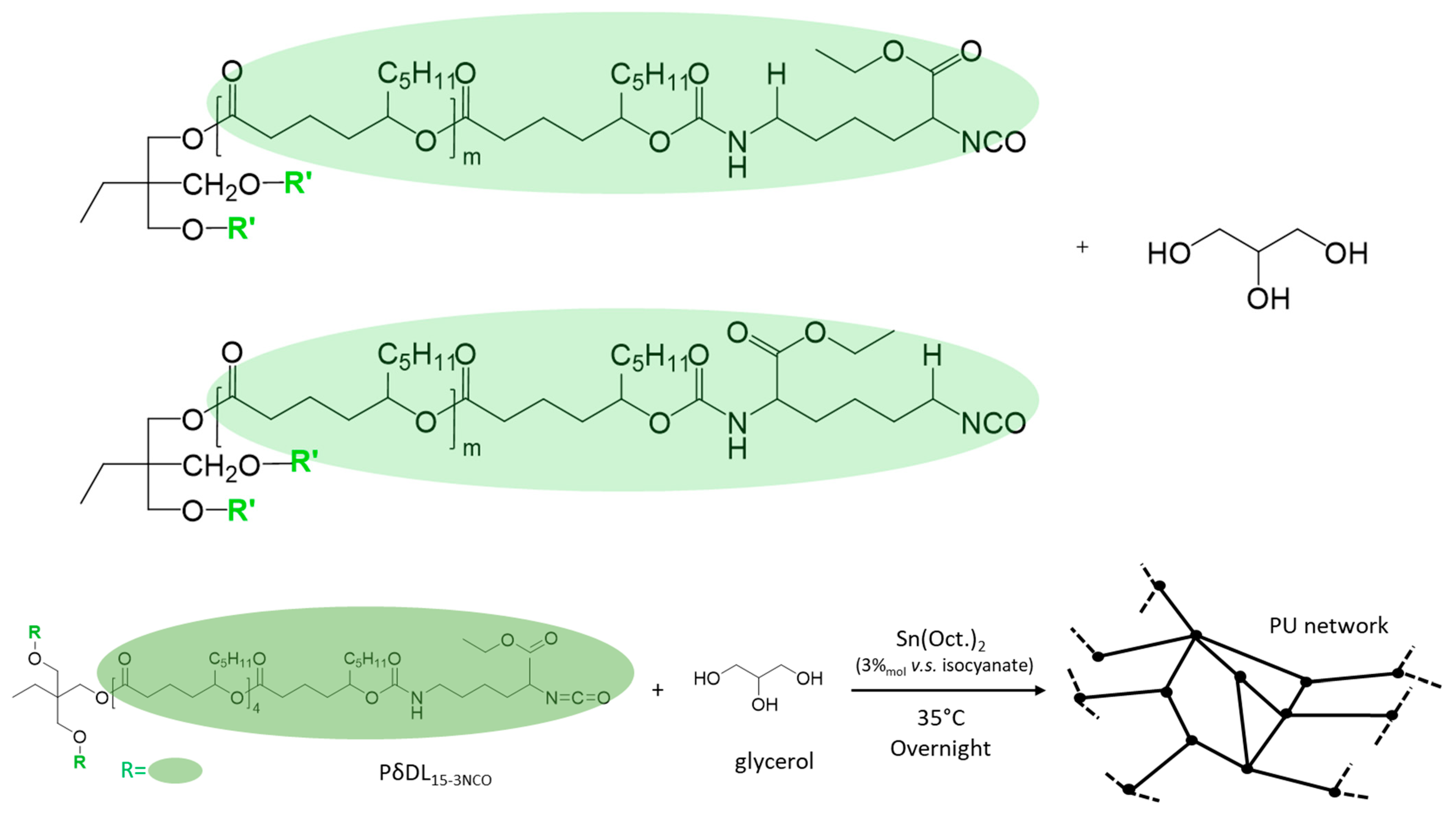
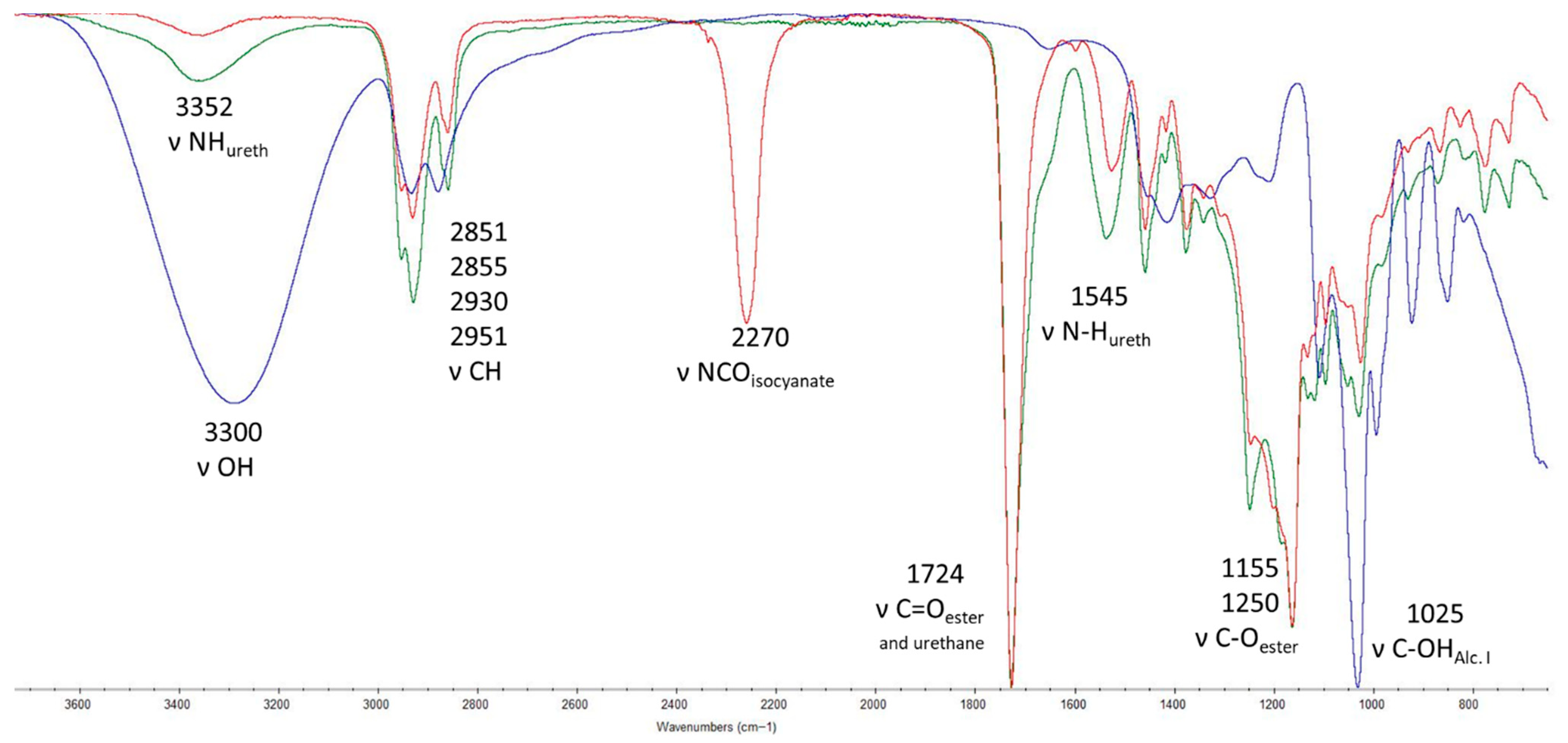
3.2. Synthesis of the Elastomer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Polyurethane Market Size, Trends, Share, Growth, Report 2032. Available online: https://www.precedenceresearch.com/polyurethane-market (accessed on 31 May 2024).
