Preparation of 3-Iodo-2-propargyl-butyl-carbamate-Loaded Microcapsules for Long-Term Mold Resistance in Bamboo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
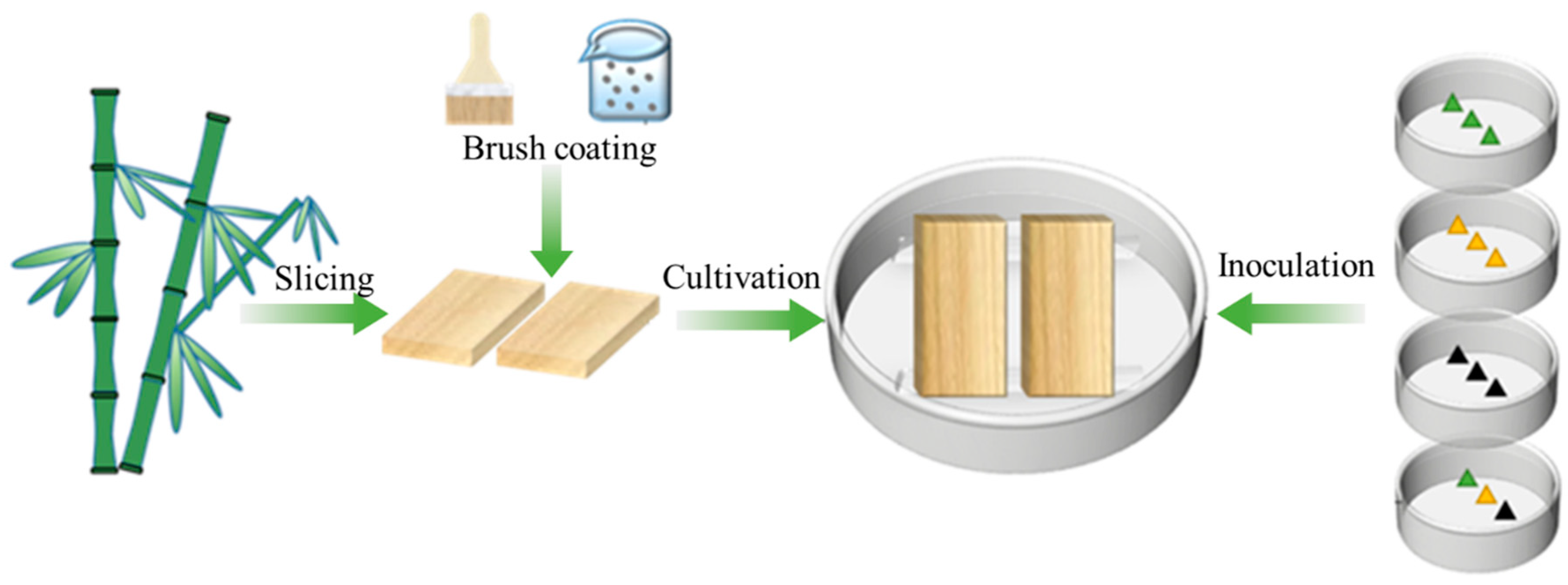
2.2. Preparation of the Microcapsules
2.3. Mold Resistance Test
2.3.1. Preparation of Fungal Mycelium and Spore Suspension
2.3.2. Sample Inoculation and Cultivation
2.3.3. Prevention and Treatment Effectiveness Calculation
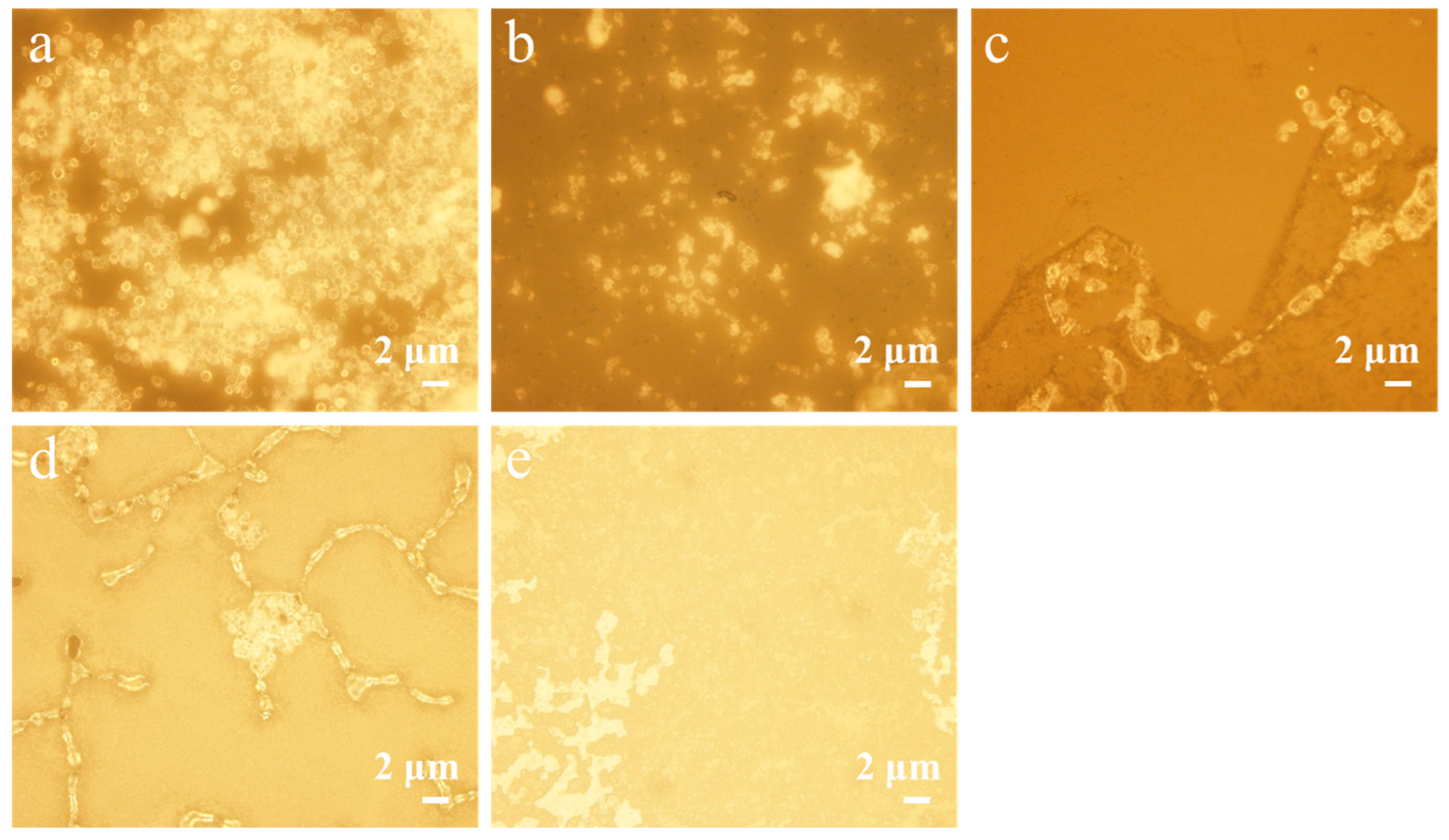
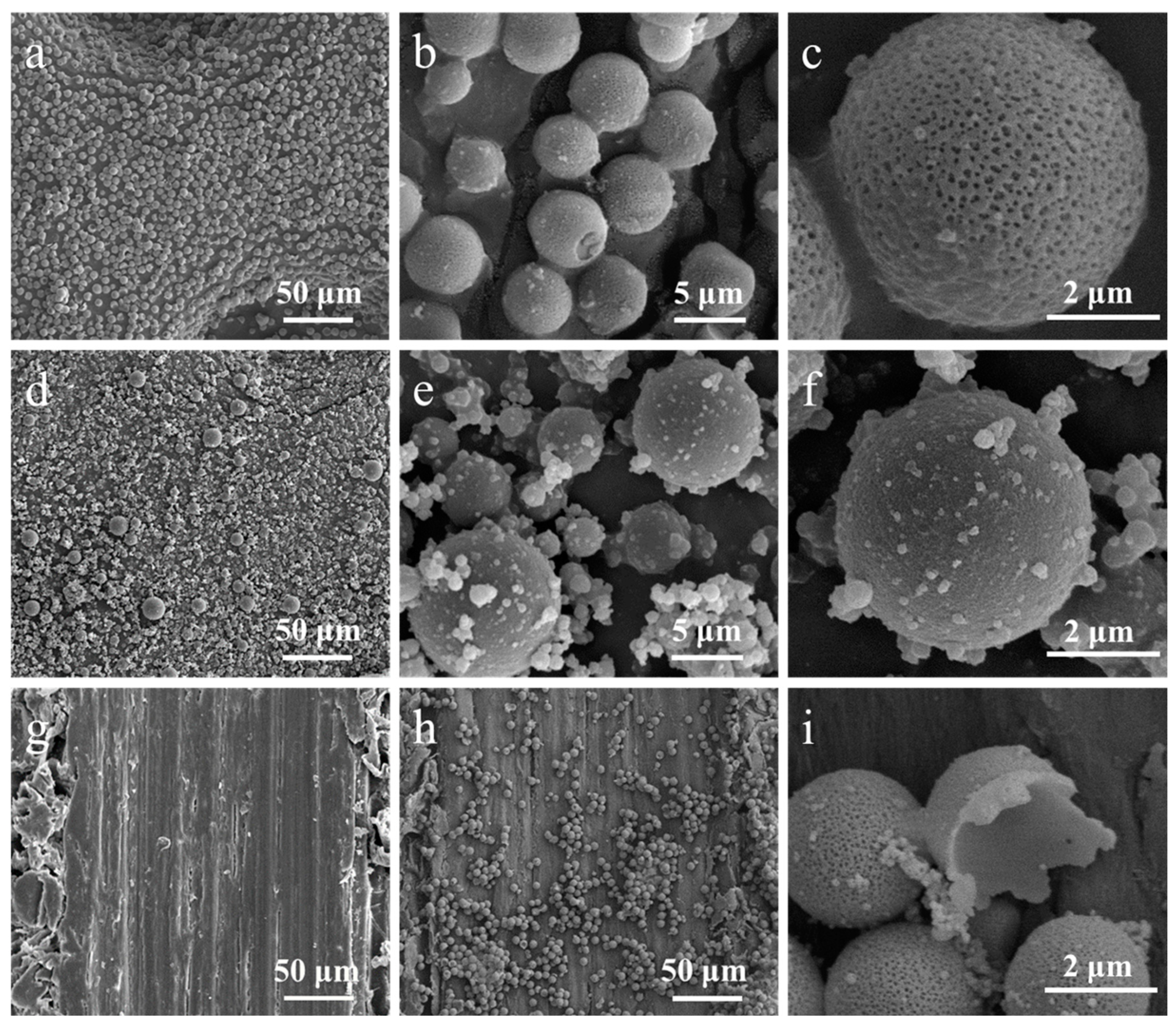
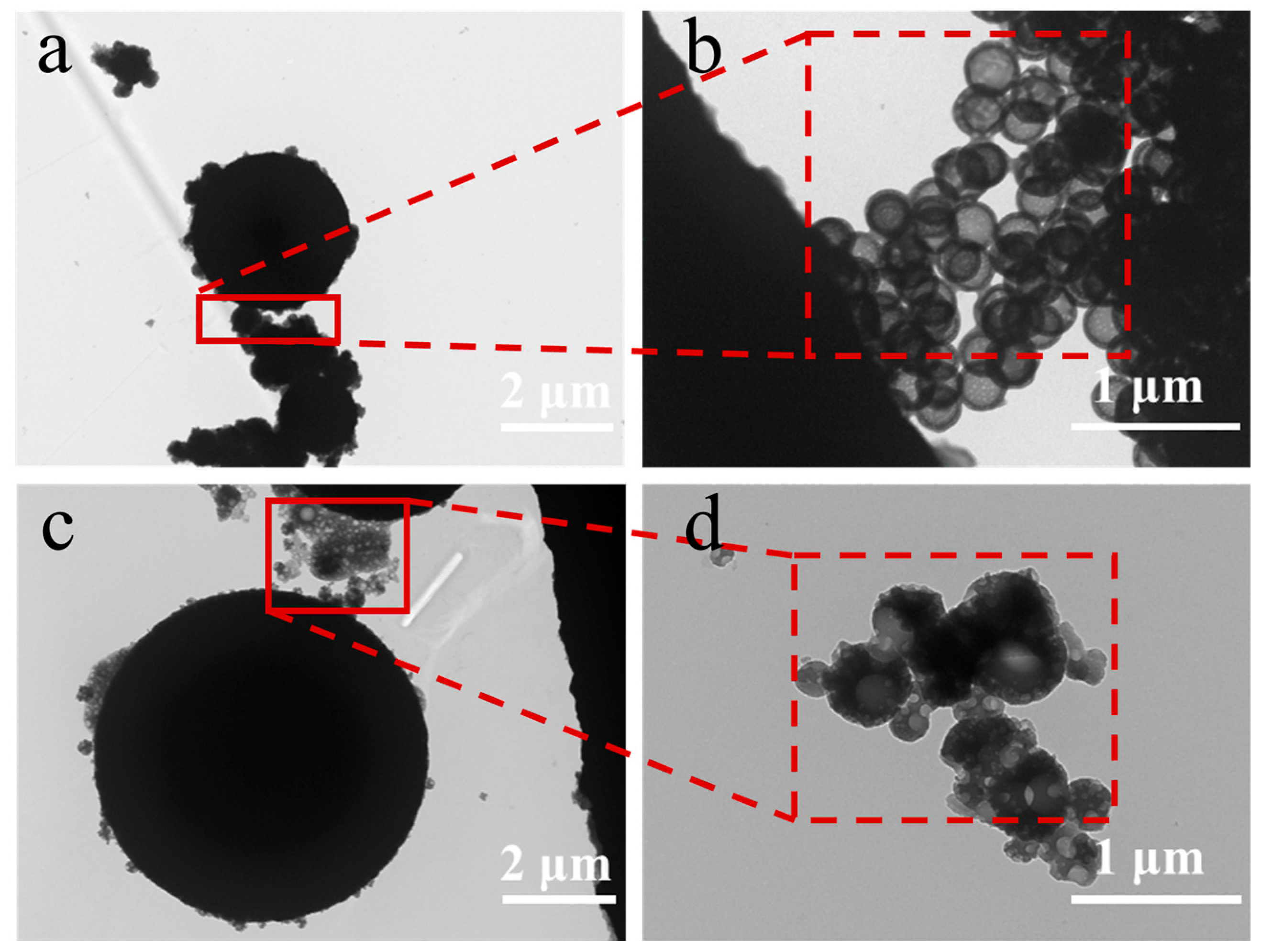
2.4. Morphological Observations
2.5. FTIR Spectroscopy
2.6. Thermogravimetric Analysis
2.7. UV Performance Test
2.8. Drug Loading and Encapsulation Rate Test
2.9. Sustained Release Performance Test
3. Result and Discussion
3.1. Morphological Analysis of Microcapsules with Different Emulsifiers
3.2. FTIR Analysis of Microcapsules
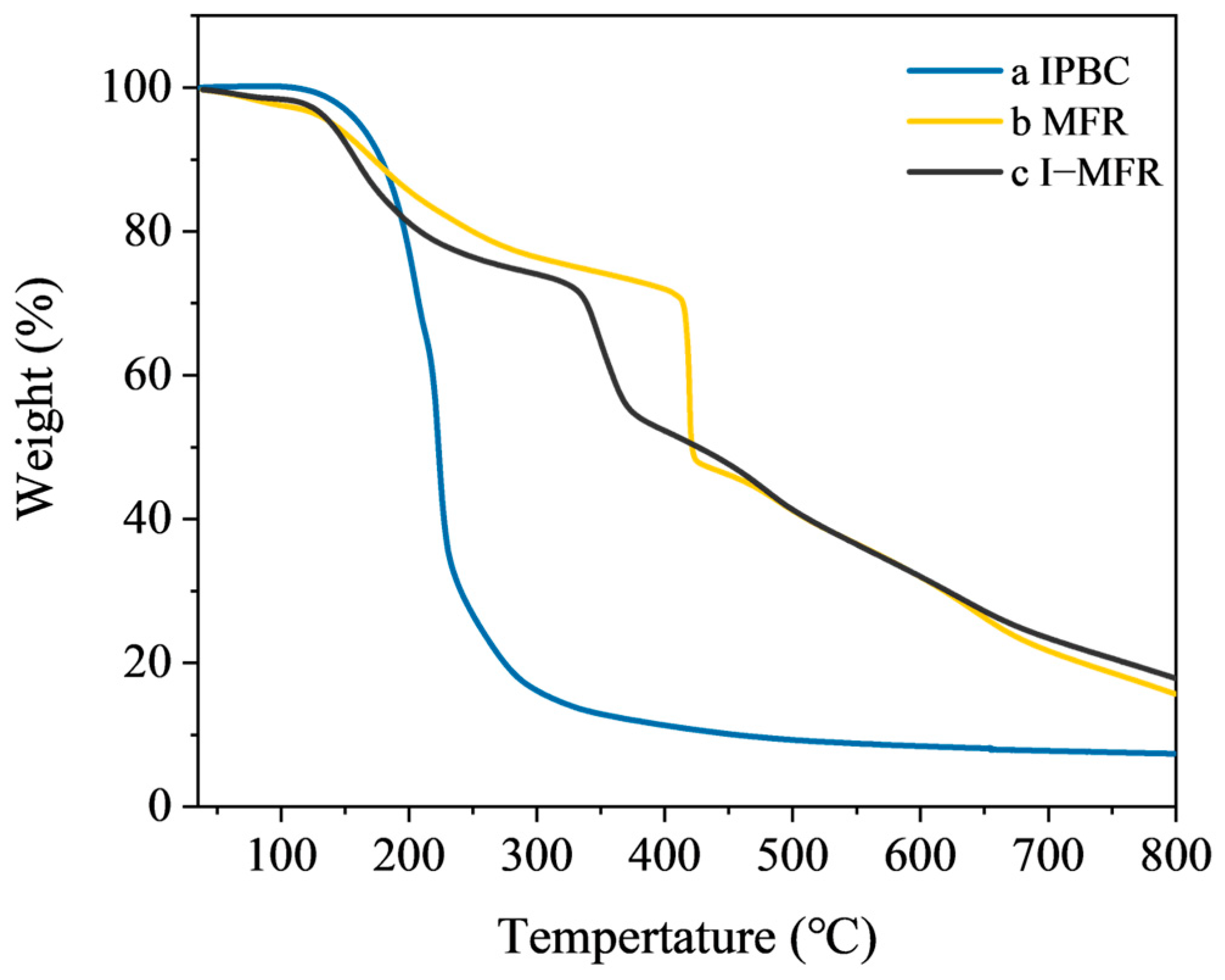
3.3. Thermal Stability Analysis of Microcapsules
3.4. UV Resistance of Microcapsules
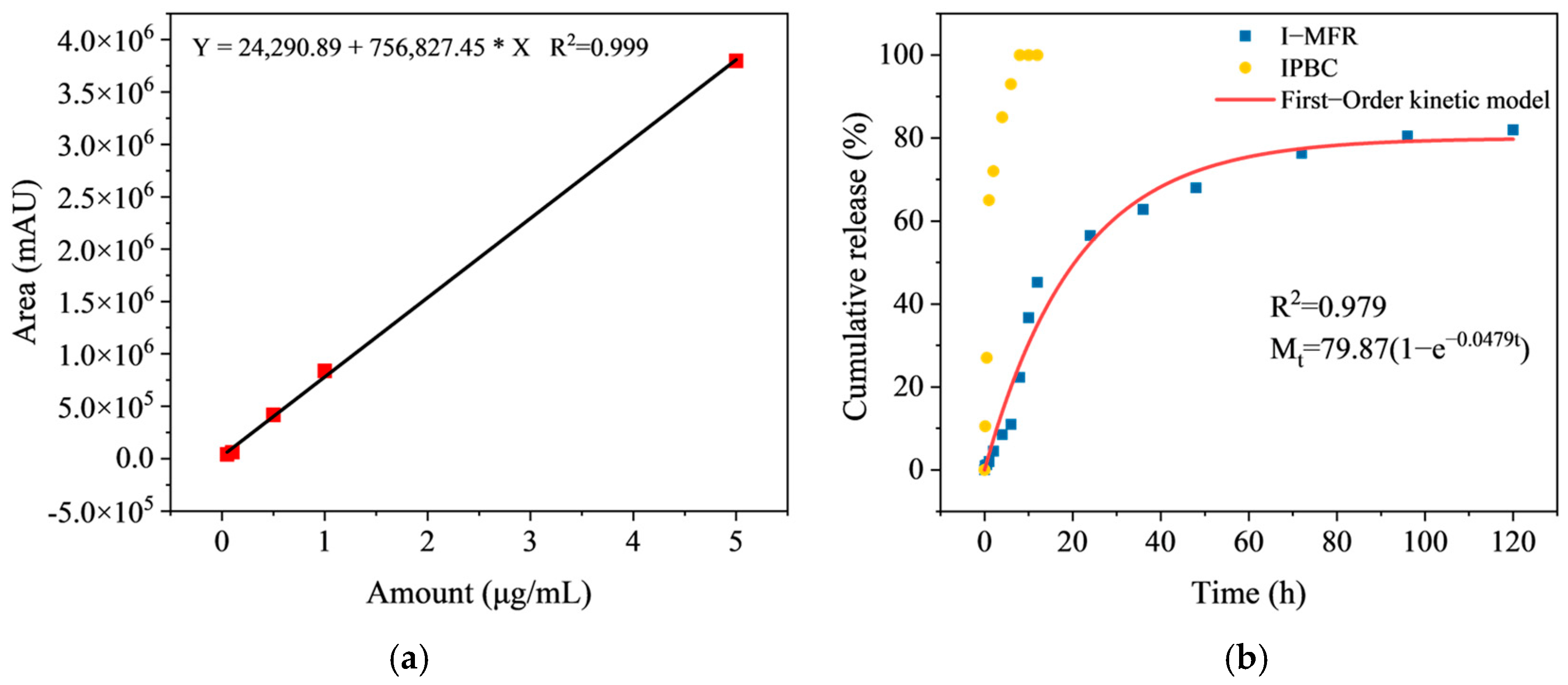
3.5. Microcapsules Sustained Release Kinetics Analysis
3.6. Analysis of Microcapsules Anti-Mold Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alamerew, A.N.; Kozak, R.; Shrestha, A.K.; Zhu, Z.; Wang, G. A Way for Livelihood Improvement: Systematic Review on Bamboo Forest Research. Trees For. People 2024, 16, 100571. [Google Scholar] [CrossRef]
- Yang, L.; Lou, Z.; Han, X.; Liu, J.; Wang, Z.; Zhang, Y.; Wu, X.; Yuan, C.; Li, Y. Fabrication of a Novel Magnetic Reconstituted Bamboo with Mildew Resistance Properties. Mater. Today Commun. 2020, 23, 101086. [Google Scholar] [CrossRef]
