Abstract
Exploring new polymerization strategies for currently available monomers is a challenge in polymer science. Herein, a bifunctional initiator (BFI) is introduced for the conventional radical polymerization of a vinyl monomer, resulting in linear radical additions-coupling polymerization (LRAsCP). In LRAsCP, the coupling reaction alongside the addition reaction of the radicals contributes to the construction of polymer chains, which leads to stepwise growth of the multiblock structure. Theoretical analysis of LRAsCP predicted variation of some structural parameters of the resulting multiblock polymer (MBP) with the extent of initiation of the BFI and the termination factor of the radicals. Simultaneous and cascade initiations of the BFI were compared. LRAsCP of styrene was conducted, and a kinetics study was carried out. The increment in Mn with polymerization time demonstrated the stepwise mechanism of the formation of the MBP. The variation of the structural parameters of MBP fitted well with the theoretical prediction. Two-step LRAsCP was conducted and multiblock copolymers (MBcP) were obtained either by in situ copolymerization of styrene and MMA or by a second copolymerization of styrene and BMA. The current results demonstrate that the introduction of a BFI to conventional radical polymerization generates a new polymerization strategy, leading to a new chain architecture, which can be extended to other radical polymerizable monomers.
1. Introduction
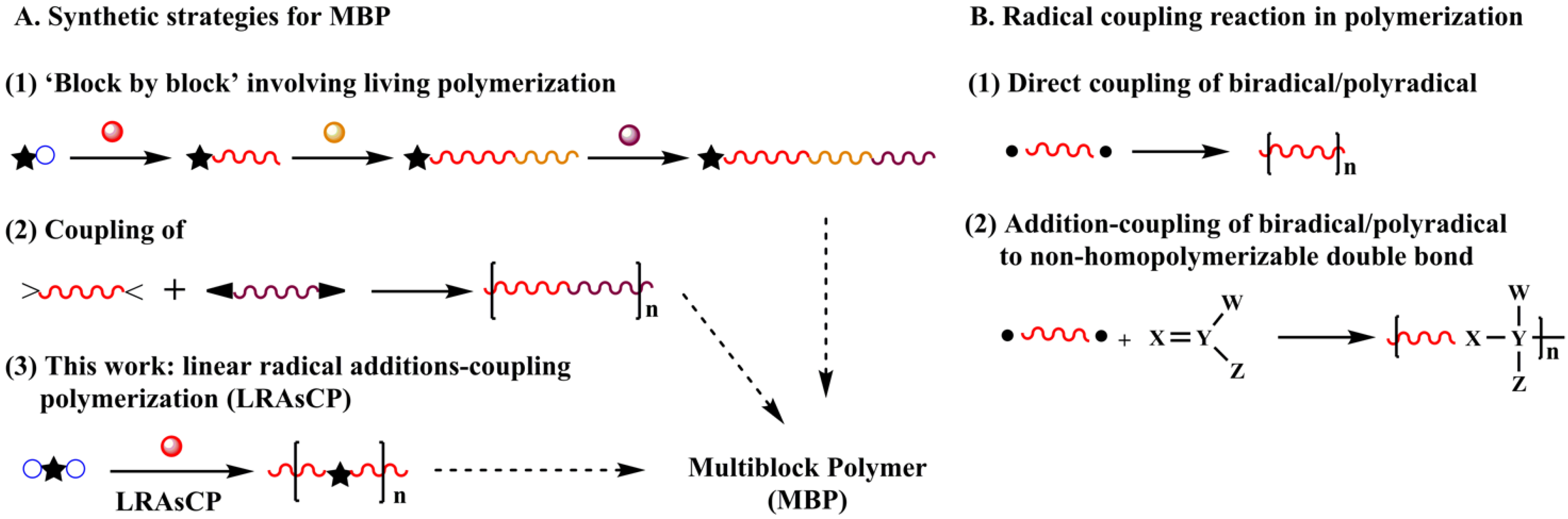
Multiblock polymer (MBP) has extensive applications in various fields [1,2,3,4,5]. Currently, its synthesis strategy includes three approaches. One is the construction of the polymer chain ‘block by block’ via sequential living polymerization [6,7,8,9,10,11] (Scheme 1A(1)), such as anionic polymerization [12,13], controlled radical polymerization [8,9,14,15,16,17,18,19,20,21] and olefin metathesis polymerization [10]. Although well-defined MBPs can be prepared under critical polymerization conditions with multiple steps, the tolerance of anionic polymerization limits its application in monomers with active groups, such as carboxyl and hydroxyl groups. While controlled radical polymerization can be applied to more types of monomers, the low polymerization rate implies that achieving the designed conversion and the molar mass of the polymer requires a long time to reach the designed conversion and molar mass of the polymer. The second approach is the coupling of α, ω-telechelic polymers prepared by living polymerization, which needs more than two steps (Scheme 1A(2)) [22,23,24,25,26,27]. Due to the relatively low activity of the terminal group of macromolecules, the efficiency of the coupling reaction is unsatisfactory. The third approach is the combination of different polymerization mechanisms, which meets the same problems existing in individual methods. Exploring simple and easy methods for building polymers with complex segmental structures is still a challenge in polymer synthesis.
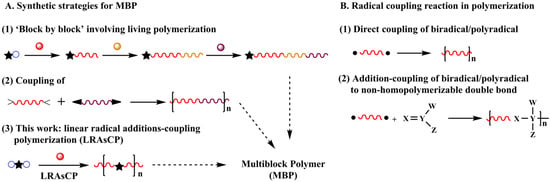
Scheme 1.
Different synthesis strategies for multiblock polymers (MBP) (A) and application of a radical coupling reaction in polymerization (B).
Radical coupling reactions are rapid reactions that have been applied in some polymerizations as a tool for building carbon–carbon bonds. Direct coupling of biradicals generates linear polymer chains (Scheme 1B(1)) [28,29,30,31,32]. The reaction between biradicals and various non-homopolymerized double bonds results in multi-segmental or multiblock linear polymers (Scheme 1B(2)) [22,23,24,25,33,34,35,36,37]. When biradicals or polyradicals are involved in radical polymerization of vinyl monomers, the radical coupling reaction is a chain extension step instead of a termination step. Radical polymerization of monovinyl monomers initiated by a polyfunctional initiator (PFI) leads to the formation of a branched polymer or polymer network. This strategy is termed non-linear radical additions-coupling polymerization (NLRAsCP) [38], in which the PFI is the source of the branching. The mechanism of NLRAsCP demonstrates that gelation is determined by the extent of initiation of the functional groups of the initiator (q) and the coupling factor of the macroradical (ϕ).
Radical polymerization is one of the oldest polymerization methods and tolerates various functional groups. It can be anticipated that when a bifunctional initiator (BFI) is introduced in the polymerization of monovinyl monomers, the coupling reaction along with the addition reaction of the radical contributes to the construction of polymer chains, producing a linear polymer composed of a multiblock structure if chain transfer to the polymer is absent. This polymerization is termed linear radical additions-coupling polymerization (LRAsCP, Scheme 1A(3)).
In this paper, we propose an efficient protocol to construct a multiblock structure via one-step LRAsCP, and two different blocks via two-step LRAsCP. The kinetic analysis of LRAsCP and its application in the synthesis of MBP and multiblock copolymers (MBcP) are investigated. Moreover, LRAsCP provides a simple approach for determining the contribution of the coupling reaction to the termination step in radical polymerization.
2. Theoretical Analysis of LRAsCP
2.1. General Process of LRAsCP
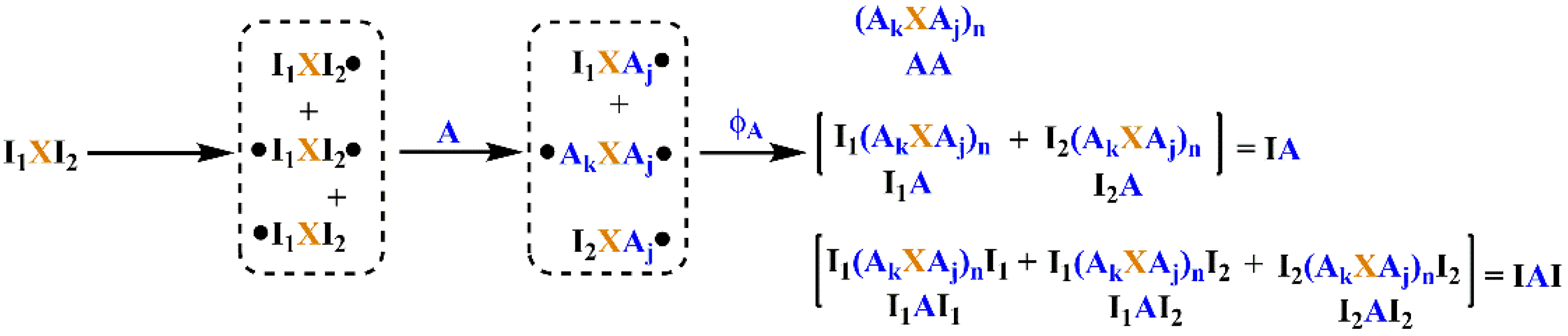
The process of LRAsCP is shown in Scheme 2. It includes three steps, such as initiation, propagation and coupling/termination, similar to normal radical polymerization. In the initiation step, the carbon radicals are formed by radical transfer reaction between bromides and pentacarbonyl manganese radical (Mn(CO)5•), which is generated by homolysis photolysis of decacarbonyl dimanganese (Mn2(CO)10) under visible light (see Scheme S1) [39,40,41]. I1XI2, the BFI, generates three kinds of small radicals, such as I2XI1•, I1XI2• and •I1XI2•. The first two radicals are monoradical, and the third one is biradical. In the propagation step, the three small radicals generate three macroradicals through the radical addition reaction with monomer A, such as I2XAj•, I1XAj• and •AkXAj•. The number of macroradicals is the same as that of the corresponding small radicals. In the coupling/termination step, the radical coupling reaction between two radicals is a chain extension step instead of the termination step in conventional radical polymerization initiated by monoradical. Other reactions, such as disproportionation and chain transfer reaction, are termination steps which result in a dead end of unit A. If radicals are considered as functional groups, the coupling reaction between the macroradicals is a kind of linear coupling polymerization.
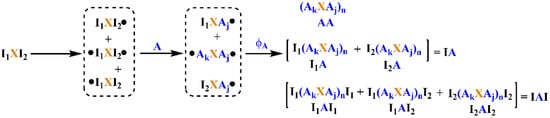
Scheme 2.
LRAsCP initiated by bifunctional initiator (BFI) I1XI2.
Let q1 and q2 be the extent of initiation of groups I1 and I2 of BFI, and q be the total extent of initiation of BFI; then, q is equal to 0.5(q1 + q2). Let ϕA be the termination factor involving radical A, which is the ratio of the rate of radical coupling reaction to the overall rate of reaction consuming radicals [38]. Let δ11, δ12, δ2 and δ0 be the molar fraction of small radicals I2XI1•, I1XI2•, •I1XI2• and free BFI, respectively. The macroradicals, I2XAj•, I1XAj• and •AkXAj•, are formed via the radical addition reaction of the corresponding small radicals to monomer A. Their molar fractions are equal to δ11, δ12 and δ2, in terms of molecule, respectively. With respect to radicals, the molar fractions of the three macroradicals are represented by θ11, θ12 and θ2. The parameters of the δ- and θ-series can be represented by q1 and q2, which are summarized in Table 1.

