Cellulose-Derived Battery Separators: A Minireview on Advances Towards Environmental Sustainability
Abstract
:1. Introduction
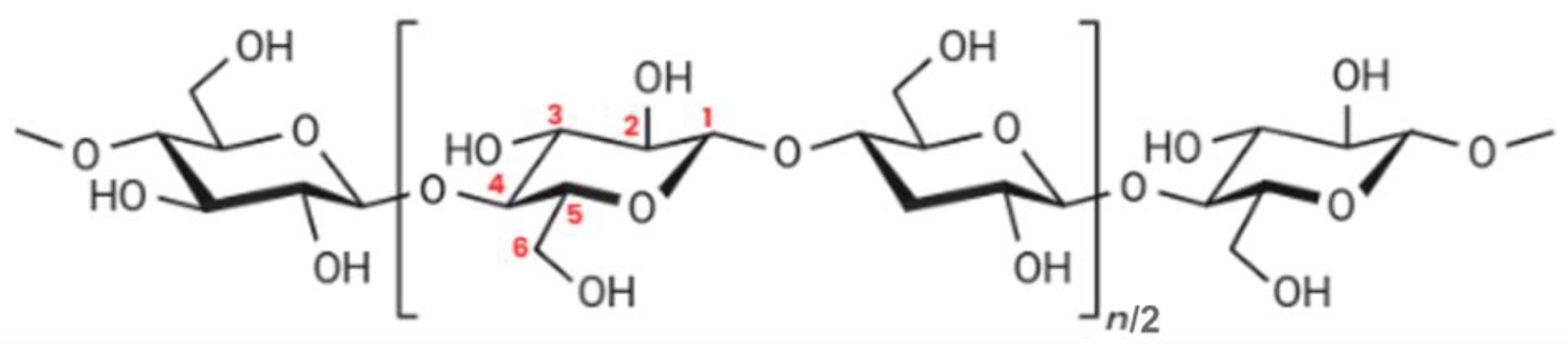
2. Cellulose and Its Derivatives
2.1. Cellulose Nanofibrils
2.2. Cellulose Nanocrystals
2.3. Bacterial Cellulose
2.4. Cellulose Acetate
2.5. Regenerated Cellulose
3. Performance of Cellulose-Derived Battery Separators
4. Environmental Impact and Sustainability of Cellulose-Derived Battery Separators
5. Conclusions
6. Challenges and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, W.; Si, Y.; Zhao, C.; Chen, T.; Li, C.; Zhang, C.; Wang, K. Biomass-derived carbono applications in the field of supercapacitores: Progress and prospects. Chem. Eng. J. 2024, 495, 153311. [Google Scholar] [CrossRef]
- Lingappan, N.; Lee, W.; Passerini, S.; Pecht, M. A comprehensive review of separator membranes in lithium-ion batteries. Renew. Sustain. Energy Rev. 2023, 187, 113726. [Google Scholar] [CrossRef]
- Babiker, D.M.D.; Usha, Z.R.; Wan, C.; Hassaan, M.M.E.; Chen, x.; Li, L. Recent progress of composite polyethylene separators for lithium/sodium batteries. J. Power Sources 2023, 564, 232853. [Google Scholar] [CrossRef]
- Bocchi, N.; Biaggio, S.R.; Filho, R.C.R. Prêmio Nobel de química 2019: Desenvolvimento das baterias íons lítio. Quím. Nova 2019, 41, 320–326. [Google Scholar]
- Joshi, S.A.; Pazhaniswamy, S.; Plank, C.; Danzer, M.A.; Hou, H.; Cheong, J.Y.; Breu, J.; Agarwal, S. High-Performance, Flame-Retardant, Binder-Free Li-Hectorite-Polybenzimidazole Fiber Separator for Li-Ion Batteries. Macromol. Mater. Eng. 2024, 309, 2300389. [Google Scholar] [CrossRef]
- Nitou, M.V.M.; Tang, M.; Niu, Y.; Pang, Y.; Wan, Z.; Mawuli, S.E.; Leoba, J.A.; Xiaodong, F.; Lv, W. Separator with active coating for fast and stable Li-ion batteries. J. Power Sources 2024, 602, 234406. [Google Scholar] [CrossRef]
- Xia, Y.; Li, X.; Zhuang, J.; Wang, W.; Abbas, S.C.; Fu, C.; Zhang, H.; Chen, T.; Yuan, Y.; Zhao, X.; et al. Exploitation of function groups in cellulose materials for lithium-ion batteries applications. Carbohydr. Polym. 2024, 325, 121570. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Sheng, J.; Zhu, H.; Yang, R. Nanoporous Regenerated Cellulose Separator for High-Performance Lithium Ion Batteries Prepared by Nonsolvent-Induced Phase Separation. ACS Sustain. Chem. Eng. 2021, 9, 14756–14765. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhang, L. Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Satchanska, G.; Davidova, S.; Petrov, P.D. Natural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers 2024, 16, 1159. [Google Scholar] [CrossRef]
