Poly(oligoethylene glycol methylether methacrylate-co-methyl methacrylate) Aggregates as Nanocarriers for Curcumin and Quercetin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Linear P(OEGMA-co-MMA)
2.3. Self-Assembly in Aqueous Solutions
2.4. Curcumin and Quercetin Nanocarrier Formulation
2.5. FBS Interaction with Nanoparticles
2.6. Characterization Methods
2.6.1. Size Exclusion Chromatography (SEC)
2.6.2. Proton Nuclear Magnetic Resonance Spectroscopy (1H-NMR)
2.6.3. Attenuated Total Reflectance (ATR)-Fourier Transform Infrared (FTIR) Spectroscopy
2.6.4. Dynamic Light Scattering (DLS)
2.6.5. Electrophoretic Light Scattering (ELS)
2.6.6. Fluorescence Spectroscopy (FS)
2.6.7. UV–Vis Absorption Spectroscopy (UV–Vis)
3. Results and Discussion
3.1. P(OEGMA-co-MMA) Molecular Characterization
3.2. Self-Assembly and Critical Aggregation Concentration in Aqueous Solutions
3.2.1. Curcumin Encapsulation
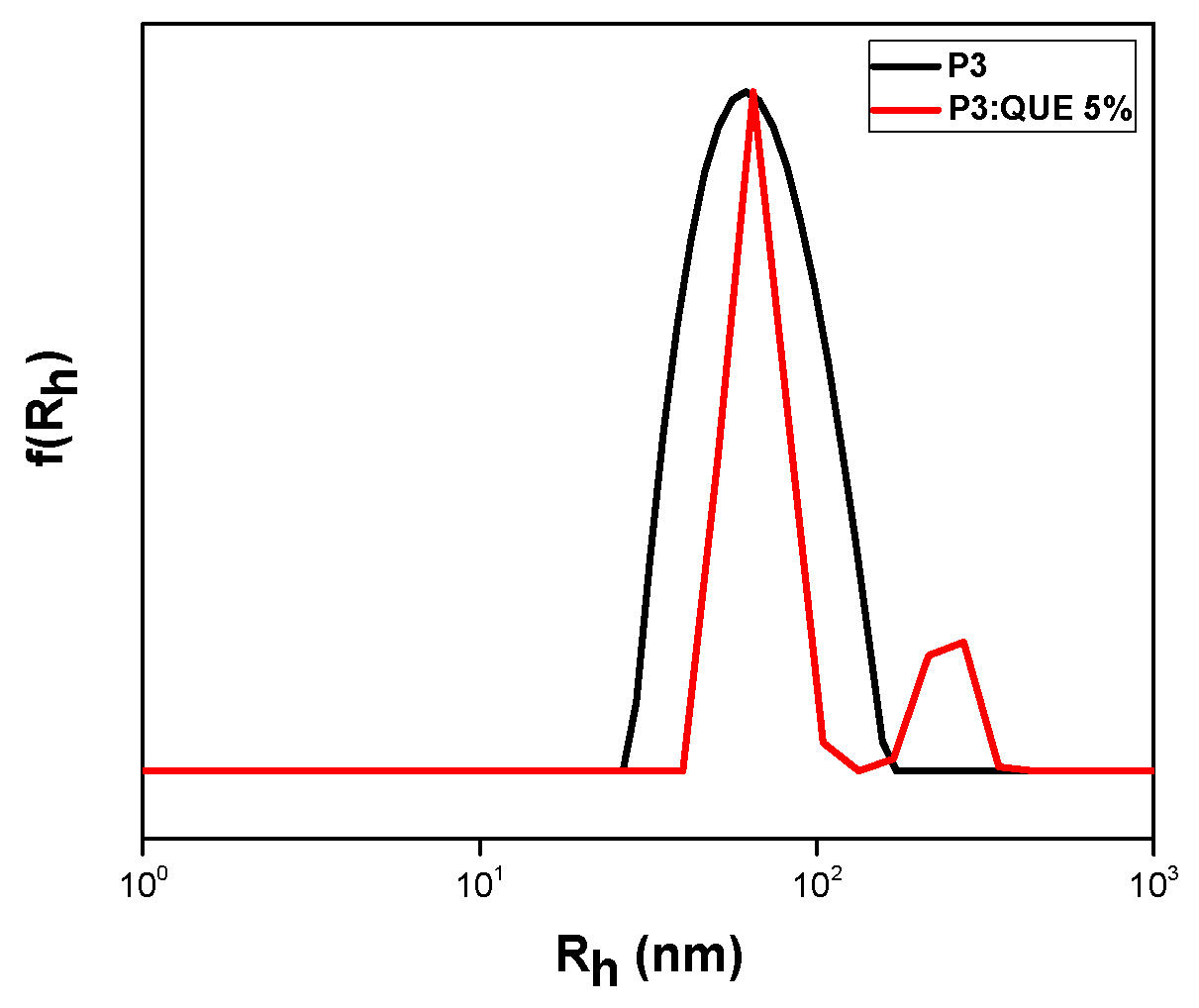
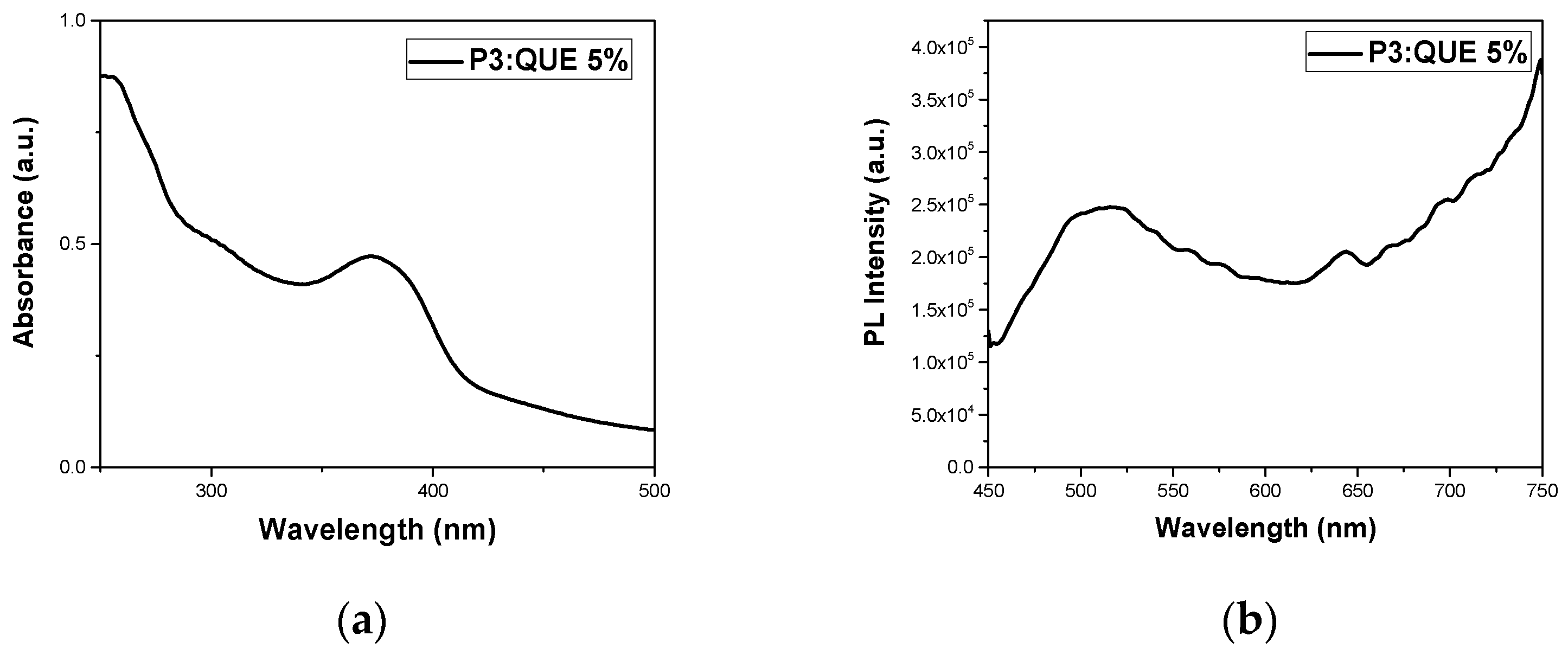
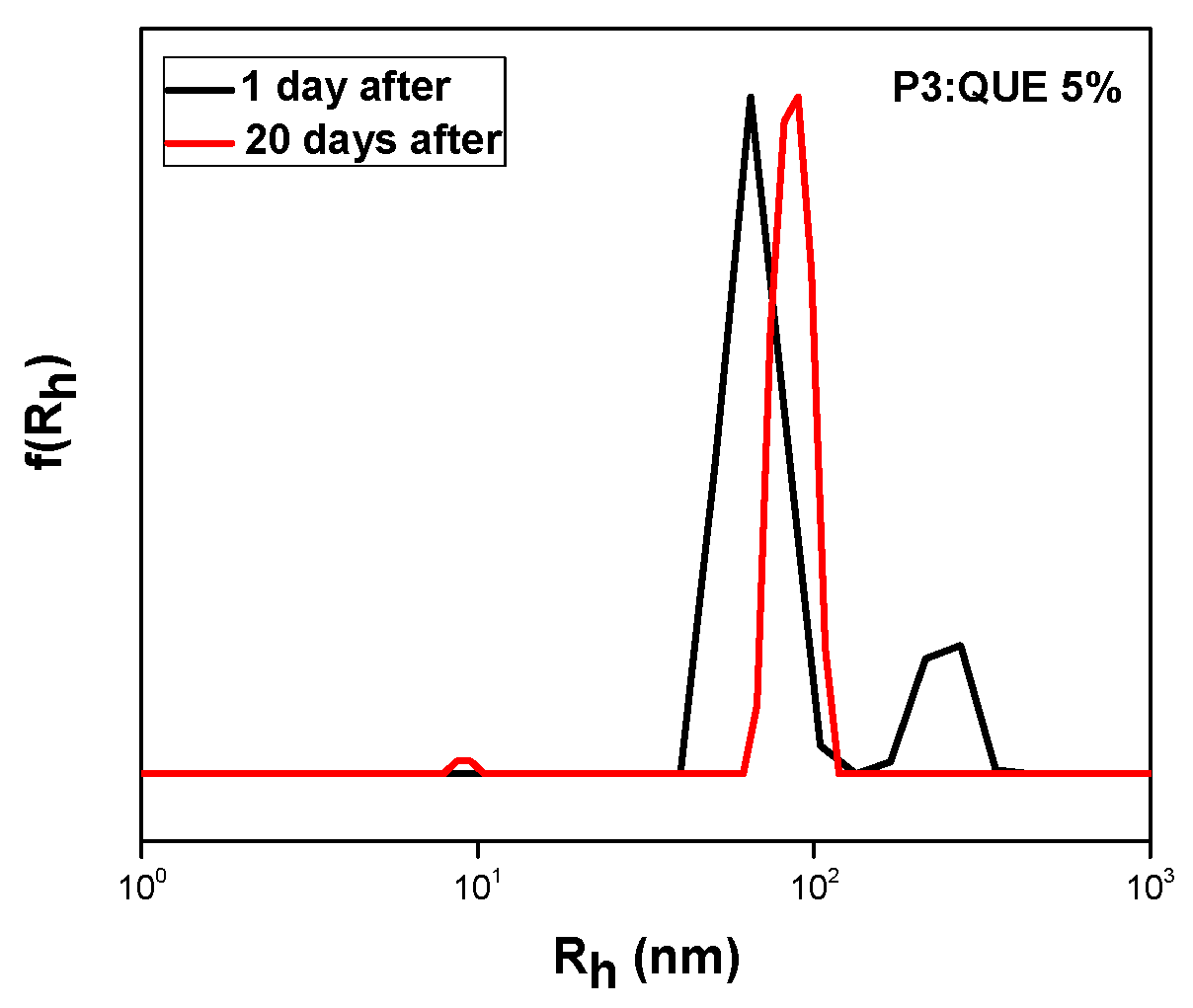
3.2.2. Quercetin Encapsulation
3.3. FBS Interactions with P(OEGMA-co-MMA) Nanoaaggregates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liao, Q.; Kim, E.J.; Tang, Y.; Xu, H.; Yu, D.-G.; Song, W.; Kim, B.J. Rational Design of Hyper-Crosslinked Polymers for Biomedical Applications. J. Polym. Sci. 2024, 62, 1517–1535. [Google Scholar] [CrossRef]
- Bhardwaj, H.; Jangde, R.K. Current Updated Review on Preparation of Polymeric Nanoparticles for Drug Delivery and Biomedical Applications. Next Nanotechnol. 2023, 2, 100013. [Google Scholar] [CrossRef]
