Chitosan (Nano)formulations as Therapeutic Tools for Neurodegenerative Diseases: A Comprehensive Review
Abstract
1. Introduction
2. Neurodegenerative Diseases
2.1. Alzheimer’s Disease
2.2. Parkinson’s Disease
2.3. Multiple Sclerosis
2.4. Huntington’s Disease
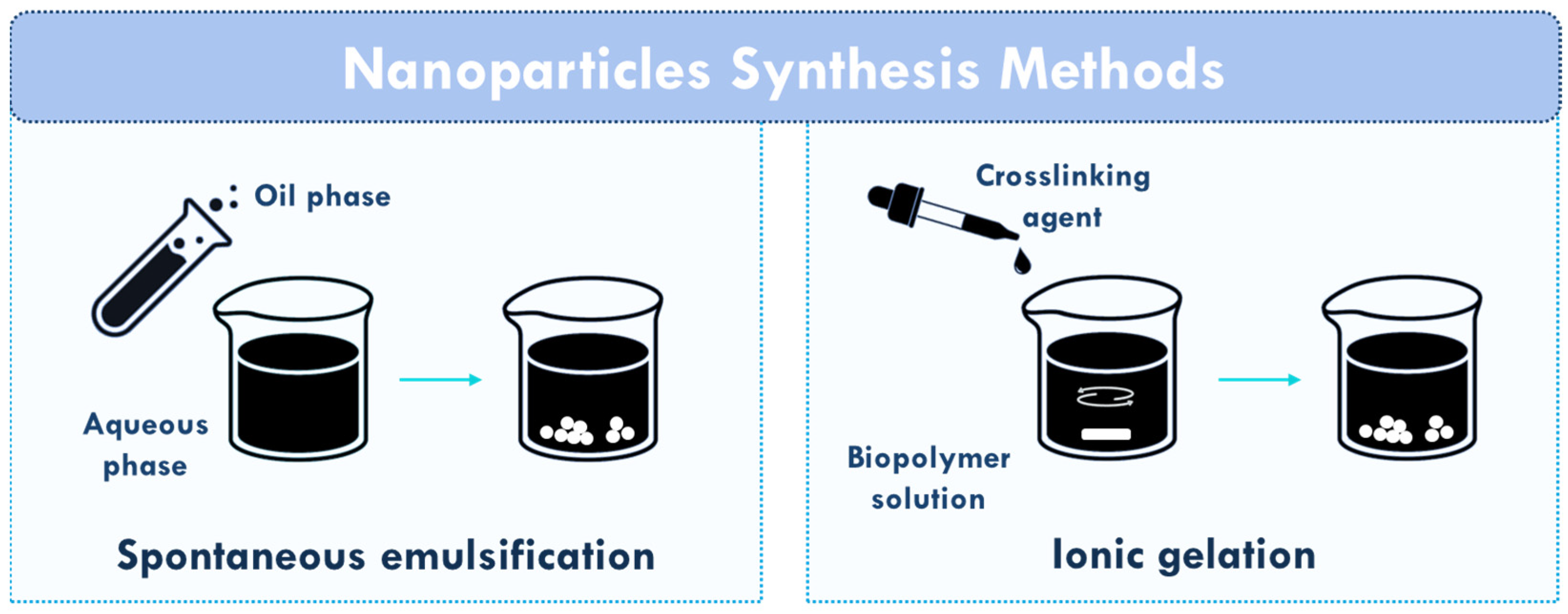
3. Chitosan (Nano)formulations for Neurodegenerative Diseases
3.1. Alzheimer’s Disease
3.2. Parkinson’s Disease
3.3. Multiple Sclerosis
3.4. Huntington’s Disease and Other Neurodegenerative Diseases
3.5. Mechanistic Insights into the Neuroprotective Effects of Chitosan
3.6. Advances, Comparative Analysis, and Translational Challenges
Critical Overview and Considerations for Clinical Translation
4. Conclusions and Future Perspectives
4.1. Conclusions
4.2. Future Directions and Clinical Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 6-OHDA | 6-hydroxydopamine |
| Aβ | Amyloid-β |
| AD | Alzheimer’s disease |
| APIs | Active pharmaceutical ingredients |
| APP | Amyloid precursor protein |
| BBB | Blood–brain barrier |
| BDNF | Brain-derived neurotrophic factor |
| BSA | Bovine serum albumin |
| CAT | Catalase |
| CN | Chitosan nanogel |
| CNS | Central nervous system |
| CP | Clobetasol propionate |
| CS | Chitosan |
| DLS | Dynamic light scattering |
| DNA | Deoxyribonucleic acid |
| DMF | Dimethyl fumarate |
| FESEM | Field emission scanning electron microscopy |
| FTIR | Fourier transform infrared spectroscopy |
| GBA | Glucocerebrosidase gene |
| GDNF | Glial cell-derived neurotrophic factor |
| GFP | Green fluorescent protein |
| GSH | Glutathione |
| HD | Huntington’s disease |
| HO-1 | Hemoxygenase-1 |
| HTT | Huntingtin gene |
| IA | Intraperitoneal administration |
| IL-6 | Interleukin-6 |
| INA | Intranasal administration |
| INFβ | Interferon-β |
| IFN-γ | Interferon-γ |
| IGF | Insulin-like growth factor |
| IVA | Intravenous administration |
| LRRK2 | Leucine-rich repeat kinase 2 gene |
| MAO-B | Monoamine oxidase-B |
| MBP | Myelin basic protein |
| MDA | Malondialdehyde |
| mHTT | Mutant huntingtin |
| MPP+ | 1-methyl-4-phenylpyridinium |
| MRI | Magnetic resonance imaging |
| MS | Multiple sclerosis |
| MSCs | Mesenchymal stem cells |
| NA | Not applicable |
| NFTs | Neurotrophic factors |
| NGF | Nerve growth factor |
| NPs | Nanoparticles |
| OA | Oral administration |
| PBS | Phosphate-buffered solution |
| PBMC | Peripheral blood mononuclear cells |
| PCCN | Platelet membrane-coated nanogel |
| PCL | Poly(caprolactone) |
| PD | Parkinson’s disease |
| pDNA | Plasmid DNA |
| PEG | Polyethylene glycol |
| PLA | Polylactic acid |
| PLGA | Poly(lactic-co-glycolic) acid |
| PS1 | Presenilin 1 |
| PS2 | Presenilin 2 |
| p-tau181 | Phosphorylated tau |
| RFP | Red fluorescent protein |
| RNA | Ribonucleic Acid |
| ROS | Reactive oxygen species |
| siRNAs | Small interfering RNAs |
| SNCA | α-synuclein gene |
| SOD | Superoxide dismutase |
| ThT | Thioflavin T |
| TNF-α | Tumour necrosis factor-α |
| t-tau | Total tau |
References
- Yacoubian, T.A. Neurodegenerative Disorders: Why Do We Need New Therapies? In Drug Discovery Approaches for the Treatment of Neurodegenerative Disorders; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–16. [Google Scholar]
- Zaib, S.; Javed, H.; Khan, I.; Jaber, F.; Sohail, A.; Zaib, Z.; Mehboob, T.; Tabassam, N.; Ogaly, H.A. Neurodegenerative Diseases: Their Onset, Epidemiology, Causes and Treatment. ChemistrySelect 2023, 8, e202300225. [Google Scholar] [CrossRef]
- WHO. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 23 September 2024).
