Ultrasonic Deposition of Cellulose Nanocrystals on Substrates for Enhanced Eradication Activity on Multidrug-Resistant Pathogens
Abstract
1. Introduction
2. Experimental
2.1. Materials and Chemicals
2.2. Preparation of CNCs
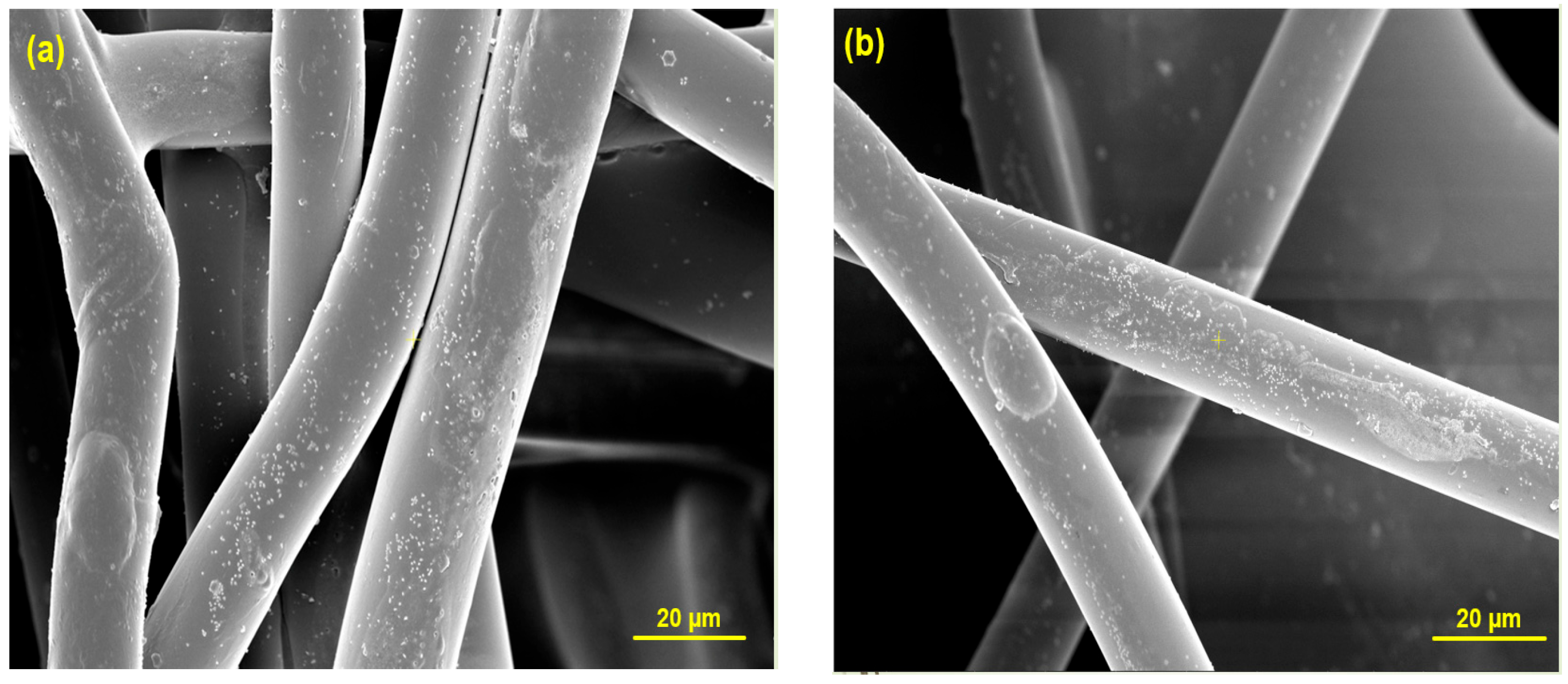
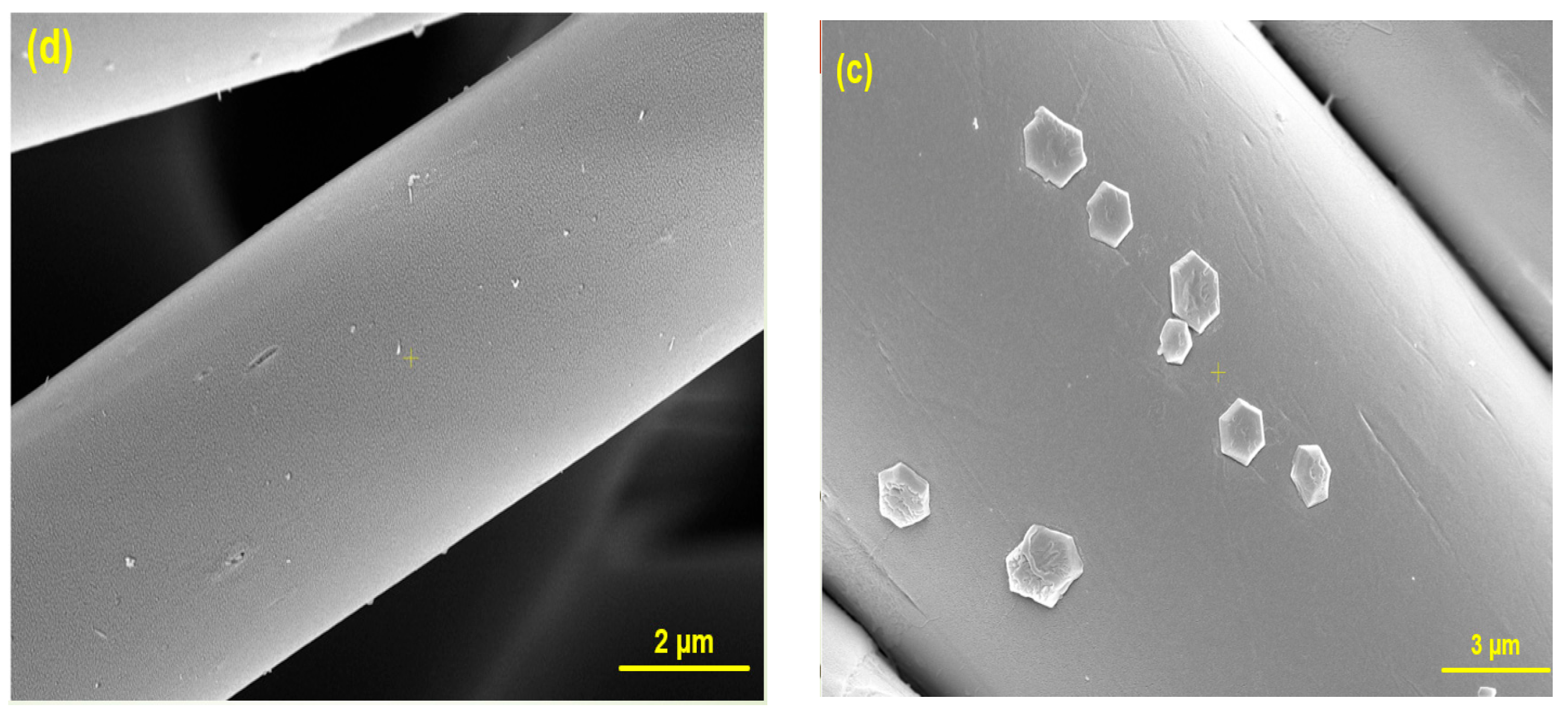
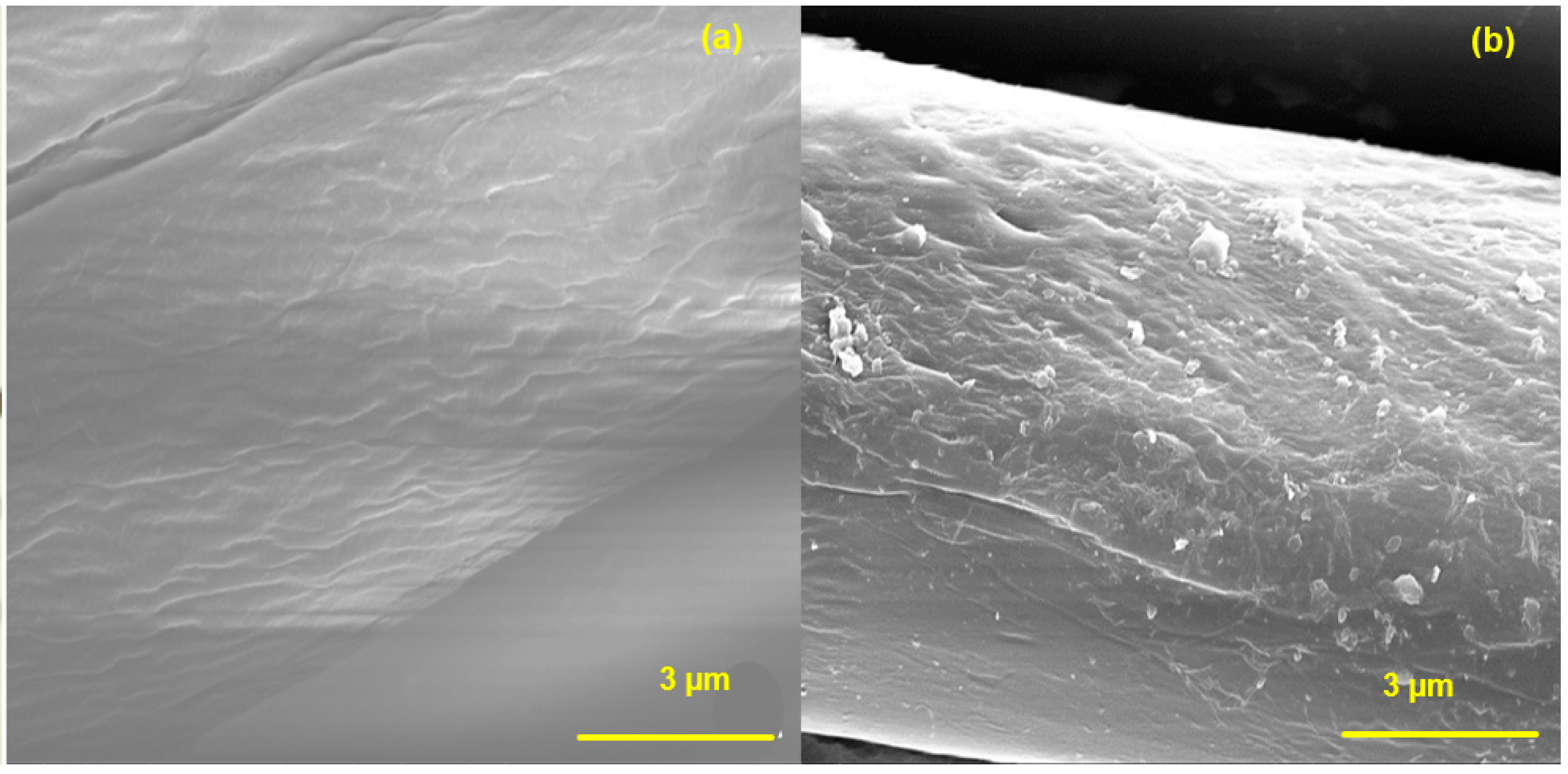
2.3. Coating Method
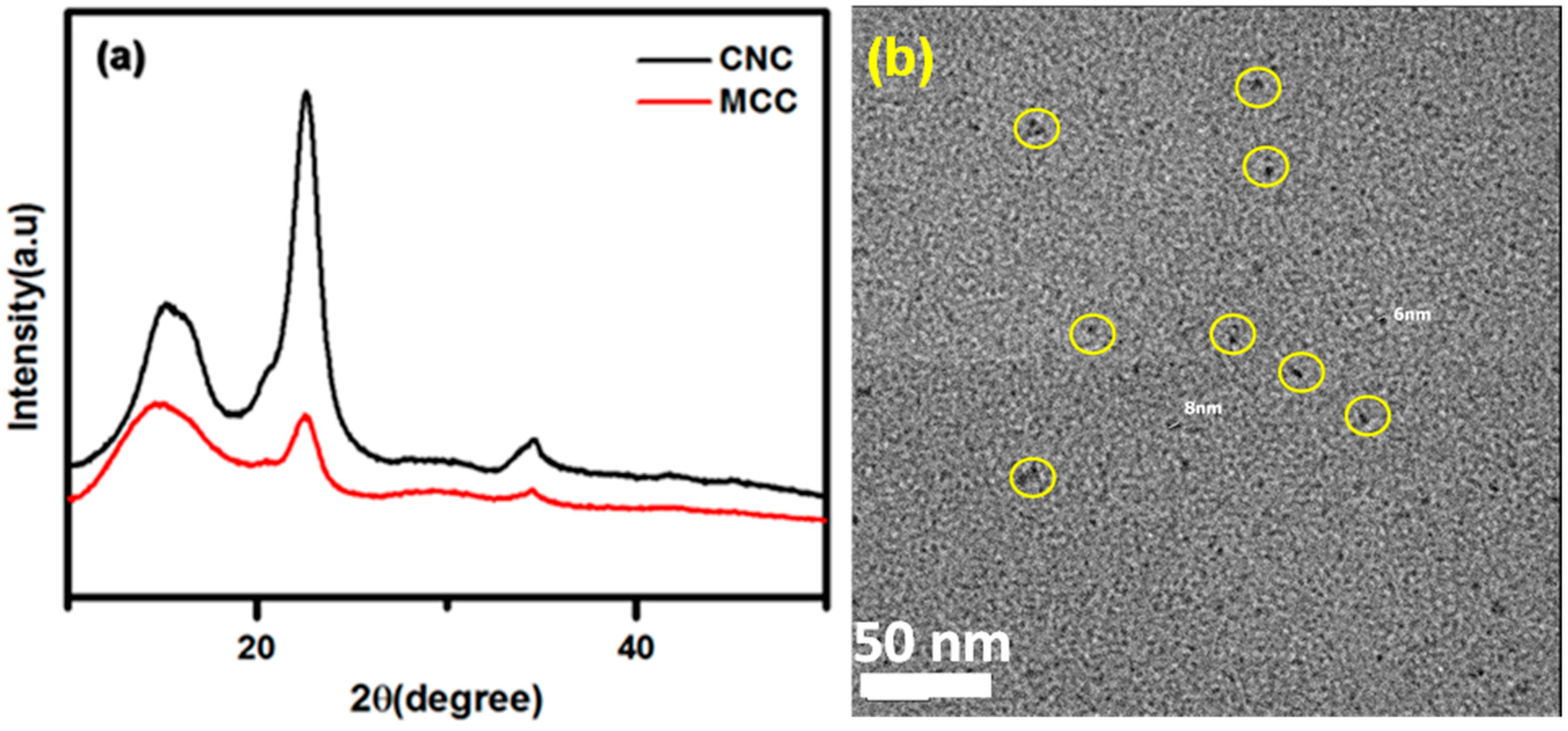
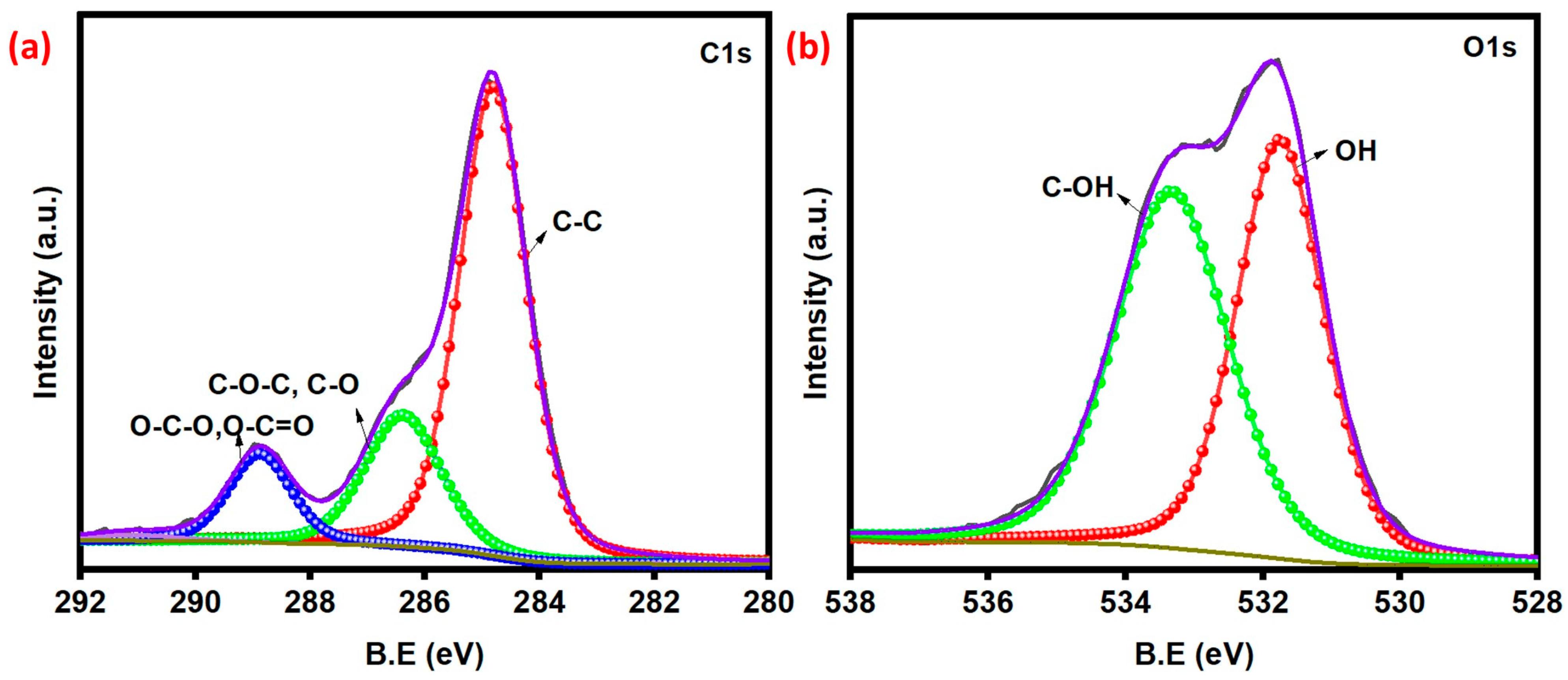
2.4. Characterization Methods
3. Results and Discussion
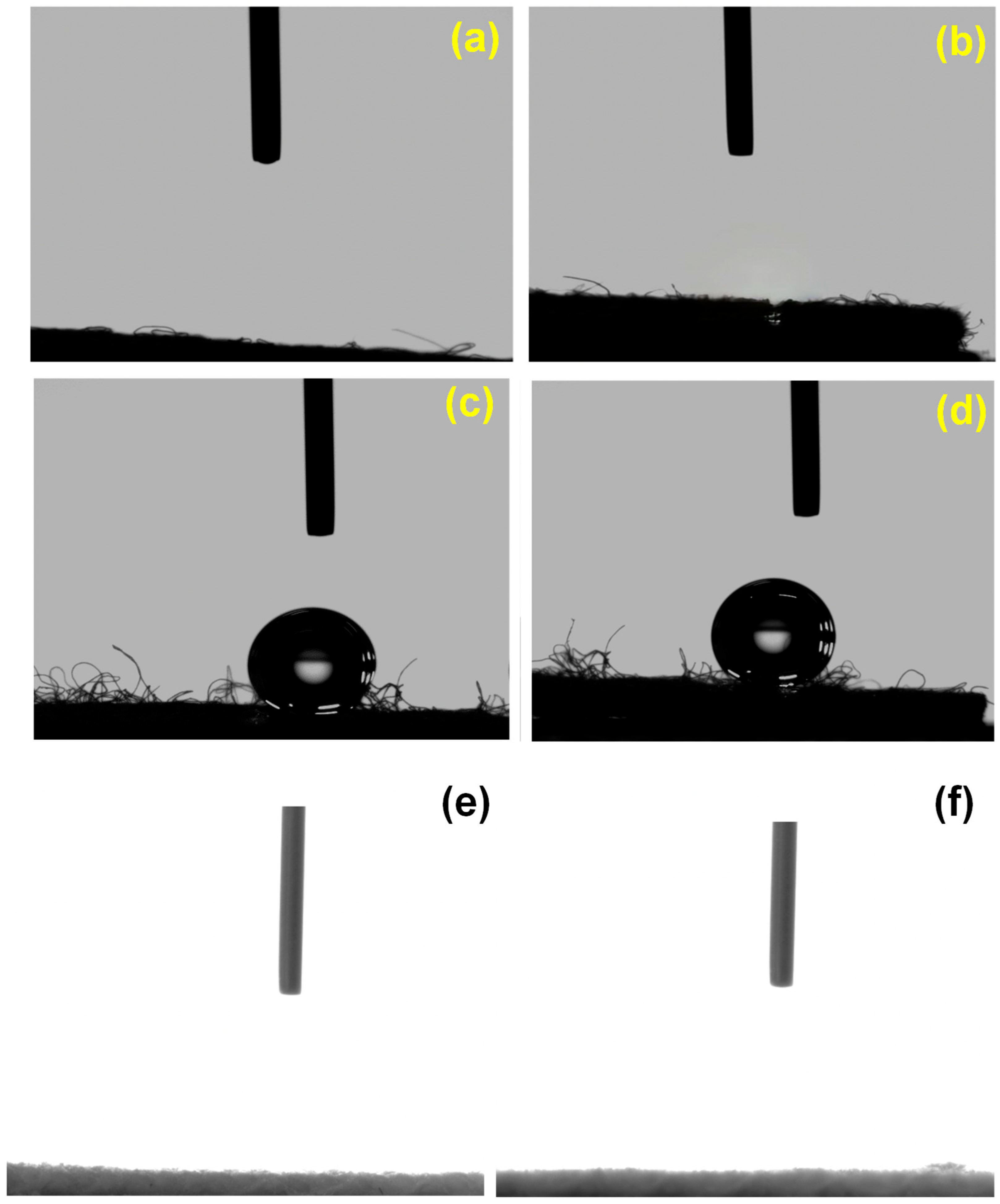
3.1. Contact Angle Measurement
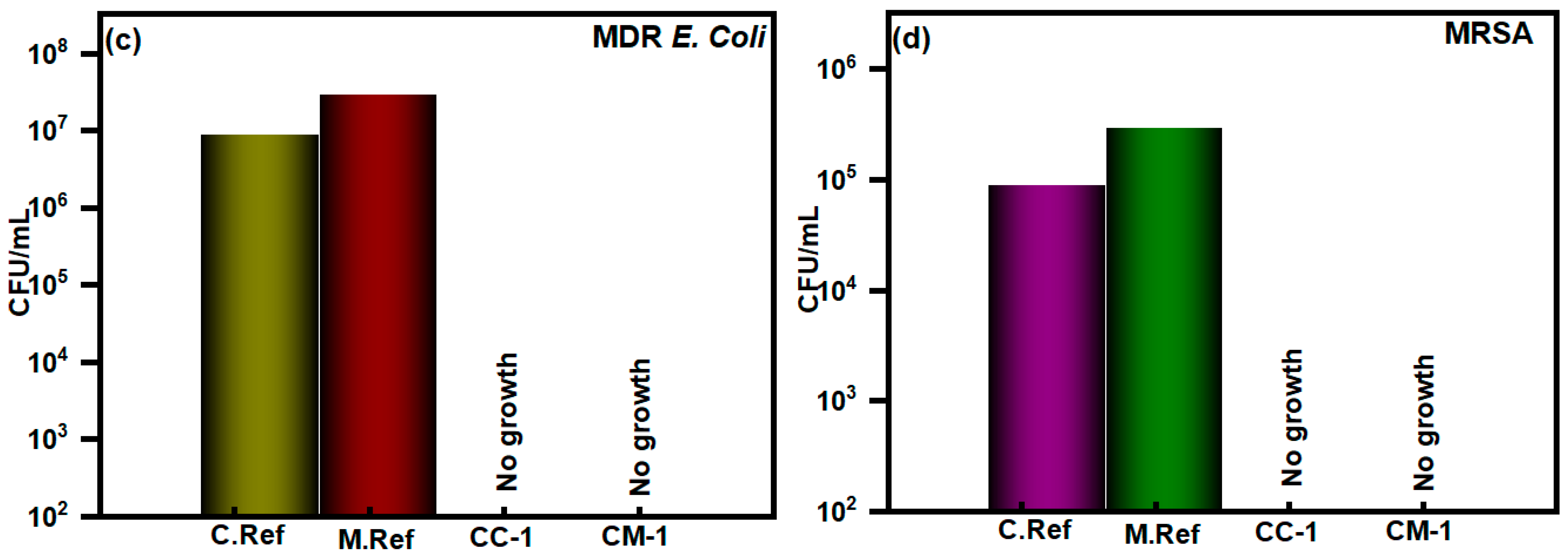
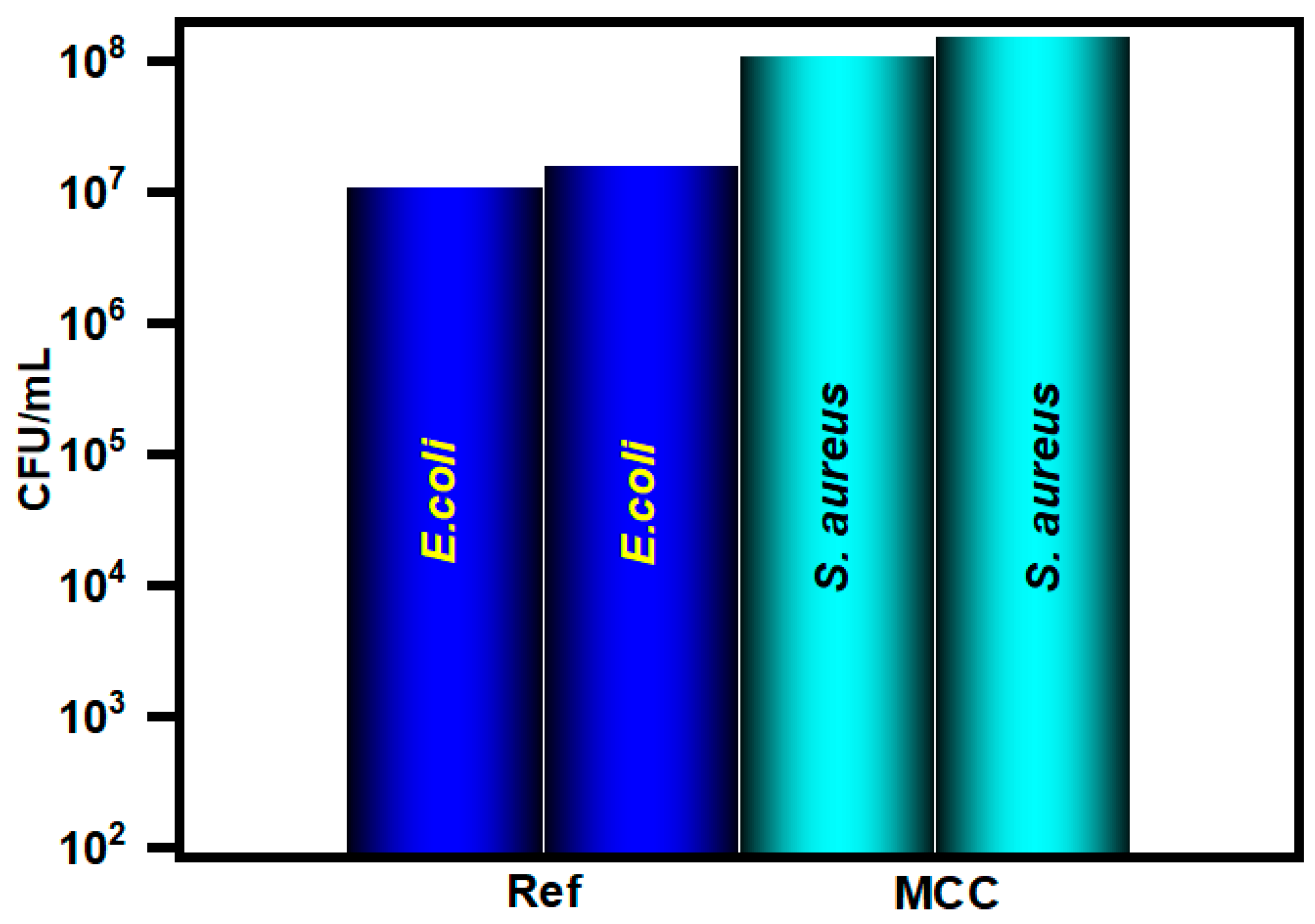
3.2. In Vitro Antibacterial Assay
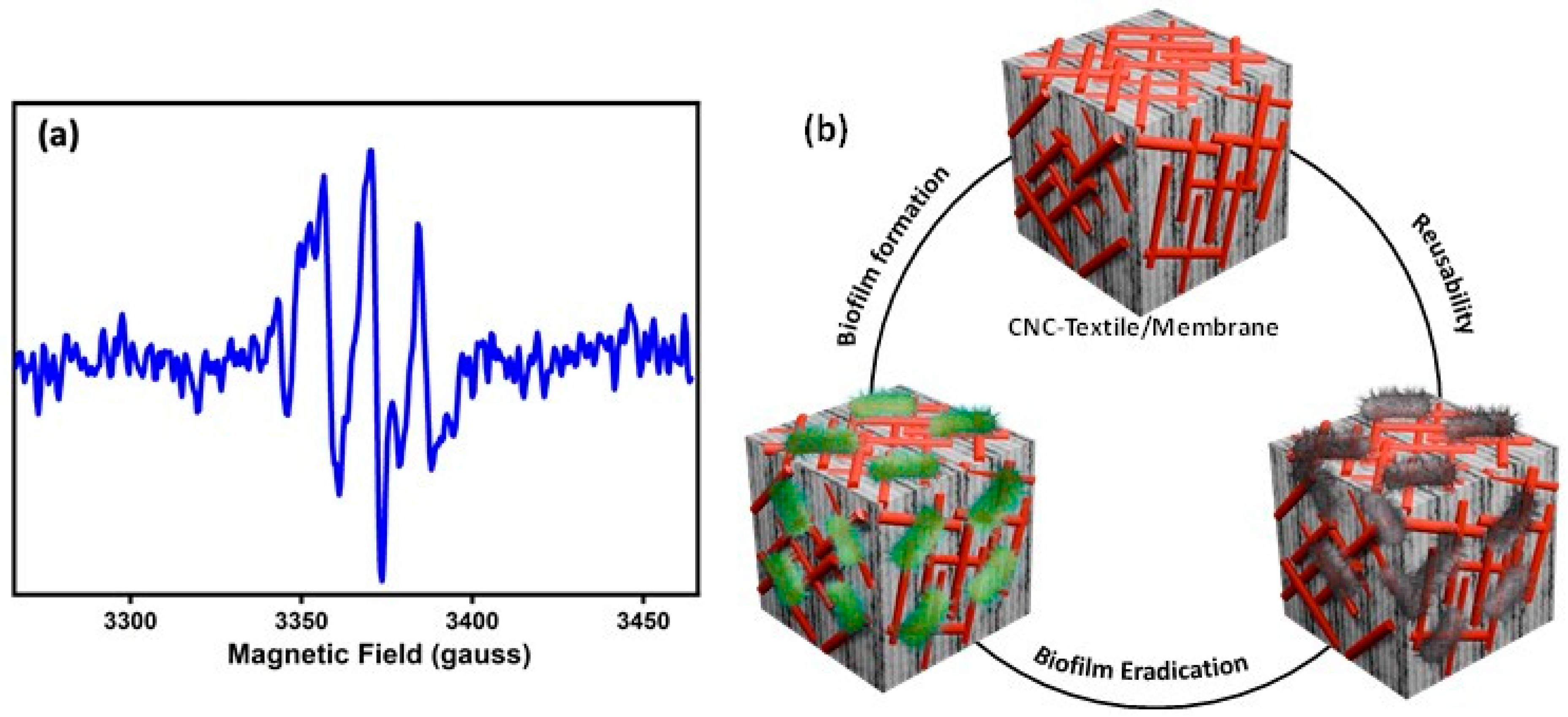
3.3. Inhibition/Rupture of Biofilm Formation
3.4. EPR Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, J.; Sisler, J.; Grishkewich, N.; Tam, K.C. Functionalization of Cellulose Nanocrystals for Advanced Applications. J. Colloid. Interface Sci. 2017, 494, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chang, R.; Ma, R.; Tian, Y. Eco-Friendly and Superhydrophobic Nano-Starch Based Coatings for Self-Cleaning Application and Oil-Water Separation. Carbohydr. Polym. 2021, 271, 118410. [Google Scholar] [CrossRef] [PubMed]
- Durairaj, A.; Maruthapandi, M.; Saravanan, A.; Luong, J.H.T.; Gedanken, A. Cellulose Nanocrystals (CNC)-Based Functional Materials for Supercapacitor Applications. Nanomaterials 2022, 12, 1828. [Google Scholar] [CrossRef]
- Nagarajan, K.J.; Ramanujam, N.R.; Sanjay, M.R.; Siengchin, S.; Surya Rajan, B.; Sathick Basha, K.; Madhu, P.; Raghav, G.R. A Comprehensive Review on Cellulose Nanocrystals and Cellulose Nanofibers: Pretreatment, Preparation, and Characterization. Polym. Compos. 2021, 42, 1588–1630. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Yang, Y.; Pang, B.; Xu, W.; Duan, G.; Jiang, S.; Zhang, K.; Chen, Y.; Duan, G.; et al. Recent Progress on Nanocellulose Aerogels: Preparation, Modification, Composite Fabrication, Applications. Adv. Mater. 2021, 33, 2005569. [Google Scholar] [CrossRef] [PubMed]
- Cherhal, F.; Cousin, F.; Capron, I. Structural Description of the Interface of Pickering Emulsions Stabilized by Cellulose Nanocrystals. Biomacromolecules 2016, 17, 496–502. [Google Scholar] [CrossRef]
- Csiszár, E.; Nagy, S. A Comparative Study on Cellulose Nanocrystals Extracted from Bleached Cotton and Flax and Used for Casting Films with Glycerol and Sorbitol Plasticisers. Carbohydr. Polym. 2017, 174, 740–749. [Google Scholar] [CrossRef]
