From Soft Lithography to 3D Printing: Current Status and Future of Microfluidic Device Fabrication
Abstract
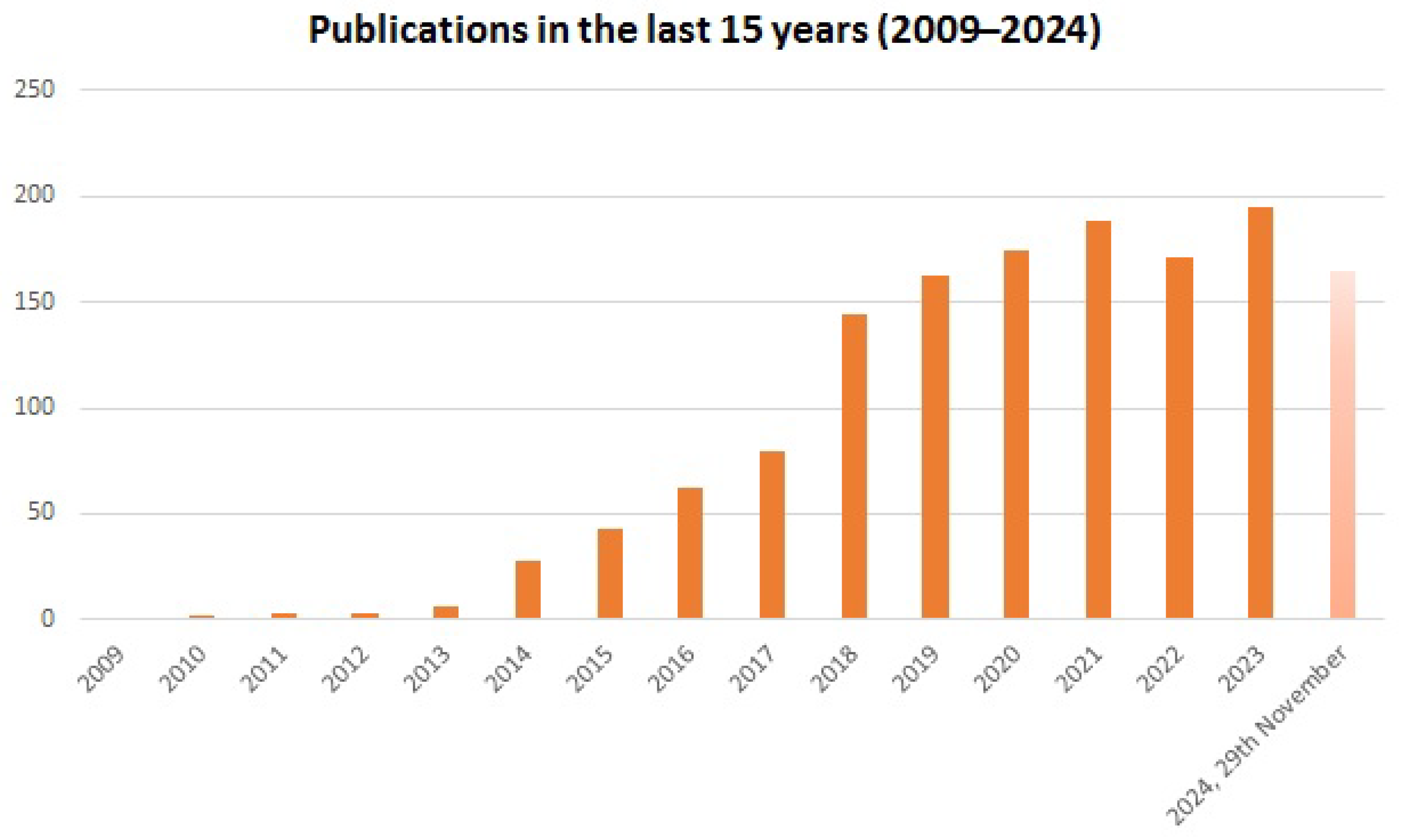
1. Introduction
2. Soft Lithography
- Master Fabrication: A silicon or SU-8 mold is created using photolithography, where a photoresist layer is patterned with UV light to form microchannel structures on the substrate. The master serves as the template for subsequent PDMS molding;
- Casting and Curing PDMS: Liquid PDMS is poured over the master mold and cured at an elevated temperature to solidify. Once cured, the PDMS is peeled off, forming a negative replica of the microchannel patterns;
- Device Assembly: The PDMS replica is bonded to a flat substrate, often a glass slide or another PDMS layer. Bonding is typically achieved via plasma treatment, which activates the surfaces and enables strong adhesion. This creates enclosed microchannels within the device;
- Integration and Functionalization: Additional components, such as inlet and outlet ports, are added to the device. The microfluidic channels can also be functionalized with coatings or molecules for specific applications, such as biological assays or chemical reactions.
