Electrically Conductive Functional Polymers and Application Progress in Lithium Batteries
Abstract
1. Introduction
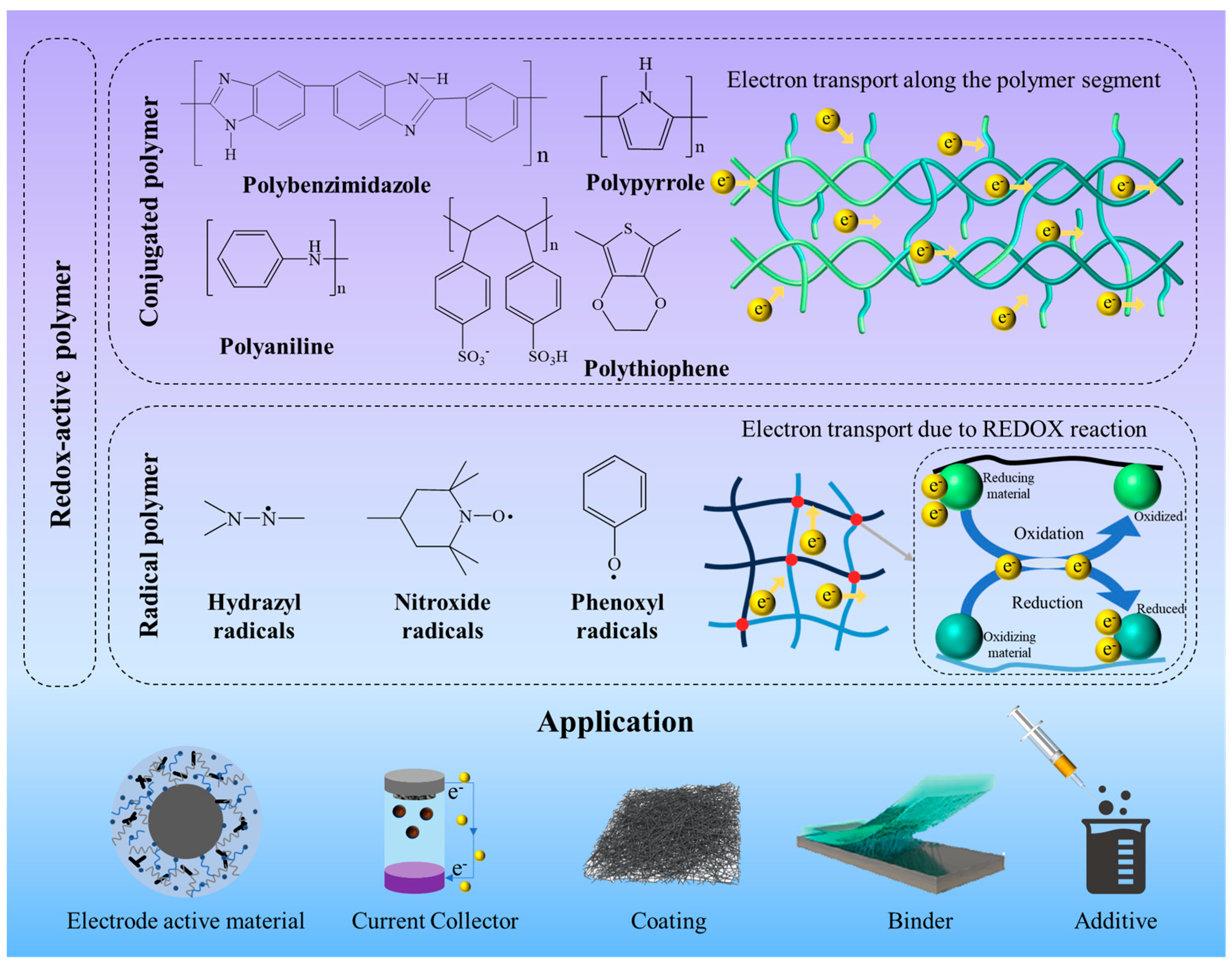
2. Conjugated Polymers
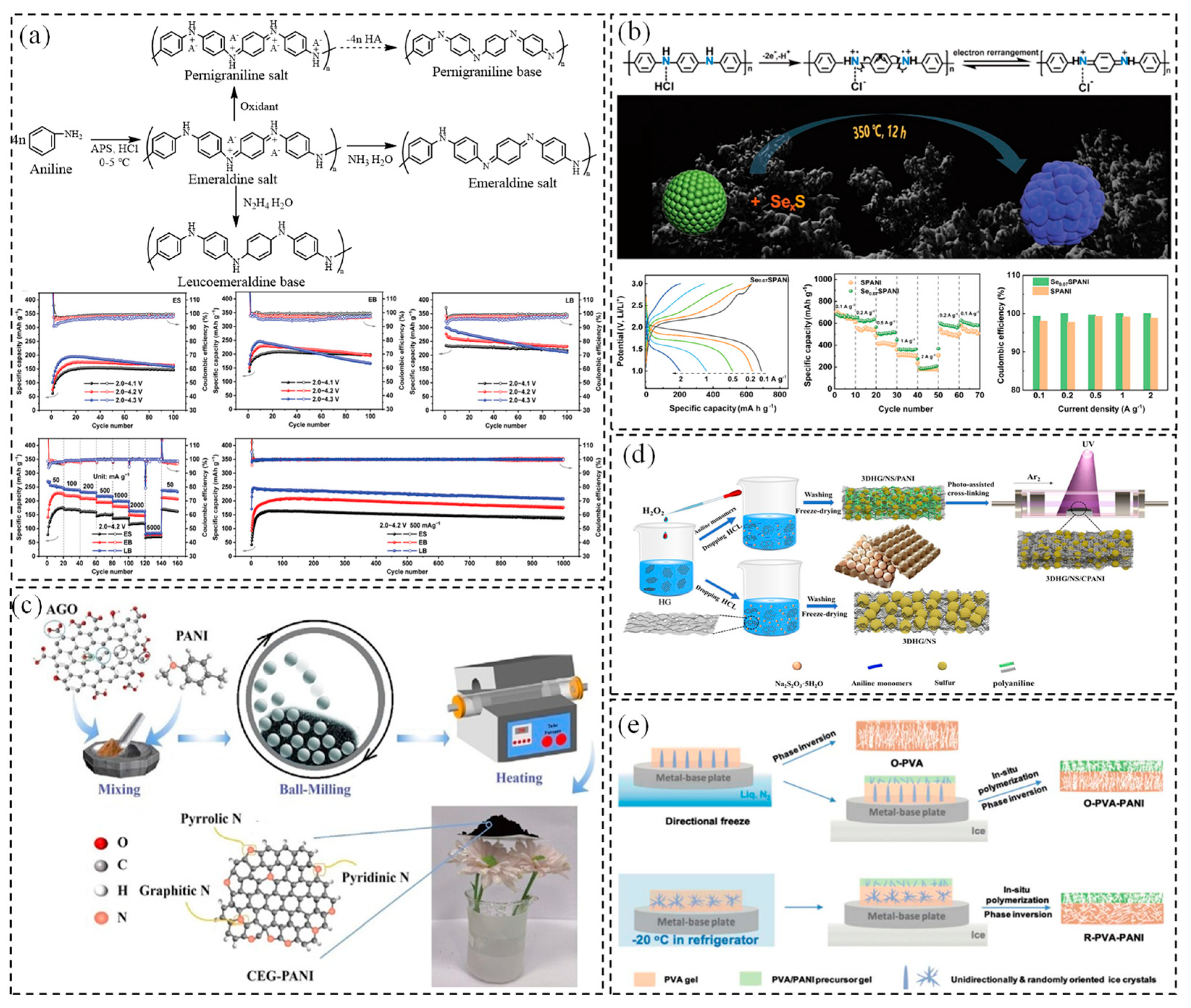
2.1. PANI
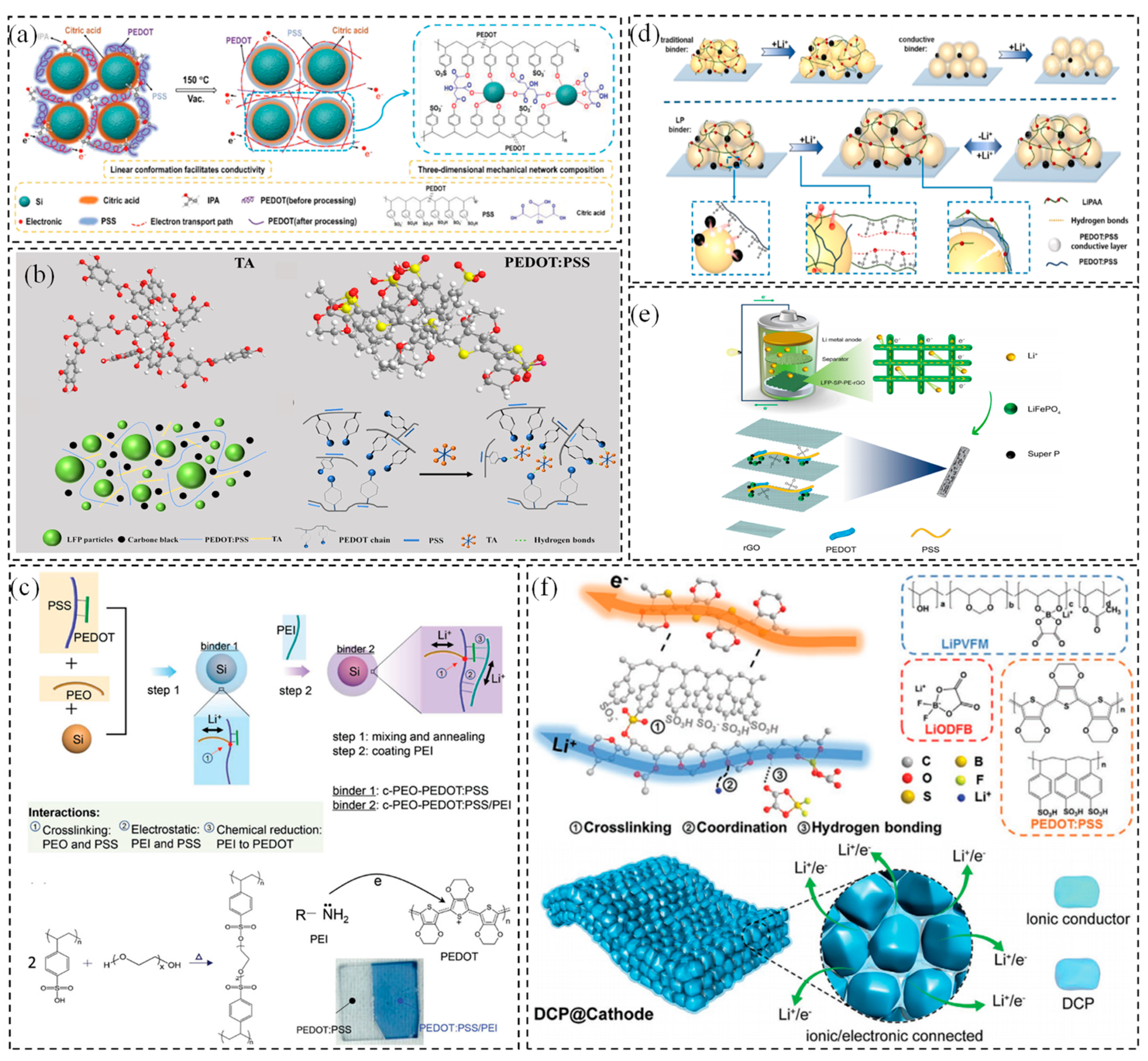
2.2. Polythiophene (PTH)—PEDOT:PSS
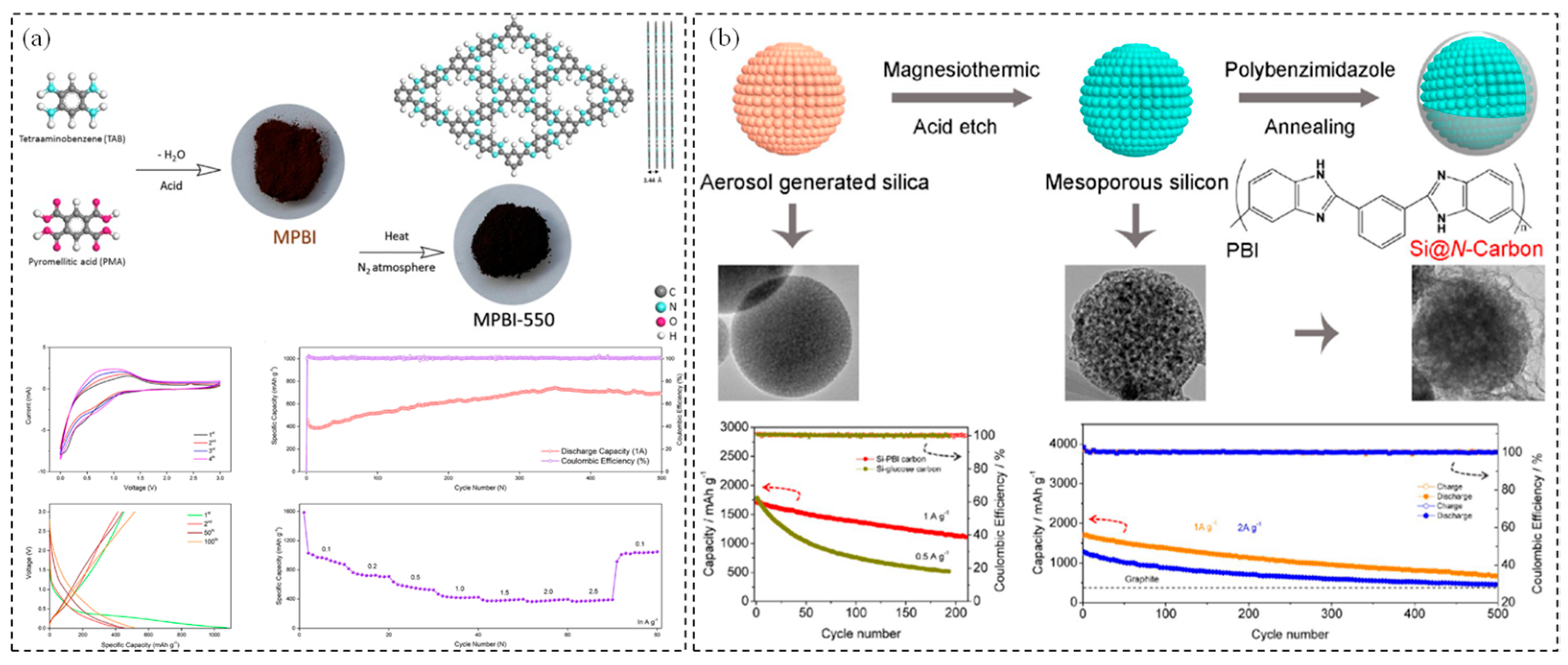
2.3. PBI
2.4. PPy
3. Radical Polymers
3.1. Nitroxide Radicals
3.1.1. PTMA
3.1.2. Polynorbornene (PNB)
3.1.3. Poly(tempo-ether-oxetane) (PTEO)
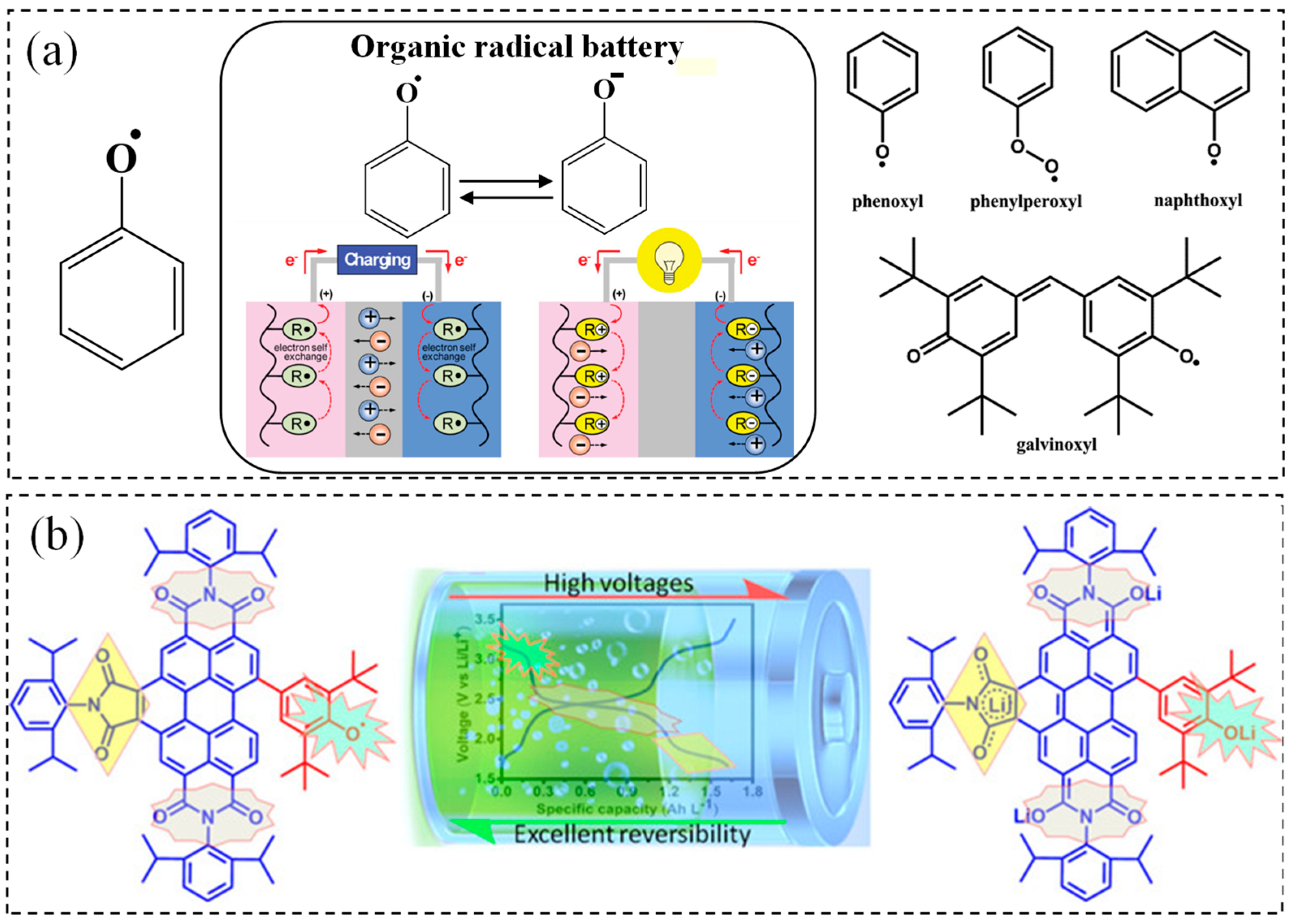
3.2. Phenoxyl Radicals
3.3. Hydrazyl Radicals
4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECFPs | Electrically conductive functional polymers |
| PANI | Polyanilines |
| PEDOT | Poly(3,4-ethylenedioxythiophene) |
| PSS | Polystyrene sulfonic acid |
| PBI | Polybenzimidazole |
| PPy | Polypyrroles |
| PVA | Polyvinyl alcohol |
| PTH | Polythiophene |
| TA | Tannic acid |
| PEO | Polyethylene oxide |
| PEI | Polyethylene imine |
| COF | Covalent organic skeletons |
| TEMPO | 2,2,6,6-tetramethylpiperidinyl-n-oxyl |
| PTEO | Poly (tempo-ether-oxetane) |
| AQ | Anthraquinone |
| PNB | Polynorbornene |
| PROXYL | 2,2,5,5-tetramethyl-1-pyrrolidinyl-N-oxyl |
| PPD | polyaniline diamine |
| MEH-PPV | Poly[(2-ethylhexyl) phenylene vinylene)] |
| PBTTT | Poly[2,5-bis(3-hexylthiophene-2-yl) thiophene] |
| PTAA | Poly(triaryl amine) |
| BBL | Poly(benzimidazobenzophenanthroline) |
References
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene, (CH)x.J. Chem. Soc. Chem. Commun. 1977, 16, 578–580. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; You, J.; Park, M.-S.; Al Hossain, S.M.; Yamauchi, Y.; Kim, J.H. Conductive polymers for next-generation energy storage systems: Recent progress and new functions. Mater. Horiz. 2016, 3, 517–535. [Google Scholar] [CrossRef]
- Otero, T.F. Biomimetic conducting polymers: Synthesis, materials, properties, functions, and devices. Polym. Rev. 2013, 53, 311–351. [Google Scholar] [CrossRef]
- Deng, H.; Lin, L.; Ji, M.; Zhang, S.; Yang, M.; Fu, Q. Progress on the morphological control of conductive network in conductive polymer composites and the use as electroactive multifunctional materials. Prog. Polym. Sci. 2014, 39, 627–655. [Google Scholar] [CrossRef]
- Huang, H.; Cong, H.T.; Lin, Z.; Liao, L.; Shuai, C.X.; Qu, N.; Luo, Y.; Guo, S.; Xu, Q.C.; Bai, H.; et al. Manipulation of conducting polymer hydrogels with different shapes and related multifunctionality. Small 2024, 20, e2309575. [Google Scholar] [CrossRef]
- Unnikrishnan, V.; Zabihi, O.; Ahmadi, M.; Li, Q.; Blanchard, P.; Kiziltas, A.; Naebe, M. Metal–organic framework structure–property relationships for high-performance multifunctional polymer nanocomposite applications. J. Mater. Chem. A 2021, 9, 4348–4378. [Google Scholar] [CrossRef]
- Zhuang, X.; Wang, F.; Hu, X. Biodegradable polymers: A promising solution for green energy devices. Eur. Polym. J. 2024, 204, 112696. [Google Scholar] [CrossRef]
- Numazawa, H.; Sato, K.; Imai, H.; Oaki, Y. Multistage redox reactions of conductive-polymer nanostructures with lithium ions: Potential for high-performance organic anodes. NPG Asia Mater. 2018, 10, 397–405. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, K.; Yamauchi, Y.; Oyaizu, K.; Jia, Z. Nitroxide radical polymers for emerging plastic energy storage and organic electronics: Fundamentals, materials, and applications. Mater. Horiz. 2021, 8, 803–829. [Google Scholar] [CrossRef]