- Durairaj, A.; Maruthapandi, M.; Luong, J.H.T.; Perelshtein, I.; Gedanken, A. Enhanced UV Protection, Heavy Metal Detection, and Antibacterial Properties of Biomass-Derived Carbon Dots Coated on Protective Fabrics. ACS Appl. Bio Mater. 2022, 5, 5790–5799. [Google Scholar] [CrossRef] [PubMed]
- Perelshtein, I.; Applerot, G.; Perkas, N.; Guibert, G.; Mikhailov, S.; Gedanken, A. Sonochemical Coating of Silver Nanoparticles on Textile Fabrics (Nylon, Polyester and Cotton) and Their Antibacterial Activity. Nanotechnology 2008, 19, 245705. [Google Scholar] [CrossRef]
- Perelshtein, I.; Ruderman, Y.; Perkas, N.; Beddow, J.; Singh, G.; Vinatoru, M.; Joyce, E.; Mason, T.J.; Blanes, M.; Mollá, K.; et al. The Sonochemical Coating of Cotton Withstands 65 Washing Cycles at Hospital Washing Standards and Retains Its Antibacterial Properties. Cellulose 2013, 20, 1215–1221. [Google Scholar] [CrossRef]
- Li, J.; Tian, X.; Hua, T.; Fu, J.; Koo, M.; Chan, W.; Poon, T. Chitosan Natural Polymer Material for Improving Antibacterial Properties of Textiles. ACS Appl. Bio Mater. 2021, 4, 4014–4038. [Google Scholar] [CrossRef] [PubMed]
- Antinate Shilpa, S.; Kavitha Sri, A.; Jeen Robert, R.B.; Subbulakshmi, M.S.; Hikku, G.S.O. A Review Focused on the Superhydrophobic Fabrics with Functional Properties. J. Appl. Polym. Sci. 2023, 140, e53664. [Google Scholar] [CrossRef]
- Zaman Khan, M.; Militky, J.; Petru, M.; Tomková, B.; Ali, A.; Tören, E.; Perveen, S. Recent Advances in Superhydrophobic Surfaces for Practical Applications: A Review. Eur. Polym. J. 2022, 178, 111481. [Google Scholar] [CrossRef]
- Tariq, H.; Rehman, A.; Kishwar, F.; Raza, Z.A. Citric Acid Cross-Linking of Chitosan Encapsulated Spearmint Oil for Antibacterial Cellulosic Fabric. Polym. Sci.-Ser. A 2022, 64, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Malucelli, G. Surface-Engineered Fire Protective Coatings for Fabrics through Sol-Gel and Layer-by-Layer Methods: An Overview. Coatings 2016, 6, 33. [Google Scholar] [CrossRef]
- Fontana, D.; Recupido, F.; Lama, G.C.; Liu, J.; Boggioni, L.; Silvano, S.; Lavorgna, M.; Verdolotti, L. Effect of Different Methods to Synthesize Polyol-Grafted-Cellulose Nanocrystals as Inter-Active Filler in Bio-Based Polyurethane Foams. Polymers 2023, 15, 923. [Google Scholar] [CrossRef]
