Formation of Nanocompounds of TiO2 Using PVA-HAp Nanofibers by Sol-Gel Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization of Nanocompounds by STM
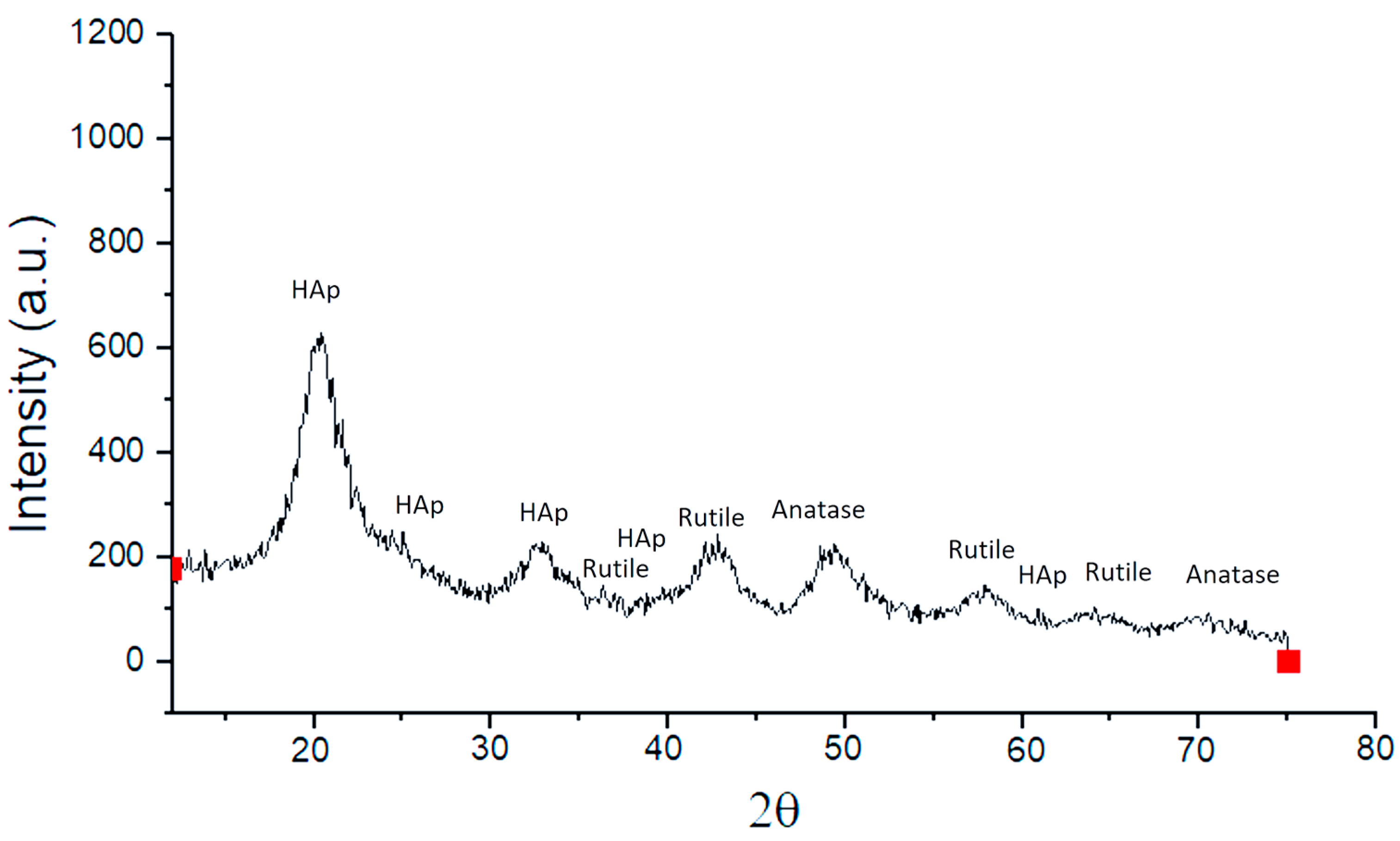
2.2. Characterization of Nanocompounds by X-Ray Diffraction Pattern
2.3. Power Spectral Density Analysis
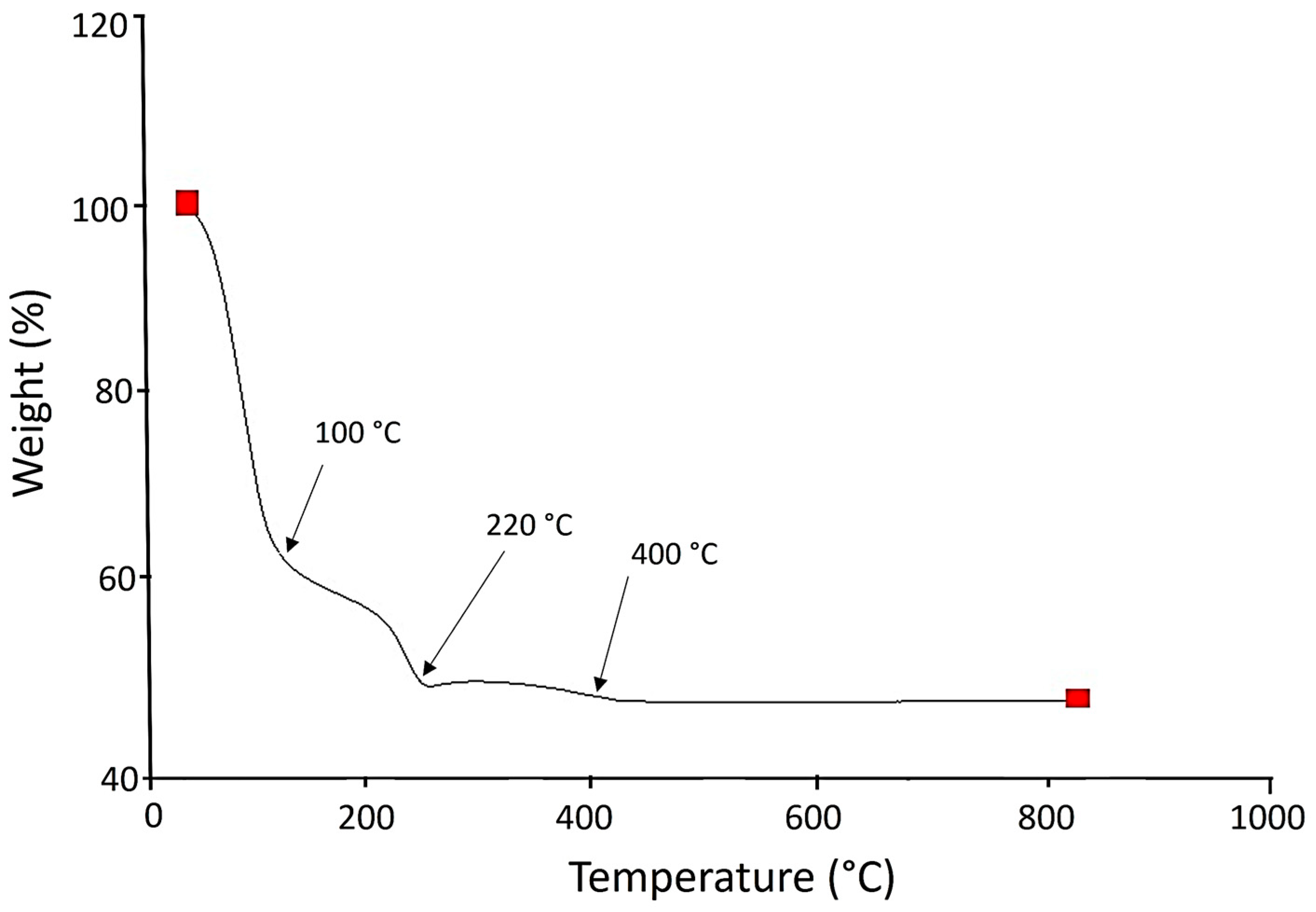
2.4. TGA Analysis
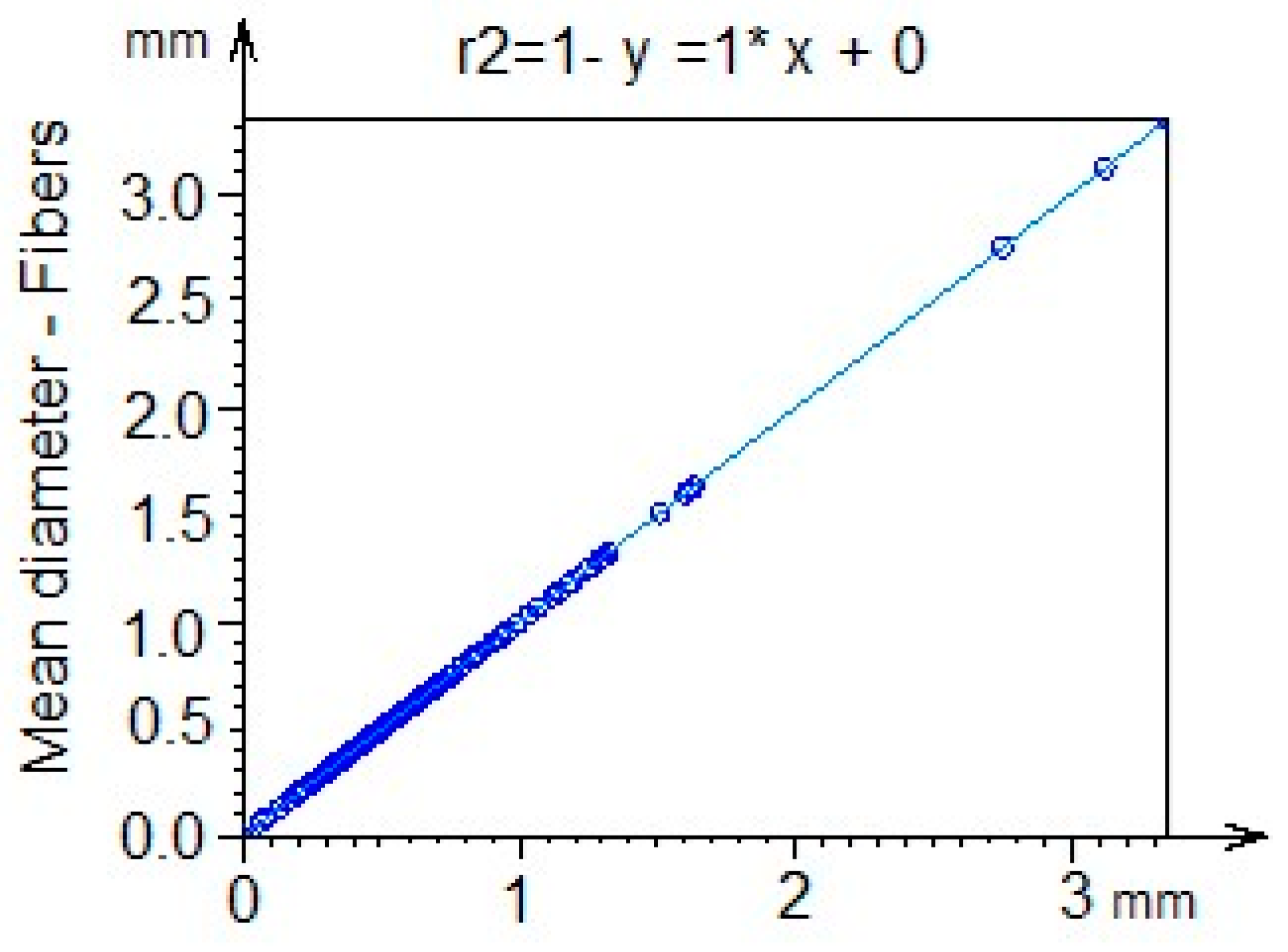
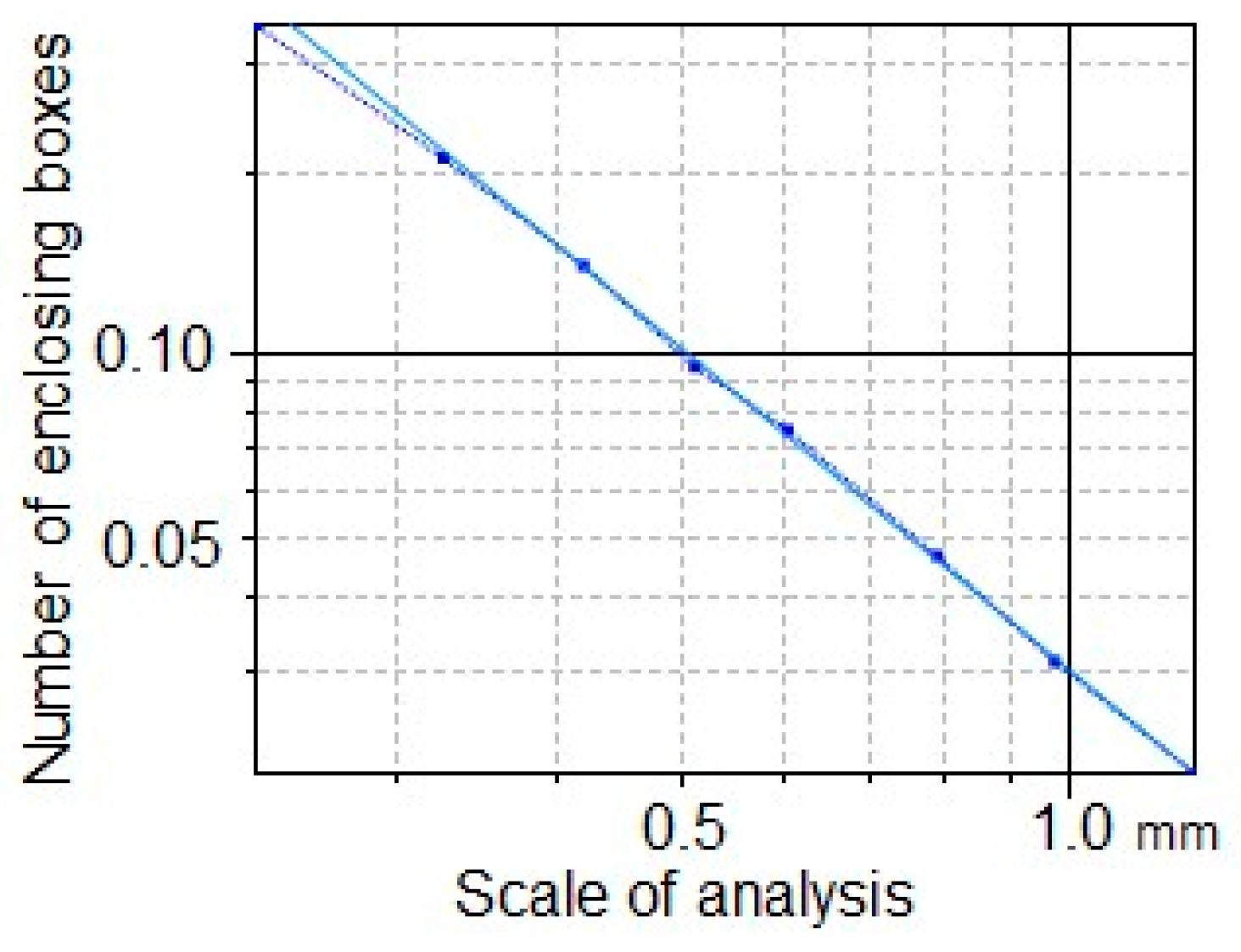
2.5. Coefficient of Determination R2 and Fractal Dimension Analysis
3. Results and Discussion
3.1. Characterization of TiO2-PVA-HAp Nanocompounds by STM
3.2. Thermo-Gravimetric Analysis
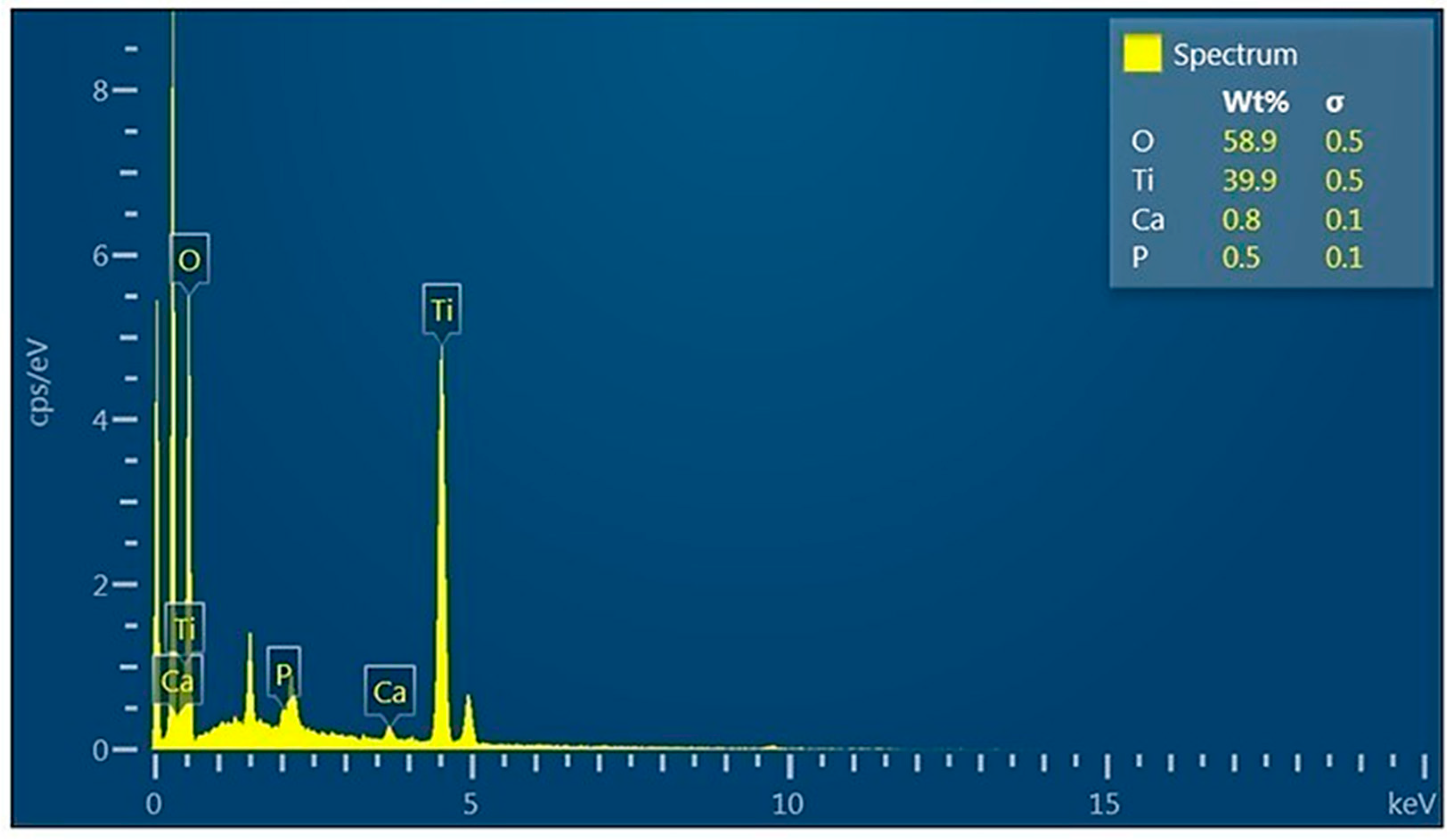
3.3. X-Ray Diffraction Pattern
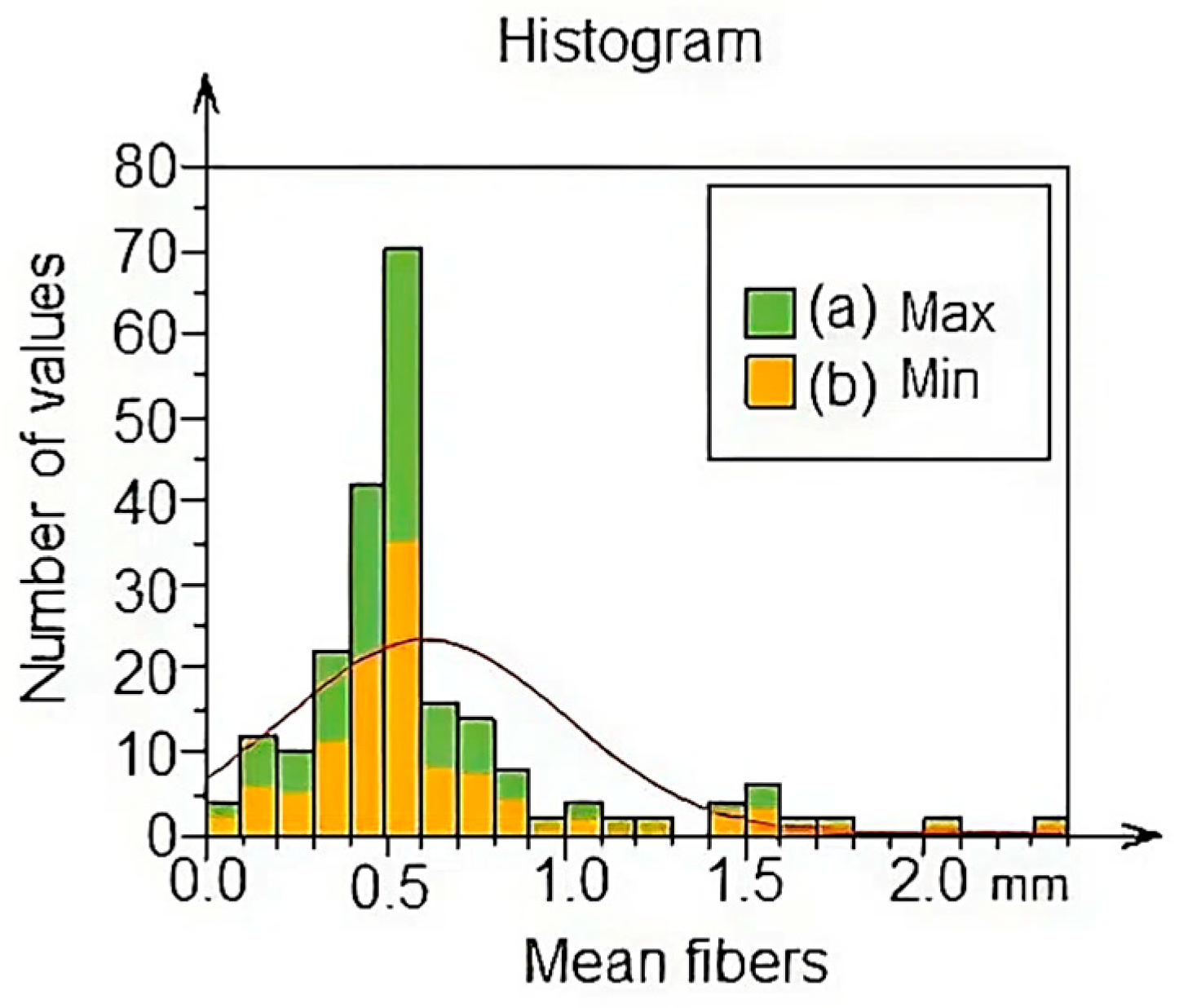
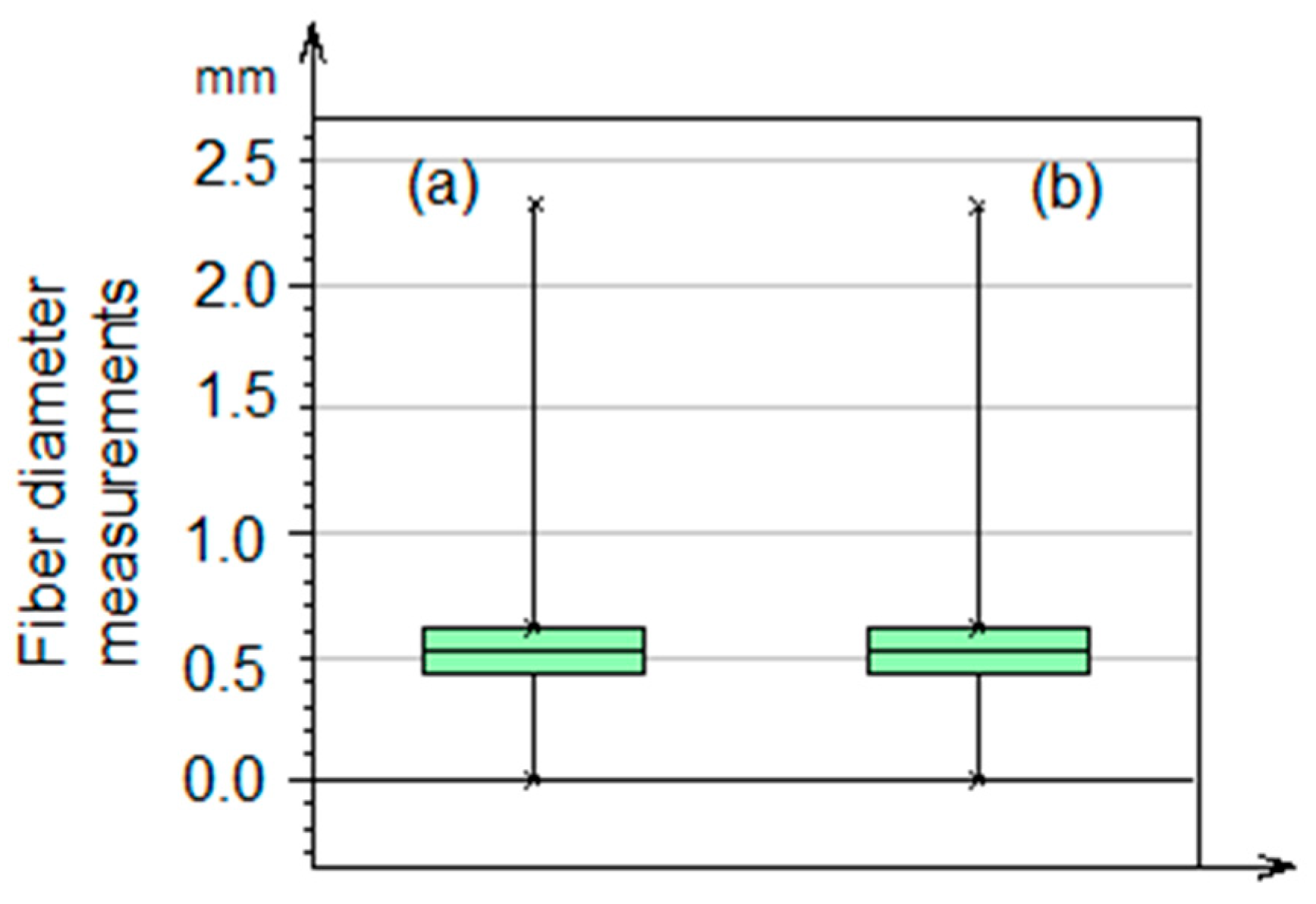
3.4. Average Diameter Analysis
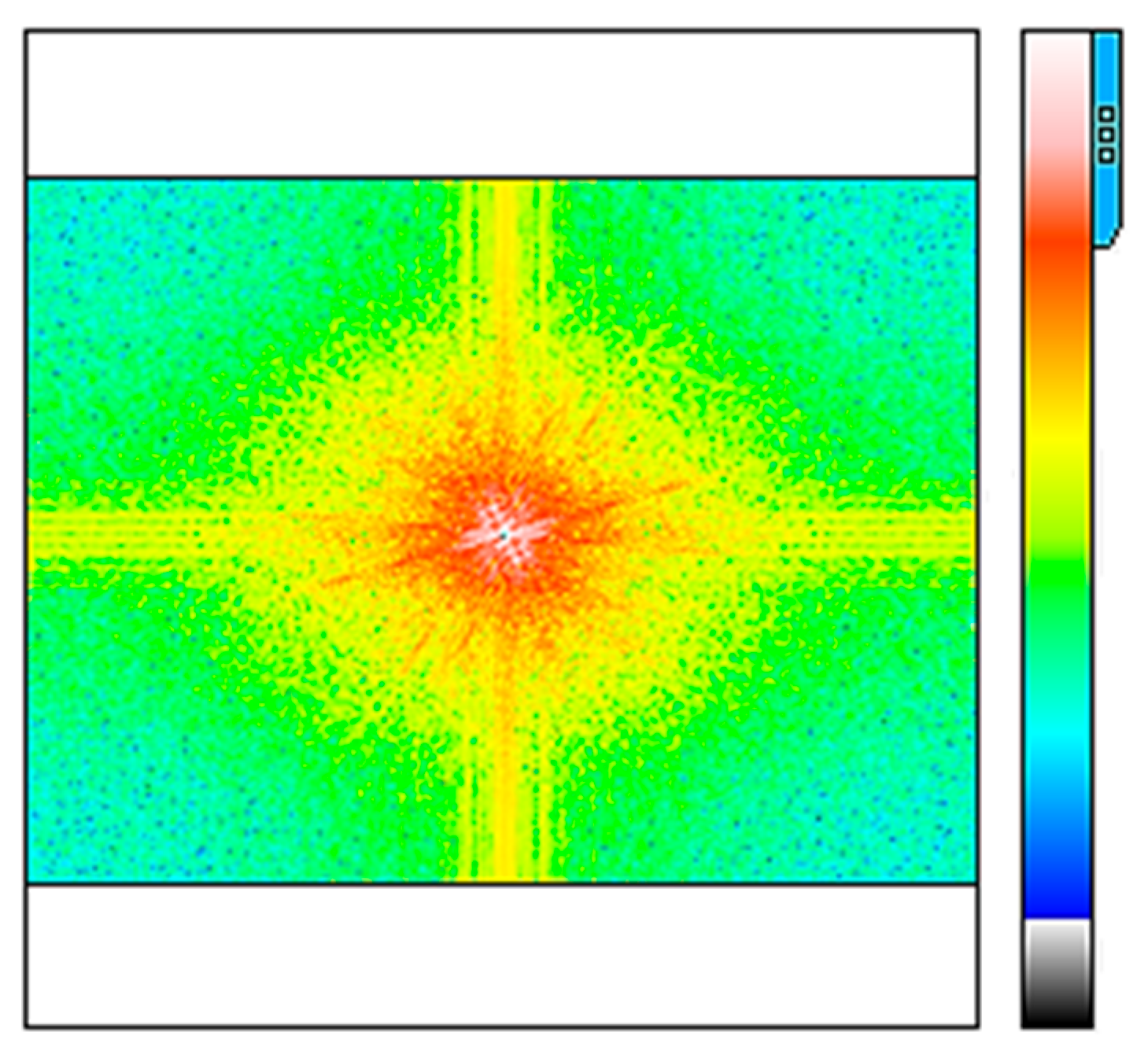
3.5. Characterization of TiO2-PVA-HAp Nanocompounds by FFT
3.6. Characterization by Power Spectral Density (PSD) Analysis of TiO2-PVA-HAp Nanocompounds
3.7. Coefficient of Determination R2
3.8. Fractal Dimension Analysis
3.9. Texture Isotropy Analysis
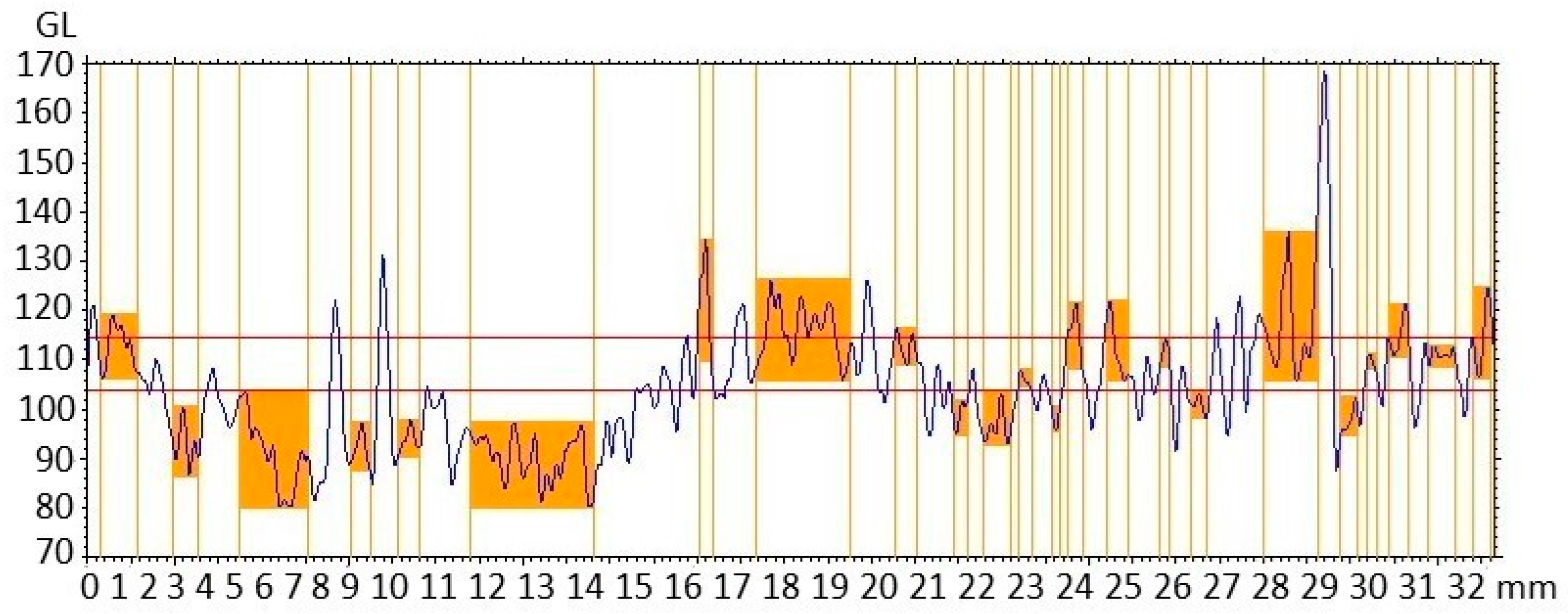
3.10. Characterization of Nanocompounds by Surface Geometry Analysis
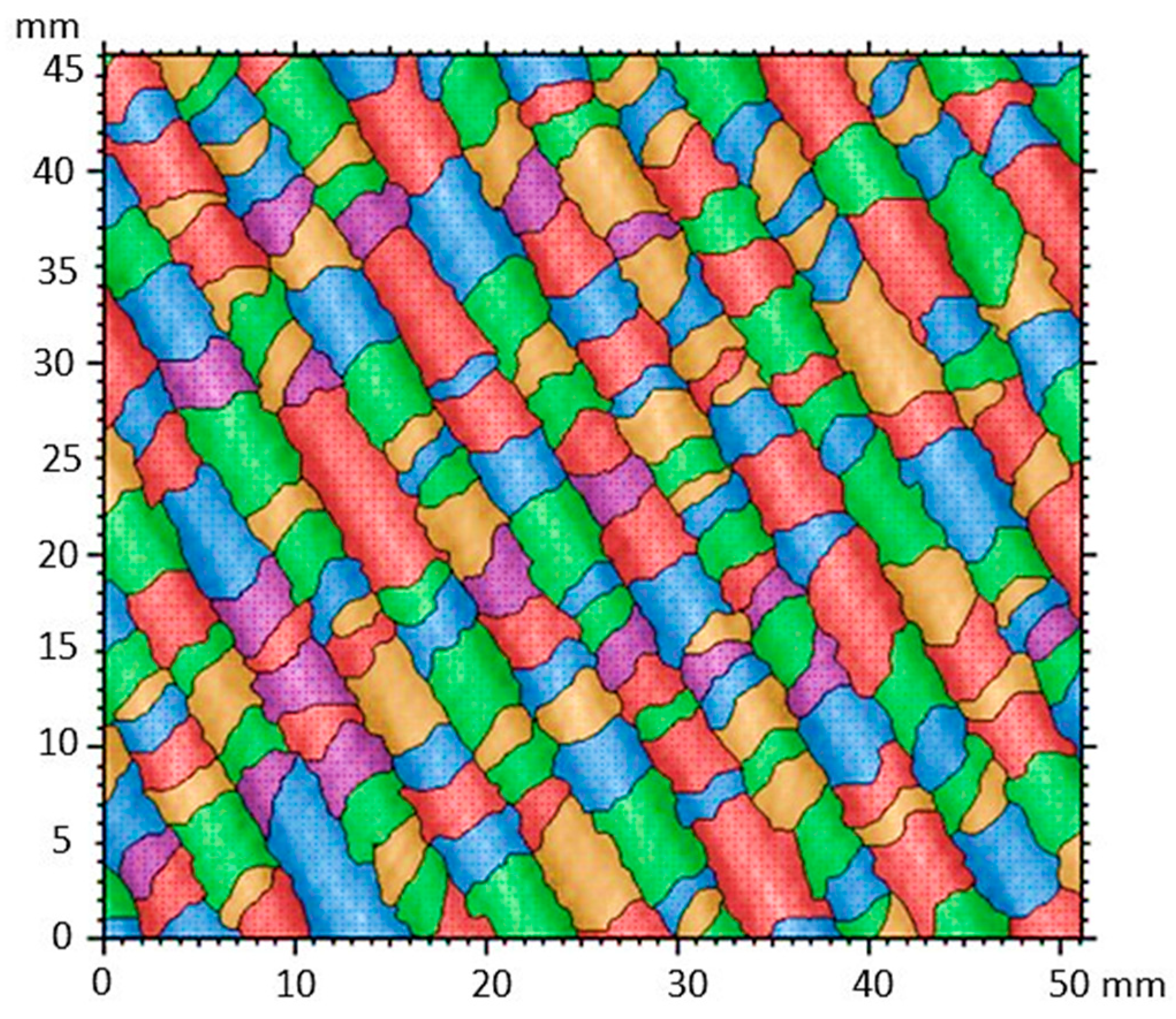
3.11. Characterization of Nanocompounds by Surface Segmentation Analysis
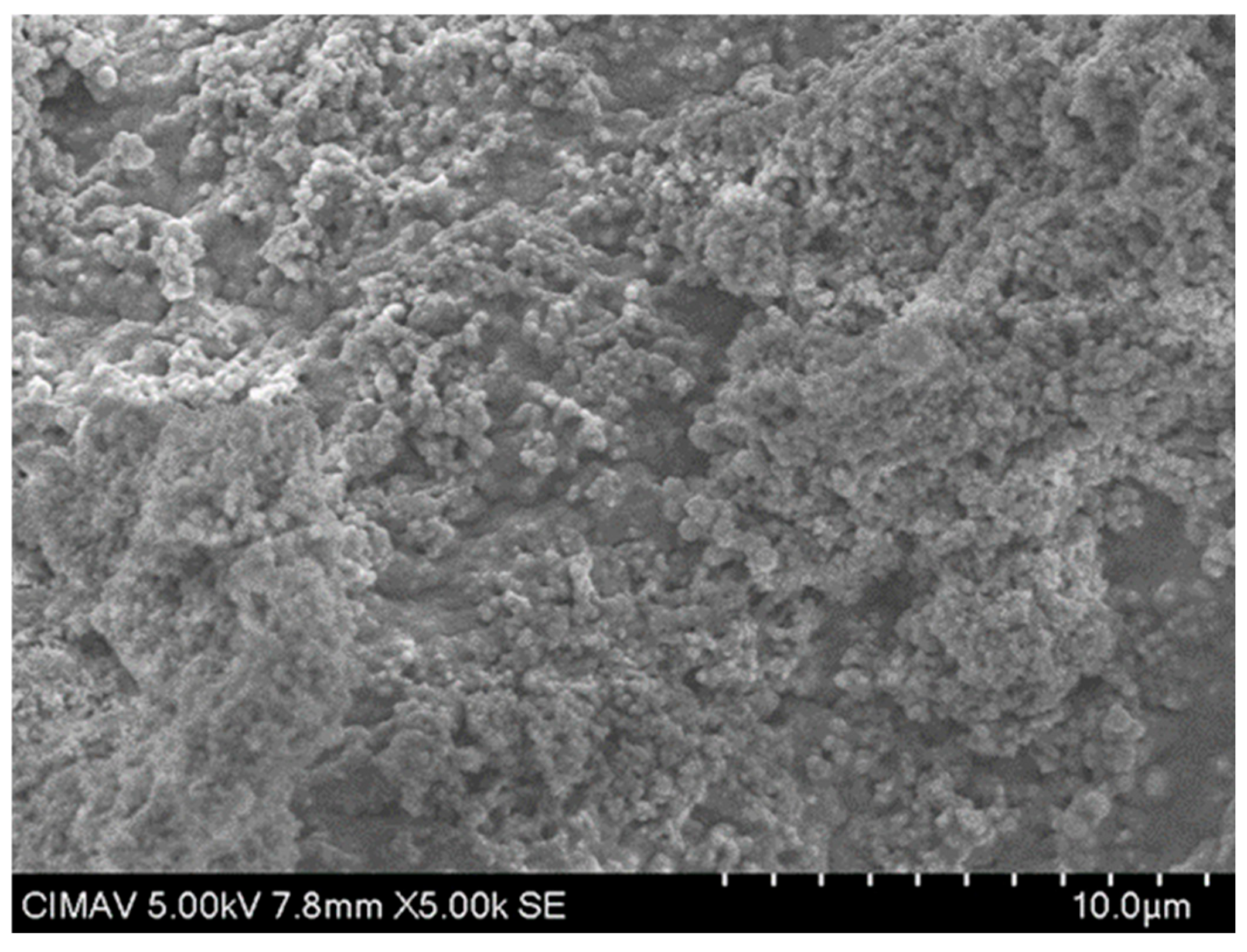
3.12. Characterization of TiO2-PVA-HAp Nanocompounds by SEM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahanty, A.; Shikha, D. Changes in the morphology, mechanical strength and biocompatibility of polymer and metal/polymer fabricated hydroxyapatite for orthopaedic implants: A review. J. Polym. Eng. 2022, 42, 298–322. [Google Scholar] [CrossRef]