- Yi, T.; Zhao, H.; Mo, Q.; Pan, D.; Liu, Y.; Huang, L.; Xu, H.; Hu, B.; Song, H. From Cellulose to Cellulose Nanofibrils—A Comprehensive Review of the Preparation and Modification of Cellulose Nanofibrils. Materials 2020, 13, 5062. [Google Scholar] [CrossRef] [PubMed]
- Lizundia, E.; Costa, C.M.; Alves, R.; Lanceros-Méndez, S. Cellulose and its derivatives for lithium ion battery separators: A review on the processing methods and properties. Carbohydr. Polym. Technol. Appl. 2020, 1, 100001. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Z.; Feng, Y.; Cai, S.; Gao, H.; Wei, Z.; Zhao, Y. Cellulose-based separators for lithium batteries: Source, preparation and performance. Chem. Eng. J. 2023, 471, 144593. [Google Scholar] [CrossRef]
- Barhoum, A.; Deshmukh, K.; García-Betancourt, M.; Alibakhshi, S.; Mousavi, S.M.; Meftahi, A.; Sabery, M.S.K.; Samyn, P. Nanocelluloses as sustainable membrane materials for separation and filtration technologies: Principles, opportunities, and challenges. Carbohydr. Polym. 2023, 317, 121057. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Liang, Y. Preparation and characterization of a Lithium-ion battery separator from cellulose nanofibers. Helyon 2015, 1, e00032. [Google Scholar] [CrossRef]
- Delaporte, N.; Perea, A.; Paolella, A.; Dubé, J.; Vigeant, M.; Demers, H.; Clément, D.; Zhu, W.; Gariépy, V.; Zaghib, K. Alumina-flame retardant separators toward safe high voltage Li-Ion batteries. J. Power Sources 2021, 506, 230189. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, X.; Wu, X.; Lu, C. Flame Retardant, Heat Insulating Cellulose Aerogels from WasteCotton Fabrics by in Situ Formation of Magnesium Hydroxide Nanoparticles in Cellulose Gel Nanostructures. ACS Sustain. Chem. Eng. 2015, 3, 1853–1859. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, X.; Liang, J.; Chen, Q.; Huang, J. Environmentally friendly separators based on cellulose diacetate-based crosslinked networks for lithium-ion batteries. Polymer 2024, 290, 126564. [Google Scholar] [CrossRef]
- Norfarhana, A.S.; Ilyas, R.A.; Ngadi, N. A review of nanocellulose adsorptive membrane as multifunctional wastewater treatment. Carbohydr. Polym. 2022, 291, 119563. [Google Scholar] [CrossRef]
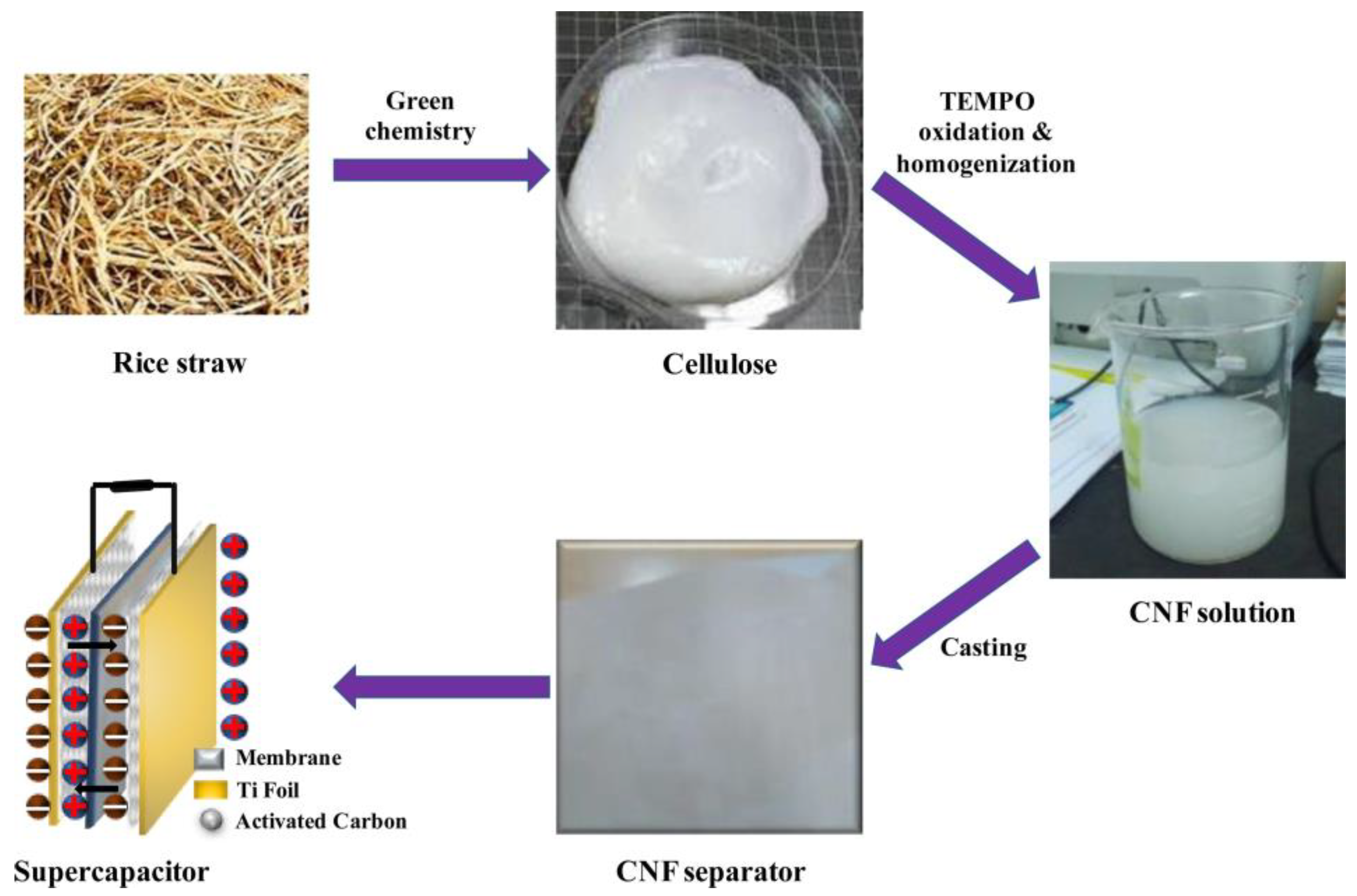
- Islam, M.A.; Ong, H.L.; Villagracia, A.R.; Halim, K.A.A.; Ganganboina, A.B.; Doong, R. Biomass–derived cellulose nanofibrils membrane from rice straw as sustainable separator for high performance supercapacitor. Ind. Crops Prod. 2021, 170, 113694. [Google Scholar] [CrossRef]