- Babaei-Ghazvini, A.; Vafakish, B.; Patel, R.; Falua, K.J.; Dunlop, M.J.; Acharya, B. Cellulose Nanocrystals in the Development of Biodegradable Materials: A Review on CNC Resources, Modification, and Their Hybridization. Int. J. Biol. Macromol. 2024, 258, 128834. [Google Scholar] [CrossRef]
- Silvano, S.; Moimare, P.; Gryshchuk, L.; Barak-Kulbak, E.; Recupido, F.; Lama, G.C.; Boggioni, L.; Verdolotti, L. Synthesis of Bio-Polyol-Functionalized Nanocrystalline Celluloses as Reactive/Reinforcing Components in Bio-Based Polyurethane Foams by Homogeneous Environment Modification. Int. J. Biol. Macromol. 2024, 278, 135282. [Google Scholar] [CrossRef]
- Wohlhauser, S.; Delepierre, G.; Labet, M.; Morandi, G.; Thielemans, W.; Weder, C.; Zoppe, J.O. Grafting Polymers from Cellulose Nanocrystals: Synthesis, Properties, and Applications. Macromolecules 2018, 51, 6157–6189. [Google Scholar] [CrossRef]
- Aliakbar Ahovan, Z.; Esmaeili, Z.; Eftekhari, B.S.; Khosravimelal, S.; Alehosseini, M.; Orive, G.; Dolatshahi-Pirouz, A.; Pal Singh Chauhan, N.; Janmey, P.A.; Hashemi, A.; et al. Antibacterial Smart Hydrogels: New Hope for Infectious Wound Management. Mater. Today Bio 2022, 17, 100499. [Google Scholar] [CrossRef]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef]
- Elena, P.; Miri, K. Formation of Contact Active Antimicrobial Surfaces by Covalent Grafting of Quaternary Ammonium Compounds. Colloids Surf. B Biointerfaces 2018, 169, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Nie, S.; Xie, M.; Hu, J. Antioxidant and Antibacterial Capabilities of Phenolic Compounds and Organic Acids from Camellia Oleifera Cake. Food Sci. Biotechnol. 2020, 29, 17–25. [Google Scholar] [CrossRef]
- Yang, W.; Owczarek, J.S.; Fortunati, E.; Kozanecki, M.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Torre, L.; Puglia, D. Antioxidant and Antibacterial Lignin Nanoparticles in Polyvinyl Alcohol/Chitosan Films for Active Packaging. Ind. Crops Prod. 2016, 94, 800–811. [Google Scholar] [CrossRef]
- Punjabi, K.; Bhatia, E.; Keshari, R.; Jadhav, K.; Singh, S.; Shastri, J.; Banerjee, R. Biopolymer Coating Imparts Sustainable Self-Disinfecting and Antimicrobial Properties to Fabric: Translated to Protective Gears for the Pandemic and Beyond. ACS Biomater. Sci. Eng. 2023, 9, 1116–1131. [Google Scholar] [CrossRef] [PubMed]