3. 3D Printing Technologies for Microfluidic Devices
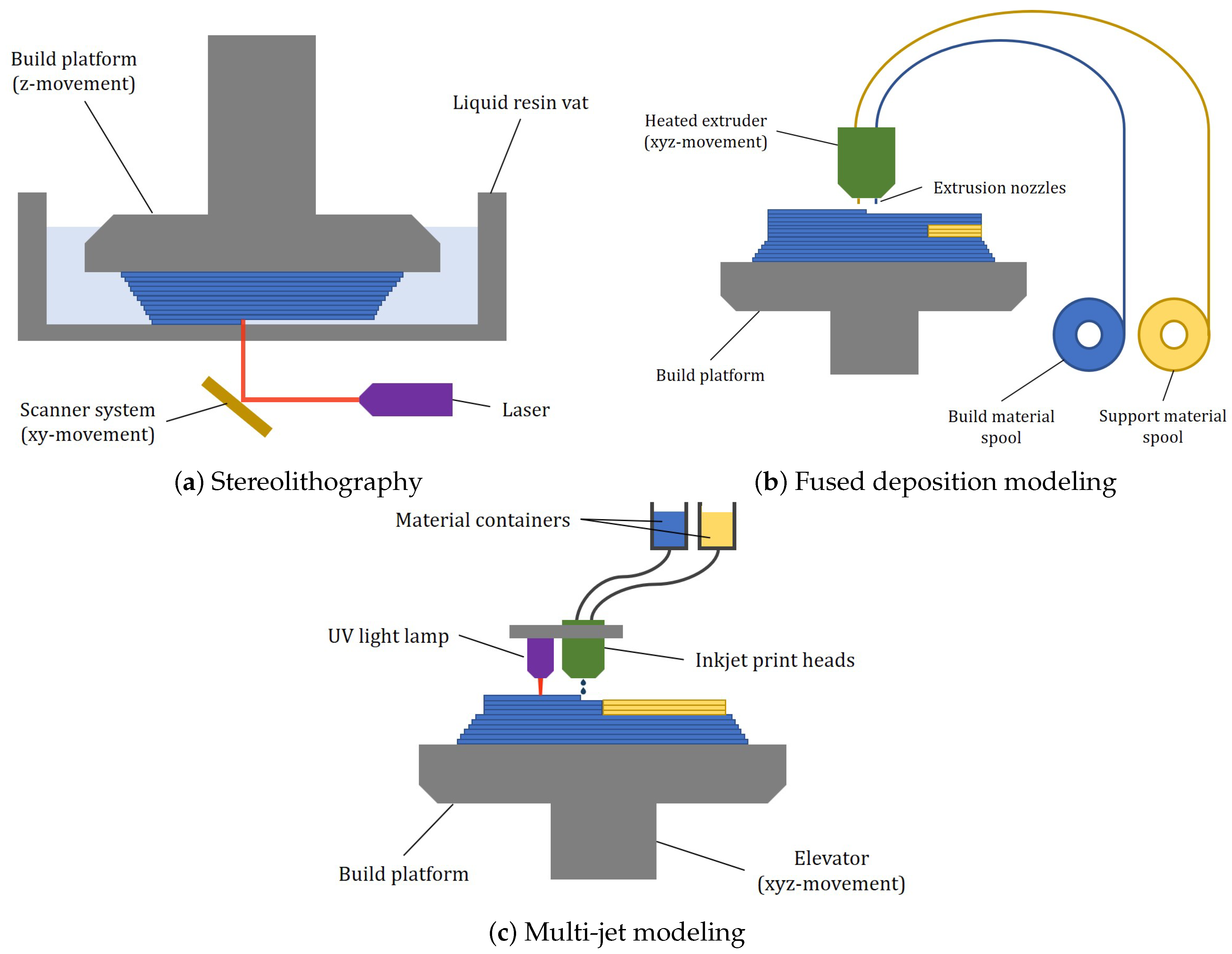
3.1. Photopolymerization
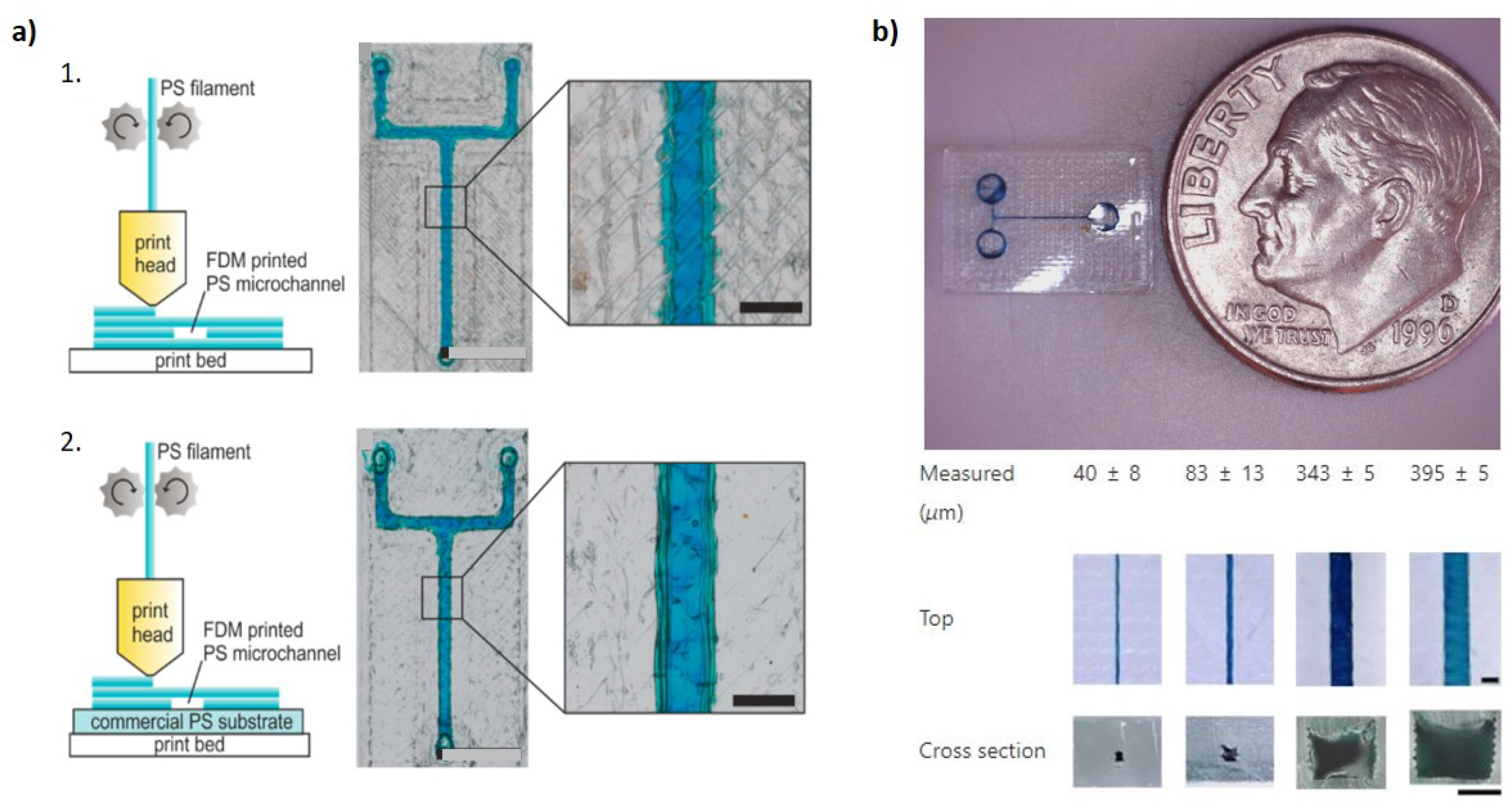
3.2. Fused Deposition Modeling
3.3. Multi-Jet Modeling
4. Comparison of 3D Printing and Soft Lithography
4.1. Cost
4.2. Geometrical Complexity
4.3. Materials
4.4. Transparency
4.5. Biocompatibility
5. Future Developments and Trends
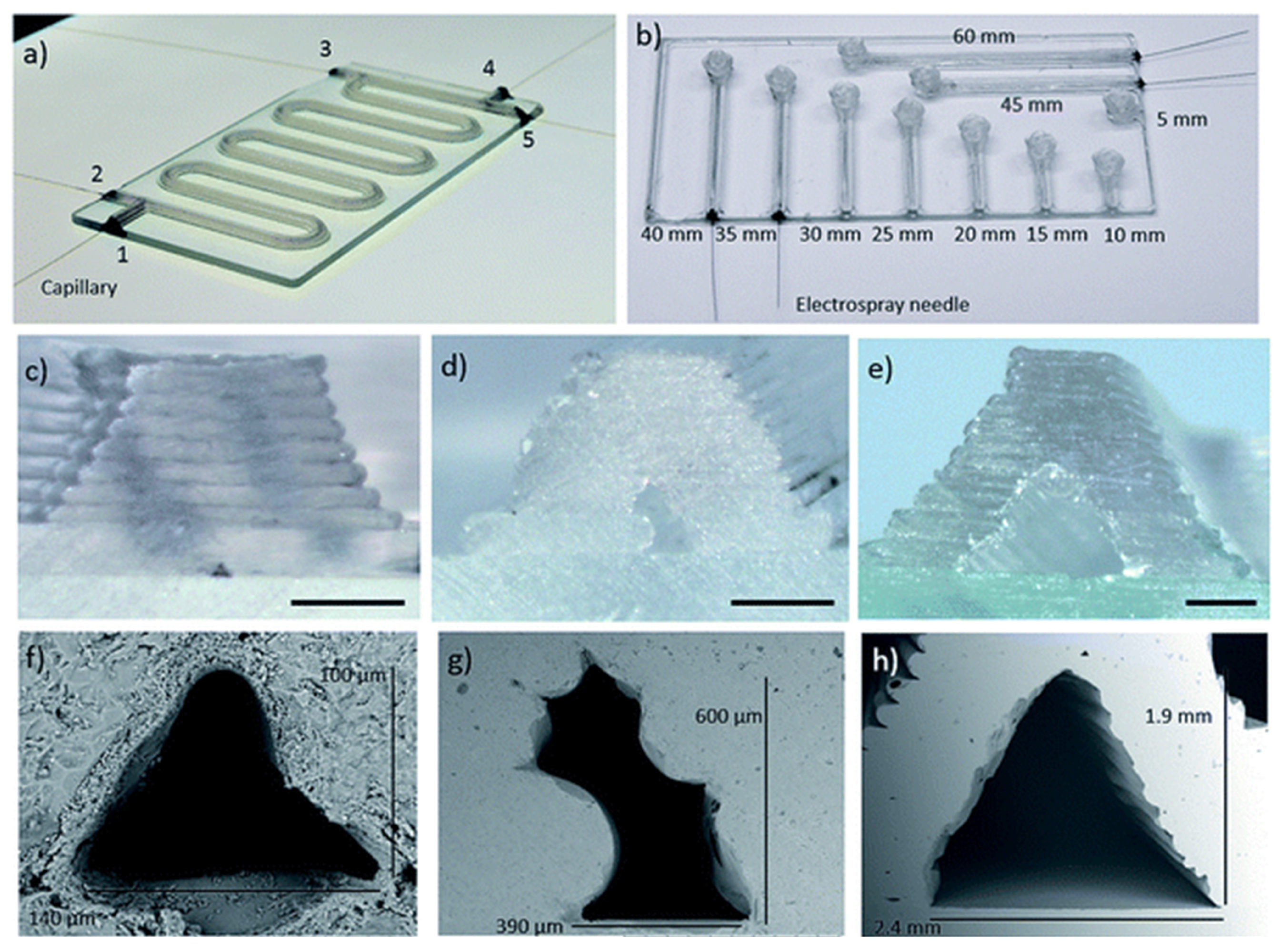
5.1. Technical Improvements in Printing Resolution and Speed
5.2. Development of New Materials for Specialized Applications
5.3. Process Standardization and Regulatory Frameworks
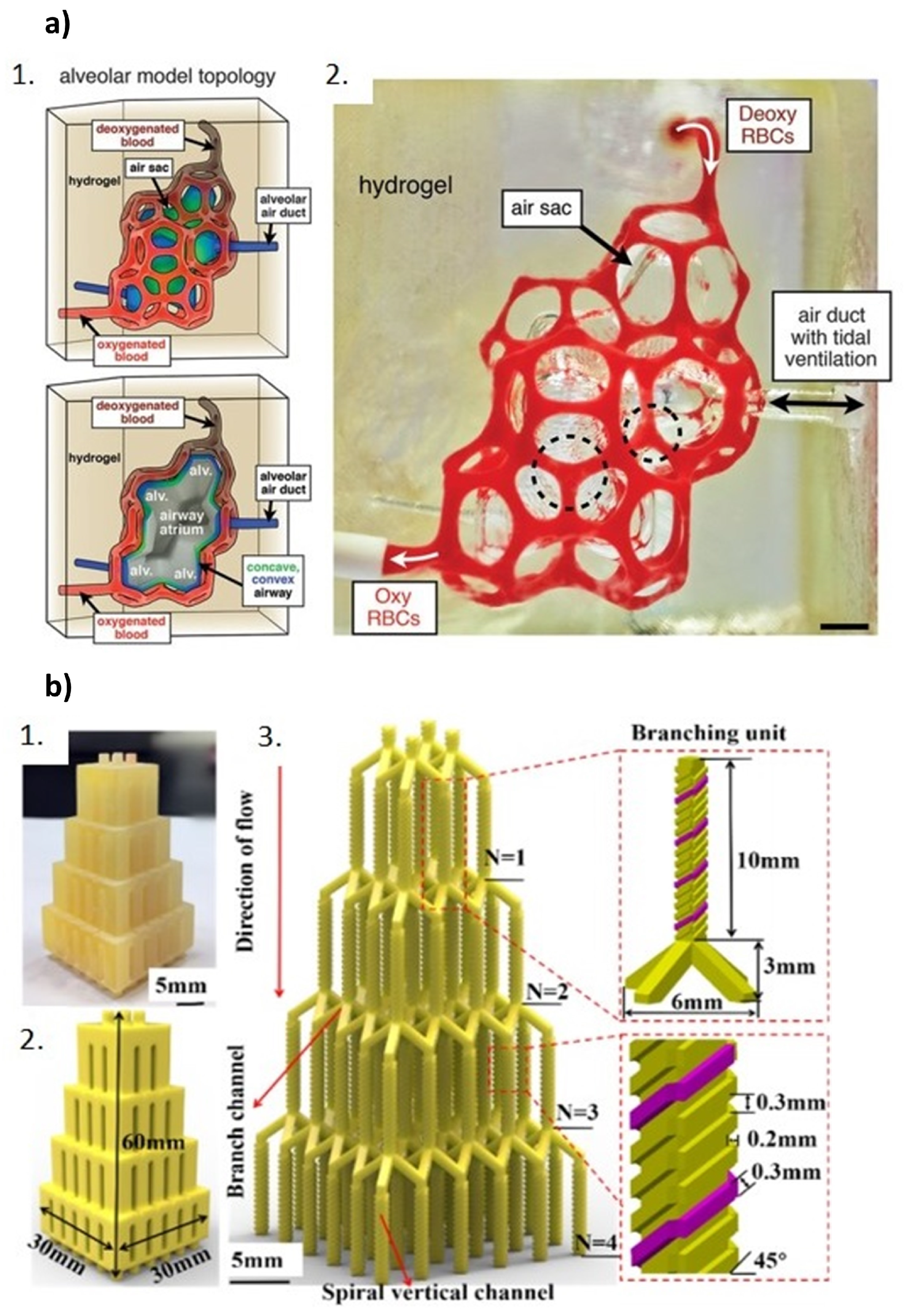
5.4. Bioprinting and Microfluidics: The Future of Organ-on-a-Chip Devices
5.5. Sustainability and Environmental Considerations
6. Conclusions
Funding
Conflicts of Interest
References
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Wereley, S.T.; Shaegh, S.A.M. Fundamentals and Applications of Microfluidics; Artech House: Norwood, MA, USA, 2019. [Google Scholar]
- Amin, R.; Knowlton, S.; Hart, A.; Yenilmez, B.; Ghaderinezhad, F.; Katebifar, S.; Messina, M.; Khademhosseini, A.; Tasoglu, S. 3D-printed microfluidic devices. Biofabrication 2016, 8, 022001. [Google Scholar] [CrossRef] [PubMed]
- Kuncová-Kallio, J.; Kallio, P.J. PDMS and its suitability for analytical microfluidic devices. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, Piscataway, NJ, USA, 28–1 September 2006; pp. 2486–2489. [Google Scholar]
- Dalvand, K.; Ghiasvand, A.; Keshan-Balavandy, S.; Li, F.; Breadmore, M. A simple 3D printed microfluidic device for point-of-care analysis of urinary uric acid. Aust. J. Chem. 2023, 76, 74–80. [Google Scholar] [CrossRef]
- Su, R.; Wang, F.; McAlpine, M.C. 3D printed microfluidics: Advances in strategies, integration, and applications. Lab Chip 2023, 23, 1279–1299. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.M.B.; Ng, S.H.; Li, K.H.H.; Yoon, Y.J. 3D printed microfluidics for biological applications. Lab Chip 2015, 15, 3627–3637. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Urrios, A.; Kang, S.; Folch, A. The upcoming 3D-printing revolution in microfluidics. Lab Chip 2016, 16, 1720–1742. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.A.; Nuzzo, R.G. Recent progress in soft lithography. Mater. Today 2005, 8, 50–56. [Google Scholar] [CrossRef]
- Kim, P.; Kwon, K.W.; Park, M.C.; Lee, S.H.; Kim, S.M.; Suh, K.Y. Soft lithography for microfluidics: A review. BioChip J. 2008, 2, 1–11. [Google Scholar]
- Rose, M.A.; Bowen, J.J.; Morin, S.A. Emergent soft lithographic tools for the fabrication of functional polymeric microstructures. ChemPhysChem 2019, 20, 909–925. [Google Scholar] [CrossRef]
- Trajkovska-Petkoska, A.; Varshneya, R.; Kosc, T.Z.; Marshall, K.L.; Jacobs, S.D. Enhanced electro-optic behavior for shaped polymer cholesteric liquid-crystal flakes made using soft lithography. Adv. Funct. Mater. 2005, 15, 217–222. [Google Scholar] [CrossRef]
- Wolfe, D.B.; Qin, D.; Whitesides, G.M. Rapid prototyping of microstructures by soft lithography for biotechnology. In Microengineering in Biotechnology; Wiley: Weinheim, Germany, 2009; pp. 81–107. [Google Scholar]
- Xia, Y.; Whitesides, G.M. Soft lithography. Angew. Chem. Int. Ed. 1998, 37, 550–575. [Google Scholar] [CrossRef]
- McDonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T.; Wu, H.; Schueller, O.J.; Whitesides, G.M. Fabrication of microfluidic systems in poly (dimethylsiloxane). Electrophor. Int. J. 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Zhao, X.M.; Xia, Y.; Whitesides, G.M. Soft lithographic methods for nano-fabrication. J. Mater. Chem. 1997, 7, 1069–1074. [Google Scholar] [CrossRef]
