Two-Dimensional Estrone-Imprinted System on a Self-Assembled Monolayer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Synthesis of Estronyl Chloroformate
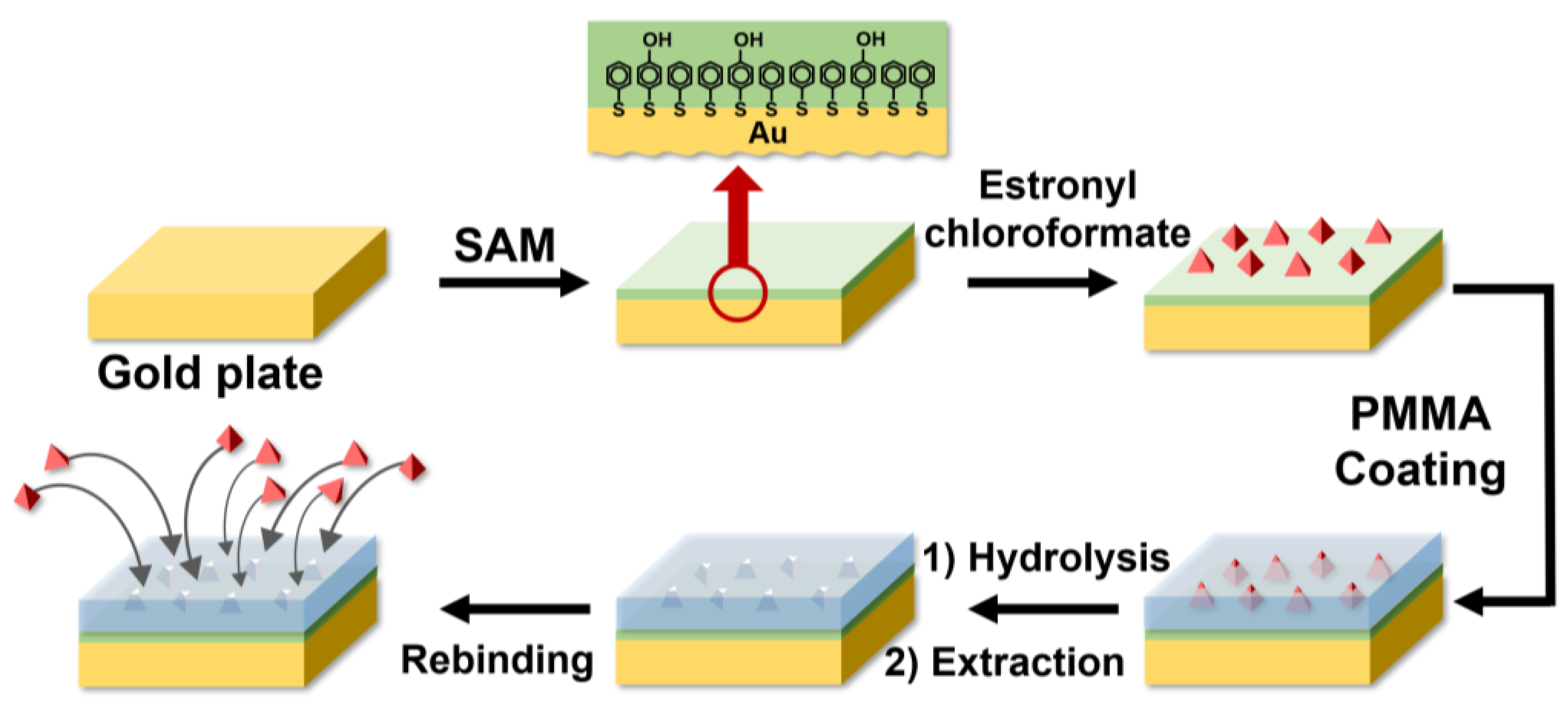
2.3. Formation of a SAM on the Surface of the Gold Plate
2.4. Reaction of Estronyl Chloroformate with 4-Mercaptophenol on the SAM
2.5. Purification of PMMA and Spin Coating of PMMA on the Gold Plate
2.6. Formation of Estrone-Imprinted Sites on the Coating Surface
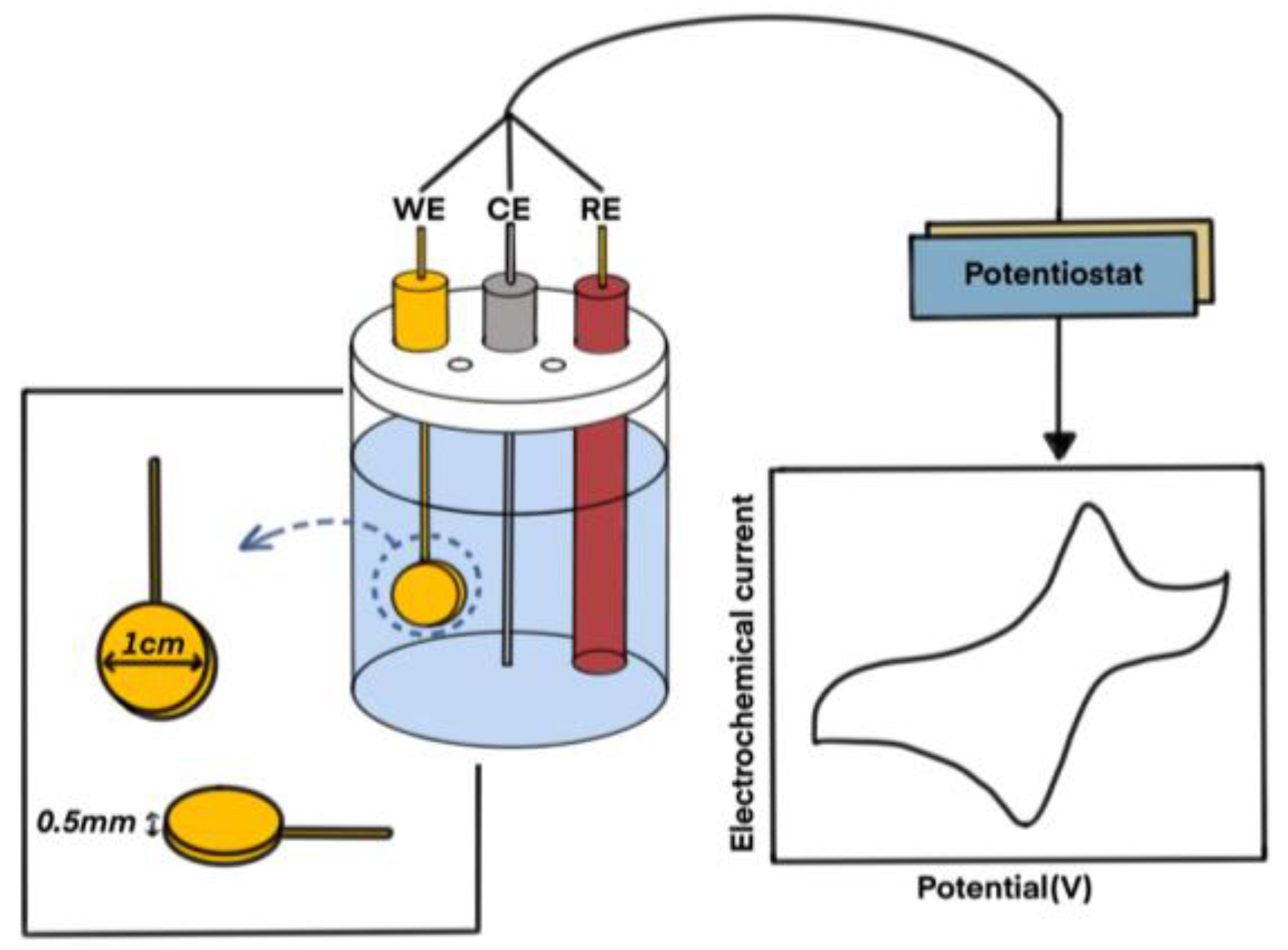
2.7. Electrochemical Measurements
2.8. Formation of a Non-Imprinted Electrode
3. Results and Discussion
3.1. Formation of Estrone-Imprinted Sites on the SAM
3.2. Recognition Ability Estimation of the Estrone-Imprinted Sites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- BelBruno, J.J. Molecularly imprinted polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef] [PubMed]
- Refaat, D.; Aggour, M.G.; Farghali, A.A.; Mahajan, R.M.; Wiklander, J.G.; Nicholls, I.A.; Piletsky, S.A. Strategies for molecular imprinting and the evolution of MIP nanoparticles as plastic antibodies-synthesis and applications. Int. J. Mol. Sci. 2019, 20, 6304. [Google Scholar] [CrossRef] [PubMed]
