Design of Functional Fluorine-Containing Coatings for 3D-Printed Items
Abstract
1. Introduction
2. Materials and Methods
2.1. The Macroscopic Texture Design of 3D-Printed Products’ Surface
2.2. The Microscopic Texture Design of the 3D-Printed Products’ Surface
2.3. The 3D-Printed Products’ Surface Structure and Properties Studying
- -
- The sample’s mass changing due to the gas-phase fluorination was determined using precision scales;
- -
- The degree of fluorination was calculated as a quotient of the induced mass increment by the sample’s surface area.
2.4. The Variation–Rotational Maps Creation Technique
3. Results and Discussion
3.1. The Surface Design Effect on the 3D-Printed Product Wettability
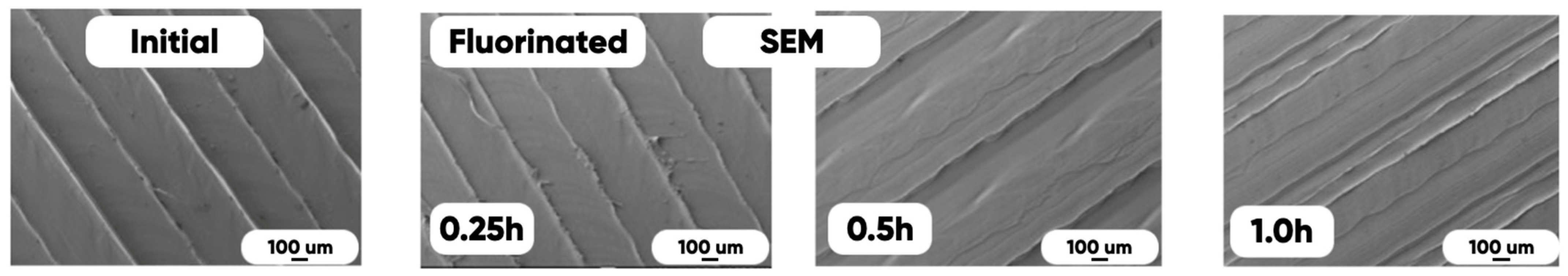
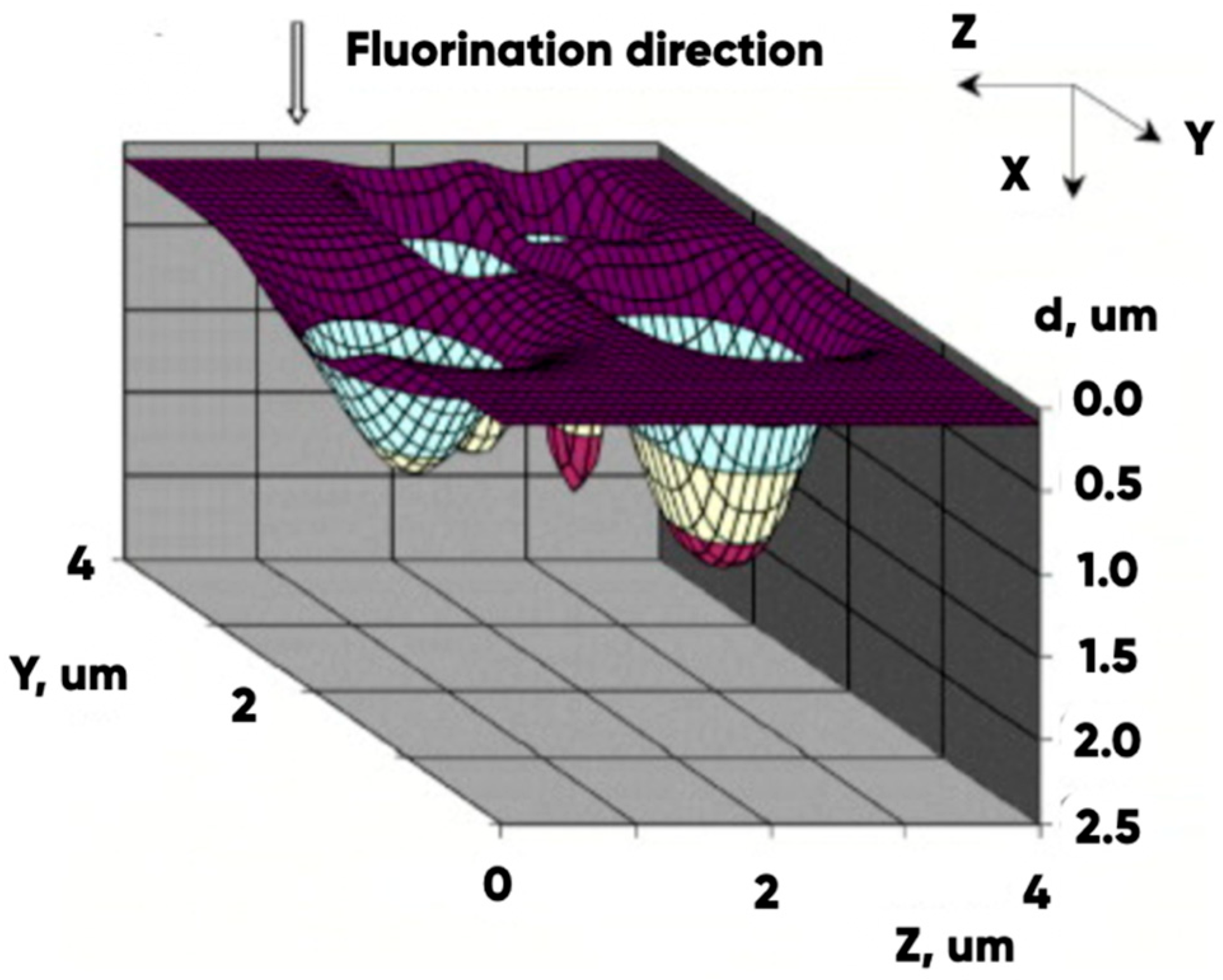
3.2. The Effect of the Fluorination on 3D-Printed Product Surface Morphology
- (a)
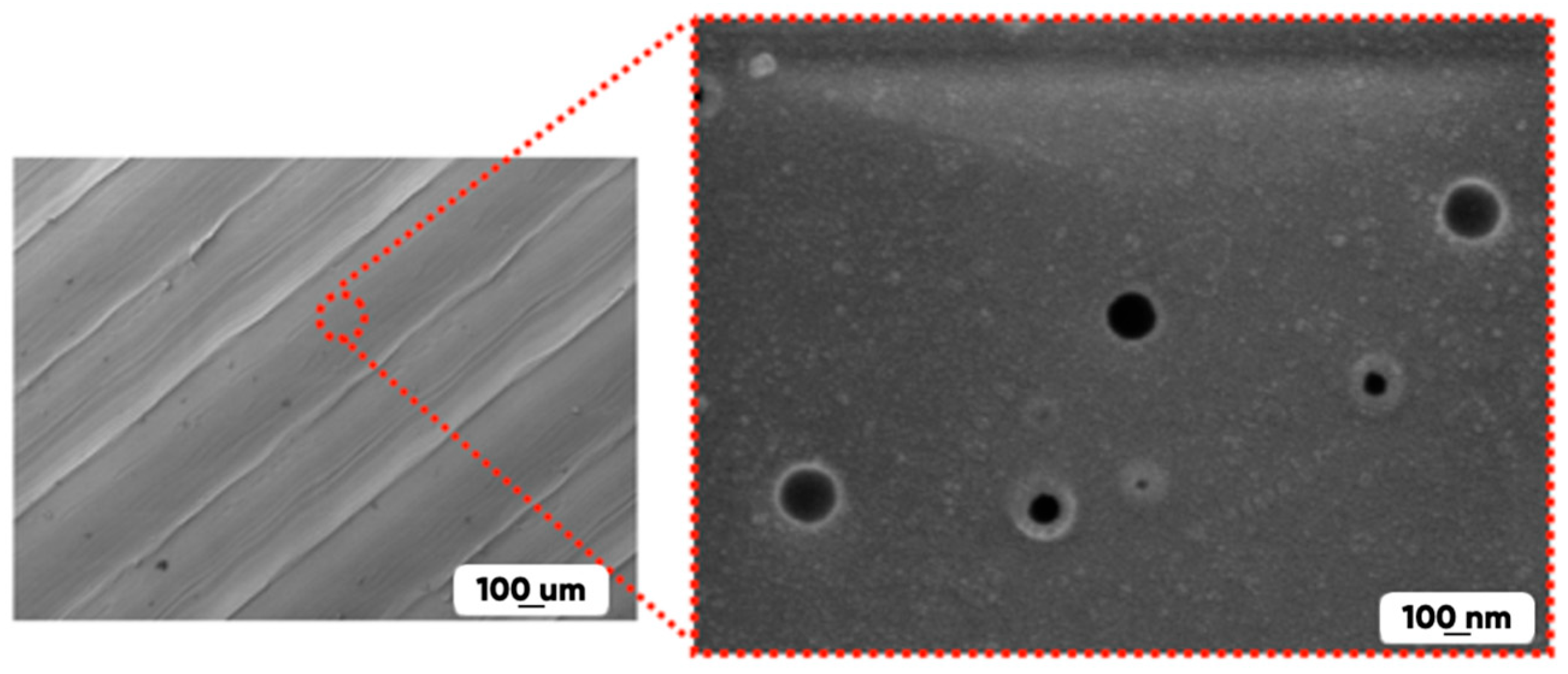
- The presence of pores (with the diameters of 50 nm or more) in the initial and in the modified experimental samples structure contributing to the effective diffusion of the active reagent (fluorine) and significantly increasing the interaction with the modified gas mixture 3D-printed product surface area (Figure 5).
- (b)
- The presence of unsaturated bonds in the 3D-printed polymers’ macromolecules (Table 5), which contribute to the intensive course of fluorine addition reactions.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Melnikov, P.; Bobrov, A.; Marfin, Y. On the use of polymer-based composites for the creation of optical sensors: A review. Polymers 2022, 14, 4448. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gou, J.; Jiang, M.; Zhou, Z.; Hui, D. 3D printing of polymer matrix composites: A review and prospective. Compos. Part B Eng. 2017, 110, 442–458. [Google Scholar] [CrossRef]
- Bauhofer, W.; Kovacs, J.Z. A review and analysis of electrical percolation in carbon nanotube polymer composites. Compos. Sci. Technol. 2009, 69, 1486–1498. [Google Scholar] [CrossRef]
- Mayandi, K.; Rajini, N.; Ayrilmis, N.; Indira Devi, M.P.; Siengchin, S.; Mohammad, F.; Al-Lohedan, H.A. An overview of endurance and ageing performance under various environmental conditions of hybrid polymer composites. J. Mater. Res. Technol. 2020, 9, 15962–15988. [Google Scholar] [CrossRef]
- Gao, J.; Wang, L.; Guo, Z.; Li, B.; Wang, H.; Luo, J.; Huang, X.; Xue, H. Flexible, superhydrophobic, and electrically conductive polymer nanofiber composite for multifunctional sensing applications. Chem. Eng. J. 2020, 381, 122778. [Google Scholar] [CrossRef]
- Shojaei, A.; Abbasi, F. Cure kinetics of a polymer-based composite friction material. J. Appl. Polym. Sci. 2006, 100, 9–17. [Google Scholar] [CrossRef]
- Montemor, M.F. Functional and smart coatings for corrosion protection: A review of recent advances. Surf. Coat. Technol. 2014, 258, 17–37. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, A.G.; Sun, B.R.; Chen, K.S.; Yu, H. Functional and versatile superhydrophobic coatings via stoichiometric silanization. Nat. Commun. 2021, 12, 982. [Google Scholar] [CrossRef] [PubMed]
- Calderon Velasco, S.; Carvalho, S.; Cavaleiro, A. Functional properties of ceramic-Ag nanocomposite coatings produced by magnetron sputtering. Prog. Mater. Sci. 2016, 84, 158–191. [Google Scholar] [CrossRef]
- Verbič, A.; Gorjanc, M.; Simončič, B. Zinc oxide for functional textile coatings: Recent advances. Coatings 2019, 9, 550. [Google Scholar] [CrossRef]
- Nazarov, V.G. Composition and dimensions of the surface and transition layers in modified polymers. Polym. Sci. Ser. B 1997, 39, 142–145. [Google Scholar]