- Zein, M.A.; Asghar, B.H.; Almohyawi, A.M.; Alqahtani, N.F.; Alharbi, A.; Alkabli, J.; Elshaarawy, R.F.M.; Ismail, L.A. Multifunctional Nanocomposites Integrated Green Synthesized Amphiphilic Chitosan/Thyme Extract/Nanosilver for Antimicrobial and Anti-Biofilm Applications. React. Funct. Polym. 2024, 194, 105791. [Google Scholar] [CrossRef]
- Jabareen, L.; Maruthapandi, M.; Saravanan, A.; Gedanken, A. Effective Degradation of Cellulose by Microwave Irradiation in Alkaline Solution. Cellulose 2021, 28, 11275–11285. [Google Scholar] [CrossRef]
- Perkas, N.; Amirian, G.; Dubinsky, S.; Gazit, S.; Gedanken, A. Ultrasound-Assisted Coating of Nylon 6,6 with Silver Nanoparticles and Its Antibacterial Activity. J. Appl. Polym. Sci. 2007, 104, 1423–1430. [Google Scholar] [CrossRef]
- Maruthapandi, M.; Saravanan, A.; Manoj, S.; Luong, J.H.T.; Gedanken, A. Facile Ultrasonic Preparation of a Polypyrrole Membrane as an Absorbent for Efficient Oil-Water Separation and as an Antimicrobial Agent. Ultrason. Sonochem 2021, 78, 105746. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Li, Y.; Huang, J.; Tang, J.; Chen, X.; Yu, H.Y.; Zhou, Y.; Tang, D. An Environmentally Friendly and Economical Strategy to Cyclically Produce Cellulose Nanocrystals with High Thermal Stability and High Yield. Green. Chemistry 2021, 23, 4866–4872. [Google Scholar] [CrossRef]
- Lam, E.; Hemraz, U.D. Preparation and Surface Functionalization of Carboxylated Cellulose Nanocrystals. Nanomaterials 2021, 11, 1641. [Google Scholar] [CrossRef] [PubMed]
- d’Errico, C.; Börjesson, J.; Ding, H.; Krogh, K.B.R.M.; Spodsberg, N.; Madsen, R.; Monrad, R.N. Improved Biomass Degradation Using Fungal Glucuronoyl—Esterases—Hydrolysis of Natural Corn Fiber Substrate. J. Biotechnol. 2016, 219, 117–123. [Google Scholar] [CrossRef]
- Leung, A.C.W.; Hrapovic, S.; Lam, E.; Liu, Y.; Male, K.B.; Mahmoud, K.A.; Luong, J.H.T. Characteristics and Properties of Carboxylated Cellulose Nanocrystals Prepared from a Novel One-Step Procedure. Small 2011, 7, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Julie Chandra, C.S.; George, N.; Narayanankutty, S.K. Isolation and Characterization of Cellulose Nanofibrils from Arecanut Husk Fibre. Carbohydr. Polym. 2016, 142, 158–166. [Google Scholar] [CrossRef]
- Muthusamy, V.P.; Krishnakumar, V. Ultrasonication-Induced Re-Dispersion of Freeze-Dried Cellulose Nanoparticles for Improving Strength and Durability of PAN Polymer Matrix. J. Dispers. Sci. Technol. 2024, 2024, 2338379. [Google Scholar] [CrossRef]
