Preparation of Chitooligosaccharides with Specific Sequence Arrangement and Their Effect on Inducing Salt Resistance in Wheat Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of COSs with Chitin Deacetylases
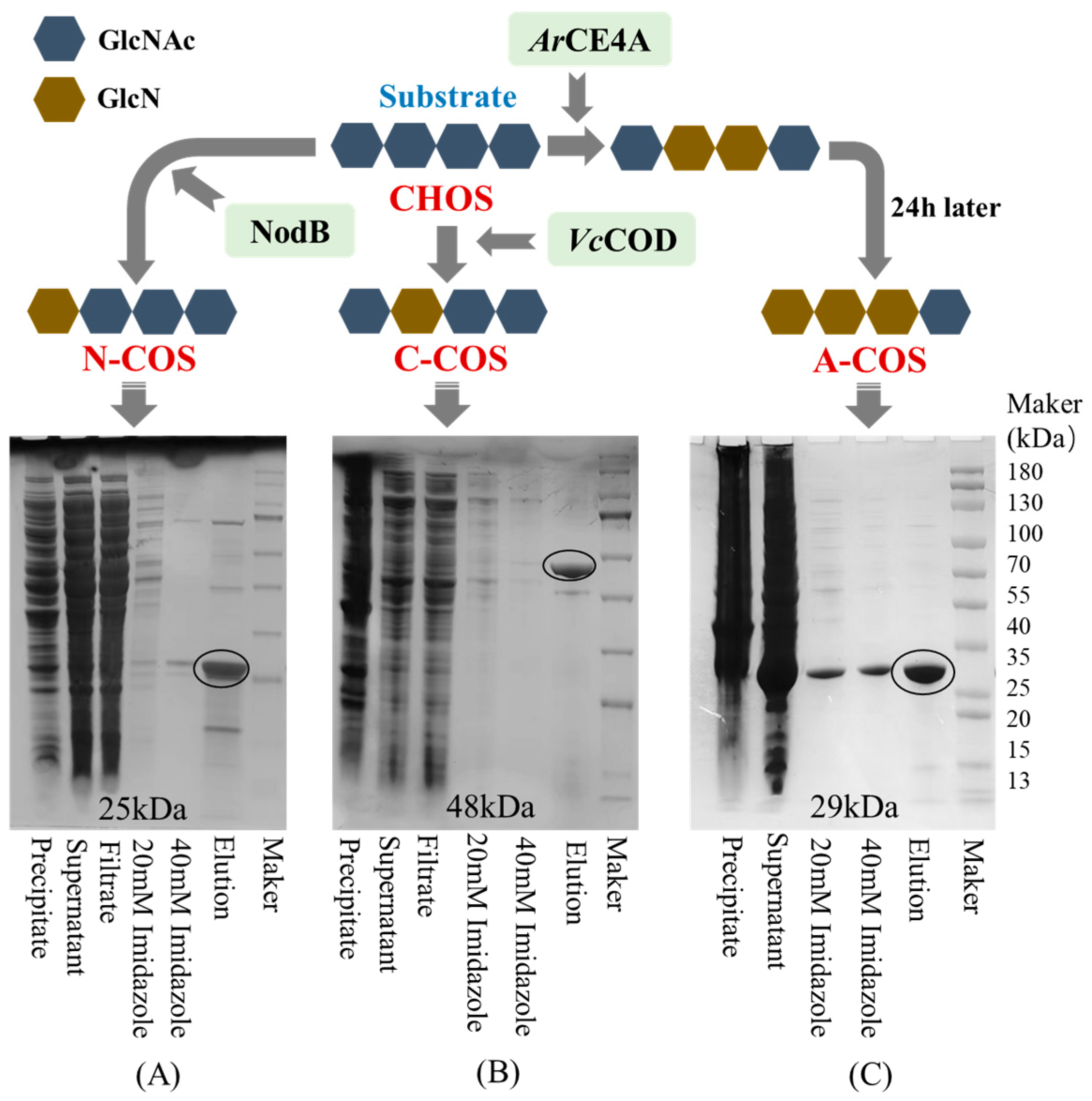
2.2.1. Heterologous Expression and Purification of Chitin Deacetylases
2.2.2. Preparation of COSs with Specific Sequence Arrangement
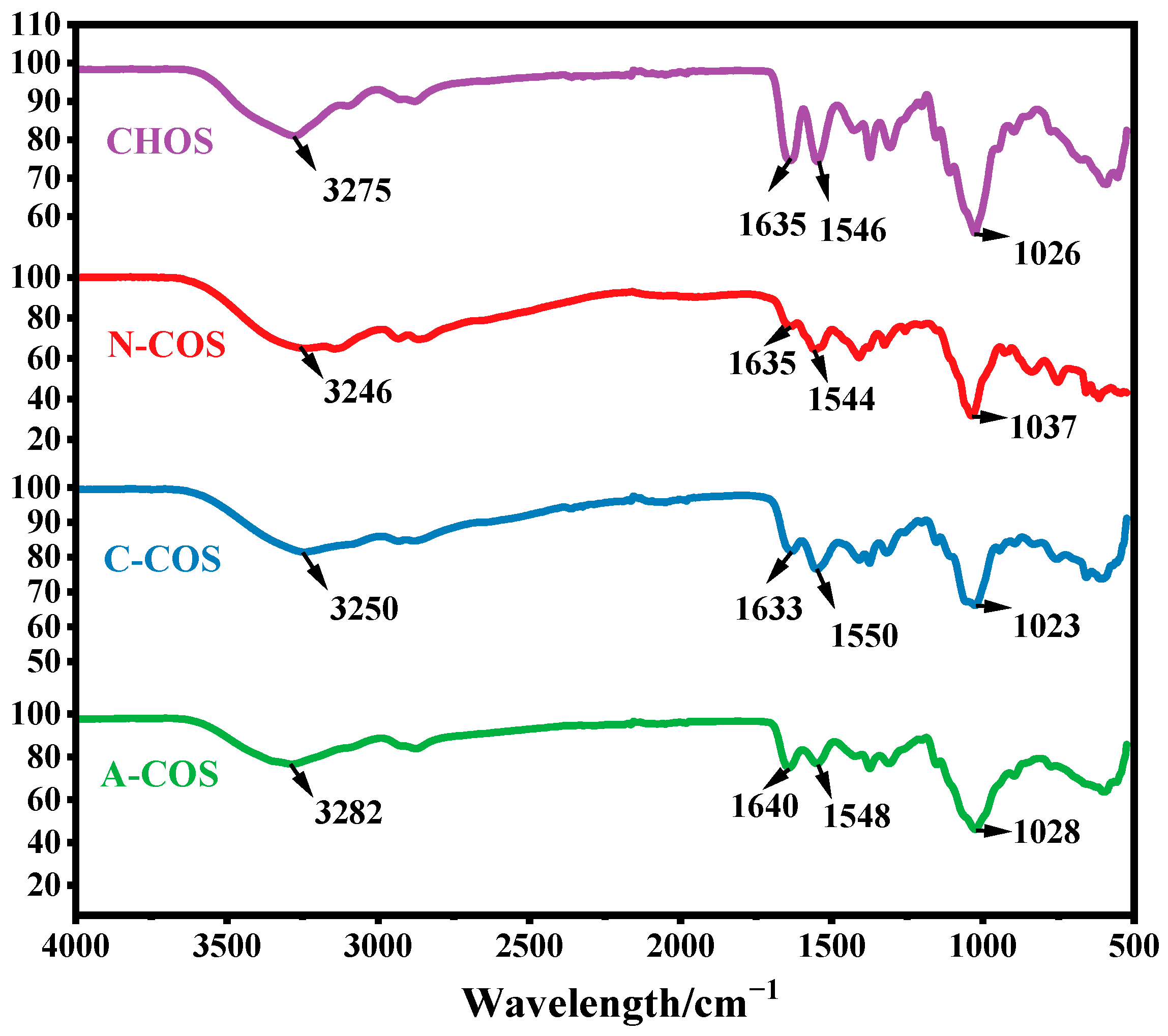
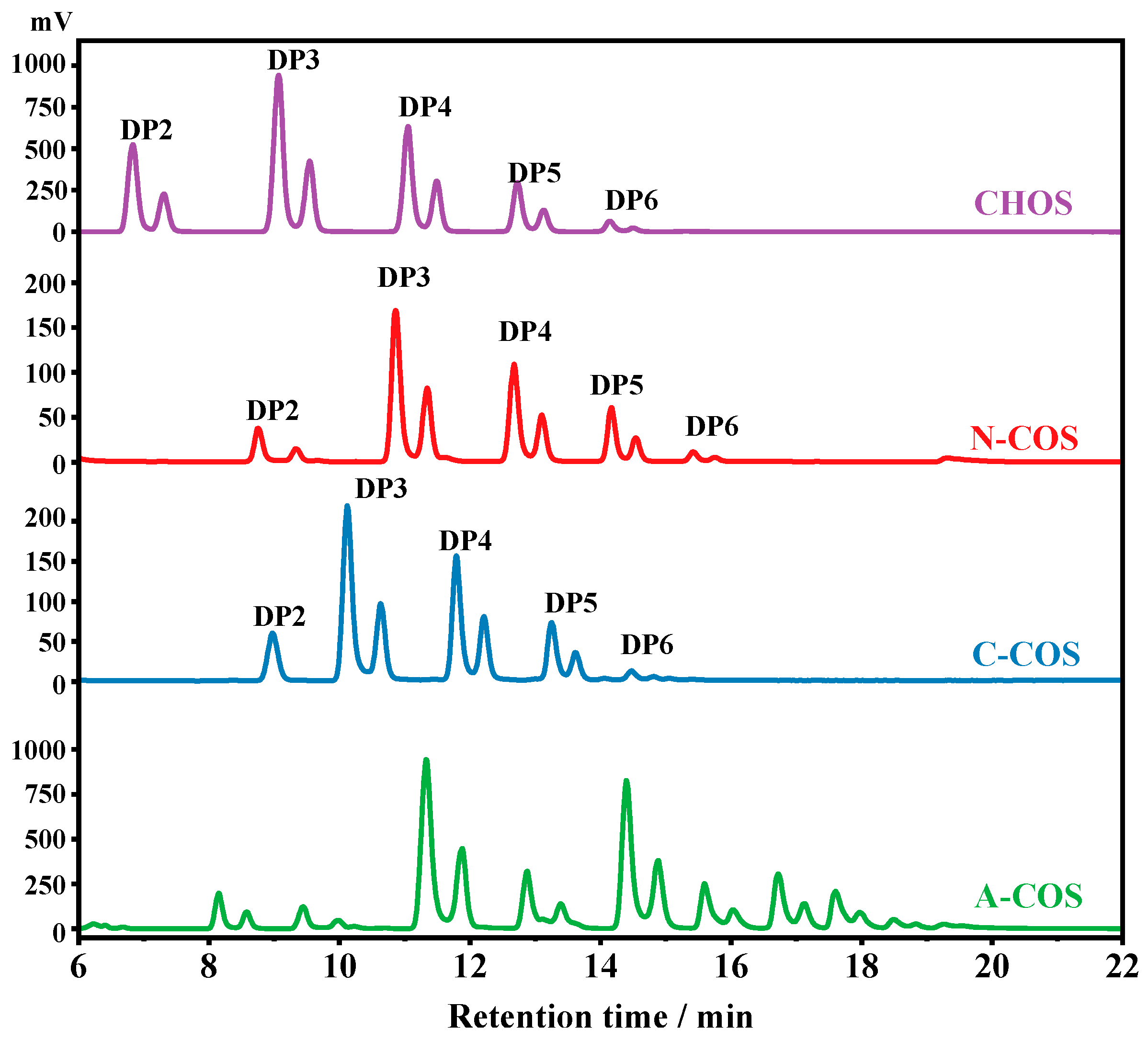
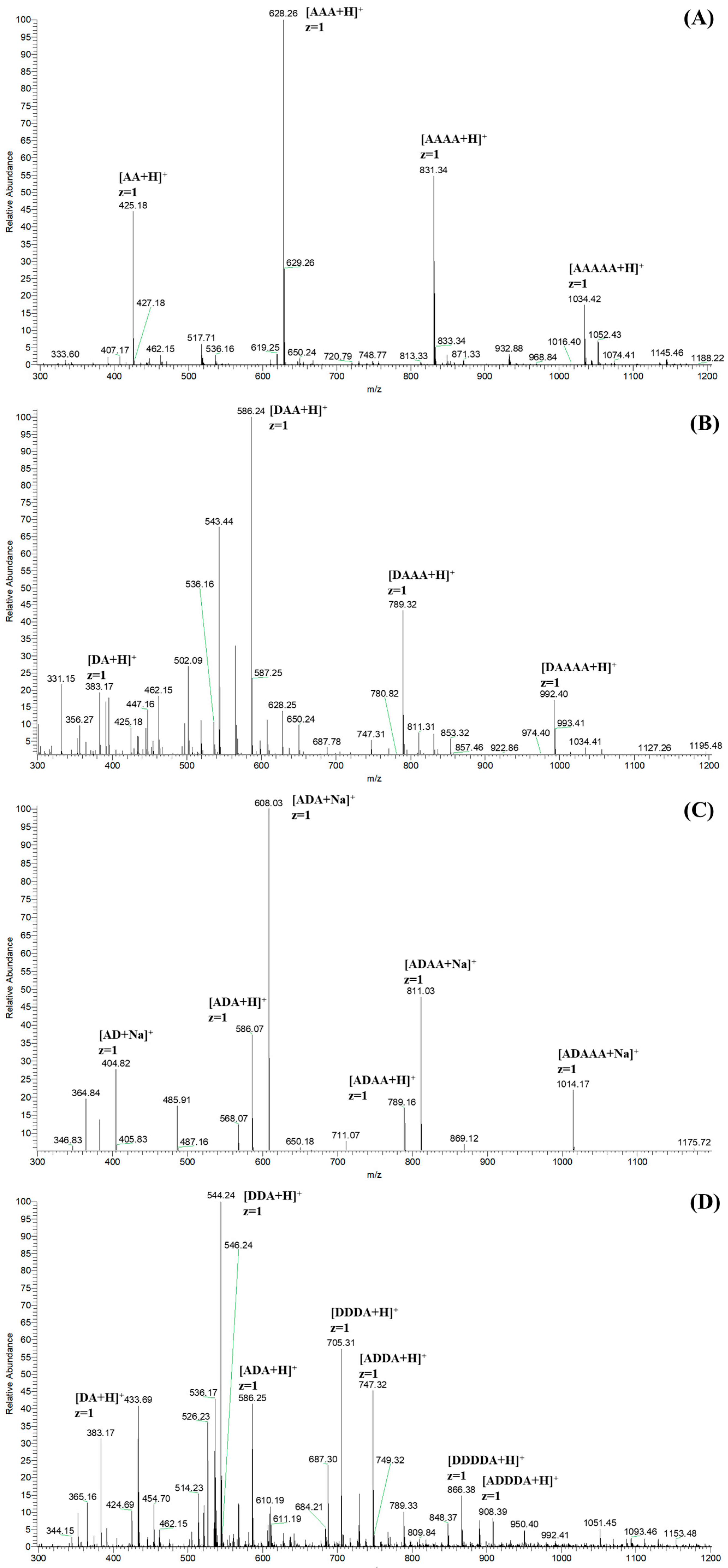
2.3. Characterization
2.4. Plant Materials and Treatment
2.5. Determination of the Growth Parameters
2.6. Determination of the Proline Content
2.7. Determination of the MDA Content
2.8. Determination of Antioxidant Enzyme Activities
2.9. Determination of Na+ and K+ Contents
2.10. Statistical Analysis
3. Results
3.1. Heterologous Expression and Purification of Deacetylase
3.2. Characterization of COSs with Specific Sequence Arrangements
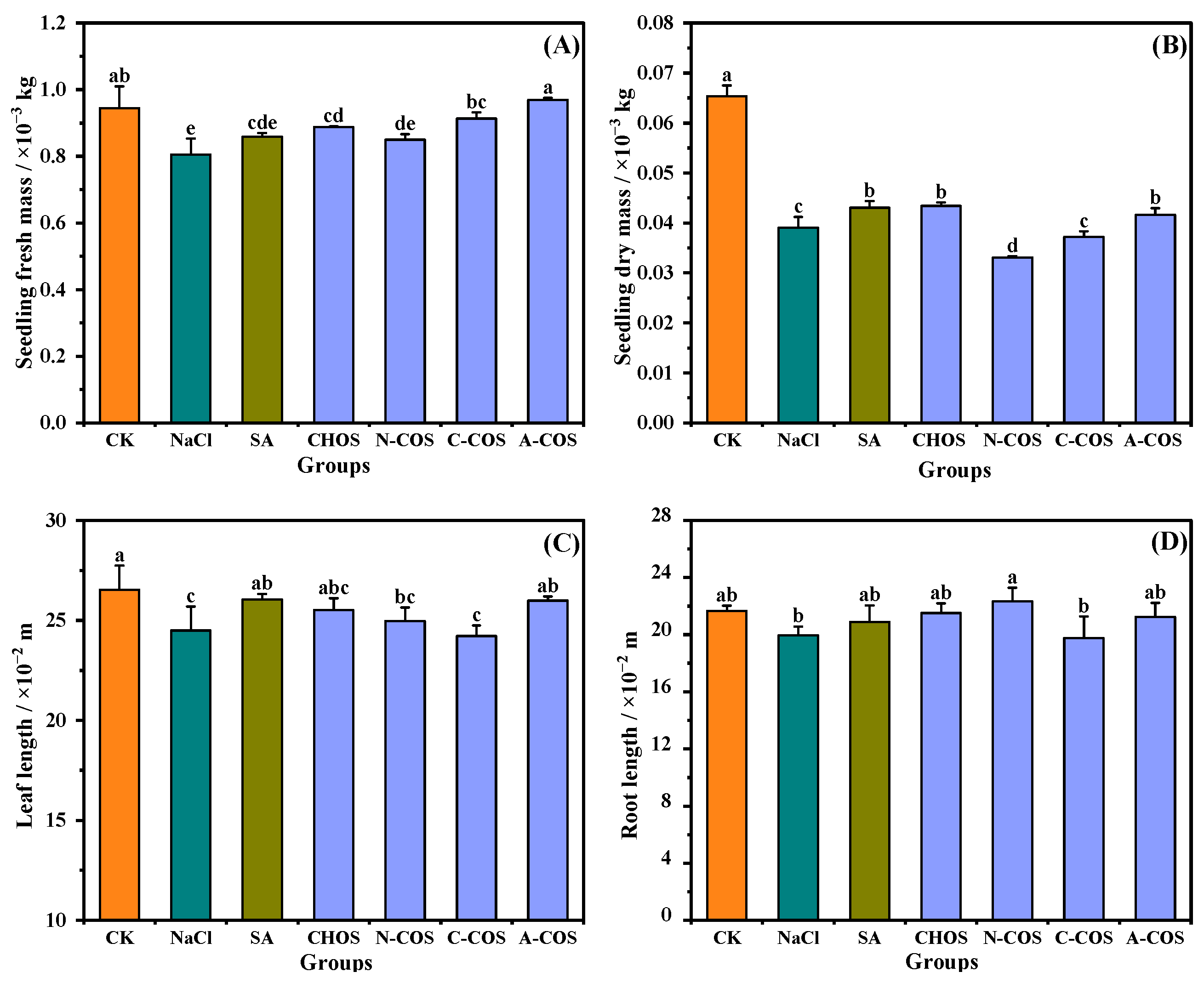
3.3. Wheat Growth Index
3.4. Proline Content
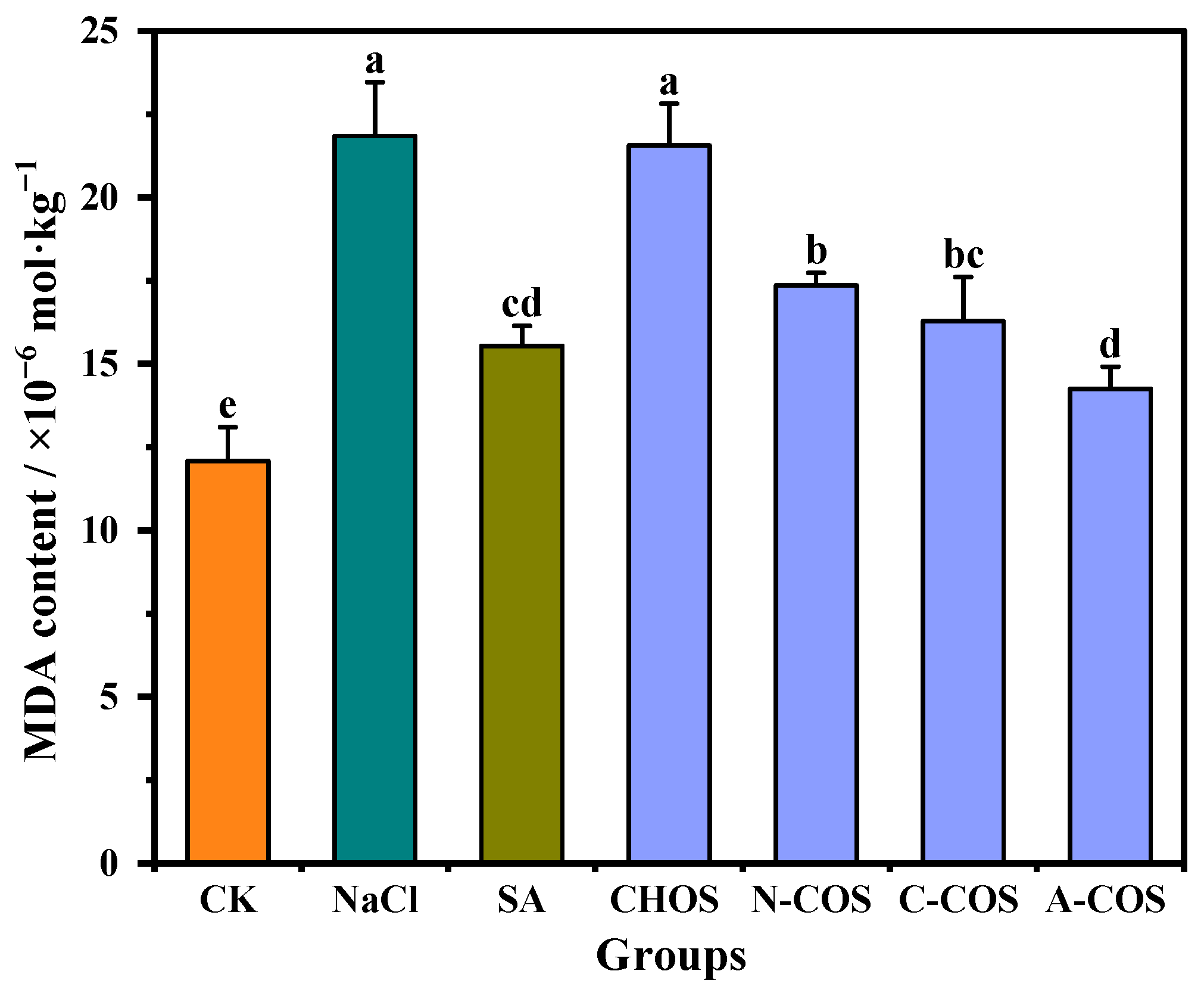
3.5. MDA Content
3.6. Antioxidant Enzyme Activities
3.7. Na+ Content and K+ Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COS | Chitooligosaccharides |
| ROS | Reactive oxygen species |
| SA | Salicylic acid |
| MDA | Malondialdehyde |
| PAMP | Pathogen-associated molecular pattern |
| A | N-acetylglucosamine |
| D | Glucosamine |
| DA | Degree of acetylation |
| CHOS | Chitin oligosaccharide |
| POD | Peroxidase |
| CAT | Catalase |
| SOD | Superoxide Dismutase |
References
- Hassani, A.; Azapagic, A.; Shokri, N. Global predictions of primary soil salinization under changing climate in the 21st century. Nat. Commun. 2021, 12, 6663. [Google Scholar] [CrossRef]