- Nazarov, V.G. Multiple surface structures in polyolefins formed by modification methods. J. Appl. Polym. Sci. 2005, 95, 1198–1208. [Google Scholar] [CrossRef]
- Mahakur, V.K.; Paul, R.; Bhowmik, S.; Patowari, P.K. Influence of surface modification on mechanical and tribology performance of jute filler polymer composites and prediction of the performance using artificial neural network. Polym. Bull. 2023, 80, 11953–11974. [Google Scholar] [CrossRef]
- Cai, S.; Wu, C.; Yang, W.; Liang, W.; Yu, H.; Liu, L. Recent advance in surface modification for regulating cell adhesion and behaviors. Nanotechnol. Rev. 2020, 9, 971–989. [Google Scholar] [CrossRef]
- Miller, D.J.; Paul, D.R.; Freeman, B.D.; Dreyer, D.R.; Bielawski, C.W. Surface modification of water purification membranes. Angew. Chem. Int. Ed. 2017, 56, 4662–4711. [Google Scholar] [CrossRef]
- Chu, P.K.; Chen, J.Y.; Wang, L.P.; Huang, N. Plasma-surface modification of biomaterials. Mater. Sci. Eng. R Rep. 2002, 36, 143–206. [Google Scholar] [CrossRef]
- Butt, M.A. Thin-film coating methods: A successful marriage of high-quality and cost-effectiveness-a brief exploration. Coatings 2022, 12, 1115. [Google Scholar] [CrossRef]
- Mbam, S.O.; Gou, X.-F.; Nwigwe, U.S.; Nwonu, S.E.; Orelaja, O.A. Thin-film coating; historical evolution, conventional deposition technologies, stress-state micro/nano-level measurement/models and prospects projection: A critical review. Mater. Res. Express 2019, 6, 122001. [Google Scholar] [CrossRef]
- Tang, C.Y.; Kwon, Y.N.; Leckie, J.O. Effect of membrane chemistry and coating layer on physiochemical properties of thin film composite polyamide Ro and Nf membranes. I. FTIR and XPS characterization of polyamide and coating layer chemistry. Desalination 2009, 242, 149–167. [Google Scholar] [CrossRef]
- Sun, T.; Jiang, L.; Feng, L.; Gao, X. Bioinspired surfaces with special wettability. Acc. Chem. Res. 2005, 38, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, X.; Wu, H.; Tian, Z.; Xin, Q.; He, G.; Peng, D.; Chen, S.; Jiang, Z.; Guiver, M.D.; et al. Advances in high permeability polymer-based membrane materials for CO2 separations. Energy Environ. Sci. 2016, 9, 1863–1890. [Google Scholar] [CrossRef]
- Zhu, T.; Li, J. Ultra-strength materials. Prog. Mater. Sci. 2010, 55, 710–757. [Google Scholar] [CrossRef]
- Nazarov, V.G.; Doronin, F.A.; Evdokimov, A.G.; Dedov, A.V. Regulation of the Wettability of Nonwoven Cloth by Oxyfluorination to Improve its Impregnation by Latex. Fibre Chem. 2020, 52, 109–111. [Google Scholar] [CrossRef]
- Li, X.; Chang, L.; Luo, S.; Yang, R.; Hou, Z.; Zou, J. Fe doping of carbon-coated manganese-rich cathode based on optimized preparation process to inhibit lattice distortion: Modification under optimal conditions. Ceram. Int. 2025, 51, 9039–9047. [Google Scholar] [CrossRef]
- Gunka, V.; Bilushchak, H.; Prysiazhnyi, Y.; Demchuk, Y.; Hrynchuk, Y.; Sidun, I.; Shyshchak, O.; Bratychak, M. Production of bitumen modified with low-molecular organic compounds from petroleum residues. 4. Determining the optimal conditions for tar modification with formaldehyde and properties of the modified products. Chem. Chem. Technol. 2022, 16, 142–149. [Google Scholar] [CrossRef]
