Modifying Cassava Starch via Extrusion with Phosphate, Erythorbate and Nitrite: Phosphorylation, Hydrolysis and Plasticization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Starch Extrusion
2.2. Characterization of TPS Extrudates
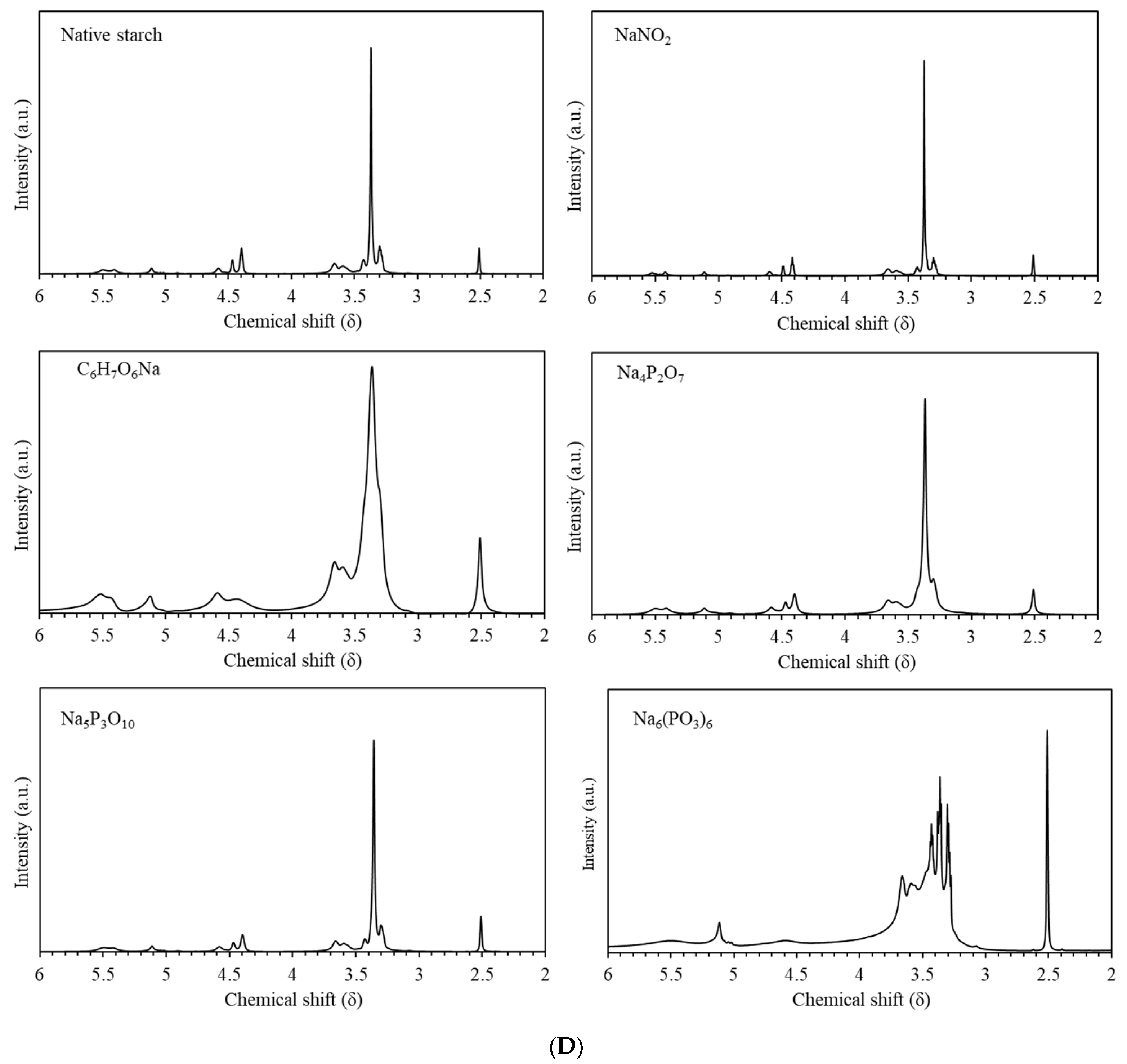
2.2.1. Nuclear Magnetic Resonance (NMR)
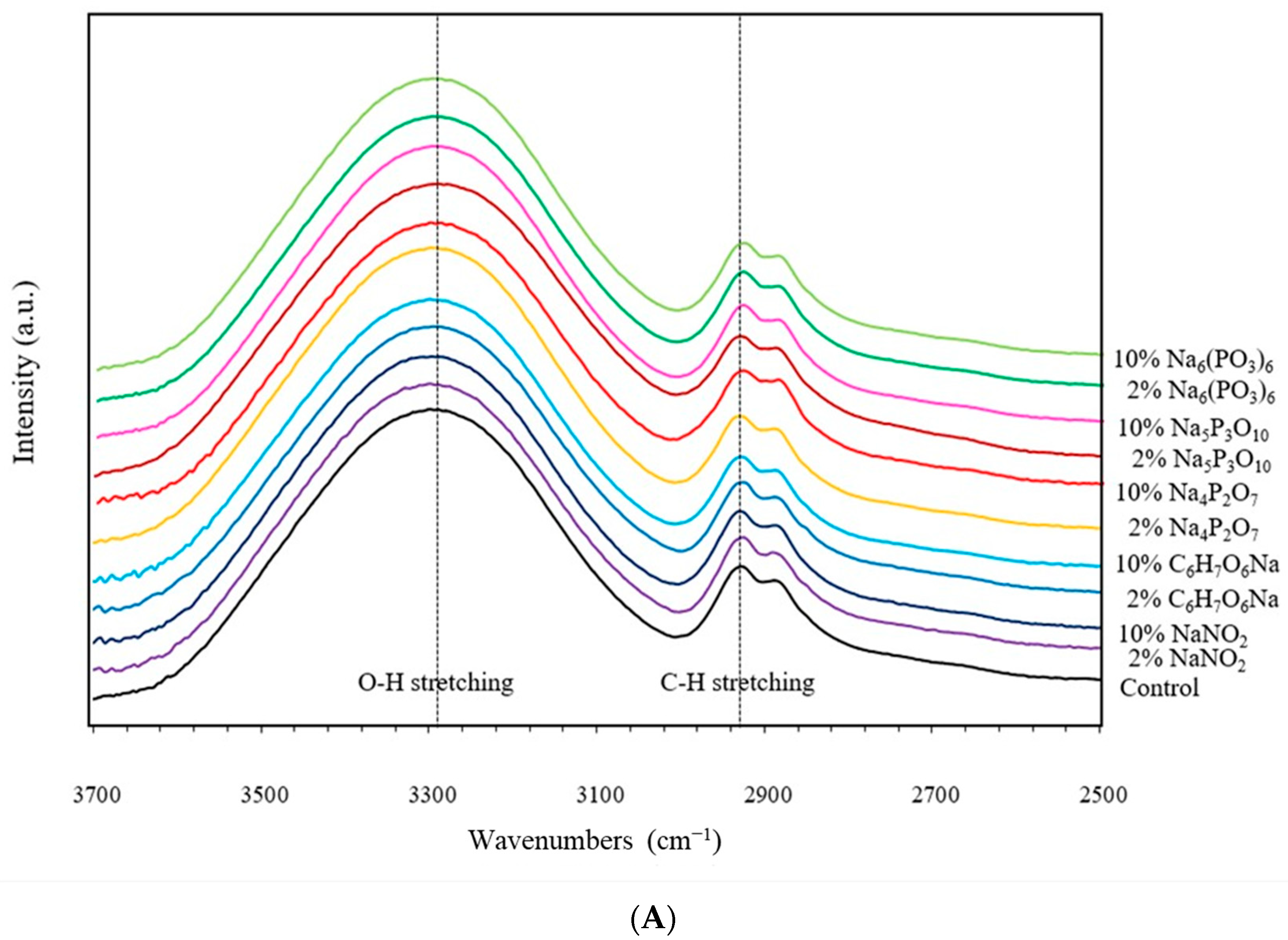
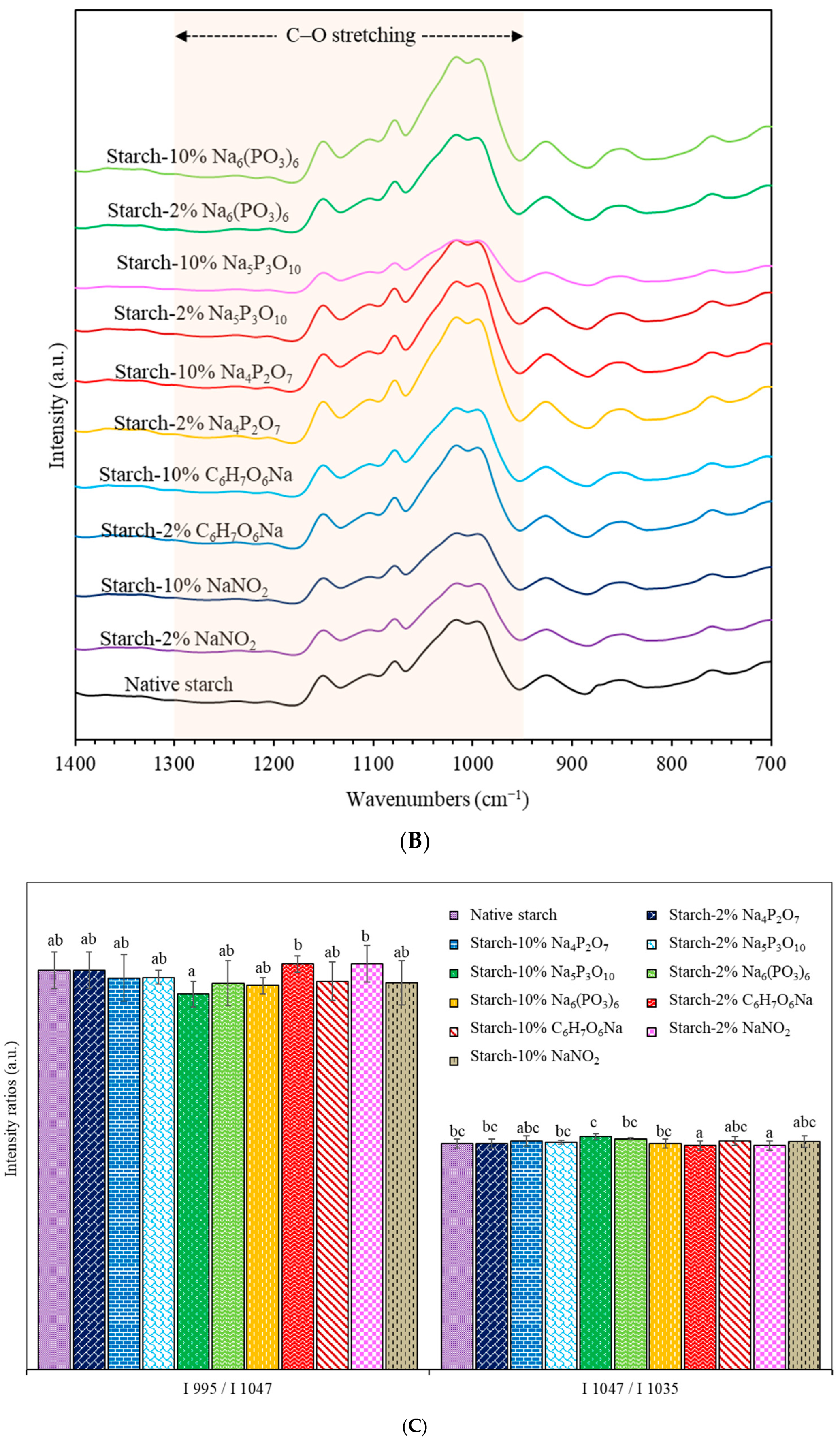
2.2.2. Fourier Transform Infrared Spectrometer (FTIR)
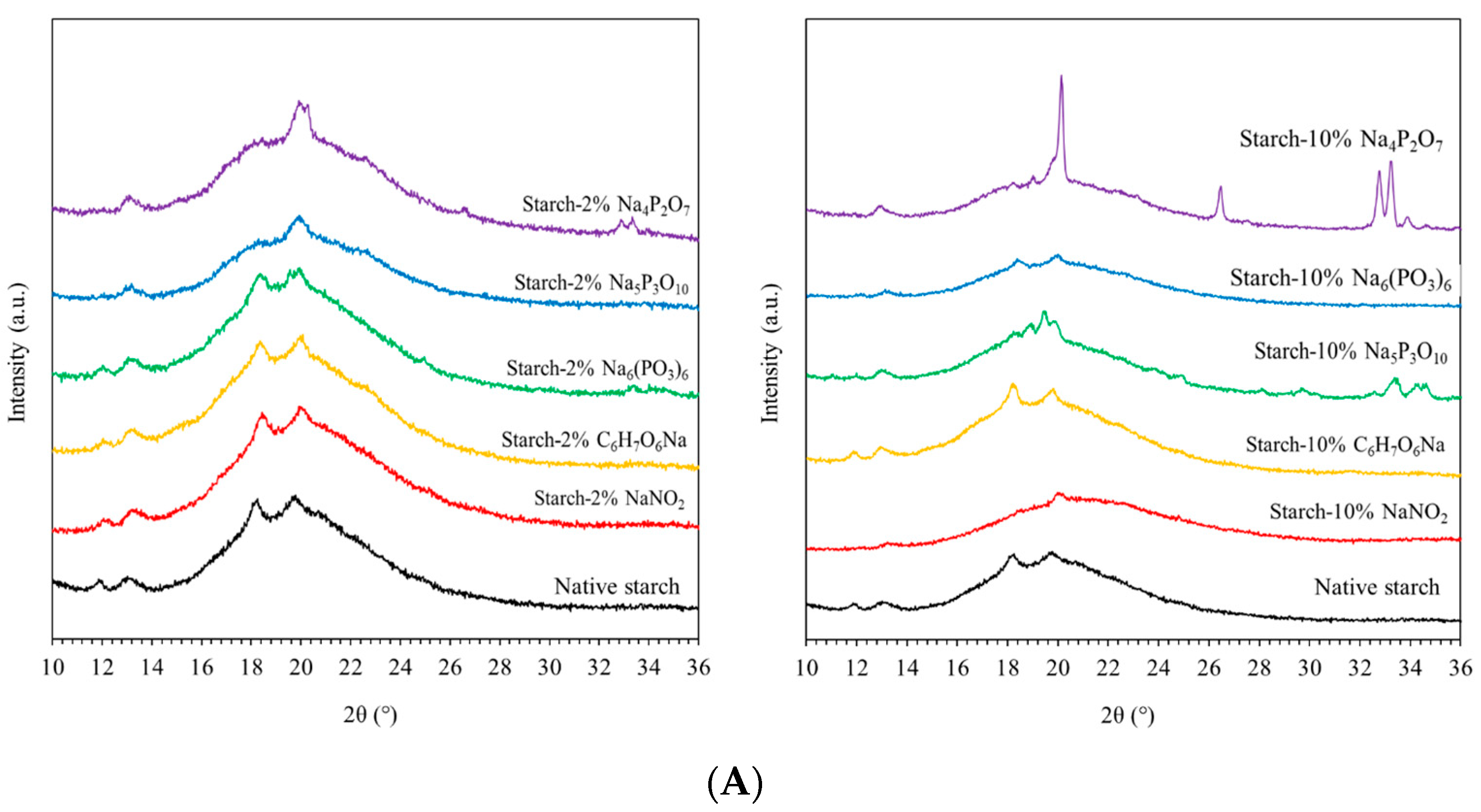
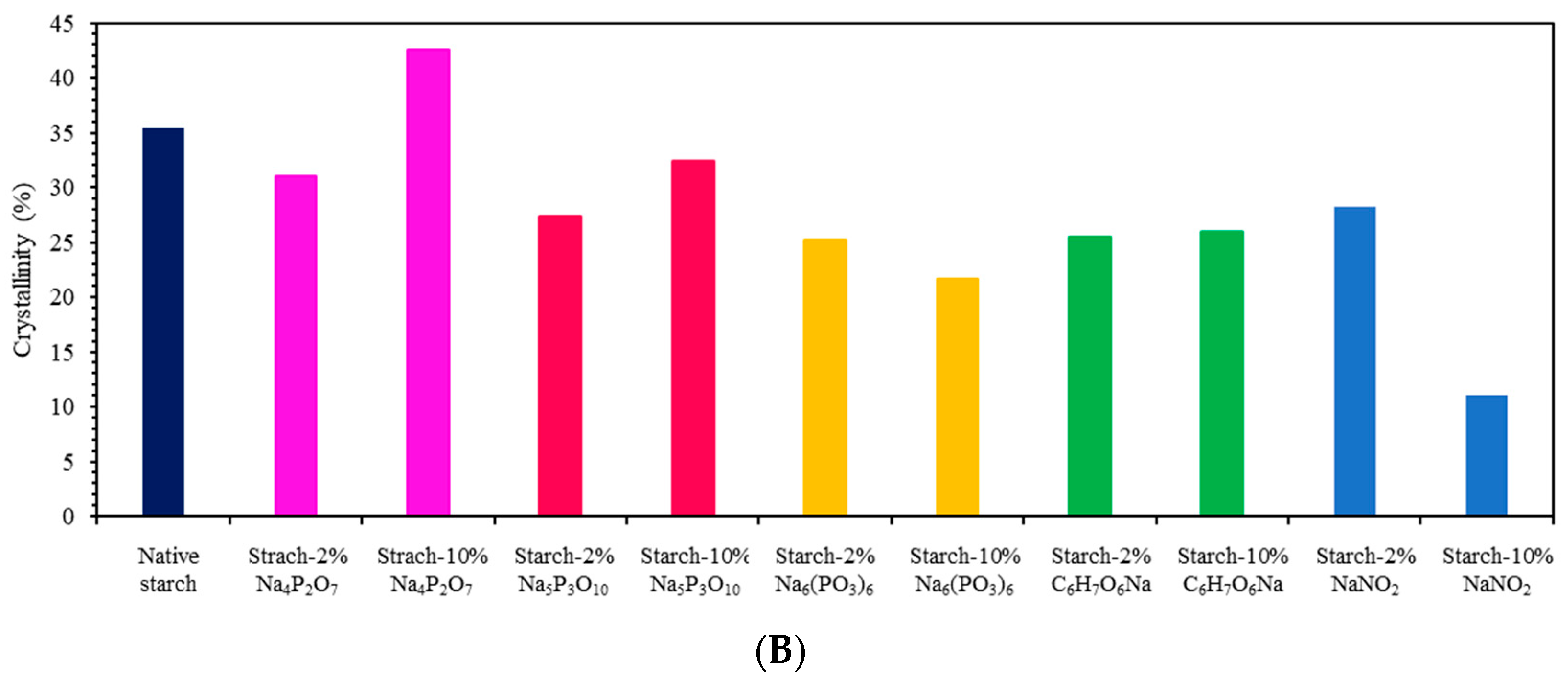
2.2.3. X-ray Diffractometer (XRD)
2.3. Thermal Properties
2.3.1. Differential Scanning Calorimetry (DSC)
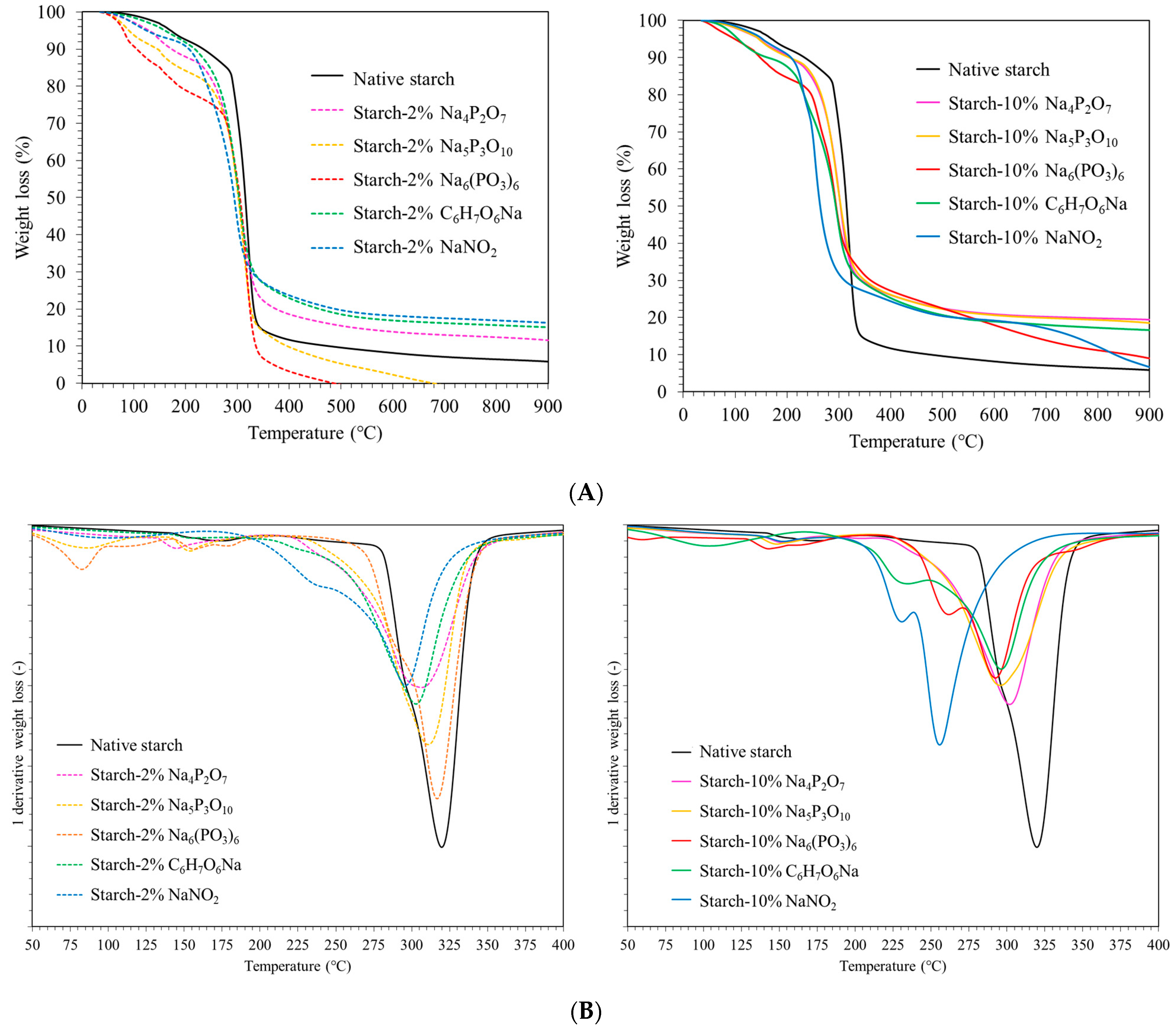
2.3.2. Thermogravimetric Analysis (TGA)
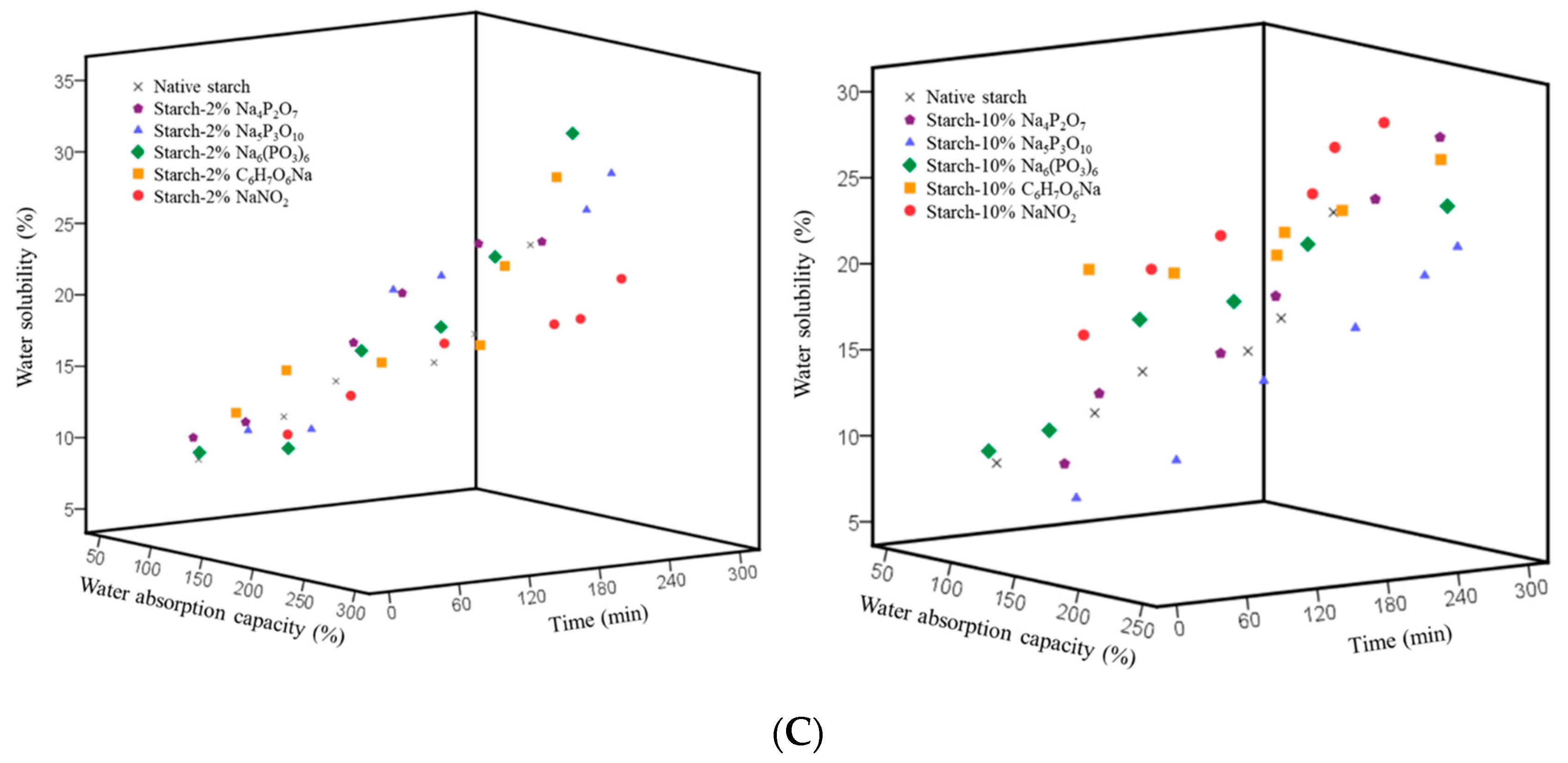
2.4. Water Absorption Capacity
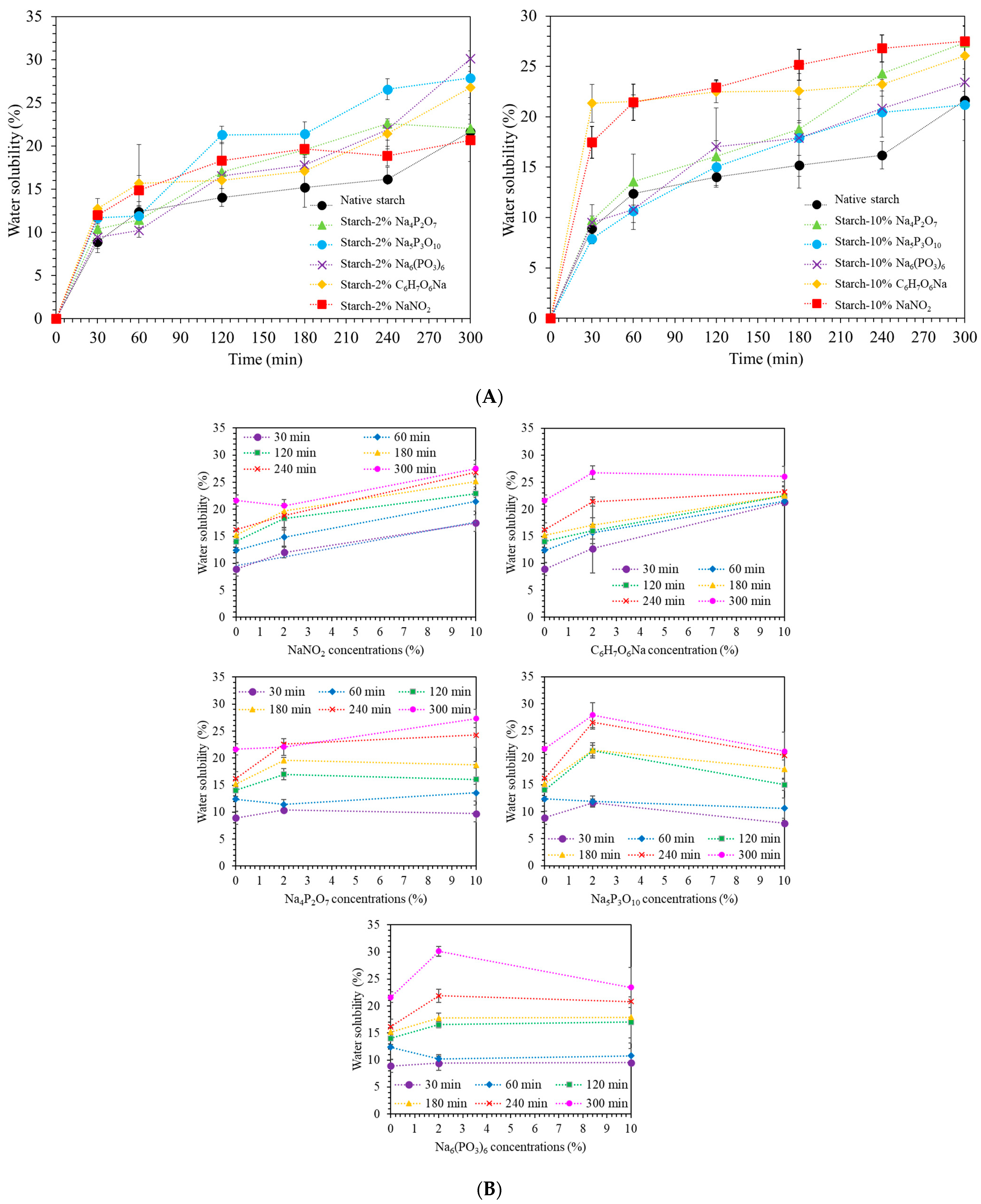
2.5. Water Solubility
2.6. Applications of TPS Extrudates
2.7. Statistical Analysis
3. Results and Discussion
3.1. X-ray Diffractometer (XRD)
3.2. Fourier Transform Infrared Spectrometer (FTIR)
3.3. Nuclear Magnetic Resonance (NMR)
3.4. Differential Scanning Calorimetry (DSC)
3.5. Thermogravimetric Analysis (TGA)
3.6. Water Absorption Capacity
3.7. Water Solubility
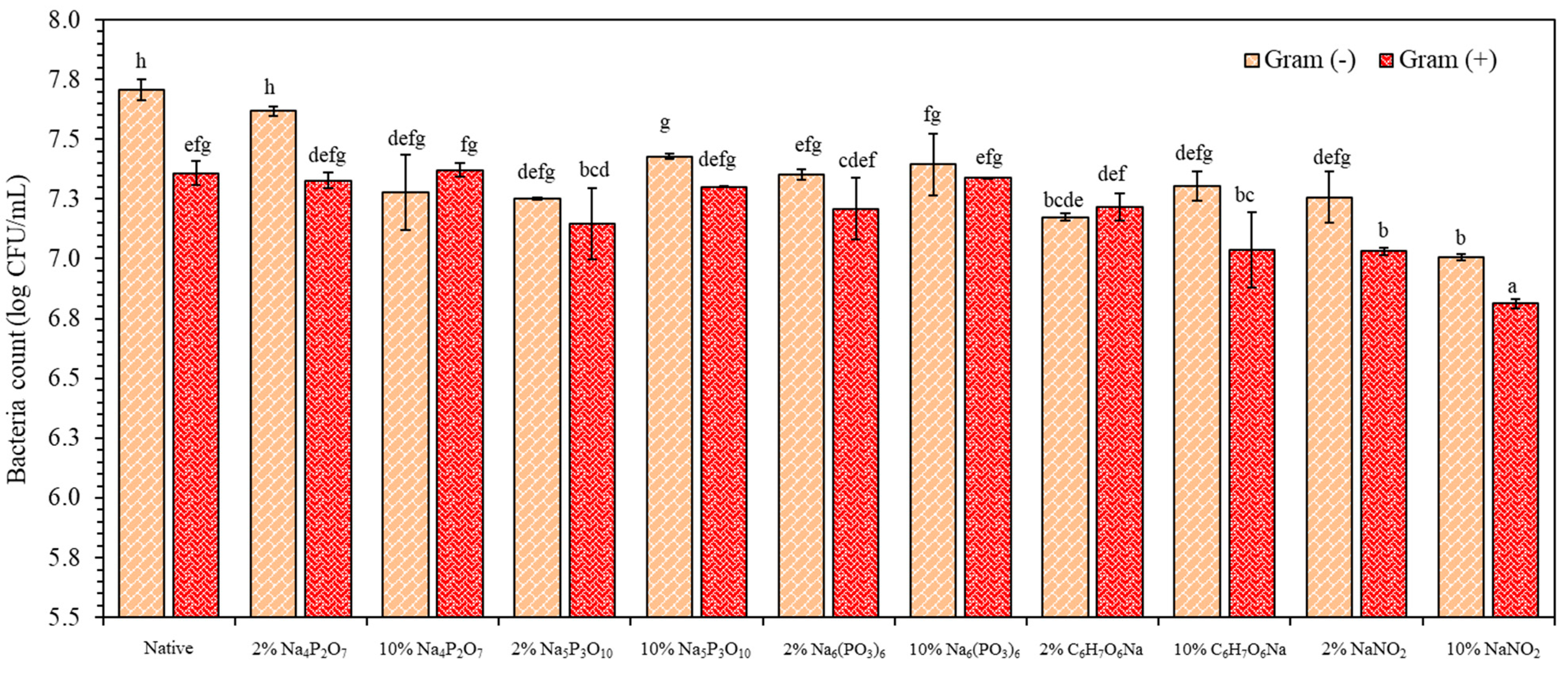
3.8. Antimicrobial Analysis of TPS Extrudates Blended PBAT as Active Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Leonel, M.; Del Bem, M.S.; Dos Santos, T.P.; Franco, C.M.L. Preparation and properties of phosphate starches from tuberous roots. Int. J. Biol. Macromol. 2021, 183, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xu, C.; Kim, S.