- Fan, Y.N.; Zhang, Y.X.; Wan, M.X.; Hu, W.Y.; Chen, Z.K.; Huang, B. Plastic shed production intensified secondary soil salinization in perennial fruit production systems. Agric. Ecosyst. Environ. 2021, 316, 107469. [Google Scholar] [CrossRef]
- Fang, X.; Mo, J.J.; Zhou, H.K.; Shen, X.F.; Xie, Y.L.; Xu, J.H.; Yang, S. Comparative transcriptome analysis of gene responses of salt-tolerant and salt-sensitive rice cultivars to salt stress. Sci. Rep. 2023, 13, 19065. [Google Scholar] [CrossRef] [PubMed]
- Ondrasek, G.; Rengel, Z. Environmental salinization processes: Detection, implications & solutions. Sci. Total Environ. 2021, 754, 142432. [Google Scholar]
- Liu, A.K.; Wang, M.Y.; Dong, J.J.; Yan, Z.Y.; Wang, X.; Li, J.; Song, H.F. Foliar application of exogenous salicylic acid mitigates the detrimental effects caused by salt stress in sunflower seedlings. Ind. Crop Prod. 2024, 222, 119854. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, Y.D.; Li, J.; Zhang, J.; Zhang, X.D.; Hu, L.X.; Ding, D.X.; Bakpa, E.P.; Xie, J.M. Trehalose Alleviated Salt Stress in Tomato by Regulating ROS Metabolism, Photosynthesis, Osmolyte Synthesis, and Trehalose Metabolic Pathways. Front. Plant Sci. 2022, 13, 772948. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.Q.; Wang, F.Z.; Li, J.F. Hide-and-Seek: Chitin-Triggered Plant Immunity and Fungal Counterstrategies. Trends Plant Sci. 2020, 25, 805–816. [Google Scholar] [CrossRef]
- Wang, X.X.; Zong, Y.Y.; Zhu, Y.T.; Zhang, X.M.; Zhang, F.; Gong, D.; Prusky, D.; Bi, Y. Chitooligosaccharide inhibits growth, pathogenicity and patulin production in Penicillium expansum by downregulating global transcription factors. Postharvest Biol. Technol. 2025, 222, 113407. [Google Scholar] [CrossRef]