- Majeed, A.H.; Mohammed, L.A.; Hammoodi, O.G.; Sehgal, S.; Alheety, M.A.; Saxena, K.K.; Dadoosh, S.A.; Mohammed, I.K.; Jasim, M.M.; Salmaan, N.U.; et al. A review on polyaniline: Synthesis, properties, nanocomposites, and electrochemical applications. Int. J. Polym. Sci. 2022, 2022, 9047554. [Google Scholar] [CrossRef]
- McGraw, M.; Kolla, P.; Yao, B.; Cook, R.; Quiao, Q.; Wu, J.; Smirnova, A. One-step solid-state in-situ thermal polymerization of silicon-PEDOT nanocomposites for the application in lithium-ion battery anodes. Polymers 2016, 99, 488–495. [Google Scholar] [CrossRef]
- Choudhary, R.B.; Ansari, S.; Purty, B. Robust electrochemical performance of polypyrrole (PPy) and polyindole (PIn) based hybrid electrode materials for supercapacitor application: A review. J. Energy Storage 2020, 29, 101302. [Google Scholar] [CrossRef]
- Charkhesht, V.; Yürüm, A.; Alkan Gürsel, S.; Yarar Kaplan, B. Titania-based freestanding electronically conductive electrospun anodes with enhanced performance for Li-Ion batteries. ACS Appl. Energy Mater. 2021, 4, 13922–13931. [Google Scholar] [CrossRef]
- Chen, R.; Li, Q.; Yu, X.; Chen, L.; Li, H. Approaching practically accessible solid-state batteries: Stability issues related to solid electrolytes and interfaces. Chem. Rev. 2019, 120, 6820–6877. [Google Scholar] [CrossRef]
- Nasreldin, M.; Delattre, R.; Calmes, C.; Ramuz, M.; Sugiawati, V.A.; Maria, S.; Tocnaye, J.-L.d.B.d.l.; Djenizian, T. High performance stretchable Li-ion microbattery. Energy Storage. Mater. 2020, 33, 108–115. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, R.; Xu, C.; Ruan, C.; Edström, K.; Strømme, M.; Nyholm, L. Conducting polymer paper-derived separators for lithium metal batteries. Energy Storage. Mater. 2018, 13, 283–292. [Google Scholar] [CrossRef]
- Guo, X.; Facchetti, A. The journey of conducting polymers from discovery to application. Nat. Mater. 2020, 19, 922–928. [Google Scholar] [CrossRef]
- Gao, H.; Xue, L.; Xin, S.; Goodenough, J.B. A high-energy-density potassium battery with a polymer-gel electrolyte and a polyaniline cathode. Angew. Chem. Int. Ed. 2018, 57, 5449–5453. [Google Scholar] [CrossRef]
- Zhang, Y.; Gou, B.; Li, Y.; Liao, Y.; Lu, J.; Wu, L.; Zhang, W.; Xu, H.; Huang, Y. Integration of gel polymer electrolytes with dry electrodes for quasi-solid-state batteries. Chem. Eng. J. 2024, 498, 155544. [Google Scholar] [CrossRef]
- Baker, C.O.; Huang, X.; Nelson, W.; Kaner, R.B. Polyaniline nanofibers: Broadening applications for conducting polymers. Chem. Soc. Rev. 2017, 46, 1510–1525. [Google Scholar] [CrossRef]
- Beygisangchin, M.; Abdul Rashid, S.; Shafie, S.; Sadrolhosseini, A.R.; Lim, H.N. Preparations, properties, and applications of polyaniline and polyaniline thin films—A review. Polymers 2021, 13, 2003. [Google Scholar] [CrossRef]
- Joo, Y.; Agarkar, V.; Sung, S.H.; Savoie, B.M.; Bryan, W. Boudouris. A nonconjugated radical polymer glass with high electrical conductivity. Science 2018, 359, 1391–1395. [Google Scholar] [CrossRef]