-J.; Ji, S.; Ren, Y.; Chen, Z.; Li, Y.; Zhou, B.; Lu, B. Investigating the evolution of the fine structure in cassava starch during growth and its correlation with gelatinization performance. Int. J. Biol. Macromol. 2024, 265, 130422. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.F.; de Oliveira, A.C.S.; de Oliveira Begali, D.; de Sena Neto, A.R.; Martins, M.A.; de Oliveira, J.E.; Borges, S.V.; Yoshida, M.I.; Tonoli, G.H.D.; Dias, M.V. Characterization of cassava starch/soy protein isolate blends obtained by extrusion and thermocompression. Ind. Crops Prod. 2021, 160, 113092. [Google Scholar] [CrossRef]
- Wang, L.; Xu, J.; Zhang, M.; Zheng, H.; Li, L. Preservation of soy protein-based meat analogues by using PLA/PBAT antimicrobial packaging film. Food Chem. 2022, 380, 132022. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.H.; Fan, Y.Y.; Liu, T.; Yang, H.; Ma, L.J.; Huang, X.J.; Liu, Y. Structural characterization of controlled decrystallization of cassava starch. Starch-Stärke 2020, 72, 1900049. [Google Scholar] [CrossRef]
- Qi, M.; Song, J.; Jiang, L.; Li, L.; Xu, M.; Li, Y.; Yu, S.; Li, H. Understanding the degradation mechanisms of cyanide and starch in cassava flour during extrusion processing. Innov. Food Sci. Emerg. Technol. 2024, 91, 103548. [Google Scholar] [CrossRef]
- Wongphan, P.; Nerin, C.; Harnkarnsujarit, N. Enhanced compatibility and functionality of thermoplastic cassava starch blended PBAT blown films with erythorbate and nitrite. Food Chem. 2023, 420, 136107. [Google Scholar] [CrossRef]