- Lutz, J.-F.; Lehn, J.-M.; Meijer, E.W.; Matyjaszewski, K. From Precision Polymers to Complex Materials and Systems. Nat. Rev. Mater. 2016, 1, 16024. [Google Scholar] [CrossRef]
- Chiefari, J.; Chong, Y.K.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living Free-Radical Polymerization by Reversible Addition−Fragmentation Chain Transfer: The RAFT Process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living Radical Polymerization by the RAFT Process—A Third Update. Aust. J. Chem. 2012, 65, 985. [Google Scholar] [CrossRef]
- Tian, X.; Ding, J.; Zhang, B.; Qiu, F.; Zhuang, X.; Chen, Y. Recent Advances in RAFT Polymerization: Novel Initiation Mechanisms and Optoelectronic Applications. Polymers 2018, 10, 318. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Discovery of the RAFT Process and Its Impact on Radical Polymerization. Macromolecules 2020, 53, 495–497. [Google Scholar] [CrossRef]
- Krishnan, A.; Roy, S.; Menon, S. Amphiphilic Block Copolymers: From Synthesis Including Living Polymerization Methods to Applications in Drug Delivery. Eur. Polym. J. 2022, 172, 111224. [Google Scholar] [CrossRef]
- Kuperkar, K.; Patel, D.; Atanase, L.I.; Bahadur, P. Amphiphilic Block Copolymers: Their Structures, and Self-Assembly to Polymeric Micelles and Polymersomes as Drug Delivery Vehicles. Polymers 2022, 14, 4702. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Thakur, A. Applications of Poly(Methyl Methacrylate) Polymer in Dentistry: A Review. Mater. Today Proc. 2022, 50, 1619–1625. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Sakib, S.; Mathews, R.; Zhitomirsky, I. Poly(Methyl Methacrylate) Coatings Containing Flame Retardant Additives from Suspensions in Water-2-Propanol. Molecules 2021, 26, 1974. [Google Scholar] [CrossRef]