- Silva, M.F.; Menis-Henrique, M.E.; Felisberto, M.H.; Goldbeck, R.; Clerici, M.T. Bamboo as an Eco-Friendly Material for Food and Biotechnology Industries. Curr. Opin. Food Sci. 2020, 33, 124–130. [Google Scholar] [CrossRef]
- He, L.; Bao, G.; Jin, X.; Zhang, R.; Qin, D. Eco-Friendly in-Situ Mineralization of Bamboo for Flame Retardancy. Ind. Crops Prod. 2023, 197, 116644. [Google Scholar] [CrossRef]
- Xu, P.; Zhu, J.; Li, H.; Xiong, Z.; Xu, X. Coupling Analysis between Cost and Carbon Emission of Bamboo Building Materials: A Perspective of Supply Chain. Energy Build. 2023, 280, 112718. [Google Scholar] [CrossRef]
- Nfornkah, B.N.; Nath, A.J.; Kaam, R.; Chimi, C.D.; Mezafack, K.L. Bamboo-Based Forest Landscape Restoration: Practical Lessons and Initiatives to Upscale in Africa. In Bamboo Science and Technology; Palombini, F.L., Nogueira, F.M., Eds.; Springer Nature: Singapore, 2023; pp. 329–356. ISBN 978-981-99-0015-2. [Google Scholar]
- Chiti, T.; Blasi, E.; Chiriacò, M.V. Carbon Sequestration in a Bamboo Plantation: A Case Study in a Mediterranean Area. J. For. Res. 2024, 35, 51. [Google Scholar] [CrossRef]
- Jember, A.A.; Taye, M.A.; Gebeyehu, G.; Mulu, G.; Long, T.T.; Jayaraman, D.; Abebe, S. Carbon Stock Potential of Highland Bamboo Plantations in Northwestern Ethiopia. Carbon Balance Manag. 2023, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Hoang, C.; Nguyen, T.; Stanley, D.; Persily, A.; Corsi, R.L. Effect of Ozonation on Fungal Resistance of Bamboo and Oak Flooring Materials. Build. Environ. 2014, 81, 226–233. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, M.; Zhang, B.; Wu, Y.; Ma, X. Mold and Stain Resistance of Bamboo Treated with Pyraclostrobin Fungicide. Polymers 2022, 14, 5537. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Ye, Z.; Patience Chizaram, E.; Jiang, J.; Liu, T.; Sun, F.; Zhang, S. Mold Resistance of Bamboo after Laccase-Catalyzed Attachment of Thymol and Proposed Mechanism of Attachment. RSC Adv. 2020, 10, 7764–7770. [Google Scholar] [CrossRef]
