A Solar-Heated Phase Change Composite Fiber with a Core–Shell Structure for the Recovery of Highly Viscous Crude Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagent
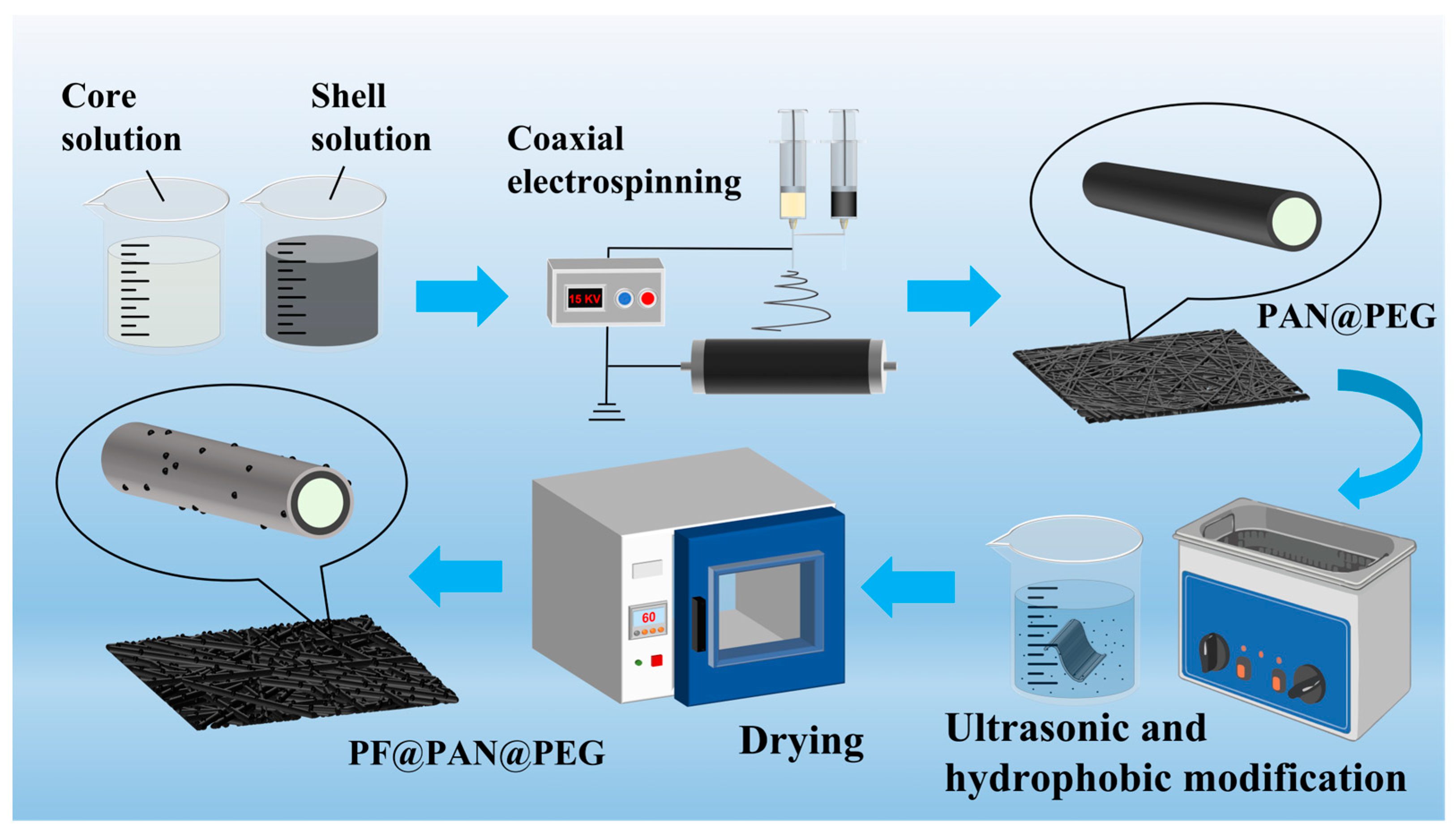
2.2. Preparation of Composite Fibers
2.2.1. Preparation of the PAN Fiber
2.2.2. Preparation of the PAN@PEG Fiber
2.2.3. Modification of the PF@PAN@PEG Fiber
2.3. Characterization
2.4. Photothermal Performance Experiment
2.5. Crude Oil Adsorption and Recovery Experiment
2.5.1. Test of Static Crude Oil Adsorption
2.5.2. Test of Continuous Crude Oil Recovery
3. Results and Discussion
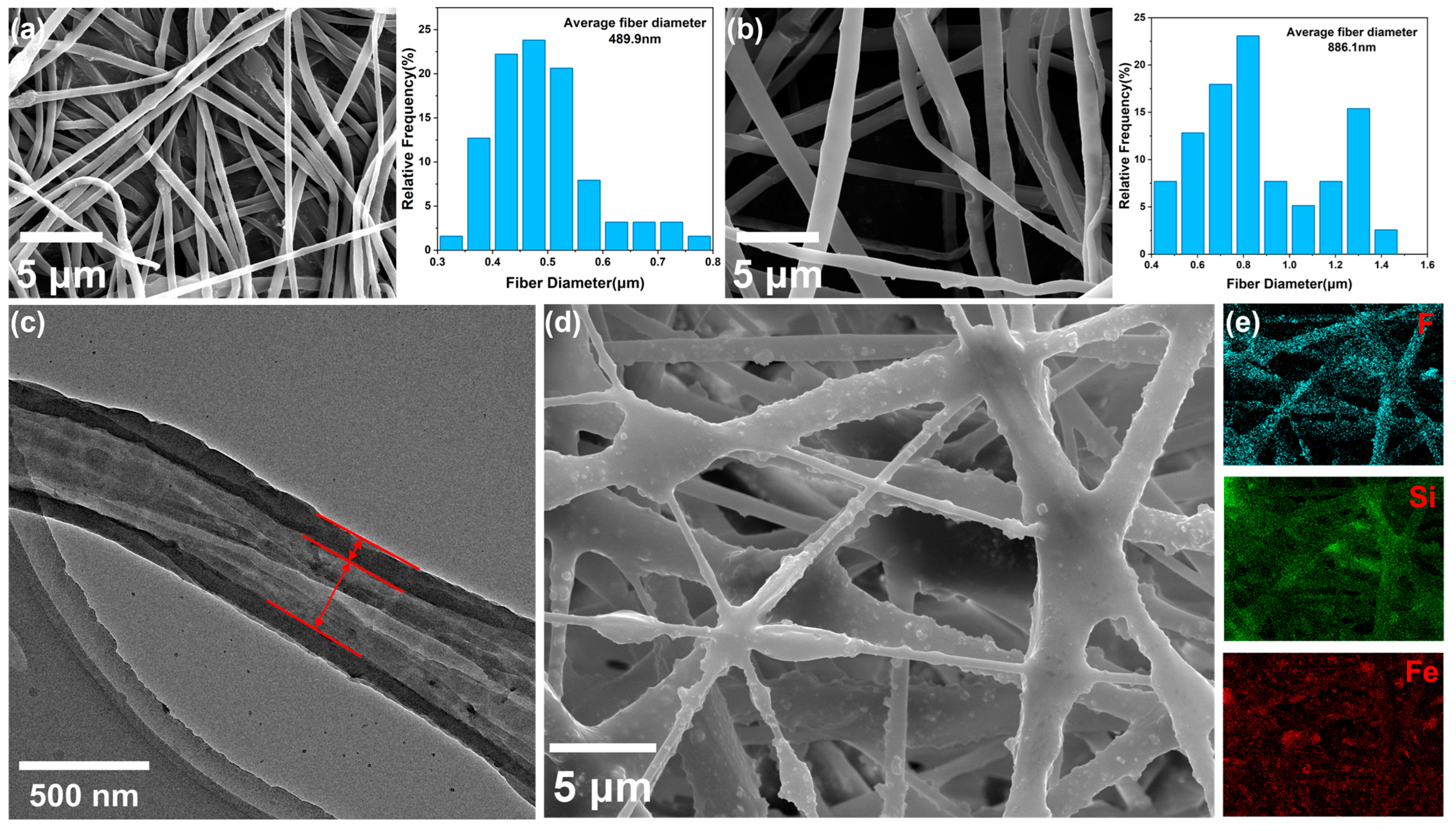
3.1. Morphology and Structure of Samples
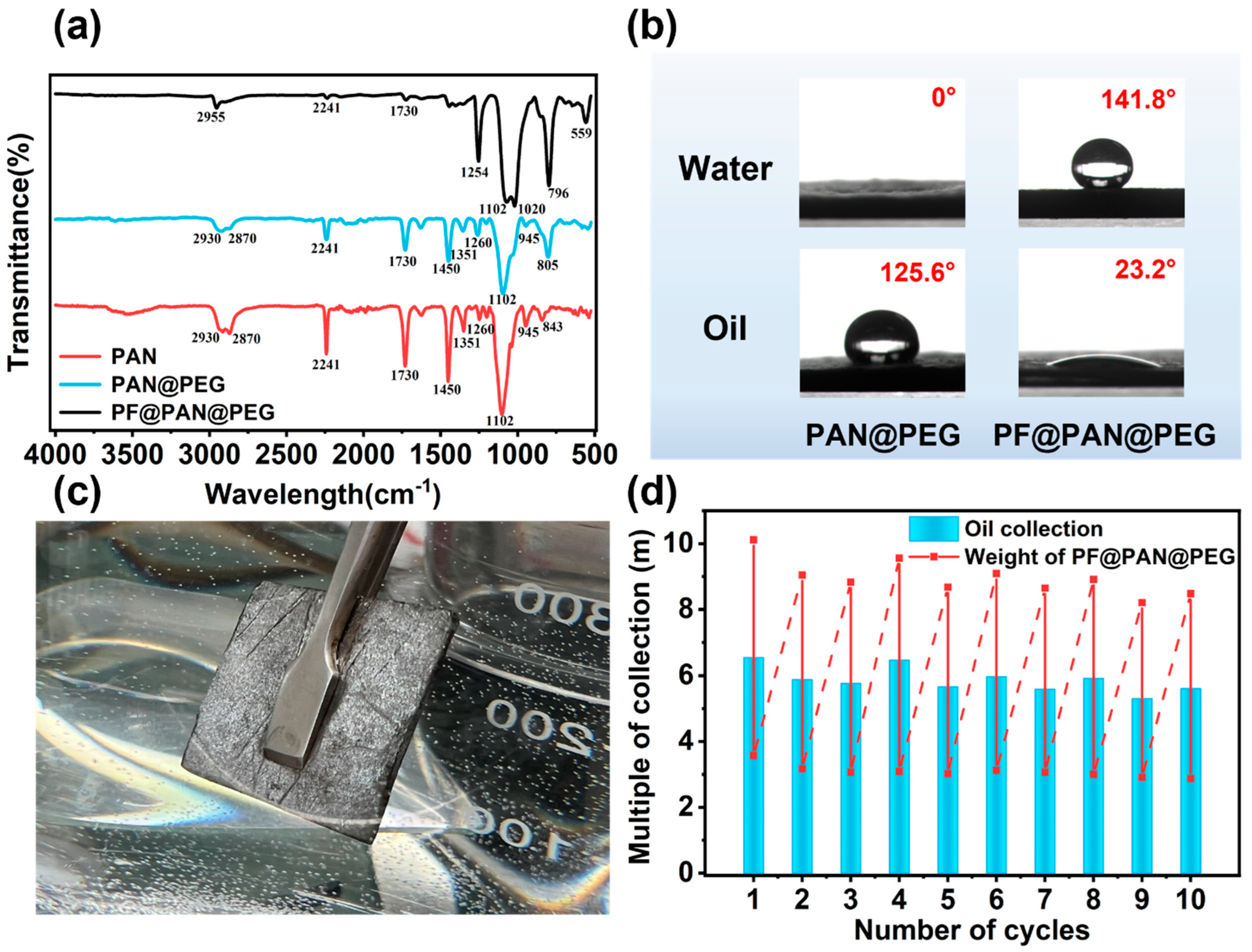
3.2. Adsorption Performance of PF@PAN@PEG for Crude Oil
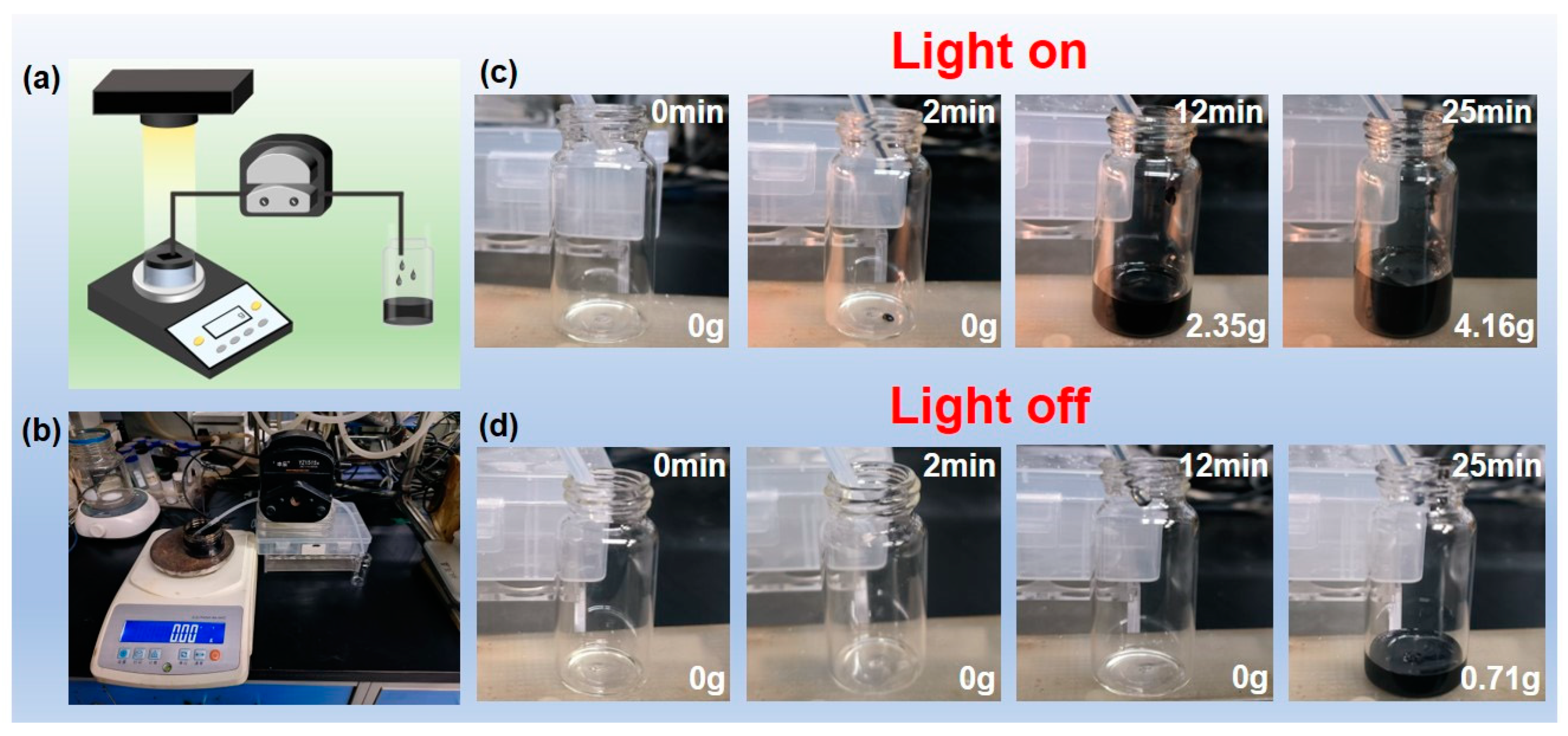
3.3. Solar Heating Conversion Properties of PF@PAN@PEG
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kai, L.; Hongliang, Y.; Xu, Y.; Liao, J. Research on Oil Spills Area Recognition Based on Thermal Infrared Images. SSRN 2024, 4699469. [Google Scholar] [CrossRef]
- Akhmedov, F.; Nasimov, R.; Abdusalomov, A. Developing a Comprehensive Oil Spill Detection Model for Marine Environments. Remote Sens. 2024, 16, 3080. [Google Scholar] [CrossRef]
- Wilson, R. Polycyclic Aromatic Hydrocarbon Concentrations in Sediment of the Huntington Beach Wetlands Complex After an Oil Spill. Bachelor’s Thesis, California State University, Long Beach, CA, USA, 2024. [Google Scholar]
- Mansurova, M.; Johann, S.; Kohlhoff, H.; Rurack, K.; Bartholmai, M.; Bell, J. On-Site Analytical Tool Based on Crude Oil Fluorescence and Chemometrics for the Rapid Determination of the Nature and Essential Properties of Oil Spills. ACS ES&T Water 2024, 4, 621–627. [Google Scholar]
