Cellulose-Based Materials and Their Application in Lithium–Sulfur Batteries
Abstract
1. Introduction
2. Working Principle of Li-S Batteries
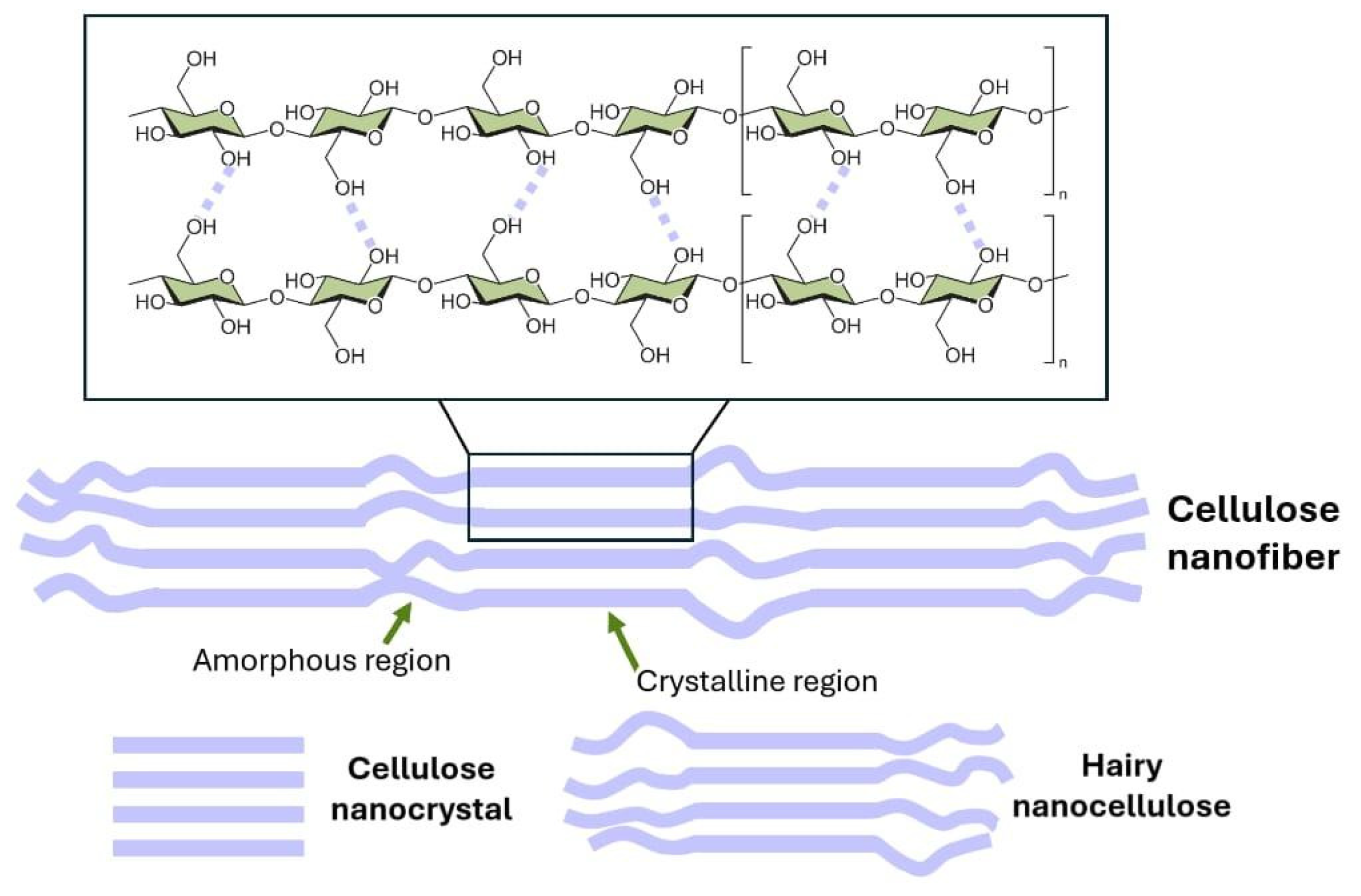
3. Cellulose’s Main Characteristics
4. Application of Cellulose-Based Materials in Li-S Batteries
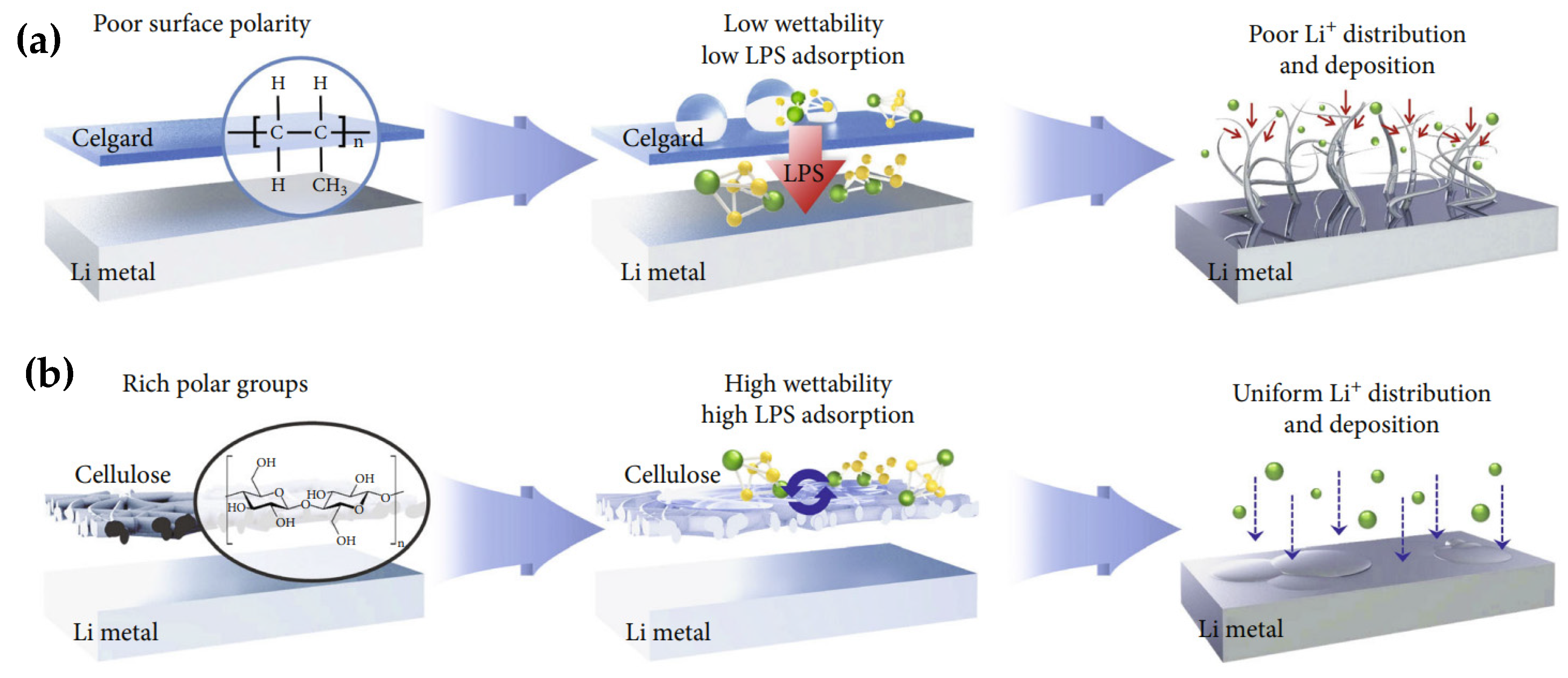
4.1. Cellulose for Lithium Metal Protection
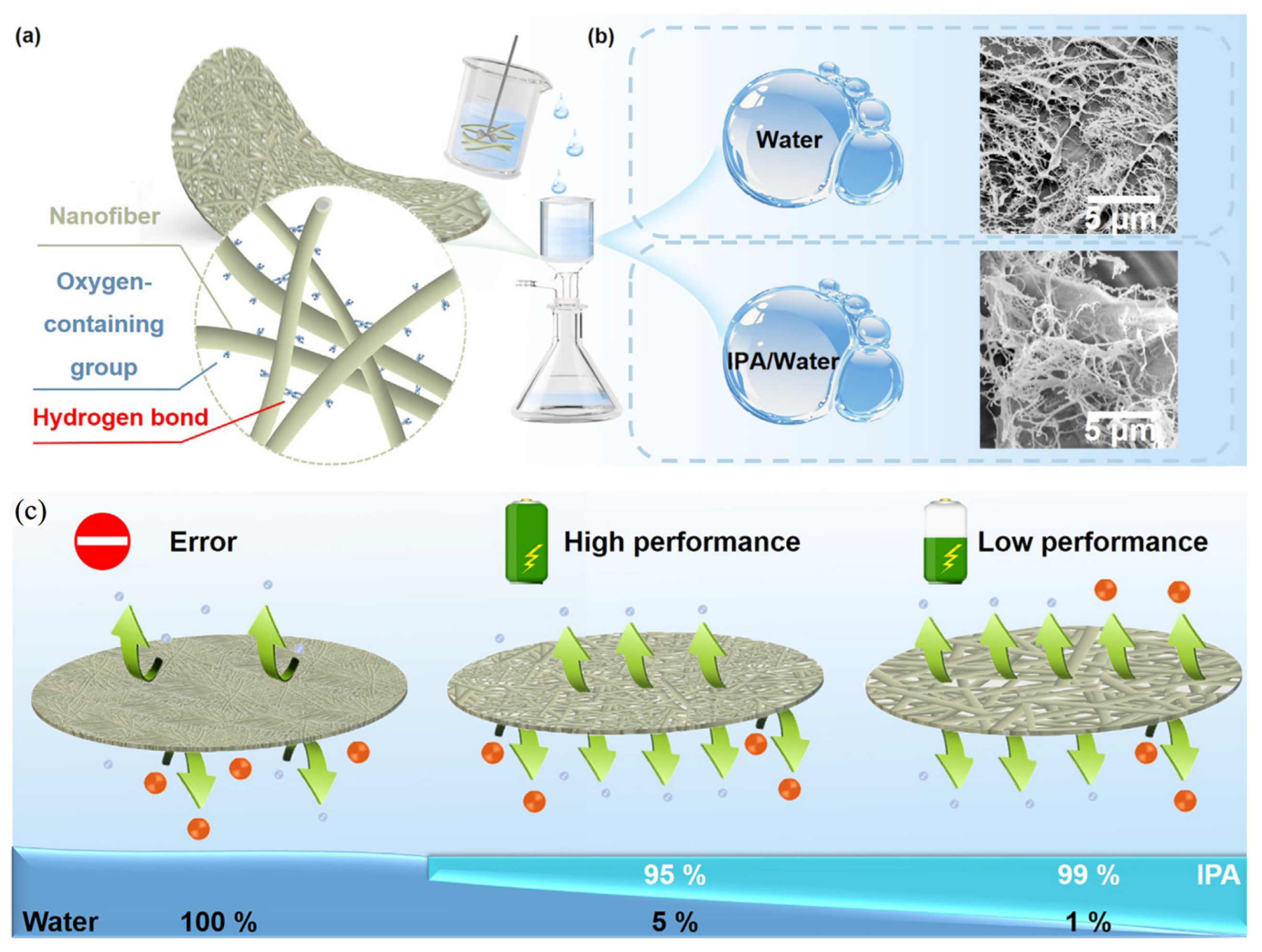
4.2. Cellulose for Separators
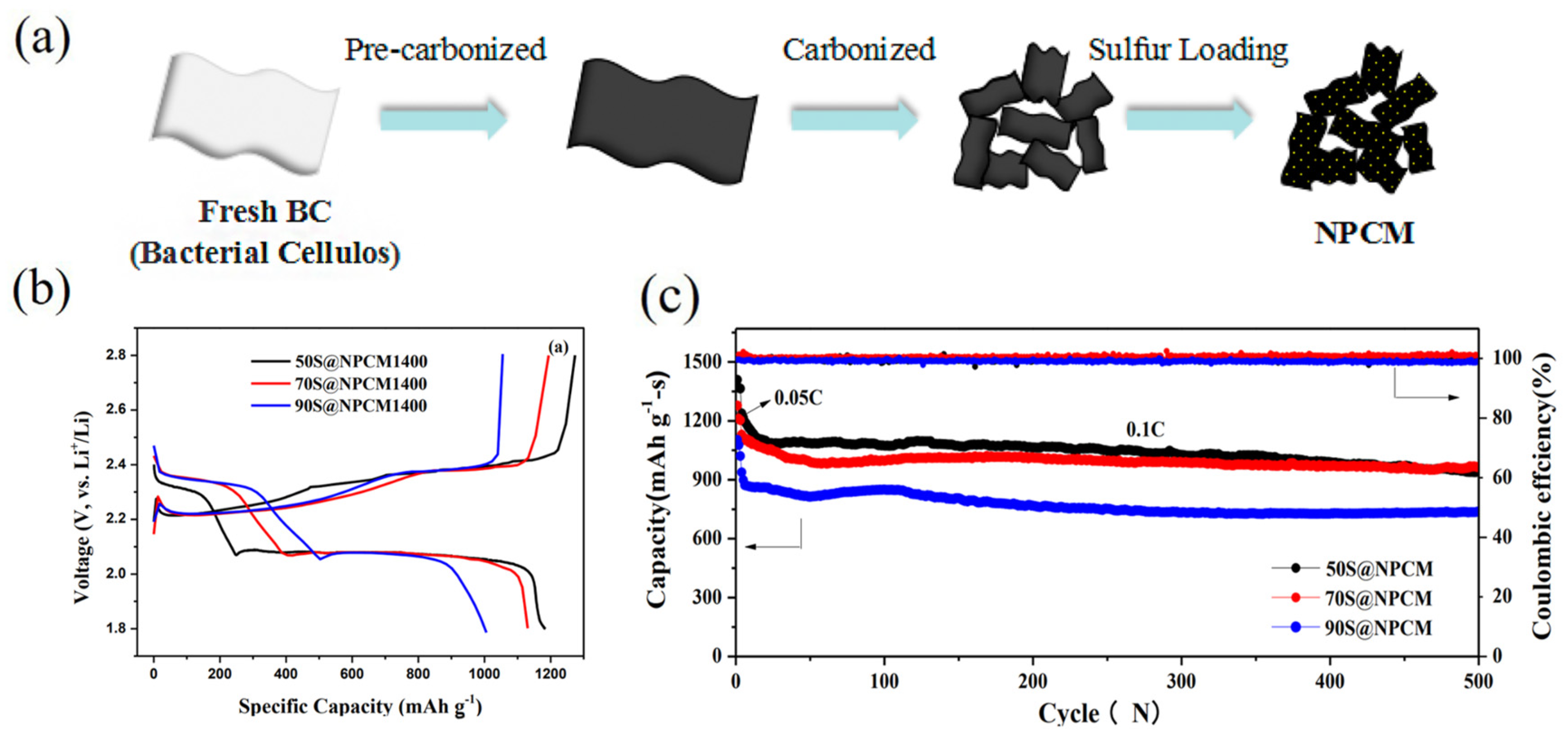
4.3. Cellulose-Based Cathode Materials for Lithium–Sulphur Batteries
5. Conclusions and Perspectives
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Fleetwood, J.; Hawley, W.B.; Kays, W. From Materials to Cell: State-of-the-Art and Prospective Technologies for Lithium-Ion Battery Electrode Processing. Chem. Rev. 2022, 122, 903–956. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.D.; Zheng, J.; Ren, X.; Xu, W.; Zhang, J.-G. Accurate Determination of Coulombic Efficiency for Lithium Metal Anodes and Lithium Metal Batteries. Adv. Energy Mater. 2018, 8, 1702097. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Chung, S.-H.; Zu, C.; Su, Y.-S. Rechargeable Lithium–Sulfur Batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.-X.; Xin, S.; Guo, Y.-G.; Wan, L.-J. Lithium–Sulfur Batteries: Electrochemistry, Materials, and Prospects. Angew. Chem. Int. Ed. 2013, 52, 13186–13200. [Google Scholar] [CrossRef]
- Cha, E.; Patel, M.; Bhoyate, S.; Prasad, V.; Choi, W. Nanoengineering to Achieve High Efficiency Practical Lithium–Sulfur Batteries. Nanoscale Horiz. 2020, 5, 808–831. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lu, A.; Zhang, L. Recent Advances in Regenerated Cellulose Materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Acharya, S.; Liyanage, S.; Parajuli, P.; Rumi, S.S.; Shamshina, J.L.; Abidi, N. Utilization of Cellulose to Its Full Potential: A Review on Cellulose Dissolution, Regeneration, and Applications. Polymers 2021, 13, 4344. [Google Scholar] [CrossRef]
- Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Green and Sustainable Pretreatment Methods for Cellulose Extraction from Lignocellulosic Biomass and Its Applications: A Review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100396. [Google Scholar] [CrossRef]
- Cheng, W.; Zhu, Y.; Jiang, G.; Cao, K.; Zeng, S.; Chen, W.; Zhao, D.; Yu, H. Sustainable Cellulose and Its Derivatives for Promising Biomedical Applications. Prog. Mater. Sci. 2023, 138, 101152. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Z.; Feng, Y.; Cai, S.; Gao, H.; Wei, Z.; Zhao, Y. Cellulose-Based Separators for Lithium Batteries: Source, Preparation and Performance. Chem. Eng. J. 2023, 471, 144593. [Google Scholar] [CrossRef]
- Grzybek, P.; Dudek, G.; van der Bruggen, B. Cellulose-Based Films and Membranes: A Comprehensive Review on Preparation and Applications. Chem. Eng. J. 2024, 495, 153500. [Google Scholar] [CrossRef]
- Son, D.; Lim, W.-G.; Lee, J. A Short Review of the Recent Developments in Functional Separators for Lithium-Sulfur Batteries. Korean J. Chem. Eng. 2023, 40, 473–487. [Google Scholar] [CrossRef]
- Lim, W.-G.; Kim, S.; Jo, C.; Lee, J. A Comprehensive Review of Materials with Catalytic Effects in Li–S Batteries: Enhanced Redox Kinetics. Angew. Chem. Int. Ed. 2019, 58, 18746–18757. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fang, Z.; Xiang, Y.; Liu, D.; Xie, Z.; Qu, D.; Sun, M.; Tang, H.; Li, J. Cellulose-Based Material in Lithium-Sulfur Batteries: A Review. Carbohydr. Polym. 2021, 255, 117469. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.T.; Hao, X.; Wang, C.; Sun, X. Cathode Materials for Single-Phase Solid-Solid Conversion Li-S Batteries. Matter 2023, 6, 316–343. [Google Scholar] [CrossRef]
- Shi, C.; Takeuchi, S.; Alexander, G.V.; Hamann, T.; O’Neill, J.; Dura, J.A.; Wachsman, E.D. High Sulfur Loading and Capacity Retention in Bilayer Garnet Sulfurized-Polyacrylonitrile/Lithium-Metal Batteries with Gel Polymer Electrolytes. Adv. Energy Mater. 2023, 13, 2301656. [Google Scholar] [CrossRef]