- Polyurethane Market Size, Share & Trends Analysis Report By Product (Rigid Foam, Flexible Foam), By Application (Construction, Furniture & Interiors), By Region, And Segment Forecasts, 2022–2030. Available online: https://www.grandviewresearch.com/industry-analysis/polyurethane-pu-market# (accessed on 1 June 2025).
- Fortune Business Insights. Polyurethane Market Size, Share & COVID-19 Impact Analysis and Regional Forecast 2021–2028; Fortune Business Insights: Pune, India, 2021. [Google Scholar]
- Das, A.; Mahanwar, P. A Brief Discussion on Advances in Polyurethane Applications. Adv. Ind. Eng. Polym. Res. 2020, 3, 93–101. [Google Scholar] [CrossRef]
- Wendels, S.; Avérous, L. Biobased Polyurethanes for Biomedical Applications. Bioact. Mater. 2021, 6, 1083–1106. [Google Scholar] [CrossRef]
- Kucinska-Lipka, J.; Gubanska, I.; Janik, H.; Sienkiewicz, M. Fabrication of Polyurethane and Polyurethane Based Composite Fibres by the Electrospinning Technique for Soft Tissue Engineering of Cardiovascular System. Mater. Sci. Eng. C 2015, 46, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Cherng, J.Y.; Hou, T.Y.; Shih, M.F.; Talsma, H.; Hennink, W.E. Polyurethane-Based Drug Delivery Systems. Int. J. Pharm. 2013, 450, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, M.; Draganova, M. Hydrolytic Stability of Polyurethane Medical Adhesive Dressings. Biomaterials 1994, 15, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Magnin, A.; Pollet, E.; Phalip, V.; Avérous, L. Evaluation of Biological Degradation of Polyurethanes. Biotechnol. Adv. 2020, 39, 107457. [Google Scholar] [CrossRef]
- Yeoh, F.H.; Lee, C.S.; Kang, Y.B.; Wong, S.F.; Cheng, S.F.; Ng, W.S. Production of Biodegradable Palm Oil-Based Polyurethane as Potential Biomaterial for Biomedical Applications. Polymers 2020, 12, 1842. [Google Scholar] [CrossRef]
- Toanen, C.; Dhollander, A.; Bulgheroni, P.; Filardo, G.; Zaffagnini, S.; Spalding, T.; Monllau, J.C.; Gelber, P.; Verdonk, R.; Beaufils, P.; et al. Polyurethane Meniscal Scaffold for the Treatment of Partial Meniscal Deficiency: 5-Year Follow-up Outcomes: A European Multicentric Study. Am. J. Sports Med. 2020, 48, 1347–1355. [Google Scholar] [CrossRef]
- van Minnen, B.S.; van Tienen, T.G. The Current State of Meniscus Replacements. Curr. Rev. Musculoskelet. Med. 2024, 17, 293–302. [Google Scholar] [CrossRef]
- Bhan, K. Meniscal Tears: Current Understanding, Diagnosis, and Management. Cureus 2020, 12, e8590. [Google Scholar] [CrossRef]
- Barber, F.A.; Herbert, M.A. Meniscal Repair Devices. Arthroscopy 2000, 16, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Li, R.; Li, W.; Sun, W.; Yan, Y.; Xiang, X.; Fang, J.; Liao, Y.; Xie, C.; Wang, X.; et al. Silk Fibroin Hydrogel Adhesive Enables Sealed-Tight Reconstruction of Meniscus Tears. Nat. Commun. 2024, 15, 2651. [Google Scholar] [CrossRef] [PubMed]