- Kim, A.; Dash, J.K.; Patel, R. Recent Development in Novel Lithium-Sulfur Nanofiber Separators: A Review of the Latest Fabrication and Performance Optimizations. Membranes 2023, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Yang, X.; Tang, Y. Nanocellulose-based electrodes and separator toward sustainable and flexible all-solid-state supercapacitor. Int. J. Biol. Macromol. 2023, 228, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.P.; Calvar, I.L.; Catchmark, J.M.; Liu, J.R.; Demirci, A.; Cheng, K.C. Biosynthesis, production and applications of bacterial cellulose. Cellulose 2013, 20, 2191–2219. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, D.; Lee, Y.H.; Chen, W.; Lee, S.Y. Nanocellulose for energy storage systems: Beyond the limits of synthetic materials. Adv. Mater. 2019, 31, 1804826. [Google Scholar] [CrossRef]
- Gregory, D.A.; Tripathi, L.; Fricker, A.; Asare, E.; Roy, L. Bacterial cellulose: A smart biomaterial with diverse applications. J. Mater. Res. 2021, 145, 100623. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods—A review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef]
- Yan, J.; Yu, D.G. Smoothening electrospinning and obtaining high-quality cellulose acetate nanofibers using a modified coaxial process. J. Mater. Sci. 2012, 47, 7138–7147. [Google Scholar] [CrossRef]
- Yang, C.; Chen, C.; Pan, Y.; Li, S.; Wang, F.; Li, J.; Li, N.; Li, X.; Zhang, Y.; Li, D. Flexible highly specific capacitance aerogel electrodes based on cellulose nanofibers, carbon nanotubes and polyaniline. Electrochim. Acta 2015, 182, 264–271. [Google Scholar] [CrossRef]
- Zhu, H.; Luo, W.; Ciesielski, P.N.; Fang, Z.; Zhu, J.Y.; Henriksson, G.; Himmel, M.E.; Hu, L. Wood-derived materials for green electronics, biological devices, and energy applications. Chem. Rev. 2016, 116, 9305–9374. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhang, L. New solvents and functional materials prepared from cellulose solutions in alkali/urea aqueous system. Food Res. Int. 2013, 52, 387–400. [Google Scholar] [CrossRef]
- Sheng, J.; Chen, T.; Wang, R.; Zhang, Z.; Hua, F.; Yang, R. Ultra-light cellulose nanofibril membrane for lithium-ion batteries. J. Memb. Sci. 2020, 595, 117550. [Google Scholar] [CrossRef]
- Kumar, S.N.; Johnsi, M.; Shalini, V.J.A.; Kavitha, N.P.; Balasubramanian, N. Cellulose nanofibril separator from Coffea arabica waste for supercapacitor applications. Ind. Crops Prod. 2024, 214, 118459. [Google Scholar] [CrossRef]