- Kumar, A.; Whitesides, G.M. Features of gold having micrometer to centimeter dimensions can be formed through a combination of stamping with an elastomeric stamp and an alkanethiol ‘‘ink’’followed by chemical etching. Appl. Phys. Lett. 1993, 63, 2002–2004. [Google Scholar] [CrossRef]
- Quake, S.R.; Scherer, A. From micro-to nanofabrication with soft materials. Science 2000, 290, 1536–1540. [Google Scholar] [CrossRef] [PubMed]
- Rankouhi, B.; Javadpour, S.; Delfanian, F.; Letcher, T. Failure analysis and mechanical characterization of 3D printed ABS with respect to layer thickness and orientation. J. Fail. Anal. Prev. 2016, 16, 467–481. [Google Scholar] [CrossRef]
- Wong, K.V.; Hernandez, A. A review of additive manufacturing. Int. Sch. Res. Not. 2012, 2012, 208760. [Google Scholar] [CrossRef]
- Gardan, J. Additive manufacturing technologies: State of the art and trends. Addit. Manuf. Handb. 2017, 2, 149–168. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.W.; Stucker, B.; Khorasani, M.; Rosen, D.; Stucker, B.; Khorasani, M. Additive Manufacturing Technologies; Springer: Berlin/Heidelberg, Germany, 2021; Volume 17. [Google Scholar]
- Su, R.; Park, S.H.; Ouyang, X.; Ahn, S.I.; McAlpine, M.C. 3D-printed flexible organic light-emitting diode displays. Sci. Adv. 2022, 8, eabl8798. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Wang, J.; Wang, X. A critical review of the use of 3-D printing in the construction industry. Autom. Constr. 2016, 68, 21–31. [Google Scholar] [CrossRef]
- Khan, S.A.; Al Rashid, A.; Muhammad, J.; Ali, F.; Koç, M. 3D Printing Technology for Rapid Response to Climate Change: Challenges and Emergency Needs. Intell. Sustain. Manuf. 2024, 1, 10004. [Google Scholar]
- Dawood, A.; Marti, B.M.; Sauret-Jackson, V.; Darwood, A. 3D printing in dentistry. Br. Dent. J. 2015, 219, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Martinez, D.W.; Espino, M.T.; Cascolan, H.M.; Crisostomo, J.L.; Dizon, J.R.C. A comprehensive review on the application of 3D printing in the aerospace industry. Key Eng. Mater. 2022, 913, 27–34. [Google Scholar] [CrossRef]
- Yan, Q.; Dong, H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A review of 3D printing technology for medical applications. Engineering 2018, 4, 729–742. [Google Scholar] [CrossRef]
- Au, A.K.; Huynh, W.; Horowitz, L.F.; Folch, A. 3D-printed microfluidics. Angew. Chem. Int. Ed. 2016, 55, 3862–3881. [Google Scholar] [CrossRef]
- Goodridge, R.; Tuck, C.; Hague, R. Laser sintering of polyamides and other polymers. Prog. Mater. Sci. 2012, 57, 229–267. [Google Scholar] [CrossRef]
- Bagheri, A.; Jin, J. Photopolymerization in 3D printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef]
- Kaur, M.; Srivastava, A. Photopolymerization: A review. J. Macromol. Sci. Part C Polym. Rev. 2002, 42, 481–512. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, L.; Li, Z.; Wang, S.; Shi, J.; Tang, W.; Li, N.; Yang, J. The recent development of vat photopolymerization: A review. Addit. Manuf. 2021, 48, 102423. [Google Scholar] [CrossRef]
- Yakovlev, A.; Veiko, V.; Yakovlev, E.; Yakhontov, Y.G. Experience in the industrial operation of an apparatus for laser-based three-dimensional synthesis by means of layer-by-layer accretion. J. Opt. Technol. 2002, 69, 183. [Google Scholar] [CrossRef]
- Touri, M.; Kabirian, F.; Saadati, M.; Ramakrishna, S.; Mozafari, M. Additive manufacturing of biomaterials—The evolution of rapid prototyping. Adv. Eng. Mater. 2019, 21, 1800511. [Google Scholar] [CrossRef]
- Raman, R.; Bashir, R. Stereolithographic 3D bioprinting for biomedical applications. In Essentials of 3D Biofabrication and Translation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 89–121. [Google Scholar]