- De Brito, J.K.S.; Campos, V.M.; Oliveira, A.H.B.; Lopes, G.S. Development of a green and low-cost method to determine mercury content in sediments affected by oil spill on the Brazilian coast. Mar. Pollut. Bull. 2024, 202, 116346. [Google Scholar] [CrossRef] [PubMed]
- Agbadiba, I.; Appah, D.; Ugwoha, E.; Patricks-E, C. Net Environmental Benefit Analysis: A Strategic Framework for Effective Oil Spill Incidents Management in the Niger Delta. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition, Lagos, Nigeria, 5–7 August 2024. [Google Scholar]
- Wilcox, G. Effect of Crude Oil Spillage and Constraints Associated with Artisanal Fishing in Oil-Spilled Areas of Bayelsa State. Afr. J. Agric. Food Sci. 2024, 7, 158–168. [Google Scholar]
- Olukaejire, S.J.; Ifiora, C.C.; Osaro, P.A.; Osuji, L.C.; Hart, A.I. Crude Oil and Refined Products Spill Incidence Impact Evaluation in Eleme; Rivers State, Nigeria. J. Energy Res. Rev. 2024, 16, 38–43. [Google Scholar]
- Guan, H.; Cheng, Z.; Wang, X. Highly compressible wood sponges with a spring-like lamellar structure as effective and reusable oil absorbents. ACS Nano 2018, 12, 10365–10373. [Google Scholar] [CrossRef]
- Kuang, Y.; Chen, C.; Chen, G.; Pei, Y.; Pastel, G.; Jia, C.; Song, J.; Mi, R.; Yang, B.; Das, S.; et al. Bioinspired solar-heated carbon absorbent for efficient cleanup of highly viscous crude oil. Adv. Funct. Mater. 2019, 29, 1900162. [Google Scholar] [CrossRef]
- Hao, W.; Xu, J.; Li, R.; Zhao, X.; Qiu, L.; Yang, W. Developing superhydrophobic rock wool for high-viscosity oil/water separation. Chem. Eng. J. 2019, 368, 837–846. [Google Scholar] [CrossRef]
- Kong, D.; He, X.; Yang, H.; Zhang, Z. Experimental study for flame base drag and burning efficiency of spilled crude oil during in-situ burning on water. Process Saf. Environ. Prot. 2019, 131, 48–54. [Google Scholar] [CrossRef]
