Cutting-Edge Sensor Design: MIP Nanoparticle-Functionalized Nanofibers for Gas-Phase Detection of Limonene in Predictive Agriculture
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Molecularly Imprinted Nanoparticles (MIP-NPs)
2.3. MWCNT Dispersion
2.4. Electrospinning Solution
2.5. Electrospinning Conditions and Device Fabrication
2.6. Interdigitated Electrodes (IDEs)
2.7. UV-Crosslinking Process
2.8. Scanning Electron Microscopy (SEM)
2.9. Atomic Force Microscopy (AFM)
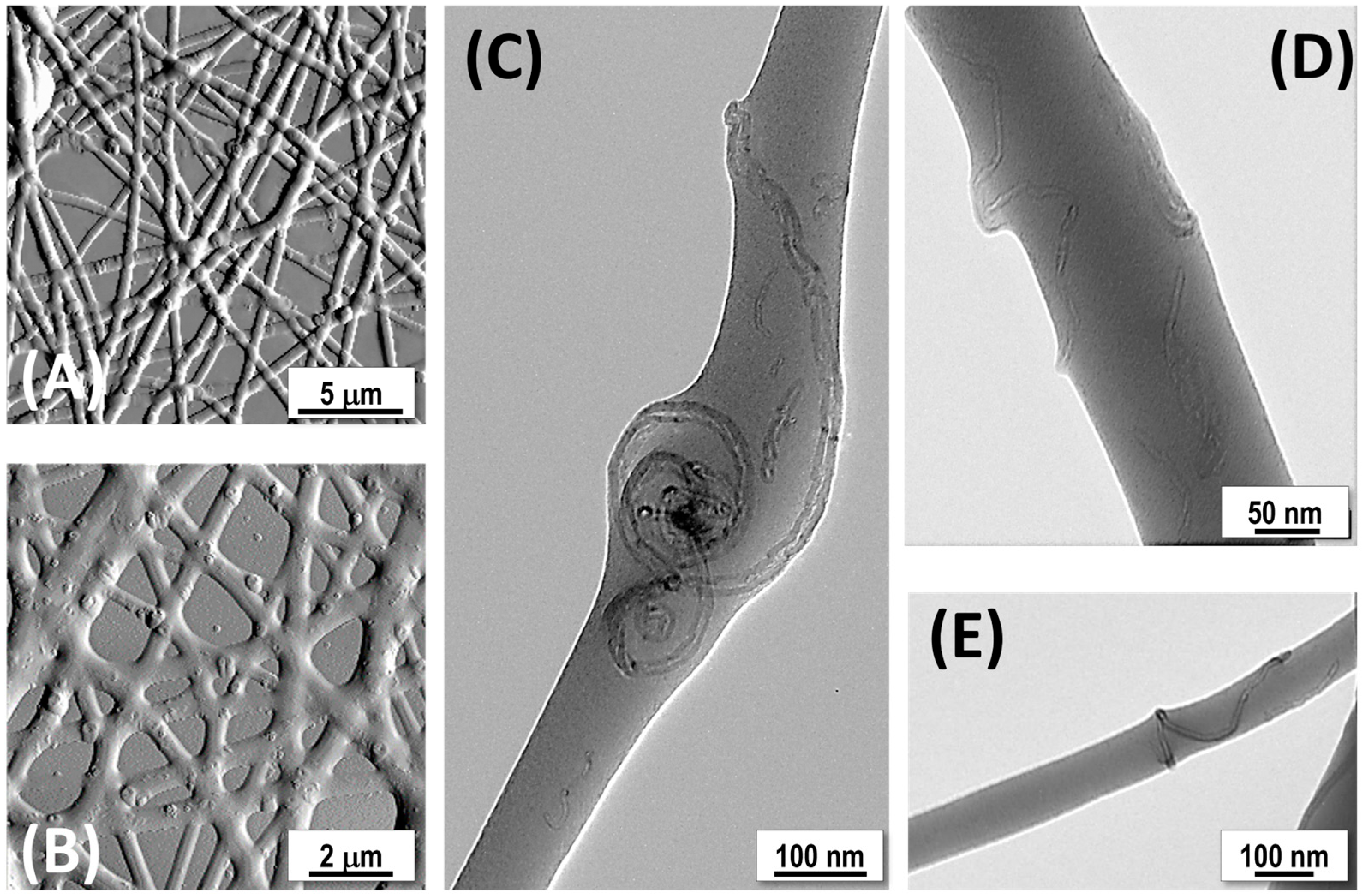
2.10. Transmission Electron Microscopy (TEM)
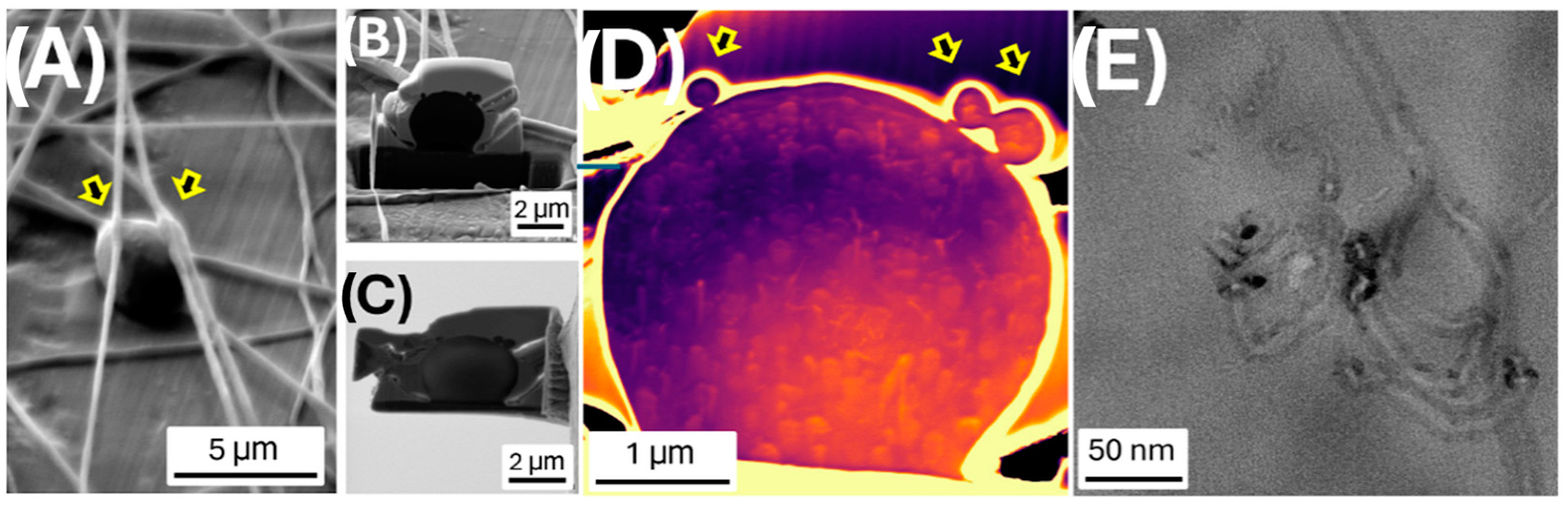
2.11. Focused Ion Beam (FIB)
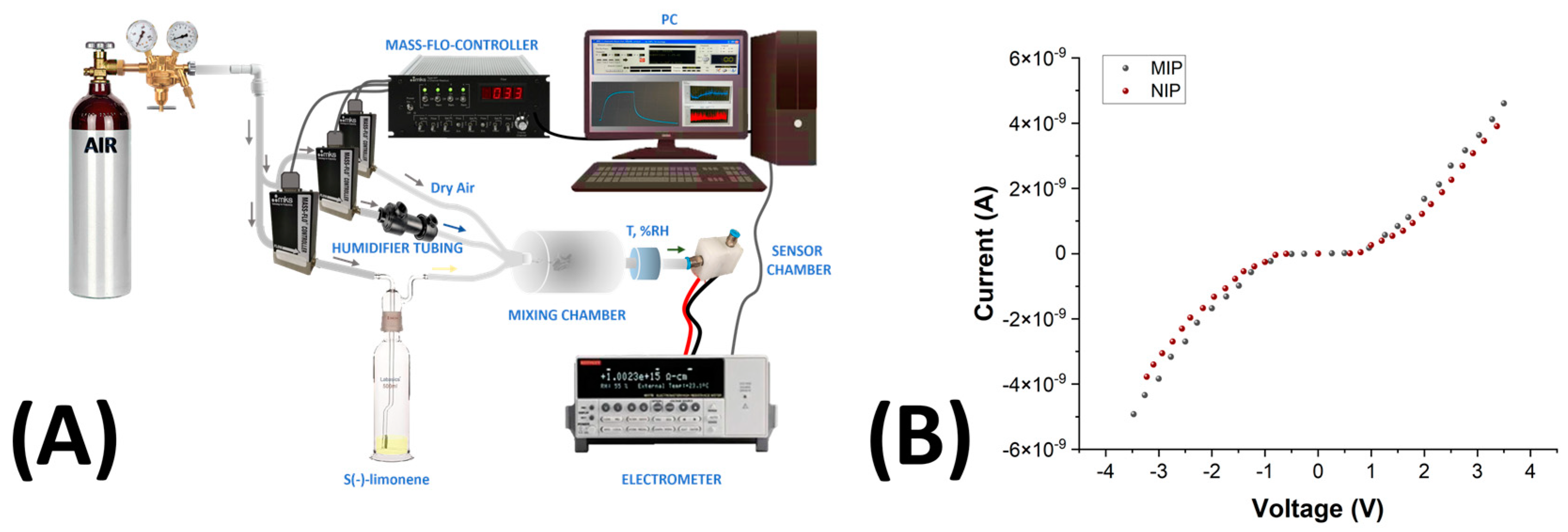
2.12. Electrical and Sensing Measurements
3. Results and Discussion
3.1. Particle Characterization
3.2. Fiber Characterization
3.3. Electrical Properties
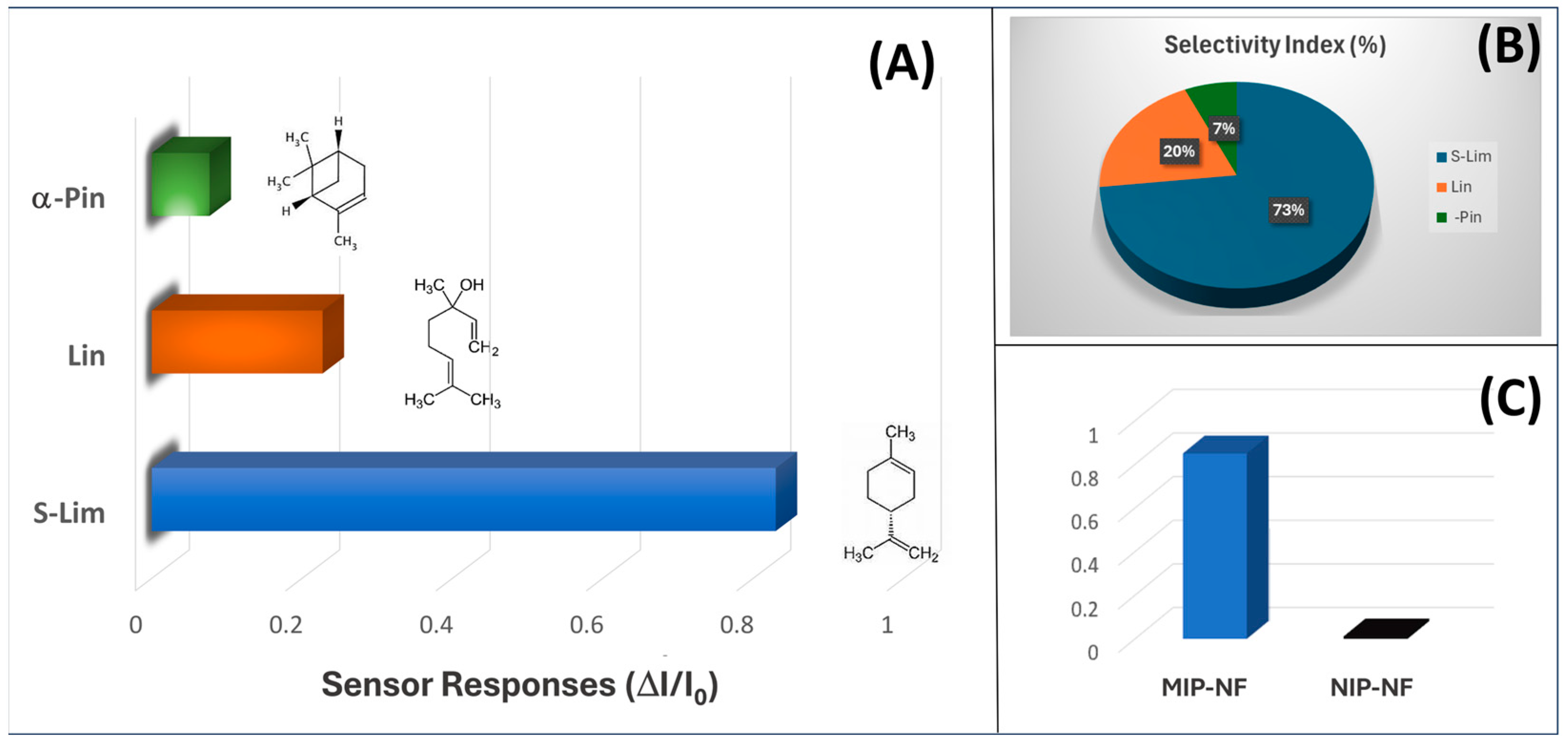
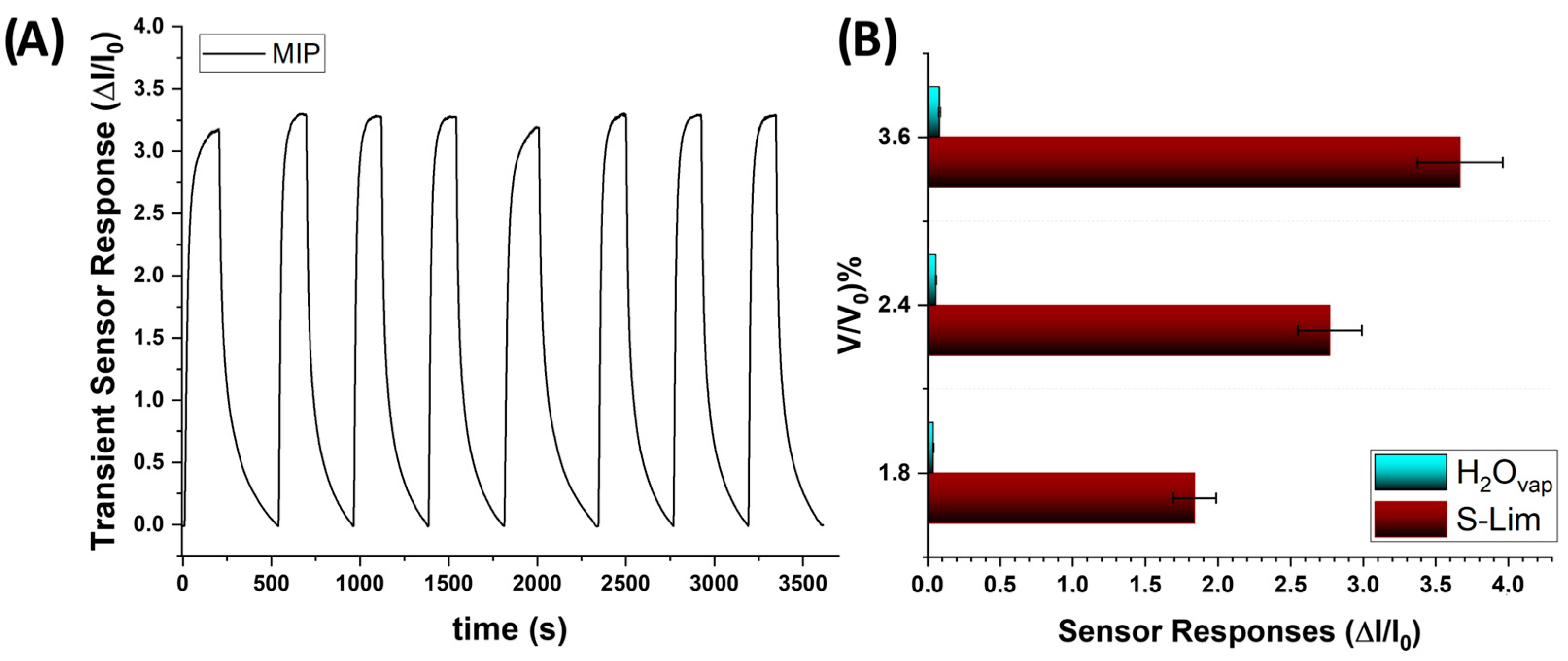
3.4. Sensor Features
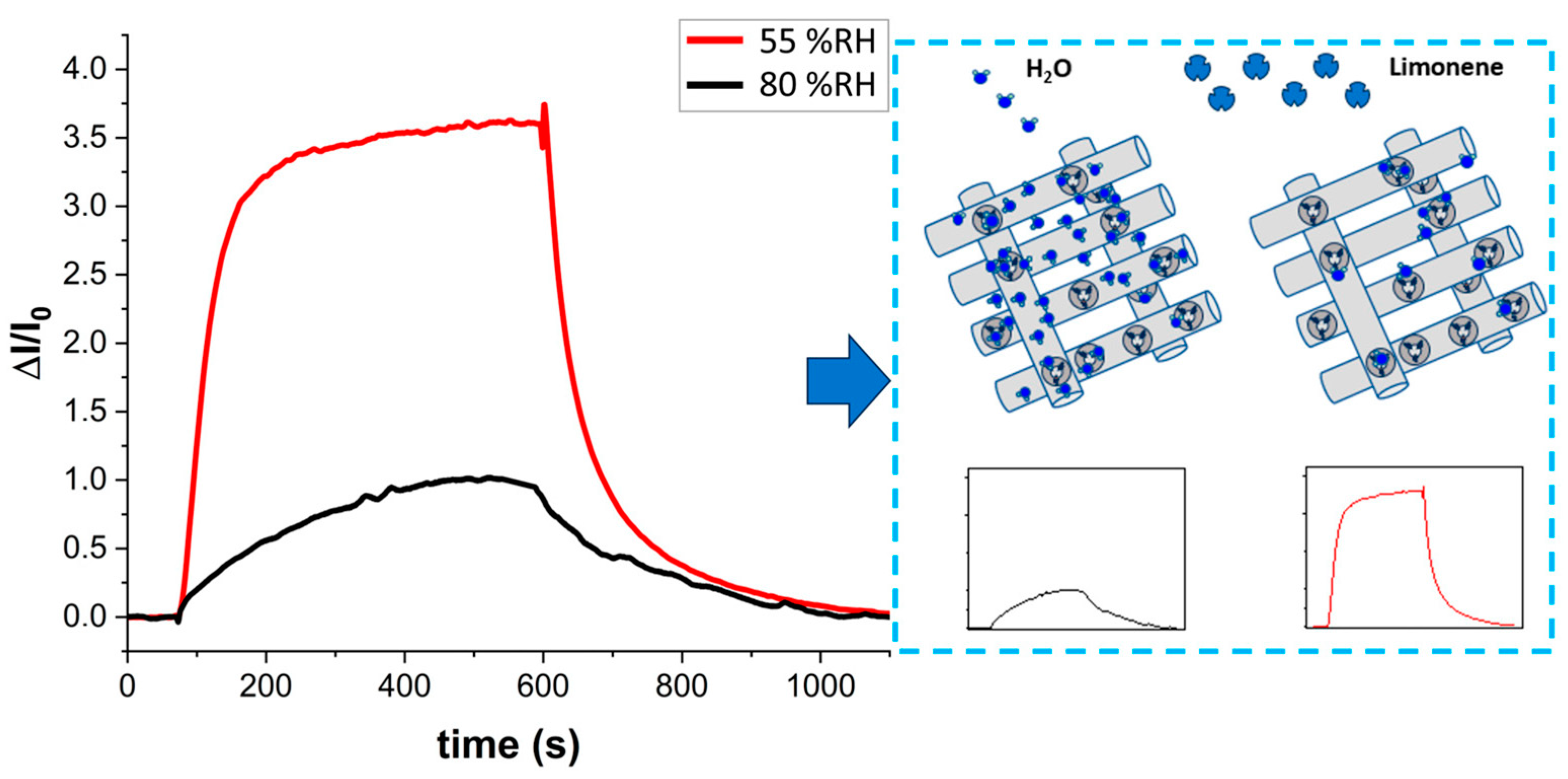
3.5. Humidity Interference
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Çakmakçı, R.; Salık, M.A.; Çakmakçı, S. Assessment and Principles of Environmentally Sustainable Food and Agriculture Systems. Agriculture 2023, 13, 1073. [Google Scholar] [CrossRef]
- Salim, N.; Raza, A. Nutrient Use Efficiency (NUE) for Sustainable Wheat Production: A Review. J. Plant Nutr. 2020, 43, 297–315. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The Global Burden of Pathogens and Pests on Major Food Crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Oerke, E.-C.; Dehne, H.-W. Safeguarding Production—Losses in Major Crops and the Role of Crop Protection. Crop Prot. 2004, 23, 275–285. [Google Scholar] [CrossRef]
- Oerke, E.-C. Crop Losses to Pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Haq, I.U.; Rahim, K.; Yahya, G.; Ijaz, B.; Maryam, S.; Paker, N.P. Eco-Smart Biocontrol Strategies Utilizing Potent Microbes for Sustainable Management of Phytopathogenic Diseases. Biotechnol. Rep. 2024, 44, e00859. [Google Scholar] [CrossRef]
- Poland, J.; Rutkoski, J. Advances and Challenges in Genomic Selection for Disease Resistance. Annu. Rev. Phytopathol. 2016, 54, 79–98. [Google Scholar] [CrossRef]
- Palmgren, M.; Shabala, S. Adapting Crops for Climate Change: Regaining Lost Abiotic Stress Tolerance in Crops. Front. Sci. 2024, 2, 1416023. [Google Scholar] [CrossRef]
- Roper, J.M.; Garcia, J.F.; Tsutsui, H. Emerging Technologies for Monitoring Plant Health in Vivo. ACS Omega 2021, 6, 5101–5107. [Google Scholar] [CrossRef]
- Oerke, E.-C. Remote Sensing of Diseases. Annu. Rev. Phytopathol. 2024, 58, 225–252. [Google Scholar] [CrossRef]
- Galieni, A.; D’Ascenzo, N.; Stagnari, F.; Pagnani, G.; Xie, Q.; Pisante, M. Past and Future of Plant Stress Detection: An Overview from Remote Sensing to Positron Emission Tomography. Front. Plant Sci. 2021, 11, 609155. [Google Scholar] [CrossRef]
- Richard, B.; Qi, A.; Fitt, B.D.L. Control of Crop Diseases through Integrated Crop Management to Deliver Climate-smart Farming Systems for Low- and High-input Crop Production. Plant Pathol. 2022, 71, 187–206. [Google Scholar] [CrossRef]
- Gupta, S.; Huang, C.H.; Singh, G.P.; Park, B.S.; Chua, N.-H.; Ram, R.J. Portable Raman Leaf-Clip Sensor for Rapid Detection of Plant Stress. Sci. Rep. 2020, 10, 20206. [Google Scholar] [CrossRef] [PubMed]
- Gouinguené, S.P.; Turlings, T.C.J. The Effects of Abiotic Factors on Induced Volatile Emissions in Corn Plants. Plant Physiol. 2002, 129, 1296–1307. [Google Scholar] [CrossRef]
- Vinicius da Silva Ferreira, M.; Barbosa, J.L.; Kamruzzaman, M.; Barbin, D.F. Low-Cost Electronic-Nose (LC-e-Nose) Systems for the Evaluation of Plantation and Fruit Crops: Recent Advances and Future Trends. Anal. Methods 2023, 15, 6120–6138. [Google Scholar] [CrossRef]
- Ali, A.; Mansol, A.S.; Khan, A.A.; Muthoosamy, K.; Siddiqui, Y. Electronic Nose as a Tool for Early Detection of Diseases and Quality Monitoring in Fresh Postharvest Produce: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2408–2432. [Google Scholar] [CrossRef]