- Paulus, W. Carbamates. In Microbicides for the Protection of Materials; Springer: Dordrecht, The Netherlands, 1993; pp. 265–287. ISBN 978-0-412-53450-8. [Google Scholar]
- Vallières, C.; Alexander, C.; Avery, S.V. Potentiated Inhibition of Trichoderma Virens and Other Environmental Fungi by New Biocide Combinations. Appl. Microbiol. Biotechnol. 2021, 105, 2867–2875. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, R.; Su, M.; Li, H.; Yue, X.; Qin, D.; Jiang, Z. Functionalization of Halloysite Nanotubes by Enlargement and Layer-by-Layer Assembly for Controlled Release of the Fungicide Iodopropynyl Butylcarbamate. RSC Adv. 2019, 9, 42062–42070. [Google Scholar] [CrossRef]
- Adam, O.; Badot, P.-M.; Degiorgi, F.; Crini, G. Mixture Toxicity Assessment of Wood Preservative Pesticides in the Freshwater Amphipod Gammarus pulex (L.). Ecotoxicol. Environ. Saf. 2009, 72, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Bailey, H.C.; Elphick, J.R.; Potter, A.; Chao, E.; Zak, B. Acute Toxicity of the Antisapstain Chemicals DDAC and IPBC, Alone and in Combination, to Rainbow Trout (Oncorhynchus mykiss). Water Res. 1999, 33, 2410–2414. [Google Scholar] [CrossRef]
- Sasseville, D. Hypersensitivity to Preservatives. Dermatol. Ther. 2004, 17, 251–263. [Google Scholar] [CrossRef]
- Palmer, K.B.; LaFon, W.; Burford, M.D. Determination of Iodopropynyl Butylcarbamate in Cosmetic Formulations Utilizing Pulsed Splitless Injection, Gas Chromatography with Electron Capture Detector. J. Chromatogr. A 2017, 1516, 131–134. [Google Scholar] [CrossRef]