- Edo, G.I.; Ndudi, W.; Ali, A.B.M.; Yousif, E.; Zainulabdeen, K.; Onyibe, P.N.; Akpoghelie, P.O.; Ekokotu, H.A.; Isoje, E.F.; Igbuku, U.A.; et al. An Updated Review on the Modifications, Recycling, Polymerization, and Applications of Polymethyl Methacrylate (PMMA). J. Mater. Sci. 2024, 59, 20496–20539. [Google Scholar] [CrossRef]
- Ramanathan, S.; Lin, Y.-C.; Thirumurugan, S.; Hu, C.-C.; Duann, Y.-F.; Chung, R.-J. Poly(Methyl Methacrylate) in Orthopedics: Strategies, Challenges, and Prospects in Bone Tissue Engineering. Polymers 2024, 16, 367. [Google Scholar] [CrossRef] [PubMed]
- Ozer, I.; Tomak, A.; Zareie, H.M.; Baran, Y.; Bulmus, V. Effect of Molecular Architecture on Cell Interactions and Stealth Properties of PEG. Biomacromolecules 2017, 18, 2699–2710. [Google Scholar] [CrossRef] [PubMed]
- Kureha, T.; Hirayama, T.; Nishi, T. Phase Diagram for the Gelation of Temperature-Responsive and Biocompatible Poly(Oligo Ethylene Glycol Methyl Ether Methacrylate) Polymers in Aqueous Free-Radical Polymerization Reactions. Polym. J. 2024, 56, 1017–1029. [Google Scholar] [CrossRef]
- Wang, L.; Constantinou, A.P.; Li, Y.; Georgiou, T.K. A Library of Thermoresponsive Diblock and Statistical Copolymers: Unravelling the Effect of Molar Mass. Eur. Polym. J. 2024, 207, 112810. [Google Scholar] [CrossRef]