- Chang, C.C.; Trinh, B.M.; Mekonnen, T.H. Robust multiphase and multilayer starch/polymer (TPS/PBAT) film with simultaneous oxygen/moisture barrier properties. J. Colloid Interface Sci. 2021, 593, 290–303. [Google Scholar] [CrossRef]
- San, H.; Harnkarnsujarit, N. Sulfite incorporated thermoplastic cassava starch blended PBAT blown films as antimicrobial and antibrowning packaging. Ind. Crops Prod. 2023, 206, 117610. [Google Scholar] [CrossRef]
- Varghese, S.A.; Phothisarattana, D.; Srisa, A.; Laorenza, Y.; Jarupan, L.; Bumbudsanpharoke, N.; Chonhenchob, V.; Harnkarnsujarit, N. Novel eco-friendly antimicrobial UV-blocking PBAT/PBS/TiO2 nanocomposite films for improved shelf-life of bananas. Food Biosci. 2023, 55, 102993. [Google Scholar] [CrossRef]
- Nisitthichai, J.; Wannaphruek, P.; Sriprablom, J.; Suphantharika, M.; Smith, S.M.; Amornsakchai, T.; Wongsagonsup, R. Impact of Oil Addition on Physicochemical Properties and In Vitro Digestibility of Extruded Pineapple Stem Starch. Polymers 2024, 16, 210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shao, F.; Wan, X.; Zhang, H.; Cai, M.; Hu, K.; Duan, Y. Effects of rapeseed protein addition on soybean protein-based textured protein produced by low-moisture extrusion: Changes in physicochemical attributes, structural properties and barrel flow behaviors. Food Hydrocoll. 2024, 149, 109631. [Google Scholar] [CrossRef]
- Wongphan, P.; Panrong, T.; Harnkarnsujarit, N. Effect of different modified starches on physical, morphological, thermomechanical, barrier and biodegradation properties of cassava starch and polybutylene adipate terephthalate blend film. Food Packag. Shelf Life 2022, 32, 100844. [Google Scholar] [CrossRef]