- Han, L.; Jiang, M.; Zhang, J.; Shao, C.; Zhang, Q. Thermal Degradation and Product Analysis of 3-Iodo-2-Propyl-Butylcarbamate as a Wood Preservative. Polymers 2022, 14, 4531. [Google Scholar] [CrossRef]
- Lobel, B.T.; Baiocco, D.; Al-Sharabi, M.; Routh, A.F.; Zhang, Z.; Cayre, O.J. Current Challenges in Microcapsule Designs and Microencapsulation Processes: A Review. ACS Appl. Mater. Interfaces 2024, 16, 40326–40355. [Google Scholar] [CrossRef]
- Paulo, F.; Santos, L. Design of Experiments for Microencapsulation Applications: A Review. Mater. Sci. Eng. C 2017, 77, 1327–1340. [Google Scholar] [CrossRef]
- Tao, R.; You, C.; Qu, Q.; Zhang, X.; Deng, Y.; Ma, W.; Huang, C. Recent Advances in the Design of Controlled- and Sustained-Release Micro/Nanocarriers of Pesticide. Environ. Sci. Nano 2023, 10, 351–371. [Google Scholar] [CrossRef]
- Tohmura, S.; Inoue, A.; Sahari, S.H. Influence of the Melamine Content in Melamine-Urea-Formaldehyde Resins on Formaldehyde Emission and Cured Resin Structure. J. Wood Sci. 2001, 47, 451–457. [Google Scholar] [CrossRef]
- Revuelta, M.V.; Bogdan, S.; Gámez-Espinosa, E.; Deya, M.C.; Romagnoli, R. Green Antifungal Waterborne Coating Based on Essential Oil Microcapsules. Prog. Org. Coat. 2021, 151, 106101. [Google Scholar] [CrossRef]
- Chang, Y.; Yan, X.; Wu, Z. Application and Prospect of Self-Healing Microcapsules in Surface Coating of Wood. Colloid Interface Sci. Commun. 2023, 56, 100736. [Google Scholar] [CrossRef]
- Sui, C.; Preece, J.A.; Zhang, Z.; Yu, S.-H. Efficient Encapsulation of Water Soluble Inorganic and Organic Actives in Melamine Formaldehyde Based Microcapsules for Control Release into an Aqueous Environment. Chem. Eng. Sci. 2021, 229, 116103. [Google Scholar] [CrossRef]