- Wu, M.B.; Huang, S.; Liu, T.Y.; Wu, J.; Agarwal, S.; Greiner, A.; Xu, Z.K. Compressible carbon sponges from delignified wood for fast cleanup and enhanced recovery of crude oil spills by joule heat and photothermal effect. Adv. Funct. Mater. 2021, 31, 2006806. [Google Scholar] [CrossRef]
- Wang, M.; Huang, J.; Li, S.; Ni, Y.; Dong, X.; Wang, X.; Chen, Z.; Li, X.; Cai, W.; Lai, Y. A sandwich-like structured superhydrophobic fabric for versatile and highly efficient emulsion separation. Sep. Purif. Technol. 2021, 275, 119253. [Google Scholar] [CrossRef]
- Zhu, Q.; Pan, Q. Mussel-inspired direct immobilization of nanoparticles and application for oil–water separation. ACS Nano 2014, 8, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.H.; Dickerson, J.H. Superhydrophobic silanized melamine sponges as high efficiency oil absorbent materials. ACS Appl. Mater. Interfaces 2014, 6, 14181–14188. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Wang, J.; Lu, X.; Zhu, Y.; Jiang, L. In situ fastening graphene sheets into a polyurethane sponge for the highly efficient continuous cleanup of oil spills. Nano Res. 2017, 10, 1756–1766. [Google Scholar] [CrossRef]
- Meng, H.; Yan, T.; Yu, J.; Jiao, F. Super-hydrophobic and super-lipophilic functionalized graphene oxide/polyurethane sponge applied for oil/water separation. Chin. J. Chem. Eng. 2018, 26, 957–963. [Google Scholar] [CrossRef]
- Wang, N.; Deng, Z. Synthesis of magnetic, durable and superhydrophobic carbon sponges for oil/water separation. Mater. Res. Bull. 2019, 115, 19–26. [Google Scholar] [CrossRef]
- Chen, J.; Ni, Y.; Gou, Y.; Zhu, T.; Sun, L.; Chen, Z.; Huang, J.; Yang, D.; Lai, Y. Hydrophobic organogel sorbent and its coated porous substrates for efficient oil/water emulsion separation and effective spilled oil remediation. J. Hazard. Mater. 2024, 461, 132674. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Shi, L.A.; Wang, Y.C.; Zhao, H.Y.; Yao, H.B.; Zhu, Y.B.; Zhang, Y.; Zhu, H.W.; Wu, H.A.; Yu, S.H. Joule-heated graphene-wrapped sponge enables fast clean-up of viscous crude-oil spill. Nat. Nanotechnol. 2017, 12, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Qiao, D.; Dong, L.; Chen, X.; Wang, Z.; Li, Y. Efficient recovery of highly viscous crude oil spill by superhydrophobic ocean biomass-based aerogel assisted with solar energy. Chem. Eng. J. 2023, 467, 143532. [Google Scholar] [CrossRef]
- Wang, P.-L.; Ma, C.; Yuan, Q.; Mai, T.; Ma, M.G. Novel Ti3C2Tx MXene wrapped wood sponges for fast cleanup of crude oil spills by outstanding Joule heating and photothermal effect. J. Colloid Interface Sci. 2022, 606, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tian, Q.; Xu, J.; Sun, S.; Cheng, Y.; Qiu, F.; Zhang, T. Easily fabricated low-energy consumption joule-heated superhydrophobic foam for fast cleanup of viscous crude oil spills. ACS Appl. Mater. Interfaces 2021, 13, 51652–51660. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Li, J.; Qiang, Z.; Ren, J. Versatile and cost-efficient cleanup of viscous crude oil by an elastic carbon sorbent from direct pyrolysis of a melamine foam. J. Mater. Chem. A 2021, 9, 11268–11277. [Google Scholar] [CrossRef]
- Wu, X.; Lei, Y.; Li, S.; Huang, J.; Teng, L.; Chen, Z.; Lai, Y. Photothermal and Joule heating-assisted thermal management sponge for efficient cleanup of highly viscous crude oil. J. Hazard. Mater. 2021, 403, 124090. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Lao, J.; Wang, B.; Li, X.; Li, G.; Gao, J.; Wan, Y.; Sun, X.; Guo, R.; Luo, J. Fast and all-weather cleanup of viscous crude-oil spills with Ti3C2TX MXene wrapped sponge. J. Mater. Chem. A 2020, 8, 20162–20167. [Google Scholar] [CrossRef]
