Palladium Membrane Applications in Hydrogen Energy and Hydrogen-Related Processes
Abstract
1. Introduction
2. Key Hydrogen Production Methods
2.1. Hydrogen Production from Natural Gas
2.2. Liquid Hydrogen Carriers
2.3. Hydrogen Production from Alcohols
2.4. Catalysts for Hydrogen Production
2.5. Water Electrolysis
3. Palladium Membranes for Hydrogen Purification
3.1. Hydrogen Purification Methods
3.2. Palladium Alloy-Based Membranes
3.3. Surface Modification of Palladium Membrane
3.4. Composite Membranes
| Membrane | T, °C | Pd Layer Thickness, μm | H2 Permeability, 10−9 mol·m−1·s−1·Pa−0.5 | H2/N2 Separation Selectivity | Ref. |
|---|---|---|---|---|---|
| Pd/Al2O3 | 350 | 5 | 5.0 | 8000–37,600 | [257] |
| Pd/Al2O3 | 400 | 5 | 6.3 | – | [257] |
| Pd-Ag/PSS | 450 | 10 | 34 | 39,000 | [194] |
| Pd/PSS-CeO2 (0.07–0.1 μm) | 400 | 12.5 | 4.5 | >10,000 | [264] |
| Pd/PSS-CeO2 (3.4 μm) | 400 | 6.3 | 3.8 | >10,000 | [264] |
| Pd/PSS-CeO2 (>10 μm) | 400 | 9.0 | 3.6 | >10,000 | [264] |
| Pd/Ta/Pd (tubular) | 450 | 1 | 60–120 | – | [273] |
| Membrane | T (°C) | P(H2), 10−16 mol m−1 s−1 Pa−1 | Q(H2), GPU | Separation Selectivity | Ref. | |
|---|---|---|---|---|---|---|
| H2/N2 | H2/CO2 | |||||
| m-PBI | 150 | 150 | – | 3.4 | 1.4 | [339] |
| 200 | 260 | – | 5.0 | 4.1 | [339] | |
| m-PBI + PEG-stabilized Pd nanoparticles (2%) | 150 | 94 | – | 68 | 9.3 | [339] |
| 200 | 180 | – | 81 | 19 | [339] | |
| m-PBI + PEG-stabilized Pd nanoparticles (3%) | 150 | 210 | – | 4.1 | 3.5 | [339] |
| 200 | 320 | – | 7.3 | 5.8 | [339] | |
| m-PBI + PEG-stabilized Pd nanoparticles (4%) | 150 | 94 | – | 75 | 6.4 | [339] |
| 200 | 210 | – | 110 | 5.0 | [339] | |
| m-PBI | 100 | 43.5 | – | 4.8 | 2.5 | [340] |
| 150 | 57 | – | 2.9 | 8.1 | [340] | |
| m-PBI + PVP (1%) | 100 | 15 | – | 5.6 | 1.1 | [340] |
| 150 | 47 | – | 3.2 | 1.8 | [340] | |
| m-PBI + PVP-stabilized Pd nanoparticles (1%) | 100 | 16 | – | 5.4 | 2.4 | [340] |
| 150 | 63.5 | – | 22 | 12 | [340] | |
| 200 | 160 | – | 39 | 21 | [340] | |
| 240 | 160 | – | 43 | 21 | [340] | |
| 260 | 315 | – | 83 | 58 | [340] | |
| 300 | 510 | – | 180 | 83 | [340] | |
| m-PBI + PVP-stabilized Pd nanoparticles (3%) | 100 | 6.0 | – | 5.9 | 2.9 | [340] |
| 150 | 30.5 | – | 8.7 | 6.0 | [340] | |
| m-PBI + Pd nanorods | 150 | 190 | – | – | 34 | [344] |
| 225 | 470 | – | – | 27 | [344] | |
| m-PBI + Pd nanoparticles (58 wt%) | 200 | 220 | – | – | 33 | [341] |
| m-PBI (in hollow fibers) | 60 | – | 0.086 | – | 5.2 | [343] |
| m-PBI + Pd nanoparticles (in hollow fibers) | 60 | – | 80 | – | 10 | [343] |
4. Membrane Reactors
4.1. Hydrogen Production Using Membrane Reactors
4.2. Polymer-Based Composite Membranes with Catalytically Active Palladium Particles for Hydrogenation/Dehydrogenation Processes
4.3. Polymer–Palladium Composites in Fuel Cells
4.4. Water Treatment: Hydrogenation with Palladium-Containing Membranes
4.4.1. Organo-Chlorinated Compounds’ Removal from Industrial and Waste Water
4.4.2. Dissolved Oxygen Removal
4.4.3. Nitrate and Nitrite Removal
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Angevine, P.J.; Oleck, S.M. Noble Metal-Containing Catalysts. U.S. Patent 4,683,214A, 28 July 1987. [Google Scholar]
- Liu, X.; Iocozzia, J.; Wang, Y.; Cui, X.; Chen, Y.; Zhao, S.; Li, Z.; Lin, Z. Noble metal-metal oxide nanohybrids with tailored nanostructures for efficient solar energy conversion, photocatalysis and environmental remediation. Energy Environ. Sci. 2017, 10, 402–434. [Google Scholar] [CrossRef]
- Stoffels, M.A.; Klauck, F.J.R.; Hamadi, T.; Glorius, F.; Leker, J. Technology trends of catalysts in hydrogenation reactions: A patent landscape analysis. Adv. Synth. Catal. 2020, 362, 1258–1274. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Gebreyohannes, T.G.; Woo lee, S.; Kim, S.K.; Kim, H.W.; Shin, J.; Kim, Y.T. Methane direct conversion to olefins, aromatics, and hydrogen over silica entrapped bimetallic MeFe-SiO2 (Me = Co, Ni, Pd, Pt) catalysts. Mol. Catal. 2023, 535, 112864. [Google Scholar] [CrossRef]
- Biffis, A.; Centomo, P.; Del Zotto, A.; Zecca, M. Pd metal catalysts for cross-couplings and related reactions in the 21st Century: A critical review. Chem. Rev. 2018, 118, 2249–2295. [Google Scholar] [CrossRef]
- McCarthy, S.; Braddock, D.C.; Wilton-Ely, J.D.E.T. Strategies for sustainable palladium catalysis. Coord. Chem. Rev. 2021, 442, 213925. [Google Scholar] [CrossRef]
- Kim, H.J.; Song, C. A refined design concept for sulfur-tolerant Pd catalyst supported on zeolite by shape-selective exclusion and hydrogen spillover for hydrogenation of aromatics. J. Catal. 2021, 403, 203–214. [Google Scholar] [CrossRef]
- Ca’, N. Della Palladium-Catalyzed Reactions. Catalysts 2021, 11, 588. [Google Scholar] [CrossRef]
- Horbaczewskyj, C.S.; Fairlamb, I.J.S. Pd-catalyzed cross-couplings: On the importance of the catalyst quantity descriptors, mol % and ppm. Org. Process Res. Dev. 2022, 26, 2240–2269. [Google Scholar] [CrossRef]
- Dong, D.-Q.; Yang, H.; Zhou, M.-Y.; Wei, Z.-H.; Wu, P.; Wang, Z.-L. Recent advances in palladium-catalyzed reactions in water. Curr. Opin. Green Sustain. Chem. 2023, 40, 100778. [Google Scholar] [CrossRef]
- Habib, M.A.; Haque, M.A.; Harale, A.; Paglieri, S.; Alrashed, F.S.; Al-Sayoud, A.; Nemitallah, M.A.; Hossain, S.; Abuelyamen, A.; Mokheimer, E.M.A.; et al. Palladium-alloy membrane reactors for fuel reforming and hydrogen production: Hydrogen production modeling. Case Stud. Therm. Eng. 2023, 49, 103359. [Google Scholar] [CrossRef]
- Gryaznov, V.M.; Smirnov, V.S. The reactions of hydrocarbons on membrane catalysts. Russ. Chem. Rev. 1974, 43, 821–834. [Google Scholar] [CrossRef]
- Gryaznov, V.M.; Mischenko, A.P.; Smirnov, V.S.; Aladyshev, S.I. Catalytic Reactor Designed for Carrying out Conjugate Chemical Reactions. U.S. Patent 3,779,711, 18 December 1973. [Google Scholar]
- Hafeez, S.; Al-Salem, S.M.; Manos, G.; Constantinou, A. Fuel production using membrane reactors: A review. Environ. Chem. Lett. 2020, 18, 1477–1490. [Google Scholar] [CrossRef]
- Vinodh, R.; Palanivel, T.; Kalanur, S.S.; Pollet, B.G. Recent advancements in catalyst coated membranes for water electrolysis: A critical review. Energy Adv. 2024, 3, 1144–1166. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, T.; Chang, Q.; Ma, C.; Yang, Y.; Wang, S.; Pan, Z.; Sun, Y.; Ding, G. Performance stability and regeneration property of catalytic membranes coupled with advanced oxidation process: A comprehensive review. Sustainability 2023, 15, 7556. [Google Scholar] [CrossRef]
- Chen, C.; Lu, L.; Fei, L.; Xu, J.; Wang, B.; Li, B.; Shen, L.; Lin, H. Membrane-catalysis integrated system for contaminants degradation and membrane fouling mitigation: A review. Sci. Total Environ. 2023, 904, 166220. [Google Scholar] [CrossRef]
- Yu, R.; Chen, W.; Zhang, J.; Liu, J.; Li, X.Y.; Lin, L. Catalytic membranes for water treatment: Perspectives and challenges. J. Hazard. Mater. Adv. 2024, 13, 100414. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Miura, T.; Shizuya, K.; Wakazono, S.; Tokunaga, K.; Kambara, S. Hydrogen production system combined with a catalytic reactor and a plasma membrane reactor from ammonia. Int. J. Hydrogen Energy 2019, 44, 9987–9993. [Google Scholar] [CrossRef]
- Stenina, I.; Yaroslavtsev, A. Modern technologies of hydrogen production. Processes 2023, 11, 56. [Google Scholar] [CrossRef]
- Gapp, E.; Pfeifer, P. Membrane reactors for hydrogen production from renewable energy sources. Curr. Opin. Green Sustain. Chem. 2023, 41, 100800. [Google Scholar] [CrossRef]
- Antolini, E. Palladium in fuel cell catalysis. Energy Environ. Sci. 2009, 2, 915–931. [Google Scholar] [CrossRef]
- Zhang, L.; Chang, Q.; Chen, H.; Shao, M. Recent advances in palladium-based electrocatalysts for fuel cell reactions and hydrogen evolution reaction. Nano Energy 2016, 29, 198–219. [Google Scholar] [CrossRef]
- Che Ramli, Z.A.; Pasupuleti, J.; Tengku Saharuddin, T.S.; Yusoff, Y.N.; Isahak, W.N.R.W.; Baharudin, L.; Tak Yaw, C.; Koh, S.P.; Tiong Kiong, S. Electrocatalytic activities of platinum and palladium catalysts for enhancement of direct formic acid fuel cells: An updated progress. Alex. Eng. J. 2023, 76, 701–733. [Google Scholar] [CrossRef]
- Schwarzer, M.; Hertl, N.; Nitz, F.; Borodin, D.; Fingerhut, J.; Kitsopoulos, T.N.; Wodtke, A.M. Adsorption and absorption energies of hydrogen with palladium. J. Phys. Chem. C 2022, 126, 14500–14508. [Google Scholar] [CrossRef] [PubMed]
- Osada, W.; Tanaka, S.; Mukai, K.; Kawamura, M.; Choi, Y.; Ozaki, F.; Ozaki, T.; Yoshinobu, J. Elucidation of the atomic-scale processes of dissociative adsorption and spillover of hydrogen on the single atom alloy catalyst Pd/Cu(111). Phys. Chem. Chem. Phys. 2022, 24, 21705–21713. [Google Scholar] [CrossRef]
- Chaplin, B.P.; Reinhard, M.; Schneider, W.F.; Schüth, C.; Shapley, J.R.; Strathmann, T.J.; Werth, C.J. Critical review of Pd-based catalytic treatment of priority contaminants in water. Environ. Sci. Technol. 2012, 46, 3655–3670. [Google Scholar] [CrossRef]
- Bhat, I.U.H.; Anwar, M.N.K.; Yusoff, N.R.B.N.; Rak, A.A.L.E. Palladium catalyst for treatment of inorganic and organic pollutants in wastewater: A short review. Desalination Water Treat. 2018, 131, 132–140. [Google Scholar] [CrossRef]
- Liu, J.; Gao, J. Catalytic reduction of water pollutants: Knowledge gaps, lessons learned, and new opportunities. Front. Environ. Sci. Eng. 2023, 17, 26. [Google Scholar] [CrossRef]
- Wang, T.; Cao, X.; Jiao, L. PEM water electrolysis for hydrogen production: Fundamentals, advances, and prospects. Carbon Neutrality 2022, 1, 21. [Google Scholar] [CrossRef]
- Hu, B.; Shu, R.; Khairun, H.S.; Tian, Z.; Wang, C.; Kumar Gupta, N. Methanol steam reforming for hydrogen production over Ni-based catalysts: State-of-the-art review and future prospects. Chem. Asian J. 2024, 19, e202400217. [Google Scholar] [CrossRef]
- Abbass, K.; Qasim, M.Z.; Song, H.; Murshed, M.; Mahmood, H.; Younis, I. A review of the global climate change impacts, adaptation, and sustainable mitigation measures. Environ. Sci. Pollut. Res. 2022, 29, 42539–42559. [Google Scholar] [CrossRef]
- Bauer, C.; Treyer, K.; Antonini, C.; Bergerson, J.; Gazzani, M.; Gencer, E.; Gibbins, J.; Mazzotti, M.; McCoy, S.T.; McKenna, R.; et al. On the climate impacts of blue hydrogen production. Sustain. Energy Fuels 2022, 6, 66–75. [Google Scholar] [CrossRef]
- Scheuing, H.; Kamm, J. The EU on the road to climate neutrality—Is the ‘Fit for 55’ package fit for purpose? Renew. Energy Law Policy Rev. 2022, 10, 4–18. [Google Scholar]
- Cho, H.H.; Strezov, V.; Evans, T.J. A review on global warming potential, challenges and opportunities of renewable hydrogen production technologies. Sustain. Mater. Technol. 2023, 35, e00567. [Google Scholar] [CrossRef]
- Pearre, N.; Swan, L. Reimagining renewable electricity grid management with dispatchable generation to stabilize energy storage. Energy 2020, 203, 117917. [Google Scholar] [CrossRef]
- Alent’ev, A.Y.; Volkov, A.V.; Vorotyntsev, I.V.; Maksimov, A.L.; Yaroslavtsev, A.B. Membrane technologies for decarbonization. Membr. Membr. Technol. 2021, 3, 255–273. [Google Scholar] [CrossRef]
- Beccarello, M.; Di Foggia, G. Review and perspectives of key decarbonization drivers to 2030. Energies 2023, 16, 1345. [Google Scholar] [CrossRef]
- Johnson, M.P.; Rötzel, T.S.; Frank, B. Beyond conventional corporate responses to climate change towards deep decarbonization: A systematic literature review. Manag. Rev. Q. 2023, 73, 921–924. [Google Scholar] [CrossRef]
- Dehghani-Sanij, A.R.; Tharumalingam, E.; Dusseault, M.B.; Fraser, R. Study of energy storage systems and environmental challenges of batteries. Renew. Sustain. Energy Rev. 2019, 104, 192–208. [Google Scholar] [CrossRef]
- Yaroslavtsev, A.B.; Stenina, I.A.; Golubenko, D.V. Membrane materials for energy production and storage. Pure Appl. Chem. 2020, 92, 1147–1157. [Google Scholar] [CrossRef]
- Song, J.; Wei, C.; Huang, Z.-F.; Liu, C.; Zeng, L.; Wang, X.; Xu, Z.J. A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 2020, 49, 2196–2214. [Google Scholar] [CrossRef]
- Kojima, H.; Nagasawa, K.; Todoroki, N.; Ito, Y.; Matsui, T.; Nakajima, R. Influence of renewable energy power fluctuations on water electrolysis for green hydrogen production. Int. J. Hydrogen Energy 2023, 48, 4572–4593. [Google Scholar] [CrossRef]
- Fan, L.; Tu, Z.; Chan, S.H. Recent development in design a state-of-art proton exchange membrane fuel cell from stack to system: Theory, integration and prospective. Int. J. Hydrogen Energy 2023, 48, 7828–7865. [Google Scholar] [CrossRef]
- Le, P.A.; Trung, V.D.; Nguyen, P.L.; Bac Phung, T.V.; Natsuki, J.; Natsuki, T. The current status of hydrogen energy: An overview. RSC Adv. 2023, 13, 28262–28287. [Google Scholar] [CrossRef] [PubMed]
- Capurso, T.; Stefanizzi, M.; Torresi, M.; Camporeale, S.M. Perspective of the role of hydrogen in the 21st century energy transition. Energy Convers. Manag. 2022, 251, 114898. [Google Scholar] [CrossRef]
- Qazi, U.Y. Future of hydrogen as an alternative fuel for next-generation industrial applications; challenges and expected opportunities. Energies 2022, 15, 4741. [Google Scholar] [CrossRef]
- Stenina, I.A.; Yaroslavtsev, A.B. Prospects for the development of hydrogen energy. Polymer membranes for fuel cells and electrolyzers. Membr. Membr. Technol. 2024, 6, 15–26. [Google Scholar] [CrossRef]
- Bloomberg Finance. Hydrogen Economy Outlook: Key Messages, 30 March 2020. Available online: https://data.bloomberglp.com/professional/sites/24/BNEF-Hydrogen-Economy-Outlook-Key-Messages-30-Mar-2020.pdf (accessed on 30 March 2020).