- Liang, X.; Dong, J.; Wei, G.; Offiong, N.A.; Yang, C. Colloidal biliquid aphron demulsification using polyaluminum chloride and density modification of DNAPLS: Optimal conditions and common ion effect. Environ. Sci. Process. Impacts 2020, 22, 1908–1915. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, V.F.; Ribeiro, C.; Fernandes, M.M.; Correia, D.M.; Lanceros-Méndez, S. Fluorinated polymers as smart materials for advanced biomedical applications. Polymers 2018, 10, 161. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Li, X.; Wu, B.; Sun, S.; Wu, P. Highly damping and self-healable ionic elastomer from dynamic phase separation of sticky fluorinated polymers. Adv. Mater. 2023, 35, 2209581. [Google Scholar] [CrossRef]
- Kim, M.P.; Lee, Y.; Park, J.; Kim, J.; Ko, H.; Hur, Y.H.; Song, S.W.; Jung, Y.S.; Ahn, C.W. Molecular structure engineering of dielectric fluorinated polymers for enhanced performances of triboelectric nanogenerators. Nano Energy 2018, 53, 37–45. [Google Scholar] [CrossRef]
- Giannetti, E. Thermal stability and bond dissociation energy of fluorinated polymers: A critical evaluation. J. Fluor. Chem. 2005, 126, 625–632. [Google Scholar] [CrossRef]
- Ciardelli, F.; Aglietto, M.; Montagnini di Mirabello, L.; Passaglia, E.; Giancristoforo, S.; Castelvetro, V.; Ruggeri, G. New fluorinated acrylic polymers for improving weatherability of building stone materials. Prog. Org. Coat. 1997, 32, 43–50. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Q.; Du, X.; Li, L.; Li, P. Surface modification of polyethylene terephthalate films by direct fluorination. AIP Adv. 2018, 8, 125333. [Google Scholar] [CrossRef]
- Pilati, F.; Montecchi, M.; Fabbri, P.; Synytska, A.; Messori, M.; Toselli, M.; Grundke, K.; Pospiech, D. Design of surface properties of pet films: Effect of fluorinated block copolymers. J. Colloid Interface Sci. 2007, 315, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.S.; Lin, H.T.; Chuang, M.J. Surface fluorination of polyethylene terephthalate films with RF plasma. Mater. Lett. 2004, 58, 650–653. [Google Scholar] [CrossRef]
- Kong, F.; Ma, Y.; Zhang, C.; Ren, C.; Shao, T.; Chang, C. Surface modifications of polystyrene and their stability: A comparison of DBD plasma deposition and direct fluorination. Appl. Surf. Sci. 2018, 459, 300–308. [Google Scholar] [CrossRef]
- Kharitonov, A.P.; Moskvin, Y.u.L. Direct fluorination of polystyrene films. J. Fluor. Chem. 1998, 91, 87–93. [Google Scholar] [CrossRef]
- Kharitonov, A.P. Direct fluorination of polymers-from fundamental research to industrial applications. Prog. Org. Coat. 2008, 61, 192–204. [Google Scholar] [CrossRef]
- Kharitonov, A.P.; Taege, R.; Ferrier, G.; Teplyakov, V.V.; Syrtsova, D.A.; Koops, G.H. Direct fluorination—Useful tool to enhance commercial properties of polymer articles. J. Fluor. Chem. 2005, 126, 251–263. [Google Scholar] [CrossRef]
- Doronin, F.; Rytikov, G.; Evdokimov, A.; Rudyak Yu Taranets, I.; Nazarov, V. The effect of electro-induced multi-gas modification on polymer substrates’ surface structure for additive manufacturing. Processes 2023, 11, 774. [Google Scholar] [CrossRef]
- Nazarov, V.G.; Stolyarov, V.P.; Doronin, F.A.; Evdokimov, A.G.; Rytikov, G.O.; Brevnov, P.N.; Zabolotnov, A.S.; Novokshonova, L.A.; Berlin, A.A. Comparison of the effects of some modification methods on the characteristics of ultrahigh-molecular-weight polyethylene and composites on its basis. Polym. Sci. Ser. A 2019, 61, 325–333. [Google Scholar] [CrossRef]
- Sevast’yanov, V.I.; Nemets, E.A.; Stolyarov, V.P.; Baranov, V.A.; Bozhko, N.N.; Nazarov, V.G. Comparative study of the influence of polyethylene film surface modification on interaction with blood components. Inorg. Mater. Appl. Res. 2011, 2, 146–152. [Google Scholar] [CrossRef]