- Skoog, S.A.; Goering, P.L.; Narayan, R.J. Stereolithography in tissue engineering. J. Mater. Sci. Mater. Med. 2014, 25, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Fabbri, P.; Leoni, E.; Mazzanti, F.; Akbari, R.; Antonini, C. Additive manufacturing by digital light processing: A review. Prog. Addit. Manuf. 2023, 8, 331–351. [Google Scholar] [CrossRef]
- Formlabs. Available online: https://formlabs.com/blog/resin-3d-printer-comparison-sla-vs-dlp/ (accessed on 17 September 2024).
- Kuo, A.P.; Bhattacharjee, N.; Lee, Y.S.; Castro, K.; Kim, Y.T.; Folch, A. High-precision stereolithography of biomicrofluidic devices. Adv. Mater. Technol. 2019, 4, 1800395. [Google Scholar] [CrossRef]
- Yao, L.; Hu, P.; Wu, Z.; Liu, W.; Lv, Q.; Nie, Z.; Zhengdi, H. Comparison of accuracy and precision of various types of photo-curing printing technology. J. Phys. Conf. Ser. 2020, 1549, 032151. [Google Scholar] [CrossRef]
- Roberson, G.A.; Sinha, P.K. 3D printing in orthodontics: A practical guide to the printer technology and selection. Proc. Semin. Orthod. 2022, 28, 100–106. [Google Scholar] [CrossRef]
- Shafique, H.; Karamzadeh, V.; Kim, G.; Shen, M.L.; Morocz, Y.; Sohrabi-Kashani, A.; Juncker, D. High-resolution low-cost LCD 3D printing for microfluidics and organ-on-a-chip devices. Lab Chip 2024, 24, 2774–2790. [Google Scholar] [CrossRef]
- Wang, Z.; Martin, N.; Hini, D.; Mills, B.; Kim, K. Rapid fabrication of multilayer microfluidic devices using the liquid crystal display-based stereolithography 3D printing system. 3D Print. Addit. Manuf. 2017, 4, 156–164. [Google Scholar] [CrossRef]
- Selimis, A.; Mironov, V.; Farsari, M. Direct laser writing: Principles and materials for scaffold 3D printing. Microelectron. Eng. 2015, 132, 83–89. [Google Scholar] [CrossRef]
- Wu, Z.L.; Qi, Y.N.; Yin, X.J.; Yang, X.; Chen, C.M.; Yu, J.Y.; Yu, J.C.; Lin, Y.M.; Hui, F.; Liu, P.L.; et al. Polymer-based device fabrication and applications using direct laser writing technology. Polymers 2019, 11, 553. [Google Scholar] [CrossRef] [PubMed]
- Balena, A.; Bianco, M.; Pisanello, F.; De Vittorio, M. Recent advances on high-speed and holographic two-photon direct laser writing. Adv. Funct. Mater. 2023, 33, 2211773. [Google Scholar] [CrossRef]
- Lin, Y.; Gao, C.; Gritsenko, D.; Zhou, R.; Xu, J. Soft lithography based on photolithography and two-photon polymerization. Microfluid. Nanofluidics 2018, 22, 1–11. [Google Scholar] [CrossRef]
- Mao, M.; He, J.; Li, X.; Zhang, B.; Lei, Q.; Liu, Y.; Li, D. The emerging frontiers and applications of high-resolution 3D printing. Micromachines 2017, 8, 113. [Google Scholar] [CrossRef]
- Kushwaha, A.K.; Rahman, M.H.; Hart, D.; Hughes, B.; Saldana, D.A.; Zollars, C.; Rajak, D.K.; Menezes, P.L. Fundamentals of stereolithography: Techniques, properties, and applications. In Tribology of Additively Manufactured Materials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 87–106. [Google Scholar]
- Chockalingam, K.; Jawahar, N.; Chandrasekhar, U. Influence of layer thickness on mechanical properties in stereolithography. Rapid Prototyp. J. 2006, 12, 106–113. [Google Scholar] [CrossRef]
- Cingesar, I.K.; Marković, M.P.; Vrsaljko, D. Effect of post-processing conditions on polyacrylate materials used in stereolithography. Addit. Manuf. 2022, 55, 102813. [Google Scholar] [CrossRef]
- Chennakesava, P.; Narayan, Y.S. Fused deposition modeling-insights. In Proceedings of the International Conference on Advances in Design and Manufacturing ICAD&M, Bangalore, India, 22–24 May 2014; Volume 14, p. 1345. [Google Scholar]