- Zhang, Y.; Poon, K.; Masonsong, G.S.P.; Ramaswamy, Y.; Singh, G. Sustainable Nanomaterials for Biomedical Applications. Pharmaceutics 2023, 15, 922. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I. Nanomaterials and Their Classification. In EMR/ESR/EPR Spectroscopy for Characterization of Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2017; pp. 3–45. [Google Scholar]
- Kreyling, W.G.; Semmler-Behnke, M.; Chaudhry, Q. A Complementary Definition of Nanomaterial. Nano Today 2010, 5, 165–168. [Google Scholar] [CrossRef]
- Biswas, M.C.; Jony, B.; Nandy, P.K.; Chowdhury, R.A.; Halder, S.; Kumar, D.; Ramakrishna, S.; Hassan, M.; Ahsan, M.A.; Hoque, M.E.; et al. Recent Advancement of Biopolymers and Their Potential Biomedical Applications. J. Polym. Environ. 2022, 30, 51–74. [Google Scholar] [CrossRef]
- Kravanja, G.; Primožič, M.; Knez, Ž.; Leitgeb, M. Chitosan-Based (Nano)Materials for Novel Biomedical Applications. Molecules 2019, 24, 1960–1982. [Google Scholar] [CrossRef]
- Iyer, M.; Elangovan, A.; Sennimalai, R.; Babu, H.W.S.; Thiruvenkataswamy, S.; Krishnan, J.; Yadav, M.K.; Gopalakrishnan, A.V.; Narayanasamy, A.; Vellingiri, B. Chitosan—An Alternative Drug Delivery Approach for Neurodegenerative Diseases. Carbohydr. Polym. Technol. Appl. 2024, 7, 100460. [Google Scholar] [CrossRef]
- Khodaverdi, K.; Bakhshi, A.; Mozafari, M.R.; Naghib, S.M. A Review of Chitosan-Based Nanocarriers as Drug Delivery Systems for Brain Diseases: Critical Challenges, Outlooks and Promises. Int. J. Biol. Macromol. 2024, 278, 134962. [Google Scholar] [CrossRef]
- Asil, S.M.; Ahlawat, J.; Barroso, G.G.; Narayan, M. Nanomaterial Based Drug Delivery Systems for the Treatment of Neurodegenerative Diseases. Biomater. Sci. 2020, 8, 4109–4128. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Cacabelos, R.; Cacabelos, P.; Carril, J.C. Epigenetics and Pharmacoepigenetics of Age-Related Neurodegenerative Disorders. In Pharmacoepigenetics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 903–950. [Google Scholar]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s Disease. Nat. Rev. Dis. Prim. 2015, 1, 15056–15073. [Google Scholar] [CrossRef] [PubMed]
- Self, W.K.; Holtzman, D.M. Emerging Diagnostics and Therapeutics for Alzheimer Disease. Nat. Med. 2023, 29, 2187–2199. [Google Scholar] [CrossRef]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s Disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer Disease. Nat. Rev. Dis. Prim. 2021, 7, 33–53. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson Disease. Nat. Rev. Dis. Prim. 2017, 3, 17013–17033. [Google Scholar] [CrossRef]
- Yarnall, A.; Archibald, N.; Burn, D. Parkinson’s Disease. Med. Baltim. 2012, 40, 529–535. [Google Scholar] [CrossRef]
- Balestrino, R.; Schapira, A.H.V. Parkinson Disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef]
- Ball, N.; Teo, W.-P.; Chandra, S.; Chapman, J. Parkinson’s Disease and the Environment. Front. Neurol. 2019, 10, 421551–421558. [Google Scholar] [CrossRef] [PubMed]
- Menozzi, E.; Schapira, A.H.V. Prospects for Disease Slowing in Parkinson Disease. Annu. Rev. Pharmacol. Toxicol. 2024, 65, 237–258. [Google Scholar] [CrossRef] [PubMed]
- Gironi, M.; Arnò, C.; Comi, G.; Penton-Rol, G.; Furlan, R. Multiple Sclerosis and Neurodegenerative Diseases. In Immune Rebalancing; Elsevier: Amsterdam, The Netherlands, 2016; pp. 63–84. [Google Scholar]
- Hauser, S.L.; Cree, B.A.C. Treatment of Multiple Sclerosis: A Review. Am. J. Med. 2020, 133, 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Mey, G.M.; Mahajan, K.R.; DeSilva, T.M. Neurodegeneration in Multiple Sclerosis. WIREs Mech. Dis. 2023, 15, e1583. [Google Scholar] [CrossRef]
- Ziemssen, T.; Akgün, K.; Brück, W. Molecular Biomarkers in Multiple Sclerosis. J. Neuroinflam. 2019, 16, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, S.J.; Flower, M.D.; Ross, C.A.; Wild, E.J. Huntington Disease: New Insights into Molecular Pathogenesis and Therapeutic Opportunities. Nat. Rev. Neurol. 2020, 16, 529–546. [Google Scholar] [CrossRef]
- McColgan, P.; Tabrizi, S.J. Huntington’s Disease: A Clinical Review. Eur. J. Neurol. 2018, 25, 24–34. [Google Scholar] [CrossRef]
- Kim, A.; Lalonde, K.; Truesdell, A.; Gomes Welter, P.; Brocardo, P.S.; Rosenstock, T.R.; Gil-Mohapel, J. New Avenues for the Treatment of Huntington’s Disease. Int. J. Mol. Sci. 2021, 22, 8363. [Google Scholar] [CrossRef]
- Bates, G.P.; Dorsey, R.; Gusella, J.F.; Hayden, M.R.; Kay, C.; Leavitt, B.R.; Nance, M.; Ross, C.A.; Scahill, R.I.; Wetzel, R.; et al. Huntington Disease. Nat. Rev. Dis. Prim. 2015, 1, 15005. [Google Scholar] [CrossRef]
- Ojeda-Hernández, D.D.; Canales-Aguirre, A.A.; Matias-Guiu, J.; Gomez-Pinedo, U.; Mateos-Díaz, J.C. Potential of Chitosan and Its Derivatives for Biomedical Applications in the Central Nervous System. Front. Bioeng. Biotechnol. 2020, 8, 389. [Google Scholar] [CrossRef]
- Caprifico, A.E.; Foot, P.J.S.; Polycarpou, E.; Calabrese, G. Overcoming the Blood-Brain Barrier: Functionalised Chitosan Nanocarriers. Pharmaceutics 2020, 12, 1013–1032. [Google Scholar] [CrossRef]
- Cortés, H.; Alcalá-Alcalá, S.; Caballero-Florán, I.H.; Bernal-Chávez, S.A.; Ávalos-Fuentes, A.; González-Torres, M.; González-Del Carmen, M.; Figueroa-González, G.; Reyes-Hernández, O.D.; Floran, B.; et al. A Reevaluation of Chitosan-Decorated Nanoparticles to Cross the Blood-Brain Barrier. Membranes 2020, 10, 212–232. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Wang, W.; Wang, S.; Zhang, L.; Guo, Y. An Overview of the Protective Effects of Chitosan and Acetylated Chitosan Oligosaccharides against Neuronal Disorders. Mar. Drugs 2017, 15, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Batra, B.; Pundir, C.S. An Amperometric Glutamate Biosensor Based on Immobilization of Glutamate Oxidase onto Carboxylated Multiwalled Carbon Nanotubes/Gold Nanoparticles/Chitosan Composite Film Modified Au Electrode. Biosens. Bioelectron. 2013, 47, 496–501. [Google Scholar] [CrossRef]
- Nazem, H.; Mohsenifar, A.; Majdi, S. Chitosan-Myristate Nanogel as an Artificial Chaperone Protects Neuroserpin from Misfolding. Adv. Biomed. Res. 2016, 5, 170. [Google Scholar] [CrossRef]
- Aluani, D.; Tzankova, V.; Yordanov, Y.; Kondeva-Burdina, M.; Yoncheva, K. In Vitro Protective Effects of Encapsulated Quercetin in Neuronal Models of Oxidative Stress Injury. Biotechnol. Biotechnol. Equip. 2017, 31, 1055–1063. [Google Scholar] [CrossRef]