- Yan, X.; Wang, Y.; Liu, H.; Li, R.; Qian, C. Synthesis and Characterization of Melamine-Formaldehyde Microcapsules Containing Pyraclostrobin by In Situ Polymerization. Polym. Sci. Ser. B 2018, 60, 798–805. [Google Scholar] [CrossRef]
- Salaün, F.; Devaux, E.; Bourbigot, S.; Rumeau, P. Influence of Process Parameters on Microcapsules Loaded with n-Hexadecane Prepared by in Situ Polymerization. Chem. Eng. J. 2009, 155, 457–465. [Google Scholar] [CrossRef]
- GB/T 18261-2013; Test Method for Anti-Mildew Agents in Controlling Wood Mould and Stain Fungi. National Forestry and Grassland Administration: Beijing, China, 2013.
- McClements, D.J.; Gumus, C.E. Natural Emulsifiers—Biosurfactants, Phospholipids, Biopolymers, and Colloidal Particles: Molecular and Physicochemical Basis of Functional Performance. Adv. Colloid Interface Sci. 2016, 234, 3–26. [Google Scholar] [CrossRef]
- Fan, C.; Zhou, X. Effect of Emulsifier on Poly(Urea–Formaldehyde) Microencapsulation of Tetrachloroethylene. Polym. Bull. 2011, 67, 15–27. [Google Scholar] [CrossRef]
- Li, Y.; Wu, C.; Wu, T.; Wang, L.; Chen, S.; Ding, T.; Hu, Y. Preparation and Characterization of Citrus Essential Oils Loaded in Chitosan Microcapsules by Using Different Emulsifiers. J. Food Eng. 2018, 217, 108–114. [Google Scholar] [CrossRef]
- Lian, H.; Peng, Y.; Shi, J.; Wang, Q. Effect of Emulsifier Hydrophilic-Lipophilic Balance (HLB) on the Release of Thyme Essential Oil from Chitosan Films. Food Hydrocoll. 2019, 97, 105213. [Google Scholar] [CrossRef]
- Schmidts, T.; Dobler, D.; Guldan, A.-C.; Paulus, N.; Runkel, F. Multiple W/O/W Emulsions—Using the Required HLB for Emulsifier Evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2010, 372, 48–54. [Google Scholar] [CrossRef]