- Arabi, M.; Ostovan, A.; Li, J.; Wang, X.; Zhang, Z.; Choo, J.; Chen, L. Molecular imprinting: Green perspectives and strategies. Adv. Mater. 2019, 33, 2100543. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef] [PubMed]
- Mtolo, S.P.; Mahlambi, P.N.; Madikizela, L.M. Synthesis and application of a molecularly imprinted polymer in selective solid-phase extraction of efavirenz from water. Water Sci. Technol. 2019, 79, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Crapnell, R.D.; Hudson, A.; Foster, C.W.; Eersels, K.; Grinsven, B.; Cleij, T.J.; Banks, C.E.; Peeters, M. Recent advances in electrosynthesized molecularly imprinted polymer sensing platforms for bioanalyte detection. Sensors 2019, 19, 1204. [Google Scholar] [CrossRef] [PubMed]
- Zarejousheghani, M.; Lorenz, W.; Vanninen, P.; Alizadeh, T.; Cammerer, M.; Borsdorf, H. Molecularly imprinted polymer materials as selective recognition sorbents for explosives: A review. Polymers 2019, 11, 888. [Google Scholar] [CrossRef] [PubMed]
- Kamel, A.H.; Mohammad, S.G.; Awwad, N.S.; Mohammed, Y.Y. Survey on the integration of molecularly imprinted polymers as artificial receptors in potentiometric transducers for pharmaceutical drugs. Int. J. Electrochem. Sci. 2019, 14, 2085–2124. [Google Scholar] [CrossRef]