- Sanchez-Ramos, J.; Song, S.; Kong, X.; Foroutan, P.; Martinez, G.; Dominguez-Viqueria, W.; Mohapatra, S.; Mohapatra, S.; Haraszti, R.A.; Khvorova, A.; et al. Chitosan-Mangafodipir Nanoparticles Designed for Intranasal Delivery of SiRNA and DNA to Brain. J. Drug Deliv. Sci. Technol. 2018, 43, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Fachel, F.N.S.; Medeiros-Neves, B.; Dal Prá, M.; Schuh, R.S.; Veras, K.S.; Bassani, V.L.; Koester, L.S.; Henriques, A.T.; Braganhol, E.; Teixeira, H.F. Box-Behnken Design Optimization of Mucoadhesive Chitosan-Coated Nanoemulsions for Rosmarinic Acid Nasal Delivery—In Vitro Studies. Carbohydr. Polym. 2018, 199, 572–582. [Google Scholar] [CrossRef]
- Rassu, G.; Porcu, E.P.; Fancello, S.; Obinu, A.; Senes, N.; Galleri, G.; Migheli, R.; Gavini, E.; Giunchedi, P. Intranasal Delivery of Genistein-Loaded Nanoparticles as a Potential Preventive System against Neurodegenerative Disorders. Pharmaceutics 2018, 11, 8–26. [Google Scholar] [CrossRef]
- Casadomé-Perales, Á.; De Matteis, L.; Alleva, M.; Infantes-Rodríguez, C.; Palomares-Pérez, I.; Saito, T.; Saido, T.C.; Esteban, J.A.; Nebreda, A.R.; de la Fuente, J.M.; et al. Inhibition of P38 MAPK in the Brain through Nasal Administration of P38 Inhibitor Loaded in Chitosan Nanocapsules. Nanomedicine 2019, 14, 2409–2422. [Google Scholar] [CrossRef]
- Mathew, S.A.; Praveena, P.; Dhanavel, S.; Manikandan, R.; Senthilkumar, S.; Stephen, A. Luminescent Chitosan/Carbon Dots as an Effective Nano-Drug Carrier for Neurodegenerative Diseases. RSC Adv. 2020, 10, 24386–24396. [Google Scholar] [CrossRef]
- Don, T.-M.; Chang, W.-J.; Jheng, P.-R.; Huang, Y.-C.; Chuang, E.-Y. Curcumin-Laden Dual-Targeting Fucoidan/Chitosan Nanocarriers for Inhibiting Brain Inflammation via Intranasal Delivery. Int. J. Biol. Macromol. 2021, 181, 835–846. [Google Scholar] [CrossRef]
- Mathew, S.A.; Prakash, P.A.; Jaabir, M.S.M.; Dhanavel, S.; Manikandan, R.; Stephen, A. Dopamine-Conjugated CuS/Chitosan Nanocomposite for Targeted Photothermal Drug Delivery: In Vitro Cytotoxicity Study to Establish Bio-Compatibility. J. Drug Deliv. Sci. Technol. 2021, 61, 102193. [Google Scholar] [CrossRef]
- Karavelioglu, Z.; Cakir-Koc, R. Preparation of Chitosan Nanoparticles as Ginkgo Biloba Extract Carrier: In Vitro Neuroprotective Effect on Oxidative Stress-Induced Human Neuroblastoma Cells (SH-SY5Y). Int. J. Biol. Macromol. 2021, 192, 675–683. [Google Scholar] [CrossRef]
- Clementino, A.R.; Marchi, C.; Pozzoli, M.; Bernini, F.; Zimetti, F.; Sonvico, F. Anti-Inflammatory Properties of Statin-Loaded Biodegradable Lecithin/Chitosan Nanoparticles: A Step Toward Nose-to-Brain Treatment of Neurodegenerative Diseases. Front. Pharmacol. 2021, 12, 716380. [Google Scholar] [CrossRef] [PubMed]
- Bashir, D.J.; Manzoor, S.; Khan, I.A.; Bashir, M.; Agarwal, N.B.; Rastogi, S.; Arora, I.; Samim, M. Nanonization of Magnoflorine-Encapsulated Novel Chitosan–Collagen Nanocapsules for Neurodegenerative Diseases: In Vitro Evaluation. ACS Omega 2022, 7, 6472–6480. [Google Scholar] [CrossRef] [PubMed]
- Sepasi, T.; Ghadiri, T.; Ebrahimi-Kalan, A.; Bani, F.; Talebi, M.; Rahbarghazi, R.; Khodakarimi, S.; Beyrampour-Basmenj, H.; Seidi, K.; Abbaspour-Ravasjani, S.; et al. CDX-Modified Chitosan Nanoparticles Remarkably Reduce Therapeutic Dose of Fingolimod in the EAE Model of Mice. Int. J. Pharm. 2023, 636, 122815–122827. [Google Scholar] [CrossRef]
- Songjiang, Z.; Lixiang, W. Amyloid-Beta Associated with Chitosan Nano-Carrier Has Favorable Immunogenicity and Permeates the BBB. AAPS PharmSciTech 2009, 10, 900–905. [Google Scholar] [CrossRef]
- Wilson, B.; Samanta, M.K.; Santhi, K.; Kumar, K.P.S.; Ramasamy, M.; Suresh, B. Chitosan Nanoparticles as a New Delivery System for the Anti-Alzheimer Drug Tacrine. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 144–152. [Google Scholar] [CrossRef]
- Wilson, B.; Samanta, M.K.; Muthu, M.S.; Vinothapooshan, G. Design and Evaluation of Chitosan Nanoparticles as Novel Drug Carrier for the Delivery of Rivastigmine to Treat Alzheimer’s Disease. Ther. Deliv. 2011, 2, 599–609. [Google Scholar] [CrossRef]
- Bhavna, S.; Ali, M.; Bhatnagar, A.; Baboota, S.; Sahni, J.K.; Ali, J. Design, Development, Optimization and Characterization of Donepezil Loaded Chitosan Nanoparticles for Brain Targeting to Treat Alzheimer’s Disease. Sci. Adv. Mater. 2014, 6, 720–735. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.R.; Etman, S.M.; Abdelmonsif, D.A.; Abdallah, O.Y. Intranasal Piperine-Loaded Chitosan Nanoparticles as Brain-Targeted Therapy in Alzheimer’s Disease: Optimization, Biological Efficacy, and Potential Toxicity. J. Pharm. Sci. 2015, 104, 3544–3556. [Google Scholar] [CrossRef]
- Hanafy, A.S.; Farid, R.M.; ElGamal, S.S. Complexation as an Approach to Entrap Cationic Drugs into Cationic Nanoparticles Administered Intranasally for Alzheimer’s Disease Management: Preparation and Detection in Rat Brain. Drug Dev. Ind. Pharm. 2015, 41, 2055–2068. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, A.S.; Farid, R.M.; Helmy, M.W.; ElGamal, S.S. Pharmacological, Toxicological and Neuronal Localization Assessment of Galantamine/Chitosan Complex Nanoparticles in Rats: Future Potential Contribution in Alzheimer’s Disease Management. Drug Deliv. 2016, 23, 3111–3122. [Google Scholar] [CrossRef]
- AnjiReddy, K.; Karpagam, S. Chitosan Nanofilm and Electrospun Nanofiber for Quick Drug Release in the Treatment of Alzheimer’s Disease: In Vitro and in Vivo Evaluation. Int. J. Biol. Macromol. 2017, 105, 131–142. [Google Scholar] [CrossRef]
- Yang, R.; Zheng, Y.; Wang, Q.; Zhao, L. Curcumin-Loaded Chitosan–Bovine Serum Albumin Nanoparticles Potentially Enhanced Aβ 42 Phagocytosis and Modulated Macrophage Polarization in Alzheimer’s Disease. Nanoscale Res. Lett. 2018, 13, 330. [Google Scholar] [CrossRef]
- Jha, A.; Ghormade, V.; Kolge, H.; Paknikar, K.M. Dual Effect of Chitosan-Based Nanoparticles on the Inhibition of β-Amyloid Peptide Aggregation and Disintegration of the Preformed Fibrils. J. Mater. Chem. B 2019, 7, 3362–3373. [Google Scholar] [CrossRef]
- Dhas, N.; Mehta, T. Cationic Biopolymer Functionalized Nanoparticles Encapsulating Lutein to Attenuate Oxidative Stress in Effective Treatment of Alzheimer’s Disease: A Non-Invasive Approach. Int. J. Pharm. 2020, 586, 119553–119566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, S.; Wong, L.R.; Xie, H.; Ho, P.C.-L. In Vitro and In Vivo Comparison of Curcumin-Encapsulated Chitosan-Coated Poly(Lactic-Co-Glycolic Acid) Nanoparticles and Curcumin/Hydroxypropyl-β-Cyclodextrin Inclusion Complexes Administered Intranasally as Therapeutic Strategies for Alzheimer’s Disease. Mol. Pharm. 2020, 17, 4256–4269. [Google Scholar] [CrossRef]