- Zhao, X.L.; Wang, Y.C.; Wang, C.Y.; Zhou, W.; Ouyang, D.; Gao, S.S.; Tan, X.F.; Huang, R.; Guo, Y. Chitooligosaccharide application enhanced the growth and phytoremediation efficiency of industrial hemp in Cd-contaminated soils. Bioresour. Technol. 2025, 418, 131998. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Li, K.C.; Liu, S.; Zou, P.; Xing, R.E.; Yu, H.H.; Chen, X.L.; Qin, Y.K.; Li, P.C. Relationship between the degree of polymerization of chitooligomers and their activity affecting the growth of wheat seedlings under salt stress. J. Agric. Food Chem. 2017, 65, 501–509. [Google Scholar] [CrossRef]
- Yu, M.L.; Wu, Q.; Zheng, D.F.; Feng, N.J.; Liang, X.L.; Liu, M.L.; Li, Y.; Mou, B.M. Plant Growth Regulators Enhance Saline–Alkali Tolerance by Upregulating the Levels of Antioxidants and Osmolytes in Soybean Seedlings. J. Plant Growth Regul. 2022, 41, 3218–3232. [Google Scholar] [CrossRef]
- Li, K.C.; Zhang, X.Q.; Yu, Y.; Xing, R.E.; Liu, S.; Li, P.C. Effect of chitin and chitosan hexamers on growth and photosynthetic characteristics of wheat seedlings. Photosynthetica 2020, 58, 819–826. [Google Scholar] [CrossRef]
- Fu, X.D.; Zhu, L.; Li, L.; Zhang, T.; Li, M.; Mou, H.J. Eco-friendly preparation of chitooligosaccharides with different degrees of deacetylation from shrimp shell waste and their effects on the germination of wheat seeds. Mar. Life Sci. Technol. 2019, 1, 95–103. [Google Scholar] [CrossRef]
- Yang, G.; Hou, X.Y.; Lu, J.; Wang, M.B.; Wang, Y.H.; Huang, Y.C.; Liu, Q.T.; Liu, S.; Fang, Y.W.; Fang, Y. Enzymatic modification of native chitin and chitin oligosaccharides by an alkaline chitin deacetylase from Microbacterium esteraromaticum MCDA02. Int. J. Biol. Macromol. 2022, 203, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Linhorst, M.; Wattjes, J.; Moerschbacher, B.M. Chitin deacetylase as a biocatalyst for the selective N-acylation of chitosan oligo-and polymers. ACS Catal. 