- Umeshbabu, E.; Zheng, B.; Yang, Y. Recent Progress in All-Solid-State Lithium−Sulfur Batteries Using High Li-Ion Conductive Solid Electrolytes. Electrochem. Energy Rev. 2019, 2, 199–230. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, L.; Bai, Z.; Lu, J.; Wu, T. Sulfide-Based All-Solid-State Lithium–Sulfur Batteries: Challenges and Perspectives. Nano-Micro Lett. 2023, 15, 75. [Google Scholar] [CrossRef]
- Chen, S.; Dai, F.; Cai, M. Opportunities and Challenges of High-Energy Lithium Metal Batteries for Electric Vehicle Applications. ACS Energy Lett. 2020, 5, 3140–3151. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, C.; Lin, Q.; Zhang, Y.; Deng, Y.; Han, J.; Wu, D.; Kang, F.; Yang, Q.-H.; Lv, W. A Protective Layer for Lithium Metal Anode: Why and How. Small Methods 2021, 5, 2001035. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Deng, S.; Li, X.; Zhang, Y.; Lv, T.; He, Y.; Mao, W.; Yang, H.; Zhang, J.; Chu, P.K.; et al. Lithiophilic and Conductive Framework of 2D MoN Nanosheets Enabling Planar Lithium Plating for Dendrite-Free and Minimum-Volume-Change Lithium Metal Anodes. Chem. Eng. J. 2023, 454, 140144. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Zhang, L.; Tao, H.; Li, Q.; Zhang, J.; Yang, X. Lithiophilicity: The Key to Efficient Lithium Metal Anodes for Lithium Batteries. J. Energy Chem. 2023, 77, 123–136. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z. Battery Separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef]
- Deimede, V.; Elmasides, C. Separators for Lithium-Ion Batteries: A Review on the Production Processes and Recent Developments. Energy Technol. 2015, 3, 453–468. [Google Scholar] [CrossRef]
- Cannon, A.; Ryan, E.M. Characterizing the Microstructure of Separators in Lithium Batteries and Their Effects on Dendritic Growth. ACS Appl. Energy Mater. 2021, 4, 7848–7861. [Google Scholar] [CrossRef]
- Zhou, H.; Fear, C.; Parekh, M.; Gray, F.; Fleetwood, J.; Adams, T.; Tomar, V.; Pol, V.G.; Mukherjee, P.P. The Role of Separator Thermal Stability in Safety Characteristics of Lithium-Ion Batteries. J. Electrochem. Soc. 2022, 169, 090521. [Google Scholar] [CrossRef]
- Tong, B.; Li, X. Towards Separator Safety of Lithium-Ion Batteries: A Review. Mater. Chem. Front. 2024, 8, 309–340. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Yang, M.; Chen, W. High-Safety Separators for Lithium-Ion Batteries and Sodium-Ion Batteries: Advances and Perspective. Energy Storage Mater. 2021, 41, 522–545. [Google Scholar] [CrossRef]
- Chen, W.; Li, Y.; Feng, D.; Lv, C.; Li, H.; Zhou, S.; Jiang, Q.; Yang, J.; Gao, Z.; He, Y.; et al. Recent Progress of Theoretical Research on Inorganic Solid State Electrolytes for Li Metal Batteries. J. Power Sources 2023, 561, 232720. [Google Scholar] [CrossRef]
- Hagen, M.; Hanselmann, D.; Ahlbrecht, K.; Maça, R.; Gerber, D.; Tübke, J. Lithium–Sulfur Cells: The Gap between the State-of-the-Art and the Requirements for High Energy Battery Cells. Adv. Energy Mater. 2015, 5, 1401986. [Google Scholar] [CrossRef]
- Rongpipi, S.; Ye, D.; Gomez, E.D.; Gomez, E.W. Progress and Opportunities in the Characterization of Cellulose—An Important Regulator of Cell Wall Growth and Mechanics. Front. Plant Sci. 2019, 9, 1894. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ioelovich, M.; Ahmad, I.; Thomas, S.; Dufresne, A. Methods for Extraction of Nanocellulose from Various Sources. In Handbook of Nanocellulose and Cellulose Nanocomposites; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 1–49. ISBN 978-3-527-68997-2. [Google Scholar]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.; da Silva, S.S.; Chandel, A.K.; da Silva, S.S. Sustainable Degradation of Lignocellulosic Biomass—Techniques, Applications and Commercialization; IntechOpen: London, UK, 2013; ISBN 978-953-51-1119-1. [Google Scholar]