- Kashyap, B.; Kumar, R. Sensing Methodologies in Agriculture for Monitoring Biotic Stress in Plants Due to Pathogens and Pests. Inventions 2021, 6, 29. [Google Scholar] [CrossRef]
- Perdomo, S.A.; Valencia, D.P.; Velez, G.E.; Jaramillo-Botero, A. Advancing Abiotic Stress Monitoring in Plants with a Wearable Non-Destructive Real-Time Salicylic Acid Laser-Induced-Graphene Sensor. Biosens. Bioelectron. 2024, 255, 116261. [Google Scholar] [CrossRef]
- Kesselmeier, J.; Staudt, M. Biogenic Volatile Organic Compounds (VOC): An Overview on Emission, Physiology and Ecology. J. Atmos Chem. 1999, 33, 23–88. [Google Scholar] [CrossRef]
- Guenther, A.; Hewitt, C.N.; Erickson, D.; Fall, R.; Geron, C.; Graedel, T.; Harley, P.; Klinger, L.; Lerdau, M.; Mckay, W.A.; et al. A Global Model of Natural Volatile Organic Compound Emissions. J. Geophys. Res. 1995, 100, 8873–8892. [Google Scholar] [CrossRef]
- Sindelarova, K.; Granier, C.; Bouarar, I.; Guenther, A.; Tilmes, S.; Stavrakou, T.; Müller, J.-F.; Kuhn, U.; Stefani, P.; Knorr, W. Global Data Set of Biogenic VOC Emissions Calculated by the MEGAN Model over the Last 30 Years. Atmos. Chem. Phys. 2014, 14, 9317–9341. [Google Scholar] [CrossRef]
- Maffei, M.E. Sites of Synthesis, Biochemistry and Functional Role of Plant Volatiles. South Afr. J. Bot. 2010, 76, 612–631. [Google Scholar] [CrossRef]
- Bracho-Nunez, A.; Welter, S.; Staudt, M.; Kesselmeier, J. Plant-Specific Volatile Organic Compound Emission Rates from Young and Mature Leaves of Mediterranean Vegetation. J. Geophys. Res. 2011, 116, D16304. [Google Scholar] [CrossRef]
- Brillante, L.; Martínez-Lüscher, J.; Kurtural, S.K. Applied Water and Mechanical Canopy Management Affect Berry and Wine Phenolic and Aroma Composition of Grapevine (Vitis vinifera L., Cv. Syrah) in Central California. Sci. Hortic. 2018, 227, 261–271. [Google Scholar] [CrossRef]
- Toffolatti, S.L.; Maddalena, G.; Passera, A.; Casati, P.; Bianco, P.A.; Quaglino, F. Role of Terpenes in Plant Defense to Biotic Stress. In Biocontrol Agents and Secondary Metabolites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 401–417. [Google Scholar]
- Kovalenko, Y.; Tindjau, R.; Madilao, L.L.; Castellarin, S.D. Regulated Deficit Irrigation Strategies Affect the Terpene Accumulation in Gewürztraminer (Vitis vinifera L.) Grapes Grown in the Okanagan Valley. Food Chem. 2021, 341, 128172. [Google Scholar] [CrossRef]
- Castorina, G.; Grassi, F.; Consonni, G.; Vitalini, S.; Oberti, R.; Calcante, A.; Ferrari, E.; Bononi, M.; Iriti, M. Characterization of the Biogenic Volatile Organic Compounds (BVOCs) and Analysis of the PR1 Molecular Marker in Vitis Vinifera L. Inoculated with the Nematode Xiphinema Index. Int. J. Mol. Sci. 2020, 21, 4485. [Google Scholar] [CrossRef]
- Malheiro, R.; Casal, S.; Cunha, S.C.; Baptista, P.; Pereira, J.A. Identification of Leaf Volatiles from Olive (Olea Europaea) and Their Possible Role in the Ovipositional Preferences of Olive Fly, Bactrocera Oleae (Rossi) (Diptera: Tephritidae). Phytochemistry 2016, 121, 11–19. [Google Scholar] [CrossRef]
- Salvatore, M.M.; Nicoletti, R.; Andolfi, A. Essential Oils in Citrus Fruit Ripening and Postharvest Quality. Horticulturae 2022, 8, 396. [Google Scholar] [CrossRef]
- Gan, Z.; Zhou, Q.; Zheng, C.; Wang, J. Challenges and Applications of Volatile Organic Compounds Monitoring Technology in Plant Disease Diagnosis. Biosens. Bioelectron. 2023, 237, 115540. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Qi, H.; Chen, Y.; Guo, W.; Yu, H.; Chen, H.; Ying, Y. Humidity-Independent Artificial Olfactory Array Enabled by Hydrophobic Core–Shell Dye/MOFs@COFs Composites for Plant Disease Diagnosis. ACS Nano 2022, 16, 14297–14307. [Google Scholar] [CrossRef]
- Chalupowicz, D.; Veltman, B.; Droby, S.; Eltzov, E. Evaluating the Use of Biosensors for Monitoring of Penicillium Digitatum Infection in Citrus Fruit. Sens. Actuators B Chem. 2020, 311, 127896. [Google Scholar] [CrossRef]