- Bochyńska, A.I.; Hannink, G.; Grijpma, D.W.; Buma, P. Tissue Adhesives for Meniscus Tear Repair: An Overview of Current Advances and Prospects for Future Clinical Solutions. J. Mater. Sci. Mater. Med. 2016, 27, 85. [Google Scholar] [CrossRef] [PubMed]
- Ghandforoushan, P.; Alehosseini, M.; Golafshan, N.; Castilho, M.; Dolatshahi-Pirouz, A.; Hanaee, J.; Davaran, S.; Orive, G. Injectable Hydrogels for Cartilage and Bone Tissue Regeneration: A Review. Int. J. Biol. Macromol. 2023, 246, 141–8130. [Google Scholar] [CrossRef]
- Olov, N.; Bagheri-Khoulenjani, S.; Mirzadeh, H. Injectable Hydrogels for Bone and Cartilage Tissue Engineering: A Review. Prog. Biomater. 2022, 11, 113–135. [Google Scholar] [CrossRef]
- Bahatibieke, A.; Wei, S.; Feng, H.; Zhao, J.; Ma, M.; Li, J.; Xie, Y.; Qiao, K.; Wang, Y.; Peng, J.; et al. Injectable and in Situ Foaming Shape-Adaptive Porous Bio-Based Polyurethane Scaffold Used for Cartilage Regeneration. Bioact. Mater. 2024, 39, 1–13. [Google Scholar] [CrossRef]
- Fan, W.; Yuan, L.; Li, J.; Wang, Z.; Chen, J.; Guo, C.; Mo, X.; Yan, Z. Injectable Double-Crosslinked Hydrogels with Kartogenin-Conjugated Polyurethane Nano-Particles and Transforming Growth Factor Β3 for in-Situ Cartilage Regeneration. Mater. Sci. Eng. C 2020, 110, 110705. [Google Scholar] [CrossRef]
- Fang, J.-L.; Vanlandingham, M.M.; Beland, F.A.; Felton, R.P.; Maisha, M.P.; Olson, G.R.; Patton, R.E.; Rosenberg, A.S.; da Costa, G.G. Toxicity of High-Molecular-Weight Polyethylene Glycols in Sprague Dawley Rats. Toxicol. Lett. 2022, 359, 22–30. [Google Scholar] [CrossRef]
- Forest, T.; Xu, Q.; Kuruvilla, S.; Vu, H.; Vlasakova, K.; Glaab, W.E.; Hines, C.; Xun, S. Magnetic Resonance and Ultrastructural Characterization of PEGylation-Associated Vacuolation in Nonclinical Models. Toxicol. Pathol. 2017, 45, 604–613. [Google Scholar] [CrossRef]
- Bansal, K.K.; Kakde, D.; Purdie, L.; Irvine, D.J.; Howdle, S.M.; Mantovani, G.; Alexander, C. New biomaterials from renewable resources—Amphiphilic block copolymers from δ-decalactone. Polym. Chem. 2015, 6, 7196–7210. [Google Scholar] [CrossRef]
- Dubey, P.; Kumar, A.; Vaiphei, K.K.; Basrani, S.; Jadhav, A.; Wilen, C.-E.; Rosenholm, J.M.; Bansal, K.K.; Chakravarti, R.; Ghosh, D.; et al. A poly-δ-decalactone (PDL) based nanoemugel for topical delivery of ketoconazole and eugenol against Candida albicans. Nanoscale Adv. 2024, 6, 5322–5336. [Google Scholar] [CrossRef]
- Speidel, A.T.; Chivers, P.R.A.; Wood, C.S.; Roberts, D.A.; Correia, I.P.; Caravaca, A.S.; Chan, Y.K.V.; Hansel, C.S.; Heimgärtner, J.; Müller, E.; et al. Tailored Biocompatible Polyurethane-Poly(Ethylene Glycol) Hydrogels as a Versatile Nonfouling Biomaterial. Adv. Healthc. Mater. 2022, 11, 2201378. [Google Scholar] [CrossRef]
- Basterretxea, A.; Haga, Y.; Sanchez-Sanchez, A.; Isik, M.; Irusta, L.; Tanaka, M.; Fukushima, K.; Sardon, H. Biocompatibility and Hemocompatibility Evaluation of Polyether Urethanes Synthesized Using DBU Organocatalyst. Eur. Polym. J. 2016, 84, 750–758. [Google Scholar] [CrossRef]