- Omran, A.A.B.; Mohammed, A.A.B.A.; Sapuan, S.M.; Ilyas, R.A.; Asyraf, M.R.M.; Rahimian Koloor, S.S.; Petrů, M. Micro- and Nanocellulose in Polymer Composite Materials: A Review. Polymers 2021, 13, 231. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of Cellulose Nanofibrils: A Review of Recent Advances. Ind. Crops Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Zeng, J.; Zeng, Z.; Cheng, Z.; Wang, Y.; Wang, X.; Wang, B.; Gao, W. Cellulose Nanofibrils Manufactured by Various Methods with Application as Paper Strength Additives. Sci. Rep. 2021, 11, 11918. [Google Scholar] [CrossRef] [PubMed]
- Abdul Khalil, H.P.S.; Davoudpour, Y.; Saurabh, C.K.; Hossain, M.S.; Adnan, A.S.; Dungani, R.; Paridah, M.T.; Islam Sarker, M.Z.; Fazita, M.R.N.; Syakir, M.I.; et al. A Review on Nanocellulosic Fibres as New Material for Sustainable Packaging: Process and Applications. Renew. Sustain. Energy Rev. 2016, 64, 823–836. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S.N. Cellulose Nanocrystals: Synthesis, Functional Properties, and Applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.; Raj, M.C.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef] [PubMed]
- van de Ven, T.G.M.; Sheikhi, A. Hairy Cellulose Nanocrystalloids: A Novel Class of Nanocellulose. Nanoscale 2016, 8, 15101–15114. [Google Scholar] [CrossRef]
- Amin Ojagh, S.M.; Amini, M.; Cranmer-Smith, S.; Vahabzadeh, F.; Arjmand, M.; Tam, K.C.; Rojas, O.J.; Kamkar, M.; van de Ven, T.G.M. Crystalline and Hairy Nanocelluloses for 3D Printed Hydrogels and Strongly Structured Cryogels. ACS Sustain. Chem. Eng. 2023, 11, 5674–5684. [Google Scholar] [CrossRef]
- Koshani, R.; Eiyegbenin, J.E.; Wang, Y.; van de Ven, T.G.M. Synthesis and Characterization of Hairy Aminated Nanocrystalline Cellulose. J. Colloid Interface Sci. 2022, 607, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C. Industrial-Scale Production and Applications of Bacterial Cellulose. Front. Bioeng. Biotechnol. 2020, 8, 605374. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Bi, Z.; Xue, Y.; Zhang, W.; Huang, Q.; Zhang, L.; Huang, Y. Bacterial Cellulose: An Encouraging Eco-Friendly Nano-Candidate for Energy Storage and Energy Conversion. J. Mater. Chem. A 2020, 8, 5812–5842. [Google Scholar] [CrossRef]
- Almihyawi, R.A.H.; Musazade, E.; Alhussany, N.; Zhang, S.; Chen, H. Production and Characterization of Bacterial Cellulose by Rhizobium Sp. Isolated from Bean Root. Sci. Rep. 2024, 14, 10848. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, X.; Ma, M.; Hu, C.; Seidi, F.; Yin, S.; Xiao, H. Recent Advances on the Bacterial Cellulose-Derived Carbon Aerogels. J. Mater. Chem. C 2021, 9, 818–828. [Google Scholar] [CrossRef]
- Xia, Y.; Li, X.; Zhuang, J.; Wang, W.; Abbas, S.C.; Fu, C.; Zhang, H.; Chen, T.; Yuan, Y.; Zhao, X.; et al. Exploitation of Function Groups in Cellulose Materials for Lithium-Ion Batteries Applications. Carbohydr. Polym. 2024, 325, 121570. [Google Scholar] [CrossRef] [PubMed]
- Szabó, L.; Milotskyi, R.; Sharma, G.; Takahashi, K. Cellulose Processing in Ionic Liquids from a Materials Science Perspective: Turning a Versatile Biopolymer into the Cornerstone of Our Sustainable Future. Green Chem. 2023, 25, 5338–5389. [Google Scholar] [CrossRef]
- Xu, D.; Teng, G.; Heng, Y.; Chen, Z.; Hu, D. Eco-Friendly and Thermally Stable Cellulose Film Prepared by Phase Inversion as Supercapacitor Separator. Mater. Chem. Phys. 2020, 249, 122979. [Google Scholar] [CrossRef]
- Wu, H.; Mu, J.; Xu, Y.; Xu, F.; Ramaswamy, S.; Zhang, X. Heat-Resistant, Robust, and Hydrophilic Separators Based on Regenerated Cellulose for Advanced Supercapacitors. Small 2023, 19, 2205152. [Google Scholar] [CrossRef]
- Chen, J.Y.; Sun, L.; Negulescu, I.I.; Xu, B. Fabrication and Evaluation of Regenerated Cellulose/Nanoparticle Fibers from Lignocellulosic Biomass. Biomass Bioenergy 2017, 101, 1–8. [Google Scholar] [CrossRef]
- Fink, H.-P.; Weigel, P.; Purz, H.J.; Ganster, J. Structure Formation of Regenerated Cellulose Materials from NMMO-Solutions. Prog. Polym. Sci. 2001, 26, 1473–1524. [Google Scholar] [CrossRef]