- Wilson, B.; Alobaid, B.N.M.; Geetha, K.M.; Jenita, J.L. Chitosan Nanoparticles to Enhance Nasal Absorption and Brain Targeting of Sitagliptin to Treat Alzheimer’s Disease. J. Drug Deliv. Sci. Technol. 2021, 61, 102176–102181. [Google Scholar] [CrossRef]
- Zameer, S.; Ali, J.; Vohora, D.; Najmi, A.K.; Akhtar, M. Development, Optimisation and Evaluation of Chitosan Nanoparticles of Alendronate against Alzheimer’s Disease in Intracerebroventricular Streptozotocin Model for Brain Delivery. J. Drug Target. 2021, 29, 199–216. [Google Scholar] [CrossRef]
- Saini, S.; Sharma, T.; Jain, A.; Kaur, H.; Katare, O.P.; Singh, B. Systematically Designed Chitosan-Coated Solid Lipid Nanoparticles of Ferulic Acid for Effective Management of Alzheimer’s Disease: A Preclinical Evidence. Colloids Surfaces B Biointerfaces 2021, 205, 111838–111849. [Google Scholar] [CrossRef] [PubMed]
- Dhas, N.; Mehta, T. Intranasal Delivery of Chitosan Decorated PLGA Core/Shell Nanoparticles Containing Flavonoid to Reduce Oxidative Stress in the Treatment of Alzheimer’s Disease. J. Drug Deliv. Sci. Technol. 2021, 61, 102242. [Google Scholar] [CrossRef]
- Nojoki, F.; Ebrahimi-Hosseinzadeh, B.; Hatamian-Zarmi, A.; Khodagholi, F.; Khezri, K. Design and Development of Chitosan-Insulin-Transfersomes (Transfersulin) as Effective Intranasal Nanovesicles for the Treatment of Alzheimer’s Disease: In Vitro, in Vivo, and Ex Vivo Evaluations. Biomed. Pharmacother. 2022, 153, 113450–113460. [Google Scholar] [CrossRef]
- Dubey, A.; Dhas, N.; Naha, A.; Rani, U.; Gs, R.; Shetty, A.; Shetty, C.R.; Hebbar, S. Cationic Biopolymer Decorated Asiatic Acid and Centella Asiatica Extract Incorporated Liposomes for Treating Early-Stage Alzheimer’s Disease: An In-Vitro and In-Vivo Investigation. F1000Research 2022, 11, 1535–1564. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.; Sayed, N.S.E.; Youssef, N.A.H.A.; Gaafar, P.M.E.; Mousa, M.R.; Fayez, A.M.; Elsheikh, M.A. Novel Luteolin-Loaded Chitosan Decorated Nanoparticles for Brain-Targeting Delivery in a Sporadic Alzheimer’s Disease Mouse Model: Focus on Antioxidant, Anti-Inflammatory, and Amyloidogenic Pathways. Pharmaceutics 2022, 14, 1003–1028. [Google Scholar] [CrossRef]
- Zhang, X.; Kang, X.; Du, L.; Zhang, L.; Huang, Y.; Wang, J.; Wang, S.; Chang, Y.; Liu, Y.; Zhao, Y. Tanshinone IIA Loaded Chitosan Nanoparticles Decrease Toxicity of β-Amyloid Peptide in a Caenorhabditis Elegans Model of Alzheimer’s Disease. Free Radic. Biol. Med. 2022, 193, 81–94. [Google Scholar] [CrossRef]
- Saleem, S.; Banerjee, R.; Rajesh Kannan, R. Chrysin-Loaded Chitosan Nanoparticle-Mediated Neuroprotection in Aβ 1–42 -Induced Neurodegenerative Conditions in Zebrafish. ACS Chem. Neurosci. 2022, 13, 2017–2034. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.; Mohamed, J.; Ruby, J.; Kanthiah, S.; Alanazi, Y.; Majrashi, K.; Alshahrani, S.; Eladl, M.; Alaryani, F.; El-Sherbiny, M.; et al. Preparation of Memantine-Loaded Chitosan Nanocrystals: In Vitro and Ex Vivo Toxicity Analysis. Crystals 2022, 13, 21–33. [Google Scholar] [CrossRef]
- Georgieva, D.; Nikolova, D.; Vassileva, E.; Kostova, B. Chitosan-Based Nanoparticles for Targeted Nasal Galantamine Delivery as a Promising Tool in Alzheimer’s Disease Therapy. Pharmaceutics 2023, 15, 829–841. [Google Scholar] [CrossRef]
- Bashir, D.J.; Manzoor, S.; Sarfaraj, M.; Afzal, S.M.; Bashir, M.; Nidhi; Rastogi, S.; Arora, I.; Samim, M. Magnoflorine-Loaded Chitosan Collagen Nanocapsules Ameliorate Cognitive Deficit in Scopolamine-Induced Alzheimer’s Disease-like Conditions in a Rat Model by Downregulating IL-1β, IL-6, TNF-α, and Oxidative Stress and Upregulating Brain-Derived Neurotrophi. ACS Omega 2023, 8, 2227–2236. [Google Scholar] [CrossRef]
- Shafqat, O.; Rehman, Z.; Shah, M.M.; Ali, S.H.B.; Jabeen, Z.; Rehman, S. Synthesis, Structural Characterization and in Vitro Pharmacological Properties of Betanin-Encapsulated Chitosan Nanoparticles. Chem. Biol. Interact. 2023, 370, 110291. [Google Scholar] [CrossRef]
- Hard, S.A.A.A.; Shivakumar, H.N.; Redhwan, M.A.M. Development and Optimization of In-Situ Gel Containing Chitosan Nanoparticles for Possible Nose-to-Brain Delivery of Vinpocetine. Int. J. Biol. Macromol. 2023, 253, 127217–127230. [Google Scholar] [CrossRef]
- Mohammadbaghban, E.; Taravati, A.; Najafzadehvarzi, H.; Khaleghzadeh-Ahangar, H.; Tohidi, F. Oral Administration of Encapsulated Catechin in Chitosan-alginate Nanoparticles Improves Cognitive Function and Neurodegeneration in an Aluminum Chloride-induced Rat Model of Alzheimer’s Disease. Physiol. Rep. 2024, 12, e16095. [Google Scholar] [CrossRef] [PubMed]
- Trapani, A.; De Giglio, E.; Cafagna, D.; Denora, N.; Agrimi, G.; Cassano, T.; Gaetani, S.; Cuomo, V.; Trapani, G. Characterization and Evaluation of Chitosan Nanoparticles for Dopamine Brain Delivery. Int. J. Pharm. 2011, 419, 296–307. [Google Scholar] [CrossRef]
- Md, S.; Khan, R.A.; Mustafa, G.; Chuttani, K.; Baboota, S.; Sahni, J.K.; Ali, J. Bromocriptine Loaded Chitosan Nanoparticles Intended for Direct Nose to Brain Delivery: Pharmacodynamic, Pharmacokinetic and Scintigraphy Study in Mice Model. Eur. J. Pharm. Sci. 2013, 48, 393–405. [Google Scholar] [CrossRef]
- Ghamami, S.; Golzani, M.; Lashgari, A. New Inorganic-Based Nanohybrids of Layered Zinc Hydroxide/Parkinson’s Disease Drug and Its Chitosan Biopolymer Nanocarriers with Controlled Release Rate. J. Incl. Phenom. Macrocycl. Chem. 2016, 86, 67–78. [Google Scholar] [CrossRef]
- Gartziandia, O.; Herrán, E.; Ruiz-Ortega, J.A.; Miguelez, C.; Igartua, M.; Lafuente, J.V.; Pedraz, J.L.; Ugedo, L.; Hernández, R.M. Intranasal Administration of Chitosan-Coated Nanostructured Lipid Carriers Loaded with GDNF Improves Behavioral and Histological Recovery in a Partial Lesion Model of Parkinson’s Disease. J. Biomed. Nanotechnol. 2016, 12, 2220–2280. [Google Scholar] [CrossRef]
- Ahmad, N. Rasagiline-Encapsulated Chitosan-Coated PLGA Nanoparticles Targeted to the Brain in the Treatment of Parkinson’s Disease. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 677–690. [Google Scholar] [CrossRef]