- Pantelaiou, M.A.; Vagenas, D.; Karvelis, E.S.; Rotas, G.; Pispas, S. Co-Assembled Nanosystems Exhibiting Intrinsic Fluorescence by Complexation of Amino Terpolymer and Its Quaternized Analog with Aggregation-Induced Emission (AIE) Dye. Nanomaterials 2024, 14, 1631. [Google Scholar] [CrossRef] [PubMed]
- Beach, M.A.; Nayanathara, U.; Gao, Y.; Zhang, C.; Xiong, Y.; Wang, Y.; Such, G.K. Polymeric Nanoparticles for Drug Delivery. Chem. Rev. 2024, 124, 5505–5616. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, M.; Fan, T.; Zhou, M.; Yin, R.; Wang, X. Advances of Stimuli-Responsive Amphiphilic Copolymer Micelles in Tumor Therapy. Int. J. Nanomed. 2025, 20, 1–24. [Google Scholar] [CrossRef]
- Kawish, S.M.; Sharma, S.; Gupta, P.; Ahmad, F.J.; Iqbal, M.; Alshabrmi, F.M.; Anwer, M.K.; Fathi-karkan, S.; Rahdar, A.; Aboudzadeh, M.A. Nanoparticle-Based Drug Delivery Platform for Simultaneous Administration of Phytochemicals and Chemotherapeutics: Emerging Trends in Cancer Management. Part. Part. Syst. Charact. 2024, 41, 2400049. [Google Scholar] [CrossRef]
- Jacob, S.; Kather, F.; Morsy, M.; Boddu, S.; Attimarad, M.; Shah, J.; Shinu, P.; Nair, A. Advances in Nanocarrier Systems for Overcoming Formulation Challenges of Curcumin: Current Insights. Nanomaterials 2024, 14, 672. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Chawra, H.S.; Dubey, G.; Alam, M.S.; Kumar, V.; Sharma, P.; Upadhayay, N.K.; Yadav, T. Herbal Based Nanoparticles as a Possible and Potential Treatment of Cancer: A Review. Explor. Target. Anti-Tumor Ther. 2025, 6, 1002285. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Pan, H.; Li, L.; Yu, Y.; Liu, B. An Insight into the in Vivo Imaging Potential of Curcumin Analogues as Fluorescence Probes. Asian J. Pharm. Sci. 2021, 16, 419–431. [Google Scholar] [CrossRef]