- Rangel, P.X.M.; Laclef, S.; Xu, J.; Panagiotopoulou, M.; Kovensky, B.; Bui, B.T.S.; Haupt, K. Solid-phase synthesis of molecularly imprinted polymer nanolabels: Affinity tools for cellular bioimaging of glycans. Sci. Rep. 2019, 9, 3923. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J. Molecular imprinting with functional DNA. Small 2019, 15, 1805246. [Google Scholar] [CrossRef]
- Sobiech, M.; Bujak, P.; Lulinski, P.; Pron, A. Semiconductor nanocrystal-polymer hybrid nanomaterials and their application in molecular imprinting. Nanoscale 2019, 11, 12030–12074. [Google Scholar] [CrossRef]
- Bukhari, A.A.H.; Minier, M.; Elsayed, N.H. Surface molecular imprinting of nylon fibers for chiral recognition of d-phenyllactic acid. Polym. Int. 2019, 68, 1460–1467. [Google Scholar] [CrossRef]
- Wang, L.; Zhi, K.; Zhang, Y.; Liu, Y.; Ahang, L.; Yasin, A.; Lin, Q. Molecularly imprinted polymers for gossypol via sol-gel, bulk, and surface layer imprinting-A comparative study. Polymers 2019, 11, 602. [Google Scholar] [CrossRef]