- Krysztof, M.; Olejnik, K.; Kulpinski, P.; Stanislawska, A.; Khadzhynova, S. Regenerated Cellulose from N-Methylmorpholine N-Oxide Solutions as a Coating Agent for Paper Materials. Cellulose 2018, 25, 3595–3607. [Google Scholar] [CrossRef]
- Gao, X.; Li, M.; Zhang, H.; Tang, X.; Chen, K. Fabrication of Regenerated Cellulose Films by DMAc Dissolution Using Parenchyma Cells via Low-Temperature Pulping from Yunnan-Endemic Bamboos. Ind. Crops Prod. 2021, 160, 113116. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, R.; Xiang, J.; Kang, H.; Liu, Z.; Huang, Y. Dissolution Mechanism of Cellulose in N,N-Dimethylacetamide/Lithium Chloride: Revisiting through Molecular Interactions. J. Phys. Chem. B 2014, 118, 9507–9514. [Google Scholar] [CrossRef] [PubMed]
- Röder, T.; Morgenstern, B.; Schelosky, N.; Glatter, O. Solutions of Cellulose in N,N-Dimethylacetamide/Lithium Chloride Studied by Light Scattering Methods. Polymer 2001, 42, 6765–6773. [Google Scholar] [CrossRef]
- Huang, K.; Maltais, A.; Liu, J.; Wang, Y. Wood Cellulose Films Regenerated from NaOH/Urea Aqueous Solution and Treated by Hot Pressing for Food Packaging Application. Food Biosci. 2022, 50, 102177. [Google Scholar] [CrossRef]
- Cai, J.; Liu, Y.; Zhang, L. Dilute Solution Properties of Cellulose in LiOH/Urea Aqueous System. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 3093–3101. [Google Scholar] [CrossRef]
- Geng, H.; Yuan, Z.; Fan, Q.; Dai, X.; Zhao, Y.; Wang, Z.; Qin, M. Characterisation of Cellulose Films Regenerated from Acetone/Water Coagulants. Carbohydr. Polym. 2014, 102, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Alsoy Altinkaya, S. A Perspective on Cellulose Dissolution with Deep Eutectic Solvents. Front. Membr. Sci. Technol. 2024, 3, 1382054. [Google Scholar] [CrossRef]
- Okwuwa, C.C.; Adam, F.; Mohd Said, F.; Ries, M.E. Cellulose Dissolution for Edible Biocomposites in Deep Eutectic Solvents: A Review. J. Clean. Prod. 2023, 427, 139166. [Google Scholar] [CrossRef]
- Zhong, Y.; Wu, J.; Kang, H.; Liu, R. Choline Hydroxide Based Deep Eutectic Solvent for Dissolving Cellulose. Green Chem. 2022, 24, 2464–2475. [Google Scholar] [CrossRef]
- Zlenko, D.V.; Vtyurina, D.N.; Usachev, S.V.; Skoblin, A.A.; Mikhaleva, M.G.; Politenkova, G.G.; Nikolsky, S.N.; Stovbun, S.V. On the Orientation of the Chains in the Mercerized Cellulose. Sci. Rep. 2021, 11, 8765. [Google Scholar] [CrossRef] [PubMed]
- Ghazali, N.A.; Salleh, K.M.; Jafri, N.F.; Khalid, K.A.M.; Zakaria, S.; Halim, N.H.A. The Dispersibility of Cellulose I and Cellulose II by Tempo-Mediated Oxidation. CERNE 2024, 30, e103320. [Google Scholar] [CrossRef]
- Shen, J.; Lei, Z.; Wang, C. An Ion Conducting ZIF-8 Coating Protected PEO Based Polymer Electrolyte for High Voltage Lithium Metal Batteries. Chem. Eng. J. 2022, 447, 137503. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, S.; Sun, X.; Ai, G.; Zhang, T.; Wu, F.; Mao, W. Metal-Organic Framework Derived Gradient Interfacial Layer for Stable Lithium Metal Anode. Electrochim. Acta 2022, 417, 140333. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, Q.; Liu, Y.; Zhang, J.; Xia, X.; Huang, H.; Gan, Y.; He, X.; Xiao, Z.; Zhang, W. Three-Dimensional Polyimide Nanofiber Framework Reinforced Polymer Electrolyte for All-Solid-State Lithium Metal Battery. J. Colloid Interface Sci. 2023, 638, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Wang, W.-P.; Yin, Y.-X.; Wan, L.-J.; Guo, Y.-G. Interfacial Design for Lithium–Sulfur Batteries: From Liquid to Solid. EnergyChem 2019, 1, 100002. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, F.; Cai, C.; Fu, Y. Simultaneously Suppressing Shuttle Effect and Dendrite Growth in Lithium–Sulfur Batteries via Building Dual-Functional Asymmetric-Cellulose Gel Electrolyte. Small 2023, 19, 2300076. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-J.; Chueh, C.-C. A Short Review and Future Prospects of Biomass-Based Solid Polymer Electrolytes for Improving the Performance of Lithium Metal Batteries. J. Chem. Eng. Jpn. 2024, 57, 2397996. [Google Scholar] [CrossRef]