- Doronin, F.; Rytikov, G.; Evdokimov, A.; Rudyak Yu Savel’ev, M.; Nazarov, V. Biostable fluorine-containing coatings on the surface of polymers. Coatings 2023, 13, 424. [Google Scholar] [CrossRef]
- Doronin, F.A.; Rudakova, A.; Rytikov, G.O.; Nazarov, V.G. A novel determination of the melt flow index of composite filaments used in extrusion additive manufacturing. Polym. Test. 2024, 133, 108376. [Google Scholar] [CrossRef]
- Song, X.; Liu, H.; Cheng, L.; Qu, Y. Surface modification of coconut-based activated carbon by liquid-phase oxidation and its effects on lead ion adsorption. Desalination 2010, 255, 78–83. [Google Scholar] [CrossRef]
- Tsubota, T.; Tanii, S.; Ida, S.; Nagata, M.; Matsumoto, Y. Chemical modification of diamond surface with various carboxylic acids by radical reaction in liquid phase. Diam. Relat. Mater. 2004, 13, 1093–1097. [Google Scholar] [CrossRef]
- Lebedev-Stepanov, P.V.; Molchanov, S.P.; Ivanov, A.A.; Mitrokhin, V.P.; Yurasik, G.A.; Alfimov, M.V.; Kadushnikov, R.M.; Rubin, N.I.; Vlasov, K.O.; Nazarov, V.G. Self-assembly of nanoparticles in the microvolume of colloidal solution: Physics, modeling, and experiment. Nanotechnologies Russ. 2013, 8, 137–162. [Google Scholar] [CrossRef]
- Nazarov, V.G.; Stolyarov, V.P. Modified polymer substrates for the formation of submicron particle ensembles from colloidal solution. Colloid J. 2016, 78, 75–82. [Google Scholar] [CrossRef]
- Kaneto, K.; Tsuruta, M.; Sakai, G.; Cho, W.Y.; Ando, Y. Electrical conductivities of multi-wall carbon nano tubes. Synth. Met. 1999, 103, 2543–2546. [Google Scholar] [CrossRef]
- Chopra, N.; Majumder, M.; Hinds, B.J. Bifunctional carbon nano tubes by side wall protection. Adv. Funct. Mater. 2005, 15, 858–864. [Google Scholar] [CrossRef]
- Joseph Berkmans, A.; Jagannatham, M.; Haridoss, P.; Priyanka, S. Synthesis of branched, nano channeled, ultrafine and nano carbon tubes from pet wastes using the arc discharge method. Waste Manag. 2014, 34, 2139–2145. [Google Scholar] [CrossRef]
- Ma, L.; Luo, P.; He, Y.; Zhang, L.; Fan, Y.; Jiang, Z. Improving the stability of multi-walled carbon nano-tubes in extremely environments: Applications as nano-plugging additives in drilling fluids. J. Nat. Gas Sci. Eng. 2020, 74, 103082. [Google Scholar] [CrossRef]
- Das, T.; Saikia, B.K.; Baruah, B.P. Formation of carbon nano-balls and carbon nano-tubes from northeast indian tertiary coal: Value added products from low grade coal. Gondwana Res. 2016, 31, 295–304. [Google Scholar] [CrossRef]
- Clarke, D.R.; Oechsner, M.; Padture, N.P. Thermal-barrier coatings for more efficient gas-turbine engines. MRS Bull. 2012, 37, 891–898. [Google Scholar] [CrossRef]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling coatings: Recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef] [PubMed]
- Musil, J. Hard and superhard nanocomposite coatings. Surf. Coat. Technol. 2000, 125, 322–330. [Google Scholar] [CrossRef]
- Nazarov, V.G.; Stolyarov, V.P.; Gagarin, M.V. Simulation of chemical modification of polymer surface. J. Fluor. Chem. 2014, 161, 120–127. [Google Scholar] [CrossRef]
- Nazarov, V.G.; Gagarin, M.V.; Stolyarov, V.P.; Evlampieva, L.A.; Baranov, V.A. Modeling of sliding friction processes in surface/spatial modified elastomer-metal couple. Inorg. Mater. Appl. Res. 2010, 1, 162–166. [Google Scholar] [CrossRef]
- Kharitonov, A.P.; Moskvin, Y.L.; Kharitonova, L.N.; Kotenko, A.A.; Tulskii, M.N. Kinetics of gas-phase fluorination of homogeneous films and composite membranes based on polycarbonate siloxane and block-copolymer of polysulfone and polybutadiene. Kinet. Catal. 1994, 35, 792. [Google Scholar]