- Uma Maheshwari, S.; Govindan, K.; Raja, M.; Raja, A.; Pravin, M.B.S.; Vasanth Kumar, S. Preliminary studies of PVA/PVP blends incorporated with HAp and β-TCP bone ceramic as template for hard tissue engineering. Bio Med. Mater. Eng. 2017, 28, 401–415. [Google Scholar] [CrossRef]
- Asran, A.S.; Henning, S.; Michler, G.H. Polyvinyl alcohol–collagen–hydroxyapatite biocomposite nanofibrous scaffold: Mimicking the key features of natural bone at the nanoscale level. Polymer 2010, 51, 868–876. [Google Scholar] [CrossRef]
- Subramanian, U.M.; Samuel, V.K.; Naveen, N. Fabrication and evaluation of (PVA/HAp/PCL) bilayer composites as potential scaffolds for bone tissue regeneration application. Ceram. Int. 2014, 40, 8469–8477. [Google Scholar]
- Forero, J.C.; Roa, E.; Reyes, J.G.; Acevedo, C.; Osses, N. Development of useful biomaterial for bone tissue engineering by incorporating nano-copper-zinc alloy (nCuZn) in chitosan/gelatin/nano-hydroxyapatite (ch/G/nHAp) scaffold. Materials 2017, 10, 1177. [Google Scholar] [CrossRef] [PubMed]
- Niroumand, H.; Zain, M.F.M.; Jamil, M. The Role of Nanotechnology in Architecture and Built Environment. Procedia Soc. Behav. Sci. 2013, 89, 10–15. [Google Scholar] [CrossRef]
- Kumar, A.; Raorane, C.J.; Syed, A.; Bahkali, A.H.; Elgorban, A.M.; Raj, V.; Kim, S.C. Synthesis of TiO2, TiO2/PAni, TiO2/PAni/GO nanocomposites and photodegradation of anionic dyes Rose Bengal and thymol blue in visible light. Environ. Res. 2023, 216, 114741. [Google Scholar] [CrossRef]
- Sadek, O.; Touhtouh, S.; Rkhis, M.; Anoua, R.; El Jouad, M.; Belhora, F.; Hajjaji, A. Synthesis by sol-gel method and characterization of nano-TiO2 powders. Mater. Today 2022, 66, 456–458. [Google Scholar] [CrossRef]