- Lei, D.; Tang, Z.; Zhao, L.; Wang, Y.; Du, K. Macroporous Cellulose Microspheres Derived from Cigarette Butts Waste: Preparation, Characterization, and Application in Proteins Adsorption. Cellulose 2023, 30, 9061–9077. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Lin, S.; Xiao, H.; Yang, Q.; Chen, S.; Yan, B.; Gu, Y. Environmentally Friendly Nanocomposites Based on Cellulose Nanocrystals and Polydopamine for Rapid Removal of Organic Dyes in Aqueous Solution. Cellulose 2020, 27, 2085–2097. [Google Scholar] [CrossRef]
- Attia, N.F.; Zakria, A.M.; Nour, M.A.; Abd El-Ghany, N.A.; Elashery, S.E.A. Rational Strategy for Construction of Multifunctional Coatings for Achieving High Fire Safety, Antibacterial, UV Protection and Electrical Conductivity Functions of Textile Fabrics. Mater. Today Sustain. 2023, 23, 100450. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Eckhard, U.; Delgado, L.M.; de Roo Puente, Y.J.D.; Hoyos-Nogués, M.; Gil, F.J.; Perez, R.A. Antibacterial Approaches in Tissue Engineering Using Metal Ions and Nanoparticles: From Mechanisms to Applications. Bioact. Mater. 2021, 6, 4470–4490. [Google Scholar] [CrossRef]
- Mulder, R.; Maboza, E.; Ahmed, R. Streptococcus Mutans Growth and Resultant Material Surface Roughness on Modified Glass Ionomers. Front. Oral. Health 2020, 1, 613384. [Google Scholar] [CrossRef]
- Villapun Puzas, V.M.; Carter, L.N.; Schröder, C.; Colavita, P.E.; Hoey, D.A.; Webber, M.A.; Addison, O.; Shepherd, D.E.T.; Attallah, M.M.; Grover, L.M.; et al. Surface Free Energy Dominates the Biological Interactions of Postprocessed Additively Manufactured Ti-6Al-4V. ACS Biomater. Sci. Eng. 2022, 8, 4311–4326. [Google Scholar] [CrossRef] [PubMed]
- Takashima, S.; Morisaki, H. Surface Characteristics of the Microbial Cell of Pseudomonas syringae and Its Relevance to Cell Attachment. Colloids Surf. B Biointerfaces 1997, 9, 205–212. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Surface Chemistry, Morphological Analysis and Properties of Cellulose Nanocrystals with Gradiented Sulfation Degrees. Nanoscale 2014, 6, 5384–5393. [Google Scholar] [CrossRef]
- El-Khoury, N.; Bennaceur, I.; Verplaetse, E.; Aymerich, S.; Lereclus, D.; Kallassy, M.; Gohar, M. Massive Integration of Planktonic Cells within a Developing Biofilm. Microorganisms 2021, 9, 298. [Google Scholar] [CrossRef]