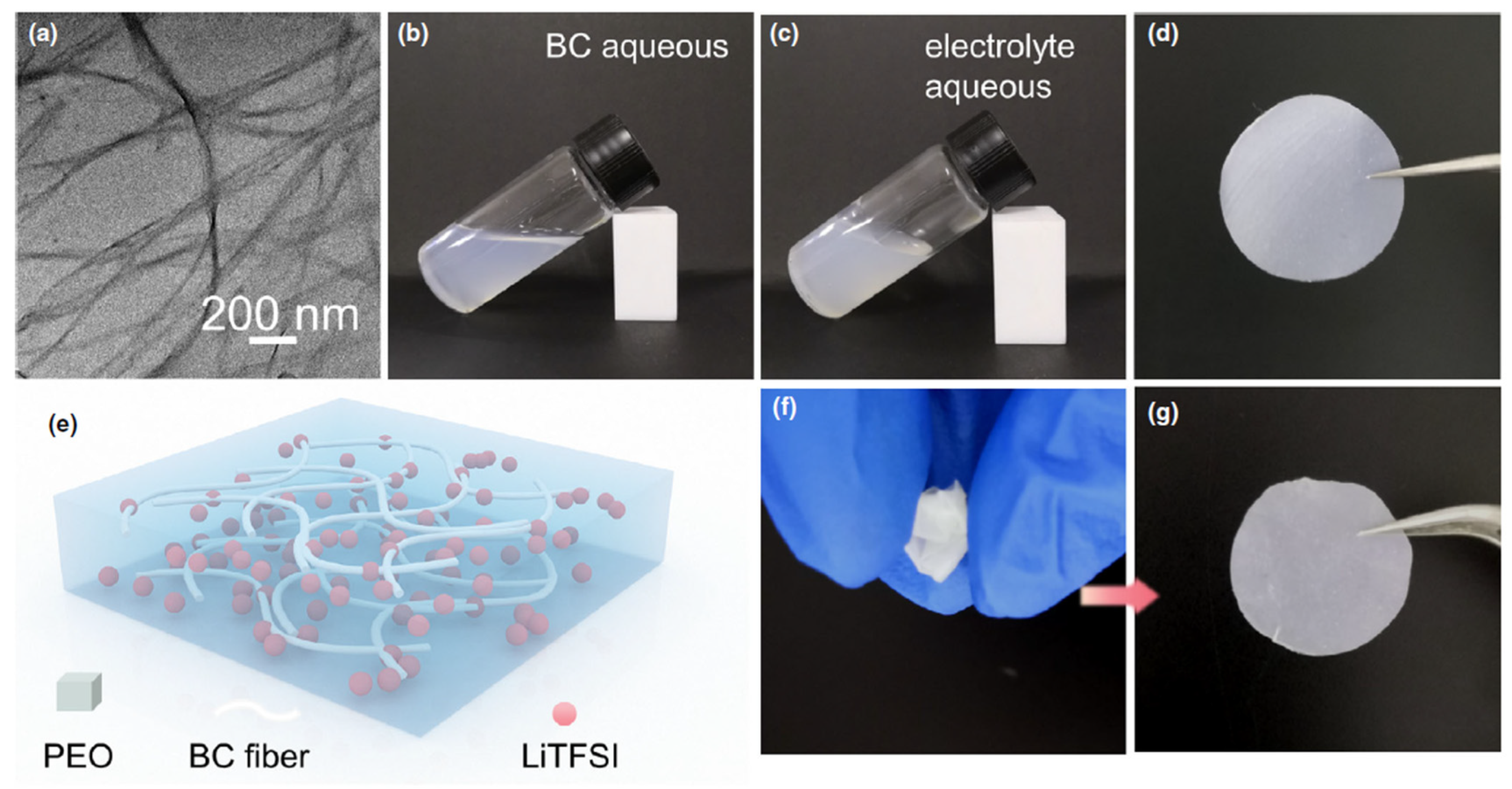
- Gong, X.; Wang, J.; Zhong, L.; Qi, G.; Liu, F.; Pan, Y.; Yang, F.; Wang, X.; Li, J.; Li, L.; et al. Recent Advances on Cellulose-Based Solid Polymer Electrolytes. Ind. Chem. Mater. 2024. [Google Scholar] [CrossRef]
- Prado-Martínez, C.; Sutton, P.; Mombrini, I.; Kamtsikakis, A.; Meesorn, W.; Weder, C.; Steiner, U.; Gunkel, I. Cellulose Nanofiber-Reinforced Solid Polymer Electrolytes with High Ionic Conductivity for Lithium Batteries. J. Mater. Chem. A 2023, 11, 9521–9529. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, J.; Zhao, Y.; Zhang, X.; Xu, L.; Liu, H.; Cui, Y.; Cui, Y.; Li, C. A Branched Cellulose-Reinforced Composite Polymer Electrolyte with Upgraded Ionic Conductivity for Anode Stabilized Solid-State Li Metal Batteries. Sustain. Energy Fuels 2019, 3, 2642–2656. [Google Scholar] [CrossRef]
- Meng, N.; Zhu, X.; Lian, F. Particles in Composite Polymer Electrolyte for Solid-State Lithium Batteries: A Review. Particuology 2022, 60, 14–36. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Z.; Liu, D.; Lu, S.; Li, F.; Gao, G.; Zhu, M.; Li, M.; Zhang, Y.; Bu, H.; et al. Bacterial Cellulose Composite Solid Polymer Electrolyte With High Tensile Strength and Lithium Dendrite Inhibition for Long Life Battery. Energy Environ. Mater. 2021, 4, 434–443. [Google Scholar] [CrossRef]
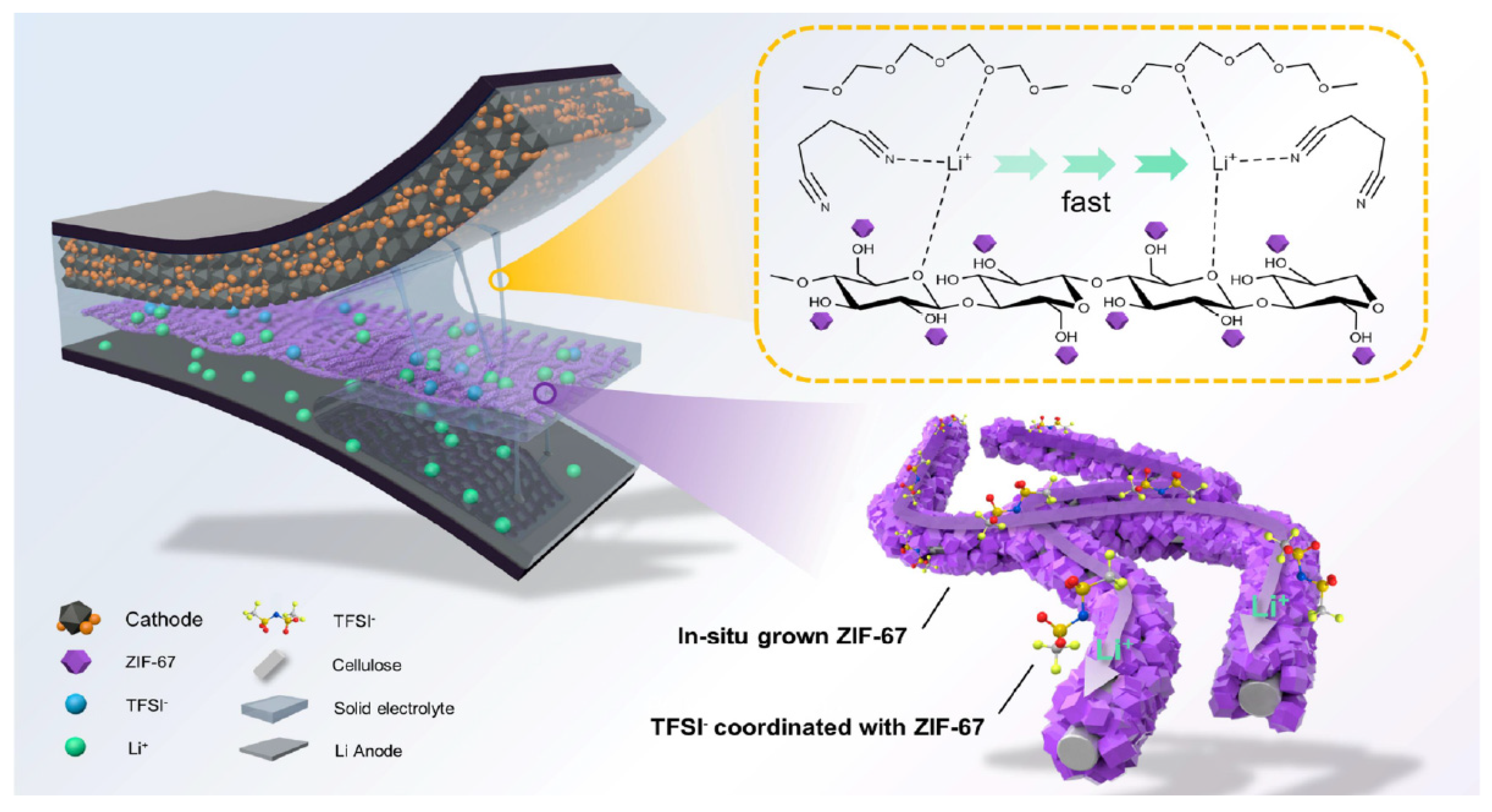
- Song, X.; Ma, K.; Wang, J.; Wang, H.; Xie, H.; Zheng, Z.; Zhang, J. Three-Dimensional Metal–Organic Framework@Cellulose Skeleton-Reinforced Composite Polymer Electrolyte for All-Solid-State Lithium Metal Battery. ACS Nano 2024, 18, 12311–12324. [Google Scholar] [CrossRef]
- Wang, H.; Wang, P.; Shi, G. Construction of Siloxane-Capped PEO Polyurethane-Cellulose Acetate Composite Electrolytes for All-Solid-State Metal Lithium Batteries. J. Phys. Chem. Solids 2024, 193, 112190. [Google Scholar] [CrossRef]
- Fu, X.; Hurlock, M.J.; Ding, C.; Li, X.; Zhang, Q.; Zhong, W.-H. MOF-Enabled Ion-Regulating Gel Electrolyte for Long-Cycling Lithium Metal Batteries Under High Voltage. Small 2022, 18, 2106225. [Google Scholar] [CrossRef] [PubMed]
- Chiappone, A.; Nair, J.R.; Gerbaldi, C.; Bongiovanni, R.; Zeno, E. Nanoscale Microfibrillated Cellulose Reinforced Truly-Solid Polymer Electrolytes for Flexible, Safe and Sustainable Lithium-Based Batteries. Cellulose 2013, 20, 2439–2449. [Google Scholar] [CrossRef]
- Zhang, J.; Yue, L.; Hu, P.; Liu, Z.; Qin, B.; Zhang, B.; Wang, Q.; Ding, G.; Zhang, C.; Zhou, X.; et al. Taichi-Inspired Rigid-Flexible Coupling Cellulose-Supported Solid Polymer Electrolyte for High-Performance Lithium Batteries. Sci. Rep. 2014, 4, 6272. [Google Scholar] [CrossRef]
- Chinnam, P.R.; Zhang, H.; Wunder, S.L. Blends of Pegylated Polyoctahedralsilsesquioxanes (POSS-PEG) and Methyl Cellulose as Solid Polymer Electrolytes for Lithium Batteries. Electrochim. Acta 2015, 170, 191–201. [Google Scholar] [CrossRef]
- Youcef, H.B.; Orayech, B.; Del Amo, J.M.L.; Bonilla, F.; Shanmukaraj, D.; Armand, M. Functionalized Cellulose as Quasi Single-Ion Conductors in Polymer Electrolyte for All-Solid–State Li/Na and LiS Batteries. Solid State Ion. 2020, 345, 115168. [Google Scholar] [CrossRef]
- Maddu, A.; Sulaeman, A.S.; Wahyudi, S.T.; Rifai, A. Enhancing Ionic Conductivity of Carboxymethyl Cellulose-Lithium Perchlorate with Crosslinked Citric Acid as Solid Polymer Electrolytes for Lithium Polymer Batteries. Int. J. Renew. Energy Dev. 2022, 11, 1002–1011. [Google Scholar] [CrossRef]
- Zhong, G.; Wang, P.; Lu, K.; Cao, H.; Shi, W.; Yan, W.; Zhu, Y. Cellulose-Based Gel-Type Electrolyte Fabricated by Lyophilization to Enable Uniform Li+ Ion Flux Distribution for Stable Li Metal Anodes with High-Rate Capability. Appl. Mater. Today 2023, 30, 101705. [Google Scholar] [CrossRef]