- Dhinakaran, V.; Kumar, K.M.; Ram, P.B.; Ravichandran, M.; Vinayagamoorthy, M. A review on recent advancements in fused deposition modeling. Mater. Today Proc. 2020, 27, 752–756. [Google Scholar] [CrossRef]
- Rehmani, M.A.A.; Jaywant, S.A.; Arif, K.M. Study of microchannels fabricated using desktop fused deposition modeling systems. Micromachines 2020, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Rajan, K.; Samykano, M.; Kadirgama, K.; Harun, W.S.W.; Rahman, M.M. Fused deposition modeling: Process, materials, parameters, properties, and applications. Int. J. Adv. Manuf. Technol. 2022, 120, 1531–1570. [Google Scholar] [CrossRef]
- Palanisamy, C.; Raman, R.; Dhanraj, P.K. Additive manufacturing: A review on mechanical properties of polyjet and FDM printed parts. Polym. Bull. 2022, 79, 7065–7116. [Google Scholar] [CrossRef]
- Bakır, A.A.; Atik, R.; Özerinç, S. Mechanical properties of thermoplastic parts produced by fused deposition modeling: A review. Rapid Prototyp. J. 2021, 27, 537–561. [Google Scholar] [CrossRef]
- Elhattab, K.; Bhaduri, S.B.; Sikder, P. Influence of fused deposition modelling nozzle temperature on the rheology and mechanical properties of 3D printed β-tricalcium phosphate (tcp)/polylactic acid (pla) composite. Polymers 2022, 14, 1222. [Google Scholar] [CrossRef] [PubMed]
- Mbow, M.M.; Marin, P.R.; Pourroy, F. Extruded diameter dependence on temperature and velocity in the fused deposition modeling process. Prog. Addit. Manuf. 2020, 5, 139–152. [Google Scholar] [CrossRef]
- Barclift, M.W.; Williams, C.B. Examining variability in the mechanical properties of parts manufactured via polyjet direct 3D printing. In Proceedings of the International Solid Freeform Fabrication Symposium, Austin, TX, USA, 6–8 August 2012; pp. 6–8. [Google Scholar]
- Schneider, K.H.; Oberoi, G.; Unger, E.; Janjic, K.; Rohringer, S.; Heber, S.; Agis, H.; Schedle, A.; Kiss, H.; Podesser, B.K.; et al. Medical 3D printing with polyjet technology: Effect of material type and printing orientation on printability, surface structure and cytotoxicity. 3D Print. Med. 2023, 9, 27. [Google Scholar] [CrossRef]
- Tee, Y.L.; Peng, C.; Pille, P.; Leary, M.; Tran, P. PolyJet 3D printing of composite materials: Experimental and modelling approach. JOM 2020, 72, 1105–1117. [Google Scholar] [CrossRef]
- Kitsakis, K.; Moza, Z.; Iakovakis, V.; Mastorakis, N.; Kechagias, J. An investigation of dimensional accuracy of multi-jet modeling parts. In Proceedings of the International Conference in Applied Mathematics, Computational Science and Engineering, Crete, Greece, 25–27 June 2015; pp. 17–19. [Google Scholar]
- Vu, I.Q.; Bass, L.B.; Williams, C.B.; Dillard, D.A. Characterizing the effect of print orientation on interface integrity of multi-material jetting additive manufacturing. Addit. Manuf. 2018, 22, 447–461. [Google Scholar] [CrossRef]
- Castiaux, A.D.; Pinger, C.W.; Hayter, E.A.; Bunn, M.E.; Martin, R.S.; Spence, D.M. PolyJet 3D-printed enclosed microfluidic channels without photocurable supports. Anal. Chem. 2019, 91, 6910–6917. [Google Scholar] [CrossRef] [PubMed]
- Selemani, M.A.; Castiaux, A.D.; Martin, R.S. PolyJet-based 3D printing against micromolds to produce channel structures for microchip electrophoresis. ACS Omega 2022, 7, 13362–13370. [Google Scholar] [CrossRef]
- Au, A.K.; Lee, W.; Folch, A. Mail-order microfluidics: Evaluation of stereolithography for the production of microfluidic devices. Lab Chip 2014, 14, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Linke, B.S.; Voet, H.; Falk, B.; Schmitt, R.; Lam, M. Cost, sustainability and surface roughness quality—A comprehensive analysis of products made with personal 3D printers. CIRP J. Manuf. Sci. Technol. 2017, 16, 1–11. [Google Scholar] [CrossRef]