- Lopresti, A.L. The Problem of Curcumin and Its Bioavailability: Could Its Gastrointestinal Influence Contribute to Its Overall Health-Enhancing Effects? Adv. Nutr. 2018, 9, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zou, L.; Yi, Z.; Zhang, Z.; Zhao, M.; Shi, S. PLGA Nanoparticles Loaded with Curcumin Produced Luminescence for Cell Bioimaging. Int. J. Pharm. 2023, 639, 122944. [Google Scholar] [CrossRef]
- Asgharian, P.; Tazekand, A.P.; Hosseini, K.; Forouhandeh, H.; Ghasemnejad, T.; Ranjbar, M.; Hasan, M.; Kumar, M.; Beirami, S.M.; Tarhriz, V.; et al. Potential Mechanisms of Quercetin in Cancer Prevention: Focus on Cellular and Molecular Targets. Cancer Cell Int. 2022, 22, 257. [Google Scholar] [CrossRef]
- Lin, J.; Yong, K.Y.A.; Zhou, Y.; Wang, Y.; Zhou, W. Improved in Vitro Bioaccessibility of Quercetin by Nanocomplexation with High-Intensity Ultrasound Treated Soy Protein Isolate. Food Chem. 2023, 406, 135004. [Google Scholar] [CrossRef]
- Tomou, E.-M.; Papakyriakopoulou, P.; Saitani, E.-M.; Valsami, G.; Pippa, N.; Skaltsa, H. Recent Advances in Nanoformulations for Quercetin Delivery. Pharmaceutics 2023, 15, 1656. [Google Scholar] [CrossRef]
- Qureshi, W.A.; Zhao, R.; Wang, H.; Ji, T.; Ding, Y.; Ihsan, A.; Mujeeb, A.; Nie, G.; Zhao, Y. Co-Delivery of Doxorubicin and Quercetin via mPEG–PLGA Copolymer Assembly for Synergistic Anti-Tumor Efficacy and Reducing Cardio-Toxicity. Sci. Bull. 2016, 61, 1689–1698. [Google Scholar] [CrossRef]
