Development of Curcumin-Loaded TiO2-Reinforced Chitosan Monofilaments for Biocompatible Surgical Sutures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of the Sutures
2.3. Characterizaton of the Produced Sutures
3. Results and Discussion
3.1. Influence of the TiO2 Addition on the Mechanical Properties of the Sutures
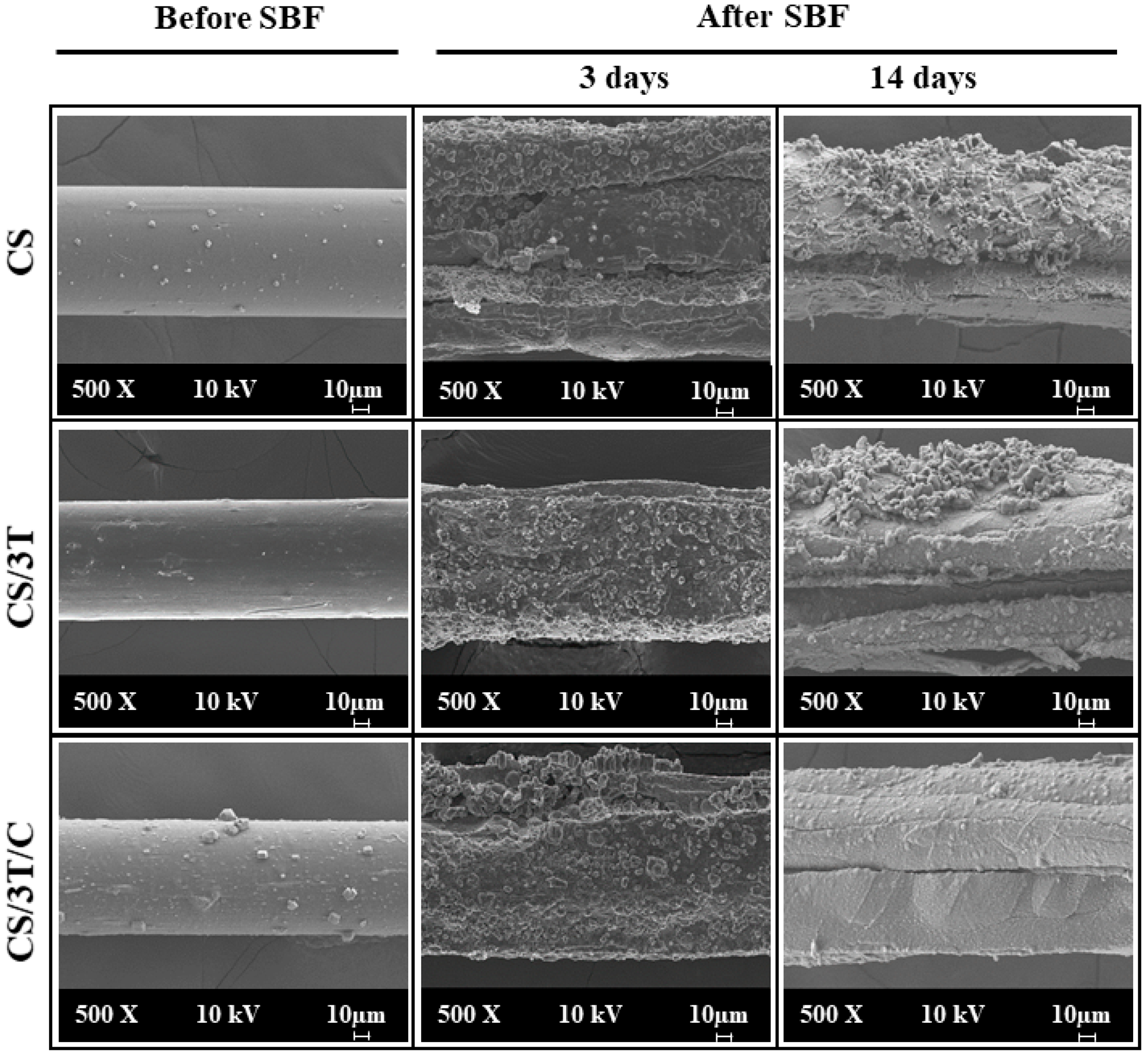
3.2. Morphological Properties of the Produced Sutures
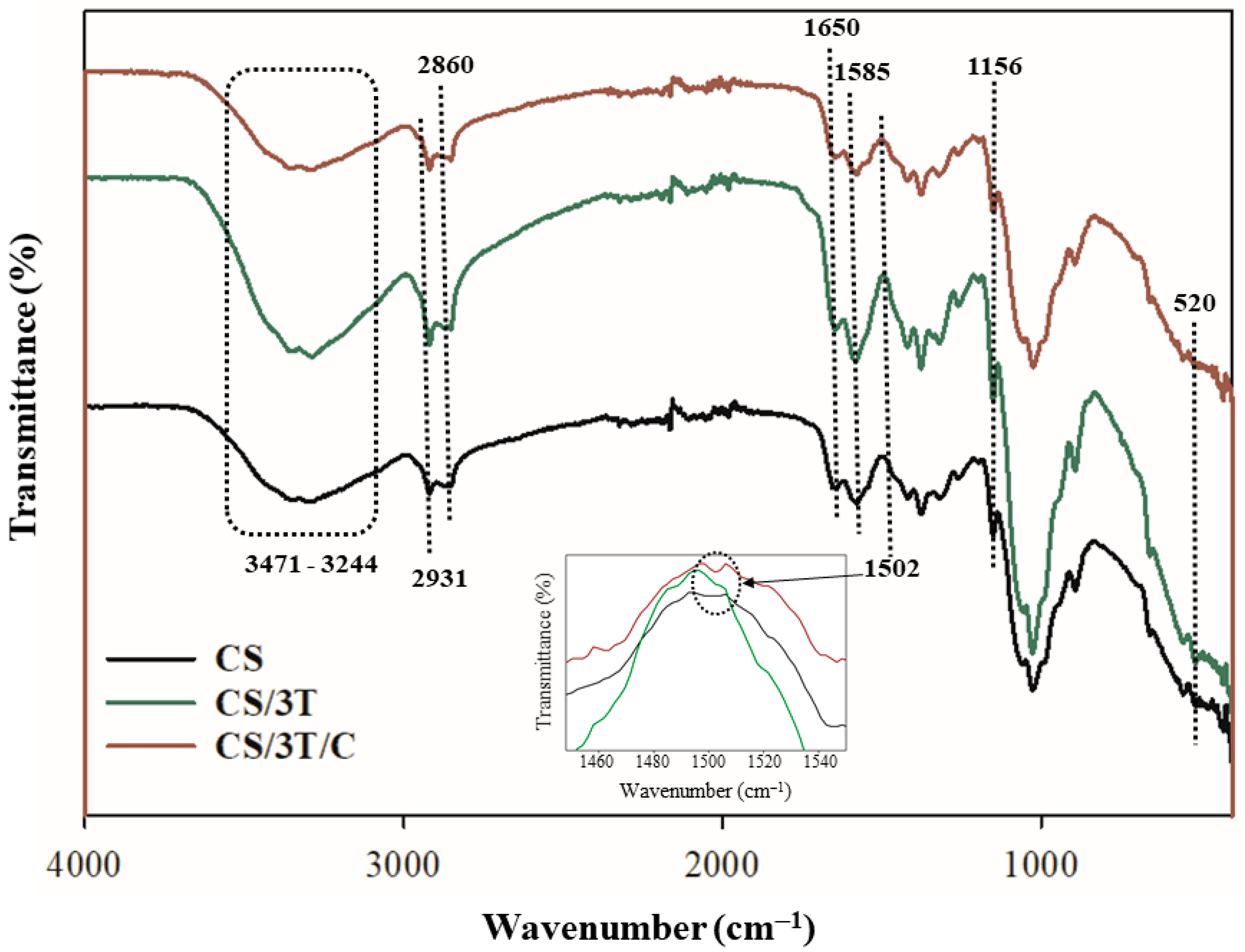
3.3. Physicochemical and Mechanical Properties of the Produced Sutures
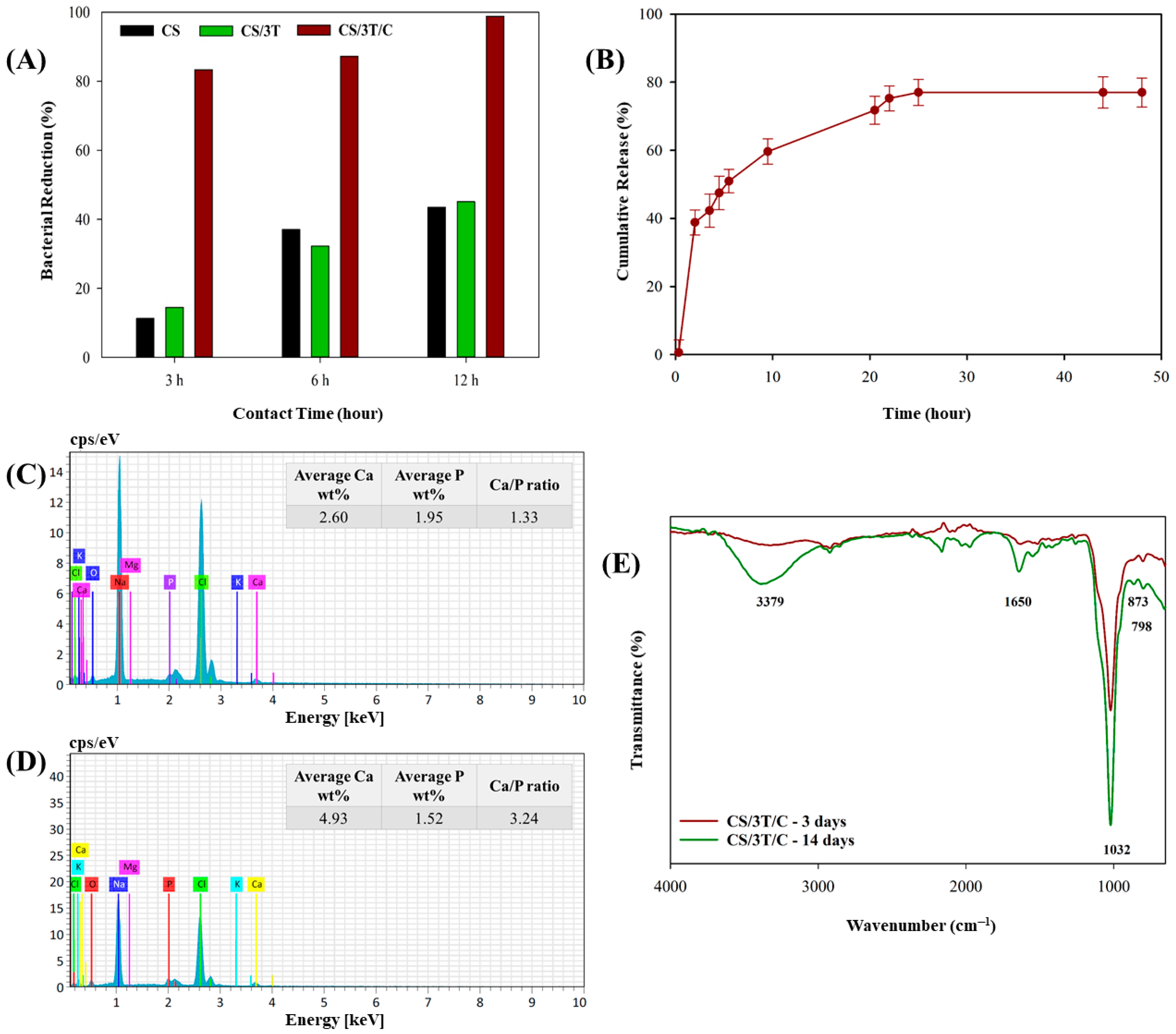
3.4. Radical Scavenging Activities of the Produced Sutures
3.5. In Vitro Characterization of the Produced Sutures
4. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joseph, B.; James, J.; Kalarikkal, N.; Thomas, S. Advances in biopolymer based surgical sutures. In Book Advanced Technologies and Polymer Materials for Surgical Sutures, 1st ed.; Thomas, S., Coates, P., Whiteside, B., Joseph, B., Nair, K., Eds.; Woodhead Publishing: Cambridge, UK, 2023; pp. 1–10. [Google Scholar]
- Öksüz, K.E.; Kurt, B.; İnan, Z.D.Ş.; Hepokur, C. Novel bioactive glass/graphene oxide-coated surgical sutures for soft tissue regeneration. ACS Omega 2023, 8, 21628–21641. [Google Scholar] [CrossRef]
- Tan, Y.; Rajoka, M.S.R.; Ke, Z.; Mehwish, H.M.; Deng, W.; Li, J.; Qin, W.; Zhao, L.; Wu, Y. Effect of squid cartilage chitosan molecular structure on the properties of its monofilament as an absorbable surgical suture. Polymers 2022, 14, 1306. [Google Scholar] [CrossRef]
- da Silva, M.C.; da Silva, H.N.; Alves Leal Cruz, R.D.C.; Sagoe Amoah, S.K.; de Lima Silva, S.M.; Lia Fook, M.V. N-acetyl-D-glucosamine-loaded chitosan filaments biodegradable and biocompatible for use as absorbable surgical suture materials. Materials 2019, 12, 1807. [Google Scholar] [CrossRef]
- Li, H.; Cheng, F.; Chávez-Madero, C.; Choi, J.; Wei, X.; Yi, X.; Zheng, T.; He, J. Manufacturing and physical characterization of absorbable oxidized regenerated cellulose braided surgical sutures. Int. J. Biol. Macromol. 2019, 134, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Dennis, C.; Sethu, S.; Nayak, S.; Mohan, L.; Morsi, Y.; Manivasagam, G. Suture materials—Current and emerging trends. J. Biomed. Mater. Res. Part A 2016, 104, 1544–1599. [Google Scholar] [CrossRef] [PubMed]
- Pillai, C.K.S.; Sharma, C.P. Absorbable polymeric surgical sutures: Chemistry, production, properties, biodegradability, and performance. J. Biomater. Appl. 2010, 25, 291–366. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.C.D.S.; Silva, H.N.D.; Lopes, D.D.S.; Wanderley, W.F.; Rosendo, R.A.; Penha, E.S.D.; de Medeiros, L.A.D.M.; Silva, S.M.d.L.; Fook, M.V.L. Biodegradable Chitosan Sutures Enhanced with N-Acetyl-D-Glucosamine: Comparative Study with Catgut Sutures. Mater. Res. 2024, 27, e20240278. [Google Scholar] [CrossRef]
- Younesi, M.; Donmez, B.O.; Islam, A.; Akkus, O. Heparinized collagen sutures for sustained delivery of PDGF-BB: Delivery profile and effects on tendon-derived cells In-Vitro. Acta Biomater. 2016, 41, 100–109. [Google Scholar] [CrossRef]