- Peterson, E.; Kaur, P. Antibiotic Resistance Mechanisms in Bacteria: Relationships between Resistance Determinants of Antibiotic Producers, Environmental Bacteria, and Clinical Pathogens. Front. Microbiol. 2018, 9, 426686. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Hua, Y.; Zhang, Y.; Lv, M.; Wang, H.; Pi, Y.; Xie, J.; Wang, C.; Yong, Y. A Biofilm Microenvironment-Activated Single-Atom Iron Nanozyme with NIR-Controllable Nanocatalytic Activities for Synergetic Bacteria-Infected Wound Therapy. Adv. Healthc. Mater. 2021, 10, 2101374. [Google Scholar] [CrossRef]
- Dai, X.; Liu, H.; Cai, B.; Liu, Y.; Song, K.; Chen, J.; Ni, S.Q.; Kong, L.; Zhan, J. A Bioinspired Atomically Thin Nanodot Supported Single-Atom Nanozyme for Antibacterial Textile Coating. Small 2023, 19, 2303901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, W.; Gao, Y.; Zhou, N.; Wang, W. Manganese-Iron Dual Single-Atom Catalyst with Enhanced Nanozyme Activity for Wound and Pustule Disinfection. ACS Appl. Mater. Interfaces 2023, 15, 42227–42240. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Wang, X.; Ye, Z.; Zhang, J.; Chen, X.; Zhou, N.; Zhang, M.; Yao, C.; Wu, F.; Shen, J. Designing Single-Atom Active Sites on Sp2-Carbon Linked Covalent Organic Frameworks to Induce Bacterial Ferroptosis-Like for Robust Anti-Infection Therapy. Adv. Sci. 2023, 10, 2207507. [Google Scholar] [CrossRef] [PubMed]
- Milosavljevic, V.; Kosaristanova, L.; Dolezelikova, K.; Adam, V.; Pumera, M.; Milosavljevic, V.; Pumera, M.; Dolezelikova, K.; Adam, V. Microrobots with Antimicrobial Peptide Nanoarchitectonics for the Eradication of Antibiotic-Resistant Biofilms. Adv. Funct. Mater. 2022, 32, 2112935. [Google Scholar] [CrossRef]
- Villa, K.; Sopha, H.; Zelenka, J.; Motola, M.; Dekanovsky, L.; Beketova, D.C.; Macak, J.M.; Ruml, T.; Pumera, M.; Villa, K.; et al. Enzyme-Photocatalyst Tandem Microrobot Powered by Urea for Escherichia Coli Biofilm Eradication. Small 2022, 18, 2106612. [Google Scholar] [CrossRef] [PubMed]
- Adam, V.; Pumera, M.; Ussia, M.; Urso, M.; Dolezelikova, K.; Michalkova, H.; Adam, V.; Pumera, M.; Ussia, M.; Urso, M.; et al. Active Light-Powered Antibiofilm ZnO Micromotors with Chemically Programmable Properties. Adv. Funct. Mater. 2021, 31, 2101178. [Google Scholar] [CrossRef]