- Nalinbenjapun, S.; Sripetthong, S.; Basit, A.; Suksuwan, A.; Sajomsang, W.; Ovatlarnporn, C. Fabrication of Curcumin-Loaded Nano-Micelles Based on Quercetin-Quarternary Ammonium-Chitosan (Qu-QCS) Conjugate and Evaluation of Synergistic Effect with Doxorubicin against Breast Cancer. Int. J. Biol. Macromol. 2024, 281, 135904. [Google Scholar] [CrossRef]
- He, T.; Niu, N.; Chen, Z.; Li, S.; Liu, S.; Li, J. Novel Quercetin Aggregation-Induced Emission Luminogen (AIEgen) with Excited-State Intramolecular Proton Transfer for In Vivo Bioimaging. Adv. Funct. Mater. 2018, 28, 1706196. [Google Scholar] [CrossRef]
- Karayianni, M.; Koufi, D.; Pispas, S. Development of Double Hydrophilic Block Copolymer/Porphyrin Polyion Complex Micelles towards Photofunctional Nanoparticles. Polymers 2022, 14, 5186. [Google Scholar] [CrossRef]
- Perrier, S. 50th Anniversary Perspective: RAFT Polymerization—A User Guide. Macromolecules 2017, 50, 7433–7447. [Google Scholar] [CrossRef]
- Akar, I.; Keogh, R.; Blackman, L.D.; Foster, J.C.; Mathers, R.T.; O’Reilly, R.K. Grafting Density Governs the Thermoresponsive Behavior of P(OEGMA-Co.-RMA) Statistical Copolymers. ACS Macro Lett. 2020, 9, 1149–1154. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, W.; Lu, Z.; Li, C.M. Photografted Poly(Methyl Methacrylate)-Based High Performance Protein Microarray for Hepatitis B Virus Biomarker Detection in Human Serum. Med. Chem. Commun. 2010, 1, 132–135. [Google Scholar] [CrossRef]
- Selianitis, D.; Pispas, S. P(MMA-co-HPMA)-b-POEGMA Copolymers: Synthesis, Micelle Formation in Aqueous Media and Drug Encapsulation. Polym. Int. 2021, 70, 1508–1522. [Google Scholar] [CrossRef]