- Shao, K.; Han, B.; Gao, J.; Jiang, Z.; Liu, W.; Liu, W.; Liang, Y. Fabrication and feasibility study of an absorbable diacetyl chitin surgical suture for wound healing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.C.; Williams, D.F. The effect of gamma irradiation on the enzymatic degradation of polyglycolic acid absorbable sutures. J. Biomed. Mater. Res. 1983, 17, 1029–1040. [Google Scholar] [CrossRef]
- Im, J.N.; Kim, J.K.; Kim, H.K.; In, C.H.; Lee, K.Y.; Park, W.H. In vitro and in vivo degradation behaviors of synthetic absorbable bicomponent monofilament suture prepared with poly (p-dioxanone) and its copolymer. Polym. Degrad. Stab. 2007, 92, 667–674. [Google Scholar] [CrossRef]
- Lim, T.Y.; Poh, C.K.; Wang, W. Poly (lactic-co-glycolic acid) as a controlled release delivery device. J. Mater. Sci. Mater. Med. 2009, 20, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.R.; Mukherjee, D.P.; Kaganov, A.L.; Gordon, S. A new synthetic monofilament absorbable suture made from polytrimethylene carbonate. Surg. Gynecol. Obstet. 1985, 161, 213–222. [Google Scholar]
- Hu, J.; Song, Y.; Zhang, C.; Huang, W.; Chen, A.; He, H.; Zhang, S.; Chen, Y.; Tu, C.; Liu, J.; et al. Highly aligned electrospun collagen/polycaprolactone surgical sutures with sustained release of growth factors for wound regeneration. ACS Appl. Bio Mater. 2020, 3, 965–976. [Google Scholar] [CrossRef]
- Viju, S.; Thilagavathi, G. Effect of chitosan coating on the characteristics of silk-braided sutures. J. Ind. Text. 2013, 42, 256–268. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Rodrigues, S.; Dionísio, M.; Remunan Lopez, C.; Grenha, A. Biocompatibility of chitosan carriers with application in drug delivery. J. Funct. Biomater. 2012, 3, 615–641. [Google Scholar] [CrossRef]
- Martău, G.A.; Mihai, M.; Vodnar, D.C. The use of chitosan, alginate, and pectin in the biomedical and food sector—Biocompatibility, bioadhesiveness, and biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef] [PubMed]
- Rizeq, B.R.; Younes, N.N.; Rasool, K.; Nasrallah, G.K. Synthesis, bioapplications, and toxicity evaluation of chitosan-based nanoparticles. Int. J. Mol. Sci. 2019, 20, 5776. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Ma, R.; Lin, C.; Liu, Z.; Tang, T. Quaternized chitosan as an antimicrobial agent: Antimicrobial activity, mechanism of action and biomedical applications in orthopedics. Int. J. Mol. Sci. 2013, 14, 1854–1869. [Google Scholar] [CrossRef]
- Panda, P.K.; Sadeghi, K.; Park, K.; Seo, J. Regeneration approach to enhance the antimicrobial and antioxidant activities of chitosan for biomedical applications. Polymers 2022, 15, 132. [Google Scholar] [CrossRef] [PubMed]