- Ansari, S.; Masoum, S. Molecularly imprinted polymers for capturing and sensing proteins: Current progress and future implications. TrAC Trends Anal. Chem. 2019, 114, 29–47. [Google Scholar] [CrossRef]
- Soman, G.; Vandana, M.; Hegde, G. Molecularly imprinted graphene based biosensor as effective tool for electrochemical sensing of uric acid. Sens. Int. 2023, 4, 100243. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, H.T.; Xie, T.J.; Yang, X.; Dong, A.J.; Zhang, H.; Wang, J.; Wang, Z.Y. Molecularly imprinted polymer on graphene surface for selective and sensitive electrochemical sensing imidacloprid. Sens. Actuators B Chem. 2017, 252, 991–1002. [Google Scholar] [CrossRef]
- Nie, D.; Jiang, D.; Zhang, D.; Liang, Y.; Xue, Y.; Zhou, T.; Jin, L.; Shi, G. Two-dimensional molecular imprinting approach for the electrochemical detection of trinitrotoluene. Sens. Actuators B Chem. 2011, 156, 43–49. [Google Scholar] [CrossRef]
- Voicu, R.; Faid, K.; Farah, A.A.; Bensebaa, F.; Barjovanu, R.; Py, C.; Tao, Y. Nanotemplating for two-dimensional molecular imprinting. Langmuir 2007, 23, 5452–5458. [Google Scholar] [CrossRef]
- Chen, F.; Zhao, W.; Zhang, J.; Kong, J. Magnetic two-dimensional molecularly imprinted materials for the recognition and separation of proteins. Phys. Chem. Chem. Phys. 2016, 18, 718–725. [Google Scholar] [CrossRef]