- Park, J.; Thomasson, J.A.; Gale, C.C.; Sword, G.A.; Lee, K.-M.; Herrman, T.J.; Suh, C.P.-C. Adsorbent-SERS Technique for Determination of Plant VOCs from Live Cotton Plants and Dried Teas. ACS Omega 2020, 5, 2779–2790. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.; Moru, S.; Schnable, P.; Dong, L. Wearable Plant Sensor for In Situ Monitoring of Volatile Organic Compound Emissions from Crops. ACS Sens 2022, 7, 2293–2302. [Google Scholar] [CrossRef]
- Kuruppuarachchi, C.; Kulsoom, F.; Ibrahim, H.; Khan, H.; Zahid, A.; Sher, M. Advancements in Plant Wearable Sensors. Comput. Electron. Agric. 2025, 229, 109778. [Google Scholar] [CrossRef]
- Chen, B.; Liu, C.; Xie, Y.; Jia, P.; Hayashi, K. Localized Surface Plasmon Resonance Gas Sensor Based on Molecularly Imprinted Polymer Coated Au Nano-Island Films: Influence of Nanostructure on Sensing Characteristics. IEEE Sens. J. 2016, 16, 3532–3540. [Google Scholar] [CrossRef]
- Shang, L.; Liu, C.; Chen, B.; Hayashi, K. Development of Molecular Imprinted Sol-Gel Based LSPR Sensor for Detection of Volatile Cis-Jasmone in Plant. Sens. Actuators B Chem. 2018, 260, 617–626. [Google Scholar] [CrossRef]
- Herrera-Chacón, A.; Cetó, X.; del Valle, M. Molecularly Imprinted Polymers-towards Electrochemical Sensors and Electronic Tongues. Anal. Bioanal. Chem. 2021, 413, 6117–6140. [Google Scholar] [CrossRef]
- Cieplak, M.; Kutner, W. Artificial Biosensors: How Can Molecular Imprinting Mimic Biorecognition? Trends Biotechnol. 2016, 34, 922–941. [Google Scholar] [CrossRef]
- Malitesta, C.; Mazzotta, E.; Picca, R.A.; Poma, A.; Chianella, I.; Piletsky, S.A. MIP Sensors–the Electrochemical Approach. Anal. Bioanal. Chem. 2012, 402, 1827–1846. [Google Scholar] [CrossRef]
- Wasilewski, T.; Orbay, S.; Brito, N.F.; Sikora, K.; Melo, A.C.A.; Melendez, M.E.; Szulczyński, B.; Sanyal, A.; Kamysz, W.; Gębicki, J. Molecularly Imprinted Polymers for the Detection of Volatile Biomarkers. TrAC Trends Anal. Chem. 2024, 177, 117783. [Google Scholar] [CrossRef]
- Fresco-Cala, B.; Batista, A.D.; Cárdenas, S. Molecularly Imprinted Polymer Micro- and Nano-Particles: A Review. Molecules 2020, 25, 4740. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular Imprinting: Perspectives and Applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef]
- Hua, Y.; Ahmadi, Y.; Kim, K.-H. Molecularly Imprinted Polymers for Sensing Gaseous Volatile Organic Compounds: Opportunities and Challenges. Environ. Pollut. 2022, 311, 119931. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Noh, B.-I.; Cha, Y.L.; Chang, Y.-H.; Dai, S.; Kim, S.H.; Kim, D.-J. Development of Molecularly Imprinted Polymer Electrochemical Sensors for Strawberry Sweetness Biomarker Detection. ACS Appl. Polym. Mater. 2024, 6, 8084–8092. [Google Scholar] [CrossRef]
- Koudehi, M.F.; Pourmortazavi, S.M.; Zibaseresht, R.; Mirsadeghi, S. MEMS-Based PVA/PPy/MIP Polymeric- Nanofiber Sensor Fabricated by LIFT-OFF Process for Detection 2,4-Dinitrotoluene Vapor. IEEE Sens. J. 2021, 21, 9492–9499. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, C.; Du, X.; Zhang, Z. Recent Advances of Nanomaterials-Based Molecularly Imprinted Electrochemical Sensors. Nanomaterials 2022, 12, 1913. [Google Scholar] [CrossRef]
- Garcia-Cruz, A.; Ahmad, O.S.; Alanazi, K.; Piletska, E.; Piletsky, S.A. Generic Sensor Platform Based on Electro-Responsive Molecularly Imprinted Polymer Nanoparticles (e-NanoMIPs). Microsyst. Nanoeng. 2020, 6, 83. [Google Scholar] [CrossRef]
- Wang, L.; Pagett, M.; Zhang, W. Molecularly Imprinted Polymer (MIP) Based Electrochemical Sensors and Their Recent Advances in Health Applications. Sens. Actuators Rep. 2023, 5, 100153. [Google Scholar] [CrossRef]
- Rahman, S.; Bozal-Palabiyik, B.; Unal, D.N.; Erkmen, C.; Siddiq, M.; Shah, A.; Uslu, B. Molecularly Imprinted Polymers (MIPs) Combined with Nanomaterials as Electrochemical Sensing Applications for Environmental Pollutants. Trends Environ. Anal. Chem. 2022, 36, e00176. [Google Scholar] [CrossRef]
- Kikuchi, M.; Tsuru, N.; Shiratori, S. Recognition of Terpenes Using Molecular Imprinted Polymer Coated Quartz Crystal Microbalance in Air Phase. Sci. Technol. Adv. Mater. 2006, 7, 156–161. [Google Scholar] [CrossRef]