- Khalil, K.D.; Bashal, A.H.; Habeeb, T.; Abu-Dief, A.M. Synergistic antibacterial and anticancer activity in gadolinium–chitosan nanocomposite films: A novel approach for biomedical applications. Appl. Organomet. Chem. 2024, 38, e7531. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a wound dressing starting material: Antimicrobial properties and mode of action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef]
- Montenegro, R.; Godeiro, J.R.G. Chitosan based suture–focusing on the real advantages of an outstanding biomaterial. Adv. Chitin Sci. 2014, 14, 211–216. [Google Scholar]
- Perrin, N.; Mohammadkhani, G.; Moghadam, F.H.; Delattre, C.; Zamani, A. Biocompatible fibers from fungal and shrimp chitosans for suture application. Curr. Res. Biotechnol. 2022, 4, 530–536. [Google Scholar] [CrossRef]
- Farrokhi, H.; Koosha, M.; Nasirizadeh, N.; Salari, M.; Abdouss, M.; Li, T.; Gong, Y. The Effect of Nanoclay Type on the Mechanical Properties and Antibacterial Activity of Chitosan/PVA Nanocomposite Films. J. Compos. Sci. 2024, 8, 255. [Google Scholar] [CrossRef]
- Aryaei, A.; Jayatissa, A.H.; Jayasuriya, A.C. Mechanical and biological properties of chitosan/carbon nanotube nanocomposite films. J. Biomed. Mater. Res. Part A 2014, 102, 2704–2712. [Google Scholar] [CrossRef] [PubMed]
- Marroquin, J.B.; Rhee, K.Y.; Park, S.J. Chitosan nanocomposite films: Enhanced electrical conductivity, thermal stability, and mechanical properties. Carbohydr. Polym. 2013, 92, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Mahalakshmi, S.; Vijaya, P. Evaluation of in-vitro biocompatibility and antimicrobial activities of titanium dioxide (TiO2) nanoparticles by hydrothermal method. Nano Biomed. Eng. 2021, 13, 36–43. [Google Scholar] [CrossRef]
- Kiran, A.S.K.; Kumar, T.S.; Sanghavi, R.; Doble, M.; Ramakrishna, S. Antibacterial and bioactive surface modifications of titanium implants by PCL/TiO2 nanocomposite coatings. Nanomaterials 2018, 8, 860. [Google Scholar] [CrossRef]
- Khan, S.; Garg, M.; Chockalingam, S.; Gopinath, P.; Kundu, P.P. TiO2 doped chitosan/poly (vinyl alcohol) nanocomposite film with enhanced mechanical properties for application in bone tissue regeneration. Int. J. Biol. Macromol. 2020, 143, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Cano, L.; Pollet, E.; Avérous, L.; Tercjak, A. Effect of TiO2 nanoparticles on the properties of thermoplastic chitosan-based nano-biocomposites obtained by mechanical kneading. Compos. Part A Appl. Sci. Manuf. 2017, 93, 33–40. [Google Scholar] [CrossRef]
- Ravandi, R.; Heris, S.Z.; Hemmati, S.; Aghazadeh, M.; Davaran, S.; Abdyazdani, N. Effects of chitosan and TiO2 nanoparticles on the antibacterial property and ability to self-healing of cracks and retrieve mechanical characteristics of dental composites. Heliyon 2024, 10, e27734. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.K.; Malaiya, A.; Kesharwani, P.; Soni, D.; Jain, A. Biomedical applications and toxicities of carbon nanotubes. Drug Chem. Toxicol. 2022, 45, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Merino, D.; Tomadoni, B.; Salcedo, M.F.; Mansilla, A.Y.; Casalongué, C.A.; Alvarez, V.A. Nanoclay as carriers of bioactive molecules applied to agriculture. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications, 1st ed.; Kharissova, O.V., Torres-Martínez, L.M., Kharisov, B.I., Eds.; Springer: Cham, Switzerland, 2022; pp. 1–22. [Google Scholar]