- Song, Y.; Shi, L.-A.; Xing, H.Y.; Jiang, K.; Ge, J.; Dong, L.; Lu, Y.; Yu, S.-H. A magneto-heated ferrimagnetic sponge for continuous recovery of viscous crude oil. Adv. Mater. 2021, 33, 2100074. [Google Scholar] [CrossRef]
- Yu, J.; Cao, C.; Liu, S.; Pan, Y. Eco-friendly magneto-photothermal sponge for the fast recovery of highly viscous crude oil spill. Sep. Purif. Technol. 2022, 298, 121668. [Google Scholar] [CrossRef]
- Shi, H.-G.; Li, S.L.; Cheng, J.B.; Zhao, H.B.; Wang, Y.Z. Multifunctional photothermal conversion nanocoatings toward highly efficient and safe high-viscosity oil cleanup absorption. ACS Appl. Mater. Interfaces 2021, 13, 11948–11957. [Google Scholar] [CrossRef]
- Li, Z.; Lin, Z.; Tian, Q.; Yue, X.; Qiu, F.; Zhang, T. Solar-heating superhydrophobic modified melamine sponge for efficient recovery of viscous crude oil. J. Hazard. Mater. 2022, 440, 129799. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cheng, S.; Chen, B.; Jiang, J.; Tu, C.; Li, W.; Yang, Y.Y.; Huang, K.W.; Wang, K.; Yuan, H.; et al. Graphene infrared radiation management targeting photothermal conversion for electric-energy-free crude oil collection. J. Am. Chem. Soc. 2022, 144, 15562–15568. [Google Scholar] [CrossRef]
- Zheng, D.; Yao, W.; Sun, C.; Chen, X.; Wang, Z.; Wang, B.; Tan, H.; Zhang, Y. Solar-assisted self-heating Ti3C2Tx-decorated wood aerogel for adsorption and recovery of highly viscous crude oil. J. Hazard. Mater. 2022, 435, 495. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, C.; Gnanasekar, P.; Xiao, P.; Luo, Q.; Li, S.; Qin, D.; Chen, T.; Chen, J.; Zhu, J.; et al. Mechanically robust, solar-driven, and degradable lignin-based polyurethane adsorbent for efficient crude oil spill remediation. Chem. Eng. J. 2021, 415, 128956. [Google Scholar] [CrossRef]