- Zainal, B.S.; Ker, P.J.; Mohamed, H.; Ong, H.C.; Fattah, I.M.R.; Rahman, S.M.A.; Nghiem, L.D.; Mahlia, T.M.I. Recent advancement and assessment of green hydrogen production technologies. Renew. Sustain. Energy Rev. 2024, 189, 113941. [Google Scholar] [CrossRef]
- Yukesh Kannah, R.; Kavitha, S.; Preethi; Parthiba Karthikeyan, O.; Kumar, G.; Dai-Viet, N.V.; Rajesh Banu, J. Techno-economic assessment of various hydrogen production methods—A review. Bioresour. Technol. 2021, 319, 124175. [Google Scholar] [CrossRef]
- Agyekum, E.B.; Nutakor, C.; Agwa, A.M.; Kamel, S. A critical review of renewable hydrogen production methods: Factors affecting their scale-up and its role in future energy generation. Membranes 2022, 12, 173. [Google Scholar] [CrossRef]
- Ishaq, H.; Dincer, I.; Crawford, C. A review on hydrogen production and utilization: Challenges and opportunities. Int. J. Hydrogen Energy 2022, 47, 26238–26264. [Google Scholar] [CrossRef]
- Sarmah, M.K.; Singh, T.P.; Kalita, P.; Dewan, A. Sustainable hydrogen generation and storage—A review. RSC Adv. 2023, 13, 25253–25275. [Google Scholar] [CrossRef] [PubMed]
- Fajín, J.L.C.; Cordeiro, M.N.D.S. Renewable hydrogen production from biomass derivatives or water on trimetallic based catalysts. Renew. Sustain. Energy Rev. 2024, 189, 113909. [Google Scholar] [CrossRef]
- Iulianelli, A.; Liguori, S.; Wilcox, J.; Basile, A. Advances on methane steam reforming to produce hydrogen through membrane reactors technology: A review. Catal. Rev. 2016, 58, 1–35. [Google Scholar] [CrossRef]
- Antzaras, A.N.; Lemonidou, A.A. Recent advances on materials and processes for intensified production of blue hydrogen. Renew. Sustain. Energy Rev. 2022, 155, 111917. [Google Scholar] [CrossRef]
- Jokar, S.M.; Farokhnia, A.; Tavakolian, M.; Pejman, M.; Parvasi, P.; Javanmardi, J.; Zare, F.; Gonçalves, M.C.; Basile, A. The recent areas of applicability of palladium based membrane technologies for hydrogen production from methane and natural gas: A review. Int. J. Hydrogen Energy 2023, 48, 6451–6476. [Google Scholar] [CrossRef]
- Jeppesen, C.; Polverino, P.; Andreasen, S.J.; Araya, S.S.; Sahlin, S.L.; Pianese, C.; Kær, S.K. Impedance characterization of high temperature proton exchange membrane fuel cell stack under the influence of carbon monoxide and methanol vapor. Int. J. Hydrogen Energy 2017, 42, 21901–21912. [Google Scholar] [CrossRef]
- Sutharssan, T.; Montalvao, D.; Chen, Y.K.; Wang, W.-C.; Pisac, C.; Elemara, H. A review on prognostics and health monitoring of proton exchange membrane fuel cell. Renew. Sustain. Energy Rev. 2017, 75, 440–450. [Google Scholar] [CrossRef]
- Li, L.; Md Dostagir, N.H.; Shrotri, A.; Fukuoka, A.; Kobayashi, H. Partial oxidation of methane to syngas via formate intermediate found for a ruthenium-rhenium bimetallic catalyst. ACS Catal. 2021, 11, 3782–3789. [Google Scholar] [CrossRef]
- Alhassan, M.; Jalil, A.A.; Nabgan, W.; Hamid, M.Y.S.; Bahari, M.B.; Ikram, M. Bibliometric studies and impediments to valorization of dry reforming of methane for hydrogen production. Fuel 2022, 328, 125240. [Google Scholar] [CrossRef]
- Khatun, R.; Bhandari, S.; Poddar, M.K.; Samanta, C.; Khan, T.S.; Khurana, D.; Bal, R. Partial oxidation of methane over high coke-resistant bimetallic Pt-Ni/CeO2 catalyst: Profound influence of Pt addition on stability. Int. J. Hydrogen Energy 2022, 47, 38895–38909. [Google Scholar] [CrossRef]
- Wachter, P.; Hödl, P.; Raic, J.; Gaber, C.; Demuth, M.; Hochenauer, C. Towards thermochemical recuperation applying combined steam reforming and partial oxidation of methane: Thermodynamic and experimental considerations. Energy Convers. Manag. 2022, 251, 114927. [Google Scholar] [CrossRef]
- Dan, M.; Mihet, M.; Borodi, G.; Lazar, M.D. Combined steam and dry reforming of methane for syngas production from biogas using bimodal pore catalysts. Catal. Today 2021, 366, 87–96. [Google Scholar] [CrossRef]
- Nedolivko, V.V.; Zasypalov, G.O.; Vutolkina, A.V.; Gushchin, P.A.; Vinokurov, V.A.; Kulikov, L.A.; Egazar’yants, S.V.; Karakhanov, E.A.; Maksimov, A.L.; Glotov, A.P. Carbon dioxide reforming of methane. Russ. J. Appl. Chem. 2020, 93, 765–787. [Google Scholar] [CrossRef]
- Wittich, K.; Krämer, M.; Bottke, N.; Schunk, S.A. Catalytic dry reforming of methane: Insights from model systems. ChemCatChem 2020, 12, 2130–2147. [Google Scholar] [CrossRef]
- Qian, J.X.; Chen, T.W.; Enakonda, L.R.; Liu, D.B.; Mignani, G.; Basset, J.M.; Zhou, L. Methane decomposition to produce COx-free hydrogen and nano-carbon over metal catalysts: A review. Int. J. Hydrogen Energy 2020, 45, 7981–8001. [Google Scholar] [CrossRef]
- Naikoo, G.A.; Arshad, F.; Hassan, I.U.; Tabook, M.A.; Pedram, M.Z.; Mustaqeem, M.; Tabassum, H.; Ahmed, W.; Rezakazemi, M. Thermocatalytic hydrogen production through decomposition of methane—A review. Front. Chem. 2021, 9, 736801. [Google Scholar] [CrossRef]
- Sánchez-Bastardo, N.; Schlögl, R.; Ruland, H. Methane pyrolysis for zero-emission hydrogen production: A potential bridge technology from fossil fuels to a renewable and sustainable hydrogen economy. Ind. Eng. Chem. Res. 2021, 60, 11855–11881. [Google Scholar] [CrossRef]
- Lee, B.; Heo, J.; Kim, S.; Sung, C.; Moon, C.; Moon, S.; Lim, H. Economic feasibility studies of high pressure PEM water electrolysis for distributed H2 refueling stations. Energy Convers. Manag. 2018, 162, 139–144. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Pinsky, R.; Sabharwall, P.; Hartvigsen, J.; O’Brien, J. Comparative review of hydrogen production technologies for nuclear hybrid energy systems. Prog. Nucl. Energy 2020, 123, 103317. [Google Scholar] [CrossRef]
- Ji, M.; Wang, J. Review and comparison of various hydrogen production methods based on costs and life cycle impact assessment indicators. Int. J. Hydrogen Energy 2021, 46, 38612–38635. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, Z.; Farouq Ali, S.M. Thermal-hydro-chemical-mechanical alteration of coal pores in underground coal gasification. Fuel 2020, 262, 116543. [Google Scholar] [CrossRef]
- Munyentwali, A.; Tan, K.C.; He, T. Advancements in the development of liquid organic hydrogen carrier systems and their applications in the hydrogen economy. Prog. Nat. Sci. Mater. Int. 2024, 34, 825–839. [Google Scholar] [CrossRef]
- Kustov, L.M.; Kalenchuk, A.N.; Bogdan, V.I. Systems for accumulation, storage and release of hydrogen. Russ. Chem. Rev. 2020, 89, 897–916. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, C.; Li, W.; Li, W.; Qiu, M.; Chen, X.; Wang, H.; Sun, Y. Ultralow Rh bimetallic catalysts with high catalytic activity for the hydrogenation of N-ethylcarbazole. ACS Sustain. Chem. Eng. 2021, 9, 5260–5267. [Google Scholar] [CrossRef]
- Mollar-Cuni, A.; Ventura-Espinosa, D.; Martín, S.; García, H.; Mata, J.A. Reduced graphene oxides as carbocatalysts in acceptorless dehydrogenation of N-heterocycles. ACS Catal. 2021, 11, 14688–14693. [Google Scholar] [CrossRef]
- Mejuto, C.; Ibáñez-Ibáñez, L.; Guisado-Barrios, G.; Mata, J.A. Visible-light-promoted iridium(III)-catalyzed acceptorless dehydrogenation of N-heterocycles at room temperature. ACS Catal. 2022, 12, 6238–6245. [Google Scholar] [CrossRef]
- Song, H.; Yang, G.; Xue, P.; Li, Y.; Zou, J.; Wang, S.; Yang, H.; Chen, H. Recent development of biomass gasification for H2 rich gas production. Appl. Energy Combust. Sci. 2022, 10, 100059. [Google Scholar] [CrossRef]
- Lim, S.; Song, Y.; Jeong, K.; Park, J.H.; Na, K. Enhanced dehydrogenative H2 release from N-containing amphicyclic LOHC boosted by Pd-supported nanosheet MFI zeolites having strong acidity and large mesoporosity. ACS Sustain. Chem. Eng. 2022, 10, 3584–3594. [Google Scholar] [CrossRef]
- Itoh, N.; Kikuchi, Y.; Furusawa, T.; Sato, T. Tube-wall catalytic membrane reactor for hydrogen production by low-temperature ammonia decomposition. Int. J. Hydrogen Energy 2021, 46, 20257–20265. [Google Scholar] [CrossRef]
- Xu, X.; Liu, E.; Zhu, N.; Liu, F.; Qian, F. Review of the current status of ammonia-blended hydrogen fuel engine development. Energies 2022, 15, 1023. [Google Scholar] [CrossRef]
- Zhai, L.; Liu, S.; Xiang, Z. Ammonia as a carbon-free hydrogen carrier for fuel cells: A perspective. Ind. Chem. Mater. 2023, 1, 332–342. [Google Scholar] [CrossRef]
- Lytkina, A.A.; Orekhova, N.V.; Yaroslavtsev, A.B. Catalysts for the steam reforming and electrochemical oxidation of methanol. Inorg. Mater. 2018, 54, 1315–1329. [Google Scholar] [CrossRef]
- Kumar, A.; Daw, P.; Milstein, D. Homogeneous catalysis for sustainable energy: Hydrogen and methanol economies, fuels from biomass, and related topics. Chem. Rev. 2022, 122, 385–441. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Darmawan, A.; Juangsa, F.B. Hydrogen production from biomasses and wastes: A technological review. Int. J. Hydrogen Energy 2021, 46, 33756–33781. [Google Scholar] [CrossRef]
- Mironova, E.Y.; Lytkina, A.A.; Ermilova, M.M.; Efimov, M.N.; Zemtsov, L.M.; Orekhova, N.V.; Karpacheva, G.P.; Bondarenko, G.N.; Muraviev, D.N.; Yaroslavtsev, A.B. Ethanol and methanol steam reforming on transition metal catalysts supported on detonation synthesis nanodiamonds for hydrogen production. Int. J. Hydrogen Energy 2015, 40, 3557–3565. [Google Scholar] [CrossRef]
- Yang, W.-W.; Ma, X.; Tang, X.-Y.; Dou, P.-Y.; Yang, Y.-J.; He, Y.-L. Review on developments of catalytic system for methanol steam reforming from the perspective of energy-mass conversion. Fuel 2023, 345, 128234. [Google Scholar] [CrossRef]
- Achomo, M.A.; Kumar, A.; Peela, N.R.; Muthukumar, P. Hydrogen production from steam reforming of methanol: A comprehensive review on thermodynamics, catalysts, reactors, and kinetic studies. Int. J. Hydrogen Energy 2024, 58, 1640–1672. [Google Scholar] [CrossRef]
- Cao, A.N.T.; Ng, K.H.; Ahmed, S.F.; Nguyen, H.T.; Kumar, P.S.; Tran, H.T.; Rajamohan, N.; Yusuf, M.; Show, P.L.; Balakrishnan, A.; et al. Hydrogen generation by heterogeneous catalytic steam reforming of short-chain alcohols: A review. Environ. Chem. Lett. 2024, 22, 561–583. [Google Scholar] [CrossRef]
- Jahromi, A.F.; Ruiz-López, E.; Dorado, F.; Baranova, E.A.; de Lucas-Consuegra, A. Electrochemical promotion of ethanol partial oxidation and reforming reactions for hydrogen production. Renew. Energy 2022, 183, 515–523. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, H.; Wang, Z. Experimental study on improving the efficiency of hydrogen production by partial oxidation of ethanol. Int. J. Hydrogen Energy 2022, 47, 18619–18628. [Google Scholar] [CrossRef]
- François, E.; Dumas, C.; Gougeon, R.D.; Alexandre, H.; Vuilleumier, S.; Ernst, B. Unexpected high production of biohydrogen from the endogenous fermentation of grape must deposits. Bioresour. Technol. 2021, 320, 124334. [Google Scholar] [CrossRef]
- Policastro, G.; Lamboglia, R.; Fabbricino, M.; Pirozzi, F. Enhancing dark fermentative hydrogen production from problematic substrates via the co-fermentation strategy. Fermentation 2022, 8, 706. [Google Scholar] [CrossRef]
- Cao, W.; Wei, X.; Jiang, Y.; Feng, J.; Gao, Z.; Tang, C. Furfural influences hydrogen evolution and energy conversion in photo-fermentation by Rhodobacter capsulatus. Catalysts 2022, 12, 979. [Google Scholar] [CrossRef]
- Guilhaume, N.; Bianchi, D.; Wandawa, R.A.; Yin, W.; Schuurman, Y. Study of CO2 and H2O adsorption competition in the combined dry/steam reforming of biogas. Catal. Today 2021, 375, 282–289. [Google Scholar] [CrossRef]
- Siang, T.J.; Jalil, A.A.; Liew, S.Y.; Owgi, A.H.K.; Rahman, A.F.A. A review on state-of-the-art catalysts for methane partial oxidation to syngas production. Catal. Rev. 2024, 66, 343–399. [Google Scholar] [CrossRef]
- Zhang, J.; Men, Y.; Wang, Y.; Liao, L.; Liu, S.; Wang, J.; An, W. Morphology effect of Pd/In2O3/CeO2 catalysts on methanol steam reforming for hydrogen production. Int. J. Hydrogen Energy 2024, 51, 1185–1199. [Google Scholar] [CrossRef]
- Vogt, C.; Kranenborg, J.; Monai, M.; Weckhuysen, B.M. Structure sensitivity in steam and dry methane reforming over nickel: Activity and carbon formation. ACS Catal. 2020, 10, 1428–1438. [Google Scholar] [CrossRef]
- Wu, Y.; Pei, C.; Tian, H.; Liu, T.; Zhang, X.; Chen, S.; Xiao, Q.; Wang, X.; Gong, J. Role of Fe species of Ni-based catalysts for efficient low-temperature ethanol steam reforming. JACS Au 2021, 1, 1459–1470. [Google Scholar] [CrossRef]
- Ye, R.; Xiao, S.; Lai, Q.; Wang, D.; Huang, Y.; Feng, G.; Zhang, R.; Wang, T. Advances in enhancing the stability of Cu-based catalysts for methanol reforming. Catalysts 2022, 12, 747. [Google Scholar] [CrossRef]
- Chernyshev, V.M.; Ananikov, V.P. Nickel and palladium catalysis: Stronger demand than ever. ACS Catal. 2022, 12, 1180–1200. [Google Scholar] [CrossRef]
- Kappis, K.; Papavasiliou, J.; Kuśmierz, M.; Słowik, G.; Li, Y.; Li, H.; Gac, W.; Avgouropoulos, G. Steam reforming of methanol over combustion synthesized CuZnOx-based catalysts for fuel cell applications. Chem. Eng. J. 2023, 461, 142098. [Google Scholar] [CrossRef]
- Mao, Q.; Gao, Z.; Liu, X.; Guo, Y.; Wang, Y.; Ma, D. The Cu–Al2O3 interface: An unignorable active site for methanol steam reforming hydrogen production. Catal. Sci. Technol. 2024, 14, 3448–3458. [Google Scholar] [CrossRef]
- Guo, C.; Li, M.; Guo, W.; Xie, J.; Qin, H.; Liao, M.; Zhang, Y.; Gao, P.; Xiao, H. Quench-induced Cu-ZnO catalyst for hydrogen production from methanol steam reforming. Chem. Eng. J. 2024, 486, 150331. [Google Scholar] [CrossRef]
- Shu, Q.; Zhang, Q.; Zhu, X. Enhancing activation and stability of core-shell CuZn catalyst by ZnOx oxygen vacancies for methanol steam reforming. Appl. Catal. A Gen. 2024, 678, 119652. [Google Scholar] [CrossRef]
- Jiang, W.; Ma, X.; Zhang, D.; Li, Z.; Fu, P. Highly efficient catalysts for hydrogen generation through methanol steam reforming: A critical analysis of modification strategies, deactivation, mechanisms and kinetics. J. Ind. Eng. Chem. 2024, 130, 54–72. [Google Scholar] [CrossRef]
- Huang, J.; Ning, S.; Luo, B.; Wang, Z.; Deng, W.; Zhao, B.; Su, Y. Copper-based catalysts supported on novel Metal-Organic Framework MIL-125(Ti) for selective catalytic reduction of NO with CO. Fuel 2024, 364, 131167. [Google Scholar] [CrossRef]
- Lytkina-Payen, A.; Tabachkova, N.; Yaroslavtsev, A. Methanol steam reforming on bimetallic catalysts based on in and nb doped titania or zirconia: A support effect. Processes 2022, 10, 19. [Google Scholar] [CrossRef]
- Mitchell, S.; Pérez-Ramírez, J. Single atom catalysis: A decade of stunning progress and the promise for a bright future. Nat. Commun. 2020, 11, 10–12. [Google Scholar] [CrossRef]
- Hannagan, R.T.; Giannakakis, G.; Flytzani-Stephanopoulos, M.; Sykes, E.C.H. Single-atom alloy catalysis. Chem. Rev. 2020, 120, 12044–12088. [Google Scholar] [CrossRef]
- Abiso, A.M.; Fasanya, O.O.; Suleiman, M.Y.; Atta, A.Y.; Dutta, J.; Jibril, B.E.Y. Advances in copper-based catalysts for sustainable hydrogen production via methanol steam reforming. Chem. Eng. J. Adv. 2024, 19, 100625. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, R.; Zhang, X.; Zhao, H. Recent advances in single-atom alloys: Preparation methods and applications in heterogeneous catalysis. RSC Adv. 2024, 14, 3936–3951. [Google Scholar] [CrossRef] [PubMed]
- Lytkina, A.A.; Orekhova, N.V.; Ermilova, M.M.; Yaroslavtsev, A.B. The influence of the support composition and structure (MXZr1-XO2-δ) of bimetallic catalysts on the activity in methanol steam reforming. Int. J. Hydrogen Energy 2018, 43, 198–207. [Google Scholar] [CrossRef]
- Taherian, Z.; Khataee, A.; Han, N.; Orooji, Y. Hydrogen production through methane reforming processes using promoted-Ni/mesoporous silica: A review. J. Ind. Eng. Chem. 2022, 107, 20–30. [Google Scholar] [CrossRef]
- Zhou, F.; Zhang, R.; Li, H.; Liu, J.; Wu, Y.; Toan, S.; Sun, Z. The critical role of Ga doped Cu/Al2O3 aerogels in carbon monoxide suppression during steam reforming of methanol. Fuel Process. Technol. 2023, 249, 107792. [Google Scholar] [CrossRef]
- De Maron, J.; Mafessanti, R.; Gramazio, P.; Orfei, E.; Fasolini, A.; Basile, F. H2 production by methane oxy-reforming: Effect of catalyst pretreatment on the properties and activity of Rh-Ce0.5Zr0.5O2 synthetized by microemulsion. Nanomaterials 2023, 13, 53. [Google Scholar] [CrossRef]
- Zhang, J.; Su, D.S.; Blume, R.; Schlögl, R.; Wang, R.; Yang, X.; Gajović, A. Surface chemistry and catalytic reactivity of a nanodiamond in the steam-free dehydrogenation of ethylbenzene. Angew. Chem. Int. Ed. 2010, 49, 8640–8644. [Google Scholar] [CrossRef]
- Bepari, S.; Khan, M.; Li, X.; Mohammad, N.; Kuila, D. Effect of Ce and Zn on Cu-based mesoporous carbon catalyst for methanol steam reforming. Top. Catal. 2023, 66, 375–392. [Google Scholar] [CrossRef]
- Lytkina, A.A.; Orekhova, N.V.; Ermilova, M.M.; Belenov, S.V.; Guterman, V.E.; Efimov, M.N.; Yaroslavtsev, A.B. Bimetallic carbon nanocatalysts for methanol steam reforming in conventional and membrane reactors. Catal. Today 2016, 268, 60–67. [Google Scholar] [CrossRef]
- Hu, G.; Wang, J.; Liu, D.; Zhang, X.; Yu, B.; Huang, T.; Zhu, M.; Yu, H. A Joule-heated carbon nanofiber aerogel-supported catalyst for hydrogen production via methanol steam reforming. Carbon 2023, 214, 118311. [Google Scholar] [CrossRef]
- Lytkina, A.A.; Orekhova, N.V.; Ermilova, M.M.; Petriev, I.S.; Baryshev, M.G.; Yaroslavtsev, A.B. Ru-Rh based catalysts for hydrogen production via methanol steam reforming in conventional and membrane reactors. Int. J. Hydrogen Energy 2019, 44, 13310–13322. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Lim, H. An overview of water electrolysis technologies for green hydrogen production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Teng, T.; Zhang, X.; Xue, Q.; Zhang, B. Research of proton exchange membrane fuel cell modeling on concentration polarization under variable-temperature operating conditions. Energies 2024, 17, 730. [Google Scholar] [CrossRef]