- Wingate, A.J.; Boudouris, B.W. Recent advances in the syntheses of radical-containing macromolecules. J. Polym. Sci. Pol. Chem. 2016, 54, 1875–1894. [Google Scholar] [CrossRef]
- Rousseau, R.; Glezakou, V.-A.; Selloni, A. Theoretical insights into the surface physics and chemistry of redox-active oxides. Nat. Rev. Mater. 2020, 5, 460–475. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.H.; Ariga, K. Redox-active polymers for energy storage nano architectonics. Joule 2017, 1, 739–768. [Google Scholar] [CrossRef]
- Kaiser, J.M.; Long, B.K. Recent developments in redox-active olefin polymerization catalysts. Coord. Chem. Rev. 2018, 372, 141–152. [Google Scholar] [CrossRef]
- Luppi, B.T.; Muralidharan, A.V.; Ostermann, N.; Cheong, I.T.; Ferguson, M.J.; Siewert, I.; Rivard, E. Redox-active heteroatom-functionalized polyacetylenes. Angew. Chem. Int. Ed. 2021, 61, e202114586. [Google Scholar] [CrossRef]
- Lutkenhaus, J. A radical advance for conducting polymers. Science 2018, 359, 1334–1335. [Google Scholar] [CrossRef]
- Tang, H.; Liang, Y.; Liu, C.; Hu, Z.; Deng, Y.; Guo, H.; Yu, Z.; Song, A.; Zhao, H.; Zhao, D.; et al. A solution-processed n-type conducting polymer with ultrahigh conductivity. Nature 2022, 611, 271–277. [Google Scholar] [CrossRef]
- Shi, Y.; Peng, L.; Ding, Y.; Zhao, Y.; Yu, G. Nanostructured conductive polymers for advanced energy storage. Chem. Soc. Rev. 2015, 44, 6684–6696. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Lv, L.; Zhu, G.; Qu, Q.; Zheng, H. Electroactive organics as promising anode materials for rechargeable lithium ion and sodium ion batteries. Energy Mater. 2022, 2, 200014. [Google Scholar] [CrossRef]
- Xie, J.; Gu, P.; Zhang, Q. Nanostructured conjugated polymers: Toward high-performance organic electrodes for rechargeable batteries. ACS Energy Lett. 2017, 2, 1985–1996. [Google Scholar] [CrossRef]
- Kashyap, Y.; Pandey, R.R.; Andola, A.; Nagaraju, D.H.; Pandey, R.K. Solid-state responses of electrochemically deposited polyaniline and polypyrrole-based symmetric supercapacitors in different pH conditions. J. Solid-State Electrochem. 2024, 28, 3357–3365. [Google Scholar] [CrossRef]
- Liao, G.; Li, Q.; Xu, Z. The chemical modification of polyaniline with enhanced properties: A review. Prog. Org. Coat. 2019, 126, 35–43. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, C.; Yang, K.; Sun, S.; Bi, J.; Zhu, N.; Cai, Q.; Wang, J.; Yan, W. Conducting polymers meet lithium–sulfur batteries: Progress, challenges, and perspectives. Energy Environ. Mater. 2023, 6, e12483. [Google Scholar] [CrossRef]
- Zhao, R.; Chang, Z.; Fu, X.; Xu, M.; Ai, X.; Qian, J. Revisit of polyaniline as a high-capacity organic cathode material for Li-ion batteries. Polymers 2024, 16, 1401. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, J.; Yu, P.; Li, M.; Huang, L.; Hu, Z.; Wang, Y.; Song, Z. Toward full utilization and stable cycling of polyaniline cathode for nonaqueous rechargeable batteries. Adv. Energy Mater. 2023, 13, 2301520. [Google Scholar] [CrossRef]
- Zou, R.; Liu, W.; Ran, F. Selenium-doped cathode materials with polyaniline skeleton for lithium-organosulfur batteries. J. Energy Chem. 2023, 79, 148–157. [Google Scholar] [CrossRef]