- Wang, D.; Xie, H.; Liu, Q.; Mu, K.; Song, Z.; Xu, W.; Tian, L.; Zhu, C.; Xu, J. Low-Cost, High-Strength Cellulose-based Quasi-Solid Polymer Electrolyte for Solid-State Lithium-Metal Batteries. Angew. Chem. Int. Ed. 2023, 62, e202302767. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Su, Y.; Zhu, W.; Li, Z.; Zhang, D.; Wang, H.; Sun, H.; Wang, B.; Zhou, D.; Fan, L.-Z. Achieving Stable Interface for Lithium Metal Batteries Using Fluoroethylene Carbonate-Modified Garnet-Type Li6.4La3Zr1.4Ta0.6O12 Composite Electrolyte. Electrochim. Acta 2023, 446, 142063. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, Y.; Huang, Y.; Huang, Z.; Li, P.; Liu, G.; Wang, Z. Recent Advances in Protecting Li-Anode via Establishing Nitrides as Stable Solid Electrolyte Interphases. J. Power Sources 2023, 579, 233274. [Google Scholar] [CrossRef]
- Chang, C.-H.; Chung, S.-H.; Manthiram, A. Dendrite-Free Lithium Anode via a Homogenous Li-Ion Distribution Enabled by a Kimwipe Paper. Adv. Sustain. Syst. 2017, 1, 1600034. [Google Scholar] [CrossRef]
- Hong, S.-H.; Jung, D.-H.; Kim, J.-H.; Lee, Y.-H.; Cho, S.-J.; Joo, S.H.; Lee, H.-W.; Lee, K.-S.; Lee, S.-Y. Electrical Conductivity Gradient Based on Heterofibrous Scaffolds for Stable Lithium-Metal Batteries. Adv. Funct. Mater. 2020, 30, 1908868. [Google Scholar] [CrossRef]
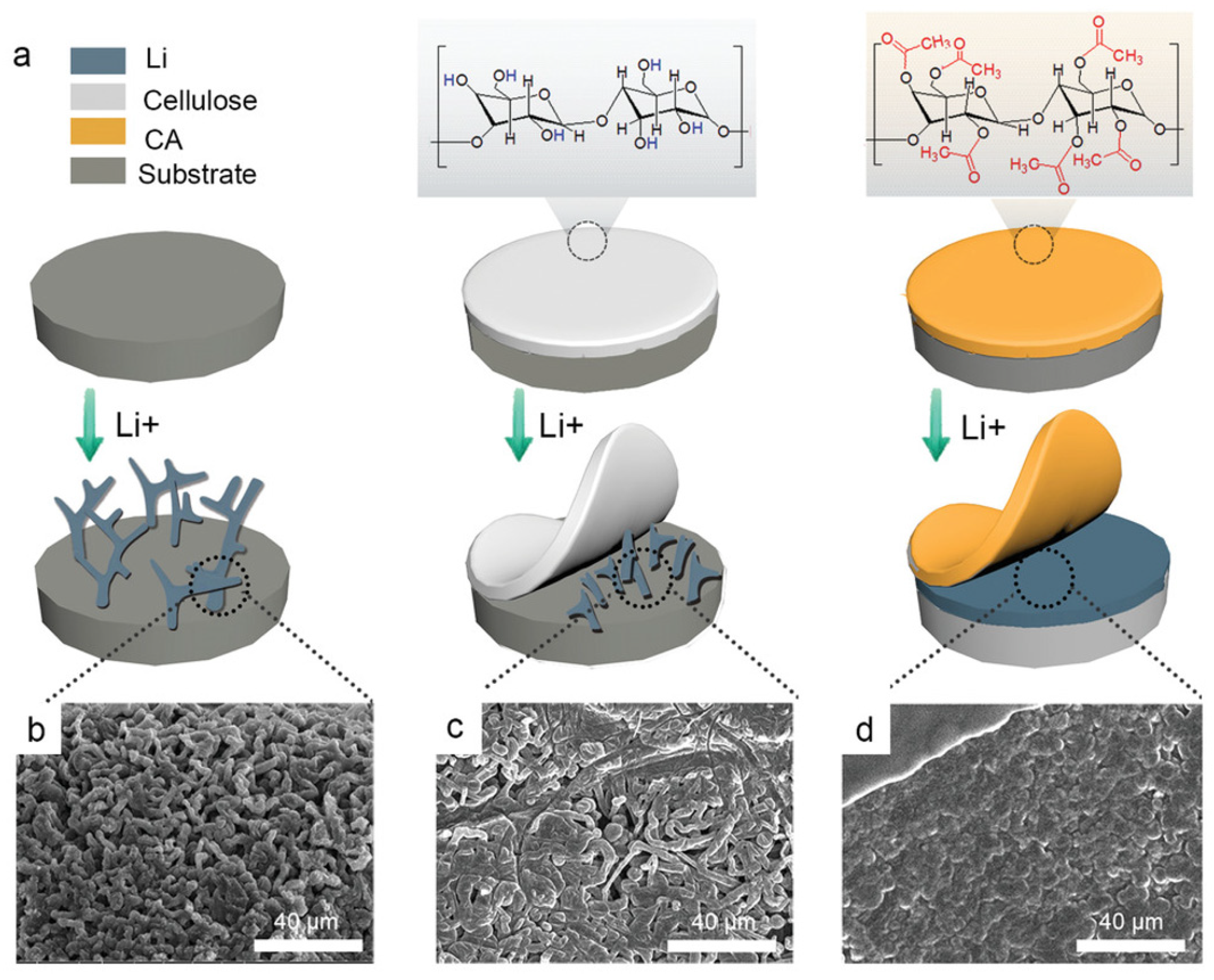
- Chen, M.; Zheng, J.; Liu, Y.; Sheng, O.; Ju, Z.; Lu, G.; Liu, T.; Wang, Y.; Nai, J.; Wang, Q.; et al. Marrying Ester Group with Lithium Salt: Cellulose-Acetate-Enabled LiF-Enriched Interface for Stable Lithium Metal Anodes. Adv. Funct. Mater. 2021, 31, 2102228. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, X.; Li, R.; Wang, C.; Song, D.; Yang, Z.; Gao, J.; Zhang, Y.; Ohsaka, T.; Matsumoto, F.; et al. In Situ Construction of Trinity Artificial Protective Layer between Lithium Metal and Sulfide Solid Electrolyte Interface. Electrochem. Commun. 2022, 142, 107377. [Google Scholar] [CrossRef]
- Zhang, J.; Yue, L.; Kong, Q.; Liu, Z.; Zhou, X.; Zhang, C.; Xu, Q.; Zhang, B.; Ding, G.; Qin, B.; et al. Sustainable, Heat-Resistant and Flame-Retardant Cellulose-Based Composite Separator for High-Performance Lithium Ion Battery. Sci. Rep. 2014, 4, 3935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Cui, X.; Guan, S.; Tu, L.; Tang, H.; Li, Z.; Li, J. Understanding the Advantageous Features of Bacterial Cellulose-Based Separator in Li–S Battery. Adv. Mater. Interfaces 2023, 10, 2201730. [Google Scholar] [CrossRef]
- Li, W.; Wang, S.; Fan, Z.; Li, S.; Newman, N. Oxidized Bacterial Cellulose Functionalized with SiO2 Nanoparticles as a Separator for Lithium-Metal and Lithium–Sulfur Batteries. Cellulose 2023, 30, 481–493. [Google Scholar] [CrossRef]
- Park, D.; Park, S.; Kim, D.-W. Electrospun Cellulose Nanofiber Membranes as Multifunctional Separators for High Energy and Stable Lithium-Sulfur Batteries. Int. J. Energy Res. 2023, 2023, 1541858. [Google Scholar] [CrossRef]
- Li, J.; Dai, L.; Wang, Z.; Wang, H.; Xie, L.; Chen, J.; Yan, C.; Yuan, H.; Wang, H.; Chen, C. Cellulose Nanofiber Separator for Suppressing Shuttle Effect and Li Dendrite Formation in Lithium-Sulfur Batteries. J. Energy Chem. 2022, 67, 736–744. [Google Scholar] [CrossRef]
- Shin, D.-M.; Son, H.; Park, K.; Choi, J.; Suk, J.; Kang, E.; Kim, D.-W.; Kim, D. Al2O3 Ceramic/Nanocellulose-Coated Non-Woven Separator for Lithium-Metal Batteries. Coatings 2023, 13, 916. [Google Scholar] [CrossRef]
- Pan, R.; Xu, X.; Sun, R.; Wang, Z.; Lindh, J.; Edström, K.; Strømme, M.; Nyholm, L. Nanocellulose Modified Polyethylene Separators for Lithium Metal Batteries. Small 2018, 14, 1704371. [Google Scholar] [CrossRef] [PubMed]
- Gou, J.; Liu, W.; Tang, A.; Wu, L. Interfacially Stable and High-Safety Lithium Batteries Enabled by Porosity Engineering toward Cellulose Separators. J. Membr. Sci. 2022, 659, 120807. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, Z.; Liu, S.; Pei, Y.; Luo, X. Polydopamine-Modified Cellulose-Based Composite Separator for Inhibiting Dendritic Growth of Lithium Metal Batteries. Electrochim. Acta 2024, 475, 143661. [Google Scholar] [CrossRef]