- Lee, J.M.; Zhang, M.; Yeong, W.Y. Characterization and evaluation of 3D printed microfluidic chip for cell processing. Microfluid. Nanofluidics 2016, 20, 1–15. [Google Scholar] [CrossRef]
- Kinstlinger, I.S.; Miller, J.S. 3D-printed fluidic networks as vasculature for engineered tissue. Lab Chip 2016, 16, 2025–2043. [Google Scholar] [CrossRef]
- Kotz, F.; Helmer, D.; Rapp, B.E. Emerging technologies and materials for high-resolution 3D printing of microfluidic chips. In Microfluidics in Biotechnology; Springer: Cham, Switzerland, 2020; pp. 37–66. [Google Scholar]
- Paulsen, S.; Miller, J. Tissue vascularization through 3D printing: Will technology bring us flow? Dev. Dyn. 2015, 244, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Hansen, C.J.; Aragón, A.M.; Geubelle, P.H.; White, S.R.; Lewis, J.A. Direct-write assembly of biomimetic microvascular networks for efficient fluid transport. Soft Matter 2010, 6, 739–742. [Google Scholar] [CrossRef]
- Kovach, K.; LaBarbera, M.; Moyer, M.; Cmolik, B.; Van Lunteren, E.; Gupta, A.S.; Capadona, J.; Potkay, J. In vitro evaluation and in vivo demonstration of a biomimetic, hemocompatible, microfluidic artificial lung. Lab Chip 2015, 15, 1366–1375. [Google Scholar] [CrossRef]
- Grigoryan, B.; Paulsen, S.J.; Corbett, D.C.; Sazer, D.W.; Fortin, C.L.; Zaita, A.J.; Greenfield, P.T.; Calafat, N.J.; Gounley, J.P.; Ta, A.H.; et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 2019, 364, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, H.; Wu, D.; Chen, Q.; Zhou, Z.; Zhang, R.; Peng, X.; Su, Y.C.; Sun, D. 3D printed microfluidic chip for multiple anticancer drug combinations. Sens. Actuators B Chem. 2018, 276, 507–516. [Google Scholar] [CrossRef]
- Au, A.K.; Bhattacharjee, N.; Horowitz, L.F.; Chang, T.C.; Folch, A. 3D-printed microfluidic automation. Lab Chip 2015, 15, 1934–1941. [Google Scholar] [CrossRef]
- Rogers, C.I.; Qaderi, K.; Woolley, A.T.; Nordin, G.P. 3D printed microfluidic devices with integrated valves. Biomicrofluidics 2015, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Beauchamp, M.; Perry, S.; Woolley, A.T.; Nordin, G.P. Optical approach to resin formulation for 3D printed microfluidics. RSC Adv. 2015, 5, 106621–106632. [Google Scholar] [CrossRef]
- Gong, H.; Woolley, A.T.; Nordin, G.P. High density 3D printed microfluidic valves, pumps, and multiplexers. Lab Chip 2016, 16, 2450–2458. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhang, Y.S.; Santiago, G.T.d.; Alvarez, M.M.; Ribas, J.; Jonas, S.J.; Weiss, P.S.; Andrews, A.M.; Aizenberg, J.; Khademhosseini, A. Interplay between materials and microfluidics. Nat. Rev. Mater. 2017, 2, 1–15. [Google Scholar] [CrossRef]
- Fu, E.; Wentland, L. A survey of 3D printing technology applied to paper microfluidics. Lab Chip 2022, 22, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Michelini, E.; Cevenini, L.; Calabria, D.; Calabretta, M.M.; Simoni, P. Integrating biochemiluminescence detection on smartphones: Mobile chemistry platform for point-of-need analysis. Anal. Chem. 2014, 86, 7299–7304. [Google Scholar] [CrossRef] [PubMed]
- Dirkzwager, R.M.; Liang, S.; Tanner, J.A. Development of aptamer-based point-of-care diagnostic devices for malaria using three-dimensional printing rapid prototyping. ACS Sens. 2016, 1, 420–426. [Google Scholar] [CrossRef]