- Wang, Z.L.; Sun, K.; Henzie, J.; Hao, X.; Li, C.; Takei, T.; Kang, Y.M.; Yamauchi, Y. Spatially confined assembly of monodisperse ruthenium nanoclusters in a hierarchically ordered carbon electrode for efficient hydrogen evolution. Angew. Chem. Int. Ed. 2018, 57, 5848–5852. [Google Scholar] [CrossRef]
- Zhou, J.; Qian, T.; Xu, N.; Wang, M.; Ni, X.; Liu, X.; Shen, X.; Yan, C. Selenium-doped cathodes for lithium–organosulfur batteries with greatly improved volumetric capacity and coulombic efficiency. Adv. Mater. 2017, 29, 1701294. [Google Scholar] [CrossRef]
- Chen, X.; Li, C.; Tang, Y.; Li, L.; Du, Y.; Li, L. Integrated optimization of cutting tool and cutting parameters in face milling for minimizing energy footprint and production time. Energy 2019, 175, 1021–1037. [Google Scholar] [CrossRef]
- Fu, H.; Gao, B.; Qiao, Y.; Zhu, W.; Liu, Z.; Wei, G.; Feng, Z.; Kamali, A.R. Graphene nanonetwork embedded with polyaniline nanoparticles as anode of Li-ion battery. Chem. Eng. J. 2023, 477, 146936. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Fan, X.; Tang, T.; Li, M.; Zeng, Y.; Wang, H.; Wen, J.; Xiao, J. Holey graphene anchoring of the monodispersed nano-sulfur with covalently-grafted polyaniline for lithium sulfur batteries. Carbon 2022, 188, 155–165. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, C.P.; Yin, Y.X.; Wan, L.J.; Guo, Y.G. Sulfur encapsulated in graphitic carbon nanocages for high-rate and long-cycle lithium–sulfur batteries. Adv. Mater. 2016, 28, 9539–9544. [Google Scholar] [CrossRef]
- Xu, L.; Daphne Ma, X.Y.; Wang, W.; Liu, J.; Wang, Z.; Lu, X. Polymeric one-side conductive Janus separator with preferably oriented pores for enhancing lithium metal battery safety. J. Mater. Chem. A 2021, 9, 3409–3417. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, X.; Chen, F.; Zhang, N.; Ma, M. Extremely strong and tough polythiophene composite for flexible electronics. Chem. Eng. J. 2019, 368, 933–940. [Google Scholar] [CrossRef]
- Gueye, M.N.; Carella, A.; Faure-Vincent, J.; Demadrille, R.; Simonato, J.-P. Progress in understanding structure and transport properties of PEDOT-based materials: A critical review. Prog. Mater. Sci. 2020, 108, 100616. [Google Scholar] [CrossRef]
- Kayser, L.V.; Lipomi, D.J. Stretchable conductive polymers and composites based on PEDOT and PEDOT: PSS. Adv. Mater. 2019, 31, e1806133. [Google Scholar] [CrossRef]
- Li, J.; Cao, J.; Lu, B.; Gu, G. 3D-printed PEDOT: PSS for soft robotics. Nat. Rev. Mater. 2023, 8, 604–622. [Google Scholar] [CrossRef]
- Ouyang, J.; Xu, Q.; Chu, C.-W.; Yang, Y.; Li, G.; Shinar, J. On the mechanism of conductivity enhancement in poly(3,4-ethylenedioxythiophene): Poly (styrene sulfonate) film through solvent treatment. Polymers 2004, 45, 8443–8450. [Google Scholar] [CrossRef]
- Park, S.; Lee, C.W.; Kim, J.-M. Highly conductive PEDOT: PSS patterns based on photo-crosslinkable and water-soluble diacetylene diol additives. Org. Electron. 2018, 58, 1–5. [Google Scholar] [CrossRef]
- Adilbekova, B.; Scaccabarozzi, A.D.; Faber, H.; Nugraha, M.I.; Bruevich, V.; Kaltsas, D.; Naphade, D.R.; Wehbe, N.; Emwas, A.H.; Alshareef, H.N.; et al. Enhancing the Electrical Conductivity and Long-Term Stability of PEDOT: PSS Electrodes through Sequential Treatment with Nitric Acid and Cesium Chloride. Adv. Mater. 2024, 36, e2405094. [Google Scholar] [CrossRef]