- AlZohbi, G. An overview of hydrogen energy generation. ChemEngineering 2024, 8, 17. [Google Scholar] [CrossRef]
- Apel, P.Y.; Biesheuvel, P.M.; Bobreshova, O.V.; Borisov, I.L.; Vasil’eva, V.I.; Volkov, V.V.; Grushevenko, E.A.; Nikonenko, V.V.; Parshina, A.V.; Pismenskaya, N.D.; et al. Concentration polarization in membrane systems. Membr. Membr. Technol. 2024, 6, 133–161. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Xu, L.; Hu, Z.; Zhao, G.; Sun, H.; Li, J.; Ouyang, M. Polarization decomposing of proton exchange membrane fuel cell considering liquid water accumulation. J. Electrochem. Soc. 2022, 169, 124517. [Google Scholar] [CrossRef]
- El-Shafie, M.; Kambara, S.; Hayakawa, Y. Hydrogen production technologies overview. J. Power Energy Eng. 2019, 7, 107–154. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Hauch, A.; Küngas, R.; Blennow, P.; Hansen, A.B.; Hansen, J.B.; Mathiesen, B.V.; Mogensen, M.B. Recent advances in solid oxide cell technology for electrolysis. Science 2020, 370, eaba6118. [Google Scholar] [CrossRef]
- Ahmad Kamaroddin, M.F.; Sabli, N.; Tuan Abdullah, T.A.; Siajam, S.I.; Abdullah, L.C.; Abdul Jalil, A.; Ahmad, A. Membrane-based electrolysis for hydrogen production: A review. Membranes 2021, 11, 810. [Google Scholar] [CrossRef]
- Mehanovic, D.; Al-Haiek, A.; Leclerc, P.; Rancourt, D.; Fréchette, L.; Picard, M. Energetic, GHG, and economic analyses of electrified steam methane reforming using conventional reformer tubes. Energy Convers. Manag. 2023, 276, 116549. [Google Scholar] [CrossRef]
- Das, G.; Choi, J.H.; Nguyen, P.K.T.; Kim, D.J.; Yoon, Y.S. Anion exchange membranes for fuel cell application: A review. Polymers 2022, 14, 1197. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Weber, A.Z. Ionomer optimization for hydroxide-exchange-membrane water electrolyzers operated with distilled water: A modeling study. J. Electrochem. Soc. 2022, 169, 054506. [Google Scholar] [CrossRef]
- Du, N.; Roy, C.; Peach, R.; Turnbull, M.; Thiele, S.; Bock, C. Anion-exchange membrane water electrolyzers. Chem. Rev. 2022, 122, 11830–11895. [Google Scholar] [CrossRef] [PubMed]
- Ďurovič, M.; Hnát, J.; Strečková, M.; Bouzek, K. Efficient cathode for the hydrogen evolution reaction in alkaline membrane water electrolysis based on NiCoP embedded in carbon fibres. J. Power Sources 2023, 556, 232506. [Google Scholar] [CrossRef]
- Fallah Vostakola, M.; Ozcan, H.; El-Emam, R.S.; Amini Horri, B. Recent advances in high-temperature steam electrolysis with solid oxide electrolysers for green hydrogen production. Energies 2023, 16, 3327. [Google Scholar] [CrossRef]
- Hammi, Z.; Labjar, N.; Lotfi, E.M.; El Hajjaji, S. Production of green hydrogen employing proton exchange membrane water electrolyzer: Characterization of electrolyte membrane. A critical review. J. Membr. Sci. Res. 2023, 9, 1978109. [Google Scholar] [CrossRef]
- Kadier, A.; Kalil, M.S.; Abdeshahian, P.; Chandrasekhar, K.; Mohamed, A.; Azman, N.F.; Logroño, W.; Simayi, Y.; Hamid, A.A. Recent advances and emerging challenges in microbial electrolysis cells (MECs) for microbial production of hydrogen and value-added chemicals. Renew. Sustain. Energy Rev. 2016, 61, 501–525. [Google Scholar] [CrossRef]
- Rousseau, R.; Etcheverry, L.; Roubaud, E.; Basséguy, R.; Délia, M.-L.; Bergel, A. Microbial electrolysis cell (MEC): Strengths, weaknesses and research needs from electrochemical engineering standpoint. Appl. Energy 2020, 257, 113938. [Google Scholar] [CrossRef]
- Durakovic, G.; del Granado, P.C.; Tomasgard, A. Are green and blue hydrogen competitive or complementary? Insights from a decarbonized European power system analysis. Energy 2023, 282, 128282. [Google Scholar] [CrossRef]
- Arcos, J.M.M.; Santos, D.M.F. The hydrogen color spectrum: Techno-economic analysis of the available technologies for hydrogen production. Gases 2023, 3, 25–46. [Google Scholar] [CrossRef]
- Filippov, S.P.; Keiko, A.V. Coal gasification: At the crossroad. technological factors. Therm. Eng. 2021, 68, 209–220. [Google Scholar] [CrossRef]
- Ustolin, F.; Paltrinieri, N.; Berto, F. Loss of integrity of hydrogen technologies: A critical review. Int. J. Hydrogen Energy 2020, 45, 23809–23840. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Hefny, M.; Al-Hinai, A.; Al-Muhtaseb, A.H.; Rooney, D.W. Hydrogen production, storage, utilisation and environmental impacts: A review. Environ. Chem. Lett. 2022, 20, 153–188. [Google Scholar] [CrossRef]
- Katebah, M.; Al-Rawashdeh, M.; Linke, P. Analysis of hydrogen production costs in Steam-Methane Reforming considering integration with electrolysis and CO2 capture. Clean. Eng. Technol. 2022, 10, 100552. [Google Scholar] [CrossRef]
- Jiang, L.; Xue, D.; Wei, Z.; Chen, Z.; Mirzayev, M.; Chen, Y.; Chen, S. Coal decarbonization: A state-of-the-art review of enhanced hydrogen production in underground coal gasification. Energy Rev. 2022, 1, 100004. [Google Scholar] [CrossRef]
- Dash, S.K.; Chakraborty, S.; Elangovan, D. A brief review of hydrogen production methods and their challenges. Energies 2023, 16, 1141. [Google Scholar] [CrossRef]
- Martínez de León, C.; Ríos, C.; Brey, J.J. Cost of green hydrogen: Limitations of production from a stand-alone photovoltaic system. Int. J. Hydrogen Energy 2023, 48, 11885–11898. [Google Scholar] [CrossRef]
- IEA. Global Hydrogen Review 2023; OECD Publishing: Paris, France, 2023. [Google Scholar] [CrossRef]
- Du, Z.; Liu, C.; Zhai, J.; Guo, X.; Xiong, Y.; Su, W.; He, G. A review of hydrogen purification technologies for fuel cell vehicles. Catalysts 2021, 11, 393. [Google Scholar] [CrossRef]
- Markowski, J.; Wojtasik, M. Piroliza metanu na tle wybranych metod otrzymywania wodoru. Nafta-Gaz 2023, 79, 428–435. [Google Scholar] [CrossRef]
- Kim, T.; Song, Y.; Kang, J.; Kim, S.K.; Kim, S. A review of recent advances in hydrogen purification for selective removal of oxygen: Deoxo catalysts and reactor systems. Int. J. Hydrogen Energy 2022, 47, 24817–24834. [Google Scholar] [CrossRef]
- Risco-Bravo, A.; Varela, C.; Bartels, J.; Zondervan, E. From green hydrogen to electricity: A review on recent advances, challenges, and opportunities on power-to-hydrogen-to-power systems. Renew. Sustain. Energy Rev. 2024, 189, 113930. [Google Scholar] [CrossRef]
- Li, H.; Liao, Z.; Sun, J.; Jiang, B.; Wang, J.; Yang, Y. Modelling and simulation of two-bed PSA process for separating H2 from methane steam reforming. Chin. J. Chem. Eng. 2019, 27, 1870–1878. [Google Scholar] [CrossRef]
- Yáñez, M.; Relvas, F.; Ortiz, A.; Gorri, D.; Mendes, A.; Ortiz, I. PSA purification of waste hydrogen from ammonia plants to fuel cell grade. Sep. Purif. Technol. 2020, 240, 116334. [Google Scholar] [CrossRef]
- Vermaak, L.; Neomagus, H.W.J.P.; Bessarabov, D.G. Recent advances in membrane-based electrochemical hydrogen separation: A review. Membranes 2021, 11, 127. [Google Scholar] [CrossRef]
- Panda, P.K.; Sahoo, B.; Ramakrishna, S. Hydrogen production, purification, storage, transportation, and their applications: A review. Energy Technol. 2023, 11, 2201434. [Google Scholar] [CrossRef]
- Sidhikku Kandath Valappil, R.; Ghasem, N.; Al-Marzouqi, M. Current and future trends in polymer membrane-based gas separation technology: A comprehensive review. J. Ind. Eng. Chem. 2021, 98, 103–129. [Google Scholar] [CrossRef]
- Alentiev, A.Y.; Ryzhikh, V.E.; Syrtsova, D.A.; Belov, N.A. Polymer materials for solving actual problems of membrane gas. Russ. Chem. Rev. 2023, 92, RCR5083. [Google Scholar] [CrossRef]
- Gao, J.; Song, Y.; Jia, C.; Sun, L.; Wang, Y.; Wang, Y.; Kipper, M.J.; Huang, L.; Tang, J. A comprehensive review of recent developments and challenges for gas separation membranes based on two-dimensional materials. FlatChem 2024, 43, 100594. [Google Scholar] [CrossRef]
- Li, P.; Wang, Z.; Qiao, Z.; Liu, Y.; Cao, X.; Li, W.; Wang, J.; Wang, S. Recent developments in membranes for efficient hydrogen purification. J. Membr. Sci. 2015, 495, 130–168. [Google Scholar] [CrossRef]
- Sazali, N.; Mohamed, M.A.; Salleh, W.N.W. Membranes for hydrogen separation: A significant review. Int. J. Adv. Manuf. Technol. 2020, 107, 1859–1881. [Google Scholar] [CrossRef]
- Lu, H.T.; Li, W.; Miandoab, E.S.; Kanehashi, S.; Hu, G. The opportunity of membrane technology for hydrogen purification in the power to hydrogen (P2H) roadmap: A review. Front. Chem. Sci. Eng. 2021, 15, 464–482. [Google Scholar] [CrossRef]
- Al- Janabi, S.K.; Barron, A.R.; Shabbani, H.J.K.; Othman, M.R.; Kim, J. Advances in hydrogen production from sustainable resources through biological and thermochemical pathways: Review and bibliometric analysis. Int. J. Hydrogen Energy 2024, 60, 28–45. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane gas separation: A review/state of the art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Robeson, L.M.; Smith, Z.P.; Freeman, B.D.; Paul, D.R. Contributions of diffusion and solubility selectivity to the upper bound analysis for glassy gas separation membranes. J. Membr. Sci. 2014, 453, 71–83. [Google Scholar] [CrossRef]
- Yaroslavtsev, A.B.; Yampolskii, Y.P. Hybrid membranes containing inorganic nanoparticles. Mendeleev Commun. 2014, 24, 319–326. [Google Scholar] [CrossRef]
- Moral, G.; Ortiz, A.; Gorri, D.; Ortiz, I. Hydrogen recovery from industrial waste streams using Matrimid®/ZIF mixed matrix membranes. Int. J. Hydrogen Energy 2024, 51, 210–224. [Google Scholar] [CrossRef]
- Mao, D.; Griffin, J.M.; Dawson, R.; Fairhurst, A.; Bimbo, N. Metal organic frameworks for hydrogen purification. Int. J. Hydrogen Energy 2021, 46, 23380–23405. [Google Scholar] [CrossRef]
- Chen, S.; Wahiduzzaman, M.; Ji, T.; Liu, Y.; Li, Y.; Wang, C.; Sun, Y.; He, G.; Maurin, G.; Wang, S.; et al. Oriented titanium-MOF membrane for hydrogen purification. Angew. Chem. Int. Ed. 2025, 64, e202413701. [Google Scholar] [CrossRef]
- Fan, H.; Peng, M.; Strauss, I.; Mundstock, A.; Meng, H.; Caro, J. MOF-in-COF molecular sieving membrane for selective hydrogen separation. Nat. Commun. 2021, 12, 38. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Zhang, N.; Li, Z.; Bao, J.; Zhang, X.; He, G.; Chen, C.; Song, Y. Tunable nano-wrinked channels of reduced graphene oxide membranes for molecular sieving gas separation. Carbon 2024, 216, 118524. [Google Scholar] [CrossRef]
- Guo, Q.; Ghalei, B.; Qin, D.; Mizutani, D.; Joko, I.; Al-Aziz, H.; Higashino, T.; Ito, M.M.; Imahori, H.; Sivaniah, E. Graphene oxide-fullerene nanocomposite laminates for efficient hydrogen purification. Chem. Commun. 2023, 59, 10012–10015. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.-W.; Ha, J.; Oruganti, Y.; Moon, H.R. Hydrogen separation and purification with MOF-based materials. Mater. Chem. Front. 2021, 5, 4022–4041. [Google Scholar] [CrossRef]
- Al-Mufachi, N.A.; Rees, N.V.; Steinberger-Wilkens, R. Hydrogen selective membranes: A review of palladium-based dense metal membranes. Renew. Sustain. Energy Rev. 2015, 47, 540–551. [Google Scholar] [CrossRef]
- Król, A.; Gajec, M.; Holewa-Rataj, J.; Kukulska-Zając, E.; Rataj, M. Hydrogen purification technologies in the context of its utilization. Energies 2024, 17, 3794. [Google Scholar] [CrossRef]
- Fernandez, E.; Helmi, A.; Medrano, J.A.; Coenen, K.; Arratibel, A.; Melendez, J.; de Nooijer, N.C.A.; Spallina, V.; Viviente, J.L.; Zuñiga, J.; et al. Palladium based membranes and membrane reactors for hydrogen production and purification: An overview of research activities at Tecnalia and TU/e. Int. J. Hydrogen Energy 2017, 42, 13763–13776. [Google Scholar] [CrossRef]
- Iulianelli, A.; Jansen, J.C.; Esposito, E.; Longo, M.; Dalena, F.; Basile, A. Hydrogen permeation and separation characteristics of a thin Pd-Au/Al2O3 membrane: The effect of the intermediate layer absence. Catal. Today 2019, 330, 32–38. [Google Scholar] [CrossRef]
- Suzuki, A.; Yukawa, H. A review for consistent analysis of hydrogen permeability through dense metallic membranes. Membranes 2020, 10, 120. [Google Scholar] [CrossRef]
- Chen, W.-H.; Escalante, J.; Chi, Y.-H.; Lin, Y.-L. Hydrogen permeation enhancement in a Pd membrane tube system under various vacuum degrees. Int. J. Hydrogen Energy 2020, 45, 7401–7411. [Google Scholar] [CrossRef]
- Bosko, M.L.; Dalla Fontana, A.; Tarditi, A.; Cornaglia, L. Advances in hydrogen selective membranes based on palladium ternary alloys. Int. J. Hydrogen Energy 2021, 46, 15572–15594. [Google Scholar] [CrossRef]
- Girotto, C.P.; Nippes, R.P.; Macruz, P.D.; Gomes, A.D.; de Souza, M.; Rodriguez, M.T. Effect of physicochemical properties on the performance of palladium-based composite membranes: A review. J. Mater. Res. 2023, 38, 4868–4891. [Google Scholar] [CrossRef]
- Cardoso, S.P.; Azenha, I.S.; Lin, Z.; Portugal, I.; Rodrigues, A.E.; Silva, C.M. Inorganic membranes for hydrogen separation. Sep. Purif. Rev. 2018, 47, 229–266. [Google Scholar] [CrossRef]
- Hadjixenophontos, E.; Mahmoudizadeh, M.; Rubin, M.; Ullmer, D.; Razmjooei, F.; Hanf, A.C.; Brien, J.; Dittmeyer, R.; Ansar, A. Palladium membrane with high density of large-angle grain boundaries to promote hydrogen diffusivity. Membranes 2022, 12, 617. [Google Scholar] [CrossRef] [PubMed]
- Gabitto, J.; Tsouris, C. Modeling sulfur poisoning of palladium membranes used for hydrogen separation. Int. J. Chem. Eng. 2019, 2019, 9825280. [Google Scholar] [CrossRef]
- Easa, J.; Yan, C.; Schneider, W.F.; O’Brien, C.P. CO and C3H6 poisoning of hydrogen permeation across Pd77Ag23 alloy membranes: A comparative study with pure palladium. Chem. Eng. J. 2022, 430, 133080. [Google Scholar] [CrossRef]
- Chen, W.-H.; Chen, Z.-Y.; Lim, S.; Park, Y.-K.; Show, P.-L. Hydrogen permeation in a palladium membrane tube: Impacts of outlet and vacuum degree. Int. J. Hydrogen Energy 2022, 47, 40787–40802. [Google Scholar] [CrossRef]
- Peters, T.; Caravella, A. Pd-based membranes: Overview and perspectives. Membranes 2019, 9, 25. [Google Scholar] [CrossRef]
- Chen, C.-H.; Ma, Y.H. The effect of H2S on the performance of Pd and Pd/Au composite membrane. J. Membr. Sci. 2010, 362, 535–544. [Google Scholar] [CrossRef]
- Bosko, M.L.; Lombardo, E.A.; Cornaglia, L.M. The effect of electroless plating time on the morphology, alloy formation and H2 transport properties of Pd–Ag composite membranes. Int. J. Hydrogen Energy 2011, 36, 4068–4078. [Google Scholar] [CrossRef]
- Peters, T.A.; Carvalho, P.A.; Stange, M.; Bredesen, R. Formation of hydrogen bubbles in Pd-Ag membranes during H2 permeation. Int. J. Hydrogen Energy 2020, 45, 7488–7496. [Google Scholar] [CrossRef]
- Nemitallah, M.A. Characteristics of hydrogen separation and methane steam reforming in a Pd-based membrane reactor of shell and tube design. Case Stud. Therm. Eng. 2023, 45, 102939. [Google Scholar] [CrossRef]
- Shi, L.; Goldbach, A.; Xu, H. High-flux H2 separation membranes from (Pd/Au)n nanolayers. Int. J. Hydrogen Energy 2011, 36, 2281–2284. [Google Scholar] [CrossRef]
- Jia, H.; Wu, P.; Zeng, G.; Salas-Colera, E.; Serrano, A.; Castro, G.R.; Xu, H.; Sun, C.; Goldbach, A. High-temperature stability of Pd alloy membranes containing Cu and Au. J. Membr. Sci. 2017, 544, 151–160. [Google Scholar] [CrossRef]
- Anzelmo, B.; Wilcox, J.; Liguori, S. Hydrogen production via natural gas steam reforming in a Pd-Au membrane reactor. Comparison between methane and natural gas steam reforming reactions. J. Membr. Sci. 2018, 568, 113–120. [Google Scholar] [CrossRef]
- Dalla Fontana, A.; Sirini, N.; Cornaglia, L.M.; Tarditi, A.M. Hydrogen permeation and surface properties of PdAu and PdAgAu membranes in the presence of CO, CO2 and H2S. J. Membr. Sci. 2018, 563, 351–359. [Google Scholar] [CrossRef]
- Iulianelli, A.; Manisco, M.; Bion, N.; Le Valant, A.; Epron, F.; Colpan, C.O.; Esposito, E.; Jansen, J.C.; Gensini, M.; Caravella, A. Sustainable H2 generation via steam reforming of biogas in membrane reactors: H2S effects on membrane performance and catalytic activity. Int. J. Hydrogen Energy 2021, 46, 29183–29197. [Google Scholar] [CrossRef]
- Pomerantz, N.; Ma, Y.H. Effect of H2S on the performance and long-term stability of Pd/Cu membranes. Ind. Eng. Chem. Res. 2009, 48, 4030–4039. [Google Scholar] [CrossRef]
- Burkhanov, B.G.S.; Gorina, N.B.; Kolchugina, N.B.; Roshan, N.R.; Slovetsky, D.I.; Chistov, E.M. Palladium-based alloy membranes for separation of high purity hydrogen from hydrogen-containing gas mixtures. Platin. Met. Rev. 2011, 55, 3–12. [Google Scholar] [CrossRef]
- Peters, T.A.; Kaleta, T.; Stange, M.; Bredesen, R. Hydrogen transport through a selection of thin Pd-alloy membranes: Membrane stability, H2S inhibition, and flux recovery in hydrogen and simulated WGS mixtures. Catal. Today 2012, 193, 8–19. [Google Scholar] [CrossRef]
- Acha, E.; van Delft, Y.C.; Cambra, J.F.; Arias, P.L. Thin PdCu membrane for hydrogen purification from in-situ produced methane reforming complex mixtures containing H2S. Chem. Eng. Sci. 2018, 176, 429–438. [Google Scholar] [CrossRef]
- Pati, S.; Ashok, J.; Dewangan, N.; Chen, T.; Kawi, S. Ultra-thin (~1 μm) Pd–Cu membrane reactor for coupling CO2 hydrogenation and propane dehydrogenation applications. J. Membr. Sci. 2020, 595, 117496. [Google Scholar] [CrossRef]
- Cerone, N.; Zito, G.D.; Florio, C.; Fabbiano, L.; Zimbardi, F. Recent advancements in Pd-based membranes for hydrogen separation. Energies 2024, 17, 4095. [Google Scholar] [CrossRef]
- Gade, S.K.; Keeling, M.K.; Davidson, A.P.; Hatlevik, O.; Way, J.D. Palladium–ruthenium membranes for hydrogen separation fabricated by electroless co-deposition. Int. J. Hydrogen Energy 2009, 34, 6484–6491. [Google Scholar] [CrossRef]
- Abu El Hawa, H.W.; Paglieri, S.N.; Morris, C.C.; Harale, A.; Douglas Way, J. Application of a Pd–Ru composite membrane to hydrogen production in a high temperature membrane reactor. Sep. Purif. Technol. 2015, 147, 388–397. [Google Scholar] [CrossRef]