- Shin, M.J.; Hong, W.H. Sensing capability of molecularly imprinted self-assembled monolayer. Biochem. Eng. J. 2011, 54, 57–61. [Google Scholar] [CrossRef]
- Shin, M.J.; Shin, Y.J.; Shin, J.S. Sensing capability of molecularly imprinted self-assembled monolayer fabricated using dithiol compound. J. Ind. Eng. Chem. 2014, 20, 91–95. [Google Scholar] [CrossRef]
- Shin, M.J.; Shin, Y.J.; Shin, J.S. Cholesterol recognition system by molecular imprinting on self-assembled monolayer. Colloid Surface A 2018, 559, 365–371. [Google Scholar] [CrossRef]
- Shin, M.J.; Shin, Y.J. Selectivity of cholesterol-imprinted system on self-assembled monolayer. Korean J. Chem. Eng. 2020, 37, 290–294. [Google Scholar] [CrossRef]
- Scholtissek, C. Syntheses using the dipyridinium salt of phosgene. Chem. Berichte 1956, 89, 2562–2565. [Google Scholar] [CrossRef]

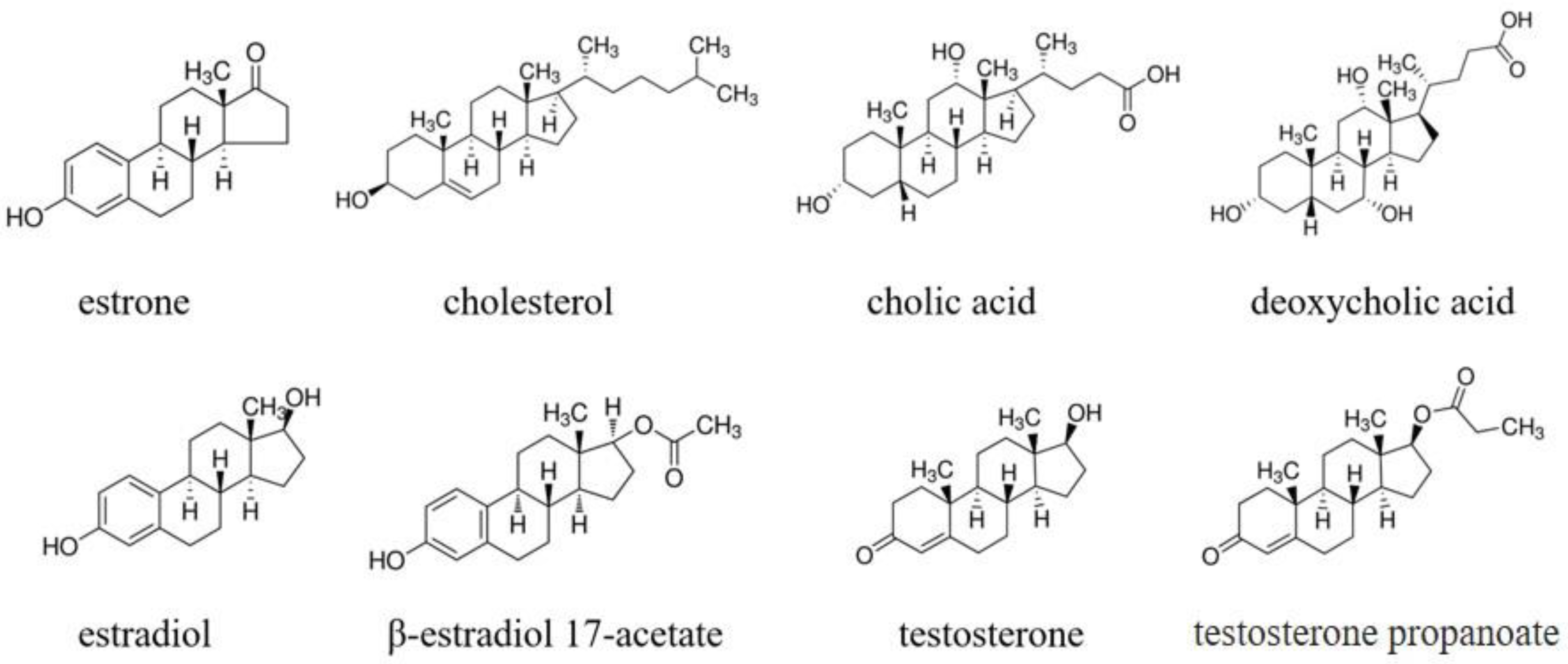

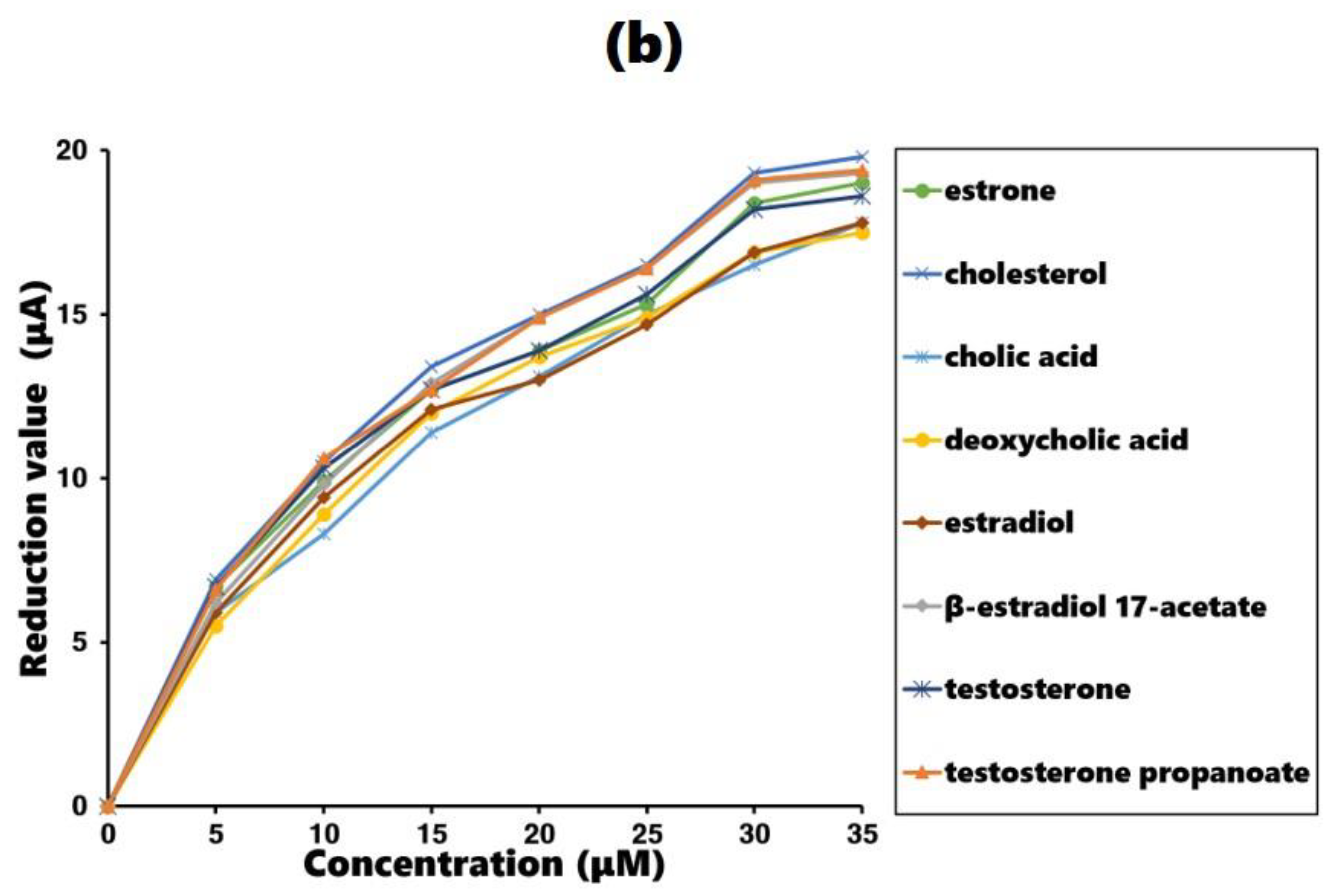
| RedMI | RedNI | ΔRed | Imprinting Factor | Selectivity Factor | The Other Selectivity Factor * | |
|---|---|---|---|---|---|---|
| estrone | 54.2 | 18.4 | 35.8 | 2.95 | 1 | 0.24 |