- Mayorga-Martinez, C.C.; Zelenka, J.; Klima, K.; Kubanova, M.; Ruml, T.; Pumera, M.; Mayorga-Martinez, C.C.; Pumera, M.; Zelenka, J.; Kubanova, M.; et al. Multimodal-Driven Magnetic Microrobots with Enhanced Bactericidal Activity for Biofilm Eradication and Removal from Titanium Mesh. Adv. Mater. 2023, 35, 2300191. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Chen, H.; Lv, Y.; Yang, W.; Zhang, F.; Wang, T.; Liu, D.; Qu, Y.; Han, L.; Fu, J.; et al. CuO2-Assisting-Zn Single Atom Hybrid Nanozymes for Biofilm-Infected Wound Healing. Chem. Eng. J. 2023, 474, 145706. [Google Scholar] [CrossRef]
- Yu, Y.; Tan, L.; Li, Z.; Liu, X.; Zheng, Y.; Feng, X.; Liang, Y.; Cui, Z.; Zhu, S.; Wu, S. Single-Atom Catalysis for Efficient Sonodynamic Therapy of Methicillin-Resistant Staphylococcus Aureus-Infected Osteomyelitis. ACS Nano 2021, 15, 10628–10639. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal Nanoparticles: Understanding the Mechanisms behind Antibacterial Activity. J. Nanobiotechnology 2017, 15, 1–20. [Google Scholar] [CrossRef]
- Hoo, D.Y.; Low, Z.L.; Low, D.Y.S.; Tang, S.Y.; Manickam, S.; Tan, K.W.; Ban, Z.H. Ultrasonic cavitation: An effective cleaner and greener intensification technology in the extraction and surface modification of nanocellulose. Ultrason. Sonochemistry 2022, 90, 106176. [Google Scholar] [CrossRef]
- You, C.; Ning, L.; Wu, H.; Huang, C.; Wang, F. A Biocompatible and PH-Responsive Nanohydrogel Based on Cellulose Nanocrystal for Enhanced Toxic Reactive Oxygen Species Generation. Carbohydr. Polym. 2021, 258, 117685. [Google Scholar] [CrossRef] [PubMed]
- Roman, M. Toxicity of Cellulose Nanocrystals: A Review. Ind. Biotechnol. 2015, 11, 25–33. [Google Scholar] [CrossRef]
- Pelegrini, B.L.; Ré, F.; de Oliveira, M.M.; Fernandes, T.; de Oliveira, J.H.; Oliveira Junior, A.G.; Girotto, E.M.; Nakamura, C.V.; Sampaio, A.R.; Valim, A.; et al. Cellulose Nanocrystals as a Sustainable Raw Material: Cytotoxicity and Applications on Healthcare Technology. Macromol. Mater. Eng. 2019, 304, 1900092. [Google Scholar] [CrossRef]
- Mahmoud, K.A.; Mena, J.A.; Male, K.B.; Hrapovic, S.; Kamen, A.; Luong, J.H.T. Effect of Surface Charge on the Cellular Uptake and Cytotoxicity of Fluorescent Labeled Cellulose Nanocrystals. ACS Appl. Mater. Interfaces 2010, 2, 2924–2932. [Google Scholar] [CrossRef] [PubMed]
- Male, K.B.; Leung, A.C.W.; Montes, J.; Kamen, A.; Luong, J.H.T. Probing Inhibitory Effects of Nanocrystalline Cellulose: Inhibition versus Surface Charge. Nanoscale 2012, 4, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; He, H.; Wan, R.; Yang, Q.; Luo, H.; Li, L.; Xiong, L. Cellulose Nanocrystals for Skin Barrier Protection by Preparing a Versatile Foundation Liquid. ACS Omega 2021, 6, 2906–2915. [Google Scholar] [CrossRef] [PubMed]