- Wategaonkar, S.; Pawar, R.; Parale, V.; Naded, D.; Sargar, B.; Mane, R. Synthesis of rutile TiO2 nanostructures by single step hydrothermal route and its characterization. Mater. Today 2020, 23, 444–451. [Google Scholar] [CrossRef]
- Pidluzhna, A. TiO2 composite electrode materials for lithium batteries. Electrochim. Acta 2021, 367, 137569. [Google Scholar] [CrossRef]
- Jesus, M.A.M.L.; Ferreira, A.M.; Lima, L.F.S.; Batista, G.F.; Mambrini, R.V.; Mohallem, N.D.S. Micro-mesoporous TiO2/SiO2 nanocomposites: Sol-gel synthesis, characterization, and enhanced photodegradation of quinoline. Ceram. Int. 2021, 47, 23844–23850. [Google Scholar] [CrossRef]
- Pragathiswaran, C.; Smitha, C.; Barabadi, H.; Al-Ansari, M.M.; Al-Humaid, L.A.; Saravanan, M. TiO2@ZnO nanocomposites decorated with gold nanoparticles: Synthesis, characterization and their antifungal, antibacterial, anti-inflammatory and anticancer activities. Inorg. Chem. Commun. 2020, 121, 108–210. [Google Scholar] [CrossRef]
- Kuda, A.; Yadav, M. Opportunities and challenges of using nanomaterials and nanotechnology in architecture: An overview. Mater. Today Proc. 2022, 65, 2102–2111. [Google Scholar] [CrossRef]
- AL-Rjoub, A.; Rebouta, L. Growth and characterization of tungsten titanium nanoparticles (WTi) produced by DC magnetron sputtering. J. Eng. 2021, 11, 34–38. [Google Scholar]
- Pasini, S.M.; Valério, A.; Yin, G.; Wang, J.; de Souza, S.M.G.U.; Hotza, D.; de Souza, A.A.U. An overview on nanostructured TiO2–containing fibers for photocatalytic degradation of organic pollutants in wastewater treatment. J. Water Process Eng. 2021, 40, 101827. [Google Scholar] [CrossRef]