| cholesterol | 42.9 | 19.3 | 23.6 | 2.22 | 0.66 | 1 |
| cholic acid | 26.8 | 16.5 | 10.3 | 1.61 | 0.29 | 0.042 |
| deoxycholic acid | 23.8 | 16.9 | 6.9 | 1.41 | 0.19 | 0.12 |
| estradiol | 28.5 | 16.9 | 11.6 | 1.69 | 0.32 | 0.24 |
| β-estradiol 17-acetate | 40.5 | 19.1 | 21.4 | 2.12 | 0.60 | 0.38 |
| testosterone | 30.8 | 18.2 | 12.6 | 1.69 | 0.35 | 0.25 |
| testosterone propanoate | 43.1 | 19.0 | 24.1 | 2.27 | 0.67 | 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, M.J.; Shin, J.S. Two-Dimensional Estrone-Imprinted System on a Self-Assembled Monolayer. Polymers 2024, 16, 2035. https://doi.org/10.3390/polym16142035
Shin MJ, Shin JS. Two-Dimensional Estrone-Imprinted System on a Self-Assembled Monolayer. Polymers. 2024; 16(14):2035. https://doi.org/10.3390/polym16142035
Chicago/Turabian StyleShin, Min Jae, and Jae Sup Shin. 2024. "Two-Dimensional Estrone-Imprinted System on a Self-Assembled Monolayer" Polymers 16, no. 14: 2035. https://doi.org/10.3390/polym16142035
APA StyleShin, M. J., & Shin, J. S. (2024). Two-Dimensional Estrone-Imprinted System on a Self-Assembled Monolayer. Polymers, 16(14), 2035. https://doi.org/10.3390/polym16142035