- Zhang, M.; Meng, Q.; Ahmad, A.; Mao, L.; Yan, W.; Wei, Z. Poly(3,4-ethylenedioxythiophene)-coated sulfur for flexible and binder-free cathodes of lithium–sulfur batteries. J. Mater. Chem. A 2017, 5, 17647–17652. [Google Scholar] [CrossRef]
- Chen, B.; Xu, D.; Chai, S.; Chang, Z.; Pan, A. Enhanced silicon anodes with robust SEI formation enabled by functional conductive binder. Adv. Funct. Mater. 2024, 34, 2401794. [Google Scholar] [CrossRef]
- Li, S.; Zhang, X.; Chen, B.; Shi, Z.; Wu, S.; Wang, C.; Tong, Q.; Zhu, M.; Weng, J. Improving LiFePO4 cathode stability in lithium-ion batteries by hybridizing activated tannic with PEDOT: PSS binders. Electrochim. Acta 2024, 483, 144037. [Google Scholar] [CrossRef]
- Sarang, K.T.; Li, X.; Miranda, A.; Terlier, T.; Oh, E.-S.; Verduzco, R.; Lutkenhaus, J.L. Tannic acid as a small-molecule binder for silicon anodes. ACS Appl. Energy Mater. 2020, 3, 6985–6994. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, L.; Peng, X.; Liu, T.; Jiang, Y.; Qin, F.; Hu, L.; Chu, P.K.; Huo, K.; Zhou, Y. Enhanced ion conductivity in conducting polymer binder for high-performance silicon anodes in advanced lithium-ion batteries. Adv. Energy Mater. 2018, 8, 1702314. [Google Scholar] [CrossRef]
- Geng, W.; Hu, X.; Zhou, Q.; Zhang, Y.; He, B.; Liu, Z.; Xiao, K.; Cai, D.; Yang, S.; Nie, H.; et al. Rational design of trifunctional conductive binder for high-performance Si anodes in lithium-ion batteries. J. Power. Sources 2024, 601, 234285. [Google Scholar] [CrossRef]
- Gao, C.; Cui, X.; Wang, C.; Wang, M.; Wu, S.; Quan, Y.; Wang, P.; Zhao, D.; Li, S. 3D-printed hierarchical porous and multidimensional conductive network based on conducting polymer/graphene oxide. J. Mater. 2024, 10, 234–244. [Google Scholar] [CrossRef]
- Li, H.; Lian, F.; Meng, N.; Xiong, C.; Wu, N.; Xu, B.; Li, Y. Constructing electronic and ionic dual conductive polymeric interface in the cathode for high-energy-density solid-state batteries. Adv. Funct. Mater. 2020, 31, 2008487. [Google Scholar] [CrossRef]
- Cao, J.; Wang, L.; Li, D.; Yuan, Z.; Xu, H.; Li, J.; Chen, R.; Shulga, V.; Shen, G.; Han, W. Ti3C2Tx MXene conductive layers supported bio-derived Fex−1Sex/MXene/carbonaceous nanoribbons for high-performance half/full sodium-ion and potassium-ion batteries. Adv. Mater. 2021, 33, e2101535. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Tang, C.; Liu, Y.; Li, H.; Du, A.; Zhang, H. Carbon-coated MoS2 nanosheets@CNTs-Ti3C2 MXene quaternary composite with the superior rate performance for sodium-ion batteries. J. Mater. Sci. Technol. 2022, 100, 101–109. [Google Scholar] [CrossRef]
- Chen, J.; Chen, H.; Chen, M.; Zhou, W.; Tian, Q.; Wong, C.-P. Nacre-inspired surface-engineered MXene/nanocellulose composite film for high-performance supercapacitors and zinc-ion capacitors. Chem. Eng. J. 2022, 428, 131380. [Google Scholar] [CrossRef]
- Cong, S.; Wang, J.; Wang, Z.; Liu, X. Polybenzimidazole (PBI) and benzimidazole-linked polymer (BILP) membranes. Green Che. Eng. 2021, 2, 44–56. [Google Scholar] [CrossRef]
- Cruz-Arzon, A.J.; Serrano-Garcia, W.; Pinto, N.J.; Gupta, N.; Johnson, A.T.C. Temperature dependent charge transport in electrostatically doped poly[benzimidazobenzophenanthroline] thin films. J. Appl. Polym. Sci. 2022, 140, e53470. [Google Scholar] [CrossRef]