- Laorenza, Y.; Harnkarnsujarit, N. Carvacrol, citral and α-terpineol essential oil incorporated biodegradable films for functional active packaging of Pacific white shrimp. Food Chem. 2021, 363, 130252. [Google Scholar] [CrossRef] [PubMed]
- Mutungi, C.; Passauer, L.; Onyango, C.; Jaros, D.; Rohm, H. Debranched cassava starch crystallinity determination by Raman spectroscopy: Correlation of features in Raman spectra with X-ray diffraction and 13C CP/MAS NMR spectroscopy. Carbohydr. Polym. 2012, 87, 598–606. [Google Scholar] [CrossRef]
- Zhu, F. Composition, structure, physicochemical properties, and modifications of cassava starch. Carbohydr. Polym. 2015, 122, 456–480. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, L.; Mo, X.; Yang, F.; Pang, J. Effects of nanosilica on retrogradation properties and structures of thermoplastic cassava starch. J. Appl. Polym. Sci. 2018, 135, 45687. [Google Scholar] [CrossRef]
- Dong, H.; Vasanthan, T. Effect of phosphorylation techniques on structural, thermal, and pasting properties of pulse starches in comparison with corn starch. Food Hydrocoll. 2020, 109, 106078. [Google Scholar] [CrossRef]
- Pozo, C.; Rodríguez-Llamazares, S.; Bouza, R.; Barral, L.; Castaño, J.; Müller, N.; Restrepo, I. Study of the structural order of native starch granules using combined FTIR and XRD analysis. J. Polym. Res. 2018, 25, 266. [Google Scholar] [CrossRef]
- Shi, R.; Zhang, Z.; Liu, Q.; Han, Y.; Zhang, L.; Chen, D.; Tian, W. Characterization of citric acid/glycerol co-plasticized thermoplastic starch prepared by melt blending. Carbohydr. Polym. 2007, 69, 748–755. [Google Scholar] [CrossRef]