- Jeon, J.D.; Kim, M.J.; Kwak, S.Y. Effects of addition of TiO2 nanoparticles on mechanical properties and ionic conductivity of solvent-free polymer electrolytes based on porous P (VdF-HFP)/P (EO-EC) membranes. J. Power Sources 2006, 162, 1304–1311. [Google Scholar] [CrossRef]
- Qu, L.; Chen, G.; Dong, S.; Huo, Y.; Yin, Z.; Li, S.; Chen, Y. Improved mechanical and antimicrobial properties of zein/chitosan films by adding highly dispersed nano-TiO2. Ind. Crops Prod. 2019, 130, 450–458. [Google Scholar] [CrossRef]
- El-Wakil, N.A.; Hassan, E.A.; Abou-Zeid, R.E.; Dufresne, A. Development of wheat gluten/nanocellulose/titanium dioxide nanocomposites for active food packaging. Carbohydr. Polym. 2015, 124, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Nocito, M.C.; De Luca, A.; Prestia, F.; Avena, P.; La Padula, D.; Zavaglia, L.; Sirianni, R.; Casaburi, I.; Puoci, F.; Chimento, A.; et al. Antitumoral activities of curcumin and recent advances to improve its oral bioavailability. Biomedicines 2021, 9, 1476. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Huang, C.; Huang, H.; Zhao, Y.; Khan, M.R.U.; Zhao, H.; Huang, L. Antibacterial mechanism of curcumin: A review. Chem. Biodivers. 2020, 17, e2000171. [Google Scholar] [CrossRef]
- Chen, J.; He, Z.M.; Wang, F.L.; Zhang, Z.S.; Liu, X.Z.; Zhai, D.D.; Chen, W.D. Curcumin and its promise as an anticancer drug: An analysis of its anticancer and antifungal effects in cancer and associated complications from invasive fungal infections. Eur. J. Pharmacol. 2016, 772, 33–42. [Google Scholar] [CrossRef]
- Jennings, M.R.; Parks, R.J. Curcumin as an antiviral agent. Viruses 2020, 12, 1242. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease, 1st ed; Aggarwal, B.B., Surh, Y., Shishodia, S., Eds.; Springer Science & Business Media: New York, NY, USA, 2007; pp. 105–125. [Google Scholar]
- Kim, D.C.; Ku, S.K.; Bae, J.S. Anticoagulant activities of curcumin and its derivative. BMB Rep. 2012, 45, 221–226. [Google Scholar] [CrossRef]
- Riyad, P.; Purohit, A.; Karishma, S.; Ram, H. Atherosclerotic plaque regression and HMG-CoA reductase inhibition potential of curcumin: An integrative omics and in-vivo study. J. Appl. Biol. Biotechnol 2022, 10, 129–135. [Google Scholar]
- Park, J.; Conteas, C.N. Anti-carcinogenic properties of curcumin on colorectal cancer. World J. Gastrointest. Oncol. 2010, 2, 169. [Google Scholar] [CrossRef]
- Puneeth, H.R.; Sharada, A.C. Antioxidant and hypoglycemic effects of curcumin pyrazole derivatives. Int. J. Pharm. Pharm. Sci. 2015, 7, 244–249. [Google Scholar]
- Bulut, B.; Duman, Ş. Effects of calcination temperature on hydrothermally synthesized titanium dioxide submicron powders. Konya J. Eng. Sci. 2021, 9, 676–685. [Google Scholar] [CrossRef]