- Niu, H.; Li, J.; Wang, X.; Luo, F.; Qiang, Z.; Ren, J. Solar-assisted, fast, and in situ recovery of crude oil spill by a superhydrophobic and photothermal sponge. ACS Appl. Mater. Interfaces 2021, 13, 21175–21185. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dong, L.; Zhang, D.; Chen, X.; Xue, Y.; Guan, Y.; Li, Y. Mesoporous carbon spheres modified polyurethane sponge/phase change material composites: Photothermal conversion, thermal storage and efficient recovery of high-viscosity crude oil. Carbon 2023, 214, 118372. [Google Scholar] [CrossRef]
- Guan, Y.; Cao, S.; Zhang, W.; Wang, Z.; Li, Y.; Zeng, H. Remediation of seawater polluted by oil spills via integrating porous materials with phase-change materials. Desalination 2024, 591, 118061. [Google Scholar] [CrossRef]
- Dong, L.; Li, Y.; Li, J.; Guan, Y.; Chen, X.; Zhang, D.; Wang, Z. Mesoporous carbon hollow spheres encapsulated phase change material for efficient emulsification of high-viscosity oil. J. Hazard. Mater. 2023, 451, 131112. [Google Scholar] [CrossRef]
- Wu, S.; Xiang, Y.; Cai, Y.; Liu, J. Superhydrophobic magnetic Fe3O4 polyurethane sponges for oil-water separation and oil-spill recovery. J. Environ. Sci. 2024, 139, 160–169. [Google Scholar] [CrossRef]
- Zhang, N.; Zhong, S.; Zhou, X.; Jiang, W.; Wang, T.; Fu, J. Superhydrophobic P (St-DVB) foam prepared by the high internal phase emulsion technique for oil spill recovery. Chem. Eng. J. 2016, 298, 117–124. [Google Scholar] [CrossRef]
- Shang, Y.; Gao, R.; Wang, Y.; Dong, X.; Li, X.; Tang, J.; Wang, X.; Liu, J.; Xie, Y.; Li, J.; et al. Scalable fabrication of efficient and recycling wood residue-derived sponge for crude oil adsorption. J. Hazard. Mater. 2023, 441, 129979. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, S.; Luo, L.; Zheng, J.; Ji, L. The controlled synthesis of porous silver structure on carbon fibres and their sterilization performance. J. Mater. Sci. 2022, 57, 6335–6344. [Google Scholar] [CrossRef]