Table 1.
Various radicals formed in LRAsCP and their molar fractions.
The coupling reaction between three macroradicals generates six MBPs, which can be categorized into three types according to their terminal groups shown in Scheme 2. The first type is a polymer with two terminal units A, denoted as AA. The second type is a polymer with one functional group I1 or I2 and one terminal unit A, denoted as I1A or I2A. Both are collectively denoted as IA. The third type is a polymer with two functional groups, I1 or I2, denoted as I1AI1, I1AI2 and I2AI2. All three are collectively denoted as IAI.
2.2. The Number-Distribution Functions of MBP
To discuss the kinetics of LRAsCP, the following assumptions should be accepted. (1) The reactivity of all radicals is equivalent. (2) The intramolecular cycling reaction is ignored. (3) The contribution of additional monoradical generated by chain transfer is neglected. Based on the above assumptions, the number-distribution functions (pMBP, n) of the six MBPs are given by
where n is the number of BFI or X (residual moiety of BFI).
For example, the coupling reaction of n biradical •AkXAj• results in the formation of AA-type of MBP containing n X units. The formation of this chain starts with one •AkXAj•, followed by (n − 1) coupling reactions of the same biradical and two non-coupling reactions of two terminal radicals. The corresponding probabilities for the three steps are , and . The product of the three probabilities yields Equation (1). The formation of I1AI2-type MBP starts with an I1XI2• radical, followed by coupling reaction with (n − 2) •AkXAj• biradicals and an I2XAj• radical. The product of the corresponding probabilities, , and θ11ϕA, yields Equation (5). Other distribution functions can be derived by the same method and are given in Equations (1)–(6). The factor of 0.5 in Equations (4)–(6) is due to the symmetry of I1AI1- and I2AI2-type MBPs. The theoretical analysis of kinetics is described in the Supplementary Materials (SM) in detail. According to Equations (1)–(6), the distribution functions depend on q, ϕA and n.
2.3. The Structural Parameters of MBP
The structural parameters of MBP can be calculated from its number-distribution functions shown in Equations (1)–(6), such as the number and the fraction of each MBP (N and F) and its number-average degree of polymerization (DPn) in terms of X. N, DPn, the number-average molar mass (Mn) and the average number of blocks per MBP (NK) of total MBPs can be obtained as well. Except Mn, all structural parameters of MBP are determined by three factors, q1, q2 and ϕ.
When more than one kind of monomer participate in LRAsCP, multiblock copolymer (MBcP) can be obtained, which is termed linear radical additions-coupling copolymerization (LRAsCcP). On the other side, at the polymerization time when the extent of initiation of I1 and I2 are q10 and q20, the addition of monomer B to the polymerization mixture leads to the second-step polymerization resulting in copolymer block. Two-step LRAsCP generates two different blocks. The structural parameters of MBcP can be derived from its number-distribution function as well, which is described in the SM in detail.
3. Discussion on Theoretical Predictions of LRAsCP
Based on the mechanism of LRAsCP, the multiblock structure is predicted to be formed during the polymerization. We briefly discuss some important aspects of LRAsCP of typical monomers, such as styrene and methyl methacrylate (MMA) with ϕA values of 0.93 and 0.33 [38].
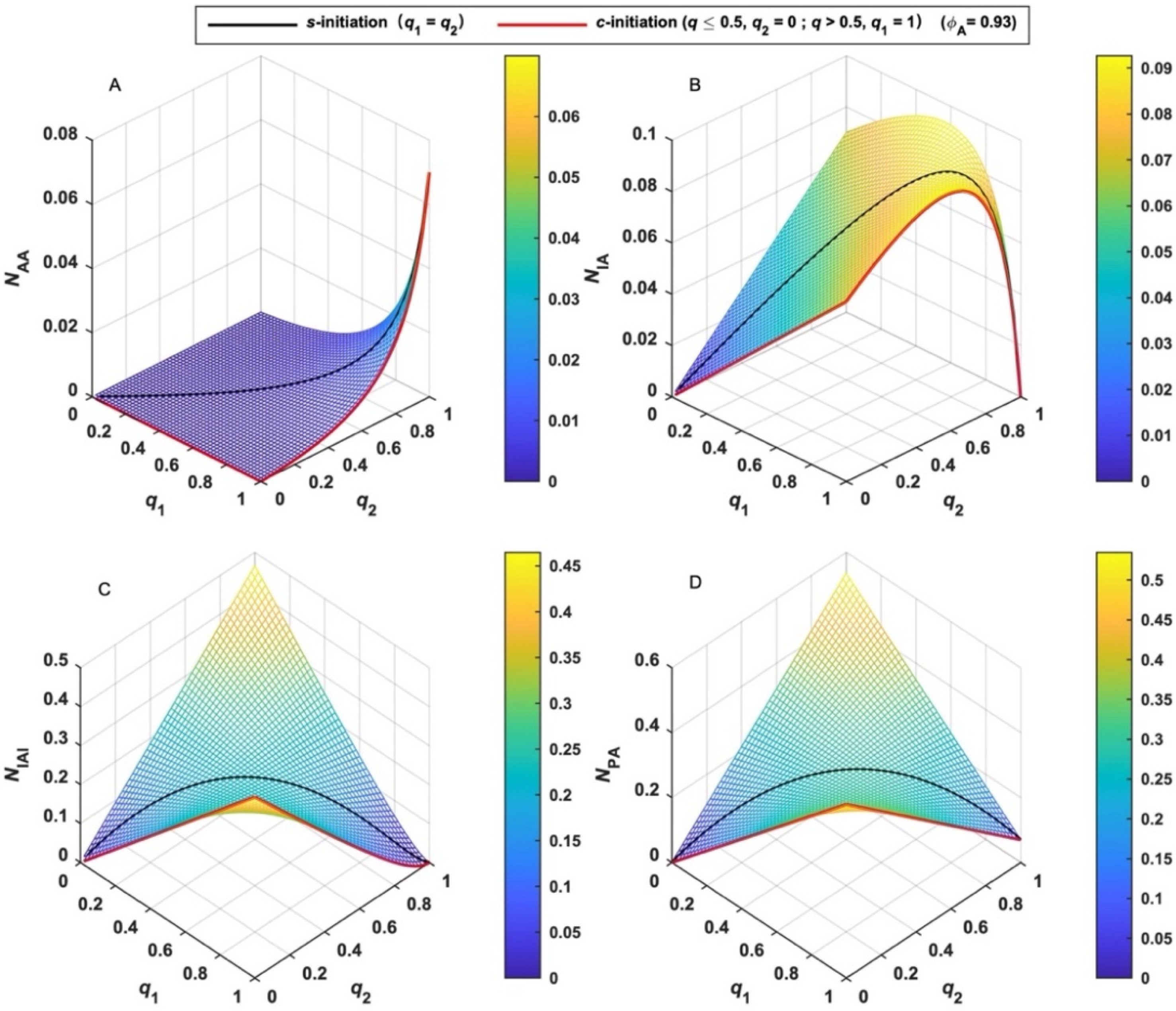
3.1. Numbers and Fractions of MBPs (NMBP and FMBP)
The numbers of MBPs (N) can be calculated by Equations (7)–(10) assuming the initial number of BFI (X0) is unity, which suggests that N depends on q1, q2 and ϕA. Taking ϕA = 0.93 [38] as an example, the variation tendency of N is shown in Figure 1. The number of AA-type MBP (NAA) monotonically increases with both q1 and q2 and increases rapidly as q is close to unity. (Figure 1A) The number of IA-type MBP (NIA) quickly increases with both q1 and q2 at the beginning of polymerization. (Figure 1B) After it reaches the maximum, it rapidly decreases to null upon complete initiation. The number of IAI-type MBP (NIAI) increases with q and decreases after it reaches the maximum. (Figure 1C) Moreover, its variation depends on the relative activity of the two functional groups of BFI. When the activities of I1 and I2 are equal (q1 = q2 = q), the case is termed simultaneous-initiation (s-initiation). When the activities of the two groups are remarkably different and the reactivity of I1 is much higher than I2, the case is termed cascade-initiation (c-initiation). Two special cases are shown in each figure by black and red lines, respectively. As shown by the black line in Figure 1C, NIAI derived from s-initiation case is lower than other cases (q1 ≠ q2). As shown by the red line in Figure 1C, NIAI derived from c-initiation case is larger than other cases. The variation tendency of the number of total MBPs (NPA) shown in Figure 1D is similar to that of NIAI.
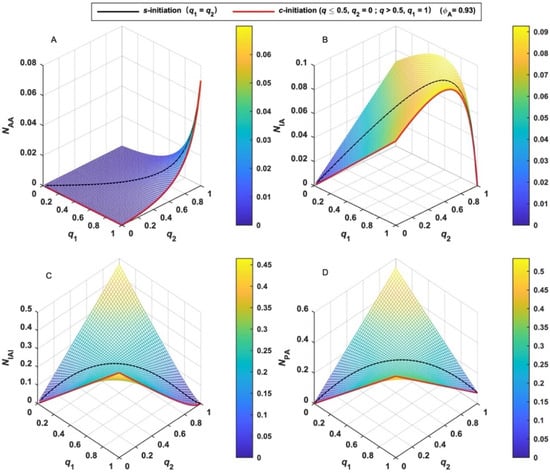
Figure 1.
Variation of the number (N) of various MBPs and their sum with the extent of initiation (q) (black line for s-initiation; red line for c-initiation). (A): AA-type MBP; (B): IA-type MBP; (C): IAI-type MBP; (D): total MBPs.