- Moussa, Z.; Chebl, M.; Patra, D. Fluorescence of Tautomeric Forms of Curcumin in Different pH and Biosurfactant Rhamnolipids Systems: Application towards on-off Ratiometric Fluorescence Temperature Sensing. J. Photochem. Photobiol. B Biol. 2017, 173, 307–317. [Google Scholar] [CrossRef]
- Subhan, M.A.; Alam, K.; Rahaman, M.S.; Rahman, M.A.; Awal, R. Synthesis and Characterization of Metal Complexes Containing Curcumin (C21H20O6) and Study of Their Anti-Microbial Activities and DNA-Binding Properties. J. Sci. Res. 2013, 6, 97–109. [Google Scholar] [CrossRef]
- Ginosati, F.; Vagenas, D.; Gerardos, A.M.; Pispas, S. Multi-Responsive Amphiphilic Hyperbranched Poly[(2-Dimethyl Aminoethyl Methacrylate)-Co-(Benzyl Methacrylate)]Copolymers: Self-Assembly and Curcumin Encapsulation in Aqueous Media. Materials 2025, 18, 513. [Google Scholar] [CrossRef] [PubMed]
- Golonka, I.; Wilk, S.; Musiał, W. The Influence of UV Radiation on the Degradation of Pharmaceutical Formulations Containing Quercetin. Molecules 2020, 25, 5454. [Google Scholar] [CrossRef]

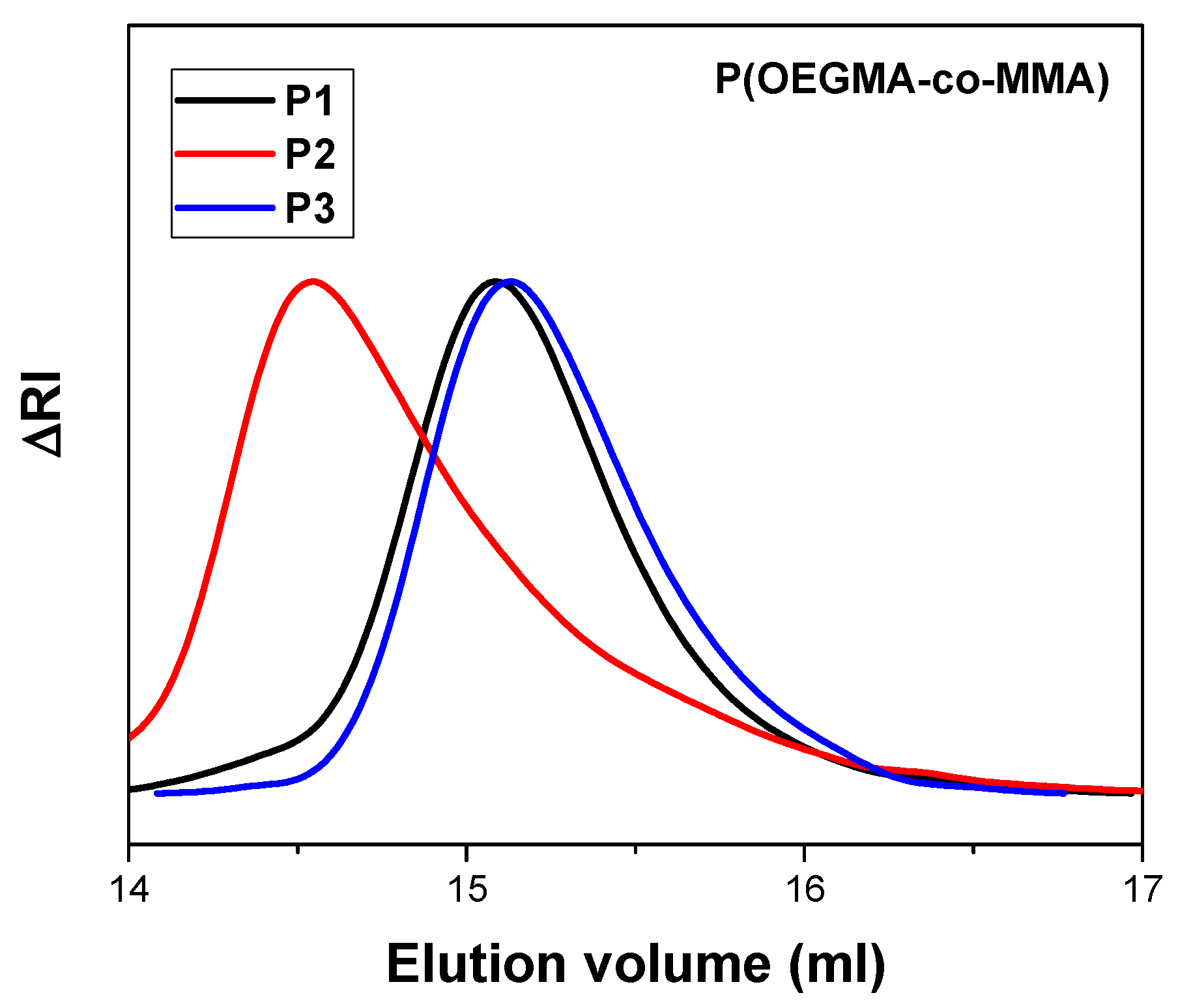
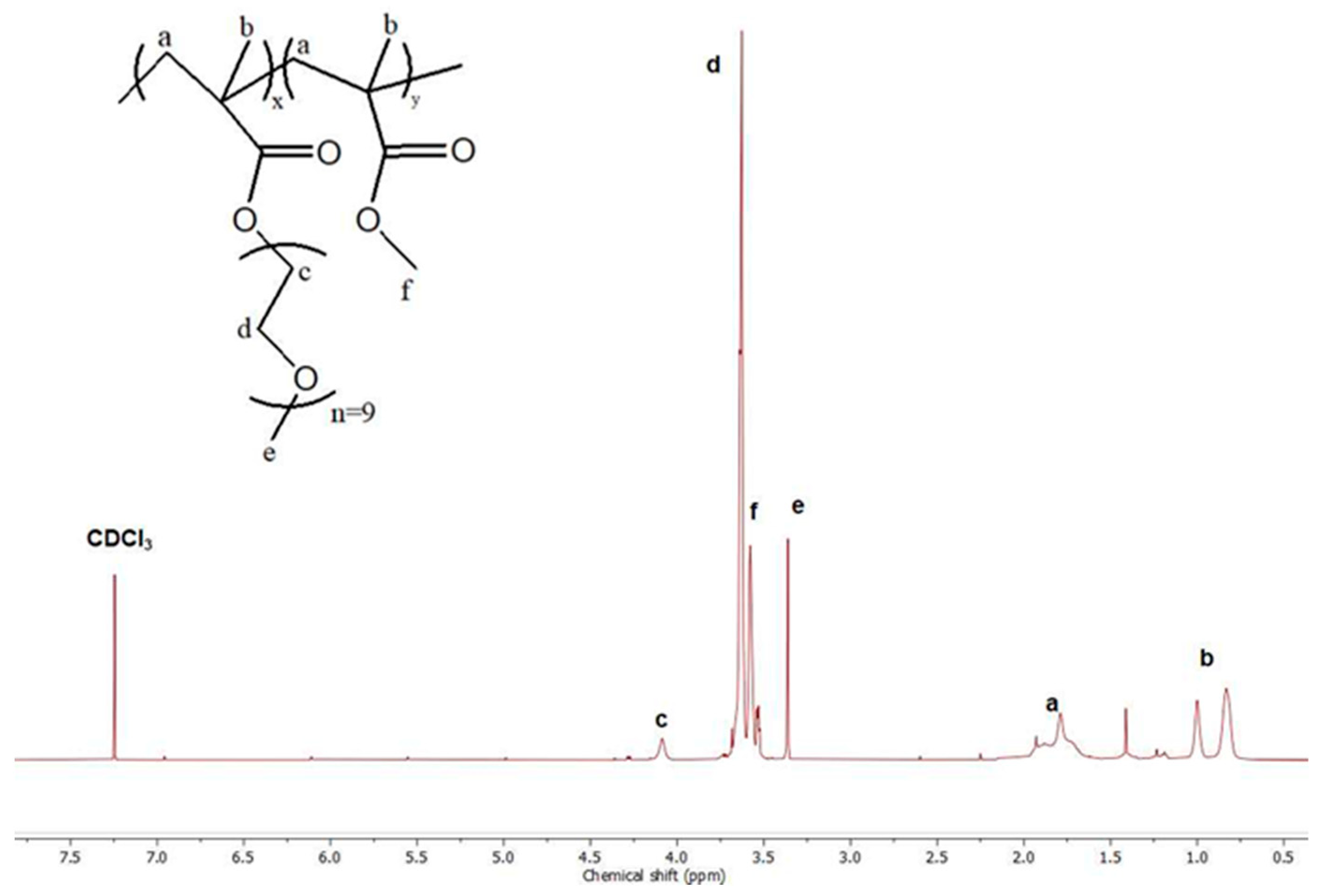


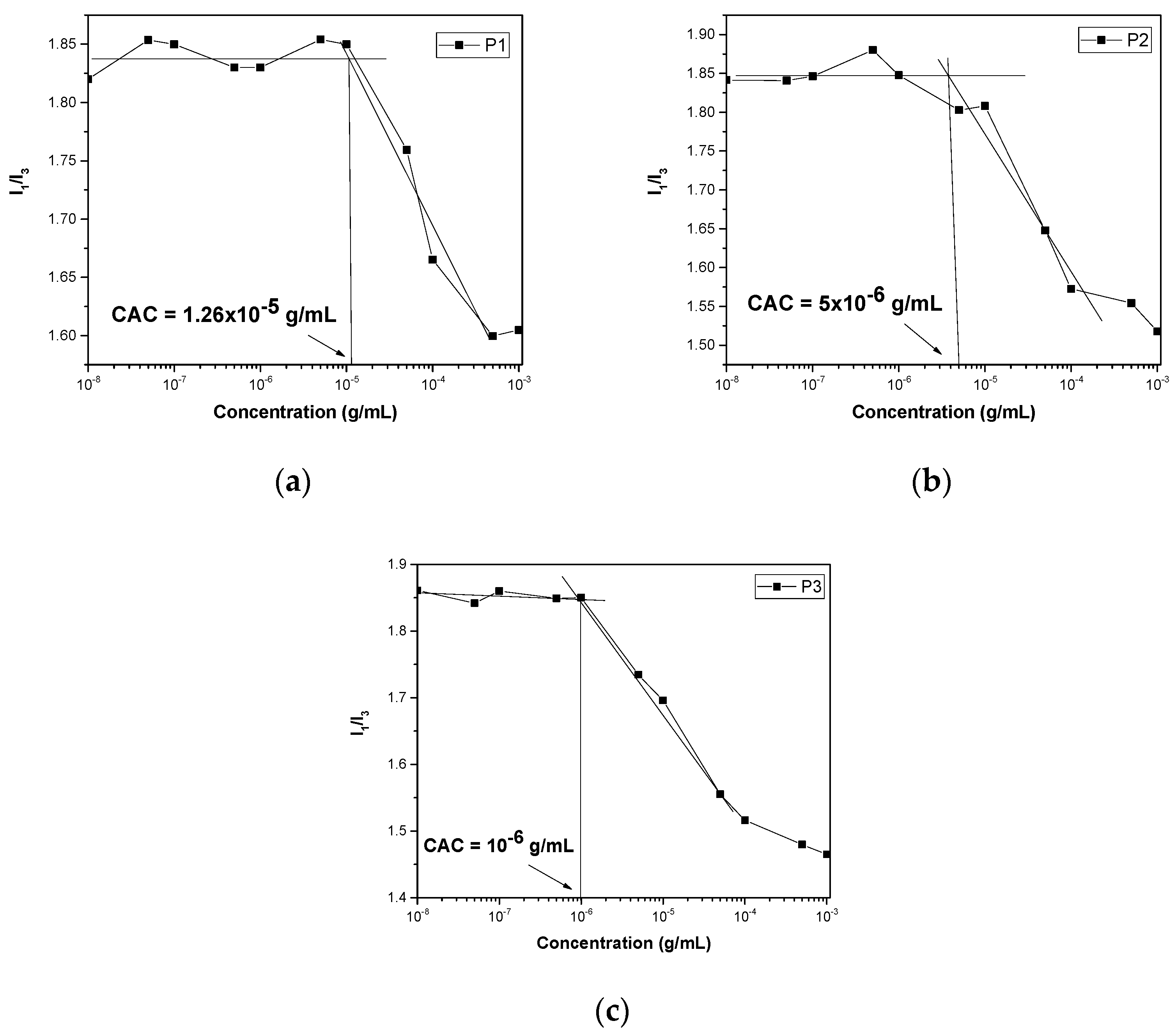
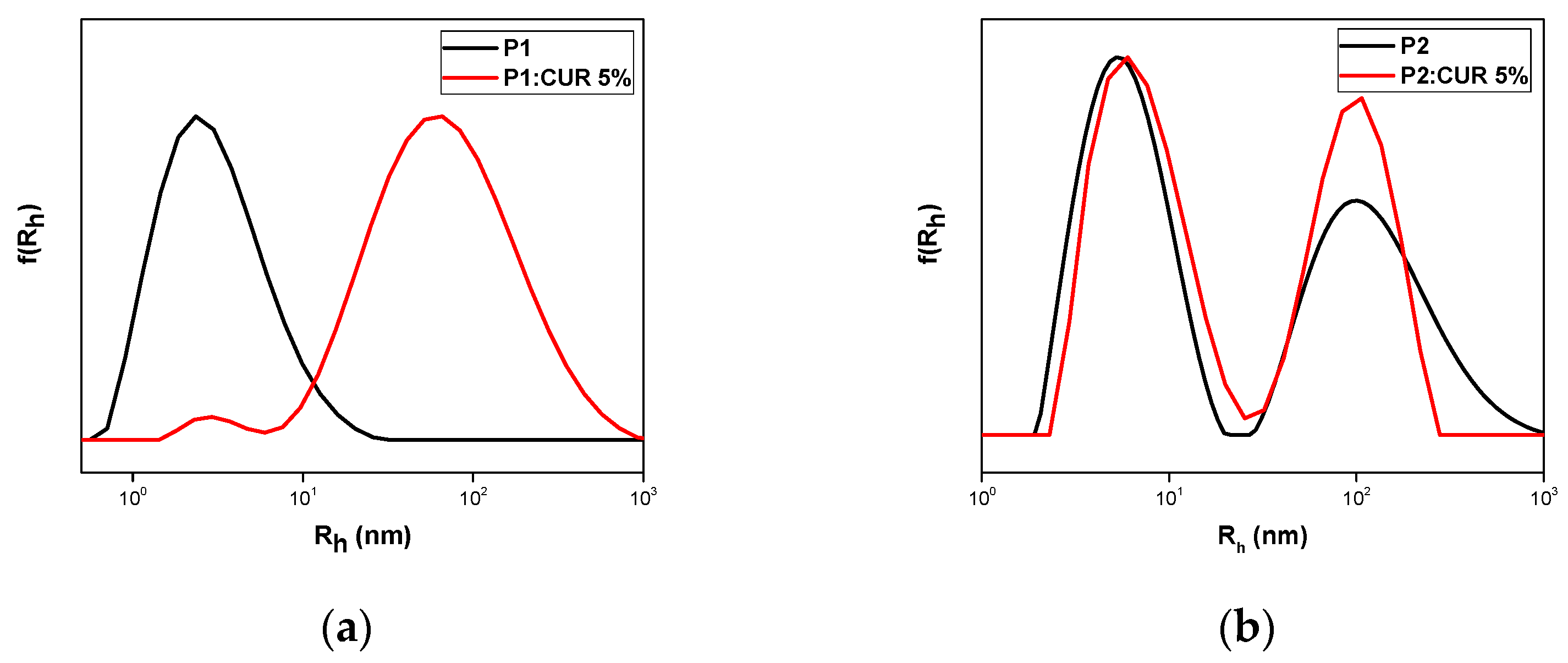





| Sample | Mw a (×104 g/mol) | Mw/Mn a | OEGMA b (wt%) | MMA b (wt%) |
|---|---|---|---|---|
| P1 | 1.73 | 1.15 | 62 | 38 |
| P2 | 2.56 | 1.29 | 56 | 44 |
| P3 | 1.58 | 1.12 | 19 | 81 |
| Sample | Int90 (Kcps) | PDI | Rh, cont (nm) | Zeta Potential (mV) |
|---|---|---|---|---|
| P1-Protocol A | 60 | 0.35 | 2.5 80 | - |
| P1-Protocol B | 24 | 0.45 | 2.9 | 3.6 |
| P2 | 79 | 0.49 | 5.7 121 | 2.3 |