2021, 11, 14456–14466. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Zhao, Y.; Zhang, H.D.; Wang, W.X.; Cong, H.H.; Yin, H. Characterization of the specific mode of action of a chitin deacetylase and separation of the partially acetylated chitosan oligosaccharides. Mar. Drugs 2019, 17, 74. [Google Scholar] [CrossRef]
- Hao, W.T.; Li, K.C.; Ma, Y.Z.; Li, R.F.; Xing, R.E.; Yu, H.H.; Li, P.C. Preparation and Antioxidant Activity of Chitosan Dimers with Different Sequences. Mar. Drugs 2021, 19, 366. [Google Scholar] [CrossRef]
- Basa, S.; Nampally, M.; Honorato, T.; Das, S.N.; Podile, A.R.; Gueddari, N.E.E.; Moerschbacher, B.M. The Pattern of Acetylation Defines the Priming Activity of Chitosan Tetramers. J. Am. Chem. Soc. 2020, 142, 1975–1986. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Sarimov, R.M.; Astashev, M.E.; Pishchalnikov, R.Y.; Yanykin, D.V.; Simakin, A.V.; Shkirin, A.V.; Serov, D.A.; Konchekov, E.M.; Ogly, G.-Z.N.G.; et al. Modern physical methods and technologies in agriculture. Phys. Usp. 2024, 67, 194–210. [Google Scholar] [CrossRef]
- Tuveng, T.R.; Rothweiler, U.; Udatha, G.; Vaaje-Kolstad, G.; Smalås, A.; Eijsink, V.G.H. Structure and function of a CE4 deacetylase isolated from a marine environment. PLoS ONE 2017, 12, e0187544. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Kashif, M.H.; Feng, J.; Dai, R.; Fan, Y.; Xia, Y.; Liu, Z. Salicylic acid-mediated alleviation of salt stress: Insights from physiological and transcriptomic analysis in Asarum sieboldii Miq. Chemosphere 2024, 362, 142604. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.A.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- John, M.; Röhrig, H.; Schmidt, J.; Wieneke, U.; Schell, J. Rhizobium NodB protein involved in nodulation signal synthesis is a chitooligosaccharide deacetylase. Proc. Natl. Acad. Sci. USA 1993, 90, 625–629. [Google Scholar] [CrossRef]