- Warren, F.J.; Gidley, M.J.; Flanagan, B.M. Infrared spectroscopy as a tool to characterise starch ordered structure--a joint FTIR-ATR, NMR, XRD and DSC study. Carbohydr. Polym. 2016, 139, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Si, W.; Weng, Y.; Tan, B.; Zhang, S. Adopted ion-pair effect to construct bicontinuous starch-based gel and its application in humidity sensitivity and strain-responsiveness. Compos. Part B Eng. 2022, 234, 109696. [Google Scholar] [CrossRef]
- Tizzotti, M.J.; Sweedman, M.C.; Tang, D.; Schaefer, C.; Gilbert, R.G. New 1H NMR procedure for the characterization of native and modified food-grade starches. J. Agric. Food Chem. 2011, 59, 6913–6919. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, R.; Lammers, G.; Janssen, L.; Beenackers, A. Quantitative analysis of chemically modified starches by 1H-NMR spectroscopy. Starch-Stärke 1995, 47, 469–475. [Google Scholar] [CrossRef]
- Xu, A.; Seib, P. Determination of the Level and Position of Substitution in Hydroxypropylated Starch by High-Resolution1H-NMR Spectroscopy ofAlpha-limit Dextrins. J. Cereal Sci. 1997, 25, 17–26. [Google Scholar] [CrossRef]
- Lendvai, L.; Apostolov, A.; Karger-Kocsis, J. Characterization of layered silicate-reinforced blends of thermoplastic starch (TPS) and poly(butylene adipate-co-terephthalate). Carbohydr. Polym. 2017, 173, 566–572. [Google Scholar] [CrossRef]
- Teodoro, A.P.; Mali, S.; Romero, N.; de Carvalho, G.M. Cassava starch films containing acetylated starch nanoparticles as reinforcement: Physical and mechanical characterization. Carbohydr. Polym. 2015, 126, 9–16. [Google Scholar] [CrossRef]