- Vandana, K.; Pandel, R. Development of TiO2/PVA nanocomposites for application in solar cells. Mater. Today Proceeding 2018, 5, 6279–6287. [Google Scholar]
- Vemulapalli, A.K.; Penmetsa, R.M.R.; Nallu, R.; Siriyala, R. HAp/TiO2 nanocomposites: Influence of TiO2 on microstructure and mechanical properties. J. Compos. Mater. 2020, 54, 765–772. [Google Scholar] [CrossRef]
- Mehrnaz, S.; Zhiqiang, W.; Tsun-Kong, S. Hydroxyapatite-TiO(2)-based nanocomposites synthesized in supercritical CO(2) for bone tissue engineering: Physical and mechanical properties. ACS Appl. Mater. Interfaces 2014, 8, 16918–16931. [Google Scholar]
- Kamoun, E.A.; Youssef, M.E.; Abu-Saied, M.A.; Fahmy, A.; Khalil, H.F.; Abdelhai, F. Ion Conducting Nanocomposite Membranes Based on PVA-HAHAP for Fuel Cell Application: II. Effect of modifier agent of PVA on membrane properties. Int. J. Electrochem. Sci. 2015, 10, 6627–6644. [Google Scholar]
- Wuriantika, M.I.; Utomo, J.; Nurhuda, M.; Santjojo, D.J.D.H. Nanostructure, porosity and tensile strength of PVA/Hydroxyapatite composite nanofiber for bone tissue engineering. Mater. Today 2021, 44, 3203–3206. [Google Scholar]
- Gyeong, M.; Ashraf, S.; Georg, H.; Paul, S.; Jeong, S. Electrospun PVA/HAp nanocomposite nanofibers: Biomimetics of mineralized hard tissues at a lower level of complexity. Bioinspir. Biomim. 2008, 3, 046003. [Google Scholar]
- Hartatiek, Y.; Nada, S.; Joko, U.; Nurhuda, M.; Dionysius, J.; Santjojo, M. Morphology, Porosity, and Biodegradation of PVA/CS/PEG/HAp Nanofiber Composites as Scaffold in Bone Tissue Engineering. AIP Conf. 2020, 2231, 040055. [Google Scholar]