- Li, X.B.; Wang, L.X.; Wang, X.S.; Roseman, S. The Chitin Catabolic Cascade in the Marine Bacterium Vibrio Cholerae: Characterization of a Unique Chitin Oligosaccharide Deacetylase. Glycobiology 2017, 17, 1377–1387. [Google Scholar] [CrossRef]
- He, X.F.; Li, K.C.; Xing, R.E.; Liu, S.; Hu, L.F.; Li, P.C. The production of fully deacetylated chitosan by compression method. Egypt. J. Aquat. Res. 2016, 42, 75–81. [Google Scholar] [CrossRef]
- Trombotto, S.; Ladavière, C.; Delolme, F.; Domard, A. Chemical Preparation and Structural Characterization of a Homogeneous Series of Chitin/Chitosan Oligomers. Biomacromolecules 2008, 9, 1731–1738. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Y.; Zhang, M.; Chen, J.G.; Liu, J.; Han, H.L.; Hua, X.J. Arabidopsis AMINO ACID PERMEASE1 Contributes to Salt Stress-Induced Proline Uptake from Exogenous Sources. Front. Plant Sci. 2017, 8, 2182. [Google Scholar] [CrossRef]
- Alvan, H.A.; Jabbarzadeh, Z.; Fard, J.R.; Noruzi, P. Selenium foliar application alleviates salinity stress in sweet william (Dianthus barbatus L.) by enhancing growth and reducing oxidative damage. Sci. Rep. 2025, 15, 5570. [Google Scholar] [CrossRef]
- Kul, R.; Arjumend, T.; Ekinci, M.; Yildirim, E.; Turan, M.; Argin, S. Biochar as an organic soil conditioner for mitigating salinity stress in tomato. Soil Sci. Plant Nutr. 2021, 67, 693–706. [Google Scholar] [CrossRef]