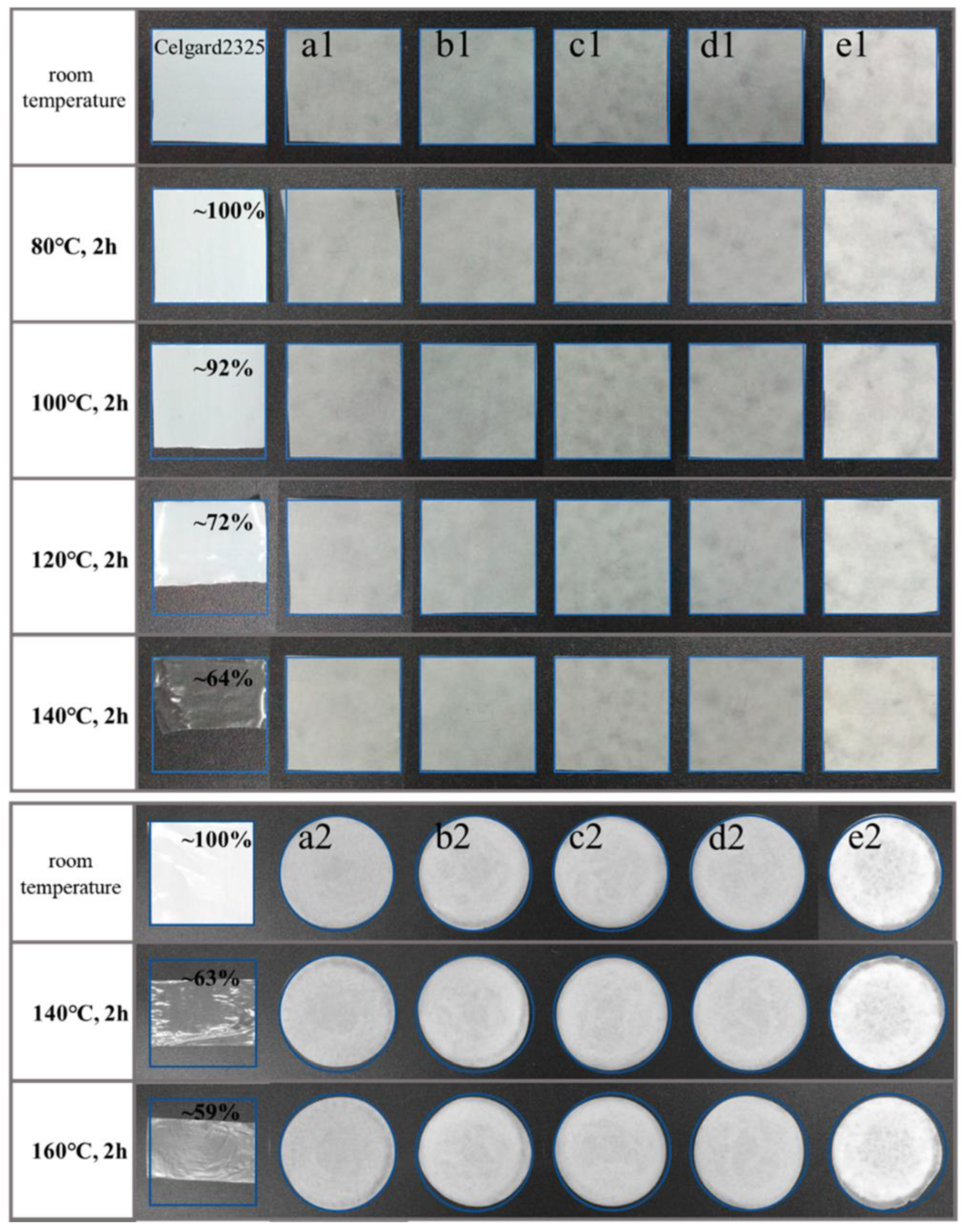
- Sheng, J.; Wang, R.; Yang, R. Physicochemical Properties of Cellulose Separators for Lithium Ion Battery: Comparison with Celgard2325. Materials 2019, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, C.; Li, C.; Liang, Z.; Hu, X.; Liu, H.; Zhang, Z.; Cui, M.; Chen, G.; Wan, J.; et al. Highly Thermally Stable, Highly Electrolyte-Wettable Hydroxyapatite/Cellulose Nanofiber Hybrid Separators for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2023, 6, 3862–3871. [Google Scholar] [CrossRef]
- Gonçalves, R.; Lizundia, E.; Silva, M.M.; Costa, C.M.; Lanceros-Méndez, S. Mesoporous Cellulose Nanocrystal Membranes as Battery Separators for Environmentally Safer Lithium-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 3749–3761. [Google Scholar] [CrossRef]
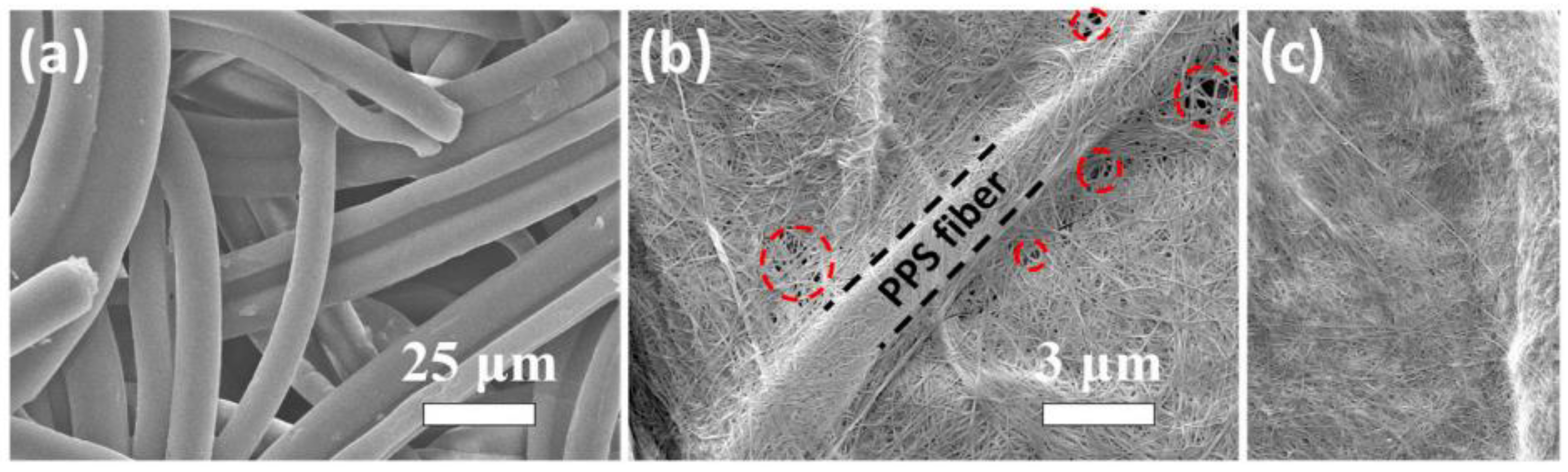
- Zhu, C.; Zhang, J.; Qiu, S.; Jia, Y.; Wang, L.; Wang, H. Tailoring the pore size of polyphenylene sulfide nonwoven with bacterial cellulose (BC) for heat-resistant and high-wettability separator in lithium-ion battery. Compos. Commun. 2021, 24, 100659. [Google Scholar] [CrossRef]
- Huang, C.; Ji, H.; Yang, Y.; Guo, B.; Luo, L.; Meng, Z.; Fan, L.; Xu, J. TEMPO-oxidized bacterial cellulose nanofiber membranes as high-performance separators for lithium-ion batteries. Carbohydr. Polym. 2020, 230, 115570. [Google Scholar] [CrossRef]
- Chen, W.; Wang, X.; Liang, J.; Chen, Y.; Ma, W.; Zhang, S. A High Performance Polyacrylonitrile Composite Separator with Cellulose Acetate and Nano-Hydroxyapatite for Lithium-Ion Batteries. Membranes 2022, 12, 124. [Google Scholar] [CrossRef]
- Chen, Y.; Qu, L.; Ma, X.; Dong, L.; Jin, Z.; Xia, G.; Du, P.; Xiong, J. Electrospun cellulose polymer nanofiber membrane with flame resistance properties for lithium-ion batteries. Carbohydr. Polym. 2020, 234, 115907. [Google Scholar] [CrossRef]
- Guo, T.; Song, J.; Jin, Y.; Sun, Z.; Li, L. Thermally stable and green cellulose-based composites strengthened by styrene-co-acrylate latex for lithium-ion battery separators. Carbohydr. Polym. 2019, 206, 801–810. [Google Scholar] [CrossRef]
- Zhou, Y. Lifecycle battery carbon footprint analysis for battery sustainability with energy digitalization and artificial intelligence. Appl. Energy 2024, 371, 123665. [Google Scholar] [CrossRef]
- Li, P.; Xia, X.; Guo, J. A review of the life cycle carbon footprint of electric vehicle batteries. Sep. Purif. Technol. 2022, 296, 121389. [Google Scholar] [CrossRef]