- Tan, J.; Saifullah, B.; Kura, A.; Fakurazi, S.; Hussein, M. Incorporation of Levodopa into Biopolymer Coatings Based on Carboxylated Carbon Nanotubes for PH-Dependent Sustained Release Drug Delivery. Nanomaterials 2018, 8, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Wairkar, S.; Sridhar, V.; Gaud, R. Pramipexole Dihydrochloride Loaded Chitosan Nanoparticles for Nose to Brain Delivery: Development, Characterization and in Vivo Anti-Parkinson Activity. Int. J. Biol. Macromol. 2018, 109, 27–35. [Google Scholar] [CrossRef]
- Rukmangathen, R.; Yallamalli, I.M.; Yalavarthi, P.R. Biopharmaceutical Potential of Selegiline Loaded Chitosan Nanoparticles in the Management of Parkinson’s Disease. Curr. Drug Discov. Technol. 2019, 16, 417–425. [Google Scholar] [CrossRef]
- Ahlawat, J.; Deemer, E.M.; Narayan, M. Chitosan Nanoparticles Rescue Rotenone-Mediated Cell Death. Materials 2019, 12, 1176–1188. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, N.; Zeng, Z.; Huang, J.; Xiang, Z.; Guan, Y.-Q. Neuroprotective Effect of Chitosan Nanoparticle Gene Delivery System Grafted with Acteoside (ACT) in Parkinson’s Disease Models. J. Mater. Sci. Technol. 2020, 43, 197–207. [Google Scholar] [CrossRef]
- Chatzitaki, A.-T.; Jesus, S.; Karavasili, C.; Andreadis, D.; Fatouros, D.G.; Borges, O. Chitosan-Coated PLGA Nanoparticles for the Nasal Delivery of Ropinirole Hydrochloride: In Vitro and Ex Vivo Evaluation of Efficacy and Safety. Int. J. Pharm. 2020, 589, 119776–119787. [Google Scholar] [CrossRef] [PubMed]
- Bhattamisra, S.K.; Shak, A.T.; Xi, L.W.; Safian, N.H.; Choudhury, H.; Lim, W.M.; Shahzad, N.; Alhakamy, N.A.; Anwer, M.K.; Radhakrishnan, A.K.; et al. Nose to Brain Delivery of Rotigotine Loaded Chitosan Nanoparticles in Human SH-SY5Y Neuroblastoma Cells and Animal Model of Parkinson’s Disease. Int. J. Biol. Macromol. 2020, 579, 119148–119158. [Google Scholar] [CrossRef]
- Nehal, N.; Nabi, B.; Rehman, S.; Pathak, A.; Iqubal, A.; Khan, S.A.; Yar, M.S.; Parvez, S.; Baboota, S.; Ali, J. Chitosan Coated Synergistically Engineered Nanoemulsion of Ropinirole and Nigella Oil in the Management of Parkinson’s Disease: Formulation Perspective and In Vitro and In Vivo Assessment. Int. J. Biol. Macromol. 2021, 167, 605–619. [Google Scholar] [CrossRef]
- Sardoiwala, M.N.; Karmakar, S.; Choudhury, S.R. Chitosan Nanocarrier for FTY720 Enhanced Delivery Retards Parkinson’s Disease via PP2A-EzH2 Signaling in Vitro and Ex Vivo. Carbohydr. Polym. 2021, 254, 117435. [Google Scholar] [CrossRef]
- Darwish, W.M.; Bayoumi, N.A.; Ebeid, N.H. Biocompatible Mucoadhesive Nanoparticles for Brain Targeting of Ropinirole Hydrochloride: Formulations, Radiolabeling and Biodistribution. Biopolymers 2022, 113, e23489. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, S.; Fracchiolla, G.; Cometa, S.; Perna, F.M.; Quivelli, A.F.; Trapani, G.; Daniello, V.; Nobile, C.; Hossain, M.N.; Trapani, A.; et al. Carboxymethyl Chitosan Dopamine Conjugates: Synthesis and Evaluation for Intranasal Anti Parkinson Therapy. Int. J. Biol. Macromol. 2023, 253, 127174. [Google Scholar] [CrossRef]
- Hassan, D.M.; El-Kamel, A.H.; Allam, E.A.; Bakr, B.A.; Ashour, A.A. Chitosan-Coated Nanostructured Lipid Carriers for Effective Brain Delivery of Tanshinone IIA in Parkinson’s Disease: Interplay between Nuclear Factor-Kappa β and Cathepsin B. Drug Deliv. Transl. Res. 2024, 14, 400–417. [Google Scholar] [CrossRef]
- Alotaibi, B.S.; Mohamed, A.A.-R.; Abd-Elhakim, Y.M.; Noreldin, A.E.; Elhamouly, M.; Khamis, T.; El-Far, A.H.; Alosaimi, M.E.; Dahran, N.; Alqahtani, L.S.; et al. Exploring the Link between Pyrethroids Exposure and Dopaminergic Degeneration through Morphometric, Immunofluorescence, and in-Silico Approaches: The Therapeutic Role of Chitosan-Encapsulated Curcumin Nanoparticles. Front. Pharmacol. 2024, 15, 1388784. [Google Scholar] [CrossRef]
- Hoveizi, E.; Tavakol, S.; Ebrahimi-Barough, S. Neuroprotective Effect of Transplanted Neural Precursors Embedded on PLA/CS Scaffold in an Animal Model of Multiple Sclerosis. Mol. Neurobiol. 2015, 51, 1334–1342. [Google Scholar] [CrossRef]
- Youssef, A.E.H.; Dief, A.E.; El Azhary, N.M.; Abdelmonsif, D.A.; El-fetiany, O.S. LINGO-1 SiRNA Nanoparticles Promote Central Remyelination in Ethidium Bromide-Induced Demyelination in Rats. J. Physiol. Biochem. 2019, 75, 89–99. [Google Scholar] [CrossRef] [PubMed]
- González, L.F.; Acuña, E.; Arellano, G.; Morales, P.; Sotomayor, P.; Oyarzun-Ampuero, F.; Naves, R. Intranasal Delivery of Interferon-β-Loaded Nanoparticles Induces Control of Neuroinflammation in a Preclinical Model of Multiple Sclerosis: A Promising Simple, Effective, Non-Invasive, and Low-Cost Therapy. J. Control. Release 2021, 331, 443–459. [Google Scholar] [CrossRef]
- Sinha, S.; Garg, V.; Sonali; Singh, R.P.; Dutt, R. Chitosan-Alginate Core-Shell-Corona Shaped Nanoparticles of Dimethyl Fumarate in Orodispersible Film to Improve Bioavailability in Treatment of Multiple Sclerosis: Preparation, Characterization and Biodistribution in Rats. J. Drug Deliv. Sci. Technol. 2021, 64, 102645–102658. [Google Scholar] [CrossRef]
- Mehdi-alamdarlou, S.; Ahmadi, F.; Azadi, A.; Shahbazi, M.-A.; Heidari, R.; Ashrafi, H. A Cell-Mimicking Platelet-Based Drug Delivery System as a Potential Carrier of Dimethyl Fumarate for Multiple Sclerosis. Int. J. Pharm. 2022, 625, 122084–122095. [Google Scholar] [CrossRef]
- Shamaeizadeh, N.; Varshosaz, J.; Mirian, M.; Aliomrani, M. Glutathione Targeted Tragacanthic Acid-Chitosan as a Non-Viral Vector for Brain Delivery of MiRNA-219a-5P: An in Vitro/in Vivo Study. Int. J. Biol. Macromol. 2022, 200, 543–556. [Google Scholar] [CrossRef]
- Khodakarimi, S.; Zarebkohan, A.; Mohaddes, G.; Shiri-Shahsavari, M.R.; Omrani, M.H.; Sepasi, T.; Beyrampour-Basmenj, H.; Ebrahimi-Kalan, A. Neurotrophic Factors Loaded TGN-Modified Chitosan Nanoparticles Ameliorate Symptoms of MS through GATA3/FOXP3 and Targeting Th1/2 Cells Pathways. J. Drug Deliv. Sci. Technol. 2023, 85, 104570–104579. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, L.; Wang, J.; Xu, X.; Ni, S.; Liu, M.; Hu, K. Nose to Brain Delivery of Astragaloside IV by β-Asarone Modified Chitosan Nanoparticles for Multiple Sclerosis Therapy. Int. J. Pharm. 2023, 644, 123351–123361. [Google Scholar] [CrossRef] [PubMed]
- Dadkhah, M.; Afshari, S.; Samizadegan, T.; Shirmard, L.R.; Barin, S. Pegylated Chitosan Nanoparticles of Fluoxetine Enhance Cognitive Performance and Hippocampal Brain Derived Neurotrophic Factor Levels in a Rat Model of Local Demyelination. Exp. Gerontol. 2024, 195, 112533. [Google Scholar] [CrossRef]