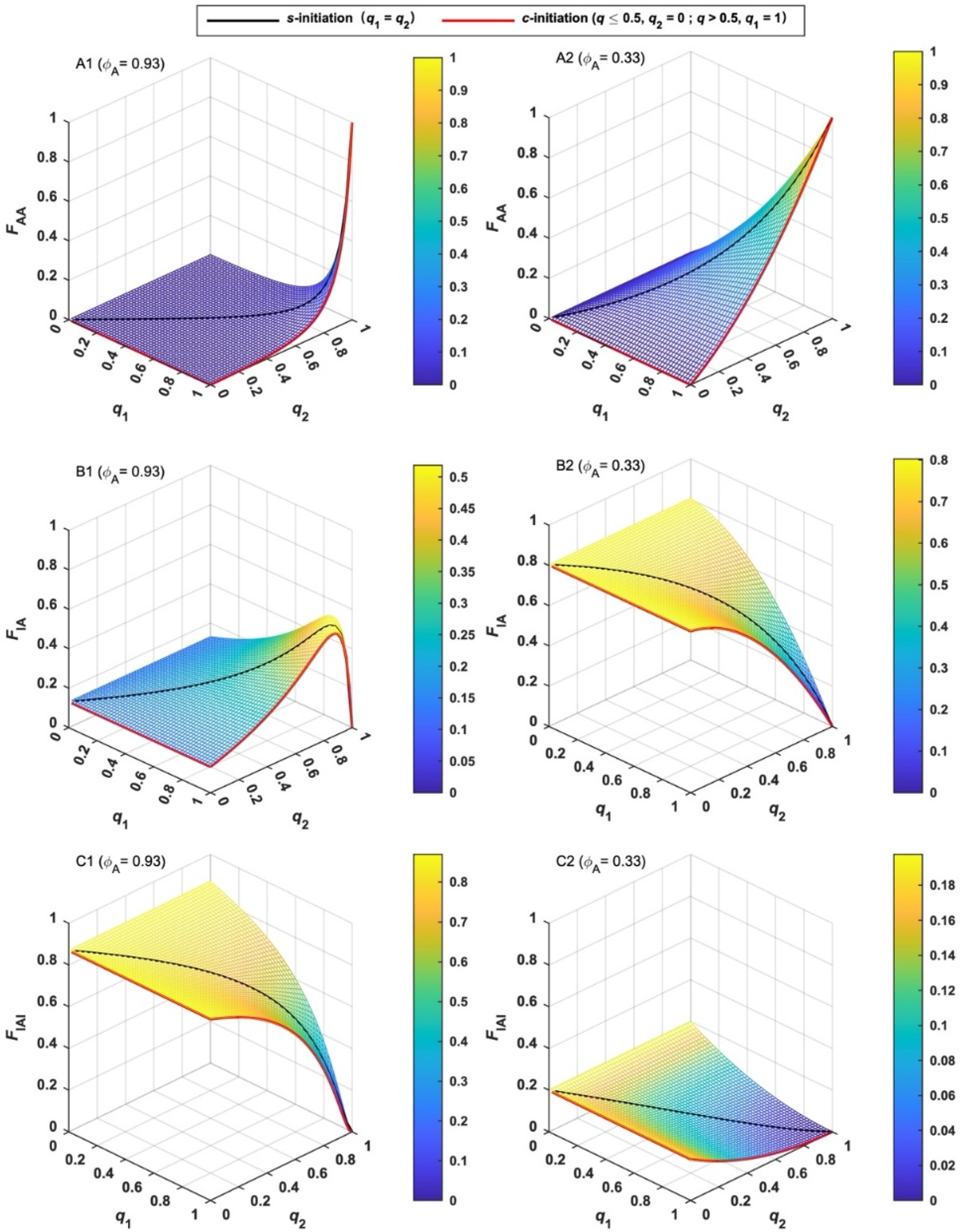
The molar fractions (F) of three MBPs are given by Equations (11)–(13), and their variation tendency is shown in Figure 2. Two typical cases, such as ϕA = 0.93 for styrene and 0.33 for MMA [38], were compared. It can be found in Figure 2A1 and A2 that the molar fraction of AA-type MBP (FAA) is very low at the beginning of polymerization and increases gradually with q for both cases. When q is close to unity, FAA increases rapidly. As shown in Figure 2C1 and C2, the variation tendency of molar fraction of IAI-type of MBP (FIAI) is opposite to FAA for both cases. The difference between the two cases is FIAI for styrene is much higher than that of MMA, which is due to the larger ϕ. The molar fraction of IA-type MBP (FIA) for styrene increases with q and reaches the maximum before the end of the initiation. (Figure 2B1) After it reaches the maximum, it decreases to null upon complete initiation. For MMA, FIA decreases with q and reaches null upon complete initiation. (Figure 2B2) The difference between the two cases is mainly due to the different values of ϕ.
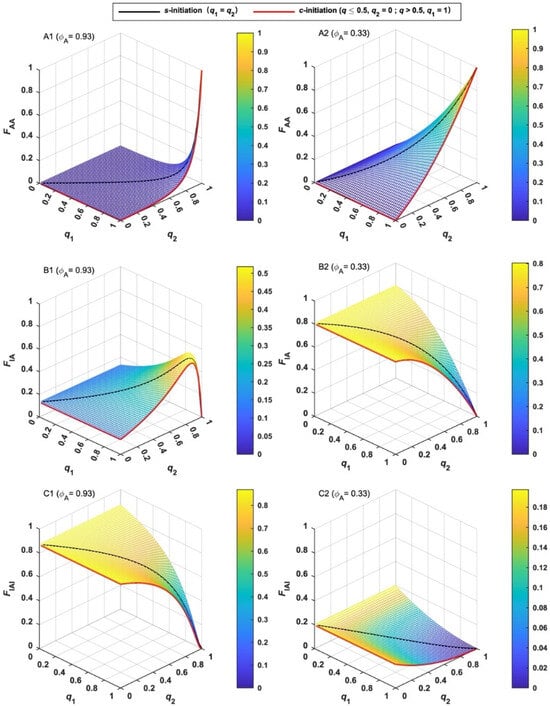
Figure 2.
Variation of the molar fraction (F) of various MBPs with the extent of initiation (q) and the different coupling factors (ϕA). ϕA = 0.93 for (A1, B1 and C1); ϕA = 0.33 for (A2, B2 and C2).
3.2. The Number-Average Degree of Polymerization in Terms of X (DPn)
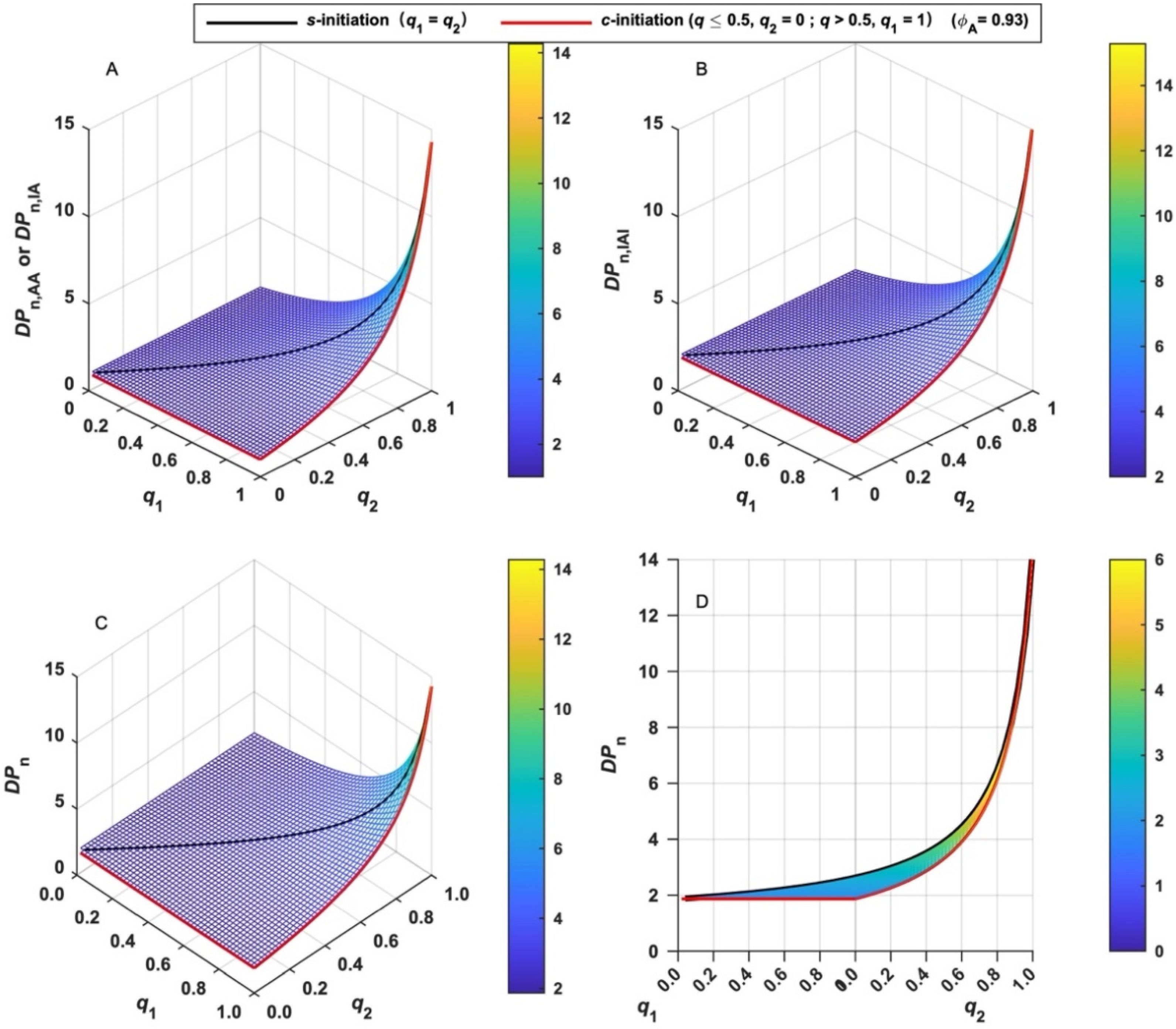
The chain length of various MBPs formed in LRAsCP can be quantitatively evaluated by the incorporated residual BFI (X). DPn in terms of X of various MBPs and their sum can be calculated by Equations (14)–(16), and the variation tendency with q is shown in Figure 3. It can be concluded that DPn increases with both q1 and q2 and increases rapidly when both q1 and q2 approach unity. This tendency is similar to the dependence of the degree of polymerization on polymerization time or the extent of reaction in stepwise polymerization [42]. When q exceeds 0.5 or both functional groups are initiated, the DPn of total MBPs gradually increases, and rapidly increases when q is close to unity. The variation tendency of DPn suggests that LRAsCP follows the stepwise mechanism since the coupling reaction is the key step in the construction of the multiblock architecture.
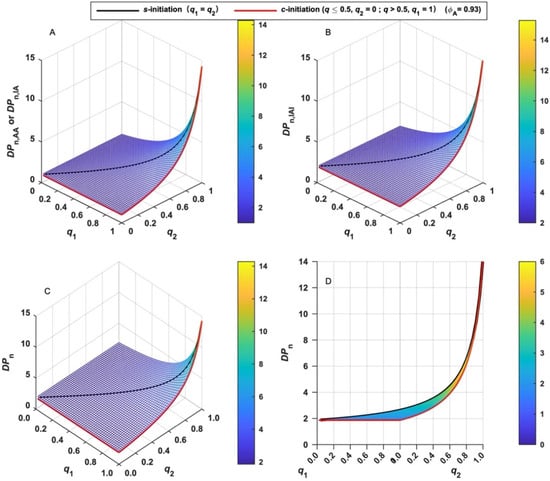
Figure 3.
Variation of the number-average degree of polymerization (DPn) of various MBPs with the extent of initiation (q). (A): AA- and IA-type MBP; (B): IAI-type MBP; (C): total MBPs; (D): total MBPs in s-initiation and c-initiation cases.
When s-initiation and c-initiation cases are compared, the difference of DPn can be found when q is less than 0.5. As shown in Figure 3D, DPn increases gradually with q and increases rapidly when q is close to unity for s-initiation case (black line), while it remains constant when q is less than 0.5 or q2 = 0 for c-initiation case (red line). This is due to the selective initiation of one functional group of BFIs and the formation of monoradical in the early stage of c-initiation case (q < 0.5), which is the same as radical polymerization initiated by monofunctional initiator (MFI). q exceeds 0.5 when the second functional group of each BFI is initiated. Initiation of two functional groups results in the continuous formation of MBPs and an increment of DPn in c-initiation case.