- Zhang, L.; Que, G. Influence of the HLB Parameter of Surfactants on the Dispersion Properties of Brine in Residue. Colloids Surf. A Physicochem. Eng. Asp. 2008, 320, 111–114. [Google Scholar] [CrossRef]
- Cingolani, A. Morphology Control of Porous Polymeric Materials via Primary Particle Architecture. Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, 2017. [Google Scholar]
- Wang, T.; Shen, Z.; Luo, J.; Sun, G.; Liu, R. Interfacial Polycondensation to Fabricate Polyurethane Microcapsules: Unraveling the Mechanism of Shell Formation and Achieving Desired Shell Thickness Based on Hansen Solubility Parameter Theory. Macromolecules 2024, 57, 7280–7292. [Google Scholar] [CrossRef]
- Elesini, U.S.; Leskovšek, M.; Bernik, S.; Šumiga, B.; Urbas, R. Influence of Co-Current Spray Drying Conditions on Agglomeration of Melamine-Formaldehyde Microcapsules. Dry. Technol. 2016, 34, 1510–1520. [Google Scholar] [CrossRef]
- Fei, X.; Zhao, H.; Zhang, B.; Cao, L.; Yu, M.; Zhou, J.; Yu, L. Microencapsulation Mechanism and Size Control of Fragrance Microcapsules with Melamine Resin Shell. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 300–306. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Rao, K.C.; Kumar, D. Preparation and Characterization of Microcapsules Containing Linseed Oil and Its Use in Self-Healing Coatings. Prog. Org. Coat. 2008, 63, 72–78. [Google Scholar] [CrossRef]
- Yuan, H.; Li, G.; Yang, L.; Yan, X.; Yang, D. Development of Melamine–Formaldehyde Resin Microcapsules with Low Formaldehyde Emission Suited for Seed Treatment. Colloids Surf. B Biointerfaces 2015, 128, 149–154. [Google Scholar] [CrossRef]