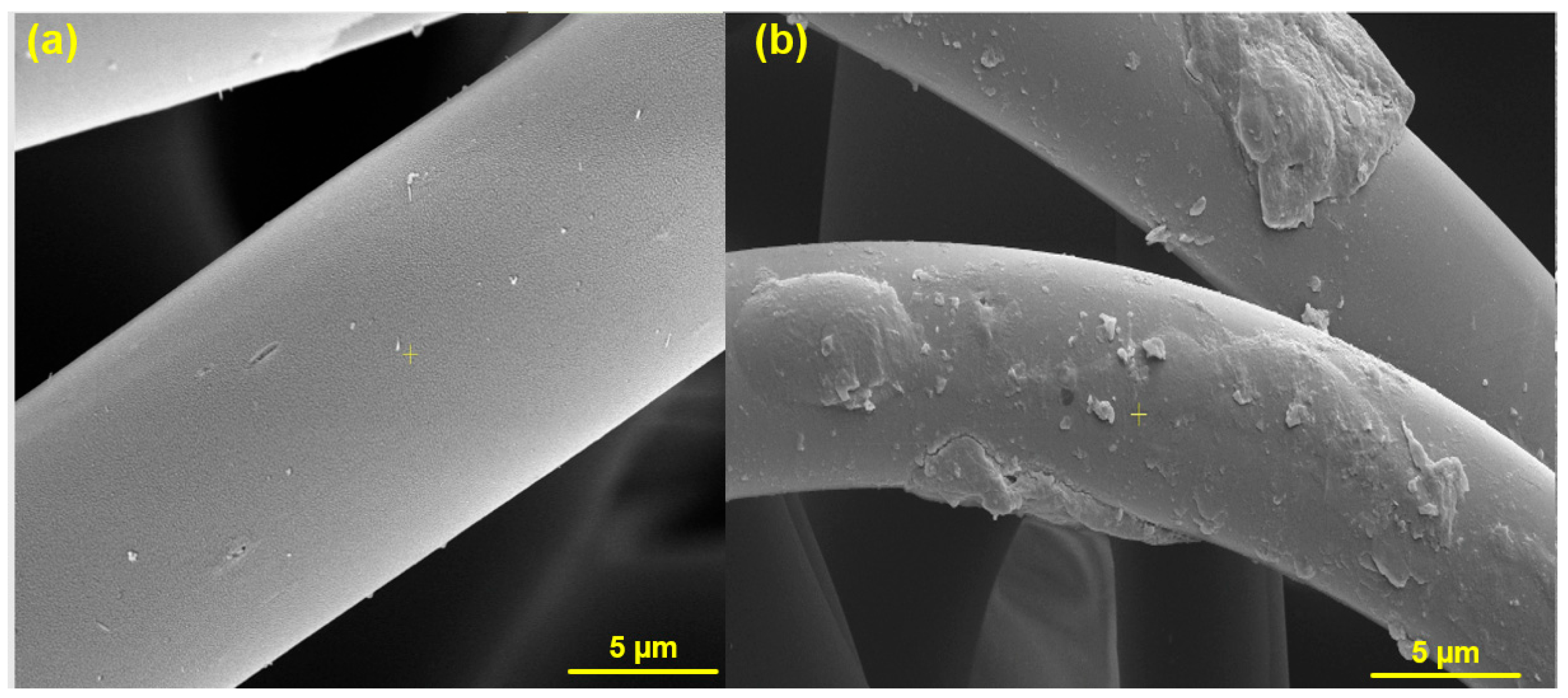
| Materials | Bacterial Strains | Treatments | Ref. |
|---|---|---|---|
| BME-activated Fe-doped polydiaminopyridine nanofusiform-mediated single-atom nanozyme | E. coli | Biofilm eradication | [46] |
| C3N4 nanodots supported single Cu atom nanozymes (Cu-CNNDs) | S. aureus/E. coli | Antibacterial activity | [47] |
| manganese-iron dual single-atom catalysts (Mn/Fe SACs) | S. aureus/E. coli | Antibacterial activity | [48] |
| (sp(2)c-COF-Ir-ppy(2) and sp(2)c-COF-Ru-bpy(2)) | MRSA | Antibacterial activity and Biofilm eradication | [49] |
| peptide/PM/Pt microrobots | MRSA | Antibacterial activity | [50] |
| TiO2/CdS nanotube | E. coli | Biofilm eradication | [51] |
| Ag-doped ZnO | MRSA and P. aeruginosa | Biofilm eradication | [52] |
| Titanium Mesh halloysite nanotubes- HNT-Fe3O4 | S.aureus | Biofilm eradication | [53] |
| CuO2-assisting-Zn single atom hybrid nanozymes | S. aureus/E. coli | Antibacterial activity | [54] |
| single-atom-doped porphyrin metal–organic framework (HNTM-Pt@Au) | MRSA-infected osteomyelitis | Antibacterial activity | [55] |
| CNC coated materials | S. aureus, E. coli, MRSA and MDR | Biofilm inhibition | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabreen, L.; Maruthapandi, M.; Durairaj, A.; Luong, J.H.T.; Gedanken, A. Ultrasonic Deposition of Cellulose Nanocrystals on Substrates for Enhanced Eradication Activity on Multidrug-Resistant Pathogens. Polymers 2025, 17, 154. https://doi.org/10.3390/polym17020154
Jabreen L, Maruthapandi M, Durairaj A, Luong JHT, Gedanken A. Ultrasonic Deposition of Cellulose Nanocrystals on Substrates for Enhanced Eradication Activity on Multidrug-Resistant Pathogens. Polymers. 2025; 17(2):154. https://doi.org/10.3390/polym17020154
Chicago/Turabian StyleJabreen, Lama, Moorthy Maruthapandi, Arulappan Durairaj, John H. T. Luong, and Aharon Gedanken. 2025. "Ultrasonic Deposition of Cellulose Nanocrystals on Substrates for Enhanced Eradication Activity on Multidrug-Resistant Pathogens" Polymers 17, no. 2: 154. https://doi.org/10.3390/polym17020154
APA StyleJabreen, L., Maruthapandi, M., Durairaj, A., Luong, J. H. T., & Gedanken, A. (2025). Ultrasonic Deposition of Cellulose Nanocrystals on Substrates for Enhanced Eradication Activity on Multidrug-Resistant Pathogens. Polymers, 17(2), 154. https://doi.org/10.3390/polym17020154