- Shen, Z.H.; Pu, X.Z.; Wang, S.M.; Dong, X.X.; Cheng, X.X.; Cheng, M.X. Silicon improves ion homeostasis and growth of liquorice under salt stress by reducing plant Na+ uptake. Sci. Rep. 2022, 12, 5089. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Quan, X.Y.; Liang, X.L.; Li, H.M.; Xie, C.J.; He, W.X.; Qin, Y.X. Identification and characterization of wheat germplasm for salt tolerance. Plants 2021, 10, 268. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Li, K.C.; Liu, S.; He, X.F.; Zhang, X.Q.; Xing, R.E.; Li, P.C. Effect of Sulfated Chitooligosaccharides on Wheat Seedlings (Triticum aestivum L.) under Salt Stress. J. Agric. Food Chem. 2016, 64, 2815–2821. [Google Scholar] [CrossRef]
- Lamnai, K.; Anaya, F.; Fghire, R.; Zine, H.; Wahbi, S.; Loutfi, K. Impact of Exogenous Application of Salicylic Acid on Growth, Water Status And Antioxidant Enzyme Activity of Strawberry Plants (Fragaria vesca L.) Under Salt Stress Conditions. Gesunde Pflanz. 2021, 73, 465–478. [Google Scholar] [CrossRef]
- Farouk, S.; Elhindi, K.M.; Alotaibi, M.A. Silicon supplementation mitigates salinity stress on Ocimum basilicum L. via improving water balance, ion homeostasis, and antioxidant defense system. Ecotoxicol. Environ. Saf. 2020, 206, 111396. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, L.Q.; Zhao, M.Y.; Chen, Q.M.; Qin, Z.; Feng, Z.H.; Fujiwara, T.; Zhao, L.M. Chitooligosaccharide plays essential roles in regulating proline metabolism and cold stress tolerance in rice seedlings. Acta Physiol. Plant. 2019, 41, 77. [Google Scholar] [CrossRef]
- Butt, M.; Sattar, A.; Abbas, T.; Sher, A.; Ijaz, M.; Ul-Allah, S.; Shaheen, M.R.; Kaleem, F. Foliage applied proline induces salt tolerance in chili genotypes by regulating photosynthetic attributes, ionic homeostasis, and antioxidant defense mechanisms. Hortic. Environ. Biotechnol. 2020, 61, 693–702. [Google Scholar] [CrossRef]