- Wang, M.; Wang, G.; Naisa, C.; Fu, Y.; Gali, S.M.; Paasch, S.; Wang, M.; Wittkaemper, H.; Papp, C.; Brunner, E.; et al. Poly(benzimidazobenzophenanthroline)-ladder-type two-dimensional conjugated covalent organic framework for fast proton storage. Angew. Chem. Int. Ed. 2023, 62, e202310937. [Google Scholar] [CrossRef]
- Kandambeth, S.; Dey, K.; Banerjee, R. Covalent organic frameworks: Chemistry beyond the structure. J. Am. Chem. Soc. 2018, 141, 1807–1822. [Google Scholar] [CrossRef]
- Roy, A.; Mondal, S.; Halder, A.; Banerjee, A.; Ghoshal, D.; Paul, A.; Malik, S. Benzimidazole linked arylimide based covalent organic framework as gas adsorbing and electrode materials for supercapacitor application. Eur. Polym. J. 2017, 93, 448–457. [Google Scholar] [CrossRef]
- Deng, Y.; Hussain, A.; Raza, W.; Cai, X.; Liu, D.; Shen, J. Review on current development of polybenzimidazole membrane for lithium battery. J. Energy Chem. 2024, 91, 579–608. [Google Scholar] [CrossRef]
- Jalees, S.; Hussain, A.; Iqbal, R.; Raza, W.; Ahmad, A.; Saleem, A.; Majeed, M.K.; Faheem, M.; Ahmad, N.; Rehman, L.N.U.; et al. Functional PBI membrane based on polyimide covalent organic framework for durable lithium metal battery. J. Energy Storage 2024, 101, 113985. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, M.; Zhao, Y.; Qiu, Y. Theoretical Studies on the Chemical Degradation and Proton Dissociation Property of PBI used in High-Temperature Polymer Electrolyte Membrane Fuel Cells. J. Phys. Chem. B 2024, 128, 6167–6177. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Meng, L.; Ma, C.; Yu, Y.; Lou, Y.; Zhang, D.; Han, Y.; Shi, Z.; Feng, S. Synthesis of a microporous poly-benzimidazole as high-performance anode materials for lithium-ion batteries. Chem. Eng. J. 2021, 405, 126621. [Google Scholar] [CrossRef]
- Nie, P.; Liu, X.; Fu, R.; Wu, Y.; Jiang, J.; Dou, H.; Zhang, X. Mesoporous silicon anodes by using polybenzimidazole derived pyrrolic N-enriched carbon toward high-energy Li-ion batteries. ACS Energy Lett. 2017, 2, 1279–1287. [Google Scholar] [CrossRef]
- Balan, B.K.; Manissery, A.P.; Chaudhari, H.D.; Kharul, U.K.; Kurungot, S. Polybenzimidazole mediated n-doping along the inner and outer surfaces of a carbon nanofiber and its oxygen reduction properties. J. Mater. Chem. C 2012, 22, 23668–23679. [Google Scholar] [CrossRef]
- Xiang, H.; Deng, N.; Zhao, H.; Wang, X.; Wei, L.; Wang, M.; Cheng, B.; Kang, W. A review on electronically conducting polymers for lithium-sulfur battery and lithium-selenium battery: Progress and prospects. J. Energy Chem. 2021, 58, 523–556. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, H.; Cheng, H.; Hu, C.; Fang, W.; Fang, J.; Xu, J.; Chen, Z. Facile large-scale synthesis of core–shell structured sulfur@polypyrrole composite and its application in lithium–sulfur batteries with high energy density. Appl. Energy 2016, 175, 522–528. [Google Scholar] [CrossRef]