- Kharitonov, A.P.; Moskvin, Y.L.; Kharitonova, L.N.; Tulskii, M.N.; Kotenko, A.A. An investigation into the direct fluorination kinetics of polymeric membranes. Mendeleev Commun. 1994, 4, 91. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Fiandini, M.; Ragadhita, R.; Sukmafitri, A.; Salam, H.; Triawan, F. Mechanical and biodegradation properties of cornstarch-based bioplastic material. Mater. Phys. Mech. 2020, 44, 380–391. [Google Scholar]
- Doronin, F.A.; Saveliev, M.A.; Taranets, I.P.; Rytikov, G.O.; Nazarov, V.G. Direct Structuring of Polymers Used in Additive Manufacturing. Russ. J. Gen. Chem. 2024, 94, 1550–1557. [Google Scholar] [CrossRef]
- Rytikov, G.O.; Pervoukhin, D.V.; Nazarov, V.G. A new approach to quantitative characterization of the material’s surface morphological heterogeneity. Lect. Notes Civ. Eng. 2022, 210, 301–307. [Google Scholar]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to read and interpret FTIR spectroscope of organic material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, I.U.; Rehman, S. Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Madejova, J. FTIR techniques in clay mineral studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Bogner, A.; Jouneau, P.H.; Thollet, G.; Basset, D.; Gauthier, C. A history of scanning electron microscopy developments: Towards “wet-stem” imaging. Micron 2007, 38, 390–401. [Google Scholar] [CrossRef]
- Brodowski, S.; Haumaier, L.; Zech, W.; Amelung, W.; Abetz, C. Morphological and chemical properties of black carbon in physical soil fractions as revealed by scanning electron microscopy and energy-dispersive x-ray spectroscopy. Geoderma 2005, 128, 116–129. [Google Scholar] [CrossRef]
- Bootz, A.; Kreuter, J.; Vogel, V.; Schubert, D. Comparison of scanning electron microscopy, dynamic light scattering and analytical ultracentrifugation for the sizing of poly(butyl cyanoacrylate) nanoparticles. Eur. J. Pharm. Biopharm. 2004, 57, 369–375. [Google Scholar] [CrossRef]
- Huckabay, H.A.; Armendariz, K.P.; Newhart, W.H.; Wildgen, S.M.; Dunn, R.C. Near-field scanning optical microscopy for high-resolution membrane studies. Methods Mol. Biol. 2013, 950, 373–394. [Google Scholar]
- García, R.; Pérez, R. Dynamic atomic force microscopy methods. Surf. Sci. Rep. 2002, 47, 197–301. [Google Scholar] [CrossRef]
- Carpick, R.W.; Salmeron, M. Scratching the surface: Fundamental investigations of tribology with atomic force microscopy. Chem. Rev. 1997, 97, 1163–1194. [Google Scholar] [CrossRef]
- Drozdov, S.A.; Nazarov, V.G.; Nozdrachev, S.A.; Rudyak, Y.u.V.; Rytikov, G.O. The polymer composites’ morphological structure simulation. Nanosyst. Phys. Chem. Math. 2017, 8, 137–145. [Google Scholar] [CrossRef]
- Rytikov, G.O.; Doronin, F.A.; Evdokimov, A.G.; Nazarov, V.G.; Savel’ev, M.A. An approach to structural and functional modeling of the surface morphology of materials based on fluorinated polymers. Russ. J. Gen. Chem. 2021, 91, 2667–2672. [Google Scholar] [CrossRef]
- Doronin, F.A.; Evdokimov, A.G.; Rudyak, Y.u.V.; Rytikov, G.O.; Taranets, I.P.; Nazarov, V.G. A new approach to function-structure modeling of the surface modified polymers. Nanosyst. Phys. Chem. Math. 2022, 13, 115–127. [Google Scholar] [CrossRef]
- Doronin, F.A.; Rytikov, G.O.; Evdokimov, A.G.; Ruduak, Y.V.; Nazarov, V.G. The synergistic effect of bulk-surface modification onto the wear resistance of the ultrahigh molecular weight polyethylene. Polym. Polym. Compos. 2023, 31, 09673911221150132. [Google Scholar] [CrossRef]