- Ghatak, B.; Ali, S.B.; Naskar, H.; Tudu, B.; Pramanik, P.; Mukherji, S.; Bandyopadhyay, R. Selective and Sensitive Detection of Limonene in Mango Using Molecularly Imprinted Polymer Based Quartz Crystal Microbalance Sensor. In Proceedings of the 2019 IEEE International Symposium on Olfaction and Electronic Nose (ISOEN), Fukuoka, Japan, 26–29 May 2019; IEEE: New York, NY, USA, 2019; pp. 1–3. [Google Scholar]
- Hawari, H.F.; Samsudin, N.M.; Ahmad, M.N.; Shakaff, A.Y.M.; Ghani, S.A.; Wahab, Y.; Za’aba, S.K.; Akitsu, T. Array of MIP-Based Sensor for Fruit Maturity Assessment. Procedia. Chem. 2012, 6, 100–109. [Google Scholar] [CrossRef]
- Iqbal, N.; Mustafa, G.; Rehman, A.; Biedermann, A.; Najafi, B.; Lieberzeit, P.A.; Dickert, F.L. QCM-Arrays for Sensing Terpenes in Fresh and Dried Herbs via Bio-Mimetic MIP Layers. Sensors 2010, 10, 6361–6376. [Google Scholar] [CrossRef]
- Chen, M.; Yazdani, M.; Murugappan, K. Non-Destructive Pest Detection: Innovations and Challenges in Sensing Airborne Semiochemicals. ACS Sens. 2024, 9, 5728–5747. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Nie, Q.; Han, L.; Gong, Z.; Li, D.; Ma, Q.; Wang, Z.; He, W.; Wen, L.; Peng, H. Molecularly Imprinted Polymers-Based Piezoelectric Coupling Sensor for the Rapid and Nondestructive Detection of Infested Citrus. Food Chem. 2022, 387, 132905. [Google Scholar] [CrossRef]
- Hawari, H.F.; Samsudin, N.M.; Md Shakaff, A.Y.; Ghani, S.A.; Ahmad, M.N.; Wahab, Y.; Hashim, U. Development of Interdigitated Electrode Molecular Imprinted Polymer Sensor for Monitoring Alpha Pinene Emissions from Mango Fruit. Procedia. Eng. 2013, 53, 197–202. [Google Scholar] [CrossRef]
- Völkle, J.; Kumpf, K.; Feldner, A.; Lieberzeit, P.; Fruhmann, P. Development of Conductive Molecularly Imprinted Polymers (CMIPs) for Limonene to Improve and Interconnect QCM and Chemiresistor Sensing. Sens. Actuators B Chem. 2022, 356, 131293. [Google Scholar] [CrossRef]
- Olsen, B.G.; Stradiotto, N.R. Enantioselective Detection of (D)- and (L)-Limonene from Essential Oil Samples Using a Molecularly Imprinted Polypyrrole Electrochemical Sensor. ECS Meet. Abstr. 2024; MA2024-01, 2667. [Google Scholar] [CrossRef]
- Macagnano, A.; Perri, V.; Zampetti, E.; Bearzotti, A.; De Cesare, F. Humidity Effects on a Novel Eco-Friendly Chemosensor Based on Electrospun PANi/PHB Nanofibres. Sens. Actuators B Chem. 2016, 232, 16–27. [Google Scholar] [CrossRef]
- Borah, A.R.; Hazarika, P.; Duarah, R.; Goswami, R.; Hazarika, S. Biodegradable Electrospun Membranes for Sustainable Industrial Applications. ACS Omega 2024, 9, 11129–11147. [Google Scholar] [CrossRef]
- Macagnano, A.; Zampetti, E.; Kny, E. Electrospinning for High Performance Sensors; Macagnano, A., Zampetti, E., Kny, E., Eds.; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-14405-4. [Google Scholar]
- Papa, P.; Zampetti, E.; Molinari, F.N.; De Cesare, F.; Di Natale, C.; Tranfo, G.; Macagnano, A. A Polyvinylpyrrolidone Nanofibrous Sensor Doubly Decorated with Mesoporous Graphene to Selectively Detect Acetic Acid Vapors. Sensors 2024, 24, 2174. [Google Scholar] [CrossRef]
- Macagnano, A.; Avossa, J. Nanostructured Composite Materials for Advanced Chemical Sensors. In Advances in Nanostructured Materials and Nanopatterning Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 297–332. [Google Scholar]
- Avossa, J.; Zampetti, E.; De Cesare, F.; Bearzotti, A.; Scarascia-Mugnozza, G.; Vitiello, G.; Zussman, E.; Macagnano, A. Thermally Driven Selective Nanocomposite PS-PHB/MGC Nanofibrous Conductive Sensor for Air Pollutant Detection. Front. Chem. 2018, 6, 432. [Google Scholar] [CrossRef]
- Patel, K.D.; Kim, H.; Knowles, J.C.; Poma, A. Molecularly Imprinted Polymers and Electrospinning: Manufacturing Convergence for Next-Level Applications. Adv. Funct. Mater. 2020, 30, 2001955. [Google Scholar] [CrossRef]