3.3. The Number-Average Molar Mass of Total MBPs (Mn)
Based on the number of all MBPs (), the monomer conversion (C) and the number-average molar mass of total MBPs (Mn) can be calculated by Equation (17).
where A0, X0, C, and mA are the initial concentration of monomer A and BFI, the monomer conversion and the molar mass of monomer, respectively. Since the Mn of MBP is determined by the feed ratio and the monomer conversion in addition to q1, q2 and ϕA, the discussion will be given in experimental studies.
3.4. The Average Number of Blocks per MBP (NK)
The average number of blocks per MBP (NK) can be evaluated by the total number of blocks and , which is given by Equation (18).
This parameter can be experimentally evaluated and will be further discussed in experimental studies.
3.5. The Second-Step Polymerization
3.5.1. Free BFI and AA-Type MBP
The molar fractions of free BFI and AA-type MBP are two parameters that need to be considered if the second LRAsCP of monomer B is conducted. Free BFI might generate BB-type MBP in the second polymerization, while AA-type MBP will not initiate the second polymerization of monomer B and cannot be incorporated into MBcP. Both AA- and BB-type MBPs are impurities with respect to MBcP.
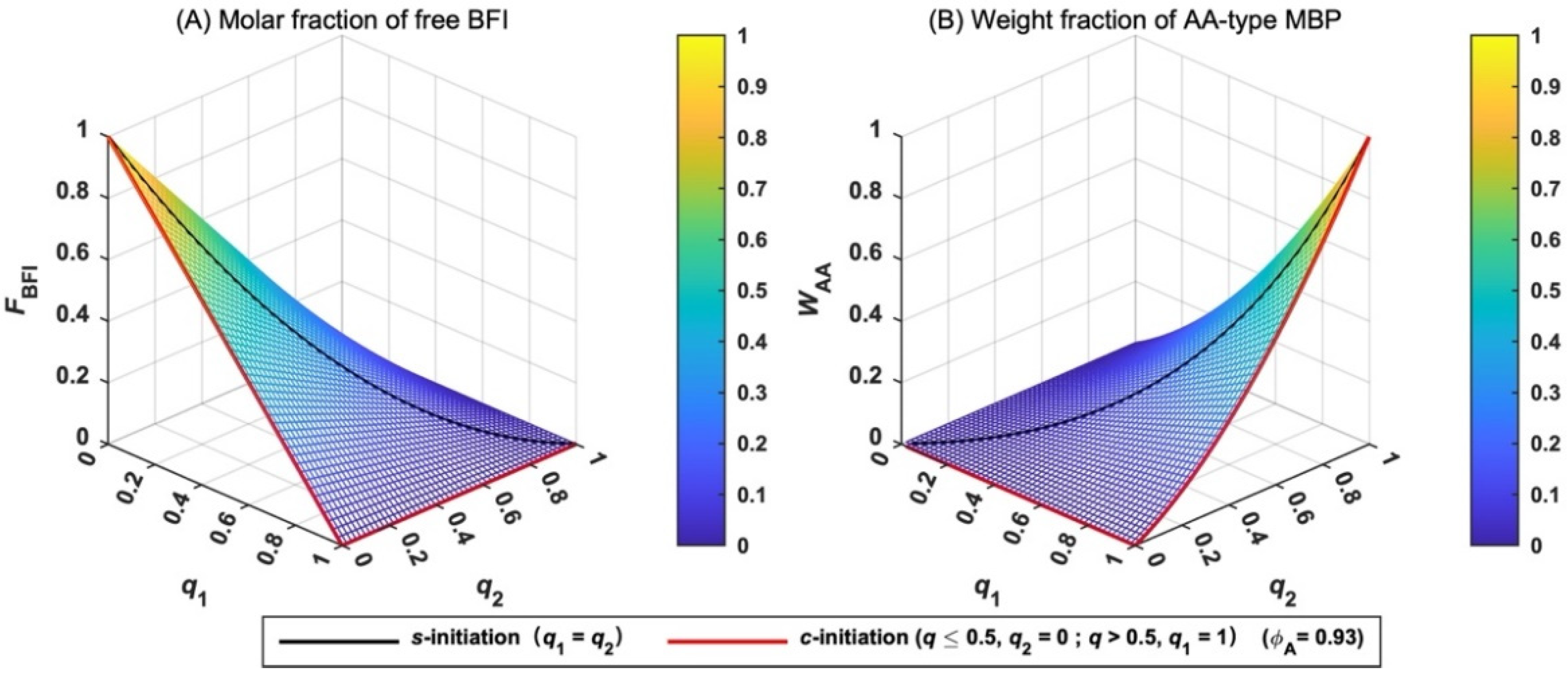
The molar fraction of free BFI (FBFI) is derived in Equation (19), and its variation with q is shown in Figure 4A. When the total extent of initiation (q) is fixed, FBFI has the maximum value when q1 and q2 are equal, and the minimum value when the difference between q1 and q2 is the largest. For example, free BFI disappears when q1 = 1 and q2 = 0 or q ≥ 0.5 for c-initiation case (red line in Figure 4A). When q1 = q2 = q = 0.5, 25% of BFI remains for s-initiation case (black line in Figure 4A). Therefore, c-initiation case has the lowest value of FBFI. In other words, c-initiation case is the best choice for the two-step polymerization.
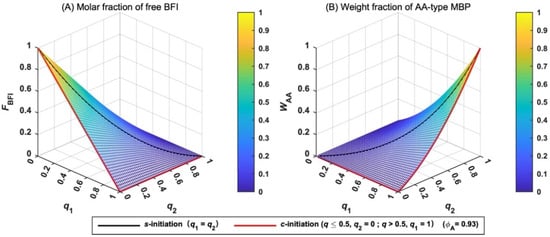
Figure 4.
Variation in the molar fraction of free BFI (FBFI) (A) and the weight fraction of AA-type MBP (WAA) (B) with the extent of initiation (q).
The weight fraction of AA-type MBP (WAA) is given by Equation (20) and its variation with q is shown in Figure 4B. When q1 and q2 are low, WAA is small. Only when q1 and q2 are close to unity, WAA increases rapidly. AA-type MBP formed in the first-step polymerization can be ignored in the two-step polymerization if monomer B is added when q1 and q2 are less than unity.
3.5.2. DPn,co of MBcP
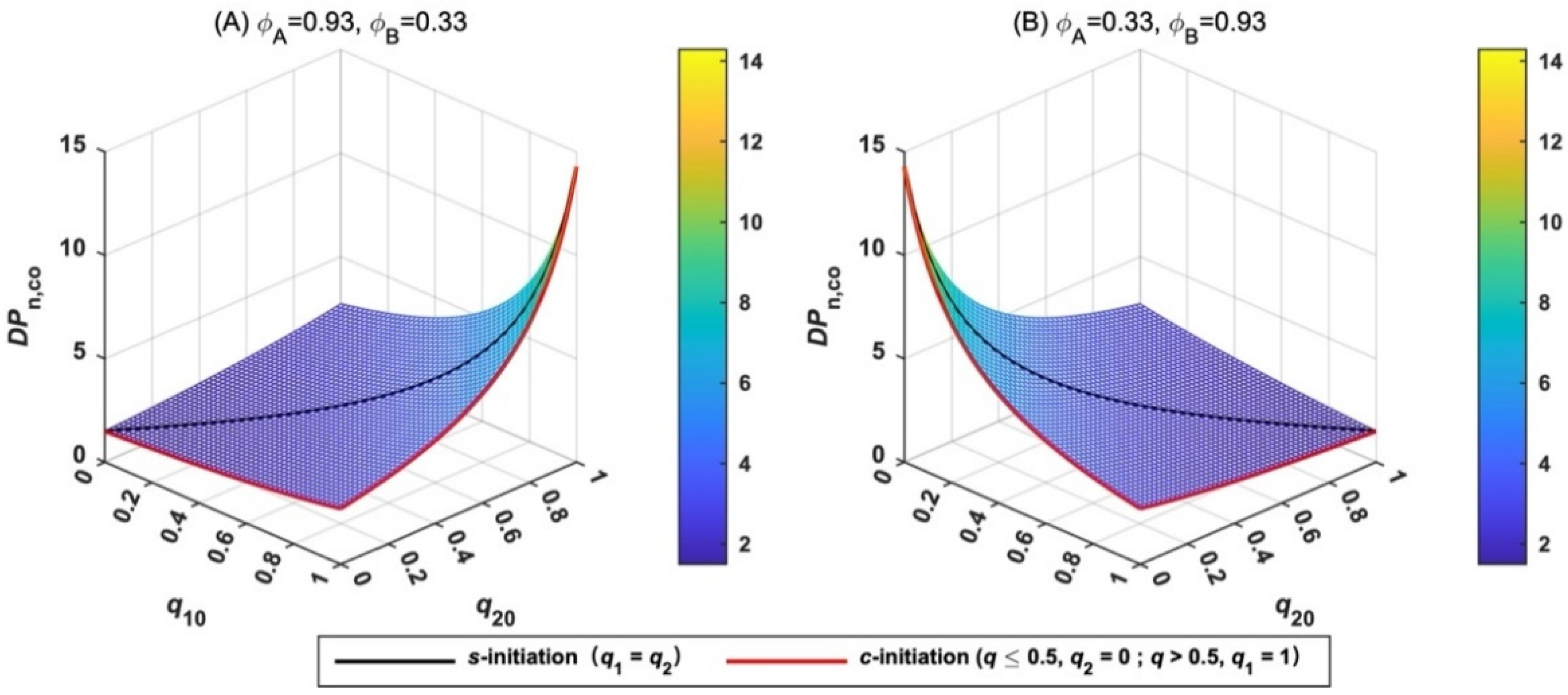
When monomer A is replaced by monomer B at the time when the extent of initiation is q10 and q20, the second LRAsCP of B occurs. The DPn,co of total MBcP is predicted by Equation (21), assuming the complete initiation of the residual function groups of BFIs in the second polymerization. The variation tendency of DPn,co is shown in Figure 5. The theoretical analysis suggests that the termination factors of two monomers, ϕA and ϕB, are the key parameters affecting DPn,co. The combination of longer polymerization of the monomer with larger ϕ and shorter polymerization of the monomer with smaller ϕ is the best way to produce MBcP with large DPn,co. For example, when styrene is first polymerized, MMA (ϕB = 0.33) is recommended to be added when q10 and q20 are close to unity. (Figure 5A) If the polymerization sequence of the two monomers is opposite, styrene is suggested to be added as early as possible (Figure 5B).
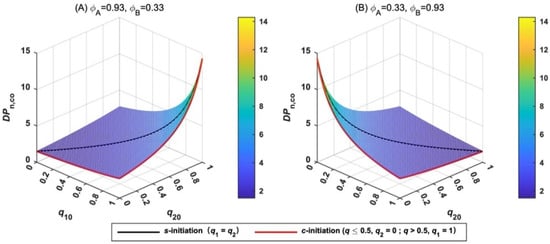
Figure 5.