- Wang, X.; Cao, L.; Hu, Y.; Chen, Y.; Jin, T.; Huang, J.; Zhang, X.; Lin, S. Highly Transparent, Hydrophobic, Hard and Flexible Coatings Based on a Novel Melamine-Formaldehyde Resin Synthesized by Hydrophobic Melamine. Prog. Org. Coat. 2023, 179, 107487. [Google Scholar] [CrossRef]
- Yin, D.; Ma, L.; Geng, W.; Zhang, B.; Zhang, Q. Microencapsulation of N--hexadecanol by in Situ Polymerization of Melamine–Formaldehyde Resin in Emulsion Stabilized by Styrene–Maleic Anhydride Copolymer. Int. J. Energy Res. 2014, 39, 661–667. [Google Scholar] [CrossRef]
- Khorasani, S.N.; Ataei, S.; Neisiany, R.E. Microencapsulation of a Coconut Oil-Based Alkyd Resin into Poly(Melamine-Urea-Formaldehyde) as Shell for Self-Healing Purposes. Prog. Org. Coat. 2017, 111, 99–106. [Google Scholar] [CrossRef]
- Siimer, K.; Christjanson, P.; Kaljuvee, T.; Pehk, T.; Lasn, I.; Saks, I. TG-DTA Study of Melamine-Urea-Formaldehyde Resins. J. Therm. Anal. Calorim. 2008, 92, 19–27. [Google Scholar] [CrossRef]
- de la Paz Miguel, M.; Vallo, C.I. Influence of the Emulsifying System to Obtain Linseed Oil-Filled Microcapsules with a Robust Poly (Melamine-Formaldehyde)-Based Shell. Prog. Org. Coat. 2019, 129, 236–246. [Google Scholar] [CrossRef]
- Kukowski, K.; Martinská, V.; Sedgeman, C.A.; Kuplic, P.; Kozliak, E.I.; Fisher, S.; Kubátová, A. Fate of Triazoles in Softwood upon Environmental Exposure. Chemosphere 2017, 184, 261–268. [Google Scholar] [CrossRef] [PubMed]



| Value | Growth of Mycelium |
|---|---|
| 0 | No hypha growth on the surface of the specimen |
| 1 | Area of mycelial infection < 25% |
| 2 | 25% < Area of mycelial infection < 50% |
| 3 | 50% < Area of mycelial infection < 75% |
| 4 | Area of mycelial infection > 75% |
| Emulsifier | HLB | Results |
|---|---|---|
| Tween 80 | 15 | The microcapsules have a porous surface, with a particle size of about 5 ± 1 μm, and the distribution is relatively uniform |