- Yudin, V.E.; Dobrovolskaya, I.P.; Neelov, I.M.; Dresvyanina, E.N.; Popryadukhin, P.V.; Ivan’kova, E.M.; Elokhovskii, V.Y.; Kasatkin, I.A.; Okrugin, B.M.; Morganti, P. Wet spinning of fibers made of chitosan and chitin nanofibrils. Carbohydr. Polym. 2014, 108, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Mohammadkhani, G.; Kumar Ramamoorthy, S.; Adolfsson, K.H.; Mahboubi, A.; Hakkarainen, M.; Zamani, A. New solvent and coagulating agent for development of chitosan fibers by wet spinning. Polymers 2021, 13, 2121. [Google Scholar] [CrossRef] [PubMed]
- Dresvyanina, E.N.; Dobrovol’Skaya, I.P.; Popryadukhin, P.V.; Yudin, V.E.; Ivan’Kova, E.M.; Elokhovskii, V.Y.; Khomenko, A.Y. Influence of spinning conditions on properties of chitosan fibers. Fibre Chem. 2013, 44, 280–283. [Google Scholar] [CrossRef]
- ASTM D2256/D2256M-21; Standard Test Method for Tensile Properties of Yarns by the Single-Strand Method. ASTM: West Conshohocken, PA, USA, 2022.
- Mavis, B.; Demirtaş, T.T.; Gümüşderelioğlu, M.; Gündüz, G.; Çolak, Ü. Synthesis, characterization and osteoblastic activity of polycaprolactone nanofibers coated with biomimetic calcium phosphate. Acta Biomater. 2009, 5, 3098–3111. [Google Scholar] [CrossRef]
- Hussein, E.M.; Desoky, W.M.; Hanafy, M.F.; Guirguis, O.W. Effect of TiO2 nanoparticles on the structural configurations and thermal, mechanical, and optical properties of chitosan/TiO2 nanoparticle composites. J. Phys. Chem. Solids 2021, 152, 109983. [Google Scholar] [CrossRef]
- Knaul, J.; Hooper, M.; Chanyi, C.; Creber, K.A. Improvements in the drying process for wet-spun chitosan fibers. J. Appl. Polym. Sci. 1998, 69, 1435–1444. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Yang Nilsson, T.; Ulmefors, H.; Köhnke, T. Wet spinning of chitosan fibers: Effect of sodium dodecyl sulfate adsorption and enhanced dope temperature. ACS Appl. Polym. Mater. 2020, 2, 3867–3875. [Google Scholar] [CrossRef]
- Pantić, M.; Maver, U.; Rožanc, J.; Vihar, B.; Andrejč, D.C.; Knez, Ž.; Novak, Z. Evaluation of ethanol-induced chitosan aerogels with human osteoblast cells. Int. J. Biol. Macromol. 2023, 253, 126694. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.F.; Teodosio Melo, K.R.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs 2014, 13, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Ibekwe, C.A.; Oyatogun, G.M.; Esan, T.A.; Oluwasegun, K.M. Synthesis and characterization of chitosan/gum arabic nanoparticles for bone regeneration. Am. J. Mater. Sci. Eng. 2017, 5, 28–36. [Google Scholar]
- Rachtanapun, P.; Klunklin, W.; Jantrawut, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Seesuriyachan, P.; Leksawasdi, N.; Chaiyaso, T.; Ruksiriwanich, W.; Phongthai, S.; et al. Characterization of chitosan film incorporated with curcumin extract. Polymers 2021, 13, 963. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, K.T.; Nithya, A.; Jothivenkatachalam, K. Photocatalytic and antimicrobial activities of chitosan-TiO2 nanocomposite. Int. J. Biol. Macromol. 2017, 104, 1762–1773. [Google Scholar] [CrossRef] [PubMed]