- Wang, Y.R.; Zhang, B.; Fan, J.L.; Yang, Q.; Chen, H.Q. Effects of sodium tripolyphosphate modification on the structural, functional, and rheological properties of rice glutelin. Food Chem. 2019, 281, 18–27. [Google Scholar] [CrossRef]
- Zhang, J.; Tao, L.; Yang, S.; Li, Y.; Wu, Q.; Song, S.; Yu, L. Water absorption behavior of starch: A review of its determination methods, influencing factors, directional modification, and food applications. Trends Food Sci. Technol. 2023, 144, 104321. [Google Scholar] [CrossRef]
- Zeng, J.-B.; Jiao, L.; Li, Y.-D.; Srinivasan, M.; Li, T.; Wang, Y.-Z. Bio-based blends of starch and poly(butylene succinate) with improved miscibility, mechanical properties, and reduced water absorption. Carbohydr. Polym. 2011, 83, 762–768. [Google Scholar] [CrossRef]
- Jaekel, L.Z.; Schmiele, M.; da Silva Rodrigues, R.; Chang, Y.K. Influence of the extrusion process on the technological properties of hydroxypropylated cross-linked cassava starch. J. Food Sci. Technol. 2015, 52, 7305–7312. [Google Scholar] [CrossRef]
- Sangokunle, O.O.; Sathe, S.K.; Singh, P. Purified starches from 18 pulses have markedly different morphology, oil absorption and water absorption capacities, swelling power, and turbidity. Starch-Stärke 2020, 72, 2000022. [Google Scholar] [CrossRef]
- Ashogbon, A.O.; Akintayo, E.T. Recent trend in the physical and chemical modification of starches from different botanical sources: A review. Starch-Stärke 2014, 66, 41–57. [Google Scholar] [CrossRef]