- Li, W.; Wang, S.; Fan, Z.; Li, S.; Bernussi, A.; Newman, N. Functionalized Bacterial Cellulose as a Separator to Address Polysulfides Shuttling in Lithium–Sulfur Batteries. Mater. Today Energy 2021, 21, 100813. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Li, X. Upcycling of Paper Waste for High-Performance Lithium-Sulfur Batteries. Mater. Today Energy 2021, 19, 100591. [Google Scholar] [CrossRef]
- Fang, Z.; Tu, L.; Zhang, Z.; Wei, J.; Xiang, Y.; Guo, W.; Li, J. Simultaneously Suppressing the Dendritic Lithium Growth and Polysulfides Migration by a Polyethyleneimine Grafted Bacterial Cellulose Membrane in Lithium-Sulfur Batteries. Appl. Surf. Sci. 2022, 597, 153683. [Google Scholar] [CrossRef]
- Li, F.; Wang, G.; Wang, P.; Yang, J.; Zhang, K.; Liu, Y.; Lai, Y. High-Performance Lithium-Sulfur Batteries with a Carbonized Bacterial Cellulose/TiO2 Modified Separator. J. Electroanal. Chem. 2017, 788, 150–155. [Google Scholar] [CrossRef]
- Yu, B.-C.; Park, K.; Jang, J.-H.; Goodenough, J.B. Cellulose-Based Porous Membrane for Suppressing Li Dendrite Formation in Lithium–Sulfur Battery. ACS Energy Lett. 2016, 1, 633–637. [Google Scholar] [CrossRef]
- Chien, Y.-C.; Pan, R.; Lee, M.-T.; Nyholm, L.; Brandell, D.; Lacey, M.J. Cellulose Separators With Integrated Carbon Nanotube Interlayers for Lithium-Sulfur Batteries: An Investigation into the Complex Interplay between Cell Components. J. Electrochem. Soc. 2019, 166, A3235. [Google Scholar] [CrossRef]
- Wu, S.; Shi, J.; Nie, X.; Yao, Y.; Jiang, F.; Wei, Q.; Huang, F. Microporous Cyclodextrin Film with Funnel-Type Channel Polymerized on Electrospun Cellulose Acetate Membrane as Separators for Strong Trapping Polysulfides and Boosting Charging in Lithium–Sulfur Batteries. Energy Environ. Mater. 2023, 6, e12319. [Google Scholar] [CrossRef]
- Li, S.; Warzywoda, J.; Wang, S.; Ren, G.; Fan, Z. Bacterial Cellulose Derived Carbon Nanofiber Aerogel with Lithium Polysulfide Catholyte for Lithium–Sulfur Batteries. Carbon 2017, 124, 212–218. [Google Scholar] [CrossRef]
- Quan, Y.; Han, D.; Feng, Y.; Wang, S.; Xiao, M.; Meng, Y. Microporous Carbon Materials from Bacterial Cellulose for Lithium−Sulfur Battery Applications. Int. J. Electrochem. Sci. 2017, 12, 5984–5997. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, M.; Lin, Z.; Zhao, B.; Zhang, S.; Yang, J.; Zhu, C.; Zhang, H.; Sun, D.; Shi, Y. Flexible Cathodes and Multifunctional Interlayers Based on Carbonized Bacterial Cellulose for High-Performance Lithium–Sulfur Batteries. J. Mater. Chem. A 2015, 3, 10910–10918. [Google Scholar] [CrossRef]
- Li, S.; Mou, T.; Ren, G.; Warzywoda, J.; Wei, Z.; Wang, B.; Fan, Z. Gel Based Sulfur Cathodes with a High Sulfur Content and Large Mass Loading for High-Performance Lithium–Sulfur Batteries. J. Mater. Chem. A 2017, 5, 1650–1657. [Google Scholar] [CrossRef]
- Bharti, V.K.; Pathak, A.D.; Sharma, C.S.; Khandelwal, M. Flexible and Free-Standing Bacterial Cellulose Derived Cathode Host and Separator for Lithium-Sulfur Batteries. Carbohydr. Polym. 2022, 293, 119731. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lin, Z.; He, G.; Huang, J. Cellulose Substance Derived Nanofibrous Activated Carbon as a Sulfur Host for Lithium-Sulfur Batteries. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125129. [Google Scholar] [CrossRef]
- Li, L.; Hou, L.; Cheng, J.; Simmons, T.; Zhang, F.; Zhang, L.T.; Linhardt, R.J.; Koratkar, N. A Flexible Carbon/Sulfur-Cellulose Core-Shell Structure for Advanced Lithium–Sulfur Batteries. Energy Storage Mater. 2018, 15, 388–395. [Google Scholar] [CrossRef]
- Li, N.; Xiu, H.; Wu, H.; Shen, M.; Huang, S.; Fan, S.; Wang, S.; Wu, M.; Li, J. Flexible Composite Fiber Paper as Robust and Stable Lithium-Sulfur Battery Cathode. Cellulose 2024, 31, 8625–8645. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M. Investigation of the Effect of Anion/Cation-Modified Cellulose Nanofibers/MXene Composite Aerogels on the High-Performance Lithium-Sulfur Batteries. Ionics 2022, 28, 2805–2815. [Google Scholar] [CrossRef]