- Abdelalim, L.R.; Elnaggar, Y.S.R.; Abdallah, O.Y. Lactoferrin, Chitosan Double-Coated Oleosomes Loaded with Clobetasol Propionate for Remyelination in Multiple Sclerosis: Physicochemical Characterization and in-Vivo Assessment in a Cuprizone-Induced Demyelination Model. Int. J. Biol. Macromol. 2024, 277, 134144. [Google Scholar] [CrossRef] [PubMed]
- Sava, V.; Fihurka, O.; Khvorova, A.; Sanchez-Ramos, J. Enriched Chitosan Nanoparticles Loaded with SiRNA Are Effective in Lowering Huntington’s Disease Gene Expression Following Intranasal Administration. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102119–102127. [Google Scholar] [CrossRef]
- Sridhar, G.R. Acetylcholinesterase Inhibitors (Galantamine, Rivastigmine, and Donepezil). In NeuroPsychopharmacotherapy; Springer: Cham, Switzerland, 2021; pp. 1–13. [Google Scholar]
- Isik, A.T.; Soysal, P.; Yay, A.; Usarel, C. The Effects of Sitagliptin, a DPP-4 Inhibitor, on Cognitive Functions in Elderly Diabetic Patients with or without Alzheimer’s Disease. Diabetes Res. Clin. Pract. 2017, 123, 192–198. [Google Scholar] [CrossRef]
- Zameer, S.; Alam, M.; Hussain, S.; Vohora, D.; Ali, J.; Najmi, A.K.; Akhtar, M. Neuroprotective Role of Alendronate against APP Processing and Neuroinflammation in Mice Fed a High Fat Diet. Brain Res. Bull. 2020, 161, 197–212. [Google Scholar] [CrossRef]
- Sava, V.; Fihurka, O.; Khvorova, A.; Sanchez-Ramos, J. Kinetics of HTT Lowering in Brain of YAC 128 Mice Following Single and Repetitive Intranasal Dosing of SiRNA Packaged in Chitosan-Based Nanoparticles. J. Drug Deliv. Sci. Technol. 2021, 63, 102517–102522. [Google Scholar] [CrossRef]
- Ait Hamdan, Y.; El-Mansoury, B.; Elouali, S.; Rachmoune, K.; Belbachir, A.; Oudadesse, H.; Rhazi, M. A Review of Chitosan Polysaccharides: Neuropharmacological Implications and Tissue Regeneration. Int. J. Biol. Macromol. 2024, 279, 135356. [Google Scholar] [CrossRef]
- Kruczkowska, W.; Gałęziewska, J.; Grabowska, K.H.; Gromek, P.; Czajkowska, K.; Rybicki, M.; Kciuk, M.; Kłosiński, K.K. From Molecules to Mind: The Critical Role of Chitosan, Collagen, Alginate, and Other Biopolymers in Neuroprotection and Neurodegeneration. Molecules 2025, 30, 1017. [Google Scholar] [CrossRef]
- Vahab, S.A.; Ki, A.; M, S.; Kumar, V.S. Exploring Chitosan Nanoparticles for Enhanced Therapy in Neurological Disorders: A Comprehensive Review. Naunyn. Schmiedebergs. Arch. Pharmacol. 2025, 398, 2151–2167. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Naki, T. Chitosan-Based Nanocarriers for Nose to Brain Delivery. Appl. Sci. 2019, 9, 2219–2245. [Google Scholar] [CrossRef]
- van der Merwe, S.M.; Verhoef, J.C.; Verheijden, J.H.M.; Kotzé, A.F.; Junginger, H.E. Trimethylated Chitosan as Polymeric Absorption Enhancer for Improved Peroral Delivery of Peptide Drugs. Eur. J. Pharm. Biopharm. 2004, 58, 225–235. [Google Scholar] [CrossRef]
- Puranik, N.; Tiwari, S.; Kumari, M.; Yadav, S.K.; Dhakal, T.; Song, M. Advanced Bioactive Polymers and Materials for Nerve Repair: Strategies and Mechanistic Insights. J. Funct. Biomater. 2025, 16, 255. [Google Scholar] [CrossRef]
- Agyare, E.K.; Curran, G.L.; Ramakrishnan, M.; Yu, C.C.; Poduslo, J.F.; Kandimalla, K.K. Development of a Smart Nano-Vehicle to Target Cerebrovascular Amyloid Deposits and Brain Parenchymal Plaques Observed in Alzheimer’s Disease and Cerebral Amyloid Angiopathy. Pharm. Res. 2008, 25, 2674–2684. [Google Scholar] [CrossRef]
- Jaruszewski, K.M.; Ramakrishnan, S.; Poduslo, J.F.; Kandimalla, K.K. Chitosan Enhances the Stability and Targeting of Immuno-Nanovehicles to Cerebro-Vascular Deposits of Alzheimer’s Disease Amyloid Protein. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 250–260. [Google Scholar] [CrossRef]
- Ebrahimi-Barough, S.; Hoveizi, E.; Javidan, A.N.; Ai, J. Investigating the Neuroglial Differentiation Effect of Neuroblastoma Conditioned Medium in Human Endometrial Stem Cells Cultured on 3D Nanofibrous Scaffold. J. Biomed. Mater. Res. Part A 2015, 103, 2621–2627. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Guan, S.; Xu, J.; Li, W.; Ge, D.; Sun, C.; Liu, T.; Ma, X. Neural Stem Cell Proliferation and Differentiation in the Conductive PEDOT-HA/Cs/Gel Scaffold for Neural Tissue Engineering. Biomater. Sci. 2017, 5, 2024–2034. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, C.; Guan, S.; Li, W.; Xu, J.; Ge, D.; Zhuang, M.; Liu, T.; Ma, X. Chitosan/Gelatin Porous Scaffolds Assembled with Conductive Poly(3,4-Ethylenedioxythiophene) Nanoparticles for Neural Tissue Engineering. J. Mater. Chem. B 2017, 5, 4774–4788. [Google Scholar] [CrossRef]
- Ghasemi Hamidabadi, H.; Rezvani, Z.; Nazm Bojnordi, M.; Shirinzadeh, H.; Seifalian, A.M.; Joghataei, M.T.; Razaghpour, M.; Alibakhshi, A.; Yazdanpanah, A.; Salimi, M.; et al. Chitosan-Intercalated Montmorillonite/Poly(Vinyl Alcohol) Nanofibers as a Platform to Guide Neuronlike Differentiation of Human Dental Pulp Stem Cells. ACS Appl. Mater. Interfaces 2017, 9, 11392–11404. [Google Scholar] [CrossRef] [PubMed]



| Disease | Nanoformulations (Size) | APIs | Results/Type of Administration | Ref. |
|---|---|---|---|---|
| Alzheimer’s disease | CS NPs (15 nm) | Amyloid-β peptide | High brain uptake efficiency (80.6% vs. 20.7% of the control group) and favourable immunogenicity (increased IgG against Aβ42)/IVA | [49] |
| CS NPs (41 and 47 nm) | Tacrine and rivastigmine | Higher concentrations of the drug in the liver, spleen and lungs high in vitro cumulative percentage release (94.64% and 97.25%) up to 12 h/IVA | [50,51] | |
| CS NPs (between 100 and 200 nm) | Donepezil | Higher drug transport efficiency (191.39%) and direct transport percentage (1834.48%) compared to donepezil solution/INA | [52] | |
| CS NPs (249 nm) | Piperine | Relief of nasal irritation, no brain toxicity, a significant reduction in the drug dose (up to 20-fold) and improvement of cognitive functions/INA | [53] | |
| CS NPs (190 nm) | Galantamine | No clinical signs of toxicity or histopathological manifestations in the brain, remarkable reduction in acetylcholinesterase activity/INA | [54,55] | |
| CS NPs nanofilm (<250 nm) and CS/PVA nanofibers (<150 nm) | Donepezil | High drug loading capacity (>99%), the nanofiber showed higher bioavailability (31.6 ng/mL vs. 25.8 ng/mL)/OA | [56] | |
| CS and BSA NPs (144 nm) | Curcumin | Increased drug penetration through the BBB (60% vs. 30% of free curcumin) and accelerated phagocytosis of the Aβ peptide/NA | [57] | |