- Hartatiek, N.; Shofura, M.; Wuriantika, Y.; Nurhuda, M.; Santjojo, D.; Ahmad, N. Oxygen Gas Vacuum Plasma Treatment on PVA/Chitosan/HAp Scaffold Nanofiber. J. Phys. Sci. Eng. 2022, 7, 108–115. [Google Scholar] [CrossRef]
- Sarigol, E.; Hascicek, C. Tissue scaffolds as a local drug delivery system for bone regeneration. In Cutting-Edge Enabling Technologies for Regenerative Medicine; Springer: Berlin/Heidelberg, Germany, 2018; Volume 25, pp. 475–493. [Google Scholar]
- Saeid, K.; Sahar, M.; Farzad, K. Hydroxyapatite Nanoparticles for Improved Cancer Theranostics. Funct. Biomater. 2022, 13, 100. [Google Scholar]
- Sheikh, F.A.; Barakat, N.A.; Kanjwal, M.A.; Park, S.J.; Park, D.K.; Kim, H.Y. Synthesis of poly(vinyl alcohol) (PVA) nanofibers incorporating hydroxyapatite nanoparticles as future implant materials. Macromol. Res. 2010, 18, 59–66. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kumar, S.; Sahu, R.K.; Nayar, S. PVA-graphene-hydroxyapatite electrospun fibres as air-filters. Mater. Res. Express 2019, 6, 125366. [Google Scholar] [CrossRef]
- Wang, B.; Chen, Z.; Zhang, J.; Cao, J.; Wang, S.; Tian, Q.; Gao, M.; Xu, Q. Fabrication of PVA/graphene oxide/TiO2 composite nanofibers through electrospinning and interface sol–gel reaction: Effect of graphene oxide on PVA nanofibers and growth of TiO2. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 318–325. [Google Scholar] [CrossRef]
- Murugapandian, R.; Clement, S.; Uthirapathy, V. Fabrication and In Vitro Drug Delivery Evaluation of Cephalexin Monohydrate-Loaded PLA: PVA/HAP: TiO2 Fibrous Scaffolds for Bone Regeneration. ACS Omega 2023, 8, 5017–5032. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Desai, T. Aligned arrays of biodegradable poly (ϵ-caprolactone) nanowires and nanofibers by template synthesis. Nano Lett. 2007, 7, 1463–1468. [Google Scholar] [CrossRef]