- Chiang, C.K.; Kurniawan, A.; Kao, C.Y.; Wang, M.J. Single step and mask-free 3D wax printing of microfluidic paper-based analytical devices for glucose and nitrite assays. Talanta 2019, 194, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tran, L.; Filipe, C.D.; Brennan, J.D.; Pelton, R.H. Printed thin films with controlled porosity as lateral flow media. Ind. Eng. Chem. Res. 2019, 58, 21014–21021. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Hanson, R.L.; Almughamsi, H.M.; Pang, C.; Fish, T.R.; Woolley, A.T. Microfluidics: Innovations in materials and their fabrication and functionalization. Anal. Chem. 2019, 92, 150–168. [Google Scholar] [CrossRef]
- Gal-Or, E.; Gershoni, Y.; Scotti, G.; Nilsson, S.M.; Saarinen, J.; Jokinen, V.; Strachan, C.J.; af Gennäs, G.B.; Yli-Kauhaluoma, J.; Kotiaho, T. Chemical analysis using 3D printed glass microfluidics. Anal. Methods 2019, 11, 1802–1810. [Google Scholar] [CrossRef]
- Li, M.; Yue, L.; Rajan, A.C.; Yu, L.; Sahu, H.; Montgomery, S.M.; Ramprasad, R.; Qi, H.J. Low-temperature 3D printing of transparent silica glass microstructures. Sci. Adv. 2023, 9, eadi2958. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Meyers, C.; Yee, T.D.; Dudukovic, N.A.; Destino, J.F.; Zhu, C.; Duoss, E.B.; Baumann, T.F.; Suratwala, T.; Smay, J.E.; et al. 3D-printed transparent glass. Adv. Mater. 2017, 29, 1701181. [Google Scholar] [CrossRef] [PubMed]
- Kotz, F.; Helmer, D.; Rapp, B.E. Additive manufacturing of microfluidic glass chips. In Proceedings of the Microfluidics, BioMEMS, and Medical Microsystems XVI, SPIE, Bellingham, WA, USA, 17–18 February 2018; Volume 10491, pp. 42–47. [Google Scholar]
- Musgrove, H.B.; Catterton, M.A.; Pompano, R.R. Applied tutorial for the design and fabrication of biomicrofluidic devices by resin 3D printing. Anal. Chim. Acta 2022, 1209, 339842. [Google Scholar] [CrossRef] [PubMed]
- Marchenkova, M.A.; Chapek, S.V.; Konarev, P.V.; Ilina, K.B.; Peters, G.S.; Pisarevsky, Y.V.; Shishkov, V.A.; Soldatov, A.V.; Kovalchuk, M.V. 3D Printed Microfluidic Cell for SAXS Time-Resolved Measurements of the Structure of Protein Crystallization Solutions. Crystals 2023, 13, 938. [Google Scholar] [CrossRef]
- Fritschen, A.; Bell, A.K.; Königstein, I.; Stühn, L.; Stark, R.W.; Blaeser, A. Investigation and comparison of resin materials in transparent DLP-printing for application in cell culture and organs-on-a-chip. Biomater. Sci. 2022, 10, 1981–1994. [Google Scholar] [CrossRef] [PubMed]
- Urrios, A.; Parra-Cabrera, C.; Bhattacharjee, N.; Gonzalez-Suarez, A.M.; Rigat-Brugarolas, L.G.; Nallapatti, U.; Samitier, J.; DeForest, C.A.; Posas, F.; Garcia-Cordero, J.L.; et al. 3D-printing of transparent bio-microfluidic devices in PEG-DA. Lab Chip 2016, 16, 2287–2294. [Google Scholar] [CrossRef] [PubMed]
- Romanov, V.; Samuel, R.; Chaharlang, M.; Jafek, A.R.; Frost, A.; Gale, B.K. FDM 3D printing of high-pressure, heat-resistant, transparent microfluidic devices. Anal. Chem. 2018, 90, 10450–10456. [Google Scholar] [CrossRef]
- Quero, R.F.; da Silveira, G.D.; da Silva, J.A.F.; de Jesus, D.P. Understanding and improving FDM 3D printing to fabricate high-resolution and optically transparent microfluidic devices. Lab Chip 2021, 21, 3715–3729. [Google Scholar] [CrossRef]
- Mader, M.; Rein, C.; Konrat, E.; Meermeyer, S.L.; Lee-Thedieck, C.; Kotz-Helmer, F.; Rapp, B.E. Fused deposition modeling of microfluidic chips in transparent polystyrene. Micromachines 2021, 12, 1348. [Google Scholar] [CrossRef]
- Nelson, M.D.; Ramkumar, N.; Gale, B.K. Flexible, transparent, sub-100 µm microfluidic channels with fused deposition modeling 3D-printed thermoplastic polyurethane. J. Micromech. Microeng. 2019, 29, 095010. [Google Scholar] [CrossRef]
- Liqcreate. Available online: https://www.liqcreate.com/supportarticles/resin-3dprinting-385nm-405nm/ (accessed on 23 September 2024).