| CS/-PLGA NPs (between 100 and 120 nm) | - | Potent inhibitor of Aβ1–42: the amplitude of ThT fluorescence is reduced to 51% by CS-PLGA NPs and to 7% by CS NPs at 20 µg/mL/NA | [58] | |
| CS/PLGA core/shell NPs (<150 nm) | Lutein | Better crossing of the BBB (20.7% vs. 7.9%) and higher concentration in the brain (0.49% vs. 0.15%) compared to free lutein/INA | [59] | |
| CS-coated PLGA NPs (200 nm) | Curcumin | In vitro studies showed reduced cytotoxicity and decreased TNF-α and IL-6 levels to approximately 70 and 40%/INA | [60] | |
| CS NPs (188 nm) | Sitagliptin | Drug release up to 73.77% over 24 h, increased brain sitagliptin concentrations by 5.07-fold compared to the free drug/INA | [61] | |
| CS NPs (136 nm) | Alendronate | High concentration in the mice’s brain, better pharmacokinetic profile (478.48 vs. 294.05 ng/mL) than the alendronate solution/INA | [62] | |
| CS-coated solid lipid NPs (185 nm) | Ferulic acid | Increased mucoadhesion after coating (from 6.88 to 8.55 N), improved biochemical parameters and cognitive abilities of rats/OA and INA | [63] | |
| CS functionalized PLGA core/shell NPs (~200 nm) | Curcumin | Enhanced permeation through the nasal mucosa (79% vs. 40%) and BBB crossing (21% vs. 9%) compared to drug suspension/INA | [64] | |
| CS nanovesicles (138 nm) | Transfersulin | Significant improvement in memory performance, neurogenesis in the hippocampus, and intranasal insulin delivery to the brain/INA | [65] | |
| CS-embedded liposomes vs. CS-coated liposomes (142–277 nm) | Centella asiatica vs. Asiatic acid | Neuroprotective role and increased bioavailability (5.32 and 9.23 μg/mL) compared to asiatic acid (3.43 μg/mL)/OA | [66] | |
| CS NPs (413 nm) | Luteolin | Improved long-term memory by 85.7%, and 0.53- and 0.54-fold reduction in the levels of Aβ1-42 and Tau compared to the control/INA | [67] | |
| CS NPs (between 50 and 80 nm) | Tanshinone IIA | Prolonged life span, attenuation of symptoms, enhanced protective effect, and inhibition of oxidative stress in a C. elegans AD model/NA | [68] | |
| CS NPs (between 100 and 120 nm) | Chrysin | In vivo studies in zebrafish showed a reduction in amyloid β aggregates, neuronal death and generation of reactive oxygen species/OA | [69] | |
| CS nanocrystals (between 153 and 310 nm) | Memantine | Prolonged in vitro release and lower cytotoxicity than the drug solution in human nasal RPMI 2650 cells and goat nasal mucosa tissue/INA | [70] | |
| CS NPs and CS/ alginate-coated NPs (240 and 286 nm) | Galantamine | Loading efficiency of 67% and 70% and release of the drug over a period of 8 and 5 h, for CS NPs and CS alginate-coated NPs, respectively/INA | [71] | |
| CS-collagen nanocapsules | Magnoflorine | Inhibitory effects against oxidative stress, acetylcholinesterase, MDA and pro-inflammatory cytokines with increased SOD levels/IA | [72] | |
| CS NPs (161 nm) | Betanin | Significant antioxidant and anti-inflammatory activities, decreased acetylcholinesterase activity (IC50 0.53 vs. 26 μg/mL of control)/NA | [73] | |
| CS NPs (131 nm) | Vinpocetine | Higher drug concentration in the brain (419 vs. 190 ng/mL) compared to oral administration, reducing systemic exposure/INA | [74] | |
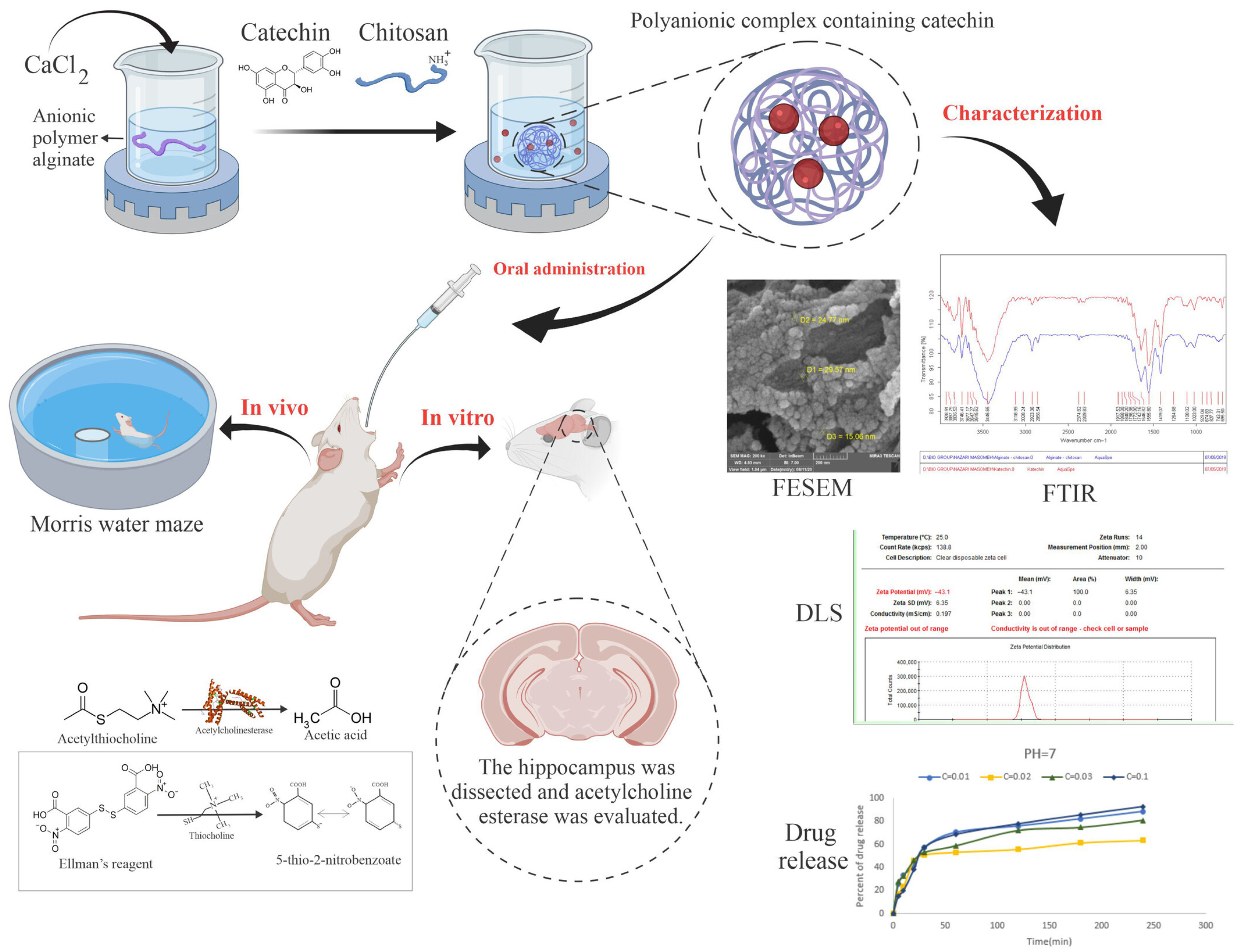
| CS-alginate NPs (between 40 and 45 nm) | Catechin | In 1 h, 68% is released in pH 7, reduced acetylcholinesterase activity (0.96 vs. 1.44 U/mg protein)/OA | [75] | |
| Parkinson’s disease | CS NPs (between 110 and 148 nm) | Dopamine | Reduced cytotoxicity and improved transport across the MDCKII-MDR1 cell line (fluorescence intensity increased 12-fold after 180 min)/IA | [76] |
| CS NPs (161 nm) | Bromocriptine | Better reversal in catalepsy behaviour (48 s vs. 153 s) and enhanced brain concentration (0.03 vs. 0.01%/g) compared to drug solution/INA | [77] | |
| Zinc hydroxide/CS nanocarrier | Carbidopa | More controlled drug release in vitro (pH 7.4: 47% in 315 min vs. 82% in 255 min) than Zn hydroxide/NA | [78] | |
| CS-coated lipid carrier (137 nm) | GDNF | Protect PC-12 cells against 6-OHDA toxin, behavioural improvement in rats, improved density of TH+ fibres in the striatum by at least 40%/INA | [79] | |
| CS-coated PLGA NPs (122 nm) | Rasagiline | Improved release on goat nasal mucosa (81% vs. 20% of the drug solution) and better bioavailability in the brain/INA | [80] | |
| Carboxylated single-walled carbon nanotubes coated with CS | Levodopa | Drug release dependent on the pH (45% at pH 7.4 and 22% at pH 4.8), non-cytotoxic to mouse embryonic 3T3 fibroblasts at 100 µg/mL/NA | [81] | |
| CS NPs (293 nm) | Pramipexole | Improvement in locomotor activity and a reduction in motor deficits, increased antioxidant effect and dopamine levels in the brain/INA | [82] | |
| CS with tripolyphosphate NPs (63 nm) | Selegiline | Higher plasma concentration (52.71 ng/mL vs. 21.69 ng/mL of the drug solution), reversible effect on catalepsy and akinesia/INA | [83] | |
| CS NPs (220 nm) | - | Reduced rotenone-initiated cytotoxicity and apoptotic cell death (by 25 to 30%) probably due to antioxidant and anti-apoptotic properties/NA | [84] | |
| CS (PEG-PLA) NPs (160 nm) | Act, NGF and pDNA | Neuroprotective effect: reduced cell viability of the PC12 + MPP+ PD cell model to 51% but were not cytotoxic in the absence of MPP+/IA | [85] | |
| CS-coated PLGA NPs (468 nm) | Ropinirole hydrochloride | Increased drug permeation through sheep nasal mucosa by 3.22-fold compared to non-coated NPs, lower Raw 264.7 and PBMC viability/INA | [86] | |
| CS NPs (<100 nm) | Rotigotine | Increased brain concentration (61.72 vs. 36.74 ng/mL) and higher SOD enzyme levels (0.248 vs. 0.147 U/mL) compared to drug solution/INA | [87] | |
| CS-coated nanoemulsion (184 nm) | Ropinirole with nigella oil | Better targeting to the brain (36,181 vs. 5680 ng/mL of the drug suspension) and improved neurobehavioral function/INA | [88] | |
| CS NPs (~100 nm) | FTY720 | 1.3-fold increase in percentage cell survival against rotenone and induction of the PP2A-EzH2 mediated pSer129 α-Syn degradation/IVA | [89] | |
| CS-alginate polyelectrolyte nanocomplex (402 nm) and CS-coated (PEG-b-PCL) nanocapsules (371 nm) | Ropinirole | IC50 against Raw 264.7 mouse macrophage cell line of 19.6 (nanocomplex) and 22.8 (nanocapsules) μg/mL, improved targeting to the brain (more than 2% compared to 0.93% radioactivity/g of ropinirole solution)/INA | [90] | |