- Chi, M.; Shi, L.; Wang, Z.; Zhu, J.; Mao, X.; Zhao, Y.; Zhang, M.; Sun, L.; Yuan, S. Excellent rate capability and cycle life of Li metal batteries with ZrO2/POSS multilayer-assembled PE separators. Nano Energy 2016, 28, 1–11. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, W.; Li, Z.; Li, Y.; Xiang, C.; Chen, M.; Wang, X.; Wang, X. Core–shell structure S@PPy/CB with high electroconductibility to effective confinement polysulfide shuttle effect for advanced lithium-sulfur batteries. Energy Fuels 2021, 35, 10181–10189. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, X.C.; Wu, Y.; Shu, Y.; Gong, X.; Ke, F.S.; Deng, H. Metal–Organic frameworks for high charge–discharge rates in lithium–sulfur batteries. Angew. Chem. Int. Ed. 2018, 130, 3980–3985. [Google Scholar] [CrossRef]
- Yi, Y.; Hai, F.; Tian, X.; Wu, Z.; Zheng, S.; Guo, J.; Chen, W.; Hua, W.; Qu, L.; Li, M. A novel sulfurized polypyrrole composite for high-performance lithium-sulfur batteries based on solid-phase conversion. Chem. Eng. J. 2023, 466, 143303. [Google Scholar] [CrossRef]
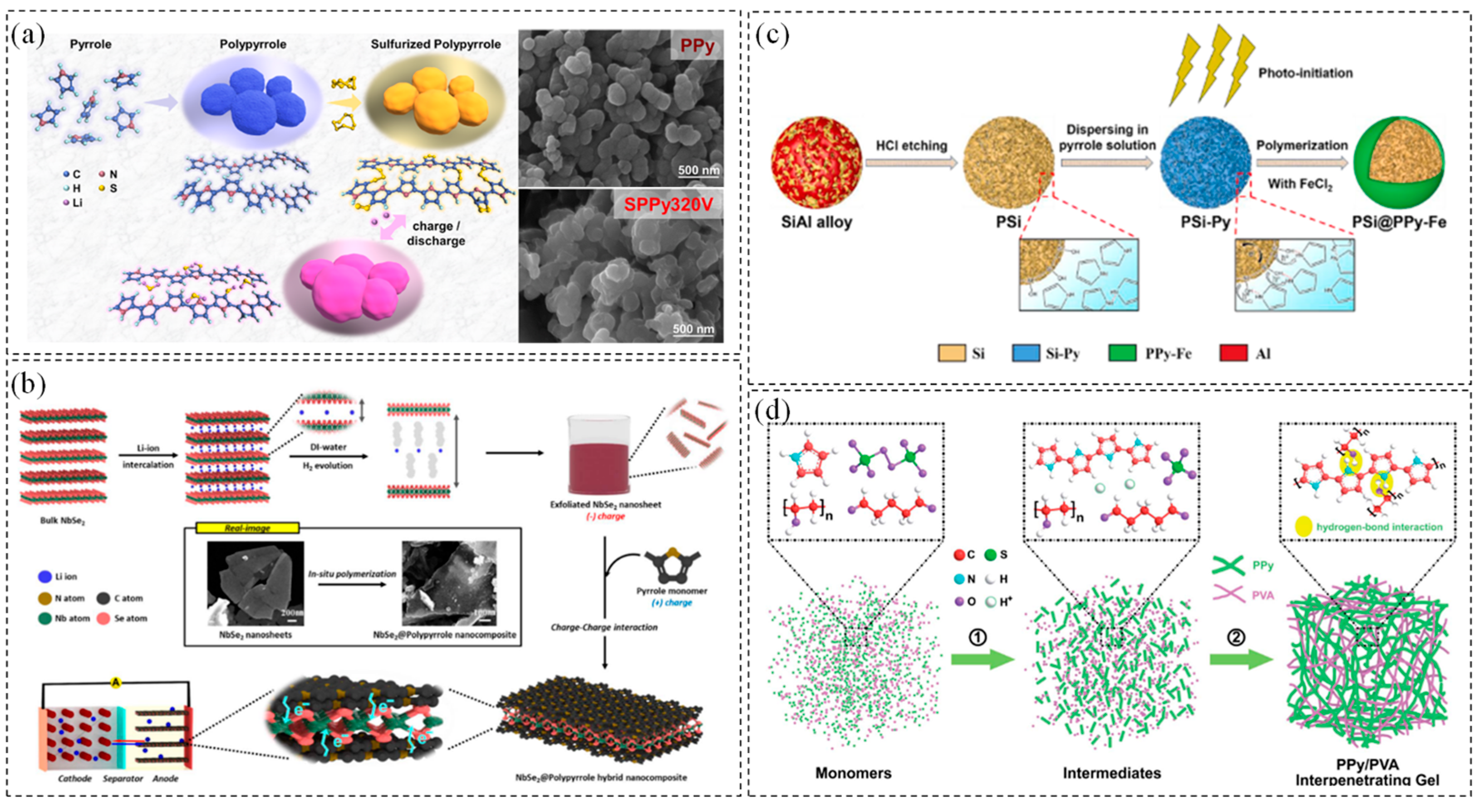
- Kang, B.-H.; Shin, S.; Nam, K.; Bae, J.; Oh, J.-M.; Koo, S.-M.; Sohn, H.; Park, S.-H.; Shin, W.H. Exfoliated NbSe2 nanosheet@polypyrrole hybrid nanocomposites as a high-performance anode of lithium-ion batteries. J. Mater. Chem. A 2023, 11, 19083–19090. [Google Scholar] [CrossRef]
- Xu, Z.; Zheng, E.; Xiao, Z.; Shao, H.; Liu, Y.; Wang, J. Photo-initiated in situ synthesis of polypyrrole Fe-coated porous silicon microspheres for high-performance lithium-ion battery anodes. Chem. Eng. J. 2023, 459, 141543. [Google Scholar] [CrossRef]
- Xia, T.; Xu, C.; Dai, P.; Li, X.; Lin, R.; Tang, Y.; Zhou, Y.; Wu, P. Interpenetrating gels as conducting/adhering matrices enabling high-performance silicon anodes. J. Mater. Chem. A 2021, 9, 12003–12008. [Google Scholar] [CrossRef]
- Lv, Y.; Shang, M.; Chen, X.; Nezhad, P.S.; Niu, J. Largely improved battery performance using a micro sized silicon skeleton caged by polypyrrole as anode. ACS Nano 2019, 13, 12032–12041. [Google Scholar] [CrossRef]
- Mao, J.; Iocozzia, J.; Huang, J.; Meng, K.; Lai, Y.; Lin, Z. Graphene aerogels for efficient energy storage and conversion. Energy Environ. Sci. 2018, 11, 772–799. [Google Scholar] [CrossRef]
- Wang, S.; Li, F.; Easley, A.D.; Lutkenhaus, J.L. Real-time insight into the doping mechanism of redox-active organic radical polymers. Nat. Mater. 2018, 18, 69–75. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, Z.; Li, Y.; Chen, Y.; Dong, L. Conjugated nitroxide radical polymer with low temperature tolerance potential for high-performance organic polymer cathode. J. Am. Chem. Soc. 2024, 146, 22777–22786. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Jiang, L.; Zhu, X.; Li, F.; Liu, X.; Mai, K.; Zhang, Z.; Fan, X.; Lv, X. Multifunctional radical polymers-enabled rapid charge/discharge and high capacity for flexible self-standing LiFePO4/PETM/SWNT hybrid electrodes. Chem. Eng. J. 2024, 482, 149008. [Google Scholar] [CrossRef]
- Nakahara, K.; Iwasa, S.; Satoh, M.; Morioka, Y.; Iriyama, J.; Suguro, M.; Hasegawa, E. Rechargeable batteries with organic radical cathodes. Chem. Phys. Lett. 2002, 359, 351–354. [Google Scholar] [CrossRef]
- Rostro, L.; Baradwaj, A.G.; Boudouris, B.W. Controlled radical polymerization and quantification of solid-state electrical conductivities of macromolecules bearing pendant stable radical groups. ACS Appl. Mater. Interfaces 2013, 5, 9896–9901. [Google Scholar] [CrossRef]