- Lee, S.M.; Xu, N.; Kim, S.S.; Li, A.; Grace, J.R.; Lim, C.J.; Boyd, T.; Ryi, S.-K.; Susdorf, A.; Schaadt, A. Palladium/ruthenium composite membrane for hydrogen separation from the off-gas of solar cell production via chemical vapor deposition. J. Membr. Sci. 2017, 541, 1–8. [Google Scholar] [CrossRef]
- Liu, J.; Bellini, S.; de Nooijer, N.C.A.; Sun, Y.; Pacheco Tanaka, D.A.; Tang, C.; Li, H.; Gallucci, F.; Caravella, A. Hydrogen permeation and stability in ultra-thin Pd Ru supported membranes. Int. J. Hydrogen Energy 2020, 45, 7455–7467. [Google Scholar] [CrossRef]
- Yin, Z.; Yang, Z.; Tong, Y.; Du, M.; Mi, J.; Yu, Q.; Li, S. Improved sulfur tolerance of Pd–Ru membranes: Influence of H2S concentration and exposure time on the hydrogen flux. Int. J. Hydrogen Energy 2023, 48, 38335–38343. [Google Scholar] [CrossRef]
- Sheu, W.-J.; Hsu, Z.-W.; Chen, W.-H.; Chen, Y.-C. Investigation of steam methane reforming in a Pd–Ru membrane reactor with a counter-current configuration. Int. J. Hydrogen Energy 2024, 52, 938–952. [Google Scholar] [CrossRef]
- Peng, L.; Rao, Y.; Luo, L.; Chen, C. The poisoning of Pd–Y alloy membranes by carbon monoxide. J. Alloys Compd. 2009, 486, 74–77. [Google Scholar] [CrossRef]
- Conde, J.J.; Maroño, M.; Sánchez-Hervás, J.M. Pd-based membranes for hydrogen separation: Review of alloying elements and their influence on membrane properties. Sep. Purif. Rev. 2017, 46, 152–177. [Google Scholar] [CrossRef]
- Tropin, E.S.; Shubnikova, E.V.; Bragina, O.A.; Nemudry, A.P. Production of ultra-pure hydrogen for fuel cells using a module based on nickel capillaries. Russ. J. Electrochem. 2024, 60, 30–35. [Google Scholar] [CrossRef]
- Omidifar, M.; Babaluo, A.A. Fabrication of thin (~2 μm) pure Ni and Pd–Ni alloy composite membranes by the organic-inorganic activation method for hydrogen separation. Int. J. Hydrogen Energy 2024, 53, 1025–1036. [Google Scholar] [CrossRef]
- Omidifar, M.; Akbar Babaluo, A.; Jamshidi, S. H2 permeance and surface characterization of a thin (2 μm) Pd-Ni composite membrane prepared by electroless plating. Chem. Eng. Sci. 2024, 283, 119370. [Google Scholar] [CrossRef]
- Amer, J.; Benguerba, Y.; Elboughdiri, N.; Albrahim, M.; Burgard, M.; Ernst, B. Enhancing hydrogen separation efficiency: Insights from the permeability study of nickel-based composite membranes. Int. J. Hydrogen Energy 2024, 66, 703–718. [Google Scholar] [CrossRef]
- Escalante, Y.; Tarditi, A.M. Thermally stable membranes based on PdNiAu systems with high nickel content for hydrogen separation. J. Membr. Sci. 2023, 676, 121581. [Google Scholar] [CrossRef]
- de Nooijer, N.; Sanchez, J.D.; Melendez, J.; Fernandez, E.; Pacheco Tanaka, D.A.; van Sint Annaland, M.; Gallucci, F. Influence of H2S on the hydrogen flux of thin-film PdAgAu membranes. Int. J. Hydrogen Energy 2020, 45, 7303–7312. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Z.; Tong, Y.; Yin, Z.; Li, S. High hydrogen permeability of Pd-Ru-In membranes prepared by electroless co-deposition. Sep. Purif. Technol. 2024, 343, 127073. [Google Scholar] [CrossRef]
- Jazani, O.; Bennett, J.; Liguori, S. Effect of temperature, air exposure and gas mixture on Pd82–Ag15–Y3 membrane for hydrogen separation. Int. J. Hydrogen Energy 2024, 51, 624–636. [Google Scholar] [CrossRef]
- Ievlev, V.M.; Dontsov, A.I.; Gorbunov, S.V.; Il’inova, T.N.; Kannykin, S.V.; Prizhimov, A.S.; Roshan, N.R.; Solntsev, K.A. Structure, texture, and substructure of foil in sequential rolling steps of Cu–36.4 at % Pd alloy. Inorg. Mater. 2021, 57, 1194–1200. [Google Scholar] [CrossRef]
- Yun, S.; Ted Oyama, S. Correlations in palladium membranes for hydrogen separation: A review. J. Membr. Sci. 2011, 375, 28–45. [Google Scholar] [CrossRef]
- Wang, M.; Ding, Y.; Hu, J.; Xu, L.; Yang, X. Numerical simulation of water and heat transport in the cathode channel of a PEM fuel cell. Int. J. Hydrogen Energy 2022, 47, 11007–11027. [Google Scholar] [CrossRef]
- Zhang, K.; Gade, S.K.; Way, J.D. Effects of heat treatment in air on hydrogen sorption over Pd–Ag and Pd–Au membrane surfaces. J. Membr. Sci. 2012, 403–404, 78–83. [Google Scholar] [CrossRef]
- Zhang, K.; Gade, S.K.; Hatlevik, Ø.; Way, J.D. A sorption rate hypothesis for the increase in H2 permeability of palladium-silver (Pd–Ag) membranes caused by air oxidation. Int. J. Hydrogen Energy 2012, 37, 583–593. [Google Scholar] [CrossRef]
- Mironova, E.Y.; Lytkina, A.A.; Ermilova, M.M.; Orekhova, N.V.; Zhilyaeva, N.A.; Roshan, N.R.; Ievlev, V.M.; Yaroslavtsev, A.B. Methanol steam reforming in a reactor with a palladium–copper membrane in the presence of a nickel-copper catalyst. Pet. Chem. 2020, 60, 1232–1238. [Google Scholar] [CrossRef]
- Ievlev, V.M.; Dontsov, A.I.; Morozova, N.B.; Roshan, N.R.; Serbin, O.V.; Prizhimov, A.S.; Solntsev, K.A. Techniques for surface cleaning of membrane foil from palladium-based solid solutions. Inorg. Mater. 2020, 56, 1059–1064. [Google Scholar] [CrossRef]
- Mironova, E.Y.; Dontsov, A.I.; Morozova, N.B.; Gorbunov, S.V.; Ievlev, V.M.; Yaroslavtsev, A.B. Lamp processing of the surface of PdCu membrane foil: Hydrogen permeability and membrane catalysis. Inorg. Mater. 2021, 57, 781–789. [Google Scholar] [CrossRef]
- Ievlev, V.M.; Solntsev, K.A.; Gorbunov, S.V.; Roshan, N.R.; Kas’yanov, V.S.; Morozova, N.B.; Dontsov, A.I. Effect of ultrasonic treatment of Pd–4 at % In–1 at % Ru membrane foil: Sorption and hydrogen permeability. Inorg. Mater. 2023, 59, 1275–1282. [Google Scholar] [CrossRef]
- Loza, S.A.; Zabolotsky, V.I.; Loza, N.V.; Fomenko, M.A. Structure, morphology, and transport characteristics of profiled bilayer membranes. Pet. Chem. 2016, 56, 1027–1033. [Google Scholar] [CrossRef]
- Pushankina, P.; Baryshev, M.; Petriev, I. Synthesis and study of palladium mono- and bimetallic (with Ag and Pt) nanoparticles in catalytic and membrane hydrogen processes. Nanomaterials 2022, 12, 4178. [Google Scholar] [CrossRef]
- Luong, H.M.; Pham, M.T.; Guin, T.; Madhogaria, R.P.; Phan, M.-H.; Larsen, G.K.; Nguyen, T.D. Sub-second and ppm-level optical sensing of hydrogen using templated control of nano-hydride geometry and composition. Nat. Commun. 2021, 12, 2414. [Google Scholar] [CrossRef]
- Petriev, I.S.; Pushankina, P.D.; Andreev, G.A. Investigation of low-temperature hydrogen permeability of surface modified Pd–Cu membranes. Membr. Membr. Technol. 2023, 5, 360–369. [Google Scholar] [CrossRef]
- Hanifah, M.F.R.; Jaafar, J.; Othman, M.H.D.; Ismail, A.F.; Rahman, M.A.; Yusof, N.; Aziz, F.; Rahman, N.A. One-pot synthesis of efficient reduced graphene oxide supported binary Pt-Pd alloy nanoparticles as superior electro-catalyst and its electro-catalytic performance toward methanol electro-oxidation reaction in direct methanol fuel cell. J. Alloys Compd. 2019, 793, 232–246. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Kanti Bera, K.; Ray, A.; Bera, P.; Maiyalagan, T.; Bhattacharya, S.K. Synergistic catalytic activity of palladium–silver alloy nanoparticle for anodic oxidation of ethanol in alkali. Int. J. Hydrogen Energy 2021, 46, 14212–14224. [Google Scholar] [CrossRef]
- Karaman, C. Engineering of N,P,S-Triple doped 3-dimensional graphene architecture: Catalyst-support for “surface-clean” Pd nanoparticles to boost the electrocatalysis of ethanol oxidation reaction. Int. J. Hydrogen Energy 2023, 48, 6691–6701. [Google Scholar] [CrossRef]
- Jung, H.; King, M.E.; Personick, M.L. Strategic synergy: Advances in the shape control of bimetallic nanoparticles with dilute alloyed surfaces. Curr. Opin. Colloid Interface Sci. 2019, 40, 104–117. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, Q.; Zhang, Q.; Huang, Y. Precisely deposited Pd on ZnO (002) facets derived from complex reduction strategy for methanol steam reforming. Int. J. Hydrogen Energy 2022, 47, 14869–14883. [Google Scholar] [CrossRef]
- Wu, H.-L.; Chen, C.-H.; Huang, M.H. Seed-mediated synthesis of branched gold nanocrystals derived from the side growth of pentagonal bipyramids and the formation of gold nanostars. Chem. Mater. 2009, 21, 110–114. [Google Scholar] [CrossRef]
- Zhang, L.; Zhong, S.; Xu, A. Highly branched concave Au/Pd bimetallic nanocrystals with superior electrocatalytic activity and highly efficient SERS enhancement. Angew. Chem. Int. Ed. 2013, 52, 645–649. [Google Scholar] [CrossRef]
- Shao, Z.; Zhu, W.; Wang, H.; Yang, Q.; Yang, S.; Liu, X.; Wang, G. Controllable synthesis of concave nanocubes, right bipyramids, and 5-fold twinned nanorods of palladium and their enhanced electrocatalytic performance. J. Phys. Chem. C 2013, 117, 14289–14294. [Google Scholar] [CrossRef]
- Petriev, I.; Pushankina, P.; Bolotin, S.; Lutsenko, I.; Kukueva, E.; Baryshev, M. The influence of modifying nanoflower and nanostar type Pd coatings on low temperature hydrogen permeability through Pd-containing membranes. J. Membr. Sci. 2021, 620, 118894. [Google Scholar] [CrossRef]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics? Angew. Chem. Int. Ed. 2009, 48, 60–103. [Google Scholar] [CrossRef] [PubMed]
- Petriev, I.S.; Pushankina, P.D.; Andreev, G.A.; Yaroslavtsev, A.B. Mechanisms of formation and shape control of pentagonal Pd-nanostars and their unique properties in electrocatalytic methanol oxidation and membrane separation of high-purity hydrogen. Int. J. Hydrogen Energy 2024, 70, 404–413. [Google Scholar] [CrossRef]
- Vicinanza, N.; Svenum, I.-H.; Peters, T.; Bredesen, R.; Venvik, H. New insight to the effects of heat treatment in air on the permeation properties of thin Pd77%Ag23% membranes. Membranes 2018, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, V.; Lin, Y.S.; Pakala, M.; Lin, R.Y. Fabrication of ultrathin metallic membranes on ceramic supports by sputter deposition. J. Membr. Sci. 1995, 99, 89–100. [Google Scholar] [CrossRef]
- Qiao, A.; Zhang, K.; Tian, Y.; Xie, L.; Luo, H.; Lin, Y.S.; Li, Y. Hydrogen separation through palladium–copper membranes on porous stainless steel with sol–gel derived ceria as diffusion barrier. Fuel 2010, 89, 1274–1279. [Google Scholar] [CrossRef]
- Toro, L.; Moscardini, E.; Baldassari, L.M.; Forte, F.; Coletta, J.; Palo, E.; Cosentino, V.; Angelini, F.; Arratibel Plazaola, A.; Pagnanelli, F.; et al. Regeneration of exhausted palladium-based membranes: Recycling process and economics. Membranes 2022, 12, 723. [Google Scholar] [CrossRef]
- Arratibel Plazaola, A.; Pacheco Tanaka, D.A.; Van Sint Annaland, M.; Gallucci, F. Recent advances in Pd-based membranes for membrane reactors. Molecules 2017, 22, 51. [Google Scholar] [CrossRef]
- Melendez, J.; Fernandez, E.; Gallucci, F.; van Sint Annaland, M.; Arias, P.L.; Pacheco Tanaka, D.A. Preparation and characterization of ceramic supported ultra-thin (~1 µm) Pd-Ag membranes. J. Membr. Sci. 2017, 528, 12–23. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, H.; Goldbach, A. Duplex Pd/ceramic/Pd composite membrane for sweep gas-enhanced CO2 capture. J. Membr. Sci. 2018, 563, 388–397. [Google Scholar] [CrossRef]
- Orakwe, I.; Shehu, H.; Gobina, E. Preparation and characterization of palladium ceramic alumina membrane for hydrogen permeation. Int. J. Hydrogen Energy 2019, 44, 9914–9921. [Google Scholar] [CrossRef]
- Alkali, A. Electroless plating of palladium membranes on porous substrates for hydrogen separation and the effects of process factors on plating rate and efficiency: A review. J. Power Energy Eng. 2020, 8, 1–19. [Google Scholar] [CrossRef]
- Magnone, E.; Lee, S.H.; Park, J.H. Relationships between electroless plating temperature, Pd film thickness and hydrogen separation performance of Pd-coated Al2O3 hollow fibers. Mater. Lett. 2020, 272, 127811. [Google Scholar] [CrossRef]
- Yue, L.; Chen, C.; Li, J.; Xiao, C.; Xia, X.; Ran, G.; Fu, X.; Hou, J.; Gong, Y.; Wang, H. Inhibition effect of CO on hydrogen permeation through a Pd/Al2O3 composite membrane: A comprehensive study on concentration polarization and competitive adsorption effect. Fusion Sci. Technol. 2020, 76, 680–689. [Google Scholar] [CrossRef]
- Lundin, S.-T.B.; Patki, N.S.; Zhang, Z.; Fuerst, T.F.; Wolden, C.A.; Way, J.D. PdAu/YSZ composite hydrogen separation membranes with enhanced stability in the presence of CO. J. Membr. Sci. 2020, 611, 118371. [Google Scholar] [CrossRef]
- Chen, W.-H.; Escalante, J. Influence of vacuum degree on hydrogen permeation through a Pd membrane in different H2/N2 gas mixtures. Renew. Energy 2020, 155, 1245–1263. [Google Scholar] [CrossRef]
- Tosto, E.; Martinez-Diaz, D.; Sanz, R.; Azzato, G.; Calles, J.A.; Medrano, J.A.; Fernandez, E.; Pacheco Tanaka, D.A.; Gallucci, F.; Alique, D.; et al. Systematic experimental assessment of concentration polarization and inhibition in Pd-based membranes for hydrogen purification. Fuel Process. Technol. 2021, 213, 106661. [Google Scholar] [CrossRef]
- Kong, S.Y.; Kim, D.H.; Henkensmeier, D.; Kim, H.J.; Ham, H.C.; Han, J.; Yoon, S.P.; Yoon, C.W.; Choi, S.H. Ultrathin layered Pd/PBI–HFA composite membranes for hydrogen separation. Sep. Purif. Technol. 2017, 179, 486–493. [Google Scholar] [CrossRef]
- Delima, R.S.; Sherbo, R.S.; Dvorak, D.J.; Kurimoto, A.; Berlinguette, C.P. Supported palladium membrane reactor architecture for electrocatalytic hydrogenation. J. Mater. Chem. A 2019, 7, 26586–26595. [Google Scholar] [CrossRef]
- Alique, D.; Martinez-Diaz, D.; Sanz, R.; Calles, J. Review of supported Pd-based membranes preparation by electroless plating for ultra-pure hydrogen production. Membranes 2018, 8, 5. [Google Scholar] [CrossRef]
- Salomé Macedo, M.; Acha Uriarte, N.; Soria, M.A.; Madeira, L.M.; Calles, J.A.; Sanz, R.; Alique, D. Effect of ceria particle size as intermediate layer for preparation of composite Pd-membranes by electroless pore-plating onto porous stainless-steel supports. Sep. Purif. Technol. 2023, 327, 124932. [Google Scholar] [CrossRef]
- Martinez-Diaz, D.; Alique, D.; Calles, J.A.; Sanz, R. Pd-thickness reduction in electroless pore-plated membranes by using doped-ceria as interlayer. Int. J. Hydrogen Energy 2020, 45, 7278–7289. [Google Scholar] [CrossRef]
- Martinez-Diaz, D.; Martínez del Monte, D.; García-Rojas, E.; Alique, D.; Calles, J.A.; Sanz, R. Comprehensive permeation analysis and mechanical resistance of electroless pore-plated Pd-membranes with ordered mesoporous ceria as intermediate layer. Sep. Purif. Technol. 2021, 258, 118066. [Google Scholar] [CrossRef]
- Martinez-Diaz, D.; Sanz, R.; Carrero, A.; Calles, J.A.; Alique, D. Effective H2 separation through electroless pore-plated Pd membranes containing graphite lead barriers. Membranes 2020, 10, 410. [Google Scholar] [CrossRef] [PubMed]
- de Moura Silva, C.L.; Ribeiro, S.R.F.L.; Terra, N.M.; Cardoso, V.L.; Reis, M.H.M. Improved hydrogen permeation through thin Pd/Al2O3 composite membranes with graphene oxide as intermediate layer. Int. J. Hydrogen Energy 2020, 45, 22990–23005. [Google Scholar] [CrossRef]
- Dehghani Kiadehi, A.; Taghizadeh, M.; Rami, M.D. Preparation of Pd/SAPO-34/PSS composite membranes for hydrogen separation: Effect of crystallization time on the zeolite growth on PSS support. J. Ind. Eng. Chem. 2020, 81, 206–218. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Q.; Jin, X.; Ding, W.; Hu, X.; Li, H. Coating the porous Al2O3 substrate with a natural mineral of Nontronite-15A for fabrication of hydrogen-permeable palladium membranes. Int. J. Hydrogen Energy 2020, 45, 7412–7422. [Google Scholar] [CrossRef]
- Sanz-Villanueva, D.; Alique, D.; Vizcaíno, A.J.; Sanz, R.; Calles, J.A. Pre-activation of SBA-15 intermediate barriers with Pd nuclei to increase thermal and mechanical resistances of pore-plated Pd-membranes. Int. J. Hydrogen Energy 2021, 46, 20198–20212. [Google Scholar] [CrossRef]
- Zito, P.F.; Brunetti, A.; Barbieri, G. Hydrogen concentration and purification by membrane process: A multistage analysis. Renew. Energy 2023, 218, 119243. [Google Scholar] [CrossRef]
- Park, Y.; Kwak, Y.; Yu, S.; Badakhsh, A.; Lee, Y.-J.; Jeong, H.; Kim, Y.; Sohn, H.; Nam, S.W.; Yoon, C.W.; et al. Degradation mechanism of a Pd/Ta composite membrane: Catalytic surface fouling with inter-diffusion. J. Alloys Compd. 2021, 854, 157196. [Google Scholar] [CrossRef]
- Ryu, S.; Badakhsh, A.; Oh, J.G.; Ham, H.C.; Sohn, H.; Yoon, S.P.; Choi, S.H. Experimental and numerical study of Pd/Ta and PdCu/Ta composites for thermocatalytic hydrogen permeation. Membranes 2022, 13, 23. [Google Scholar] [CrossRef]
- Alimov, V.N.; Bobylev, I.V.; Busnyuk, A.O.; Notkin, M.E.; Peredistov, E.Y.; Livshits, A.I. Hydrogen transport through the tubular membranes of V-Pd alloys: Permeation, diffusion, surface processes and WGS mixture test of membrane assembly. J. Membr. Sci. 2018, 549, 428–437. [Google Scholar] [CrossRef]
- Alimov, V.N.; Bobylev, I.V.; Busnyuk, A.O.; Kolgatin, S.N.; Kuzenov, S.R.; Peredistov, E.Y.; Livshits, A.I. Extraction of ultrapure hydrogen with V-alloy membranes: From laboratory studies to practical applications. Int. J. Hydrogen Energy 2018, 43, 13318–13327. [Google Scholar] [CrossRef]
- Alimov, V.N.; Busnyuk, A.O.; Kuzenov, S.R.; Peredistov, E.U.; Livshits, A.I. Bcc V–Fe alloys for the hydrogen separation membranes: Hydrogen solubility and global character of alloying effect. J. Membr. Sci. 2022, 644, 120159. [Google Scholar] [CrossRef]
- Kuzenov, S.R.; Alimov, V.N.; Busnyuk, A.O.; Peredistov, E.U.; Livshits, A.I. Hydrogen transport through V–Fe alloy membranes: Permeation, diffusion, effects of deviation from Sieverts’ law. J. Membr. Sci. 2023, 674, 121504. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, Y.; Zou, D.; Pan, Q.; Jiang, C.; Li, Y.; Chen, C. Effect of single atomic layer graphene film on the thermal stability and hydrogen permeation of Pd-coated Nb composite membrane. Int. J. Hydrogen Energy 2022, 47, 8359–8371. [Google Scholar] [CrossRef]
- Liang, X.; Li, X.; Chen, R.; Nagaumi, H.; Guo, J.; Liu, D. Enhancement of hydrogen permeation stability at high temperatures for Pd/Nb30Ti35Co35/Pd composite membranes by HfN intermediate layer. J. Membr. Sci. 2022, 643, 120062. [Google Scholar] [CrossRef]