- Lu, H.; Zhao, X.M.; Wang, W.X.; Yin, H.; Xu, J.G.; Bai, X.F.; Du, Y.G. Inhibition effect on tobacco mosaic virus and regulation effect on calreticulin of oligochitosan in tobacco by induced Ca2+ influx. Carbohyd. Polym. 2010, 82, 136–142. [Google Scholar] [CrossRef]
- Brulé, D.; Villano, C.; Davies, L.J.; Trdá, L.; Claverie, J.; Héloir, M.C.; Chiltz, A.; Adrian, M.; Darblade, B.; Tornero, P.; et al. The grapevine (Vitis vinifera) LysM receptor kinases VvLYK1-1 and VvLYK1-2 mediate chitooligosaccharide-triggered immunity. Plant Biotechnol. J. 2019, 17, 812–825. [Google Scholar] [CrossRef]
- Al-Huqail, A.A.; Aref, N.M.A.; Khan, F.; Sobhy, S.E.; Hafez, E.E.; Khalifa, A.M.; Saad-Allah, K.M. Azolla filiculoides extract improved salt tolerance in wheat (Triticum aestivum L.) is associated with prompting osmostasis, antioxidant potential and stress-interrelated genes. Sci. Rep. 2024, 14, 11100. [Google Scholar]
- Wang, W.Y.; Liu, S.; Yan, M.Y. Synthesis of γ-Aminobutyric Acid-Modified Chitooligosaccharide Derivative and Enhancing Salt Resistance of Wheat Seedlings. Molecules 2022, 27, 3068. [Google Scholar] [CrossRef]
- Zou, P.; Li, K.C.; Liu, S.L.; Xing, R.E.; Qin, Y.K.; Yu, H.H.; Zhou, M.M.; Li, P.P. Effect of chitooligosaccharides with different degrees of acetylation on wheat seedlings under salt stress. Carbohyd. Polym. 2015, 126, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Chatelain, P.G.; Pintado, M.E.; Vasconcelos, M.W. Evaluation of chitooligosaccharide application on mineral accumulation and plant growth in Phaseolus vulgaris. Plant Sci. 2014, 215, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.M.; Xu, X.S.; Kong, X.L. K+-Selectivity Due to Coordination with a D4d-Symmetric Homochiral Proline Octamer Verified by Mass Spectrometry and Infrared Photodissociation Spectroscopy. J. Phys. Chem. Lett. 2023, 14, 2660–2664. [Google Scholar] [CrossRef] [PubMed]