- Hartati, S.; Zulfi, A.; Maulida, P.Y.D.; Yudhowijoyo, A.; Dioktyanto, M.; Saputro, K.E.; Noviyanto, A.; Rochman, N.T. Synthesis of electrospun PAN/TiO2/Ag nanofibers membrane as potential air filtration media with photocatalytic activity. ACS Omega 2022, 7, 10516–10525. [Google Scholar] [CrossRef] [PubMed]
- Kayan, G.Ö.; Kayan, A. Polyhedral oligomeric silsesquioxane and polyorganosilicon hybrid materials and their usage in the removal of methylene blue dye. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2781–2792. [Google Scholar] [CrossRef]
- Atta, A.; Abdeltwab, E. Influence of ion irradiation on the surface properties of silver-coated flexible PDMS polymeric films. Braz. J. Phys. 2022, 52, 3. [Google Scholar] [CrossRef]
- Ramlan, N.; Zubairi, S.I.; Maskat, M.Y. Response surface optimisation of polydimethylsiloxane (PDMS) on borosilicate glass and stainless steel (SS316) to increase hydrophobicity. Molecules 2022, 27, 3388. [Google Scholar] [CrossRef]
- Ma, Q.; Yu, Y.; Sindoro, M.; Fane, A.G.; Wang, R.; Zhang, H. Carbon-based functional materials derived from waste for water remediation and energy storage. Adv. Mater. 2017, 29, 1605361. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.; Hossain, S.M.; Seo, D.H.; McDonagh, A.; Foster, T.; Shon, H.K.; Tijing, L. Insight into the role of polydopamine nanostructures on nickel foam-based photothermal materials for solar water evaporation. Sep. Purif. Technol. 2022, 293, 121054. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, J.; Mersal, G.A.; Zuo, J.; Li, J.; Wang, Q.; Feng, Y.; Liu, J.; Liu, Z.; Wang, B.; et al. “Several birds with one stone” strategy of pH/thermoresponsive flame- retardant/photothermal bactericidal oil-absorbing material for recovering complex spilled oil. J. Mater. Sci. Technol. 2022, 128, 82–97. [Google Scholar] [CrossRef]