- Mercea, P.; Mureşan, L.; Mecea, V.; Silipaş, D.; Ursu, I. Permeation of gases through poly (ethylene terephthalate) membranes metallized with palladium. J. Membr. Sci. 1988, 35, 291–300. [Google Scholar] [CrossRef]
- Mercea, P.V.; Silipas, D.; Mecea, V.; Ursu, I. The separation of a gas mixture through a metallized polymer membrane. Gas Sep. Purif. 1990, 4, 137–140. [Google Scholar] [CrossRef]
- Mercea, P.V.; Mecea, V. Gas separation through a high-flux asymmetric polymer membrane metallized with palladium. Gas Sep. Purif. 1991, 5, 267–272. [Google Scholar] [CrossRef]
- Gryaznov, V.M.; Serebryannikova, O.S.; Serov, Y.M.; Ermilova, M.M.; Karavanov, A.N.; Mischenko, A.P.; Orekhova, N.V. Preparation and catalysis over palladium composite membranes. J. Membr. Sci. 1993, 77, 284. [Google Scholar] [CrossRef]
- Gryaznov, V.M.; Yermilova, M.M.; Zavodchenko, S.I.; Orekhova, N.V. Hydrogen permeability of some metal-polymer membranes. Vysokomol. Soedininiya 1993, 35, 325–329. (In Russian) [Google Scholar]
- Patel, A.K.; Acharya, N.K. Metal coated and nanofiller doped polycarbonate membrane for hydrogen transport. Int. J. Hydrogen Energy 2018, 43, 21675–21682. [Google Scholar] [CrossRef]
- Kamakshi; Kumar, R.; Saraswat, V.K.; Kumar, M.; Awasthi, K. Palladium nanoparticle binding in functionalized track etched PET membrane for hydrogen gas separation. Int. J. Hydrogen Energy 2017, 42, 16186–16194. [Google Scholar] [CrossRef]
- Saini, N.; Agarwal, S.; Awasthi, K. Bimetallic PdPt alloy nanoparticle-decorated track-etched polyethylene terephthalate membranes for efficient H2 separation. Mater. Adv. 2024, 5, 2906–2916. [Google Scholar] [CrossRef]
- Kumar, R.; Kamakshi; Shisodia, S.; Kumar, M.; Awasthi, K. Effect of UV irradiation on PC membrane and use of Pd nanoparticles with/without PVP for H2 selectivity enhancement over CO2 and N2 gases. Int. J. Hydrogen Energy 2018, 43, 21690–21698. [Google Scholar] [CrossRef]
- Lin, Z.; Yuan, Z.; Dai, Z.; Shao, L.; Eisen, M.S.; He, X. A review from material functionalization to process feasibility on advanced mixed matrix membranes for gas separations. Chem. Eng. J. 2023, 475, 146075. [Google Scholar] [CrossRef]
- Sun, S.; Li, S.; Wang, S.; Chen, Y. Design and development of highly selective and permeable membranes for H2/CO2 separation—A review. Chem. Eng. J. 2024, 494, 152972. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, X.; Zhai, J.; Li, X.; Guo, X.; He, G. Research progress and prospects on hydrogen separation membranes. Clean Energy 2023, 7, 217–241. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Sadrzadeh, M.; Matsuura, T. Thermally stable polymers for advanced high-performance gas separation membranes. Prog. Energy Combust. Sci. 2018, 66, 1–41. [Google Scholar] [CrossRef]
- Rusanov, A.L.; Komarova, L.G. 5.21—High-Performance Heterocyclic Polymers; Elsevier B.V.: Amsterdam, The Netherlands, 2012; pp. 537–596. ISBN 9780080878621. [Google Scholar] [CrossRef]
- Yampolskii, Y.; Belov, N.; Alentiev, A. Perfluorinated polymers as materials of membranes for gas and vapor separation. J. Membr. Sci. 2020, 598, 117779. [Google Scholar] [CrossRef]
- Karpov, G.O.; Bermeshev, M.V.; Borisov, I.L.; Sterlin, S.R.; Tyutyunov, A.A.; Yevlampieva, N.P.; Bulgakov, B.A.; Volkov, V.V.; Finkelshtein, E.S. Metathesis-type poly-exo-tricyclononenes with fluoroorganic side substituents: Synthesis and gas-transport properties. Polymer 2018, 153, 626–636. [Google Scholar] [CrossRef]
- Borisov, I.L.; Grushevenko, E.A.; Anokhina, T.S.; Bakhtin, D.S.; Levin, I.S.; Bondarenko, G.N.; Volkov, V.V.; Volkov, A.V. Influence of side chains assembly on the structure and transport properties of comb-like polysiloxanes in hydrocarbon separation. Mater. Today Chem. 2021, 22, 100598. [Google Scholar] [CrossRef]
- Ershova, T.; Anisimov, A.; Krylov, F.; Polshchikova, N.; Temnikov, M.; Shchegolikhina, O.; Muzafarov, A. A new highly efficient method for the preparation of phenyl-containing siloxanes by condensation of phenylsilanols in liquid ammonia. Chem. Eng. Sci. 2022, 247, 116916. [Google Scholar] [CrossRef]
- Anokhina, T.S.; Ershova, T.O.; Anisimov, A.A.; Temnikov, M.N.; Grushevenko, E.A.; Borisov, I.L.; Volkov, A.V.; Muzafarov, A.M. Pervaporation and Gas Separation Properties of High-Molecular Ladder-like Polyphenylsilsesquioxanes. Polymers 2023, 15, 3277. [Google Scholar] [CrossRef] [PubMed]
- Kryzhanovskii, I.N.; Temnikov, M.N.; Anisimov, A.A.; Ratnikov, A.K.; Frank, I.V.; Naumkin, A.V.; Chistovalova, S.M.; Muzafarov, A.M. From silicon to silicones without dimethyldichlorosilane: Direct green mechanochemical synthesis of methylmethoxysilanes from silicon and dimethyl ether. Green Chem. 2024, 26, 6656–6665. [Google Scholar] [CrossRef]
- Han, Y.; Ho, W.S.W. Polymeric membranes for CO2 separation and capture. J. Membr. Sci. 2021, 628, 119244. [Google Scholar] [CrossRef]
- Bera, D.; Chatterjee, R.; Banerjee, S. Aromatic polyamide nonporous membranes for gas separation application. e-Polymers 2021, 21, 108–130. [Google Scholar] [CrossRef]
- Suhail, F.; Batool, M.; Anjum, T.; Shah, A.T.; Tabassum, S.; Khan, A.L.; AlMohamadi, H.; Najam, M.; Gilani, M.A. Enhanced CO2 separation performance of polysulfone membranes via incorporation of pyrazole modified MCM-41 mesoporous silica as a nano-filler. Fuel 2023, 350, 128840. [Google Scholar] [CrossRef]
- Singh, S.; Varghese, A.M.; Reddy, K.S.K.; Romanos, G.E.; Karanikolos, G.N. Polysulfone mixed-matrix membranes comprising poly(ethylene glycol)-grafted carbon nanotubes: Mechanical properties and CO2 separation performance. Ind. Eng. Chem. Res. 2021, 60, 11289–11308. [Google Scholar] [CrossRef]
- Patdiya, J.; Gavane, G.B.; Kandasubramanian, B. A review on polybenzimidazoles blends and nanocomposites for engineering applications. Polym. Technol. Mater. 2022, 61, 1411–1438. [Google Scholar] [CrossRef]
- Aili, D.; Yang, J.; Jankova, K.; Henkensmeier, D.; Li, Q. From polybenzimidazoles to polybenzimidazoliums and polybenzimidazolides. J. Mater. Chem. A 2020, 8, 12854–12886. [Google Scholar] [CrossRef]
- Bitter, J.H.; Tashvigh, A.A.; Asadi Tashvigh, A. Recent advances in polybenzimidazole membranes for hydrogen purification. Ind. Eng. Chem. Res. 2022, 61, 6125–6134. [Google Scholar] [CrossRef]
- Xu, Z.; Croft, Z.L.; Guo, D.; Cao, K.; Liu, G. Recent development of polyimides: Synthesis, processing, and application in gas separation. J. Polym. Sci. 2021, 59, 943–962. [Google Scholar] [CrossRef]
- Wang, X.; Wilson, T.J.; Alentiev, D.; Gringolts, M.; Finkelshtein, E.; Bermeshev, M.; Long, B.K. Substituted polynorbornene membranes: A modular template for targeted gas separations. Polym. Chem. 2021, 12, 2947–2977. [Google Scholar] [CrossRef]
- Wozniak, A.I.; Bermesheva, E.V.; Petukhov, D.I.; Lunin, A.O.; Borisov, I.L.; Shantarovich, V.P.; Bekeshev, V.G.; Alentiev, D.A.; Bermeshev, M.V. The magic of spiro-epoxy moiety: An easy way to improve CO2-separation performance of polymer membrane. Adv. Funct. Mater. 2024, 34, 2405461. [Google Scholar] [CrossRef]
- Sen, S.K.; Dasgupta, B.; Banerjee, S. Effect of introduction of heterocyclic moieties into polymer backbone on gas transport properties of fluorinated poly(ether imide) membranes. J. Membr. Sci. 2009, 343, 97–103. [Google Scholar] [CrossRef]
- Chatfield, D.A.; Einhorn, I.N. Stepwise Thermal Degradation of a Polybenzimidazole Foam. J. Polym. Sci. 1981, 19, 601–618. [Google Scholar] [CrossRef]
- Kumbharkar, S.C.; Karadkar, P.B.; Kharul, U.K. Enhancement of gas permeation properties of polybenzimidazoles by systematic structure architecture. J. Membr. Sci. 2006, 286, 161–169. [Google Scholar] [CrossRef]
- Tanaka, K.; Okano, M.; Toshino, H.; Kita, H.; Okamoto, K.-I. Effect of methyl substituents on permeability and permselectivity of gases in polyimides prepared from methyl-substituted phenylenediamines. J. Polym. Sci. Part B Polym. Phys. 1992, 30, 907–914. [Google Scholar] [CrossRef]
- Frazer, A.H.; Sweeny, W.; Wallenberger, F.T. Poly(1,3,4-oxadiazoles): A new class of polymers by cyclodehydration of polyhydrazides. J. Polym. Sci. Part A Gen. Pap. 1964, 2, 1157–1169. [Google Scholar] [CrossRef]
- Alentiev, A.Y.; Syrtsova, D.A.; Nikiforov, R.Y.; Ryzhikh, V.E.; Belov, N.A.; Skupov, K.M.; Volkova, Y.A.; Ponomarev, I.I. Polynaphthoylenebenzimidazoles as polymer materials for high-temperature membrane gas separation. Polymer 2024, 308, 127394. [Google Scholar] [CrossRef]
- Nazarov, I.V.; Khrychikova, A.P.; Medentseva, E.I.; Bermesheva, E.V.; Borisov, I.L.; Yushkin, A.A.; Volkov, A.V.; Wozniak, A.I.; Petukhov, D.I.; Topchiy, M.A.; et al. CO2-selective vinyl-addition polymers from nadimides: Synthesis and performance for membrane gas separation. J. Membr. Sci. 2023, 677, 121624. [Google Scholar] [CrossRef]
- Alentiev, A.Y.; Yampolskii, Y.P.; Shantarovich, V.P.; Nemser, S.M.; Platé, N.A. High transport parameters and free volume of perfluorodioxole copolymers. J. Membr. Sci. 1997, 126, 123–132. [Google Scholar] [CrossRef]
- Guo, L.; Shi, Y.; Wu, S.; Jin, J.; Wang, Z. Poly(hydrazide-imide) membranes with enhanced interchain interaction for highly selective H2/CO2 separation. Macromolecules 2023, 56, 3430–3439. [Google Scholar] [CrossRef]
- Park, H.B.; Han, S.H.; Jung, C.H.; Lee, Y.M.; Hill, A.J. Thermally rearranged (TR) polymer membranes for CO2 separation. J. Membr. Sci. 2010, 359, 11–24. [Google Scholar] [CrossRef]
- Ramimoghadam, D.; Boyd, S.E.; Brown, C.L.; Mac, A.; Gray, E.; Webb, C.J. The Effect of Thermal Treatment on the Hydrogen-Storage Properties of PIM-1. ChemPhysChem 2019, 20, 1613–1623. [Google Scholar] [CrossRef]
- Alentiev, A.Y.; Chirkov, S.V.; Nikiforov, R.Y.; Levin, I.A.; Kechekyan, A.S.; Kechekyan, P.A.; Belov, N.A. Poly(2,6-Dimethyl-1,4-Phenylene Oxide) as a Polymer-Polymer Nanocomposite: Mechanical and Gas Transport Characteristics. Membr. Membr. Technol. 2022, 4, 3–14. [Google Scholar] [CrossRef]
- Lee, W.H.; Seong, J.G.; Hu, X.; Lee, Y.M. Recent progress in microporous polymers from thermally rearranged polymers and polymers of intrinsic microporosity for membrane gas separation: Pushing performance limits and revisiting trade-off lines. J. Polym. Sci. 2020, 58, 2450–2466. [Google Scholar] [CrossRef]
- Guo, L.; Liu, W.; Yang, Y.; Ali, A.; Lau, C.H.; Bermeshev, M.V.; Shao, L. Recent progress in thermally rearranged (TR) polymer based membranes for sustainable gas separations. Sep. Purif. Technol. 2025, 355, 129690. [Google Scholar] [CrossRef]
- Borisov, I.; Bakhtin, D.; Luque-Alled, J.M.; Rybakova, A.; Makarova, V.; Foster, A.B.; Harrison, W.J.; Volkov, V.; Polevaya, V.; Gorgojo, P.; et al. Synergistic enhancement of gas selectivity in thin film composite membranes of PIM-1. J. Mater. Chem. A 2019, 7, 6417–6430. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Y.; Guo, L.; Di, J.; Lau, C.H.; Bermeshev, M.V.; Shao, L. Recent advances in porous organic polymers for sustainable gas separations. Chem. Eng. J. 2024, 498, 155569. [Google Scholar] [CrossRef]
- Almansour, F.; Alberto, M.; Foster, A.B.; Mohsenpour, S.; Budd, P.M.; Gorgojo, P. Thin film nanocomposite membranes of superglassy PIM-1 and amine-functionalised 2D fillers for gas separation. J. Mater. Chem. A 2022, 10, 23341–23351. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Fan, B.; Carta, M.; Malpass-Evans, R.; McKeown, N.B.; Marken, F. Polymer of intrinsic microporosity (PIM) films and membranes in electrochemical energy storage and conversion: A mini-review. Electrochem. Commun. 2020, 118, 106798. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Volkova, Y.A.; Vtyurina, E.S.; Skupov, K.M. Porous organic polymers based on a polymer of intrinsic microporosity. INEOS OPEN 2023, 6, 144–149. [Google Scholar] [CrossRef]
- Zotkin, M.A.; Alentiev, D.A.; Borisov, R.S.; Kozlova, A.A.; Borisov, I.L.; Shalygin, M.G.; Bermeshev, M.V. Polynorbornenes with carbocyclic substituents: A perspective approach to highly permeable gas separation membranes. J. Membr. Sci. 2024, 702, 122786. [Google Scholar] [CrossRef]
- Medentseva, E.I.; Khrychikova, A.P.; Bermesheva, E.V.; Borisov, I.L.; Petukhov, D.I.; Karpov, G.O.; Morontsev, A.A.; Nesterova, O.V.; Bermeshev, M.V. CO2-separation performance of vinyl-addition polynorbornenes with ester functionalities. J. Membr. Sci. 2024, 705, 122916. [Google Scholar] [CrossRef]
- Wang, X.; Wilson, T.J.; Maroon, C.R.; Laub, J.A.; Rheingold, S.E.; Vogiatzis, K.D.; Long, B.K. Vinyl-addition fluoroalkoxysilyl-substituted polynorbornene membranes for CO2/CH4 separation. ACS Appl. Polym. Mater. 2022, 4, 7976–7988. [Google Scholar] [CrossRef]
- Chae, C.G.; Go, Y.; Choi, J.; Park, D.A.; Ho, L.N.T.; Bak, I.G.; Park, J.W.; Jung, K.; Park, S.; Lee, W.; et al. Palladium(II)-catalyzed synthesis of a vinyl-addition ultrahigh-molecular-weight polynorbornene copolymer with an entanglement network for enhanced fracture resistance. Macromolecules 2024, 57, 565–573. [Google Scholar] [CrossRef]
- Suslov, D.S.; Pakhomova, M.V.; Bykov, M.V.; Orlov, T.S.; Abramov, Z.D.; Suchkova, A.V.; Abramov, P.A. Novel catalyst systems based on cationic palladium cyclopentadienyl complexes for the polymerization of norbornene and norbornene derivatives. Kinet. Catal. 2024, 65, 40–56. [Google Scholar] [CrossRef]
- Omidvar, M.; Mansouri, S.; Mortazavi, S.M.M.; Ahmadjo, S. Shedding light on the poly(5-ethylidene-2-norbornene) microstructural differences: Skeleton rearrangements during polymerization. J. Appl. Polym. Sci. 2024, 141, e54775. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, G.; Tang, H.; Wang, G.; Xiao, L.; Zhuang, L. An efficient approach towards highly chemically stable poly(norbornene) membrane for alkaline polyelectrolyte fuel cells. J. Membr. Sci. 2024, 709, 123057. [Google Scholar] [CrossRef]
- Sun, X.; Cao, D.; Liu, M.; Wang, B.; Song, D.; Pan, L.; Li, N.; Li, Y. Remarkable impact of chain backbone on the performance of poly(norbornene derivatives)-based anion exchange membranes. J. Membr. Sci. 2024, 703, 122830. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Volkova, Y.A.; Ponomarev, I.I.; Razorenov, D.Y.; Skupov, K.M.; Nikiforov, R.Y.; Chirkov, S.V.; Ryzhikh, V.E.; Belov, N.A.; Alentiev, A.Y. Polynaphthoylenebenzimidazoles for gas separation—Unexpected PIM relatives. Polymer 2022, 238, 124396. [Google Scholar] [CrossRef]
- Suhaimi, H.S.M.; Leo, C.P.; Ahmad, A.L. Hydrogen Purification Using Polybenzimidazole Mixed-Matrix Membranes with Stabilized Palladium Nanoparticles. Chem. Eng. Technol. 2017, 40, 631–638. [Google Scholar] [CrossRef]
- Suhaimi, H.S.M.; Leo, C.P.; Ahmad, A.L. Hydrogen separation using polybenzimidazole membrane with palladium nanoparticles stabilized by polyvinylpyrrolidone. Int. J. Energy Res. 2021, 45, 15171–15181. [Google Scholar] [CrossRef]
- Zhu, L.; Yin, D.; Qin, Y.; Konda, S.; Zhang, S.; Zhu, A.; Liu, S.; Xu, T.; Swihart, M.T.; Lin, H. Sorption-enhanced mixed matrix membranes with facilitated hydrogen transport for hydrogen purification and CO2 capture. Adv. Funct. Mater. 2019, 29, 1904357. [Google Scholar] [CrossRef]
- Kumar, A.; Huang, L.; Hu, L.; Yin, D.; Lin, H.; Swihart, M.T. Facile one-pot synthesis of PdM (M = Ag, Ni, Cu, Y) nanowires for use in mixed matrix membranes for efficient hydrogen separation. J. Mater. Chem. A 2021, 9, 12755–12762. [Google Scholar] [CrossRef]
- Villalobos, L.F.; Hilke, R.; Akhtar, F.H.; Peinemann, K.V. Fabrication of polybenzimidazole/palladium nanoparticles hollow fiber membranes for hydrogen purification. Adv. Energy Mater. 2018, 8, 1701567. [Google Scholar] [CrossRef]
- Hu, L.; Chen, K.; Lee, W.-I.; Kisslinger, K.; Rumsey, C.; Fan, S.; Bui, V.T.; Esmaeili, N.; Tran, T.; Ding, Y.; et al. Palladium-percolated networks enabled by low loadings of branched nanorods for enhanced H2 separations. Adv. Mater. 2023, 35, 2301007. [Google Scholar] [CrossRef]
- Suhaimi, H.S.M.; Khir, M.N.I.M.; Leo, C.P.; Ahmad, A.L. Preparation and characterization of polysulfone mixed-matrix membrane incorporated with palladium nanoparticles dispersed in polyvinylpyrrolidone for hydrogen separation. J. Polym. Res. 2014, 21, 428. [Google Scholar] [CrossRef]
- Mohd Suhaimi, H.S.; Peng, L.C.; Ahmad, A.L. Preparation and characterization of polysulfone mixed matrix membrane incorporated with thermodynamically stable nano-palladium for hydrogen separation. Adv. Mater. Res. 2014, 832, 143–148. [Google Scholar] [CrossRef]
- Kumar, R.; Kamakshi; Kumar, M.; Awasthi, K. Functionalized Pd-decorated and aligned MWCNTs in polycarbonate as a selective membrane for hydrogen separation. Int. J. Hydrogen Energy 2016, 41, 23057–23066. [Google Scholar] [CrossRef]
- Mirzaei, A.; Navarchian, A.H.; Tangestaninejad, S. Mixed matrix membranes on the basis of Matrimid and palladium-zeolitic imidazolate framework for hydrogen separation. Iran. Polym. J. 2020, 29, 479–491. [Google Scholar] [CrossRef]
- Pal, N.; Saini, N.; Agarwal, M.; Awasthi, K. Experimental investigation of natural polysaccharide-based mixed matrix membrane modified with graphene oxide and Pd-nanoparticles for enhanced gas separation performance. Int. J. Hydrogen Energy 2022, 47, 41820–41832. [Google Scholar] [CrossRef]
- Pal, N.; Agarwal, M. Performance evaluation of biopolymer mixed matrix membrane for CO2/H2 separation. Int. J. Hydrogen Energy 2023, 48, 37762–37773. [Google Scholar] [CrossRef]
- Sajjan, P.; Nayak, V.; Padaki, M.; Zadorozhnyy, V.Y.; Klyamkin, S.N.; Konik, P.A. Fabrication of cellulose acetate film through blending technique with palladium acetate for hydrogen gas separation. Energy Fuels 2020, 34, 11699–11707. [Google Scholar] [CrossRef]
- Markov, A.A.; Merkulov, O.V.; Suntsov, A.Y. Development of membrane reactor coupling hydrogen and syngas production. Membranes 2023, 13, 626. [Google Scholar] [CrossRef]
- Basov, N.L.; Ermilova, M.M.; Orekhova, N.V.; Yaroslavtsev, A.B. Membrane catalysis in the dehydrogenation and hydrogen production processes. Russ. Chem. Rev. 2013, 82, 352–368. [Google Scholar] [CrossRef]