- Jorge, A.M.; Coutinho, J.A.; Pereira, J.F. Owens-Wendt method as a tool to evaluate polarity of polymer-polymer aqueous two-phase systems. Colloids Surf. A Physicochem. Eng. Asp. 2025, 726, 138080. [Google Scholar] [CrossRef]
- Hong, G.; Cheng, H.; Meng, Y.; Lin, J.; Chen, Z.; Zhang, S.; Song, W. Mussel-Inspired Polydopamine as a Green, Efficient, and Stable Platform to Functionalize Bamboo Fiber with Amino-Terminated Alkyl for High Performance Poly(butylene succinate) Compo-sites. Polymers 2018, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Waldner, C.; Hirn, U. Modeling liquid penetration into porous materials based on substrate and liquid surface energies. J. Colloid Interface Sci. 2023, 640, 445–455. [Google Scholar] [CrossRef]
- Fowkes, F.M. Additivity of Intermolecular Forces at Interfaces. I. Determination of the Contribution to Surface and Interfacial Tensions of Dispersion Forces in Various Liquids1. J. Phys. Chem. 1963, 67, 2538–2541. [Google Scholar] [CrossRef]
- Good, R.J. Contact Angle, Wettability and Adhesion; Mittal, K.L., Ed.; VSP: Utrecht, The Netherlands, 1993. [Google Scholar]
- Parale, V. Effect of surface composition and roughness on the apparent surface free energy of silica aerogel materials. Appl. Phys. Lett. 2011, 99, 104104. [Google Scholar] [CrossRef]
- Selvakumar, N.; Barshilia, H.C.; Rajam, K.S. Effect of substrate roughness on the apparent surface free energy of sputter deposited superhydrophobic polytetrafluoroethylene coatings: A com-parison of experimental data with different theoretical models. J. Appl. Phys. 2010, 108, 013505. [Google Scholar] [CrossRef]
- Laput, O.; Vasenina, I.; Salvadori, M.C.; Savkin, K.; Zuza, D.; Kurzina, I. Low-temperature plasma treatment of polylactic acid and PLA/HA composite material. J. Mater. Sci. 2019, 54, 11726–11738. [Google Scholar] [CrossRef]
- Xie, Y.; Zhu, J.; Fu, L.; Yang, W.; Li, D.; Zhou, L. TPU with outstanding wettability and hydrophilic stability is obtained by plasma-induced graft polymerization. Appl. Surf. Sci. 2024, 654, 159509. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, J. Surface hydrophilic modification of acrylonitrile-butadiene-styrene terpolymer by poly(ethylene glycol-co-1,4-cyclohexanedimethanol terephthalate): Preparation, characterization, and properties studies. Appl. Surf. Sci. 2016, 388 Pt A, 133–140. [Google Scholar] [CrossRef]
- Nazarov, V.G.; Doronin, F.A.; Evdokimov, A.G.; Rytikov, G.O.; Stolyarov, V.P. Oxyfluorination-Controlled Variations in the Wettability of Polymer Film Surfaces. Colloid J. 2019, 81, 146–157. [Google Scholar] [CrossRef]
- Taranets, I.P.; Rytikov, G.O.; Doronin, F.A.; Saveliev, M.A.; Nazarov, V.G. Structural-Functional Mathematical Modeling of Additive Manufacturing Polymers. Russ. J. Gen. Chem. 2024, 94, 1558–1563. [Google Scholar] [CrossRef]
- Datta, J.; Kasprzyk, P. Thermoplastic Polyurethanes Derived From Petrochemical or Renewable Resources: A Comprehensive Review. Polym. Eng. Sci. 2017, 58, E14–E35. [Google Scholar] [CrossRef]
- Suthapakti, K.; Molloy, R.; Leejarkpai, T. Disintegration Testing of Biodegradable Poly(L-lactide)/Thermoplastic Polyurethane Melt Blended Films. Chiang Mai J. Sci. 2018, 45, 2079–2091. [Google Scholar]
- Beeker, L.Y.; Pringle, A.M.; Pearce, J.M. Open-source parametric 3-D printed slot die system for thin film semiconductor processing. Addit. Manuf. 2018, 20, 90–100. [Google Scholar] [CrossRef]
- Jin, X.; Wang, F.; Wang, Z.; Yang, Y.; Chu, Z.; Guo, N.; Lv, X. Research on Micro-Mechanics Modelling of TPU-Modified Asphalt Mastic. Coatings 2022, 12, 1029. [Google Scholar] [CrossRef]