- de Pedro, M.B.; Ponce, C.H.; de Meatza, I.; Frax, L.M.; Peidro, C.S.; Boyano, I.; Díaz, M.Y. Environmental and economic assessment of a higher energy density and safer operation lithium-ion cell for stationary applications. Sustain. Mater. Technol. 2023, 37, e00704. [Google Scholar] [CrossRef]
- Dai, J.; Shi, C.; Li, C.; Shen, X.; Peng, L.; Wu, D.; Sun, D.; Zhang, P.; Zhao, J. A rational design of separator with substantially enhanced thermal features for lithium-ion batteries by the polydopamine–ceramic composite modification of polyolefin membranes. Energy Environ. Sci. 2016, 9, 3252–3261. [Google Scholar] [CrossRef]
- Wang, C.; Wu, K.; Cui, J.; Fang, X.; Li, J.; Zheng, N. Robust Room-Temperature Sodium-Sulfur Batteries Enabled by a Sandwich- Structured MXene@C/Polyolefin/MXene@C Dual-functional Separator. Small 2022, 18, 2106983. [Google Scholar] [CrossRef]
- Wang, E.; Chiu, C.; Chou, P. Safety assessment of polyolefin and nonwoven separators used in lithium-ion batteries. J. Power Sources 2020, 461, 228148. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, W.; Meng, G.; Zhang, J. Function-directed design of battery separators based on microporous polyolefin membranes. J. Mater. Chem. A 2022, 10, 14137–14170. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, C.; Dong, C.; Shen, C.; Shuai, B.; Li, C.; Li, Y.; Na, Q.; Xu, X.; Mai, L. Polydopamine-assisted in-situ formation of dense MOF layer on polyolefin separator for synergistic enhancement of lithium-sulfur battery. Nano Res. 2022, 15, 8048–8055. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, Z.; Xiang, Y.; Liu, D.; Xie, Z.; Qu, D.; Sun, M.; Tang, H.; Li, J. Cellulose-based material in lithium-sulfur batteries: A review. Carbohydr. Polym. 2021, 255, 117469. [Google Scholar] [CrossRef]
- Sheng, J.; Tong, S.; He, Z.; Yang, R. Recent developments of cellulose materials for lithium-ion battery separators. Cellulose 2017, 24, 4103–4122. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.; Kong, Q.; Zhang, C.; Pang, S.; Yue, L.; Wang, X.; Yao, J.; Cui, G. Renewable and superior thermal-resistant cellulose-based composite nonwoven as lithium-ion battery separator. ACS Appl. Mater. Interfaces 2013, 5, 128–134. [Google Scholar]
- Jabbour, L.; Bongiovanni, R.; Chaussy, D.; Gerbaldi, C.; Beneventi, D. Cellulose-based Li-ion batteries: A review. Cellulose 2013, 20, 1523–1545. [Google Scholar] [CrossRef]
- Atila, D.; Keskin, D.; Tezcaner, A. Cellulose acetate based 3-dimensional electrospun scaffolds for skin tissue engineering applications. Carbohydr. Polym. 2015, 133, 251–261. [Google Scholar] [CrossRef]