- Musielak, E.; Feliczak-Guzik, A.; Jaroniec, M.; Nowak, I. Photodynamic light-triggered release of curcumin from hierarchical FAU zeolite. Catalysts 2023, 13, 394. [Google Scholar] [CrossRef]
- Notin, L.; Viton, C.; David, L.; Alcouffe, P.; Rochas, C.; Domard, A. Morphology and mechanical properties of chitosan fibers obtained by gel-spinning: Influence of the dry-jet-stretching step and ageing. Acta Biomater. 2006, 2, 387–402. [Google Scholar] [CrossRef]
- Albanna, M.Z.; Bou-Akl, T.H.; Walters, H.L., III; Matthew, H.W. Improving the mechanical properties of chitosan-based heart valve scaffolds using chitosan fibers. J. Mech. Behav. Biomed. Mater. 2012, 5, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, F.; Özarslan, A.C.; Evcimen Duygulu, N. Production and comprehensive characterization of PVA/chitosan transdermal composite mats loaded with bioactive curcumin; evaluation of its release kinetics, antioxidant, antimicrobial, and biocompatibility features. J. Appl. Polym. Sci. 2024, 141, e55874. [Google Scholar] [CrossRef]
- Abd El-Hady, M.M.; Saeed, S.E.S. Antibacterial properties and pH sensitive swelling of insitu formed silver-curcumin nanocomposite based chitosan hydrogel. Polymers 2020, 12, 2451. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. IJBS 2008, 4, 89. [Google Scholar] [CrossRef]
- Yuan, Y.; Tan, W.; Zhang, J.; Li, Q.; Guo, Z. Water-soluble amino functionalized chitosan: Preparation, characterization, antioxidant and antibacterial activities. Int. J. Biol. Macromol. 2022, 217, 969–978. [Google Scholar] [CrossRef]
- Rasheed, U.; Kiani, M.N.; Butt, M.S.; Saeed, H.; Hanif, R.; Anwar, S. Fabrication and biocompatibility of neem/chitosan coated silk sutures for infection control and wound healing. J. King Saud Univ. -Sci. 2024, 36, 103435. [Google Scholar] [CrossRef]
- Shanmugam, A.; Subhapradha, N.; Suman, S.; Ramasamy, P.; Saravanan, R.; Shanmugam, V.; Srinivasan, A. Characterization of biopolymer “chitosan” from the shell of donacid clam Donax scortum (Linnaeus, 1758) and its antioxidant activity. Int. J. Pharm. Pharm. Sci 2012, 4, 460–465. [Google Scholar]
- Si Trung, T.; Bao, H.N.D. Physicochemical properties and antioxidant activity of chitin and chitosan prepared from pacific white shrimp waste. Int. J. Carbohydr. Chem. 2015, 2015, 706259. [Google Scholar] [CrossRef]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Yong, H.; Qin, Y.; Liu, J.; Liu, J. Development of multifunctional food packaging films based on chitosan, TiO2 nanoparticles and anthocyanin-rich black plum peel extract. Food Hydrocoll. 2019, 94, 80–92. [Google Scholar] [CrossRef]
- Ullattil, S.G.; Narendranath, S.B.; Pillai, S.C.; Periyat, P. Black TiO2 nanomaterials: A review of recent advances. Chem. Eng. J. 2018, 343, 708–736. [Google Scholar] [CrossRef]
- Kazan, A.; Demirci, F. Olive leaf extract incorporated chitosan films for active food packaging. Konya J. Eng. Sci. 2023, 11, 1061–1072. [Google Scholar] [CrossRef]