- Sevilla, M.; Díez, N.; Fuertes, A.B. Cellulose as a Precursor of High-Performance Energy Storage Materials in Li–S Batteries and Supercapacitors. Energy Technol. 2021, 9, 2100268. [Google Scholar] [CrossRef]
- Mao, H.; Liu, L.; Shi, L.; Wu, H.; Lang, J.; Wang, K.; Zhu, T.; Gao, Y.; Sun, Z.; Zhao, J.; et al. High Loading Cotton Cellulose-Based Aerogel Self-Standing Electrode for Li-S Batteries. Sci. Bull. 2020, 65, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhou, X.; Zeng, P.; Guo, C.; Liu, H.; Guo, X.; Chang, B.; Chen, M.; Su, J.; Wang, X. 3D Cellulose Graphene Aerogel with Self-Redox CeO2 as Li2S Host for High-Performance Li–S Battery. Energy Technol. 2022, 10, 2200616. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Xuan, Y.; Zhou, L.; Wang, D.; Li, Z.; Lin, H.; Tretiak, S.; Wang, H.; Wang, L.; et al. Multifunctional Cellulose Nanocrystals as a High-Efficient Polysulfide Stopper for Practical Li–S Batteries. ACS Appl. Mater. Interfaces 2020, 12, 17592–17601. [Google Scholar] [CrossRef]








| Type of Cellulose | Composite | Electrochemical Window [V] | Li Transfer Number | Li ionic Conductivity [Scm−1] [MPa] | Tensile Stenght [MPa] | Storage Modulus [MPa] | Ref. |
|---|---|---|---|---|---|---|---|
| Methyl 2-hydroxyethyl cellulose (HEMC) | PEO-LiTFSI-20HEMC | 0–5.56 | 0.467 | 1.30 × 10−4 at 30 °C 1.68 × 10−3 at 60 °C | 3.26 | 46.95 | [74] |
| Bacterial cellulose (BC) | BC as a polymer filler incorporated with PEO in a LiTFSI based electrolyte | 0–1.43 | 0.57 | 0.56 × 10−4 at 30 °C | 4.43 | 76.7 | [76] |
| Cellulose nanofibers (CNFs) | Comprised soft poly(ethylene oxide co-epichlorohydrin) (EO-co-EPI) with CNF | 0–4 | - | 6 × 10−5 at 25 °C | - | 64 | [73] |
| Commercial membrane cellulose (CF) | Succinonitrile (SN) plasticizer in addition to zeolitic imidazolate frameworks (ZIFs) grown in situ on the CF skeleton, all into PEO | 0–5 | 0.40 | 1.17 × 10−4 at 30 °C | 18.7 | 295.85 | [77] |
| Polyurethane-cellulose acetate (PCA) | Blend of PCA with mono-hydroxysiloxane-capped polyethylene oxide (PU-CA) | - | - | 1.5 × 10−5 at 25 °C | - | - | [78] |
| Nano scale micro-fibrillated cellulose (MCF) | MCF fibers reinforced fully-solid methacrylic-based thermo-set polymer electrolyte membranes | 0–4.7 | - | 0.1 × 10−3 at 50 °C | 2.3 | 32 | [80] |
| Robust cellulose nonwoven (RCNW) | Poly (ethylene oxide), poly (cyano acrylate), lithium bis(oxalate)borate with RCNW | 0–4.6 | - | 3 × 10−4 at 60 °C | 43 | - | [81] |
| Methyl cellulose (MC) | Blending of LiClO4, MC and Pegylated Polyoctahedralsilsesquioxane | 1.5–4.2 | 0.25 | 1.6 × 10−5 at 30 °C and 1.1 × 10−5 at 0 °C | - | 32 | [82] |
| Ethyl cellulose (EC) | PEO, LiTFSI and EC mixture | 0–4 | 0.9 | 0.52 × 10−4 at 70 °C | - | - | [83] |
| Carboxymethyl cellulose (CMC) | CMC-lithium perchlorate and citric acid | 0–2.15 | - | 1.24 × 10−7 | - | - | [84] |
| Methyl cellulose (MC) | Blend of MC with LiPF6-based organic electrolyte | 0–4.8 | 0.48 | 0.7 × 10−3 at 30 °C | 5.8 | - | [85] |
| Cellulose acetate (CA) | Mixture of CA with inorganic NASICO-type electrolyte Li1.3Al0.3Ti1.7(PO4)3 | 0–4.6 | 0.85 | 6.17 × 10−4 at 30 °C | 1.3 | - | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zampieri, M.; Tommasone, G.; Morel, L.; Luque, G.L. Cellulose-Based Materials and Their Application in Lithium–Sulfur Batteries. Polymers 2025, 17, 164. https://doi.org/10.3390/polym17020164
Zampieri M, Tommasone G, Morel L, Luque GL. Cellulose-Based Materials and Their Application in Lithium–Sulfur Batteries. Polymers. 2025; 17(2):164. https://doi.org/10.3390/polym17020164
Chicago/Turabian StyleZampieri, Muriel, Guillermina Tommasone, Luciana Morel, and Guillermina Leticia Luque. 2025. "Cellulose-Based Materials and Their Application in Lithium–Sulfur Batteries" Polymers 17, no. 2: 164. https://doi.org/10.3390/polym17020164
APA StyleZampieri, M., Tommasone, G., Morel, L., & Luque, G. L. (2025). Cellulose-Based Materials and Their Application in Lithium–Sulfur Batteries. Polymers, 17(2), 164. https://doi.org/10.3390/polym17020164