- Helmi, A.; Fernandez, E.; Melendez, J.; Pacheco Tanaka, D.; Gallucci, F.; Van Sint Annaland, M. Fluidized bed membrane reactors for ultra pure H2 production—A step forward towards commercialization. Molecules 2016, 21, 376. [Google Scholar] [CrossRef]
- Iulianelli, A.; Ghasemzadeh, K.; Basile, A. Progress in methanol steam reforming modelling via membrane reactors technology. Membranes 2018, 8, 65. [Google Scholar] [CrossRef]
- Franchi, G.; Capocelli, M.; De Falco, M.; Piemonte, V.; Barba, D. Hydrogen production via steam reforming: A critical analysis of MR and RMM technologies. Membranes 2020, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Alrashed, F.; Zahid, U. Comparative analysis of conventional steam methane reforming and PdAu membrane reactor for the hydrogen production. Comput. Chem. Eng. 2021, 154, 107497. [Google Scholar] [CrossRef]
- Lytkina, A.A.; Orekhova, N.V.; Yaroslavtsev, A.B. Methanol steam reforming in membrane reactors. Pet. Chem. 2018, 58, 911–922. [Google Scholar] [CrossRef]
- Kim, D.; Kellogg, A.; Livaich, E.; Wilhite, B.A. Towards an integrated ceramic micro-membrane network: Electroless-plated palladium membranes in cordierite supports. J. Membr. Sci. 2009, 340, 109–116. [Google Scholar] [CrossRef]
- Tan, X.; Li, K. Membrane microreactors for catalytic reactions. J. Chem. Technol. Biotechnol. 2013, 88, 1771–1779. [Google Scholar] [CrossRef]
- Kurokawa, H.; Yakabe, H.; Yasuda, I.; Peters, T.; Bredesen, R. Inhibition effect of CO on hydrogen permeability of Pd–Ag membrane applied in a microchannel module configuration. Int. J. Hydrogen Energy 2014, 39, 17201–17209. [Google Scholar] [CrossRef]
- Cechetto, V.; Di Felice, L.; Gutierrez Martinez, R.; Arratibel Plazaola, A.; Gallucci, F. Ultra-pure hydrogen production via ammonia decomposition in a catalytic membrane reactor. Int. J. Hydrogen Energy 2022, 47, 21220–21230. [Google Scholar] [CrossRef]
- Pişkin, F.; Öztürk, T. Combinatorial screening of Pd-Ag-Ni membranes for hydrogen separation. J. Membr. Sci. 2017, 524, 631–636. [Google Scholar] [CrossRef]
- Amiri, T.Y.; Ghasemzageh, K.; Iulianelli, A. Membrane reactors for sustainable hydrogen production through steam reforming of hydrocarbons: A review. Chem. Eng. Process. Process Intensif. 2020, 157, 108148. [Google Scholar] [CrossRef]
- Gobina, E.; Hughes, R. Reaction coupling in catalytic membrane reactors. Chem. Eng. Sci. 1996, 51, 3045–3050. [Google Scholar] [CrossRef]
- Kim, C.-H.; Han, J.-Y.; Kim, S.; Lee, B.; Lim, H.; Lee, K.-Y.; Ryi, S.-K. Hydrogen production by steam methane reforming in a membrane reactor equipped with a Pd composite membrane deposited on a porous stainless steel. Int. J. Hydrogen Energy 2018, 43, 7684–7692. [Google Scholar] [CrossRef]
- Fedotov, A.S.; Tsodikov, M.V.; Yaroslavtsev, A.B. Hydrogen production in catalytic membrane reactors based on porous ceramic converters. Processes 2022, 10, 2060. [Google Scholar] [CrossRef]
- Fernandez, E.; Sanchez-Garcia, J.A.; Melendez, J.; Spallina, V.; van Sint Annaland, M.; Gallucci, F.; Pacheco Tanaka, D.A.; Prema, R. Development of highly permeable ultra-thin Pd-based supported membranes. Chem. Eng. J. 2016, 305, 149–155. [Google Scholar] [CrossRef]
- Upadhyay, M.; Lee, H.; Kim, A.; Lee, S.; Lim, H. CFD simulation of methane steam reforming in a membrane reactor: Performance characteristics over range of operating window. Int. J. Hydrogen Energy 2021, 46, 30402–30411. [Google Scholar] [CrossRef]
- Anzelmo, B.; Liguori, S.; Mardilovich, I.; Iulianelli, A.; Ma, Y.-H.; Wilcox, J.; Basile, A. Fabrication & performance study of a palladium on alumina supported membrane reactor: Natural gas steam reforming, a case study. Int. J. Hydrogen Energy 2018, 43, 7713–7721. [Google Scholar] [CrossRef]
- Pati, S.; Jangam, A.; Wang, Z.; Dewangan, N.; Wai, M.H.; Kawi, S. Catalytic Pd0.77Ag0.23 alloy membrane reactor for high temperature water-gas shift reaction: Methane suppression. Chem. Eng. J. 2019, 362, 116–125. [Google Scholar] [CrossRef]
- Didenko, L.P.; Sementsova, L.A.; Babak, V.N.; Chizhov, P.E.; Dorofeeva, T.V.; Kvurt, J.P. Steam reforming of n-butane in membrane reactor with industrial nickel catalyst and foil made of Pd-Ru alloy. Membr. Membr. Technol. 2020, 2, 85–97. [Google Scholar] [CrossRef]
- Alrashed, F.S.; Paglieri, S.N.; Alismail, Z.S.; Khalaf, H.; Harale, A.; Overbeek, J.P.; van Veen, H.M.; Hakeem, A.S. Steam reforming of simulated pre-reformed naphtha in a PdAu membrane reactor. Int. J. Hydrogen Energy 2021, 46, 21939–21952. [Google Scholar] [CrossRef]
- Bang, G.; Moon, D.-K.; Kang, J.-H.; Han, Y.-J.; Kim, K.-M.; Lee, C.-H. High-purity hydrogen production via a water-gas-shift reaction in a palladium-copper catalytic membrane reactor integrated with pressure swing adsorption. Chem. Eng. J. 2021, 411, 128473. [Google Scholar] [CrossRef]
- Fasolini, A.; Mafessanti, R.; Abate, S.; Gramazio, P.; De Maron, J.; Centi, G.; Basile, F. Integration of catalytic methane oxy-reforming and water gas shift membrane reactor for intensified pure hydrogen production and methanation suppression over Ce0.5Zr0.5O2 based catalysts. Catal. Today 2023, 418, 114047. [Google Scholar] [CrossRef]
- Galucci, F.; Basile, A.; Tosti, S.; Iulianelli, A.; Drioli, E. Methanol and ethanol steam reforming in membrane reactors: An experimental study. Int. J. Hydrogen Energy 2007, 32, 1201–1210. [Google Scholar] [CrossRef]
- Shi, Z.; Peng, Q.; Wang, H.; Huang, Z.; Liu, H.; Tian, X.; Yan, F.; Yin, R. Catalyst, reactor, reaction mechanism and CO remove technology in methanol steam reforming for hydrogen production: A review. Fuel Process. Technol. 2023, 252, 108000. [Google Scholar] [CrossRef]
- Mohammed Abbas, A.H.; Cheralathan, K.K.; Porpatham, E.; Arumugam, S.K. Hydrogen generation using methanol steam reforming—Catalysts, reactors, and thermo-chemical recuperation. Renew. Sustain. Energy Rev. 2024, 191, 114147. [Google Scholar] [CrossRef]
- Murmura, M.A.; Patrascu, M.; Annesini, M.C.; Palma, V.; Ruocco, C.; Sheintuch, M. Directing selectivity of ethanol steam reforming in membrane reactors. Int. J. Hydrogen Energy 2015, 40, 5837–5848. [Google Scholar] [CrossRef]
- Liguori, S.; Iulianelli, A.; Dalena, F.; Piemonte, V.; Huang, Y.; Basile, A. Methanol steam reforming in an Al2O3 supported thin Pd-layer membrane reactor over Cu/ZnO/Al2O3 catalyst. Int. J. Hydrogen Energy 2014, 39, 18702–18710. [Google Scholar] [CrossRef]
- Ghasemzadeh, K.; Harasi, J.N.; Amiri, T.Y.; Basile, A.; Iulianelli, A. Methanol steam reforming for hydrogen generation: A comparative modeling study between silica and Pd-based membrane reactors by CFD method. Fuel Process. Technol. 2020, 199, 106273. [Google Scholar] [CrossRef]
- Wang, C.; Weng, J.; Liao, M.; Luo, Q.; Luo, X.; Tian, Z.; Shu, R.; Chen, Y.; Du, Y. Configuration of coupling methanol steam reforming over Cu-based catalyst in a synthetic palladium membrane for one-step high purity hydrogen production. J. Energy Inst. 2023, 108, 101245. [Google Scholar] [CrossRef]
- Di Nardo, A.; Portarapillo, M.; Russo, D.; Di Benedetto, A. Hydrogen production via steam reforming of different fuels: Thermodynamic comparison. Int. J. Hydrogen Energy 2024, 55, 1143–1160. [Google Scholar] [CrossRef]
- Borgognoni, F.; Tosti, S.; Vadrucci, M.; Santucci, A. Combined methane and ethanol reforming for pure hydrogen production through Pd-based membranes. Int. J. Hydrogen Energy 2013, 38, 1430–1438. [Google Scholar] [CrossRef]
- Jia, H.; Zhang, J.; Yu, J.; Yang, X.; Sheng, X.; Xu, H.; Sun, C.; Shen, W.; Goldbach, A. Efficient H2 production via membrane-assisted ethanol steam reforming over Ir/CeO2 catalyst. Int. J. Hydrogen Energy 2019, 44, 24733–24745. [Google Scholar] [CrossRef]
- Lytkina, A.A.; Mironova, E.Y.; Orekhova, N.V.; Ermilova, M.M.; Yaroslavtsev, A.B. Ru-containing catalysts for methanol and ethanol steam reforming in conventional and membrane reactors. Inorg. Mater. 2019, 55, 547–555. [Google Scholar] [CrossRef]
- Jia, H.; Xu, H.; Sheng, X.; Yang, X.; Shen, W.; Goldbach, A. High-temperature ethanol steam reforming in PdCu membrane reactor. J. Membr. Sci. 2020, 605, 118083. [Google Scholar] [CrossRef]
- Martinelli, M.; Castro, J.D.; Alhraki, N.; Matamoros, M.E.; Kropf, A.J.; Cronauer, D.C.; Jacobs, G. Effect of sodium loading on Pt/ZrO2 during ethanol steam reforming. Appl. Catal. A Gen. 2021, 610, 117947. [Google Scholar] [CrossRef]
- Viviente, J.L.; Meléndez, J.; Pacheco Tanaka, D.A.; Gallucci, F.; Spallina, V.; Manzolini, G.; Foresti, S.; Palma, V.; Ruocco, C.; Roses, L. Advanced m-CHP fuel cell system based on a novel bio-ethanol fluidized bed membrane reformer. Int. J. Hydrogen Energy 2017, 42, 13970–13987. [Google Scholar] [CrossRef]
- Iulianelli, A.; Palma, V.; Bagnato, G.; Ruocco, C.; Huang, Y.; Veziroğlu, N.T.; Basile, A. From bioethanol exploitation to high grade hydrogen generation: Steam reforming promoted by a Co-Pt catalyst in a Pd-based membrane reactor. Renew. Energy 2018, 119, 834–843. [Google Scholar] [CrossRef]
- Li, C.; He, Z.; Ban, X.; Li, N.; Chen, C.; Zhan, Z. Membrane-based catalytic partial oxidation of ethanol coupled with steam reforming for solid oxide fuel cells. J. Membr. Sci. 2021, 622, 119032. [Google Scholar] [CrossRef]
- Eremeev, N.; Krasnov, A.; Bespalko, Y.; Bobrova, L.; Smorygo, O.; Sadykov, V. An experimental performance study of a catalytic membrane reactor for ethanol steam reforming over a metal honeycomb catalyst. Membranes 2021, 11, 790. [Google Scholar] [CrossRef]
- Iulianelli, A.; Longo, T.; Basile, A. CO-free hydrogen production by steam reforming of acetic acid carried out in a Pd–Ag membrane reactor: The effect of co-current and counter-current mode. Int. J. Hydrogen Energy 2008, 33, 4091–4096. [Google Scholar] [CrossRef]
- Jiang, J.; Dong, Q.; McCullough, K.; Lauterbach, J.; Li, S.; Yu, M. Novel hollow fiber membrane reactor for high purity H2 generation from thermal catalytic NH3 decomposition. J. Membr. Sci. 2021, 629, 119281. [Google Scholar] [CrossRef]
- Cerrillo, J.L.; Morlanés, N.; Kulkarni, S.R.; Realpe, N.; Ramírez, A.; Katikaneni, S.P.; Paglieri, S.N.; Lee, K.; Harale, A.; Solami, B.; et al. High purity, self-sustained, pressurized hydrogen production from ammonia in a catalytic membrane reactor. Chem. Eng. J. 2022, 431, 134310. [Google Scholar] [CrossRef]
- Fritsch, D.; Bengtson, G. Development of catalytically reactive porous membranes for the selective hydrogenation of sunflower oil. Catal. Today 2006, 118, 121–127. [Google Scholar] [CrossRef]
- Beyer, A.; Schomäcker, R.; Reichert, K.H. Synthesis and characterization of palladium containing membranes based upon polyacrylic acid. Colloid Polym. Sci. 2003, 281, 862–868. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; Comite, A.; Di Felice, R. Polymeric and ceramic membranes in three-phase catalytic membrane reactors for the hydrogenation of methylenecyclohexane. Desalination 2002, 144, 411–416. [Google Scholar] [CrossRef]
- Brandão, L.; Madeira, L.M.; Mendes, A.M. Propyne hydrogenation in a continuous polymeric catalytic membrane reactor. Chem. Eng. Sci. 2007, 62, 6768–6776. [Google Scholar] [CrossRef]
- Brandão, L.; Fritsch, D.; Mendes, A.M.; Madeira, L.M. Propylene hydrogenation in a continuous polymeric catalytic membrane reactor. Ind. Eng. Chem. Res. 2007, 46, 5278–5285. [Google Scholar] [CrossRef]
- Ciebien, J.F.; Cohen, R.E.; Duran, A. Catalytic properties of palladium nanoclusters synthesized within diblock copolymer films: Hydrogenation of ethylene and propylene. Supramol. Sci. 1998, 5, 31–39. [Google Scholar] [CrossRef]
- Ciebien, J.F.; Cohen, R.E.; Duran, A. Membrane catalysts for partial hydrogenation of 1,3-butadiene: Catalytic properties of palladium nanoclusters synthesized within diblock copolymer films. Mater. Sci. Eng. C 1999, 7, 45–50. [Google Scholar] [CrossRef]
- Fritsch, D.; Peinemann, K.V. Catalysis with homogeneous membranes loaded with nanoscale metallic clusters and their preparation. Catal. Today 1995, 25, 277–283. [Google Scholar] [CrossRef]
- Gao, H.; Xu, Y.; Liao, S.; Liu, R.; Liu, J.; Li, D.; Yu, D.; Zhao, Y.; Fan, Y. Catalytic polymeric hollow-fiber reactors for the selective hydrogenation of conjugated dienes. J. Membr. Sci. 1995, 106, 213–219. [Google Scholar] [CrossRef]
- Gröschel, L.; Haidar, R.; Beyer, A.; Cölfen, H.; Frank, B.; Schomäcker, R. Hydrogenation of propyne in palladium-containing polyacrylic acid membranes and its characterization. Ind. Eng. Chem. Res. 2005, 44, 9064–9070. [Google Scholar] [CrossRef]
- Liguori, F.; Barbaro, P.; Giordano, C.; Sawa, H. Partial hydrogenation reactions over Pd-containing hybrid inorganic/polymeric catalytic membranes. Appl. Catal. A Gen. 2013, 459, 81–88. [Google Scholar] [CrossRef]
- Liu, C.; Xu, Y.; Liao, S.; Yu, D.; Zhao, Y.; Fan, Y. Selective hydrogenation of propadiene and propyne in propene with catalytic polymeric hollow-fiber reactor. J. Membr. Sci. 1997, 137, 139–144. [Google Scholar] [CrossRef]
- Liu, C.; Xu, Y.; Liao, S.; Yu, D. Mono- and bimetallic catalytic hollow-fiber reactors for the selective hydrogenation of butadiene in 1-butene. Appl. Catal. A Gen. 1998, 172, 23–29. [Google Scholar] [CrossRef]
- López-Viveros, M.; Favier, I.; Gómez, M.; Lahitte, J.F.; Remigy, J.C. Remarkable catalytic activity of polymeric membranes containing gel-trapped palladium nanoparticles for hydrogenation reactions. Catal. Today 2021, 364, 263–269. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, Y.; Liao, S.; Liu, R. Catalytic behaviors and gas permeation properties of palladium-containing phenophthalein poly(ether sulfone). J. Appl. Polym. Sci. 1996, 61, 599–605. [Google Scholar] [CrossRef]
- Ziegler, S.; Theis, J.; Fritsch, D. Palladium modified porous polymeric membranes and their performance in selective hydrogenation of propyne. J. Membr. Sci. 2001, 187, 71–84. [Google Scholar] [CrossRef]
- Bengtson, G.; Scheel, H.; Theis, J.; Fritsch, D. Catalytic membrane reactor to simultaneously concentrate and react organics. Chem. Eng. J. 2002, 85, 303–311. [Google Scholar] [CrossRef]
- Fritsch, D.; Kuhr, K.; Mackenzie, K.; Kopinke, F.D. Hydrodechlorination of chloroorganic compounds in ground water by palladium catalysts: Part 1. Development of polymer-based catalysts and membrane reactor tests. Catal. Today 2003, 82, 105–118. [Google Scholar] [CrossRef]
- Yamada, Y.M.A.; Watanabe, T.; Ohno, A.; Uozumi, Y. Development of polymeric palladium-nanoparticle membrane-installed microflow devices and their application in hydrodehalogenation. ChemSusChem 2012, 5, 293–299. [Google Scholar] [CrossRef]
- Domènech, B.; Muñoz, M.; Muraviev, D.N.; Macanás, J. Catalytic membranes with palladium nanoparticles: From tailored polymer to catalytic applications. Catal. Today 2012, 193, 158–164. [Google Scholar] [CrossRef]
- He, Y.; Cheshomi, N.; Lawson, S.M.; Itta, A.K.; Rezaei, F.; Kapila, S.; Rownaghi, A.A. PDMS/PAI-HF composite membrane containing immobilized palladium nanoparticles for 4-nitrophenol reduction. Chem. Eng. J. 2021, 410, 128326. [Google Scholar] [CrossRef]
- Rajlaxmi; Gupta, N.; Behere, R.P.; Layek, R.K.; Kuila, B.K. Polymer nanocomposite membranes and their application for flow catalysis and photocatalytic degradation of organic pollutants. Mater. Today Chem. 2021, 22, 100600. [Google Scholar] [CrossRef]
- Gu, Y.; Favier, I.; Pradel, C.; Gin, D.L.; Lahitte, J.F.; Noble, R.D.; Gómez, M.; Remigy, J.C. High catalytic efficiency of palladium nanoparticles immobilized in a polymer membrane containing poly(ionic liquid) in Suzuki-Miyaura cross-coupling reaction. J. Membr. Sci. 2015, 492, 331–339. [Google Scholar] [CrossRef]
- Bahadorikhalili, S.; Mahdavi, H. Palladium magnetic nanoparticle-polyethersulfone composite membrane as an efficient and versatile catalytic membrane reactor. Polym. Adv. Technol. 2018, 29, 1138–1149. [Google Scholar] [CrossRef]
- Ahmed, M.; Dincer, I. A review on methanol crossover in direct methanol fuel cells: Challenges and achievements. Int. J. Energy Res. 2011, 35, 1213–1228. [Google Scholar] [CrossRef]
- Kusoglu, A.; Weber, A.Z. New insights into perfluorinated sulfonic-acid ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef]
- Sengupta, S.; Lyulin, A.V. Molecular modeling of structure and dynamics of Nafion protonation states. J. Phys. Chem. B 2019, 123, 6882–6891. [Google Scholar] [CrossRef]
- Prikhno, I.A.; Safronova, E.Y.; Stenina, I.A.; Yurova, P.A.; Yaroslavtsev, A.B. Dependence of the transport properties of perfluorinated sulfonated cation-exchange membranes on ion-exchange capacity. Membr. Membr. Technol. 2020, 2, 265–271. [Google Scholar] [CrossRef]
- Islam, M.N.; Mansoor Basha, A.B.; Kollath, V.O.; Soleymani, A.P.; Jankovic, J.; Karan, K. Designing fuel cell catalyst support for superior catalytic activity and low mass-transport resistance. Nat. Commun. 2022, 13, 6157. [Google Scholar] [CrossRef]
- Ayoub, M.; Böhm, T.; Bierling, M.; Thiele, S.; Brodt, M. Review—Graded catalyst layers in hydrogen fuel cells—A pathway to application-tailored cells. J. Electrochem. Soc. 2024, 171, 094503. [Google Scholar] [CrossRef]
- Ghosh, S.; Das, S.; Mosquera, M.E.G. Conducting polymer-based nanohybrids for fuel cell application. Polymers 2020, 12, 2993. [Google Scholar] [CrossRef] [PubMed]
- Sajid, A.; Pervaiz, E.; Ali, H.; Noor, T.; Baig, M.M. A perspective on development of fuel cell materials: Electrodes and electrolyte. Int. J. Energy Res. 2022, 46, 6953–6988. [Google Scholar] [CrossRef]
- Halim, E.M.; Chemchoub, S.; El Attar, A.; Salih, F.E.; Oularbi, L.; EL RHAZI, M. Recent Advances in Anode Metallic Catalysts Supported on Conducting Polymer-Based Materials for Direct Alcohol Fuel Cells. Front. Energy Res. 2022, 10, 843736. [Google Scholar] [CrossRef]
- Long, N.V.; Thi, C.M.; Yong, Y.; Nogami, M.; Ohtaki, M. Platinum and palladium nano-structured catalysts for polymer electrolyte fuel cells and direct methanol fuel cells. J. Nanosci. Nanotechnol. 2013, 13, 4799–4824. [Google Scholar] [CrossRef]