Variation of the degree of polymerization of the copolymerization (DPn,co) with the extent of initiation (q10, q20) and the termination factors of two monomers (ϕA, ϕB). (A) ϕA = 0.93, ϕB = 0.33; (B) ϕA = 0.33, ϕB = 0.93.
In summary, the structural parameters of MBPs are determined by q and ϕ according to theoretical analysis of LRAsCP. The value of q is determined by the initiation rate and polymerization time. Φ is determined by the nature of the monomer and is the key factor to constructing the multiblock structure.
4. Experimental Study
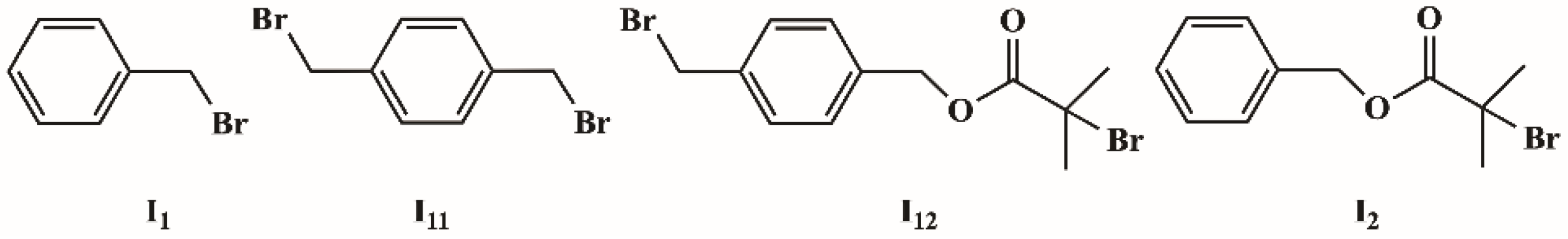
Two initiators, I12 and I2, shown in Scheme 3, were prepared from commercially available two benzylbromides I11 and I1 by published methods [43]. I11 is a symmetrical dibromide corresponding to s-initiation case, and I12 is an unsymmetrical dibromide corresponding to c-initiation case.
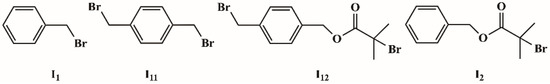
Scheme 3.
The structure of initiators.
4.1. Homopolymerization of Styrene
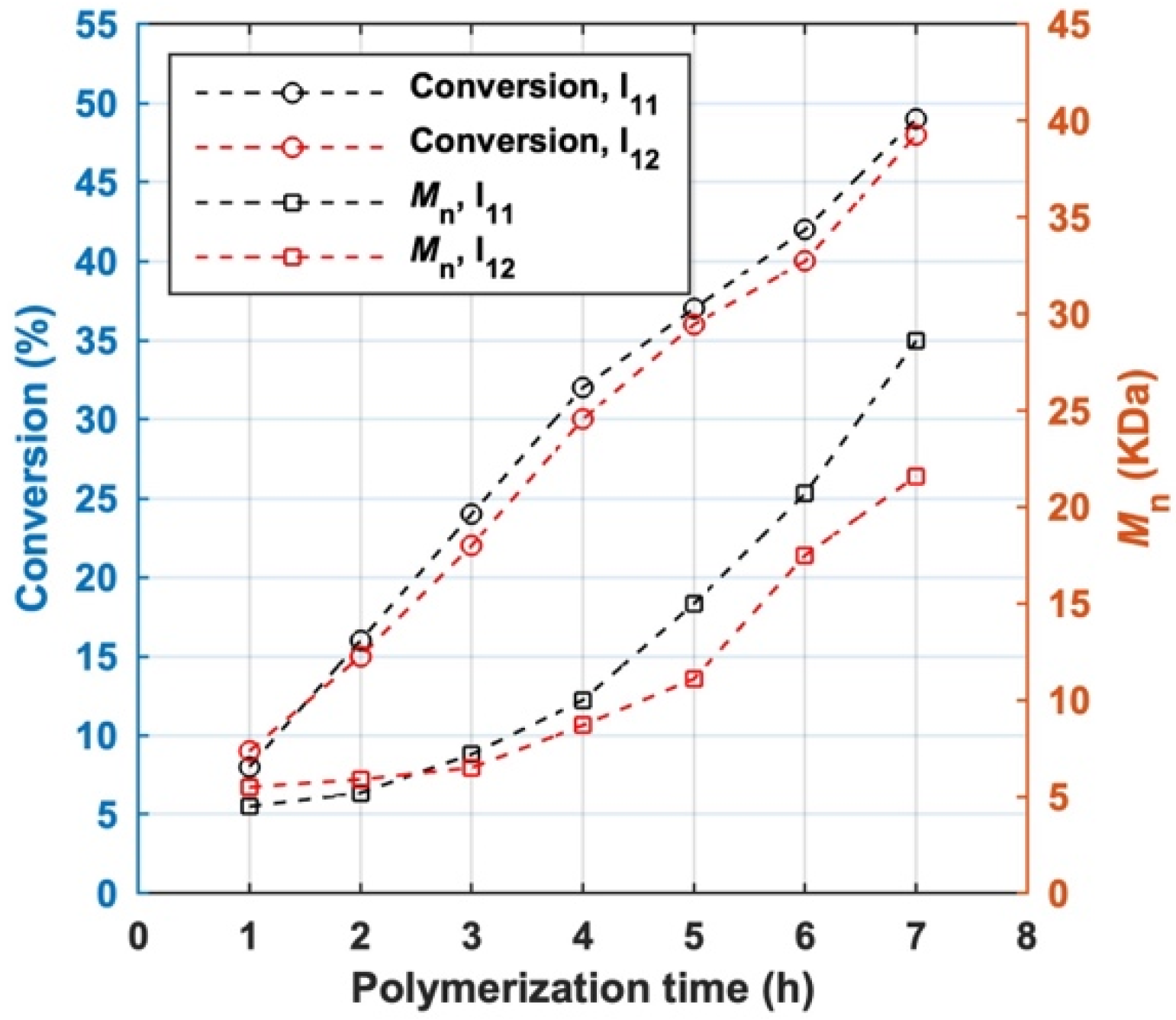
The styrene homopolymerization initiated by I11 and I12 were conducted under the same conditions. The monomer conversion, the number-average molar mass (Mn) and its distribution of all MBPs measured by GPC are shown in Figure 6 and Tables S2 and S3.
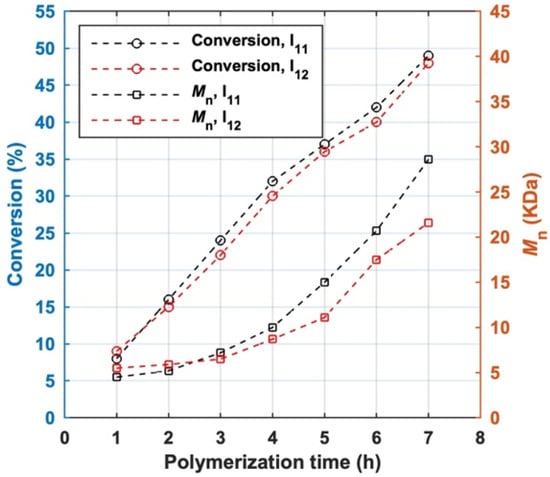
Figure 6.
The variation of monomer conversion and number-average molar mass of MBP (Mn) with polymerization time.
The conversion of styrene increased with the polymerization time for both BFIs. Mn gradually increased with polymerization time before 3 h and accelerated afterwards. The variation tendency of Mn of total MBP prepared by two BFIs suggest that the coupling of macroradicals occurred in LRAsCP. Accelerated growth of Mn of total MBP after 3 h reflects the stepwise construction of the multiblock architecture. The Mn of MBPs prepared by I11 (s-initiation case) grows slightly faster than those obtained by I12 in the early period of polymerization, which is consistent with the tendency predicted by Equations (14)–(16) and Figure 3D. When I12 was used as initiator, Mn of total MBP did not remain constant at the early stage of polymerization, which suggests that LRAsCP initiated by I12 is not exactly c-initiation case, but a case between s-initiation and c-initiation. Although I12 is not a typical c-initiation case, its ester moiety introduces cleavable sites into MBPs, making the resultant MBPs degradable.
The Mn values of total MBP for s-initiation and c-initiation cases are further derived from Equations (17)–(22) and (23), respectively. The ratio, A0CmA/X0, in Equation (17) is the molar mass of polymer upon one chain per BFI, which is defined as the apparent molar mass (Mn,0) and is given by Equation (24). Since the parameters A0, X0, C, mA and ϕA are available, the extent of initiation of the two cases at different times can be solved by Equations (22)–(24), which are given by Equations (25)–(27), respectively.
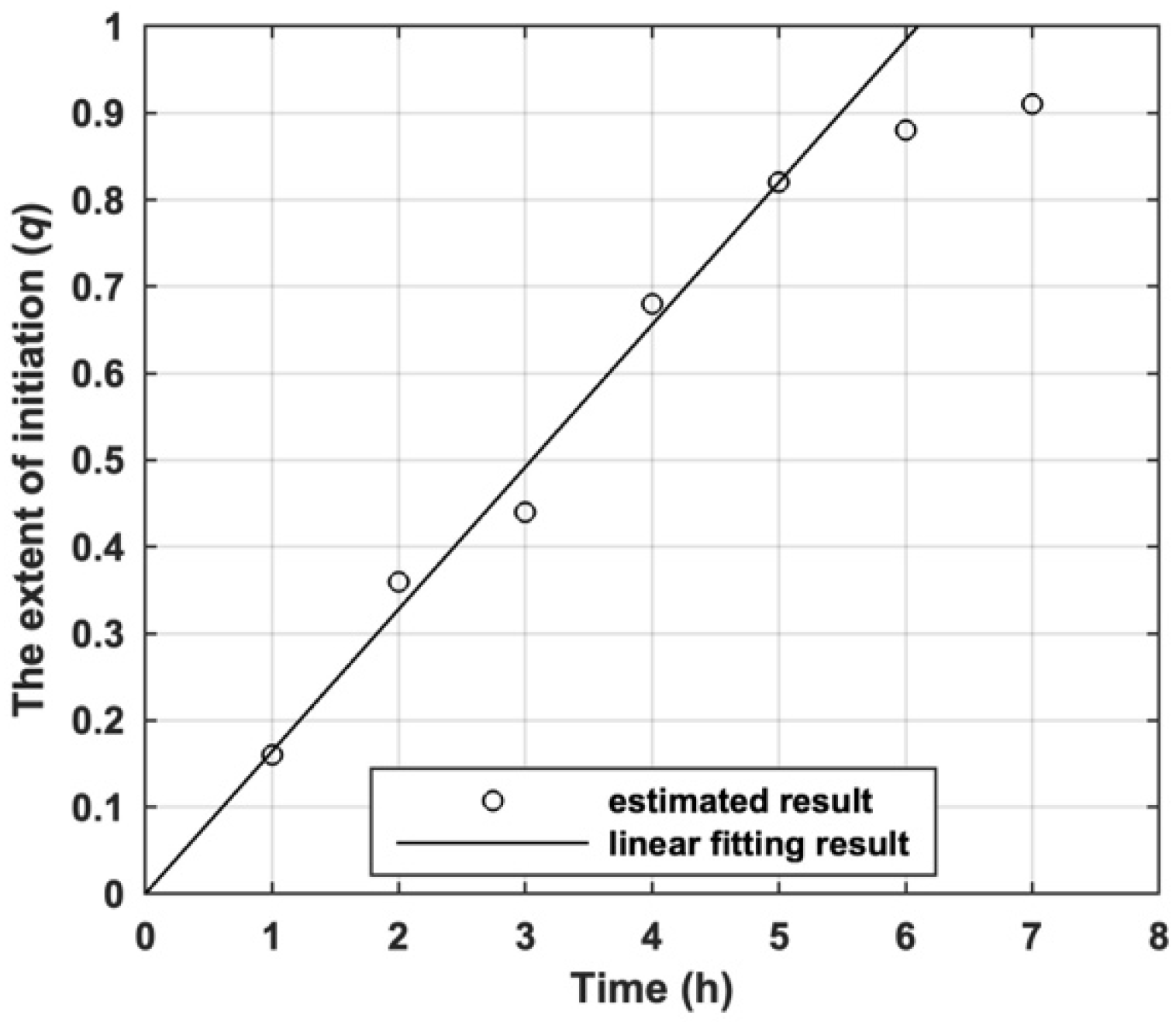
Based on the feed ratio, the monomer conversion and Mn of product prepared by I11, the variation of q with the polymerization time estimated by Equation (25) is shown by Figure 7. It can be found that q of I11 for s-initiation case increased linearly with polymerization time because the polymerization is promoted by Mn2(CO)10/visible light. The decay of q when it was over 0.8 is due to the high monomer conversion and the gel effect observed in the polymerization [42].