- Grzybek, P.; Dudek, G.; Brugge, B. Cellulose-based films and membranes: A comprehensive review on preparation and applications. Chem. Eng. J. 2024, 495, 153500. [Google Scholar] [CrossRef]
- Ratri, C.R.; Sabrina, Q.; Nugraha, A.F.; Astutiningsih, S.; Chalid, M. Green Chemistry of Cellulose Acetate Membrane Plasticized by Citric Acid and Succinonitrile for Lithium-Ion Battery Application. J. Renew. Mater. 2024, 12, 1863–1878. [Google Scholar] [CrossRef]
- Ren, J.; Guo, J.; Luo, X.; Dai, X.; Zhang, G.; Ping, P.; Kong, D. A sustainable green strategy: Flame-retardant cellulose-based separators for enhancing the safety and cycle stability of lithium-ion batteries. J. Power Sources 2025, 628, 235832. [Google Scholar] [CrossRef]
- Chen, M.; Xu, Y.; Dong, M.; Chen, K.; Zhang, Z.; Yang, L.; Chai, J.; Zheng, Y.; Li, Y.; Liu, Z.; et al. A safe composite cellulose membrane for quasi-solid-state lithium-ion batteries. Next Mater. 2024, 5, 100266. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, L.; Liu, C.; Feng, X.; Zhang, J.; Guan, L.; Mi, L.; Cui, S. Electrospun Flexible Cellulose Acetate-Based Separators for Sodium-Ion Batteries with Ultralong Cycle Stability and Excellent Wettability: The Role of Interface Chemical Groups. ACS Appl. Mater. Interfaces 2018, 10, 23883–23890. [Google Scholar] [CrossRef]
- Chen, W.; Shi, L.; Wang, Z.; Zhu, J.; Yang, H.; Mao, X.; Chi, M.; Sun, L.; Yuan, S. Porous cellulose diacetate-SiO2 composite coating on polyethylene separator for high-performance lithium-ion battery. Carbohydr. Polym. 2016, 147, 517–524. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Yuan, T.; Sun, R. Comparison of cellulose and chitin nanocrystals for reinforcing regenerated cellulose fibers. J. Appl. Polym. Sci. 2017, 134, 44880. [Google Scholar] [CrossRef]
- Hu, C.; Zhou, Y.; Zhang, T.; Jiang, T.; Meng, C.; Zeng, G. Morphological, Thermal, Mechanical, and Optical Properties of Hybrid Nanocellulose Film Containing Cellulose Nanofiber and Cellulose Nanocrystals. Fibers Polym. 2021, 22, 2187–2193. [Google Scholar] [CrossRef]
- Islam, M.S.; Chen, L.; Sisler, J.; Tam, K.C. Cellulose nanocrystal (CNC)–inorganic hybrid systems: Synthesis, properties and applications. J. Mater. Chem. B 2018, 6, 864–883. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhu, W.; Tang, Q.; Huang, Z.; Yu, P.; Gui, X.; Lin, S.; Hu, J.; Tu, Y. Mini Review on Cellulose-Based Composite Separators for Lithium-Ion Batteries: Recent Progress and perspectives. Energy Fuels 2021, 35, 12938–12947. [Google Scholar] [CrossRef]