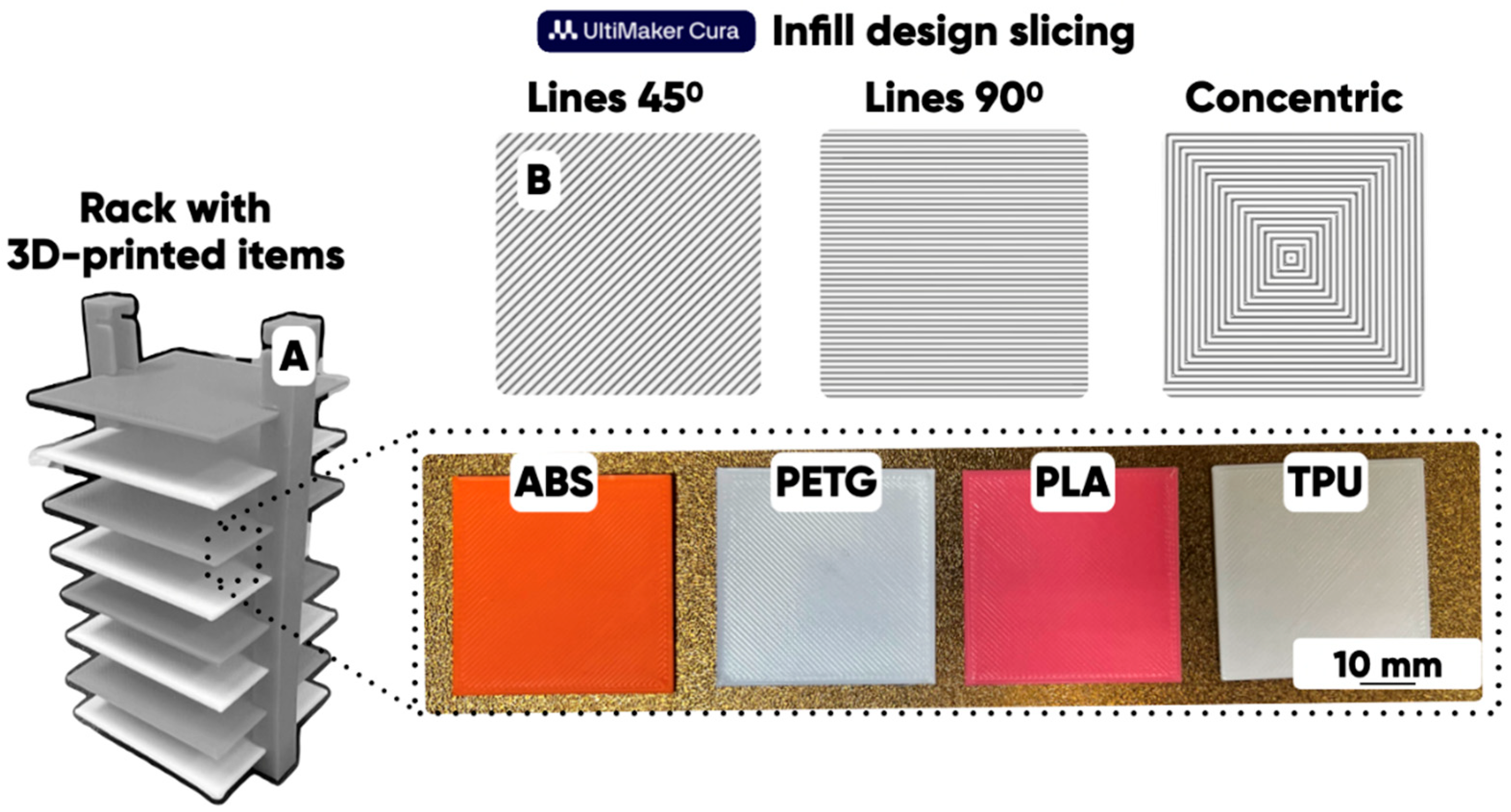

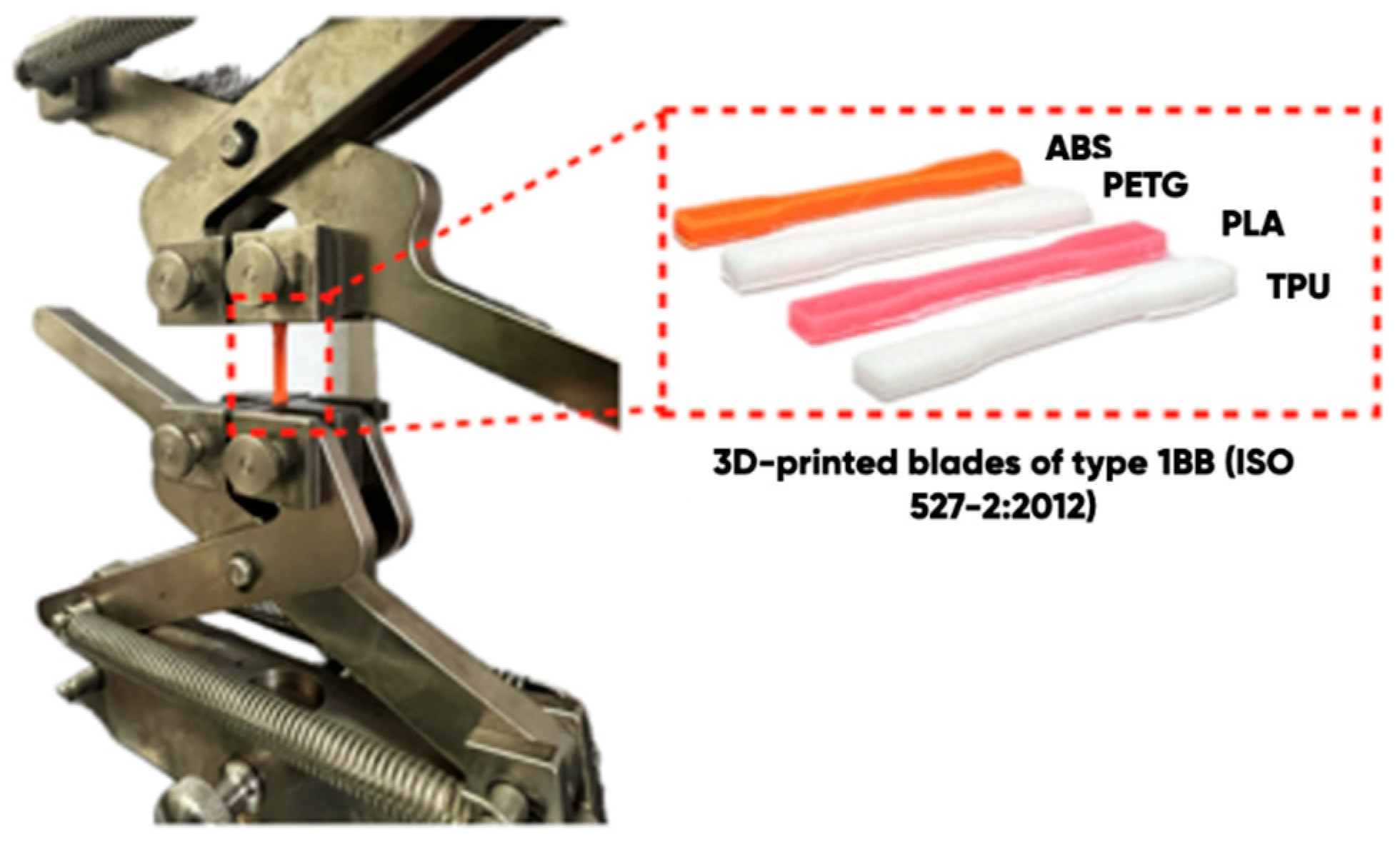



| Type of Polymer (Filament) | Manufacturer | 3D Printing Options | ||
|---|---|---|---|---|
| Nozzle Temperature, °C | Printing Platform Temperature, °C | Printing Speed, mm/min | ||
| Acrylonitrile, butadiene and styrene copolymer (ABS) | Shenzhen Esun Industrial Co., Shenzhen, China | 250 | 80 | 40 |
| Polylactide (PLA) | 210 | 50 | 40 | |
| Polyethylene Terephthalate Glycol (PETG) | 235 | 60 | 40 | |
| Thermopolastic polyurethane (TPU) | U3print, Moscow, Russia | 210 | 60 | 30 |
| 3D-Printed Product Surface Macroscopic Textural Design | Wetting Edge Angle, ° | ||||
|---|---|---|---|---|---|
 | 74 ± 7 | 46 ± 6 | 35 ± 4 | 24 ± 2 | 11 ± 2 |
 | 76 ± 8 | 59 ± 6 | 31 ± 3 | 12 ± 1 | 16 ± 2 |
 | 116 ± 8 | 91 ± 8 | 19 ± 3 | 18 ± 3 | 1 ± 1 |
 | 106 ± 8 | 65 ± 7 | 52 ± 5 | 51 ± 1 | 1 ± 1 |
| 3D-Printed Product Surface Macroscopic Textural Design | Filament Type | Wetting Edge Angle, ° | ||||
|---|---|---|---|---|---|---|
| Fluorination Duration (0 h) | ||||||
 | TPU | 85 ± 9 | 48 ± 5 | 42 ± 4 | 40 ± 4 | 2.0 ± 0.5 |
| ABS | 67 ± 7 | 62 ± 6 | 37 ± 4 | 3.2 ± 0.5 | 35 ± 4 | |
| PETG | 79 ± 8 | 51 ± 5 | 41 ± 4 | 8.0 ± 0.8 | 33 ± 4 | |
| PLA | 74 ± 7 | 46 ± 5 | 35 ± 4 | 24 ± 2 | 11 ± 2 | |
| Fluorination duration (0.25 h) | ||||||
| TPU | 49 ± 5 | 22 ± 2 | 50 ± 5 | 15 ± 2 | 36 ± 4 | |
| ABS | 59 ± 6 | 19 ± 2 | 46 ± 5 | 27 ± 3 | 19 ± 2 | |
| PETG | 68 ± 7 | 28 ± 2 | 44 ± 4 | 34 ± 3 | 10 ± 2 | |
| PLA | 56 ± 6 | 26 ± 2 | 45 ± 4 | 19 ± 2 | 26 ± 3 | |
| Fluorination duration (0.5 h) | ||||||
| TPU | 59 ± 6 | 24 ± 2 | 44 ± 5 | 24 ± 3 | 20 ± 2 | |
| ABS | 51 ± 5 | 14 ± 2 | 49 ± 5 | 20 ± 2 | 29 ± 3 | |
| PETG | 58 ± 6 | 22 ± 2 | 45 ± 5 | 24 ± 3 | 21 ± 2 | |
| PLA | 63 ± 6 | 34 ± 3 | 41 ± 4 | 21 ± 2 | 19 ± 2 | |
| Fluorination duration (1 h) | ||||||
| TPU | 48 ± 5 | 20 ± 2 | 51 ± 5 | 15 ± 2 | 36 ± 4 | |
| ABS | 45 ± 5 | 18 ± 2 | 53 ± 5 | 13 ± 2 | 40 ± 4 | |
| PETG | 52 ± 5 | 23 ± 2 | 33 ± 4 | 25 ± 3 | 7.7 ± 0.8 | |
| PLA | 43 ± 4 | 17 ± 2 | 55 ± 6 | 12 ± 1 | 43 ± 4 | |
| Modification Duration, h | ||||
|---|---|---|---|---|
| ABS | PLA | TPU | PETG | |
| 0.25 | 4.1 ± 0.4 | 2.8 ± 0.2 | 2.3 ± 0.2 | 5.3 ± 0.4 |
| 0.5 | 17.5 ± 0.8 | 7.0 ± 0.5 | 8.0 ± 0.5 | 7.3 ± 0.5 |
| 1.0 | 21.9 ± 0.8 | 13.3 ± 0.5 | 22.2 ± 0.8 | 13.0 ± 0.5 |
| Abbreviation | Chemical Formula |
|---|---|
| ABS [88] | 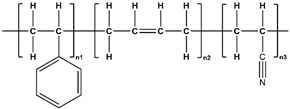 |
| PLA [89] |  |
| PETG [90] |  |
| TPU [91] |  |
| Fluorination Duration, Hour | ||||
|---|---|---|---|---|
| Initial | 0.25 | 0.5 | 1.0 | |
| C |  |  |  |  |
 |  |  |  | |
| O |  |  |  |  |
 |  |  |  | |
| N |  |  |  |  |
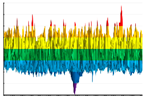 | 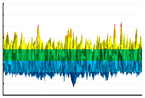 |  |  | |
| F | - |  |  |  |
 | 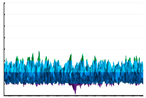 | 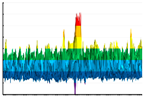 | ||
| Gas-Phase Fluorination Duration, Hour | 0.00 | 0.25 | 0.5 | 1.0 |
|---|---|---|---|---|
| Carbon Planar Distribution Quantitative Characteristics | ||||
| Average value of the variation coefficient | 0.13 | 0.14 | 0.12 | 0.21 |
| Standard deviation of the variation coefficient | 0.04 | 0.03 | 0.03 | 0.05 |
| Asymmetry of the variation coefficient distribution | 0.77 | 0.37 | 0.28 | 0.36 |
| Kurtosis of the variation coefficient distribution | 0.87 | 0.34 | 0.25 | 0.07 |
| Structural domain characteristic size (at 0.05 level), nm | 120 | 130 | 290 | 60 |
| Structural domain characteristic size (at 0.5 level), nm | 5000 | 5000 | 5000 | 5000 |