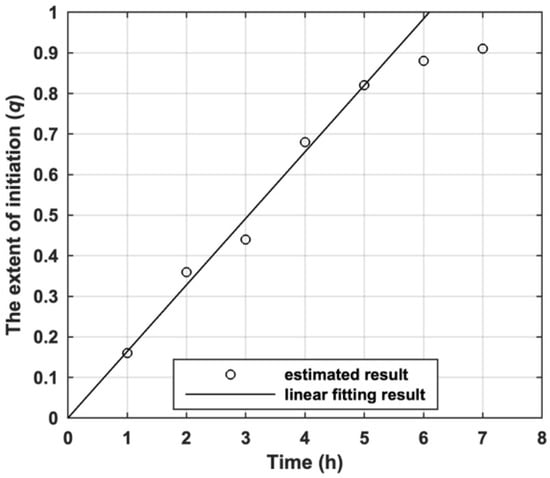
Figure 7.
The variation of estimated the extent of initiation (q) of I11 with polymerization time.
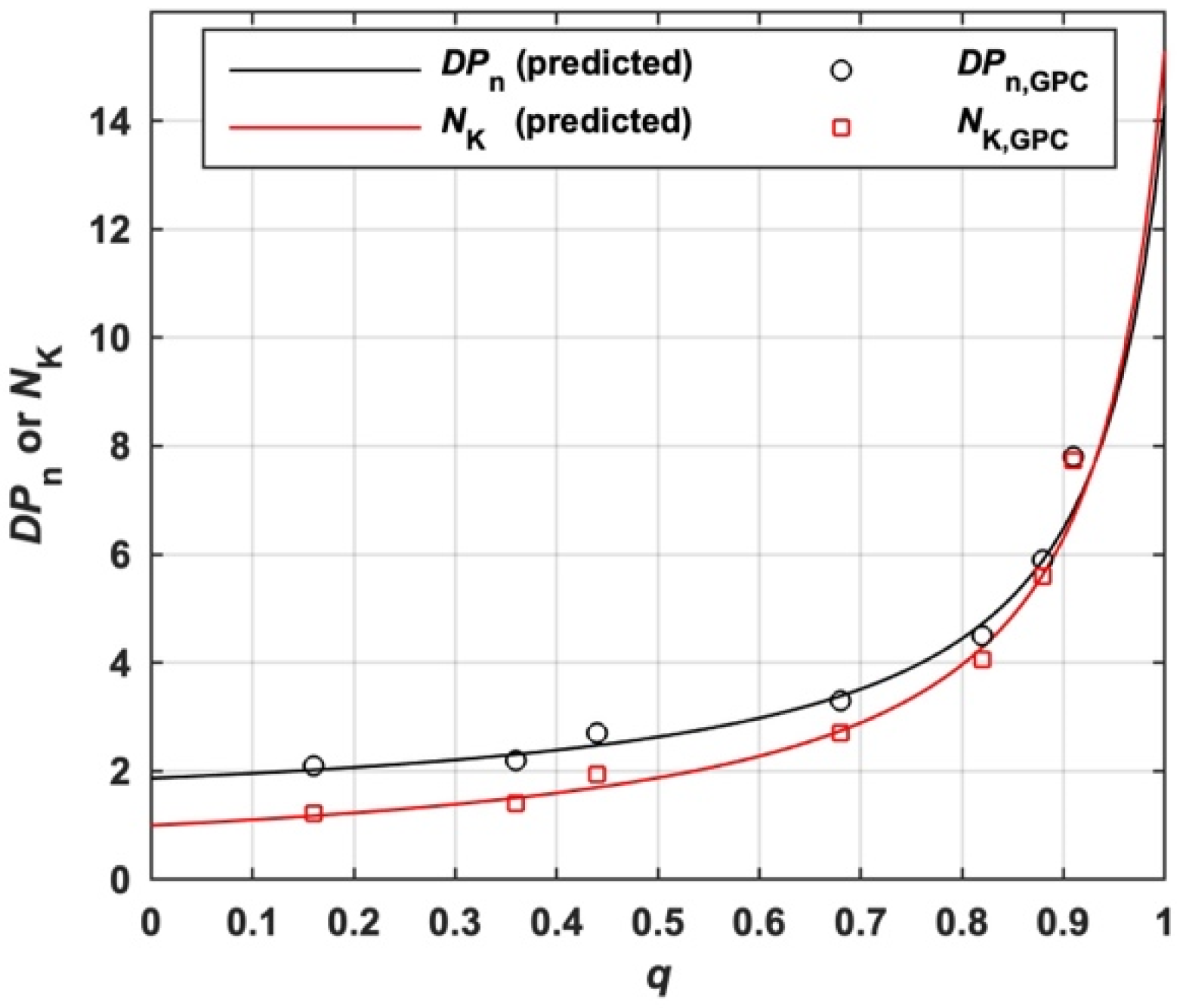
As shown in Scheme 1 and Scheme S2, MBP is a chain composed of several segmental chains linked by moiety X. The average number of blocks per MBP (NK) for the s-initiation case can be predicted by Equation (28) derived from Equation (18). The molar mass of the block is equal to the molar mass of the polymer generated by I1 () in conventional radical polymerization under the same polymerization conditions if the kinetics chain length (ν) generated from two initiators are supposed to be the same. Therefore, NK can be estimated by the ratio of Mn to measured by GPC, which is denoted as NK,GPC in Equation (29). As shown in Figure 8, the predicted NK according to Equation (28) and its measured value according to Equation (29) are in good agreement.
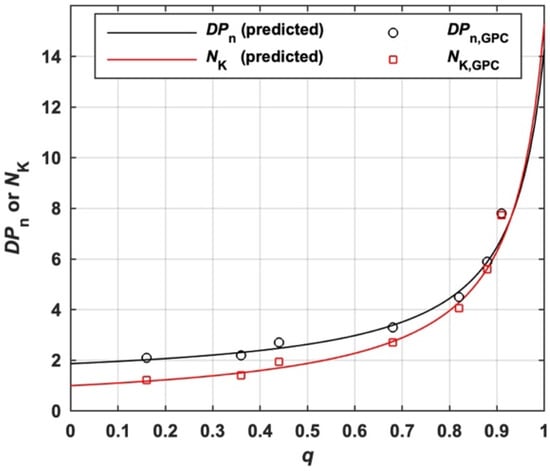
Figure 8.
The variation of predicted and measured NK and DPn with q of I11 (s-initiation case).
On the other hand, the relationship between ν and in conventional polymerization is given by Equation (30) [42]. Therefore, ν can be quantified by the polymerization of monomer with known ϕ initiated by I1.
As shown in Scheme S2, the relationship between DPn and the number of kinetic chains per MBP (Nν) varies with its type due to the different terminal units. If ν generated by BFI is the same as that by MFI under the same polymerization conditions, the relationship between DPn of MBP and ν is deduced as
Equation (31) can be used to experimentally estimate the DPn of MBP based on Mn, , FIA and FIAI, which is denoted as DPn,GPC. For the s-initiation case, Equation (31) is reduced to Equation (32) and Equation (16) is reduced to Equation (33). DPn,GPC of total MBP prepared by I11 experimentally estimated by Equation (32) at various values of q is shown in Figure 8, which fits well with DPn predicted by Equation (33).