- Yeoh, F.H.; Lee, C.S.; Kang, Y.B.; Wong, S.F.; Cheng, S.F. One-Pot Synthesis of Palm Oil-Based Polyester Polyol for Production of Biodegradable and Biocompatible Polyurethane. J. Appl. Polym. Sci. 2018, 135, 46861. [Google Scholar] [CrossRef]
- Delavarde, A.; Savin, G.; Derkenne, P.; Boursier, M.; Morales-Cerrada, R.; Nottelet, B.; Pinaud, J.; Caillol, S. Sustainable Polyurethanes: Toward New Cutting-Edge Opportunities. Prog. Polym. Sci. 2024, 151, 101805. [Google Scholar] [CrossRef]
- Eling, B.; Tomović, Ž.; Schädler, V. Current and Future Trends in Polyurethanes: An Industrial Perspective. Macromol. Chem. Phys. 2020, 221, 2000114. [Google Scholar] [CrossRef]
- Wegener, G.; Brandt, M.; Duda, L.; Hofmann, J.; Klesczewski, B.; Koch, D.; Kumpf, R.-J.; Orzesek, H.; Pirkl, H.-G.; Six, C.; et al. Trends in Industrial Catalysis in the Polyurethane Industry. Appl. Catal. A Gen. 2001, 221, 303–335. [Google Scholar] [CrossRef]
- Ferreira, P.; Pereira, R.; Coelho, J.F.J.; Silva, A.F.M.; Gil, M.H. Modification of the Biopolymer Castor Oil with Free Isocyanate Groups to Be Applied as Bioadhesive. Int. J. Biol. Macromol. 2007, 40, 144–152. [Google Scholar] [CrossRef]
- Villegas-Villalobos, S.; Díaz, L.E.; Vilariño-Feltrer, G.; Vallés-Lluch, A.; Gómez-Tejedor, J.A.; Valero, M.F. Effect of an Organotin Catalyst on the Physicochemical Properties and Biocompatibility of Castor Oil-Based Polyurethane/Cellulose Composites. J. Mater. Res. 2018, 33, 2598–2611. [Google Scholar] [CrossRef]
- Van Maris, R.; Tamano, Y.; Yoshimura, H.; Gay, K.M. Polyurethane Catalysis by Tertiary Amines. J. Cell. Plast. 2005, 41, 305–322. [Google Scholar] [CrossRef]
- Sardon, H.; Engler, A.C.; Chan, J.M.W.; García, J.M.; Coady, D.J.; Pascual, A.; Mecerreyes, D.; Jones, G.O.; Rice, J.E.; Horn, H.W.; et al. Organic Acid-Catalyzed Polyurethane Formation via a Dual-Activated Mechanism: Unexpected Preference of n-Activation over o-Activation of Isocyanates. J. Am. Chem. Soc. 2013, 135, 16235–16241. [Google Scholar] [CrossRef]
- Alsarraf, J.; Ammar, Y.A.; Robert, F.; Cloutet, E.; Cramail, H.; Landais, Y. Cyclic Guanidines as Efficient Organocatalysts for the Synthesis of Polyurethanes. Macromolecules 2012, 45, 2249–2256. [Google Scholar] [CrossRef]
- Arnould, P.; Simon, F.; Fouquay, S.; Pardal, F.; Michaud, G.; Gajan, D.; Raynaud, J.; Monteil, V. Harnessing Catalysis Selectivity and Isophorone Diisocyanate Asymmetry for Tailored Polyurethane Prepolymers and Networks. Macromolecules 2022, 55, 3344–3352. [Google Scholar] [CrossRef]
- Arnould, P.; Bosco, L.; Sanz, F.; Simon, F.N.; Fouquay, S.; Michaud, G.; Raynaud, J.; Monteil, V. Identifying Competitive Tin-or Metal-Free Catalyst Combinations to Tailor Polyurethane Prepolymer and Network Properties. Polym. Chem. 2020, 11, 5725–5734. [Google Scholar] [CrossRef]