| Oxygen planar distribution quantitative characteristics | ||||
| Average value of the variation coefficient | 0.40 | 0.29 | 0.23 | 0.47 |
| Standard deviation of the variation coefficient | 0.09 | 0.07 | 0.05 | 0.11 |
| Asymmetry of the variation coefficient distribution | 0.53 | 0.34 | 0.32 | 0.29 |
| Kurtosis of the variation coefficient distribution | 0.46 | 0.14 | 0.29 | 0.05 |
| Structural domain characteristic size (at 0.05 level), nm | 40 | 40 | 100 | 40 |
| Structural domain characteristic size (at 0.5 level), nm | 4640 | 4980 | 4990 | 3930 |
| Nitrogen planar distribution quantitative characteristics | ||||
| Average value of the variation coefficient | 0.89 | 0.78 | 0.66 | 0.66 |
| Standard deviation of the variation coefficient | 0.19 | 0.16 | 0.14 | 0.14 |
| Asymmetry of the variation coefficient distribution | 0.49 | 0.50 | 0.38 | 0.39 |
| Kurtosis of the variation coefficient distribution | 0.78 | 0.67 | 0.64 | 0.34 |
| Structural domain characteristic size (at 0.05 level), nm | 50 | 40 | 40 | 40 |
| Structural domain characteristic size (at 0.5 level), nm | 510 | 830 | 1710 | 1760 |
| Fluorine planar distribution quantitative characteristics | ||||
| Average value of the variation coefficient | - | 0.70 | 0.87 | 1.16 |
| Standard deviation of the variation coefficient | - | 0.20 | 0.19 | 0.29 |
| Asymmetry of the variation coefficient distribution | - | 0.78 | 0.57 | 1.13 |
| Kurtosis of the variation coefficient distribution | - | 1.06 | 0.90 | 3.43 |
| Structural domain characteristic size (at 0.05 level), nm | - | 40 | 40 | 60 |
| Structural domain characteristic size (at 0.5 level), nm | - | 1940 | 550 | 120 |
| Fluorination Duration, Hour | C (at. %) | O (at. %) | N (at. %) | F (at. %) |
|---|---|---|---|---|
| 0 | 67.0 ± 0.8 | 25.0 ± 0.8 | 8.0 ± 0.1 | - |
| 0.25 | 66.0 ± 0.7 | 24.0 ± 0.7 | 8.0 ± 0.1 | 2 ± 0.1 |
| 0.5 | 66.0 ± 0.7 | 23.0 ± 0.6 | 7.0 ± 0.1 | 4 ± 0.2 |
| 1 | 66.0 ± 0.5 | 18.0 ± 0.4 | 10.0 ± 0.2 | 6 ± 0.4 |
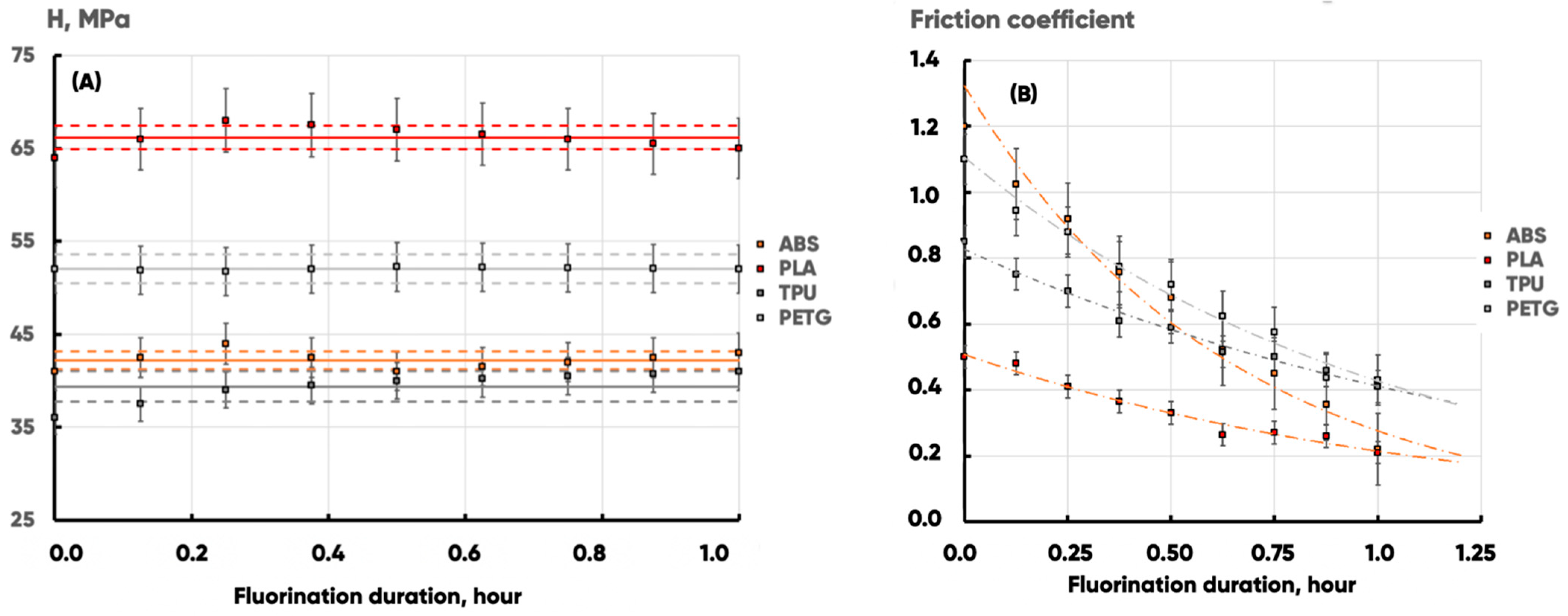
| Fluorination Duration, Hour | 3D-Printing Material | |||||||
|---|---|---|---|---|---|---|---|---|
| ABS | PLA | TPU | PETG | |||||
| H, MPa | H, MPa | H, MPa | H, MPa | |||||
| 0 | 41 ± 3 | 1.20 ± 0.10 | 64 ± 6 | 0.50 ± 0.05 | 36 ± 3 | 0.85 ± 0.08 | 52 ± 5 | 1.10 ± 0.10 |
| 0.25 | 44 ± 4 | 0.92 ± 0.09 | 68 ± 7 | 0.41 ± 0.05 | 39 ± 4 | 0.70 ± 0.07 | 52 ± 4 | 0.88 ± 0.09 |
| 0.5 | 41 ± 3 | 0.68 ± 0.07 | 67 ± 7 | 0.33 ± 0.04 | 40 ± 4 | 0.59 ± 0.06 | 52 ± 3 | 0.72 ± 0.08 |
| 1 | 43 ± 3 | 0.22 ± 0.02 | 65 ± 6 | 0.21 ± 0.02 | 41 ± 4 | 0.41 ± 0.04 | 52 ± 4 | 0.43 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doronin, F.; Rytikov, G.; Evdokimov, A.; Savel’ev, M.; Rudyak, Y.; Nazarov, V. Design of Functional Fluorine-Containing Coatings for 3D-Printed Items. Polymers 2025, 17, 2824. https://doi.org/10.3390/polym17212824
Doronin F, Rytikov G, Evdokimov A, Savel’ev M, Rudyak Y, Nazarov V. Design of Functional Fluorine-Containing Coatings for 3D-Printed Items. Polymers. 2025; 17(21):2824. https://doi.org/10.3390/polym17212824
Chicago/Turabian StyleDoronin, Fedor, Georgy Rytikov, Andrey Evdokimov, Mikhail Savel’ev, Yuriy Rudyak, and Victor Nazarov. 2025. "Design of Functional Fluorine-Containing Coatings for 3D-Printed Items" Polymers 17, no. 21: 2824. https://doi.org/10.3390/polym17212824
APA StyleDoronin, F., Rytikov, G., Evdokimov, A., Savel’ev, M., Rudyak, Y., & Nazarov, V. (2025). Design of Functional Fluorine-Containing Coatings for 3D-Printed Items. Polymers, 17(21), 2824. https://doi.org/10.3390/polym17212824