4.2. Two-Step LRAsCP
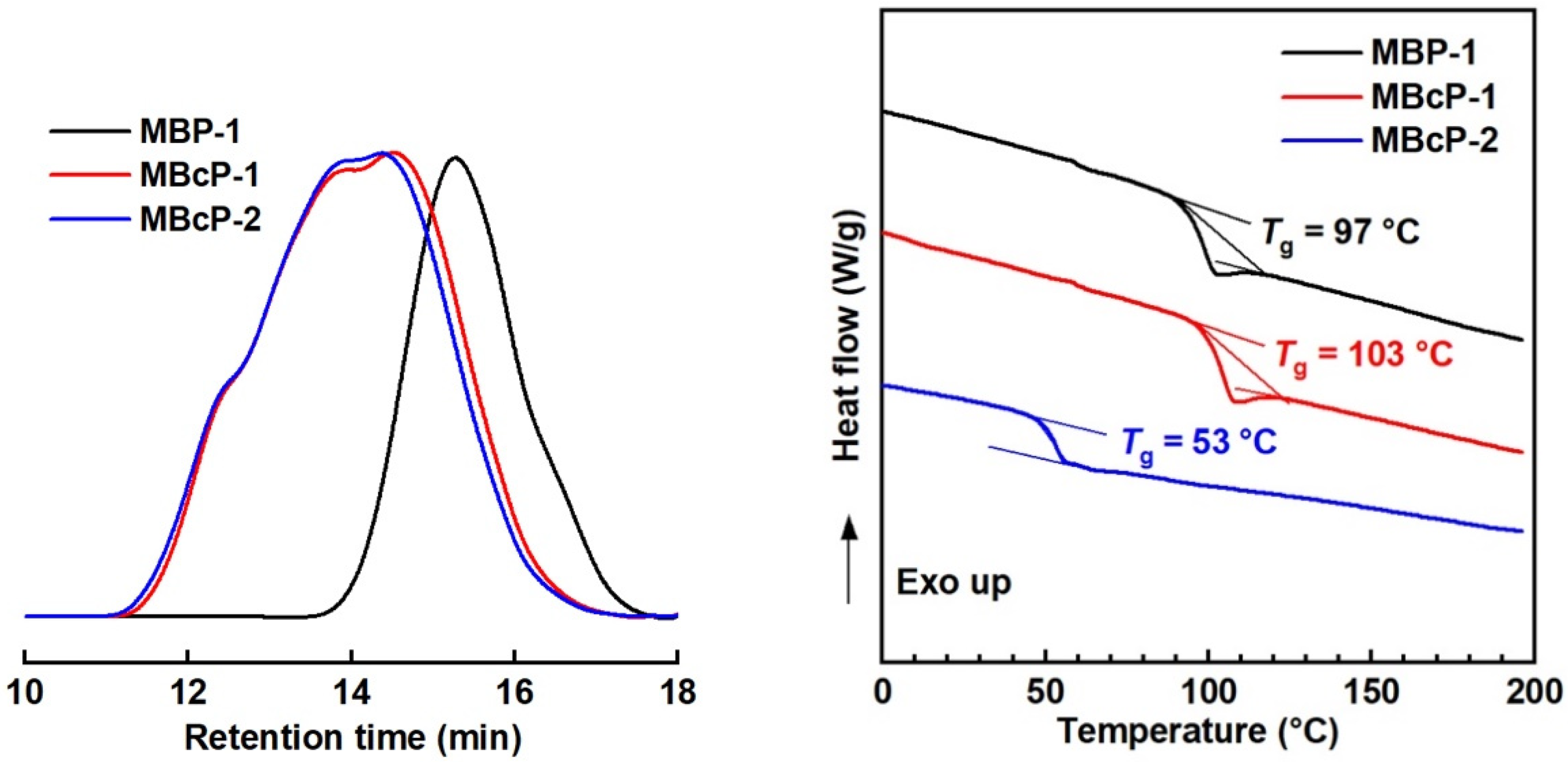
Two-step LRAsCP was conducted in two ways. One is one-pot polymerization via the addition of comonomer at a certain time. LRAsCP under the same polymerization condition as Run 4 in Table S2 was conducted. After 4 h, the polymerization was suspended by turning off the light and half of the polymer solution was collected and precipitated. The resultant sample was denoted as MBP-1 (Mn = 9.4 KDa, Ð = 2.0 in Figure 9 and Table S4). MMA was added to the remaining half of the solution, and the second LRAsCP started by turning on the light for an additional 190 min. Sample MBcP-1 (Mn = 34.7 KDa, Ð = 5.1 in Figure 9 and Table S4) composed of 81 mol% styrene was obtained. This is in situ two-step LRAsCP and the second LRAsCP is the copolymerization of styrene and MMA resulting in St-MMA copolymer block. The obtained MBP-1 contains both IA- and IAI-type MBPs and was used as macro-initiator for the copolymerization of styrene and butyl methacrylate (BMA) for 4 h. Sample MBcP-2 (Mn = 38.6 KDa, Ð = 5.0 in Figure 9 and Table S4) composed of 89 mol% styrene was obtained. The improved molar masses of MBcP-1 and MBcP-2 compared with that of MBP-1 demonstrates that LRAsCP continues in the second-step polymerizations in two different ways. MBcP-2 is composed of 75 wt% polystyrene block and 25 wt% styrene-BMA (49/51, mol/mol) copolymer block and the glass transition temperature (Tg) of 53 °C was detected by DSC, which is different from the Tg of PS (100 °C) and PBMA (21 °C). This confirms that a copolymer block was formed during the second-step LRAsCP.
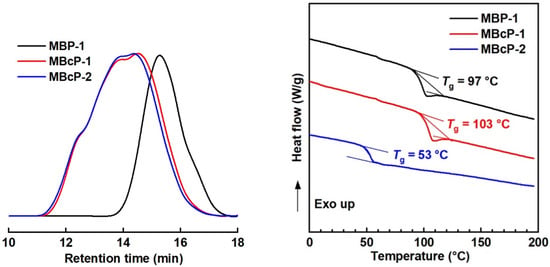
Figure 9.
GPC (left) and DSC (right) curves of MBP-1, MBcP-1 and MBcP-2 prepared by I11.
4.3. Determination of ϕ of Different Monomers
The termination mode in terms of kt,c/(kt,c + kt,d) is a key parameter in radical polymerization kinetics. Several experimental methods, such as isotopic labeling of the initiator [44], transformation of initiator fragments after polymerization [45], gelation technique [46,47] and MALDI analysis of polymer [48], have been proposed to determine the termination mode. These methods are based on quantitative analysis of the chain end, which is still difficult because the concentration of initiator fragments is low. Since the NK of MBP produced in LRAsCP is determined by ϕ, it is possible to acquire the value of ϕ in LRAsCP. Replacing NK in Equation (28) with NK,GPC in Equation (29) yields Equation (34).
If the same q is achieved under the same polymerization conditions, Equation (34) can be applied to measure the factor ϕ of any polymerization based on NK,GPC and known ϕ of styrene. The polymerization of styrene, MMA and BMA initiated by I11 and I1 were conducted at the same conditions for 3 h and the polymerization results are given in Table S5. The number-average molar mass of the six samples prepared by I11 (Mn) and I1 () results in different NK,GPC. With known ϕ of styrene (0.93) [38], the calculated value of ϕ of MMA is 0.33 according to Equation (34), which is the same as that determined by the measurement of gelation point in NLRAsCP of MMA [38]. The calculated ϕ of BMA is −0.25, which is obviously unreasonable. This is probably due to the gelation effect caused by the high monomer conversion and the chain transfer reaction to the polymer. Although the factor ϕ is not the same as kt,c/(kt,c + kt,d), LRAsCP provides an easy method of quantitative determination of the contribution of the coupling reaction in the termination step of radical polymerization independent of the initiator fragment.
5. Conclusions
LRAsCP initiated by BFI and its kinetics modal have been proposed. Theoretical analysis of LRAsCP provides the quantitative relationships between some key parameters of MBP, such as the molar fraction of various MBPs, DPn, Mn, NK with q and ϕ. The s-initiation case of LRAsCP of styrene initiated by I11/Mn2(CO)10/visible light were intensively investigated. The experimental results fit well with the theoretical predictions, which approve the stepwise construction of the multiblock structure of MBP. MBcP composed of two types of blocks, PS block and P(St-MMA/BMA) block, can be produced by two-step LRAsCP. Furthermore, LRAsCP can be applied to the measurement of ϕ of radicals when ϕ of one specific monomer is known. The current results demonstrate that the introduction of BFI to conventional radical polymerization generates a new polymerization strategy, which is a facile method to construct the multiblock structure. Since radical polymerization is tolerant to various monomers, LRAsCP can be extended to other monomers that are difficult to be polymerized by living and controlled radical polymerizations.
6. Experimental Section
Two initiators, I2 and I12, shown in Scheme 2 were prepared by the reaction between I1 or I11 and 2-bromoisobutanoic acid by published methods [43]. The structure of two compounds was confirmed by NMR, HRMS. The homopolymerization of styrene and copolymerization of styrene with MMA and BMA promoted by Mn2(CO)10/visible light were conducted. MBPs were characterized by GPC, and MBcPs were characterized by GPC and NMR. Full descriptions of the methods and analytical data for the compounds and polymer used and generated in the study are given in the Supplementary Materials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polym17060741/s1. Theoretical analysis of LRAsCP; all experimental and analytical data for the compounds and polymer used and generated in the study. Figure S1: 1H-NMR (400 MHz) and 13C-NMR (100 MHz) spectra of I2; Figure S2: 1H-NMR (400 MHz) and 13C-NMR (100 MHz) spectra of I12; Figure S3: 1H-NMR (400 MHz) spectra of MBcP-1 (top) and MBcP-2 (bottom); Figure S4: GPC curves of polystyrenes prepared by I11 and I1 at various times; Figure S5: GPC curves of polystyrene prepared by I12 and I2 at various times; Table S1: The parameters of various MBP; Table S2: LRAsCP of styrene initiated by BFI I11 and I1; Table S3: LRAsCP of styrene initiated by BFI I12 and I2; Table S4: Two-step LRAsCP initiated by I11; Table S5: LRAsCP of various monomers initiated by I11 and I1. (PDF); Scheme S1 Initiation reaction of visible-light induced decomposition of decacarbonyl dimanganese (Mn2(CO)10); Scheme S2 The quantitively relationship between DPn and Nv for various MBPs; Scheme S3 Preparation of initiators.
Author Contributions
Conceptualization, Q.W.; Formal analysis, Y.J.; Investigation, Y.J.; Writing—original draft, Y.J.; Writing—review and editing, K.C. and Q.W.; Supervision, Q.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are available within the article and Supplementary Materials.
Conflicts of Interest
There are no conflicts to declare.