- Nateghi, L.; Zarei, F.; Afshari, K.P. The Effect of Sodium Nitrite Replacement with Lycopene Pigment in German Sausage and Evaluation of Its Physicochemical, Antimicrobial and Sensory Properties. J. Nutr. Food Secur. 2024, 9, 265–274. [Google Scholar] [CrossRef]
- Chatkitanan, T.; Harnkarnsujarit, N. Effects of nitrite incorporated active films on quality of pork. Meat Sci. 2021, 172, 108367. [Google Scholar] [CrossRef] [PubMed]
- Karpińska-Tymoszczyk, M. The effect of oil-soluble rosemary extract, sodium erythorbate, their mixture, and packaging method on the quality of Turkey meatballs. J. Food Sci. Technol. 2013, 50, 443–454. [Google Scholar] [CrossRef]
- Laorenza, Y.; Harnkarnsujarit, N. Ginger oil and lime peel oil loaded PBAT/PLA via cast-extrusion as shrimp active packaging: Microbial and melanosis inhibition. Food Packag. Shelf Life 2023, 38, 101116. [Google Scholar] [CrossRef]
- Leelaphiwat, P.; Pechprankan, C.; Siripho, P.; Bumbudsanpharoke, N.; Harnkarnsujarit, N. Effects of nisin and EDTA on morphology and properties of thermoplastic starch and PBAT biodegradable films for meat packaging. Food Chem. 2022, 369, 130956. [Google Scholar] [CrossRef]
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Biodegradable Poly(Butylene Adipate-Co-Terephthalate) and Thermoplastic Starch-Blended TiO2 Nanocomposite Blown Films as Functional Active Packaging of Fresh Fruit. Polymers 2021, 13, 4192. [Google Scholar] [CrossRef]
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Blown film extrusion of PBAT/TPS/ZnO nanocomposites for shelf-life extension of meat packaging. Colloids Surf. B Biointerfaces 2022, 214, 112472. [Google Scholar] [CrossRef]

| Samples | Tg (°C) | To (°C) | Tp (°C) |