| P3 | 384 | 0.17 | 65 | −7.3 |
| Sample | Curcumin Maximum Loading wt% | Rh, cont (nm) Day 1 | Rh, cont (nm) Day 20 | Encapsulation Efficiency % | Encapsulation Loading % |
|---|---|---|---|---|---|
| P1 | 5 | 61 | 242 | 42 | 2.1 |
| P2 | 5 | 6 107 | 5 37 351 | 54 | 2.6 |
| P2 | 10 | 8 113 | 6 41 450 | 50 | 4.8 |
| P3 | 5 | 85 | 85 | 39 | 1.9 |
| Sample | Quercetin Maximum Loading wt% | Rh, cont (nm) Day 1 | Rh, cont (nm) Day 20 | Encapsulation Efficiency % | Encapsulation Loading % |
|---|---|---|---|---|---|
| P3 | 5 | 66 241 | 81 | 49 | 2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pantelaiou, M.A.; Vagenas, D.; Pispas, S. Poly(oligoethylene glycol methylether methacrylate-co-methyl methacrylate) Aggregates as Nanocarriers for Curcumin and Quercetin. Polymers 2025, 17, 635. https://doi.org/10.3390/polym17050635
Pantelaiou MA, Vagenas D, Pispas S. Poly(oligoethylene glycol methylether methacrylate-co-methyl methacrylate) Aggregates as Nanocarriers for Curcumin and Quercetin. Polymers. 2025; 17(5):635. https://doi.org/10.3390/polym17050635
Chicago/Turabian StylePantelaiou, Michaila Akathi, Dimitrios Vagenas, and Stergios Pispas. 2025. "Poly(oligoethylene glycol methylether methacrylate-co-methyl methacrylate) Aggregates as Nanocarriers for Curcumin and Quercetin" Polymers 17, no. 5: 635. https://doi.org/10.3390/polym17050635
APA StylePantelaiou, M. A., Vagenas, D., & Pispas, S. (2025). Poly(oligoethylene glycol methylether methacrylate-co-methyl methacrylate) Aggregates as Nanocarriers for Curcumin and Quercetin. Polymers, 17(5), 635. https://doi.org/10.3390/polym17050635