- Dong, H.; Tong, L.; Cheng, M.; Hou, S. Utilizing Electrospun Molecularly Imprinted Membranes for Food Industry: Opportunities and Challenges. Food Chem. 2024, 460, 140695. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Dai, Y.; Rong, J.; Zhang, T.; Xue, S.; Yang, D.; Pan, J.; Qiu, F. “Sandwich-like” Electrospinning Fiber-Based Molecularly Imprinted Membrane Constructed with Electrospun Polyethyleneimine as the Multifunction Interlayer for the Selective Separation of Shikimic Acid. Chem. Eng. J. 2023, 463, 142501. [Google Scholar] [CrossRef]
- Moraes Segundo, J. de D.P. de; Moraes, M.O.S. de; Brito, W.R.; Matos, R.S.; Salerno, M.; Barcelay, Y.R.; Segala, K.; Fonseca Filho, H.D. da; d’Ávila, M.A. Molecularly Imprinted Membrane Produced by Electrospinning for β-Caryophyllene Extraction. Materials 2022, 15, 7275. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Liu, H.; He, R.; Zhao, X.; Wang, Y.; Qin, W.; Yin, S. Molecularly Imprinted Sensors Based on Highly Stretchable Electrospun Membranes for Cortisol Detection. Microchem. J. 2024, 207, 112115. [Google Scholar] [CrossRef]
- Demirkurt, M.; Olcer, Y.A.; Demir, M.M.; Eroglu, A.E. Electrospun Polystyrene Fibers Knitted around Imprinted Acrylate Microspheres as Sorbent for Paraben Derivatives. Anal. Chim. Acta. 2018, 1014, 1–9. [Google Scholar] [CrossRef]
- Hassanzadeh, M.; Ghaemy, M.; Amininasab, S.M.; Shami, Z. An Effective Approach for Fast Selective Separation of Cr(VI) from Water by Ion-Imprinted Polymer Grafted on the Electro-Spun Nanofibrous Mat of Functionalized Polyacrylonitrile. React. Funct. Polym. 2018, 130, 70–80. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Zhang, M.; Liu, F.; Wan, Y.; Huang, Z.; Ye, L.; Zhou, Q.; Shi, Y.; Lu, B. Selective and Simultaneous Determination of Trace Bisphenol A and Tebuconazole in Vegetable and Juice Samples by Membrane-Based Molecularly Imprinted Solid-Phase Extraction and HPLC. Food Chem. 2014, 164, 527–535. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Q.; Zhang, Y.; Liu, Y.; Wan, Y.; Gao, K.; Huang, Y.; Xia, W.; Wang, H.; Shi, Y.; et al. Molecularly Imprinted Nanofiber Membranes Enhanced Biodegradation of Trace Bisphenol A by Pseudomonas Aeruginosa. Chem. Eng. J. 2015, 262, 989–998. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, Y.; Chi, W.; Shi, H.; Ren, H.; Guo, T. Electrospun Nanofibers Containing P-Nitrophenol Imprinted Nanoparticles for the Hydrolysis of Paraoxon. Chin. J. Polym. Sci. 2014, 32, 1469–1478. [Google Scholar] [CrossRef]
- Molinari, F.N.; De Cesare, F.; Macagnano, A. Housing Molecularly Imprinted Polymer Nanoparticles in Polyvinylpyrrolidone/Multiwall Carbon Nanotube Nanofibers to Detect Chiral Terpene Vapors. Proceedings 2024, 97, 96. [Google Scholar] [CrossRef]
- Macagnano, A.; Molinari, F.N.; Papa, P.; Mancini, T.; Lupi, S.; D’Arco, A.; Taddei, A.R.; Serrecchia, S.; De Cesare, F. Nanofibrous Conductive Sensor for Limonene: One-Step Synthesis via Electrospinning and Molecular Imprinting. Nanomaterials 2024, 14, 1123. [Google Scholar] [CrossRef] [PubMed]
- Pratama, K.F.; Manik, M.E.R.; Rahayu, D.; Hasanah, A.N. Effect of the Molecularly Imprinted Polymer Component Ratio on Analytical Performance. Chem. Pharm. Bull. 2020, 68, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Molinari, F.; Medrano, A.V.; Bacigalupe, A.; Escobar, M.M.; Monsalve, L.N. Different Dispersion States of MWCNT in Aligned Conductive Electrospun PCL/MWCNT Composites. Fuller. Nanotub. Carbon Nanostructures 2018, 26, 667–674. [Google Scholar] [CrossRef]
- Ricca, M.; Foderà, V.; Giacomazza, D.; Leone, M.; Spadaro, G.; Dispenza, C. Probing the Internal Environment of PVP Networks Generated by Irradiation with Different Sources. Colloid. Polym. Sci. 2010, 288, 969–980. [Google Scholar] [CrossRef]
- Biran, I.; Houben, L.; Kossoy, A.; Rybtchinski, B. Transmission Electron Microscopy Methodology to Analyze Polymer Structure with Submolecular Resolution. J. Phys. Chem. C 2024, 128, 5988–5995. [Google Scholar] [CrossRef]
- Martin, H.J.; White, B.T.; Wang, H.; Mays, J.; Saito, T.; Dadmun, M.D. Effect of Solvent Quality and Monomer Water Solubility on Soft Nanoparticle Morphology. In Gels and Other Soft Amorphous Solids; American Chemical Society: Knoxville, TN, USA, 2018; pp. 117–137. [Google Scholar]