- Liao, H.; Lin, J.; Liu, Y.; Huang, P.; Jin, A.; Chen, X. Self-assembly mechanisms of nanofibers from peptide amphiphiles in solution and on substrate surfaces. Nanoscale 2016, 8, 14814–14820. [Google Scholar] [CrossRef]
- Otieno, O.V.; Csáki, E.; Kéri, O.; Simon, L.; Lukács, I.E.; Szécsényi, K.M.; Szilágyi, I.M. Synthesis of TiO2 nanofibers by electrospinning using water-soluble Ti-precursor. J. Therm. Anal. Calorim. 2020, 139, 57–66. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, J. Recent advances in the synthesis of defective TiO2 nanofibers and their applications in energy and catalysis. Chem. Eng. J. 2023, 472, 144831. [Google Scholar] [CrossRef]
- Song, J.; Guan, R.; Xie, M.; Dong, P.; Yang, X.; Zhang, J. Advances in electrospun TiO2 nanofibers: Design, construction, and applications. Chem. Eng. J. 2022, 431, 134343. [Google Scholar] [CrossRef]
- Priyadarshini, B.; Ramya, S.; Shinyjoy, E.; Kavitha, L.; Gopi, D.; Vijayalakshmi, U. Structural, morphological and biological evaluations of cerium incorporated hydroxyapatite sol–gel coatings on Ti–6Al–4V for orthopaedic applications. J. Mater. Res. Technol. 2021, 12, 1319–1338. [Google Scholar] [CrossRef]
- Sisy, A.; Diah, H. Fabrication of PVA/TiO2 Nanofibers by Electrospinning Method as Photocatalytic Degradation Indon. Phys. Rev. 2022, 5, 86–97. [Google Scholar]
- Hoang, T.D.; Nguyen, T.C.; Doan, T.T.; Ngo, T.D.; Nguyen, T.Y.; Ngo, B.T.; Tran, T.T.; Le, T.L. Preparation and photocatalytic and antibacterial properties of polyvinyl alcohol/chitosan/TiO2@Ag electrospun nanofibers. Thin Solid Films 2024, 797, 140344. [Google Scholar] [CrossRef]