- Ma, J.; Ma, S.; Xue, J.; Xu, M.; Zhang, J.; Li, J.; Zhao, Z.; Zhao, S.; Pan, J.; Ye, Z. Synthesis of elastic hydrophobic biomass sponge for rapid solar-driven viscous crude-oil cleanup absorption, oil-water separation and organic pollutants treating. Sep. Purif. Technol. 2023, 305, 122512. [Google Scholar] [CrossRef]


| Sample | Melting | Crystallizing | ||||||
|---|---|---|---|---|---|---|---|---|
| Tm (°C) | ΔHm (J g−1) | ΔHmT (J g−1) | λ (%) | Tc (°C) | ΔHc (J g−1) | ΔHcT (J g−1) | λ (%) | |
| PEG | 70.00 | 189.3 | - | - | 41.24 | 178.2 | - | - |
| PAN@PEG | 69.50 | 87.66 | 103.93 | 84.34 | 41.98 | 86.21 | 97.84 | 88.11 |
| PF@PAN@PEG | 69.20 | 77.12 | 88.77 | 86.74 | 41.04 | 75.21 | 83.57 | 90.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.; Wang, Y.; Liu, C.; Meng, K.; Chang, E.; Wu, X.; Wang, J. A Solar-Heated Phase Change Composite Fiber with a Core–Shell Structure for the Recovery of Highly Viscous Crude Oil. Polymers 2025, 17, 135. https://doi.org/10.3390/polym17020135
Lin C, Wang Y, Liu C, Meng K, Chang E, Wu X, Wang J. A Solar-Heated Phase Change Composite Fiber with a Core–Shell Structure for the Recovery of Highly Viscous Crude Oil. Polymers. 2025; 17(2):135. https://doi.org/10.3390/polym17020135
Chicago/Turabian StyleLin, Chenxin, Yifan Wang, Cenyu Liu, Kaiyue Meng, Endong Chang, Xiaowen Wu, and Jiancheng Wang. 2025. "A Solar-Heated Phase Change Composite Fiber with a Core–Shell Structure for the Recovery of Highly Viscous Crude Oil" Polymers 17, no. 2: 135. https://doi.org/10.3390/polym17020135
APA StyleLin, C., Wang, Y., Liu, C., Meng, K., Chang, E., Wu, X., & Wang, J. (2025). A Solar-Heated Phase Change Composite Fiber with a Core–Shell Structure for the Recovery of Highly Viscous Crude Oil. Polymers, 17(2), 135. https://doi.org/10.3390/polym17020135