- Mueller, A. A Note about Crosslinking Density in Imprinting Polymerization. Molecules 2021, 26, 5139. [Google Scholar] [CrossRef]
- Andriotis, E.G.; Papi, R.M.; Paraskevopoulou, A.; Achilias, D.S. Synthesis of D-Limonene Loaded Polymeric Nanoparticles with Enhanced Antimicrobial Properties for Potential Application in Food Packaging. Nanomaterials 2021, 11, 191. [Google Scholar] [CrossRef]
- Golker, K.; Nicholls, I.A. The Effect of Crosslinking Density on Molecularly Imprinted Polymer Morphology and Recognition. Eur. Polym. J. 2016, 75, 423–430. [Google Scholar] [CrossRef]
- Patel, B.; Pearson, R.; Watanabe, M. Bright Field and Dark Field STEM-IN-SEM Imaging of Polymer Systems. J. Appl. Polym. Sci. 2014, 131, 40851. [Google Scholar] [CrossRef]
- Raymo, F. Nanomaterials Synthesis and Applications: Molecule-Based Devices. In Springer Handbook of Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2007; pp. 13–42. [Google Scholar]
- Salalha, W.; Dror, Y.; Khalfin, R.L.; Cohen, Y.; Yarin, A.L.; Zussman, E. Single-Walled Carbon Nanotubes Embedded in Oriented Polymeric Nanofibers by Electrospinning. Langmuir 2004, 20, 9852–9855. [Google Scholar] [CrossRef] [PubMed]
- Dror, Y.; Salalha, W.; Khalfin, R.L.; Cohen, Y.; Yarin, A.L.; Zussman, E. Carbon Nanotubes Embedded in Oriented Polymer Nanofibers by Electrospinning. Langmuir 2003, 19, 7012–7020. [Google Scholar] [CrossRef]
- Sarabia-Riquelme, R.; Craddock, J.; Morris, E.A.; Eaton, D.; Andrews, R.; Anthony, J.; Weisenberger, M.C. Simple, Low-Cost, Water-Processable n -Type Thermoelectric Composite Films from Multiwall Carbon Nanotubes in Polyvinylpyrrolidone. Synth. Met. 2017, 225, 86–92. [Google Scholar] [CrossRef]
- Macagnano, A.; Molinari, F.N.; Mancini, T.; Lupi, S.; De Cesare, F. UV Light Stereoselective Limonene Sensor Using Electrospun PVP Composite Nanofibers. Proceedings 2024, 97, 131. [Google Scholar] [CrossRef]
- Habboush, S.; Rojas, S.; Rodríguez, N.; Rivadeneyra, A. The Role of Interdigitated Electrodes in Printed and Flexible Electronics. Sensors 2024, 24, 2717. [Google Scholar] [CrossRef]
- Khan, W.S.; Asmatulu, R.; Eltabey, M.M. Electrical and Thermal Characterization of Electrospun PVP Nanocomposite Fibers. J. Nanomater. 2013, 2013, 160931. [Google Scholar] [CrossRef]
- Li, J.; Ma, P.C.; Chow, W.S.; To, C.K.; Tang, B.Z.; Kim, J. -K. Correlations between Percolation Threshold, Dispersion State, and Aspect Ratio of Carbon Nanotubes. Adv. Funct. Mater. 2007, 17, 3207–3215. [Google Scholar] [CrossRef]
- Behnam, A.; Guo, J.; Ural, A. Effects of Nanotube Alignment and Measurement Direction on Percolation Resistivity in Single-Walled Carbon Nanotube Films. J. Appl. Phys. 2007, 102, 044313. [Google Scholar] [CrossRef]
- Bauhofer, W.; Kovacs, J.Z. A Review and Analysis of Electrical Percolation in Carbon Nanotube Polymer Composites. Compos. Sci. Technol. 2009, 69, 1486–1498. [Google Scholar] [CrossRef]
- Kovacs, J.Z.; Velagala, B.S.; Schulte, K.; Bauhofer, W. Two Percolation Thresholds in Carbon Nanotube Epoxy Composites. Compo.s Sci. Technol. 2007, 67, 922–928. [Google Scholar] [CrossRef]
- Penu, C.; Hu, G.; Fernandez, A.; Marchal, P.; Choplin, L. Rheological and Electrical Percolation Thresholds of Carbon Nanotube/Polymer Nanocomposites. Polym. Eng. Sci. 2012, 52, 2173–2181. [Google Scholar] [CrossRef]
- Serban, B.-C.; Cobianu, C.; Dumbravescu, N.; Buiu, O.; Bumbac, M.; Nicolescu, C.M.; Cobianu, C.; Brezeanu, M.; Pachiu, C.; Serbanescu, M. Electrical Percolation Threshold and Size Effects in Polyvinylpyrrolidone-Oxidized Single-Wall Carbon Nanohorn Nanocomposite: The Impact for Relative Humidity Resistive Sensors Design. Sensors 2021, 21, 1435. [Google Scholar] [CrossRef] [PubMed]
- Shanmgam, G.; Mathew, V.; Selvaraj, B.; Thanikachalam, P.M.; Kim, J.; Pichai, M.; Natarajan, A.; Almansour, A.I. Influence of Limonene from Orange Peel in Poly (Ethylene Oxide) PEO/I − /I 3 − Based Nanocrystalline Dye-Sensitized Solar Cell. Chem. Sel. 2022, 7, e202103007. [Google Scholar] [CrossRef]