- van der Linden, P.J.; Popov, A.M.; Pontoni, D. Accurate and rapid 3D printing of microfluidic devices using wavelength selection on a DLP printer. Lab Chip 2020, 20, 4128–4140. [Google Scholar] [CrossRef]
- Rupal, B.S.; Garcia, E.A.; Ayranci, C.; Qureshi, A.J. 3D printed 3D-microfluidics: Recent developments and design challenges. J. Integr. Des. Process Sci. 2018, 22, 5–20. [Google Scholar] [CrossRef]
- Guttridge, C.; Shannon, A.; O’Sullivan, A.; O’Sullivan, K.J.; O’Sullivan, L.W. Biocompatible 3D printing resins for medical applications: A review of marketed intended use, biocompatibility certification, and post-processing guidance. Ann. 3D Print. Med. 2022, 5, 100044. [Google Scholar] [CrossRef]
- Alifui-Segbaya, F.; Varma, S.; Lieschke, G.J.; George, R. Biocompatibility of photopolymers in 3D printing. 3D Print. Addit. Manuf. 2017, 4, 185–191. [Google Scholar] [CrossRef]
- Sta. Agueda, J.R.H.; Chen, Q.; Maalihan, R.D.; Ren, J.; da Silva, Í.G.; Dugos, N.P.; Caldona, E.B.; Advincula, R.C. 3D printing of biomedically relevant polymer materials and biocompatibility. MRS Commun. 2021, 11, 197–212. [Google Scholar] [CrossRef]
- Warr, C.; Valdoz, J.C.; Bickham, B.P.; Knight, C.J.; Franks, N.A.; Chartrand, N.; Van Ry, P.M.; Christensen, K.A.; Nordin, G.P.; Cook, A.D. Biocompatible PEGDA resin for 3D printing. ACS Appl. Bio Mater. 2020, 3, 2239–2244. [Google Scholar] [CrossRef]
- Boaks, M.; Roper, C.; Viglione, M.; Hooper, K.; Woolley, A.T.; Christensen, K.A.; Nordin, G.P. Biocompatible High-Resolution 3D-Printed Microfluidic Devices: Integrated Cell Chemotaxis Demonstration. Micromachines 2023, 14, 1589. [Google Scholar] [CrossRef] [PubMed]
- Kotz, F.; Mader, M.; Dellen, N.; Risch, P.; Kick, A.; Helmer, D.; Rapp, B.E. Fused deposition modeling of microfluidic chips in polymethylmethacrylate. Micromachines 2020, 11, 873. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, B.; Xiao, J.; Zhang, Q. An approach to improve the resolution of DLP 3D printing by parallel mechanism. Appl. Sci. 2022, 12, 12905. [Google Scholar] [CrossRef]
- Saska, S.; Pilatti, L.; Blay, A.; Shibli, J.A. Bioresorbable polymers: Advanced materials and 4D printing for tissue engineering. Polymers 2021, 13, 563. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.M.; Amaro, A.M.; Piedade, A.P. 3D printing of polymeric bioresorbable stents: A strategy to improve both cellular compatibility and mechanical properties. Polymers 2022, 14, 1099. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; He, C.; Fu, J.; Han, Q.; He, Y. Balancing the customization and standardization: Exploration and layout surrounding the regulation of the growing field of 3D-printed medical devices in China. Bio-Des. Manuf. 2022, 5, 580–606. [Google Scholar] [CrossRef]
- Arcot, Y.; Samuel, G.; Kong, L. Manufacturability and surface characterisation of polymeric microfluidic devices for biomedical applications. Int. J. Adv. Manuf. Technol. 2022, 121, 3093–3110. [Google Scholar] [CrossRef]
- Yi, H.G.; Lee, H.; Cho, D.W. 3D printing of organs-on-chips. Bioengineering 2017, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Choudhury, D. Microfluidic bioprinting for organ-on-a-chip models. Drug Discov. Today 2019, 24, 1248–1257. [Google Scholar] [CrossRef]
- Carvalho, V.; Gonçalves, I.; Lage, T.; Rodrigues, R.O.; Minas, G.; Teixeira, S.F.; Moita, A.S.; Hori, T.; Kaji, H.; Lima, R.A. 3D printing techniques and their applications to organ-on-a-chip platforms: A systematic review. Sensors 2021, 21, 3304. [Google Scholar] [CrossRef]
- van Berlo, D.; van de Steeg, E.; Amirabadi, H.E.; Masereeuw, R. The potential of multi-organ-on-chip models for assessment of drug disposition as alternative to animal testing. Curr. Opin. Toxicol. 2021, 27, 8–17. [Google Scholar] [CrossRef]
- Bourhis, F.L.; Kerbrat, O.; Hascoet, J.Y.; Mognol, P. Sustainable manufacturing: Evaluation and modeling of environmental impacts in additive manufacturing. Int. J. Adv. Manuf. Technol. 2013, 69, 1927–1939. [Google Scholar] [CrossRef]
- Shuaib, M.; Haleem, A.; Kumar, S.; Javaid, M. Impact of 3D Printing on the environment: A literature-based study. Sustain. Oper. Comput. 2021, 2, 57–63. [Google Scholar] [CrossRef]
- Nyika, J.; Mwema, F.M.; Mahamood, R.; Akinlabi, E.T.; Jen, T. Advances in 3D printing materials processing-environmental impacts and alleviation measures. Adv. Mater. Process. Technol. 2022, 8, 1275–1285. [Google Scholar] [CrossRef]
- Khosravani, M.R.; Reinicke, T. On the environmental impacts of 3D printing technology. Appl. Mater. Today 2020, 20, 100689. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Harasek, M.; Gföhler, M. From Soft Lithography to 3D Printing: Current Status and Future of Microfluidic Device Fabrication. Polymers 2025, 17, 455. https://doi.org/10.3390/polym17040455
Xu J, Harasek M, Gföhler M. From Soft Lithography to 3D Printing: Current Status and Future of Microfluidic Device Fabrication. Polymers. 2025; 17(4):455. https://doi.org/10.3390/polym17040455
Chicago/Turabian StyleXu, Jingjing, Michael Harasek, and Margit Gföhler. 2025. "From Soft Lithography to 3D Printing: Current Status and Future of Microfluidic Device Fabrication" Polymers 17, no. 4: 455. https://doi.org/10.3390/polym17040455
APA StyleXu, J., Harasek, M., & Gföhler, M. (2025). From Soft Lithography to 3D Printing: Current Status and Future of Microfluidic Device Fabrication. Polymers, 17(4), 455. https://doi.org/10.3390/polym17040455