- Montes, D.; Henao, J.; Taborda, E.A.; Gallego, J.; Cortés, F.B.; Franco, C.A. Effect of Textural Properties and Surface Chemical Nature of Silica Nanoparticles from Different Silicon Sources on the Viscosity Reduction of Heavy Crude Oil. ACS Omega 2020, 5, 5085–5097. [Google Scholar] [CrossRef]
- Arantxa, D.; Magdaleno, R. Fabrication of PVA/Ag-TiO2 nanofiber mats for visible-light-active photocatalysis. Res. Phys. 2021, 25, 104205. [Google Scholar]
- Ataf, A.; Muneeb, A.; Muhammad, H.; Samia, K.; Muhammad, W.; Amin, B. Titania nano-fibers: A review on synthesis and utilities. Inorg. Chim. Acta 2020, 501, 119268. [Google Scholar] [CrossRef]
- Alexander, Y.; Tatiana, B. TiO2 and TiO2/Ag nanofibers: Template synthesis, structure, and photocatalytic properties. J. Porous Mater. 2021, 28, 1023–1030. [Google Scholar]
- Treacy, M.; Bellessa, J. On the measurement of surface step heights by low-loss imaging in stem. Ultramicroscopy 1993, 11, 173–178. [Google Scholar] [CrossRef]
- Sho, H.; Toru, K.; Shohei, O.; Yuya, O.; Hiroaki, I. Fibrous nanocrystals of hydroxyapatite loaded with TiO2 nanoparticles for the capture and photocatalytic decomposition of specific proteins. Colloids Surf. B Biointerfaces 2010, 79, 131–135. [Google Scholar]
- Sorin, J.; Andreea, Z.; Georgeta, V.; Monica, E.; Alexandru, E.; Cristina, B. PCL-ZnO/TiO2/HAp Electrospun Composite Fibers with Applications in Tissue Engineering. Polymers 2019, 11, 1793. [Google Scholar]