- Asghar, M.R.; Xu, Q. A review of advancements in commercial and non-commercial Nafion-based proton exchange membranes for direct methanol fuel cells. J. Polym. Res. 2024, 31, 125. [Google Scholar] [CrossRef]
- Yaroslavtsev, A.B.; Karavanova, Y.A.; Safronova, E.Y. Ionic conductivity of hybrid membranes. Pet. Chem. 2011, 51, 473–479. [Google Scholar] [CrossRef]
- Kim, D.; Sauk, J.; Byun, J.; Lee, K.S.; Kim, H. Palladium composite membranes using supercritical CO2 impregnation method for direct methanol fuel cells. Solid State Ionics 2007, 178, 865–870. [Google Scholar] [CrossRef]
- Das, P.; Mandal, B.; Gumma, S. L-tyrosine grafted palladium graphite oxide and sulfonated poly(ether ether ketone) based novel composite membrane for direct methanol fuel cell. Chem. Eng. J. 2021, 423, 130235. [Google Scholar] [CrossRef]
- Kim, Y.M.; Park, K.W.; Choi, J.H.; Park, I.S.; Sung, Y.E. A Pd-impregnated nanocomposite Nafion membrane for use in high-concentration methanol fuel in DMFC. Electrochem. Commun. 2003, 5, 571–574. [Google Scholar] [CrossRef]
- Kim, Y.J.; Choi, W.C.; Woo, S.I.; Hong, W.H. Evaluation of a palladinized NafionTM for direct methanol fuel cell application. Electrochim. Acta 2004, 49, 3227–3234. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, Y.M.; Lee, J.S.; Cho, K.Y.; Jung, H.Y.; Park, J.K.; Park, I.S.; Sung, Y.E. A polyaniline supported PtRu nanocomposite anode and a Pd-impregnated nanocomposite Nafion membrane for DMFCs. Solid State Ionics 2005, 176, 3031–3034. [Google Scholar] [CrossRef]
- Jiang, R.; Zhang, Y.; Swier, S.; Wei, X.; Erkey, C.; Kunz, H.R.; Fenton, J.M. Preparation via supercritical fluid route of Pd-impregnated nafion membranes which exhibit reduced methanol crossover for DMFC. Electrochem. Solid-State Lett. 2005, 8, 611–615. [Google Scholar] [CrossRef]
- Tian, A.H.; Kim, J.Y.; Shi, J.Y.; Kim, K. Poly(1-vinylimidazole)/Pd-impregnated Nafion for direct methanol fuel cell applications. J. Power Sources 2008, 183, 1–7. [Google Scholar] [CrossRef]
- Iwai, Y.; Ikemoto, S.; Haramaki, K.; Hattori, R.; Yonezawa, S. Influence of ligands of palladium complexes on palladium/Nafion composite membranes for direct methanol fuel cells by supercritical CO2 impregnation method. J. Supercrit. Fluids 2014, 94, 48–58. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Nie, L.; Liu, X.; Chang, L. Modification of Nafion membrane by Pd-impregnation via electric field. J. Power Sources 2012, 216, 526–529. [Google Scholar] [CrossRef]
- Shim, J.H.; Koo, I.G.; Lee, W.M. Nafion-impregnated polyethylene-terephthalate film used as the electrolyte for direct methanol fuel cells. Electrochim. Acta 2005, 50, 2385–2391. [Google Scholar] [CrossRef]
- Yoon, S.R.; Hwang, G.H.; Cho, W.I.; Oh, I.H.; Hong, S.A.; Ha, H.Y. Modification of polymer electrolyte membranes for DMFCs using Pd films formed by sputtering. J. Power Sources 2002, 106, 215–223. [Google Scholar] [CrossRef]
- Ma, Z.Q.; Cheng, P.; Zhao, T.S. A palladium-alloy deposited Nafion membrane for direct methanol fuel cells. J. Membr. Sci. 2003, 215, 327–336. [Google Scholar] [CrossRef]
- Hejze, T.; Gollas, B.R.; Sauerbrey, R.K.; Schmied, M.; Hofer, F.; Besenhard, J.O. Preparation of Pd-coated polymer electrolyte membranes and their application in direct methanol fuel cells. J. Power Sources 2005, 140, 21–27. [Google Scholar] [CrossRef]
- Prabhuram, J.; Zhao, T.S.; Liang, Z.X.; Yang, H.; Wong, C.W. Pd and Pd-Cu Alloy Deposited Nafion Membranes for Reduction of Methanol Crossover in Direct Methanol Fuel Cells. J. Electrochem. Soc. 2005, 152, A1390. [Google Scholar] [CrossRef]
- Sun, H.; Sun, G.; Wang, S.; Liu, J.; Zhao, X.; Wang, G.; Xu, H.; Hou, S.; Xin, Q. Pd electroless plated Nafion® membrane for high concentration DMFCs. J. Membr. Sci. 2005, 259, 27–33. [Google Scholar] [CrossRef]
- Tian, A.H.; Kim, J.Y.; Shi, J.Y.; Kim, K.; Lee, K. Surface-modified Nafion membrane by oleylamine-stabilized Pd nanoparticles for DMFC applications. J. Power Sources 2007, 167, 302–308. [Google Scholar] [CrossRef]
- Tian, A.H.; Kim, J.Y.; Shi, J.Y.; Lee, K.; Kim, K. Surface-modified Nafion membrane by trioctylphosphine-stabilized palladium nanoparticles for DMFC applications. J. Phys. Chem. Solids 2009, 70, 1207–1212. [Google Scholar] [CrossRef]
- Tang, H.; Pan, M.; Jiang, S.; Wan, Z.; Yuan, R. Self-assembling multi-layer Pd nanoparticles onto NafionTM membrane to reduce methanol crossover. Colloids Surfaces A Physicochem. Eng. Asp. 2005, 262, 65–70. [Google Scholar] [CrossRef]
- Brandão, L.; Rodrigues, J.; Madeira, L.M.; Mendes, A. Methanol crossover reduction by Nafion modification with palladium composite nanoparticles: Application to direct methanol fuel cells. Int. J. Hydrogen Energy 2010, 35, 11561–11567. [Google Scholar] [CrossRef]
- Thiam, H.S.; Daud, W.R.W.; Kamarudin, S.K.; Mohamad, A.B.; Kadhum, A.A.H.; Loh, K.S.; Majlan, E.H. Nafion/Pd-SiO2 nanofiber composite membranes for direct methanol fuel cell applications. Int. J. Hydrogen Energy 2013, 38, 9474–9483. [Google Scholar] [CrossRef]
- Pethaiah, S.S.; Ulaganathan, M.; Ramalinga Viswanathan, M.; Chan, S.H. Fabrication and electrochemical characterization of Pt-Pd impregnated nanocomposite polymer electrolyte membranes for high concentration DMFCs. RSC Adv. 2015, 5, 981–987. [Google Scholar] [CrossRef]
- Casalegno, A.; Bresciani, F.; Di Noto, V.; Casari, C.S.; Li Bassi, A.; Negro, E.; Marchesi, R.; Di Fonzo, F. Nanostructured Pd barrier for low methanol crossover DMFC. Int. J. Hydrogen Energy 2014, 39, 2801–2811. [Google Scholar] [CrossRef]
- Datta, J.; Dutta, A.; Biswas, M. Enhancement of functional properties of PtPd nano catalyst in metal-polymer composite matrix: Application in direct ethanol fuel cell. Electrochem. Commun. 2012, 20, 56–59. [Google Scholar] [CrossRef]
- Siwal, S.; Matseke, S.; Mpelane, S.; Hooda, N.; Nandi, D.; Mallick, K. Palladium-polymer nanocomposite: An anode catalyst for the electrochemical oxidation of methanol. Int. J. Hydrogen Energy 2017, 42, 23599–23605. [Google Scholar] [CrossRef]
- Kannan, R.; Kim, A.R.; Yoo, D.J. Enhanced electrooxidation of methanol, ethylene glycol, glycerol, and xylitol over a polypyrrole/manganese oxyhydroxide/palladium nanocomposite electrode. J. Appl. Electrochem. 2014, 44, 893–902. [Google Scholar] [CrossRef]
- Arukula, R.; Vinothkannan, M.; Kim, A.R.; Yoo, D.J. Cumulative effect of bimetallic alloy, conductive polymer and graphene toward electrooxidation of methanol: An efficient anode catalyst for direct methanol fuel cells. J. Alloys Compd. 2019, 771, 477–488. [Google Scholar] [CrossRef]
- Boulaghi, M.; Ghafouri Taleghani, H.; Soleimani Lashkenari, M.; Ghorbani, M. Platinum-palladium nanoparticles-loaded on N-doped graphene oxide/polypyrrole framework as a high performance electrode in ethanol oxidation reaction. Int. J. Hydrogen Energy 2018, 43, 15164–15175. [Google Scholar] [CrossRef]
- De, A.; Adhikary, R.; Datta, J. Transition metal oxide-polymer composite supported PtPd/PNVC-WO3 nano-catalyst: Multifaceted functional behavior boosting the performance of ethanol oxidation kinetics. Mater. Chem. Phys. 2024, 313, 128794. [Google Scholar] [CrossRef]
- Mozafari, V.; Basiri Parsa, J. Electrochemical synthesis of Pd supported on PANI-MWCNTs-SnO2 nanocomposite as a novel catalyst towards ethanol oxidation in alkaline media. Synth. Met. 2020, 259, 116214. [Google Scholar] [CrossRef]
- Yaroslavtsev, A.B.; Dobrovolsky, Y.A.; Shaglaeva, N.S.; Frolova, L.A.; Gerasimova, E.V.; Sanginov, E.A. Nanostructured materials for low-temperature fuel cells. Russ. Chem. Rev. 2012, 81, 191–220. [Google Scholar] [CrossRef]
- Lashkenari, M.S.; Rezaei, S.; Fallah, J. Experimental and theoretical studies of methanol oxidation in the presence of Co-Pd @ polyaniline/N-doped reduced graphene oxide electrocatalyst. J. Appl. Electrochem. 2021, 51, 1267–1278. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y.; Liu, Y.; Zhou, W.; Zhen, X.; Lang, M.F. Facile fabrication of a flexible electrode by electrodeposition of palladium on silver nanowires for ethanol oxidation. Int. J. Hydrogen Energy 2019, 44, 5990–5996. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, H.; Lin, Z.; Ma, S.; Ren, L.; Ren, L. High specific surface area Pd/Pt electrode-based ionic polymer–metal composite for high-performance biomimetic actuation. ACS Sustain. Chem. Eng. 2022, 10, 2645–2652. [Google Scholar] [CrossRef]
- Xu, G.; Du, X.; Ding, W.; Ma, S.; Zhang, L.; Li, J.; Huang, J.; Song, J.; Liang, D. Nano-Pd loaded composite membrane for reduced hydrogen crossover in proton exchange membrane water electrolysis via recasting method. Renew. Energy 2024, 235, 121285. [Google Scholar] [CrossRef]
- Tai, H.; Duan, Z.; Wang, Y.; Wang, S.; Jiang, Y. Paper-based sensors for gas, humidity, and strain detections: A review. ACS Appl. Mater. Interfaces 2020, 12, 31037–31053. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.S.; Kim, K.H.; Lee, J.S.; Kim, S.H.; Yoo, J.Y.; Choi, K.W.; Kim, B.J.; Kwon, D.S.; Yoo, I.; Yang, J.S.; et al. Ultrafast (∼0.6 s), robust, and highly linear hydrogen detection up to 10% using fully suspended pure Pd nanowire. ACS Nano 2023, 17, 23649–23658. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Thundat, T.; Swihart, M.T. Ultrathin palladium nanowires for fast and hysteresis-free H2 sensing. ACS Appl. Nano Mater. 2022, 5, 5895–5905. [Google Scholar] [CrossRef]
- Kumar, A.; Chen, K.; Thundat, T.; Swihart, M.T. Paper-based hydrogen sensors using ultrathin palladium nanowires. ACS Appl. Mater. Interfaces 2023, 15, 5439–5448. [Google Scholar] [CrossRef]
- Kumar, A.; Zhao, Y.; Abraham, S.R.; Thundat, T.; Swihart, M.T. Pd alloy nanosheet inks for inkjet-printable H2 sensors on paper. Adv. Mater. Interfaces 2022, 9, 2200363. [Google Scholar] [CrossRef]
- Kumar, A.; Zhao, Y.; Mohsenifard, S.; Maheshkar, V.; Thundat, T.; Swihart, M.T. Platinum decorated palladium nanowires for room-temperature hydrogen detection. Adv. Sens. Res. 2024, 3, 2400013. [Google Scholar] [CrossRef]
- Mahadeva, S.K.; Walus, K.; Stoeber, B. Paper as a platform for sensing applications and other devices: A review. ACS Appl. Mater. Interfaces 2015, 7, 8345–8362. [Google Scholar] [CrossRef]
- Darmadi, I.; Nugroho, F.A.A.; Langhammer, C. High-performance nanostructured palladium-based hydrogen sensors—Current limitations and strategies for their mitigation. ACS Sens. 2020, 5, 3306–3327. [Google Scholar] [CrossRef]
- Koo, W.T.; Kim, Y.; Kim, S.; Suh, B.L.; Savagatrup, S.; Kim, J.; Lee, S.J.; Swager, T.M.; Kim, I.D. Hydrogen sensors from composites of ultra-small bimetallic nanoparticles and porous ion-exchange polymers. Chem 2020, 6, 2746–2758. [Google Scholar] [CrossRef]
- Yuan, S.; Zeng, S.; Hu, Y.; Kong, W.; Yang, H.; Gong, P.; Xiao, T.; Wang, H.; Wan, H.; Li, Q.; et al. Epitaxial metal-organic framework-mediated electron relay for H2 detection on demand. ACS Nano 2024, 18, 19723–19731. [Google Scholar] [CrossRef]
- Abascal, E.; Gómez-Coma, L.; Ortiz, I.; Ortiz, A. Global diagnosis of nitrate pollution in groundwater and review of removal technologies. Sci. Total Environ. 2022, 810, 152233. [Google Scholar] [CrossRef] [PubMed]
- Mutegoa, E. Efficient techniques and practices for wastewater treatment: An update. Discov. Water 2024, 4, 69. [Google Scholar] [CrossRef]
- Kordbacheh, F.; Heidari, G. Water pollutants and approaches for their removal. Mater. Chem. Horizons 2023, 2, 139–153. [Google Scholar] [CrossRef]
- Saravanan, A.; Senthil Kumar, P.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P.R.; Reshma, B. Effective water/wastewater treatment methodologies for toxic pollutants removal: Processes and applications towards sustainable development. Chemosphere 2021, 280, 130595. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Duttagupta, S.; Mukherjee, A. Emerging organic contaminants in global community drinking water sources and supply: A review of occurrence, processes and remediation. J. Environ. Chem. Eng. 2022, 10, 107560. [Google Scholar] [CrossRef]
- Farghali, M.; Chen, Z.; Osman, A.I.; Ali, I.M.; Hassan, D.; Ihara, I.; Rooney, D.W.; Yap, P.-S. Strategies for ammonia recovery from wastewater: A review. Environ. Chem. Lett. 2024, 22, 2699–2751. [Google Scholar] [CrossRef]
- Lapworth, D.J.; Boving, T.B.; Kreamer, D.K.; Kebede, S.; Smedley, P.L. Groundwater quality: Global threats, opportunities and realising the potential of groundwater. Sci. Total Environ. 2022, 811, 152471. [Google Scholar] [CrossRef]
- Kianpour, A.; Yargholi, B.; Shrafati, A.; Akhavan, K. Examining the efficacy of biological filters in the removal of agricultural pesticides and nutrient elements from agricultural drainage water. Sci. Rep. 2024, 14, 21507. [Google Scholar] [CrossRef]
- Lee, J.; Baek, S.-M.; Boo, C.; Son, A.; Jung, H.; Park, S.S.; Hong, S.W. Water deoxygenation using a hollow fiber membrane contactor to prevent pipe corrosion for sustainable management of district heating systems: A pilot-scale study. J. Clean. Prod. 2020, 277, 124049. [Google Scholar] [CrossRef]
- Rongwong, W.; Goh, K. Resource recovery from industrial wastewaters by hydrophobic membrane contactors: A review. J. Environ. Chem. Eng. 2020, 8, 104242. [Google Scholar] [CrossRef]
- Ran, W.; Zhao, H.; Zhang, X.; Li, S.; Sun, J.-F.; Liu, J.; Liu, R.; Jiang, G. Critical review of Pd-catalyzed reduction process for treatment of waterborne pollutants. Environ. Sci. Technol. 2024, 58, 3079–3097. [Google Scholar] [CrossRef] [PubMed]
- Marks, R.; Seaman, J.; Perez-Calleja, P.; Kim, J.; Nerenberg, R.; Doudrick, K. Catalytic hydrogel membrane reactor for treatment of aqueous contaminants. Environ. Sci. Technol. 2019, 53, 6492–6500. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, V.I.; Gryaznov, V.M.; Petrova, I.V.; Volkov, V.V.; Tereshchenko, G.F.; Shkol’nikov, E.I.; Plyasova, L.M.; Kochubey, D.I.; van der Vaart, R.; van Soest-Verecammen, E.L.J. Porous Pd-containing polypropylene membranes for catalytic water deoxygenation. Kinet. Catal. 2006, 47, 867–872. [Google Scholar] [CrossRef]
- Du, Z.; Ran, W.; Zhao, H.; Ma, J.; Sun, J.; Liu, B.; Lin, S.; Liu, R. Fully exposed Pd ensembles on ultrathin Co3O4 nanosheets: A reductive–oxidative dual-active catalyst for the detoxification of chlorophenol. ACS ES&T Eng. 2024, 4, 1401–1411. [Google Scholar] [CrossRef]
- Shenoy, C.S.; Khan, T.S.; Verma, K.; Tsige, M.; Jha, K.C.; Haider, M.A.; Gupta, S. Understanding the origin of structure sensitivity in hydrodechlorination of trichloroethylene on a palladium catalyst. React. Chem. Eng. 2021, 6, 2270–2279. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Li, J.; Wang, S.; Li, G.; Liu, X. Hydrodechlorination and deep hydrogenation on single-palladium-atom-based heterogeneous catalysts. Appl. Catal. B Environ. 2021, 282, 119518. [Google Scholar] [CrossRef]
- Lin, Y.; Cao, Y.; Yao, Q.; Chai, O.J.H.; Xie, J. Engineering noble metal nanomaterials for pollutant decomposition. Ind. Eng. Chem. Res. 2020, 59, 20561–20581. [Google Scholar] [CrossRef]
- Liu, M.; Chen, G.; Song, Z.; He, Z.; Zhong, A.; Cui, M. Catalytic dechlorination of three organochlorides by recyclable nano-palladium-engineered natural sponge with formic acid. Catalysts 2024, 14, 424. [Google Scholar] [CrossRef]
- Fan, B.; Chen, S.; Zhu, C.; Zhu, F.; Gong, Z.; Wang, Y.; Mao, Y.; Lei, B.; Zhou, D.; He, F.; et al. Mechanochemical synthesis of microscale carbon-modified Fe/Cu bimetallic particles for perchloroethylene degradation. ACS ES&T Water 2025, 5, 197–208. [Google Scholar] [CrossRef]
- Gong, L.; Qiu, X.; Cheng, D.; Hu, Y.; Zhang, Z.; Yuan, Q.; Yang, D.; Liu, C.; Liang, L.; He, F. Coincorporation of N and S into zero-valent iron to enhance TCE dechlorination: Kinetics, electron rfficiency, and dechlorination capacity. Environ. Sci. Technol. 2021, 55, 16088–16098. [Google Scholar] [CrossRef]
- Fan, B.; Zhou, B.; Chen, S.; Zhu, F.; Chen, B.; Gong, Z.; Wang, X.; Zhu, C.; Zhou, D.; He, F.; et al. Preparation of Fe/Cu bimetals by ball milling iron powder and copper sulfate for trichloroethylene degradation: Combined effect of FeS and Fe/Cu alloy. J. Hazard. Mater. 2023, 460, 132402. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Iglesias-Juez, A.; Hungría, A.B.; Martin-Martinez, M.; Bedia, J.; Rodriguez, J.J.; Gómez-Sainero, L.M. Upgrading of dichloromethane to olefins by hydrodechlorination: Improving process efficiency by the addition of Fe to carbon nanotubes-supported Pd catalyst. Chem. Eng. J. 2024, 492, 152128. [Google Scholar] [CrossRef]
- Tsu, Y.-T.; Chen, Y.-W. Preparation of gold-containing binary metal clusters by co-deposition-precipitation method and for hydrogenation of chloronitrobenzene. AIMS Mater. Sci. 2017, 4, 738–754. [Google Scholar] [CrossRef]
- Li, D.-C.; Tian, Z.; Huang, X.; Zhang, W.; Wang, W.; Zhang, Q.; Deng, X.; Wang, G.-H. Hierarchically porous and flexible chitin-fiber/melamine-sponge composite filter with high-loading of PdAu nanoparticles for effective hydrodechlorination of chlorophenols. J. Hazard. Mater. 2024, 479, 135683. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, J.; Meng, F.; Yang, K.; Lin, D. Modification of Pd nanoparticles with lower work function elements for enhanced formic acid dehydrogenation and trichloroethylene dechlorination. ACS Appl. Mater. Interfaces 2022, 14, 30735–30745. [Google Scholar] [CrossRef]
- Martin-Martinez, M.; Gómez-Sainero, L.M. Progress in catalytic hydrodechlorination. Catalysts 2021, 11, 272. [Google Scholar] [CrossRef]
- Flid, M.R.; Kartashov, L.M.; Treger, Y.A. Theoretical and applied aspects of hydrodechlorination processes—Catalysts and technologies. Catalysts 2020, 10, 216. [Google Scholar] [CrossRef]
- Tian, S.; Chen, Y.; Wen, X.; Li, B.; Lu, J.; Li, Z.; Feng, F.; Wang, Q.; Zhang, Q.; Li, X. Hydrodechlorination of trifluoro-trichloroethane to chlorotrifluoroethylene: Revealing the deactivation mechanism and regeneration strategy of Pd-Cu/AC catalyst. Chin. J. Chem. Eng. 2024, 70, 261–268. [Google Scholar] [CrossRef]