| Carboxymethyl CS NPs (292 and 459 nm) | Dopamine | Antioxidant activity, no cytotoxicity against neuroblastoma SH-SY5Y cells and increased cell uptake demonstrated in 40 to 66% of cells/INA | [91] | |
| CS-coated nanostructured lipid carriers (<200 nm) | Tanshinone IIA | Significant increase in GSH levels by 154.6% with 48% and 84.6% reduction in MDA and HO-1 levels compared to the control/INA | [92] | |
| CS NPs | Curcumin | Restored ATP production by 195.5% and mitigated oxidative stress (by reducing ROS and MDA levels by 43.05% and 56.89%, respectively)/OA | [93] | |
| Multiple sclerosis | CS/PLA nanofibrous scaffold (diameter 100 nm) | PC12 cells | PC12 cells attach, grow, and differentiate into neural-like cells that further reduce symptoms, axonal damage and demyelination/NA | [94] |
| CS NPs | siRNA | Improved motor performance and coordination, neuroprotection and improvement of remyelination/INA | [95] | |
| CS/sulfobutylether-β cyclodextrin NPs (between 202 and 280 nm) | INFβ | No toxicity towards L(tk−) mouse fibroblasts and mouse splenocytes, in vivo studies in a MS model in mice showed lower clinical symptoms/INA | [96] | |
| CS-alginate NPs (561 nm) | DMF | Sustained drug release (18% in 30 min vs. 80% in 15 min) and 0.6-fold higher bioavailability compared to an oral DMF film formulation/OA | [97] | |
| CS nanogel (111 nm) and platelet membrane-coated nanogel (118 nm) | DMF | Sustained drug release, in vivo pharmacokinetic studies in rats indicated higher plasma and brain concentrations compared to the free drug/IVA | [98] | |
| CS, tragacanthic acid and glutathione NPs (between 227 and 558 nm) | miR-219 | Improved myelin sheaths, reduced inflammation and increased cell regeneration in the brain/IVA | [99] | |
| TGN-modified CS NPs (72 nm) | NFTs | Neuroprotection and reduced inflammation (to 27% compared to 100% of the non-treated group)/IA | [100] | |
| CS NPs (120 nm) | β-asarone and astragaloside IV | Reduced behavioural scores, suppressed inflammatory infiltration and astrocyte/microglial activation, and increased remyelination/INA | [101] | |
| CS/PEG NPs (240 nm) | Fluoxetine | Reduced anxiety, improved memory, increased BDNF levels, and reduced extent of demyelination, with no change in IGF-levels/OA | [102] | |
| Lactoferrin/CS double-coated oleosomes (220 nm) | Clobetasol propionate | Improved functions of mice, 2.3 folds increase in corpus callosum thickness, remyelination with 6.6 folds reduction in CP dose/INA | [103] | |
| Huntington’s disease | CS NPs (between 104 and 205 nm) | anti-HTT siRNA | Reduced expression of HTT mRNA in the brain by at least 50%/INA | [104] |
| Other neurodegenerative diseases | Biosensor: carboxylated multi-walled carbon nanotubes, gold NPs, CS film, Au electrode | Glutamate oxidase | Response within 2 s at pH 7.5 and 35 °C, high sensitivity (155 nA/μM/cm2), low detection limit (1.6 μM) and wide linear range (5–500 μM)/NA | [35] |
| CS-myristate nanogel (<50 nm) | - | Protection of neuroserpin from misfolding and aggregation/NA | [36] | |
| CS-alginate NPs | Quercetin | Neuroprotection in a model of H2O2-induced oxidative stress in neuroblastoma SH-SY5Y cells and of 6-OHDA in rat brain synaptosomes/NA | [37] | |
| CS-mangafodipir NPs (between 90 and 114 nm) | siRNA and dsDNA | Reduced GFP mRNA (by at least 50%) and RFP expression in multiple brain regions (e.g., cerebral cortex, hippocampus, and stratium)/INA | [38] | |
| CS-coated nanoemulsions (258 nm) | Rosmarinic acid | Sustained permeation through porcine nasal mucosa (47 vs. 132 µg cm−2 after 8 h) compared to the drug solution, non-cytotoxic to fibroblasts/INA | [39] | |
| CS NPs (between 300 and 400 nm) | Genistein | 60% of the drug permeated through the nasal mucosa compared to none of the drug solution, and showed no cytotoxicity to PC12 cells/INA | [40] | |
| CS nanocapsules (406 nm) | p38 MAPK inhibitor | Reduced enzymatic activity of p38 MAPK in microglial and neuronal cells in vitro and ex vivo, as well as in a mouse model for AD/INA | [41] | |
| CS/carbon dots (144 nm) | Dopamine | In vitro drug release is pH dependent (60% at pH 4 and 4.5% at pH 7), not cytotoxic to IC-21 and SH-SY5Y cell lines/NA | [42] | |
| CS/fucoidan nanocarriers (150 nm) | Curcumin | In vivo studies in mice showed increased accumulation of curcumin in the brain (20.4% compared to 0.8% of free curcumin)/INA | [43] | |
| Nanospheres of covellite copper sulphide with CS (15 nm) | Dopamine | Photo-controlled drug release (from 6% to 50% of drug release in 5 h), non-cytotoxic to A549, L132 and SH-SY5Y cell lines at 100 μg/NA | [44] | |
| CS NPs (104 nm) | Ginkgo Biloba extract | Neuroprotective activity by increasing the viability of SY5Y cells from 60% to 92.3% (also higher than that of the free extract (83.9%))/NA | [45] | |
| CS/lecithin NPs (218 nm) | Statin | 11-fold increase in drug permeation across a human cell model of the nasal epithelium, stronger suppression of pro-inflammatory signalling/INA | [46] | |
| CS–collagen nanocapsules (12 nm) | Magnoflorine | Good antioxidant potential (IC50 < 25 μg/mL), 85.50% viability of SH-SY5Y cells at 50 μg/mL and good acetylcholinesterase inhibitor (85.20%)/NA | [47] | |
| CDX-modified CS NPs (110 nm) | Fingolimod | Reduced INF-γ levels, decreased expression of TBX21, GATA3, FOXP3 and Rorc, efficient cellular uptake, and regulation inflammation/IA | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, A.C.C.; Almeida, A.; Freire, C.S.R.; Ferreira, B.L. Chitosan (Nano)formulations as Therapeutic Tools for Neurodegenerative Diseases: A Comprehensive Review. Polymers 2025, 17, 2838. https://doi.org/10.3390/polym17212838
Gomes ACC, Almeida A, Freire CSR, Ferreira BL. Chitosan (Nano)formulations as Therapeutic Tools for Neurodegenerative Diseases: A Comprehensive Review. Polymers. 2025; 17(21):2838. https://doi.org/10.3390/polym17212838
Chicago/Turabian StyleGomes, Adriana C. C., Adelaide Almeida, Carmen S. R. Freire, and Bárbara Leite Ferreira. 2025. "Chitosan (Nano)formulations as Therapeutic Tools for Neurodegenerative Diseases: A Comprehensive Review" Polymers 17, no. 21: 2838. https://doi.org/10.3390/polym17212838
APA StyleGomes, A. C. C., Almeida, A., Freire, C. S. R., & Ferreira, B. L. (2025). Chitosan (Nano)formulations as Therapeutic Tools for Neurodegenerative Diseases: A Comprehensive Review. Polymers, 17(21), 2838. https://doi.org/10.3390/polym17212838