- Cai, M.; Yuan, D.; Zhang, X.; Pu, Y.; Liu, X.; He, H.; Zhang, L.; Ning, X. Lithium ion battery separator with improved performance via side-by-side bicomponent electrospinning of PVDF-HFP/PI followed by 3D thermal crosslinking. J. Power Sources 2020, 461, 228123. [Google Scholar] [CrossRef]
- Lv, D.; Chai, J.; Wang, P.; Zhu, L.; Liu, C.; Nie, S.; Li, B.; Cui, G. Pure cellulose lithium-ion battery separator with tunable pore size and improved working stability by cellulose nanofibrils. Carbohydr. Polym. 2021, 251, 116975. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, H.; Huang, H.; Wei, Z.; Zhao, Y. Water-soluble ammonium polyphosphate synchronously enables mechanically robust and flame-retardant cellulose composite separator for high safety lithium batteries. J. Power Sources 2023, 558, 232627. [Google Scholar] [CrossRef]
- Qian, J.; Chen, Q.; Hong, M.; Xie, W.; Jing, S.; Bao, Y.; Chen, G.; Pang, Z.; Hu, L.; Li, T. Toward stretchable batteries: 3D-printed deformable electrodes and separator enabled by nanocellulose. Mater. Today 2022, 54, 18–26. [Google Scholar] [CrossRef]


| Parameter | Objective |
|---|---|
| Pore size | <1.0 µm |
| Porosity | 40.0–60.0% |
| Thickness | 20.0–25.0 µm |
| Tensile strength | 98.06 (>1000 kg.cm−1) MPa |
| Thermal shrinkage | <5.0% at 90 °C for 60 min |
| High-temperature melt integrity | >150 °C |
| Ionic conductivity | 10−3–10−1 S.cm−1 |
| Type of Cellulose | Production Method | Advantages | Disadvantages |
|---|---|---|---|
| CNF | Mechanical defibrillation | Enhances mechanical strength, flexibility, thermal stability, ionic conductivity, and electrolyte retention | Tendency to aggregate, structural fragility |
| CNC | Acid hydrolysis and oxidation (e.g., TEMPO reagent) | Provides matrix reinforcement, better control of porosity, and improved ionic conductivity | High cost |
| BC | Synthesized by bacteria (e.g., Gluconacetobacter xylinus) | Excellent thermal stability, long cycle life, and potential for energy efficiency improvement with conductive additives | Slow production process, high cost |
| CA | Esterification of cellulose with acetic acid and anhydride | Flexibility, good electrical insulation, and can be modified to improve electrochemical properties | Limited chemical stability |
| RC | Chemical coagulation and regeneration processes | Flexibility, porosity, and enhances battery performance and lifespan | High hygroscopicity |