- del Olmo, R.B.; Torres, M.; Nieto-Sandoval, J.; Munoz, M.; de Pedro, Z.M.; Casas, J.A. Precious metal-based catalytic membrane reactors for continuous flow catalytic hydrodechlorination. J. Environ. Chem. Eng. 2024, 12, 112754. [Google Scholar] [CrossRef]
- Wang, W.; Nadagouda, M.N.; Mukhopadhyay, S.M. Advances in matrix-supported palladium nanocatalysts for water treatment. Nanomaterials 2022, 12, 3593. [Google Scholar] [CrossRef]
- Kowalewski, E.; Zienkiewicz-Machnik, M.; Lisovytskiy, D.; Nikiforov, K.; Matus, K.; Śrębowata, A.; Sá, J. Turbostratic carbon supported palladium as an efficient catalyst for reductive purification of water from trichloroethylene. AIMS Mater. Sci. 2017, 4, 1276–1288. [Google Scholar] [CrossRef]
- Meduri, K.; Stauffer, C.; Qian, W.; Zietz, O.; Barnum, A.; O’Brien Johnson, G.; Fan, D.; Ji, W.; Zhang, C.; Tratnyek, P.; et al. Palladium and gold nanoparticles on carbon supports as highly efficient catalysts for effective removal of trichloroethylene. J. Mater. Res. 2018, 33, 2404–2413. [Google Scholar] [CrossRef]
- Jeon, J.; Park, Y.; Hwang, Y. Catalytic hydrodechlorination of 4-chlorophenol by palladium-based catalyst supported on alumina and graphene materials. Nanomaterials 2023, 13, 1564. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Sandoval, J.; Gomez-Herrero, E.; Munoz, M.; de Pedro, Z.M.; Casas, J.A. Palladium-based catalytic membrane reactor for the continuous flow hydrodechlorination of chlorinated micropollutants. Appl. Catal. B Environ. 2021, 293, 120235. [Google Scholar] [CrossRef]
- Dittmeyer, R.; Höllein, V.; Daub, K. Membrane reactors for hydrogenation and dehydrogenation processes based on supported palladium. J. Mol. Catal. A Chem. 2001, 173, 135–184. [Google Scholar] [CrossRef]
- Zhou, D.; Luo, Y.-H.; Zheng, C.-W.; Long, M.; Long, X.; Bi, Y.; Zheng, X.; Zhou, C.; Rittmann, B.E. H2-based membrane catalyst-film reactor (H2-MCfR) loaded with palladium for removing oxidized contaminants in water. Environ. Sci. Technol. 2021, 55, 7082–7093. [Google Scholar] [CrossRef]
- Xu, J.; Bhattacharyya, D. Fe/Pd nanoparticle immobilization in microfiltration membrane pores: Synthesis, characterization, and application in the dechlorination of polychlorinated biphenyls. Ind. Eng. Chem. Res. 2007, 46, 2348–2359. [Google Scholar] [CrossRef]
- Wan, H.; Islam, M.S.; Briot, N.J.; Schnobrich, M.; Pacholik, L.; Ormsbee, L.; Bhattacharyya, D. Pd/Fe nanoparticle integrated PMAA-PVDF membranes for chloro-organic remediation from synthetic and site groundwater. J. Membr. Sci. 2020, 594, 117454. [Google Scholar] [CrossRef]
- Wan, H.; Saiful Islam, M.; Tarannum, T.; Shi, K.; Mills, R.; Yi, Z.; Fang, F.; Lei, L.; Li, S.; Ormsbee, L.; et al. Reactive membranes for groundwater remediation of chlorinated aliphatic hydrocarbons: Competitive dechlorination and cost aspects. Sep. Purif. Technol. 2023, 320, 123955. [Google Scholar] [CrossRef]
- Luo, Y.-H.; Zhou, C.; Bi, Y.; Long, X.; Wang, B.; Tang, Y.; Krajmalnik-Brown, R.; Rittmann, B.E. Long-term continuous co-reduction of 1,1,1-trichloroethane and trichloroethene over palladium nanoparticles spontaneously deposited on H2-transfer membranes. Environ. Sci. Technol. 2021, 55, 2057–2066. [Google Scholar] [CrossRef]
- Cai, Y.; Long, X.; Luo, Y.-H.; Zhou, C.; Rittmann, B.E. Stable dechlorination of Trichloroacetic Acid (TCAA) to acetic acid catalyzed by palladium nanoparticles deposited on H2-transfer membranes. Water Res. 2021, 192, 116841. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lin, M.; Cai, Y.; Luo, Y.-H.; Zhou, D.; Rittmann, B.E. Complete dehalogenation of chloramphenicol by bimetallic alloy Pd-Au nanoparticles in a H2-Based membrane Catalyst-Film reactor. Chem. Eng. J. 2024, 497, 154758. [Google Scholar] [CrossRef]
- Petrova, I.V.; Anokhina, T.S.; Borisov, R.S.; Volkov, V.V.; Yaroslavtsev, A.B. Removal of trichloroethylene from water in the catalytic membrane reactor. Catal. Today 2016, 268, 150–155. [Google Scholar] [CrossRef]
- Wongwailikhit, K.; Warunyuwong, P.; Chawaloesphonsiya, N.; Dietrich, N.; Hébrard, G.; Painmanakul, P. Gas sparger orifice sizes and solid particle characteristics in a bubble column—Relative effect on hydrodynamics and mass transfer. Chem. Eng. Technol. 2018, 41, 461–468. [Google Scholar] [CrossRef]
- Jun, Y.-D. Degassing dissolved oxygen through bubbling: The contribution and control of vapor bubbles. Processes 2023, 11, 3158. [Google Scholar] [CrossRef]
- Jokar, S.; Aghel, B.; Fathi, S.; Karimi, M. Removal of dissolved oxygen from industrial raw water in a microchannel. Environ. Technol. Innov. 2021, 23, 101672. [Google Scholar] [CrossRef]
- Wang, Y.; Du, C.; Yan, Z.; Duan, W.; Deng, J.; Luo, G. Fast deoxygenation in a miniaturized annular centrifugal device. Sep. Purif. Technol. 2022, 297, 121546. [Google Scholar] [CrossRef]
- Shindo, M.; Yamamoto, T.; Kamada, K. Gas Transfer Process with Hollow Fiber Membrane. U.S. Patent 4,268,279, 19 May 1981. [Google Scholar]
- Moradian, A.; Delijani, F.; Ekhtiary, F. The effect of different parameters on the efficiency of the catalytic reduction of dissolved oxygen. In Thermal Power Plants—Advanced Applications; InTech: Rijeka, Croatia, 2013; pp. 143–152. [Google Scholar]
- Qing, W.; Li, X.; Shao, S.; Shi, X.; Wang, J.; Feng, Y.; Zhang, W.; Zhang, W. Polymeric catalytically active membranes for reaction-separation coupling: A review. J. Membr. Sci. 2019, 583, 118–138. [Google Scholar] [CrossRef]
- Tai, M.S.L.; Chua, I.; Li, K.; Ng, W.J.; Teo, W.K. Removal of dissolved oxygen in ultrapure water production using microporous membrane modules. J. Membr. Sci. 1994, 87, 99–105. [Google Scholar] [CrossRef]
- Volkov, V.V.; Lebedeva, V.I.; Petrova, I.V.; Bobyl, A.V.; Konnikov, S.G.; Roldughin, V.I.; van Erkel, J.; Tereshchenko, G.F. Adlayers of palladium particles and their aggregates on porous polypropylene hollow fiber membranes as hydrogenization contractors/reactors. Adv. Colloid Interface Sci. 2011, 164, 144–155. [Google Scholar] [CrossRef]
- Pich, R.; Lahitte, J.-F.; Remigy, J.-C.; Pla, D.; Gómez, M. Metal nanoparticles on polymeric membranes applied in catalytic hydrogenations. In Surface Functionalized Metal Catalysts. Topics in Organometallic Chemistry; Martínez-Prieto, L.M., Ed.; Springer: Cham, Switzerland, 2024; Volume 75, pp. 47–104. [Google Scholar]
- Zhou, C.; Wang, Z.; Ontiveros-Valencia, A.; Long, M.; Lai, C.; Zhao, H.; Xia, S.; Rittmann, B.E. Coupling of Pd nanoparticles and denitrifying biofilm promotes H2-based nitrate removal with greater selectivity towards N2. Appl. Catal. B Environ. 2017, 206, 461–470. [Google Scholar] [CrossRef]
- Hörold, S.; Vorlop, K.-D.; Tacke, T.; Sell, M. Development of catalysts for a selective nitrate and nitrite removal from drinking water. Catal. Today 1993, 17, 21–30. [Google Scholar] [CrossRef]
- Boasiako, C.A.; Zhou, Z.; Huo, X.; Ye, T. Development of Pd-based catalysts for hydrogenation of nitrite and nitrate in water: A review. J. Hazard. Mater. 2023, 446, 130661. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.; Ortiz, A.; Ortiz, I. State-of-the-art and perspectives of the catalytic and electrocatalytic reduction of aqueous nitrates. Appl. Catal. B Environ. 2017, 207, 42–59. [Google Scholar] [CrossRef]
- Marí, A.; Baeza, J.A.; Calvo, L.; Gilarranz, M.A. Catalytic membrane reactor based on Pd-Sn supported on nanocarbons for the reduction of nitrate in water. J. Environ. Chem. Eng. 2022, 10, 108011. [Google Scholar] [CrossRef]
- González, D.T.; Marí, A.; Baeza, J.A.; Calvo, L.; Gilarranz, M.A. Enhancement of activity and selectivity to nitrogen in catalytic nitrate reduction by use of conductive carbon catalytic supports and control of hydrogen mass transfer regime. J. Environ. Chem. Eng. 2021, 9, 106419. [Google Scholar] [CrossRef]
- Dagan-Jaldety, C.; Fridman-Bishop, N.; Gendel, Y. Nitrate hydrogenation by microtubular CNT-made catalytic membrane contactor. Chem. Eng. J. 2020, 401, 126142. [Google Scholar] [CrossRef]
- Marchesini, F.A.; Aghemo, V.; Moreno, I.; Navascués, N.; Irusta, S.; Gutierrez, L. Pd and Pd, In nanoparticles supported on polymer fibres as catalysts for the nitrate and nitrite reduction in aqueous media. J. Environ. Chem. Eng. 2020, 8, 103651. [Google Scholar] [CrossRef]
- Guate, M.; Ortiz, A.; Ortiz, I. New polymer catalytic membranes for nitrite reduction: Experimental assessment. J. Membr. Sci. Res. 2019, 5, 157–164. [Google Scholar] [CrossRef]
- Levi, J.; Guo, S.; Kavadiya, S.; Luo, Y.; Lee, C.-S.; Jacobs, H.P.; Holman, Z.; Wong, M.S.; Garcia-Segura, S.; Zhou, C.; et al. Comparing methods to deposit Pd-In catalysts on hydrogen-permeable hollow-fiber membranes for nitrate reduction. Water Res. 2023, 235, 119877. [Google Scholar] [CrossRef]
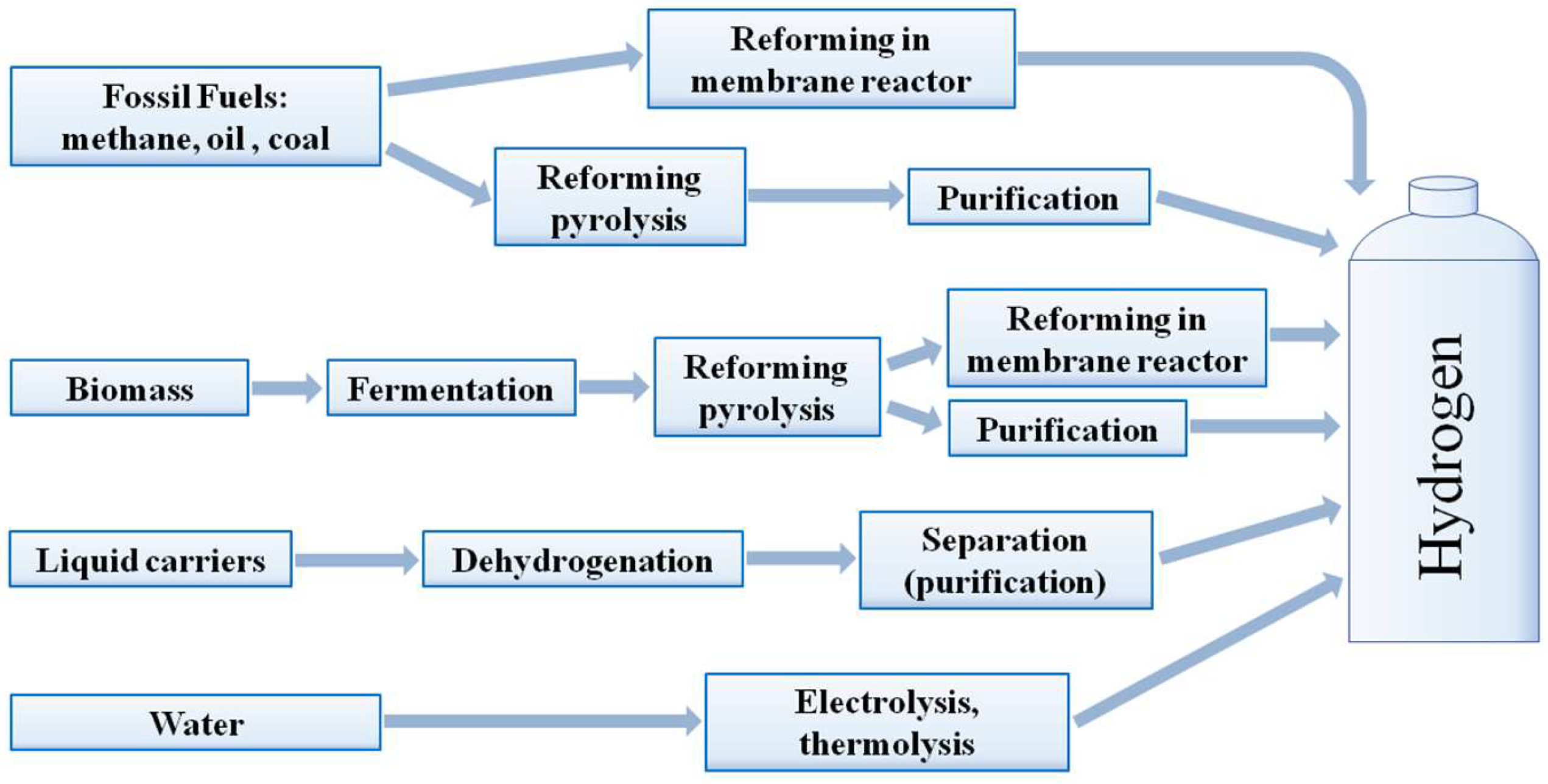
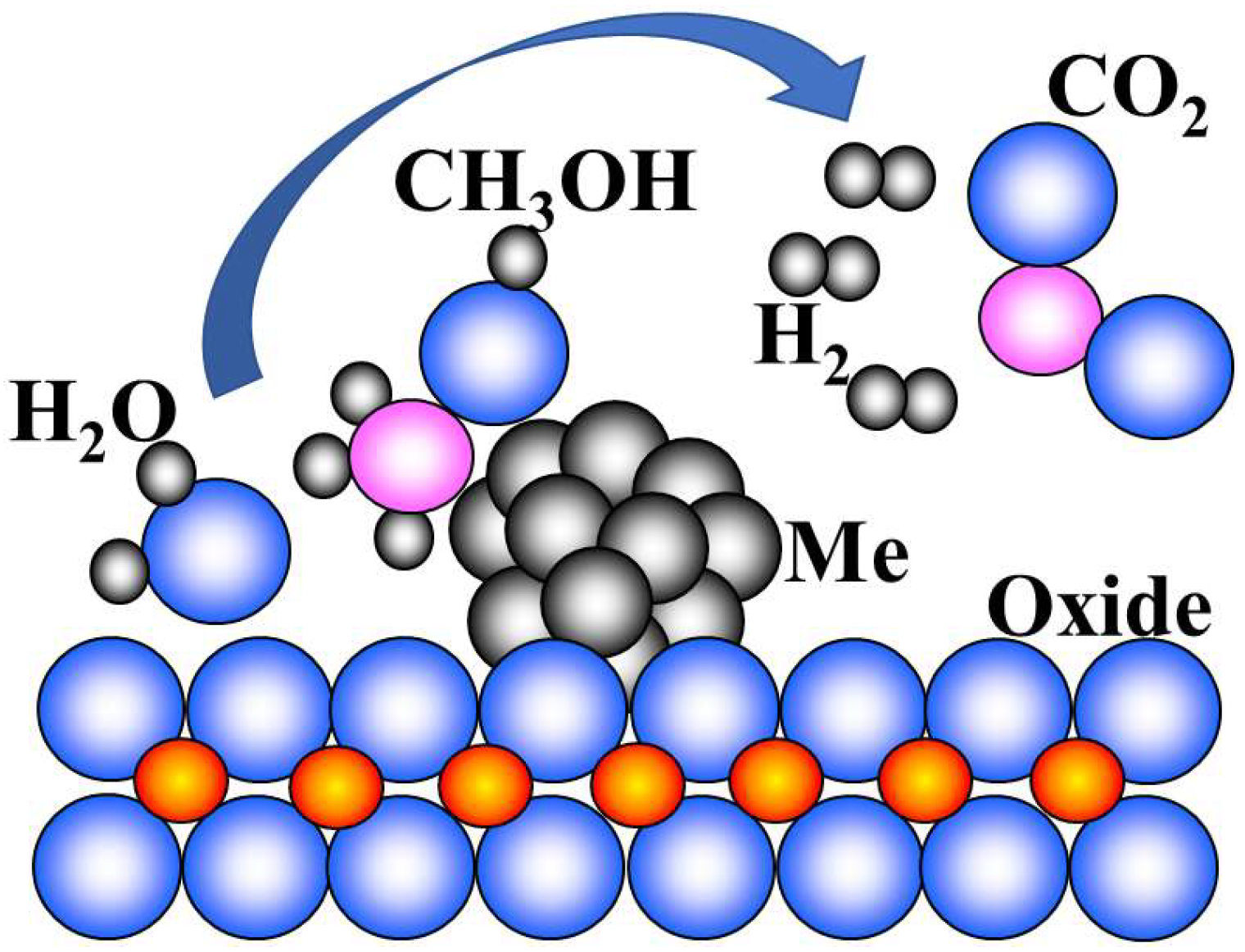

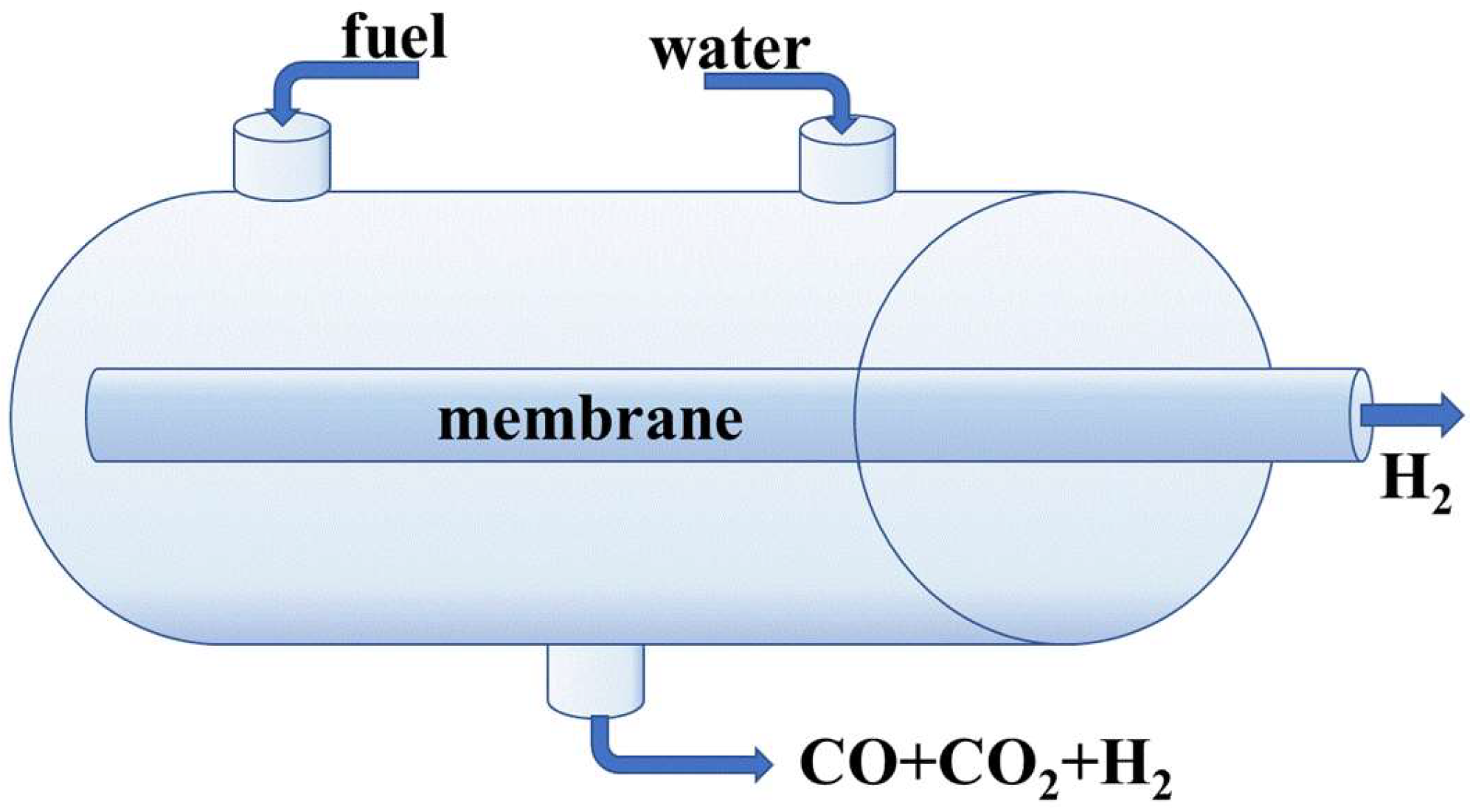


| Hydrogen Production Method | Energy Efficiency, % | Carbon Footprint, kg(CO2)/kg(H2) | Costs of Hydrogen, €/kg |
|---|---|---|---|
| Methane stream reforming | 75 | 10 | 4 |
| Methane pyrolysis | 55 | 3 | 7 |
| Biomass gasification | 40 | 6 | 8 |
| Coal gasification | 70 | 25 | 1.5 |
| Water electrolysis | 70 | 0 | 6 |
| Pd Layer Thickness (nm) | Polymer Surface Treatment | Pd Coating Method a | P, 10−16 mol m−1 s−1 Pa−1 | Separation Selectivity | ||||
|---|---|---|---|---|---|---|---|---|
| H2 | N2 | CO2 | CO | H2/N2 | H2/CO2 | |||
| 0 | none | none | 925 | 33 | 200 | 41 | 28 | 4.5 |
| 313 | none | CELP | 800 | 24 | 150 | 0 | 33 | 5.4 |
| 417 | none | VELP | 785 | 23 | 135 | 0 | 33.5 | 5.8 |
| 656 | H2O2 | VELP | 870 | 0 | 120 | 0 | ∞ | 7.1 |
| 188 | H2O2 | VELP | 780 | 29.5 | 34.5 | 0 | 27 | 23 |
| 130 | H2O2 | VELP | 585 | 34 | 92 | 0 | 17 | 6.4 |
| 159 | CO2 Plasma | VELP | 880 | 21 | 39.5 | 0 | 41 | 22 |
| Polymer | Tg (°C) | Tm (°C) | Td (°C) | Reference |
|---|---|---|---|---|
 AF-2400 | 240 | – | >360 | [318] |
 | – | – | >300 | [319] |
 | 420 | – | 450–500 | [314] |
 | 273 | – | 526 | [311] |
 m-PBI | 450 | – | >450 | [312,313] |
 | – | – | 520 | [294] |
 | – | >400 | 450 | [315] |
 | – | – | >400 | [320] |
 | >330 | – | >400 | [317] |
 | – | – | ~450 | [321] |
 | 206–230 | 240–280 | >300 | [322] |
| Membrane Material | Membrane w/o Pd | Pd-Polymer Composite | Ref. | ||||
|---|---|---|---|---|---|---|---|
| P(H2), 10−16 mol m−1 s−1 Pa−1 | α(H2/CO2) | α(H2/N2) | P(H2), 10−16 mol m−1 s−1 Pa−1 | α(H2/CO2) | α(H2/N2) | ||
| Polysulfone + 1% Pd Polysulfone + 2% Pd Polysulfone + 3% Pd | – | 4.4 | 6.2 | – | 3.7 | 15.2 | [345] |
| – | 6.2 | 20.2 | |||||
| – | 5.2 | 15.7 | |||||
| Polycarbonate + CNT-ox a + Pd | 33,000 | 6.1 | 3.3 | 16,000 | 3.9 | 2.2 | [347] |
| Polycarbonate + CNT-ox a + Pd | 40,000 | 6.4 | 4.1 | 16,000 | 8.0 | 4.2 | [347] |
| Matrimid + ZIF-8 + Pd | 150 | 3.3 | 87 | 230 | 5.1 | 140 | [348] |
| Membrane Material | Palladium Nanoparticles’ Introduction Method | Process | Ref. |
|---|---|---|---|
| Polyacrylonitrile, polyetherimide, and polyamidimide modified with palladium nanoclusters | Introduction of TiO2 into the membrane pores and further membrane treatment with a Pd(OAc)2 solution in methyl-ethyl ketone and subsequent NaBH4 reduction | Selective hydrogenation of propyne to propene | [411] |
| Polydimethylsiloxane with Pd nanoclusters in a polymer matrix | Introduction of Pd(OAc)2 solution and subsequent NaBH4 reduction | Hydrogenation of propyne to propene and propane | [399] |
| Propene hydrogenation | [400] | ||
| Polyacrylic acid with Pd nanoparticles in a polymer matrix | Introduction of Pd(OAc)2 solution and subsequent NaBH4 reduction | Hydrogenation of propyne to propene and propane | [405] |
| Cyclohexene to cyclohexane and propyne to propene and propane hydrogenation | [397] | ||
| Metathesis diblock copolymer based on norbornene with complex palladium substituent and methyltetracyclodecene | Polymerization of a monomer containing palladium in substituent and subsequent polymer treatment with hydrogen while heating | Ethylene and propene hydrogenation | [401] |
| 1,3-Butadiene hydrogenation | [402] | ||
| Polyamidimides with Pd nanoclusters in a polymer matrix | Introduction of Pd(OAc)2 solution and subsequent NaBH4 reduction | N2O hydrogenation to nitrogen | [403] |
| Polyvinylidene fluoride with Pd nanoclusters in a polymer matrix | Introduction of PdCl2 solution and subsequent NaBH4 reduction | Methylenecyclohexane to methylcyclohexane hydrogenation | [398] |
| Polyamidimide or polyether sulfone surface-modified with palladium deposited on an alumina substrate | Introduction of Pd salt solution and subsequent NaBH4 reduction; introduction of Pd salts solution and subsequent calcination in air | Sunflower oil hydrogenation | [396] |
| Phenolphthalein polyethersulfone modified with palladium nanoparticles in a polymer matrix | Injection of PdCl2 solution and drying at 110 °C | 1-Octene hydrogenation | [410] |
| Cellulose acetate-based hollow fibers surface-modified with palladium nanoparticles | Hollow fibers’ immersion in PdCl2 solution stabilized with poly-vinylpyrrolidone and reduction with hydrazine | Selective hydrogenation of propadiene and propine to propene | [407] |
| Selective hydrogenation of 1,3-butadiene to 1-butene | [408] | ||
| Cellulose acetate, polyacrylonitrile, and polysulfone-based hollow fibers surface-modified with palladium nanoparticles | Hollow fibers’ immersion in PdCl2 solution stabilized with polyvinylpyrrolidone and reduction with hydrazine | Hydrogenation of conjugated dienes: cyclopentadiene, 1,3-butadiene and isoprene | [404] |
| Hybrid membrane based on polyvinyl alcohol, ZrO2, and palladium nanoparticles | NaBH4 reduction of PdO contained in membrane | Hydrogenation of 1,5-cyclooctadiene, 3-hexyn-1-ol, 4-phenyl-3-buten-2-one, and methyl 2-acetamidoacrylate | [406] |
| Polyethersulfone-based hollow fibers and flat membranes surface-modified with palladium nanoparticles | Immersion of membranes in [Pd(NH3)4]Cl2 solution and NaBH4 reduction | Hydrogenation of indene, 1-dodecene, 4-isopropenyl-1-methylcyclohexene, diphenyl acetylene, nitrobenzene, and 4-nitrobenzonitrile | [409] |
| Polymer Matrix | Modification Method | Impact of Pd on Methanol Permeability | Effect of Pd on Proton Conductivity | Ref. |
|---|---|---|---|---|
| Nafion 117 | Pd impregnation | 7.4x reduction | 1.5x reduction | [434] |
| Nafion 117 | Pd impregnation | 1.4–1.5x reduction | 1.1x reduction at low Pd content and increase at high Pd content | [435] |
| Nafion 117 | Pd impregnation | – | 1.5x reduction | [436] |
| Nafion 117 | Pd impregnation in SC-CO2 | 15–30% reduction | 10–20% reduction (depending on temperature) | [437] |
| Nafion 117 | Pd impregnation in SC-CO2 | 1.8–7.5x reduction | Minor change or minor increase | [432] |
| Nafion 115 | Pd/poly(1-vinylimidazole) impregnation | 1.3–1.8x reduction | up to 2x reduction | [438] |
| Nafion | Pd impregnation in SC-CO2 using Pd salts with different ligands | 1.2–1.7x reduction | up to 1.2x reduction | [439] |
| Nafion 117 | Pd impregnation using an electrolytic cell | 14–60% increase | 5–23% increase | [440] |
| PETE composite with Nafion | Pd spraying (layer thickness of 20 nm) | – | 5x reduction | [441] |
| Nafion 115 and Nafion 117 | Pd spraying (layer thickness of 0.05–0.1 μm) | 23–44% reduction | up to 30% reduction | [442] |
| Nafion 117 | Pt/Pd-Ag/Pt spraying (Pd-Ag layer thickness of 0.1–1 μm) | – | – | [443] |
| Nafion 117 | Chemical Pd coating on anode side | In 5 h methanol concentration on cathode less 2 g/L (vs. pure Nafion 117, for which it is over 10 g/L in 2 h and ramp) | – | [444] |
| Nafion 115 | Pd spraying | 1.5x reduction | – | [445] |
| Nafion 115 | Pd-Cu spraying | 1.3x reduction | – | [445] |
| Nafion 115 | Pd spraying | 1.6x reduction (with high methanol concentration) | 18% increase | [446] |
| Nafion 115 | Nano-Pd coating stabilized by 9-octadecene-1-ylamine | 20% reduction | Minor increase | [447] |
| Nafion 115 | Nano-Pd coating stabilized by trioctylphosphine | 16% reduction | 20–25% reduction | [448] |
| Nafion 112 | Self-assembly of nano-Pd coating on surface | Reduction by 10+ times with 1 nano-layer and 100+ times with 5 layers | Minor reduction with 1 nano-layer and 30% reduction with 5 layers | [449] |
| Nafion | Nano-Pd coating stabilized by poly(diallyldimethylammonium) | 10–35% reduction | Minor change | [450] |
| Nafion 117 | Pd-SiO2 nanofibers introduction into polymer matrix | up to 1.5x reduction | up to 1.3x increase | [451] |
| Nafion 117 | Pt-Pd (50–90% Pt) impregnation | – | – | [452] |
| Nafion 117 | PLD (pulsed laser deposition) coating of Pd | More than 50% reduction | – | [453] |
| SPEEK a | Introduction of graphite oxide nanocomposite with Pd into polymer matrix | 1.2x reduction | 1.2x increase | [433] |
| SPEEK a | Introduction of graphite oxide nanocomposite with Pd grafted with L-tyrosine into a polymer matrix | 1.7x reduction | 1.8x increase | [433] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alentiev, D.A.; Bermeshev, M.V.; Volkov, A.V.; Petrova, I.V.; Yaroslavtsev, A.B. Palladium Membrane Applications in Hydrogen Energy and Hydrogen-Related Processes. Polymers 2025, 17, 743. https://doi.org/10.3390/polym17060743
Alentiev DA, Bermeshev MV, Volkov AV, Petrova IV, Yaroslavtsev AB. Palladium Membrane Applications in Hydrogen Energy and Hydrogen-Related Processes. Polymers. 2025; 17(6):743. https://doi.org/10.3390/polym17060743
Chicago/Turabian StyleAlentiev, Dmitry A., Maxim V. Bermeshev, Alexey V. Volkov, Inna V. Petrova, and Andrey B. Yaroslavtsev. 2025. "Palladium Membrane Applications in Hydrogen Energy and Hydrogen-Related Processes" Polymers 17, no. 6: 743. https://doi.org/10.3390/polym17060743
APA StyleAlentiev, D. A., Bermeshev, M. V., Volkov, A. V., Petrova, I. V., & Yaroslavtsev, A. B. (2025). Palladium Membrane Applications in Hydrogen Energy and Hydrogen-Related Processes. Polymers, 17(6), 743. https://doi.org/10.3390/polym17060743