- Kandhasamy, M.; Shanmugam, G.; Kamaraj, S.; Selvaraj, B.; Gunasekeran, A.; Sambandam, A. Effect of D-Limonene Additive in Copper Redox-Based Quasi-Solid-State Electrolytes on the Performance of Dye-Sensitized Solar Cells. Mater. Today Commun. 2023, 35, 105505. [Google Scholar] [CrossRef]
- Peveler, W.J.; Yazdani, M.; Rotello, V.M. Selectivity and Specificity: Pros and Cons in Sensing. ACS Sens. 2016, 1, 1282–1285. [Google Scholar] [CrossRef]
- Pan, X.; Xue, Q.; Zhang, J.; Guo, Q.; Jin, Y.; Lu, W.; Li, X.; Ling, C. Effective Enhancement of Humidity Sensing Characteristics of Novel Thermally Treated MWCNTs/Polyvinylpyrrolidone Film Caused by Interfacial Effect. Adv. Mater. Interfaces 2016, 3, 1600153. [Google Scholar] [CrossRef]
- Shinde, D.B.; Vlassiouk, I.V.; Talipov, M.R.; Smirnov, S.N. Exclusively Proton Conductive Membranes Based on Reduced Graphene Oxide Polymer Composites. ACS Nano 2019, 13, 13136–13143. [Google Scholar] [CrossRef]
- Venkatasetty, H.V. Electrochemical Amperometric Gas Sensors for Environmental Monitoring and Control. SAE Tech. Pap. 1990, 901296. [Google Scholar] [CrossRef]
- Rutolo, M.F.; Clarkson, J.P.; Covington, J.A. The use of an electronic nose to detect early signs of soft-rot infection in potatoes. Biosyst. Eng. 2018, 167, 137–143. [Google Scholar] [CrossRef]
- MacDougall, S.; Bayansal, F.; Ahmadi, A. Emerging Methods of Monitoring Volatile Organic Compounds for Detection of Plant Pests and Disease. Biosensors 2022, 12, 239. [Google Scholar] [CrossRef]
- Cui, S.; Ling, P.; Zhu, H.; Keener, H. Plant Pest Detection Using an Artificial Nose System: A Review. Sensors 2018, 18, 378. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A Survey on Gas Sensing Technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.B.; Ghatak, B.; Debabhuti, N.; Pal, S.; Sharma, P.; Tudu, B.; Bhattacharyya, N.; Bandyopadhyay, R. Determination of Alpha-Myrcene Volatile in Mango by Quartz Crystal Microbalance Sensor. IEEE Sens. J. 2019, 19, 893–900. [Google Scholar] [CrossRef]
- Zhou, Q.; Zheng, C.; Zhu, L.; Wang, J. A Review on Rapid Detection of Modified Quartz Crystal Microbalance Sensors for Food: Contamination, Flavour and Adulteration. TrAC Trends Anal. Chem. 2022, 157, 116805. [Google Scholar] [CrossRef]
- Dickert, F.L.; Hayden, O.; Bindeus, R.; Mann, K.-J.; Blaas, D.; Waigmann, E. Bioimprinted QCM Sensors for Virus Detection?Screening of Plant Sap. Anal. Bioanal. Chem. 2004, 378, 1929–1934. [Google Scholar] [CrossRef]
- Choi, Y.S.; Son, W.K.; Kwak, H.; Park, J.; Choi, S.; Sim, D.; Kim, M.G.; Kimm, H.; Son, H.; Jeong, D.H.; et al. Real-Time Monitoring of Volatile Organic Compound-Mediated Plant Intercommunication Using Surface-Enhanced Raman Scattering Nanosensor. Adv. Sci. 2024. [Google Scholar] [CrossRef]
- Nikoutadbir, A.; Tarmian, A.; Mohtasebi, S.S.; Abdulkhani, A. Emission of Volatile Organic Compounds from Heat-Treated Scots Pine Wood as Affected by Wood Drying Method: Results Obtained with Olfactory Machine and Headspace Gas Chromatography-Mass Spectrometry. Dry. Technol. 2023, 41, 577–589. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, J.; Cheng, S.; Wang, Y. Detection of Pest Species with Different Ratios in Tea Plant Based on Electronic Nose. Ann. Appl. Biol. 2019, 174, 209–218. [Google Scholar] [CrossRef]
- Kresnawaty, I.; Mulyatni, A.S.; Eris, D.D.; Prakoso, H.T.; Tri-Panji; Triyana, K.; Widiastuti, H. Electronic Nose for Early Detection of Basal Stem Rot Caused by Ganoderma in Oil Palm. IOP Conf. Ser. Earth. Environ. Sci. 2020, 468, 012029. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor Metal Oxide Gas Sensors: A Review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Kataria, S.; Chandel, M.; Kumar, P.; Palanisami, M.; Moun, N.; Kanagarajan, S.; Shanmugam, V. Irrigation-Friendly Sensor to Manage Drought in Crops through Carbon-Based Signature Volatile Sensing. Sens. Actuators B Chem. 2024, 403, 134975. [Google Scholar] [CrossRef]