- Bi, F.; Qin, Y.; Chen, D.; Kan, J.; Liu, J. Development of active packaging films based on chitosan and nano-encapsulated luteolin. Int. J. Biol. Macromol. 2021, 182, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Qiao, Y.; Li, C.; Lin, J.; Han, H.; Li, X.; Mao, J.; Wang, F.; Wang, L. Chitosan/gelatin-tannic acid decorated porous tape suture with multifunctionality for tendon healing. Carbohydr. Polym. 2021, 268, 118246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Qiao, Y.; Zhu, J.; Li, Y.; Li, C.; Lin, J.; Li, X.; Han, H.; Mao, J.; Wang, F.; et al. Electroactive and antibacterial surgical sutures based on chitosan-gelatin/tannic acid/polypyrrole composite coating. Compos. Part B Eng. 2021, 223, 109140. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, G.; Wang, Y.; Zhao, Y.; Su, H.; Tan, T. Preparation of chitosan-TiO2 composite film with efficient antimicrobial activities under visible light for food packaging applications. Carbohydr. Polym. 2017, 169, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Sagadevan, S.; Lett, J.A.; Vennila, S.; Prasath, P.V.; Kaliaraj, G.S.; Fatimah, I.; Léonard, E.; Mohammad, F.; Al-Lohedan, H.A.; Alshahateet, S.F.; et al. Photocatalytic activity and antibacterial efficacy of titanium dioxide nanoparticles mediated by Myristica fragrans seed extract. Chem. Phys. Lett. 2021, 771, 138527. [Google Scholar] [CrossRef]
- Li, H.; Jiang, Y.; Yang, J.; Pang, R.; Chen, Y.; Mo, L.; Jiang, Q.; Qin, Z. Preparation of curcumin-chitosan composite film with high antioxidant and antibacterial capacity: Improving the solubility of curcumin by encapsulation of biopolymers. Food Hydrocoll. 2023, 145, 109150. [Google Scholar] [CrossRef]
- Rezaei, A.; Nasirpour, A. Evaluation of release kinetics and mechanisms of curcumin and curcumin-β-cyclodextrin inclusion complex incorporated in electrospun almond gum/PVA nanofibers in simulated saliva and simulated gastrointestinal conditions. BioNanoScience 2019, 9, 438–445. [Google Scholar] [CrossRef]
- Cam, M.E.; Yildiz, S.; Alenezi, H.; Cesur, S.; Ozcan, G.S.; Erdemir, G.; Edirisinghe, U.; Akakin, D.; Kuruca, D.S.; Kabasakal, L.; et al. Evaluation of burst release and sustained release of pioglitazone-loaded fibrous mats on diabetic wound healing: An in vitro and in vivo comparison study. J. R. Soc. Interface 2020, 17, 20190712. [Google Scholar] [CrossRef]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef]
- Bui, H.T.; Chung, O.H.; Park, J.S. Fabrication of electrospun antibacterial curcumin-loaded zein nanofibers. Polymer 2014, 38, 744–751. [Google Scholar] [CrossRef]
- Houshyar, S.; Yin, H.; Pope, L.; Zizhou, R.; Dekiwadia, C.; Hill-Yardin, E.L.; MC Yeung, J.; John, S.; Fox, K.; Tran, N.; et al. Smart suture with iodine contrasting nanoparticles for computed tomography. OpenNano 2023, 9, 100120. [Google Scholar] [CrossRef]