|---|---|---|---|
| Native starch | 67.08 | 180.60 | 183.17 |
| Starch-2% Na4P2O7 | 56.17 | 182.66 | 187.50 |
| Starch-10% Na4P2O7 | 66.75 | 182.35 | 185.33 |
| Starch-2% Na5P3O10 | 74.43 | 179.24 | 183.00 |
| Starch-10% Na5P3O10 | 71.59 | 190.26 | 193.17 |
| Starch-2% Na6(PO3)6 | 71.98 | 179.13 | 182.17 |
| Starch-10% Na6(PO3)6 | 66.87 | 183.83 | 186.00 |
| Starch-2% C6H7O6Na | 70.98 | 182.77 | 187.17 |
| Starch-10% C6H7O6Na | 67.46 | 174.77 | 178.50 |
| Starch-2% NaNO2 | 66.07 | 183.52 | 187.50 |
| Starch-10% NaNO2 | 49.88 | 183.87 | 186.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wongphan, P.; Nerin, C.; Harnkarnsujarit, N. Modifying Cassava Starch via Extrusion with Phosphate, Erythorbate and Nitrite: Phosphorylation, Hydrolysis and Plasticization. Polymers 2024, 16, 2787. https://doi.org/10.3390/polym16192787
Wongphan P, Nerin C, Harnkarnsujarit N. Modifying Cassava Starch via Extrusion with Phosphate, Erythorbate and Nitrite: Phosphorylation, Hydrolysis and Plasticization. Polymers. 2024; 16(19):2787. https://doi.org/10.3390/polym16192787
Chicago/Turabian StyleWongphan, Phanwipa, Cristina Nerin, and Nathdanai Harnkarnsujarit. 2024. "Modifying Cassava Starch via Extrusion with Phosphate, Erythorbate and Nitrite: Phosphorylation, Hydrolysis and Plasticization" Polymers 16, no. 19: 2787. https://doi.org/10.3390/polym16192787
APA StyleWongphan, P., Nerin, C., & Harnkarnsujarit, N. (2024). Modifying Cassava Starch via Extrusion with Phosphate, Erythorbate and Nitrite: Phosphorylation, Hydrolysis and Plasticization. Polymers, 16(19), 2787. https://doi.org/10.3390/polym16192787