| SDBS | 10.6 | Resin particles are adhered to the surface of the microcapsules; the particle size is between 2 and 16 μm, and the size is relatively uneven |
| SDS | 40 | Uncapsulated |
| GA | —— | Uncapsulated |
| Span 80 | 4.3 | Uncapsulated |
| Wavenumber (cm−1) | Functional Group | Vibration Type |
|---|---|---|
| 3414/3399 | N-H and O-H | Stretching vibrations |
| 2940/2930 | C-H | Stretching vibrations |
| 2199/2198 | Alkynyl C≡C | Stretching vibration |
| 1557/1553 | Triazine ring C=N | Stretching vibration and N-H shear bending vibrations |
| 1340/1351 | Tertiary amines C-N | Stretching vibrations |
| 813 | N-H | Shear bending vibrations |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, G.; He, L.; Zhang, X.; Yu, X.; Li, J.; Qin, D. Preparation of 3-Iodo-2-propargyl-butyl-carbamate-Loaded Microcapsules for Long-Term Mold Resistance in Bamboo. Polymers 2025, 17, 679. https://doi.org/10.3390/polym17050679
Bao G, He L, Zhang X, Yu X, Li J, Qin D. Preparation of 3-Iodo-2-propargyl-butyl-carbamate-Loaded Microcapsules for Long-Term Mold Resistance in Bamboo. Polymers. 2025; 17(5):679. https://doi.org/10.3390/polym17050679
Chicago/Turabian StyleBao, Gege, Lu He, Xiaofeng Zhang, Xi Yu, Jingpeng Li, and Daochun Qin. 2025. "Preparation of 3-Iodo-2-propargyl-butyl-carbamate-Loaded Microcapsules for Long-Term Mold Resistance in Bamboo" Polymers 17, no. 5: 679. https://doi.org/10.3390/polym17050679
APA StyleBao, G., He, L., Zhang, X., Yu, X., Li, J., & Qin, D. (2025). Preparation of 3-Iodo-2-propargyl-butyl-carbamate-Loaded Microcapsules for Long-Term Mold Resistance in Bamboo. Polymers, 17(5), 679. https://doi.org/10.3390/polym17050679