- Rutolo, M.; Covington, J.; Clarkson, J.; Iliescu, D. Detection of Potato Storage Disease via Gas Analysis: A Pilot Study Using Field Asymmetric Ion Mobility Spectrometry. Sensors 2014, 14, 15939–15952. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.T.; Boock, J.J.; Kemperman, R.H.J.; Wei, M.S.; Beekman, C.R.; Yost, R.A. Portable FAIMS: Applications and Future Perspectives. Int. J. Mass Spectrom. 2017, 422, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Khot, L.R.; Schroeder, B.K. FAIMS Based Sensing of Burkholderia Cepacia Caused Sour Skin in Onions under Bulk Storage Condition. J. Food Meas. Charact. 2017, 11, 1578–1585. [Google Scholar] [CrossRef]
- Sinha, R.; Khot, L.R.; Schroeder, B.K.; Si, Y. Rapid and Non–Destructive Detection of Pectobacterium Carotovorum Causing Soft Rot in Stored Potatoes through Volatile Biomarkers Sensing. Crop Prot. 2017, 93, 122–131. [Google Scholar] [CrossRef]
- Li, Z.; Paul, R.; Ba Tis, T.; Saville, A.C.; Hansel, J.C.; Yu, T.; Ristaino, J.B.; Wei, Q. Non-Invasive Plant Disease Diagnostics Enabled by Smartphone-Based Fingerprinting of Leaf Volatiles. Nat. Plants 2019, 5, 856–866. [Google Scholar] [CrossRef]
- Hassan, M.H.; Omar, A.M.; Daskalakis, E.; Mohamed, A.A.; Boyd, L.A.; Blanford, C.; Grieve, B.; Bartolo, P. JDS. Multi-Layer Biosensor for Pre-Symptomatic Detection of Puccinia Strifformis, the Causal Agent of Yellow Rust. Biosensors 2022, 12, 829. [Google Scholar] [CrossRef]
- Fang, Y.; Ramasamy, R.P. A Portable Electrochemical System for Plant Volatile Detection. ECS Trans. 2018, 85, 1359–1367. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.; Kim, D.; Park, H.; Myoung, S.; Han, J.; Park, C.; Kim, Y.; Choi, C.; Lee, G. Wearable Volatile Organic Compound Sensors for Plant Health Monitoring. Adv. Sustain. Syst. 2024, 8. [Google Scholar] [CrossRef]
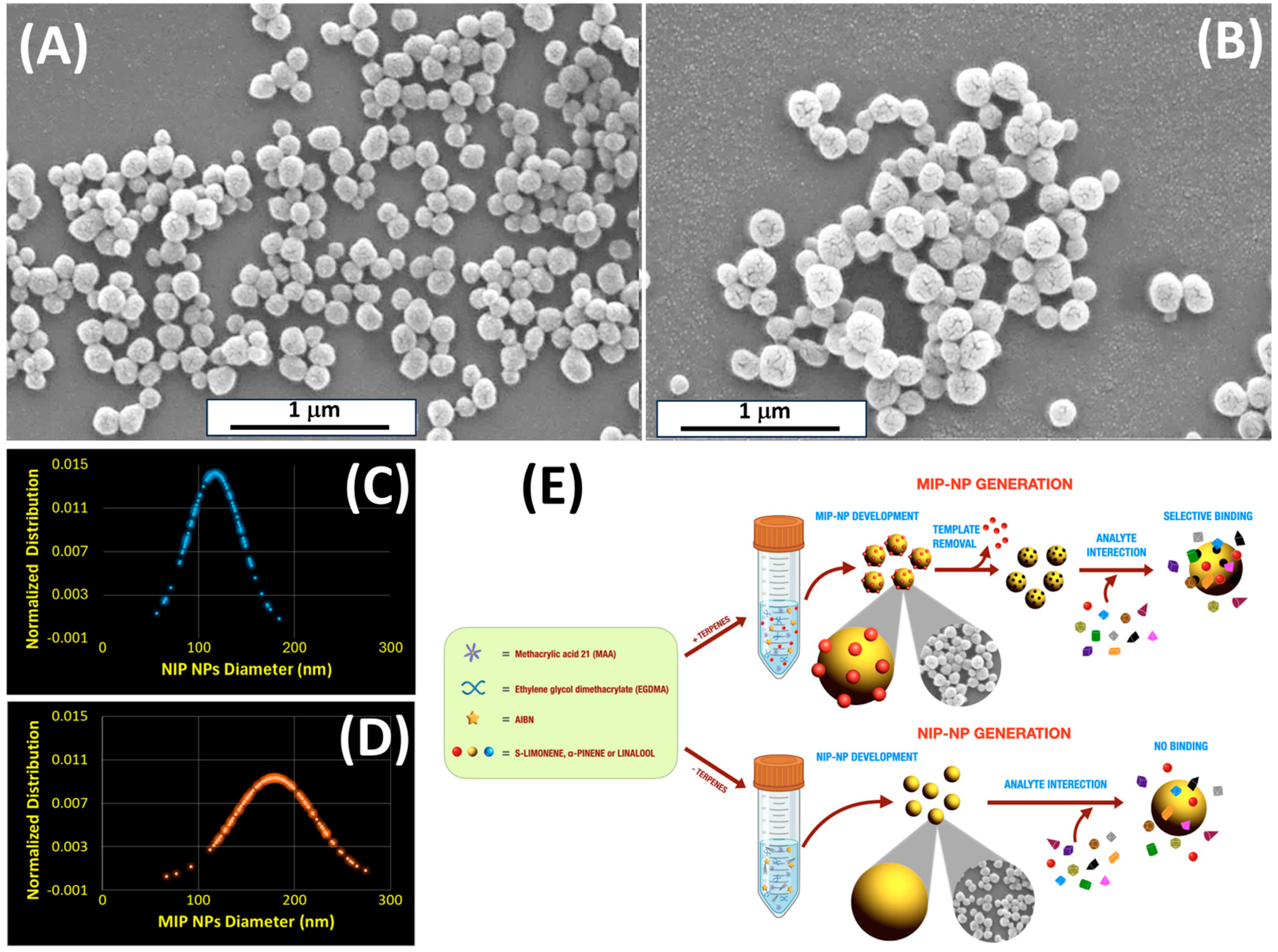

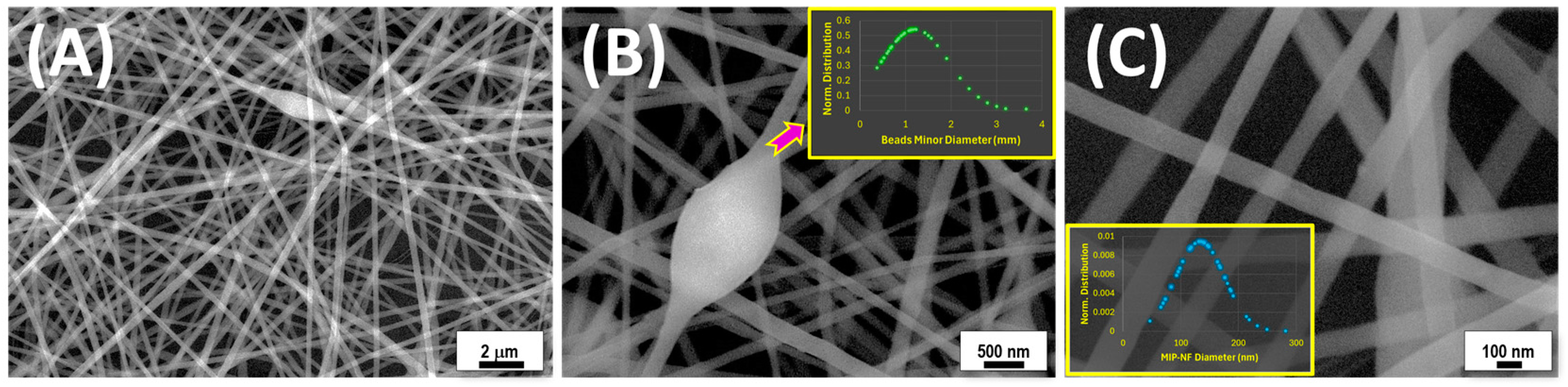

| Mean Diameter (nm) | |
|---|---|
| MIP-NPs | 179.40 ± 43.00 |
| NIP-NPs | 117.15 ± 28.00 |
| R (Ohm) | W/L (µm) | Rs (Ohm/□) | |
|---|---|---|---|
| MIP-NFs | 8.35∙108 ± 1.00∙108 | 2.86∙10−3 | 9.95∙103 ± 1.20∙103 |
| NIP-NFs | 1.54∙109 ± 1.85∙108 | 2.86∙10−3 | 18.35∙103 ± 2.30∙103 |
| Type | Molar Ratio | Sensing Layer | Transducer | Linear Range (ppm) | LOD (ppm) | Reference |
|---|---|---|---|---|---|---|
| T:Styrene:DVB | 0.06:1:1.5 | Film | QCM | 20–250 | 20 | [54] |
| T:MAA:EGDMA | 1:5:20 | Film | QCM | - | - | [53] |
| T:MAA:EGDMA | 1:4:20 | Film | QCM | 300–2100 | 7.43 | [56] |
| T:Styrene:DVB | 0.06:1:1.5 | Film | QCM/IDE | 50 | [58] | |
| T:MAA:EGDMA | 1:4:20 | Film | QCM | 1–1000 | - | [52] |
| T:MAA:EGDMA | 1:4:20 | Film | QCM | 10 | [51] | |
| MAA:EGDMA | 1:5:20 | Film | IDE | 1–400 | - | [57] |
| PAA:PVP:MWCNT | 1:4:8 | Nanofibers | IDE | 1–60 | 0.23 | [77] |
| Sensors | SENS (ppm−1) | LOD (ppb) | LOQ (ppb) | Reference |
|---|---|---|---|---|
| MIP-NF sensors | 0.102 ± 0.022 | 190 | 630 | This study |
| MINF sensors | 0.037 ± 0.001 | 226 | - | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molinari, F.N.; Marelli, M.; Berretti, E.; Serrecchia, S.; Coppola, R.E.; De Cesare, F.; Macagnano, A. Cutting-Edge Sensor Design: MIP Nanoparticle-Functionalized Nanofibers for Gas-Phase Detection of Limonene in Predictive Agriculture. Polymers 2025, 17, 326. https://doi.org/10.3390/polym17030326
Molinari FN, Marelli M, Berretti E, Serrecchia S, Coppola RE, De Cesare F, Macagnano A. Cutting-Edge Sensor Design: MIP Nanoparticle-Functionalized Nanofibers for Gas-Phase Detection of Limonene in Predictive Agriculture. Polymers. 2025; 17(3):326. https://doi.org/10.3390/polym17030326
Chicago/Turabian StyleMolinari, Fabricio Nicolàs, Marcello Marelli, Enrico Berretti, Simone Serrecchia, Roxana Elisabeth Coppola, Fabrizio De Cesare, and Antonella Macagnano. 2025. "Cutting-Edge Sensor Design: MIP Nanoparticle-Functionalized Nanofibers for Gas-Phase Detection of Limonene in Predictive Agriculture" Polymers 17, no. 3: 326. https://doi.org/10.3390/polym17030326
APA StyleMolinari, F. N., Marelli, M., Berretti, E., Serrecchia, S., Coppola, R. E., De Cesare, F., & Macagnano, A. (2025). Cutting-Edge Sensor Design: MIP Nanoparticle-Functionalized Nanofibers for Gas-Phase Detection of Limonene in Predictive Agriculture. Polymers, 17(3), 326. https://doi.org/10.3390/polym17030326