- Guelcher, S.A.; Srinivasan, A.; Dumas, J.E.; Didier, J.E.; McBride, S.; Hollinger, J.O. Synthesis, Mechanical Properties, Biocompatibility, and Biodegradation of Polyurethane Networks from Lysine Polyisocyanates. Biomaterials 2008, 29, 1762–1775. [Google Scholar] [CrossRef] [PubMed]
- Bondaug, J.C.S.; Dingcong, R.G.; Hipulan, L.N.; Ochigue, P.C.; Dumancas, G.G.; Alguno, A.C.; Malaluan, R.M.; Lubguban, A.A.; Bacosa, H.P. Development of a Catalyst System for Enhanced Properties of Coconut Diethanolamide-Based Rigid Poly(Urethane-Urea) Foam. ACS Appl. Polym. Mater. 2024, 6, 6875–6887. [Google Scholar] [CrossRef]
- Storey, R.F.; Wiggins, J.S.; Puckett, A.D. Hydrolyzable Poly (Ester-Urethane) Networks from L-Lysine Diisocyanate and D,L-Lactide/e-Caprolactone Homo- and Copolyester. J. Polym. Sci. Part A Polym. Chem. 1994, 32, 2345–2363. [Google Scholar] [CrossRef]
- Sardon, H.; Pascual, A.; Mecerreyes, D.; Taton, D.; Cramail, H.; Hedrick, J.L. Synthesis of Polyurethanes Using Organocatalysis: A Perspective. Macromolecules 2015, 48, 3153–3165. [Google Scholar] [CrossRef]
- Belmokaddem, F.-Z.; Dagonneau, J.; Lhomme, J.; Blanc, R.; Garduno-Alva, A.; Maliverney, C.; Baceiredo, A.; Maerten, E.; Fleury, E.; Méchin, F. Novel Nucleophilic/Basic and Acidic Organocatalysts for Reaction between Poorly Reactive Diisocyanate and Diols. Des. Monomers Polym. 2016, 19, 347–360. [Google Scholar] [CrossRef]
- Lhomme, J. Nouveaux Catalyseurs et Systèmes Catalytiques Appliqués à La Synthèse Du Polyuréthane via La Réaction Isocyanate Alcool, Université Claude Bernard Lyon 1—Institut National des Sciences Appliquées. 2013. Available online: https://tel.archives-ouvertes.fr/tel-01077954 (accessed on 1 June 2025).
- Havlickova, K.; Kostakova, E.K.; Lisnenko, M.; Hauzerova, S.; Stuchlik, M.; Vrchovecka, S.; Vistejnova, L.; Molacek, J.; Lukas, D.; Prochazkova, R.; et al. The Impacts of the Sterilization Method and the Electrospinning Conditions of Nanofibrous Biodegradable Layers on Their Degradation and Hemocompatibility Behavior. Polymers 2024, 16, 1029. [Google Scholar] [CrossRef]
- Feng, Y.; Xiao, K.; He, Y.; Du, B.; Hong, J.; Yin, H.; Lu, D.; Luo, F.; Li, Z.; Li, J.; et al. Tough and Biodegradable Polyurethane-Curcumin Composited Hydrogel with Antioxidant, Antibacterial and Antitumor Properties. Mater. Sci. Eng. C 2021, 121, 111820. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C. Polyurethane for Biomedical Applications: A Review of Recent Developments; Elsevier Masson SAS: Amsterdam, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Gangolli, S. Dictionary of Substances and Their Effects (DOSE, 3rd Electronic Edition); Royal Society of Chemistry: London, UK, 2005. [Google Scholar]
- Available online: https://www.chemicalbook.com/article/trifluoroacetic-acid-physicochemical-property-uses-and-nmr-challenge.htm (accessed on 8 September 2025).