Abbreviations
| Abbreviation/symbol | Definition |
| A, A0 | the concentration and initial concentration of monomer |
| B(M)FI | bi(mono)functional initiator |
| MB(c)P | multiblock (co)polymer |
| C | the conversion of monomer |
| f | the functionality of MBP |
| FAA, FIA, FIAI | the molar fraction of three types of MBPs |
| Mn | the number-average molar mass of MBP prepared by BFI |
| Mn,0 | the apparent number-average molar mass |
| mA | the molar mass of monomer A |
| NAA, NIA, NIAI | the number of three types of MBPs |
| NK | the average number of blocks per MBP |
| NK,GPC | the average number of blocks per MBP estimated by GPC |
| the total number of MBPs | |
| Nv | the number of kinetic chains per MBP |
| , , | the number-distribution functions of different kinds of MBPs |
| q | the extent of initiation of functional group |
| W | the weight fraction of MBP |
| X, X0 | residual fragment of BFI, the concentration and initial concentration of BFI |
| DPn | the number-average degree of polymerization in terms of X |
| DPn,GPC | DPn estimated by molar mass of MBP (GPC) |
| DPn,co | DPn of MBcP |
| δ | the molar fraction of small radicals |
| θ | the molar fractions of macroradicals |
| ϕ | termination factor |
| ν | kinetic chain length |
References
- Qiang, X.; Chakroun, R.; Janoszka, N.; Groeschel, A.H. Self-Assembly of Multiblock Copolymers. Isr. J. Chem. 2019, 59, 945–958. [Google Scholar] [CrossRef]
- Steube, M.; Johann, T.; Barent, R.D.; Mueller, A.H.E.; Frey, H. Rational design of tapered multiblock copolymers for thermoplastic elastomers. Prog. Polym. Sci. 2022, 124, 101488. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, Y.; Gao, X.; Li, B.-G.; Xie, T. A General Approach Towards Thermoplastic Multishape-Memory Polymers via Sequence Structure Design. Adv. Mater. 2013, 25, 743–748. [Google Scholar] [CrossRef]
- Self, J.L.; Zervoudakis, A.J.; Peng, X.; Lenart, W.R.; Macosko, C.W.; Ellison, C.J. Linear, Graft, and Beyond: Multiblock Copolymers as Next-Generation Compatibilizers. JACS Au 2022, 2, 310–321. [Google Scholar] [CrossRef]
- Constantinou, A.P.; Sam-Soon, N.F.; Carroll, D.R.; Georgio, T.K. Thermoresponsive Tetrablock Terpolymers: Effect of Architecture and Composition on Gelling Behavior. Macromolecules 2018, 51, 7019–7031. [Google Scholar] [CrossRef]
- Bates, F.S.; Hillmyer, M.A.; Lodge, T.P.; Bates, C.M.; Delaney, K.T.; Fredrickson, G.H. Multiblock Polymers: Panacea or Pandora’s Box? Science 2012, 336, 434–440. [Google Scholar] [CrossRef]
- Beyer, V.P.; Kim, J.; Becer, C. R. Synthetic approaches for multiblock copolymers. Polym. Chem. 2020, 11, 1271–1291. [Google Scholar] [CrossRef]
- Tang, X.; Fan, X.; Chen, X.; Zhou, Q. Progress of Atom Transfer Radical Polymerization (ATRP) Applied to the Synthesis of Multiblock Copolymers. Polym. Bull. 2006, 6, 36–43. [Google Scholar]
- Clothier, G.K.K.; Guimaraes, T.R.; Thompson, S.W.; Rho, J.Y.; Perrier, S.; Moad, G.; Zetterlund, P.B. Multiblock copolymer synthesis via RAFT emulsion polymerization. Chem. Soc. Rev. 2023, 52, 3438–3469. [Google Scholar] [CrossRef]
- Gringolts, M.L.; Denisova, Y.I.; Finkelshtein, E.S.; Kudryavtsev, Y.V. Olefin metathesis in multiblock copolymer synthesis. Beil. J. Org. Chem. 2019, 15, 218–235. [Google Scholar] [CrossRef]
- Diaz, C.; Mehrkhodavandi, P. Strategies for the synthesis of block copolymers with biodegradable polyester segments. Polym. Chem. 2021, 12, 783–806. [Google Scholar] [CrossRef]
- Steube, M.; Johann, T.; Galanos, E.; Appold, M.; Ruettiger, C.; Mezger, M.; Gallei, M.; Müller, A.H.E.; Floudas, G.; Frey, H. Isoprene/Styrene Tapered Multiblock Copolymers with up to Ten Blocks: Synthesis, Phase Behavior, Order, and Mechanical Properties. Macromolecules 2018, 51, 10246–10258. [Google Scholar] [CrossRef]
- Gleede, T.; Rieger, E.; Blankenburg, J.; Klein, K.; Wurm, F.R. Fast Access to Amphiphilic Multiblock Architectures by the Anionic Copolymerization of Aziridines and Ethylene Oxide. J. Am. Chem. Soc. 2018, 140, 13407–13412. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, J.; Gurnani, P.; Cook, A.B.; Haekkinen, S.; Zhang, J.; Yang, J.; Kerr, A.; Haddleton, D.M.; Perrier, S.; Wilson, P. Microscale synthesis of multiblock copolymers using ultrafast RAFT polymerisation. Polym. Chem. 2019, 10, 1186–1191. [Google Scholar] [CrossRef]
- Nishimura, S.-n.; Higashi, N.; Koga, T. Facile Synthesis of Multiblock Copolymers Containing Sequence-Controlled Peptides and Well-Defined Vinyl Polymers by Nitroxide-Mediated Polymerization. Chem. A Eur. J. 2017, 23, 15050–15058. [Google Scholar] [CrossRef]
- Kuroki, A.; Martinez-Botella, I.; Hornung, C.H.; Martin, L.; Williams, E.G.L.; Locock, K.E.S.; Hartlieb, M.; Perrier, S. Looped flow RAFT polymerization for multiblock copolymer synthesis. Polym. Chem. 2017, 8, 3249–3254. [Google Scholar] [CrossRef]
- Kerr, A.; Hartlieb, M.; Sanchis, J.; Smith, T.; Perrier, S. Complex multiblock bottle-brush architectures by RAFT polymerization. Chem. Commun. 2017, 53, 11901–11904. [Google Scholar] [CrossRef]
- Engelis, N.G.; Anastasaki, A.; Nurumbetov, G.; Truong, N.P.; Nikolaou, V.; Shegiwal, A.; Whittaker, M.R.; Davis, T.P.; Haddleton, D.M. Sequence-controlled methacrylic multiblock copolymers via sulfur-free RAFT emulsion polymerization. Nat. Chem. 2017, 9, 171–178. [Google Scholar] [CrossRef]
- Anastasaki, A.; Oschmann, B.; Willenbacher, J.; Melker, A.; Van Son, M.H.C.; Truong, N.P.; Schulze, M.W.; Discekici, E.H.; McGrath, A.J.; Davis, T.P.; et al. One-Pot Synthesis of ABCDE Multiblock Copolymers with Hydrophobic, Hydrophilic, and Semi-Fluorinated Segments. Angew. Chem. Int. Ed. 2017, 56, 14483–14487. [Google Scholar] [CrossRef]
- Aksakal, R.; Resmini, M.; Becer, C.R. Pentablock star shaped polymers in less than 90 minutes Via aqueous SET-LRP. Polym. Chem. 2016, 7, 171–175. [Google Scholar] [CrossRef]
- Gody, G.; Barbey, R.; Danial, M.; Perrier, S. Ultrafast RAFT polymerization: Multiblock copolymers within minutes. Polym. Chem. 2015, 6, 1502–1511. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Q. Degradable Multisegmented Polymers Synthesized by Consecutive Radical Addition-Coupling Reaction of alpha,omega-Macrobiradicals and Nitroso Compound. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 612–618. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, Q. Thermodegradable multisegmented polymer synthesized by consecutive radical addition-coupling reaction of α, ω-macrobiradicals and dithioester. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 2029–2036. [Google Scholar] [CrossRef]
- Tao, F.; Li, J.; Wang, Q. Aqueous radical addition-coupling polymerization for the synthesis of hydrophilic periodic polymer. RSC Adv. 2014, 4, 53253–53256. [Google Scholar] [CrossRef]
- Tao, F.; Wang, Q. Aqueous radical addition-coupling polymerization using a nitroso benzene/cyclodextrin complex for the synthesis of a hydrophilic periodic polymer. RSC Adv. 2015, 5, 46007–46010. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Z.; Liu, J.; Min, X.; Wang, T.; Fan, X. A New Strategy to Synthesize α,ω-Dihydroxy Multiblock Copolymers via CpRu(CH3CN)3 PF6/Quinaldic Acid Catalyst. Macromol. Rapid Commun. 2019, 40, 1900135. [Google Scholar] [CrossRef]
- Beyer, V.P.; Cattoz, B.; Strong, A.; Phillips, D.J.; Schwarz, A.; Becer, C. R. Fast track access to multi-block copolymers via thiol-bromo click reaction of telechelic dibromo polymers. Polym. Chem. 2019, 10, 4259–4270. [Google Scholar] [CrossRef]
- Cianga, I.; Yagci, Y. First polyrecombination reaction via atom transfer radical coupling (ATRC), a new way for the synthesis of poly(p-xylylene). Des. Monomers Polym. 2007, 10, 575–584. [Google Scholar] [CrossRef][Green Version]
- Durmaz, Y.Y.; Aydogan, B.; Cianga, I.; Yagci, Y. The Use of Atom Transfer Radical Coupling Reactions for the Synthesis of Various Macromolecular Structures. Polym. Prepr. 2008, 49, 382–383. [Google Scholar]
- Wang, C.H.; Song, Z.Y.; Deng, X.X.; Zhang, L.J.; Du, F.S.; Li, Z.C. Combination of ATRA and ATRC for the Synthesis of Periodic Vinyl Copolymers. Macromol. Rapid Commun. 2014, 35, 474–478. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Q. Radical coupling copolymerization (RCCP) for synthesis of various polymers. Polymer 2016, 100, 56–59. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Q. Radical coupling polymerization (RCP) for synthesis of various polymers. RSC Adv. 2016, 6, 39568–39572. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Q. Step-Growth Radical Addition-Coupling Polymerization (RACP) for Synthesis of Alternating Copolymers. Macromol. Rapid Commun. 2011, 32, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ling, J.; Wang, Q. Radical Addition-Coupling Polymerization (RACP) toward Periodic Copolymers. Macromolecules 2011, 44, 8739–8743. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Q. Block copolymers prepared by polymeric radical addition cross-coupling reaction to different double bonds. J. Polym. Sci. Part. A Polym. Chem. 2013, 51, 2817–2823. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q. Radical Addition-Coupling Polymerization with Various Nitroso Compounds. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 810–815. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Q. Radical addition-coupling polymerization (RACP) of various benzyl-type biradical toward periodic polymers. Polymer 2016, 94, 14–18. [Google Scholar] [CrossRef]
- Ren, L.M.; Li, C.L.; Wang, Q. Non-linear Radical Additions-Coupling Polymerization of Monovinyl Monomers towards Polymer Networks: Theory, Tunability and Heritable Architecture. Chin. J. Polym. Sci. 2022, 40, 1623–1630. [Google Scholar] [CrossRef]
- Gilbert, B.C.; Harrison, R.J.; Lindsay, C.I.; McGrail, P.T.; Parsons, A.F.; Southward, R.; Irvine, D. J. Polymerization of methyl methacrylate using dimanganese decacarbonyl in the presence of organohalides. Macromolecules 2003, 36, 9020–9023. [Google Scholar] [CrossRef]
- Haines, L.I.B.; PoË, A.J. Initiation of Vinyl Polymerization by Manganese Carbonyl and Carbon Tetrachloride. Nature 1967, 215, 699–701. [Google Scholar] [CrossRef]
- Ciftci, M.; Tasdelen, M.A.; Yagci, Y. Sunlight induced atom transfer radical polymerization by using dimanganese decacarbonyl. Polym. Chem. 2014, 5, 600–606. [Google Scholar] [CrossRef]
- Odian, G. Principles of Polymerization, 4th ed.; Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Dewkar, G.K.; Carneiro, P.B.; Hartman, M.C.T. Synthesis of Novel Peptide Linkers: Simultaneous Cyclization and Labeling. Org. Lett. 2009, 11, 4708–4711. [Google Scholar] [CrossRef] [PubMed]
- Arnett, L.M.; Peterson, J.H. Vinyl Polymerization with Radioactive Aliphatic Azobisnitrile Initiators. J. Am. Chem. Soc. 1952, 74, 2031–2033. [Google Scholar] [CrossRef]
- Bamford, C.H.; Jenkins, A.D. Termination Reaction in Vinyl Polymerization: Preparation of Block Copolymers. Nature 1955, 176, 78. [Google Scholar] [CrossRef]
- Bamford, C.H.; Dyson, R.W.; Eastmond, G.C. Studies in network formation. J. Polym. Sci. Part C Polym. Symp. 1967, 16, 2425–2434. [Google Scholar] [CrossRef]
- Bamford, C.H.; Eastmond, G.C.; Whittle, D. Network formation III—Influence of organometallic initiator on network structure. Polymer 1969, 10, 771–783. [Google Scholar] [CrossRef]
- Zammit, M.D.; Davis, T.P.; Haddleton, D.M.; Suddaby, K.G. Evaluation of the mode of termination for a thermally initiated free-radical polymerization via matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Macromolecules 1997, 30, 1915–1920. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).