- Bojórquez-Quintal, E.; Ruiz-Lau, N.; Velarde-Buendía, A.; Echevarría-Machado, I.; Pottosin, I.; Martínez-Estévez, M. Natural variation in primary root growth and K+ retention in roots of habanero pepper (Capsicum chinense) under salt stress. Funct. Plant Biol. 2016, 43, 1114–1125. [Google Scholar] [CrossRef]
- Hayafune, M.; Berisio, R.; Marchetti, R.; Silipo, A.; Kayama, M.; Desaki, Y.; Arima, S.; Squeglia, F.; Ruggiero, A.; Tokuyasu, K.; et al. Chitin-induced activation of immune signaling by the rice receptor CEBiP relies on a unique sandwich-type dimerization. Proc. Natl. Acad. Sci. USA 2014, 111, E404–E413. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, A.; Li, Y.; Zhu, S.; Song, L.; Liu, S.; Xing, R.; Li, K. Preparation of Chitooligosaccharides with Specific Sequence Arrangement and Their Effect on Inducing Salt Resistance in Wheat Seedlings. Polymers 2025, 17, 1194. https://doi.org/10.3390/polym17091194
Li J, Li A, Li Y, Zhu S, Song L, Liu S, Xing R, Li K. Preparation of Chitooligosaccharides with Specific Sequence Arrangement and Their Effect on Inducing Salt Resistance in Wheat Seedlings. Polymers. 2025; 17(9):1194. https://doi.org/10.3390/polym17091194
Chicago/Turabian StyleLi, Jingwen, Anbang Li, Yupeng Li, Siqi Zhu, Lin Song, Song Liu, Ronge Xing, and Kecheng Li. 2025. "Preparation of Chitooligosaccharides with Specific Sequence Arrangement and Their Effect on Inducing Salt Resistance in Wheat Seedlings" Polymers 17, no. 9: 1194. https://doi.org/10.3390/polym17091194
APA StyleLi, J., Li, A., Li, Y., Zhu, S., Song, L., Liu, S., Xing, R., & Li, K. (2025). Preparation of Chitooligosaccharides with Specific Sequence Arrangement and Their Effect on Inducing Salt Resistance in Wheat Seedlings. Polymers, 17(9), 1194. https://doi.org/10.3390/polym17091194