- Blaker, J.J.; Nazhat, S.N.; Boccaccini, A.R. Development and characterisation of silver-doped bioactive glass-coated sutures for tissue engineering and wound healing applications. Biomaterials 2004, 25, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, B.; Demirci, F.; Duman, Ş. Investigating the impact of coagulation bath temperature on the properties of biphasic calcium phosphate/poly (vinylidene fluoride)-based membrane scaffold via immersion precipitation. J. Appl. Polym. Sci. 2024, 142, e56375. [Google Scholar] [CrossRef]
- Duman, Ş.; Bulut, B. Effect of akermanite powders on mechanical properties and bioactivity of chitosan-based scaffolds produced by 3D-bioprinting. Ceram. Int. 2021, 47, 13912–13921. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Stamboulis, A.G.; Rashid, A.; Roether, J.A. Composite surgical sutures with bioactive glass coating. J. Biomed. Mater. Res. Part B Appl. Biomater. 2003, 67, 618–626. [Google Scholar] [CrossRef]
- Sierra, L.A.Q.; Escobar, D.M. Characterization and bioactivity behavior of sol–gel derived bioactive vitroceramic from non-conventional precursors. Boletín De La Soc. Española De Cerámica Y Vidr. 2019, 58, 85–92. [Google Scholar] [CrossRef]
- Wei, D.; Zhou, Y.; Jia, D.; Wang, Y. Biomimetic apatite deposited on microarc oxidized anatase-based ceramic coating. Ceram. Int. 2008, 34, 1139–1144. [Google Scholar] [CrossRef]
- Mutlu, B.; Demirci, F.; Duman, Ş. Influence of boron incorporated biphasic calcium phosphate on mechanical, thermal, and biological properties of poly (vinylidene fluoride) membrane scaffold. J. Am. Ceram. Soc. 2024, 107, 7274–7288. [Google Scholar] [CrossRef]

| Sample Code | Chitosan (g) | TiO2 (g) | Curcumin (g) |
|---|---|---|---|
| CS | 0.800 | 0 | 0 |
| CS/1T | 0.792 | 0.008 | 0 |
| CS/3T | 0.776 | 0.024 | 0 |
| CS/5T | 0.760 | 0.040 | 0 |
| CS/3T/C | 0.768 | 0.024 | 0.008 |
| Sample | Breaking Strength (N) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|
| CS | 0.74 ± 0.02 | 168.63 ± 5.17 | 2.55 ± 0.18 |
| CS/1T | 0.77 ± 0.05 | 176.17 ± 12.23 | 2.63 ± 0.52 |
| CS/3T | 0.84 ± 0.04 | 189.41 ± 8.21 | 3.37 ± 0.49 |
| CS/5T | 0.66 ± 0.02 | 148.83 ± 5.39 | 2.77 ± 0.30 |
| CS/3T/C | 0.57 ± 0.01 | 128.10 ± 2.67 | 2.5 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demirci, F. Development of Curcumin-Loaded TiO2-Reinforced Chitosan Monofilaments for Biocompatible Surgical Sutures. Polymers 2025, 17, 484. https://doi.org/10.3390/polym17040484
Demirci F. Development of Curcumin-Loaded TiO2-Reinforced Chitosan Monofilaments for Biocompatible Surgical Sutures. Polymers. 2025; 17(4):484. https://doi.org/10.3390/polym17040484
Chicago/Turabian StyleDemirci, Fatma. 2025. "Development of Curcumin-Loaded TiO2-Reinforced Chitosan Monofilaments for Biocompatible Surgical Sutures" Polymers 17, no. 4: 484. https://doi.org/10.3390/polym17040484
APA StyleDemirci, F. (2025). Development of Curcumin-Loaded TiO2-Reinforced Chitosan Monofilaments for Biocompatible Surgical Sutures. Polymers, 17(4), 484. https://doi.org/10.3390/polym17040484