- Hood, G.C.; Redlich, O.; Reilly, C.A. Ionization of Strong Electrolytes. IV. Nuclear Magnetic Resonance and Dissociation of Trifluoroacetic Acid. J. Chem. Phys. 1955, 23, 2229–2230. [Google Scholar] [CrossRef]
- Shertzer, H.G. Patty’s Toxicology CD-ROM; Org. Sulfur Compd. Online Post. Date April 16, 2001; John Wiley sons: New York, NY, USA, 2000. [Google Scholar] [CrossRef]
- Serjeant, E.P.; Dempsey, B. Ionisation Constants of Organic Acids in Aqueous Solution; Elsevier: Amsterdam, The Netherlands, 1979; p. 989. [Google Scholar]
- Uma, K.; Rajapriya, V.; Reksha, S. The Effect of Inhibitor on the Corrosion of Aluminium in Acidic Solutions. SOJ Mater. Sci. Eng. 2016, 4, 1–6. [Google Scholar] [CrossRef][Green Version]
- 2-ethylhexanoic Acid Tin(II) Salt|C16H30O4Sn|CID 9318—PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/9318#section=Hazards-Summary (accessed on 8 September 2025).[Green Version]
- N,N-Dimethylcyclohexylamine|C8H17N|CID 7415—PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/7415#section=Physical-Description (accessed on 8 September 2025).[Green Version]
- Juhász, A.; Serra, Ü.H.; Lakatos, C.; Vadkerti, B.; Rágyanszki, A.; Farkas, Ö.; Kéki, S.; Nagy, L. The Kinetics of Uncatalyzed and Catalyzed Urethane Forming Reactions of Aliphatic Diisocyanates with Butan-1-Ol. New J. Chem. 2023, 47, 16096–16107. [Google Scholar] [CrossRef]
- Zeng, Y.; Chen, X.; Zhao, D.; Li, H.; Zhang, Y.; Xiao, X. Estimation of PKa Values for Carboxylic Acids, Alcohols, Phenols and Amines Using Changes in the Relative Gibbs Free Energy. Fluid Phase Equilib. 2012, 313, 148–155. [Google Scholar] [CrossRef]
- Waleed, H.Q.; Viskolcz, B.; Fiser, B. Urethane Synthesis in the Presence of Organic Acid Catalysts—A Computational Study. Molecules 2024, 29, 2375. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.C. Infrared Spectroscopy of Polymers XIII: Polyurethanes. Spectroscopy 2023, 38, 14–17. [Google Scholar] [CrossRef]
- Shi, Y.; Zhan, X.; Luo, Z.; Zhang, Q.; Chen, F. Quantitative IR Characterization of Urea Groups in Waterborne Polyurethanes. J. Polym. Sci. Part A Polym. Chem. 2007, 46, 2433–2444. [Google Scholar] [CrossRef]
- Heijkants, R.G.J.C.; van Calck, R.V.; de Groot, J.H.; Pennings, A.J.; Schouten, A.J.; van Tienen, T.G.; Ramrattan, N.; Buma, P.; Veth, R.P.H. Design, Synthesis and Properties of a Degradable Polyurethane Scaffold for Meniscus Regeneration. J. Mater. Sci. Mater. Med. 2004, 15, 423–427. [Google Scholar] [CrossRef]
- Asadi, N.; Alizadeh, E.; Rahmani Del Bakhshayesh, A.; Mostafavi, E.; Akbarzadeh, A.; Davaran, S. Fabrication and in Vitro Evaluation of Nanocomposite Hydrogel Scaffolds Based on Gelatin/PCL-PEG-PCL for Cartilage Tissue Engineering. ACS Omega 2019, 4, 449–457. [Google Scholar] [CrossRef]