| Separator | Type of Cellulose | Methodology | Thickness (µm) | Porosity (%) | Ionic Conductivity (mS.cm−1) | Electrochemical Perfomance | Battery (Cathode/Anode) | Reference |
|---|---|---|---|---|---|---|---|---|
| CNF | Coffee waste | Casting | 25 | 55 | 3.00 | Specific capacitance retention of 47.1% | Zn/SS | [32] |
| CNF | Rice straw | Casting | 30 | 51 | 3.40 | 100% after 5000 cycles at 0.5C | Activated carbon | [20] |
| CNF | Bamboo pulp, hardwood pulp, softwood pulp, cotton pulp, and hemp pulp | Vacuum filtration | 20–30 | - | - | - | - | [33] |
| HAP/CNC | CNC | Vacuum filtration | 28 | 76 | 0.81 | 67.1% after 100 cycles at 2C | LiFePO4/Li | [34] |
| CNC | CNC | Casting | 150 | 75.3 | 2.7 | 91 mAhg−1 after 10 cycles at C/8 | LiFePO4/Li | [35] |
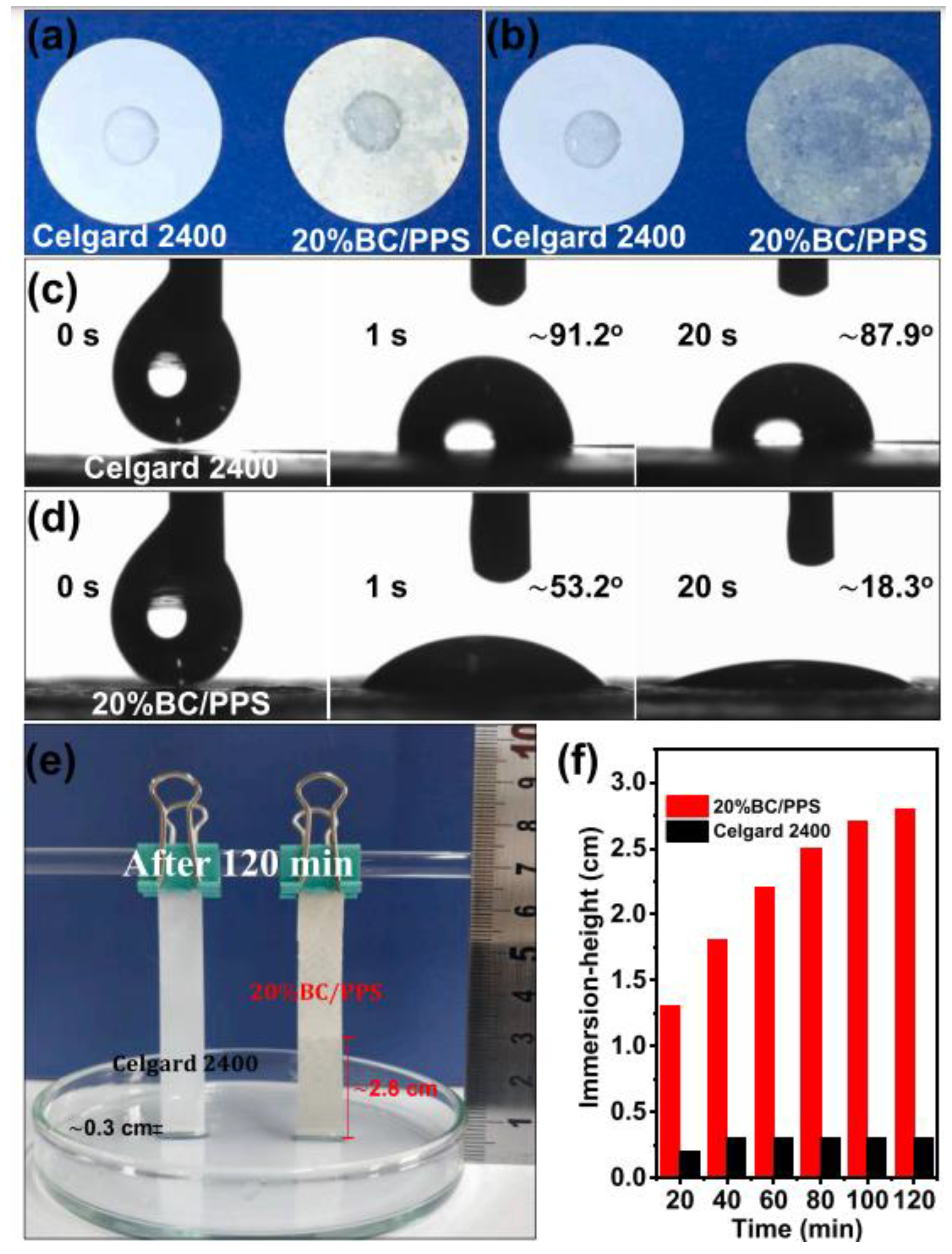
| BC/PPS | BC | Vacuum filtration | - | 62.7 | 1.55 | 91.3% after 100 cycles at 0.5C | LiFePO4/Li | [36] |
| TOBC | BC | Vacuum filtration | 29 | 88.3 | 13.45 | 94% after 100 cycles at 0.2C | LiFePO4/Li | [37] |
| PAN/CA/HAP | AC | Eletrospinning | 46 | 61 | 3.02 | 157.6 mAhg−1 after 50 cycles at 0.5C | LiFePO4/Li | [38] |
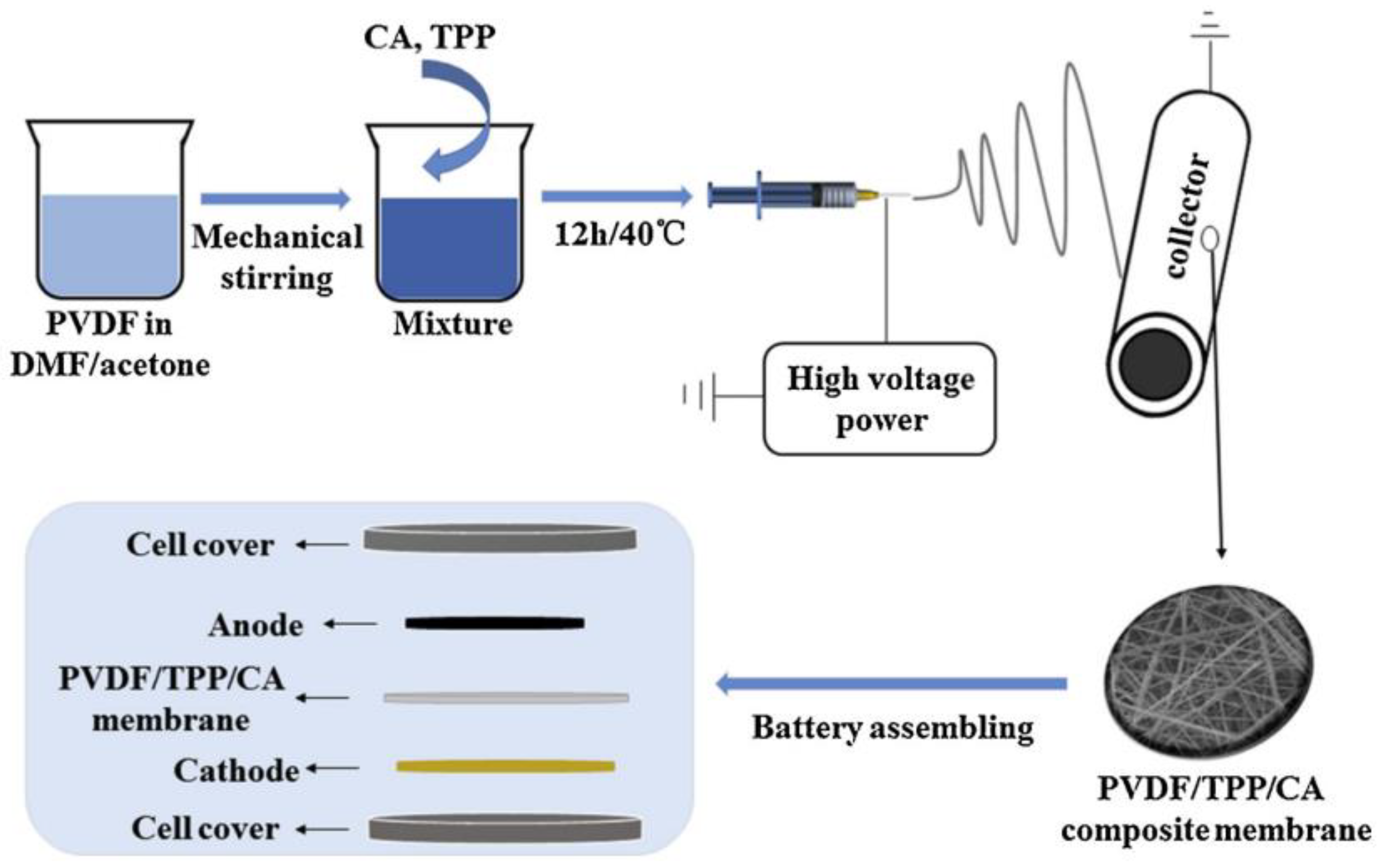
| PVDF/CA/TPP | AC | Eletrospinning | 58 | 90 | 4.4 | 86.9% after 100 cycles at 0.2C | LiFePO4/Li | [39] |
| RCS | Cotton pulp | Phase inversion | 19.74 | 61 | 1.25 | 72% after 100 cycles at 0.2C | LiFePO4/Li | [8] |
| CSA | Cotton pulp | Phase inversion | 109 | 58.43 | 1.34 | 78.7% after 80 cycles at 0.5C | LiFe0.2Mn0.8PO4/Li | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turossi, T.C.; Júnior, H.L.O.; Monticeli, F.M.; Dias, O.T.; Zattera, A.J. Cellulose-Derived Battery Separators: A Minireview on Advances Towards Environmental Sustainability. Polymers 2025, 17, 456. https://doi.org/10.3390/polym17040456
Turossi TC, Júnior HLO, Monticeli FM, Dias OT, Zattera AJ. Cellulose-Derived Battery Separators: A Minireview on Advances Towards Environmental Sustainability. Polymers. 2025; 17(4):456. https://doi.org/10.3390/polym17040456
Chicago/Turabian StyleTurossi, Tayse Circe, Heitor Luiz Ornaghi Júnior, Francisco Maciel Monticeli, Otávio Titton Dias, and Ademir José Zattera. 2025. "Cellulose-Derived Battery Separators: A Minireview on Advances Towards Environmental Sustainability" Polymers 17, no. 4: 456. https://doi.org/10.3390/polym17040456
APA StyleTurossi, T. C., Júnior, H. L. O., Monticeli, F. M., Dias, O. T., & Zattera, A. J. (2025). Cellulose-Derived Battery Separators: A Minireview on Advances Towards Environmental Sustainability. Polymers, 17(4), 456. https://doi.org/10.3390/polym17040456