- Loganathan, M.; Durgalakshmi, D.; Geetha, M.; Sankara, T. Electrophoretic deposition of nanocomposite (HAp + TiO2) on ti-tanium alloy for biomedical applications. Ceram. Int. 2012, 38, 3435–3443. [Google Scholar]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estrada Macias, M.E.; Monreal Romero, H.A.; Mata, G.M.; Pacheco Santiesteban, R.; Meléndez, C.L.; López Aguilar, H.A.; Chávez Acosta, O.; Martínez-Pérez, C.A.; Carreño-Gallardo, C.; Chacón-Nava, J.G. Formation of Nanocompounds of TiO2 Using PVA-HAp Nanofibers by Sol-Gel Technique. Polymers 2025, 17, 2796. https://doi.org/10.3390/polym17202796
Estrada Macias ME, Monreal Romero HA, Mata GM, Pacheco Santiesteban R, Meléndez CL, López Aguilar HA, Chávez Acosta O, Martínez-Pérez CA, Carreño-Gallardo C, Chacón-Nava JG. Formation of Nanocompounds of TiO2 Using PVA-HAp Nanofibers by Sol-Gel Technique. Polymers. 2025; 17(20):2796. https://doi.org/10.3390/polym17202796
Chicago/Turabian StyleEstrada Macias, Marvin Elco, Humberto Alejandro Monreal Romero, Guillermo Martínez Mata, Rosaura Pacheco Santiesteban, Claudia López Meléndez, Héctor Alfredo López Aguilar, Oscar Chávez Acosta, Carlos A. Martínez-Pérez, Caleb Carreño-Gallardo, and José Guadalupe Chacón-Nava. 2025. "Formation of Nanocompounds of TiO2 Using PVA-HAp Nanofibers by Sol-Gel Technique" Polymers 17, no. 20: 2796. https://doi.org/10.3390/polym17202796
APA StyleEstrada Macias, M. E., Monreal Romero, H. A., Mata, G. M., Pacheco Santiesteban, R., Meléndez, C. L., López Aguilar, H. A., Chávez Acosta, O., Martínez-Pérez, C. A., Carreño-Gallardo, C., & Chacón-Nava, J. G. (2025). Formation of Nanocompounds of TiO2 Using PVA-HAp Nanofibers by Sol-Gel Technique. Polymers, 17(20), 2796. https://doi.org/10.3390/polym17202796