| Volume (μL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Experiment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| LDI | 117 | 117 | 117 | 117 | 117 | 117 | 117 | 117 | 117 | 117 |
| 1-Butanol | 111 | 111 | 111 | 111 | 111 | |||||
| 3-Pentanol | 132 | 132 | 132 | 132 | 132 | |||||
| TFA solution | 20 | 20 | ||||||||
| MSA solution | 20 | 20 | ||||||||
| Sn Oct solution | 20 | 20 | ||||||||
| DMCHA solution | 20 | 20 | ||||||||
| CDCl3 | 382 | 361 | 362 | 341 | 362 | 341 | 362 | 341 | 362 | 341 |
| Catalyst | Formula | LD50 (rat, oral) (mg·kg−1) a | pKa b |
|---|---|---|---|
| TFA |  | 500/1000 [47] | 0.23/0.26 [48,49] |
| MSA | 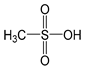 | 648 [50] | −1.9 [51,52] |
| Sn(Oct.)2 | 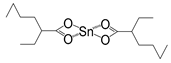 | 5870 [53] | - |
| DMCHA | 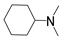 | 348 [54] | 10.9 [54] |
| Exp. | Alcohol | Catalyst 1%mol vs. Iso | Convalcohol a (%) t = 60 min | ConvNCO-I b (%) t = 60 min | ConvNCO-II c (%) t = 60 min | TOF d (s−1) |
|---|---|---|---|---|---|---|
| 1 | 1-butanol | none | 25 | 13 | 12 | 37 |
| 2 | 3-pentanol | 17 | 9 | 8 | 23 | |
| 3 | 1-butanol | TFA | 29 | 9 | 20 | 42 |
| 4 | 3-pentanol | 14 | 7 | 7 | 18 | |
| 5 | 1-butanol | MSA | 37 | 21 | 16 | 44 |
| 6 | 3-pentanol | 22 | 15 | 7 | 18 | |
| 7 | 1-butanol | DMCHA | 25 | 6 | 18 | 36 |
| 8 | 3-pentanol | 9 | 1 | 8 | 17 | |
| 9 | 1-butanol | Sn(Oct.)2 | 96 | 49 | 47 | 1083 |
| 10 | 3-pentanol | 73 | 36 | 37 | 224 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boursier, M.; Lebrun, A.; Parra, K.; Caillol, S.; Negrell, C.; Pinaud, J. Catalyst Selection for Body-Temperature Curable Polyurethane Networks from Poly(δ-Decalactone) and Lysine Diisocyanate. Polymers 2025, 17, 2548. https://doi.org/10.3390/polym17182548
Boursier M, Lebrun A, Parra K, Caillol S, Negrell C, Pinaud J. Catalyst Selection for Body-Temperature Curable Polyurethane Networks from Poly(δ-Decalactone) and Lysine Diisocyanate. Polymers. 2025; 17(18):2548. https://doi.org/10.3390/polym17182548
Chicago/Turabian StyleBoursier, Marine, Aurelien Lebrun, Karine Parra, Sylvain Caillol, Claire Negrell, and Julien Pinaud. 2025. "Catalyst Selection for Body-Temperature Curable Polyurethane Networks from Poly(δ-Decalactone) and Lysine Diisocyanate" Polymers 17, no. 18: 2548. https://doi.org/10.3390/polym17182548
APA StyleBoursier, M., Lebrun, A., Parra, K., Caillol, S., Negrell, C., & Pinaud, J. (2025). Catalyst Selection for Body-Temperature Curable Polyurethane Networks from Poly(δ-Decalactone) and Lysine Diisocyanate. Polymers, 17(18), 2548. https://doi.org/10.3390/polym17182548