- Lee, S.; Kwon, G.; Ku, K.; Yoon, K.; Jung, S.K.; Lim, H.D.; Kang, K. Recent progress in organic electrodes for Li and Na rechargeable batteries. Adv. Mater. 2018, 30, e1704682. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, Y.; Yoo, J.; Kwon, G.; Ko, Y.; Kang, K. Organic batteries for a greener rechargeable world. Nat. Rev. Mater. 2022, 8, 54–70. [Google Scholar] [CrossRef]
- Jiang, S.; Xie, Y.; Xie, Y.; Yu, L.-J.; Yan, X.; Zhao, F.-G.; Mudugamuwa, C.J.; Coote, M.L.; Jia, Z.; Zhang, K. Lewis Acid-Induced Reversible Disproportionation of TEMPO Enables Aqueous Aluminum Radical Batteries. J. Am. Chem. Soc. 2023, 145, 14519–14528. [Google Scholar] [CrossRef]
- Chen, B.; Lu, Z.; Feng, S.; Zhou, Z.; Lu, C. Redox-active nitroxide radicals grafted onto MXene: Boosting energy storage via improved charge transfer and surface capacitance. ACS Energy Lett. 2023, 8, 1096–1106. [Google Scholar] [CrossRef]
- Bugnon, L.; Morton, C.J.; Novak, P.; Vetter, J.; Nesvadba, P. Synthesis of poly(4-methacryloyloxy-TEMPO) via group-transfer polymerization and its evaluation in organic radical battery. Chem. Mater. 2007, 19, 2910–2914. [Google Scholar] [CrossRef]
- Nakahara, K.; Iriyama, J.; Iwasa, S.; Suguro, M.; Satoh, M.; Cairns, E.J. High-rate capable organic radical cathodes for lithium rechargeable batteries. J. Power Sources 2007, 165, 870–873. [Google Scholar] [CrossRef]
- Nakahara, K.; Iriyama, J.; Iwasa, S.; Suguro, M.; Satoh, M.; Cairns, E.J. Cell properties for modified PTMA cathodes of organic radical batteries. J. Power Sources 2007, 165, 398–402. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.; Dai, Y.; Ge, J.; Lu, W.; Yang, J.; Chen, L. In-situ activated polycation as a multifunctional additive for Li-S batteries. Nano Energy 2016, 26, 43–49. [Google Scholar] [CrossRef]
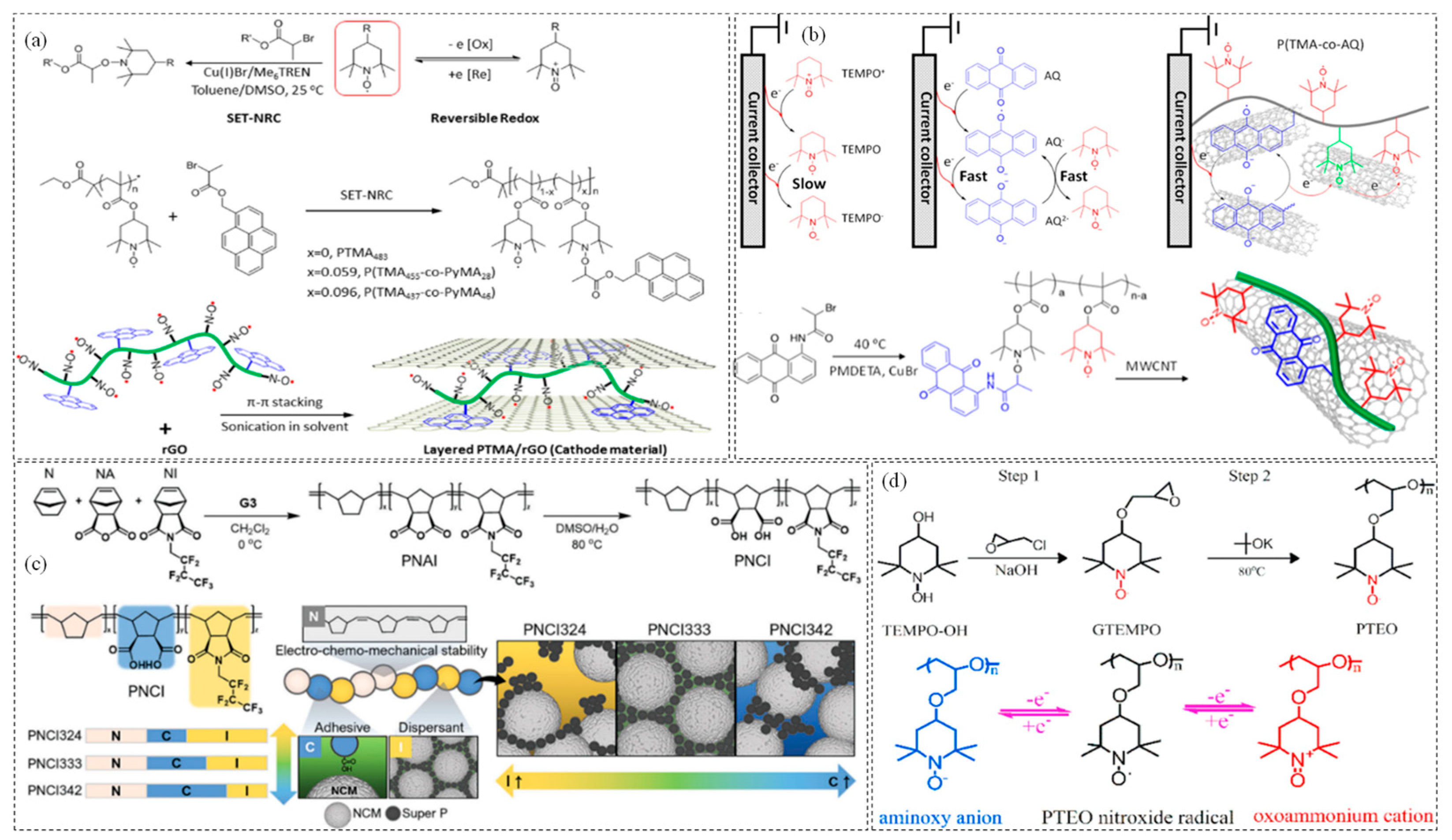
- Zhang, K.; Hu, Y.; Wang, L.; Monteiro, M.J.; Jia, Z. Pyrene-functionalized PTMA by NRC for Greater π–π stacking with rGO and enhanced electrochemical properties. ACS Appl. Mater. Interfaces 2017, 9, 34900–34908. [Google Scholar] [CrossRef]
- Li, W.; Jiang, S.; Xie, Y.; Yan, X.; Zhao, F.; Pang, X.; Zhang, K.; Jia, Z. Anthraquinone-catalyzed TEMPO reduction to realize two-electron energy storage of poly (TEMPO-methacrylate). ACS Energy Lett. 2022, 7, 1481–1489. [Google Scholar] [CrossRef]
- Jeong, D.; Kwon, D.S.; Kim, H.J.; Shim, J. Striking a balance: Exploring optimal functionalities and composition of highly adhesive and dispersing binders for high-nickel cathodes in lithium-ion batteries. Adv. Energy Mater. 2023, 13, 2302845. [Google Scholar] [CrossRef]
- Deng, W.; Shi, W.; Liu, Q.; Jiang, J.; Wang, Q.; Guo, C. Conductive nonconjugated radical polymer as high-capacity organic cathode material for high-energy Li/Na ion batteries. J. Power Sources 2020, 479, 228796. [Google Scholar] [CrossRef]
- Hatakeyama-Sato, K.; Matsumoto, S.; Takami, H.; Nagatsuka, T.; Oyaizu, K. A PROXYL-type norbornene polymer for high-voltage cathodes in lithium batteries. Macromol. Rapid Commun. 2021, 42, 2100374. [Google Scholar] [CrossRef]
- Yoshida, L.; Hakari, T.; Matsui, Y.; Deguchi, M.; Yamamoto, H.; Inoue, M.; Ishikawa, M. Understanding the improved performances of Lithium–Sulfur batteries containing oxidized microporous carbon with an affinity-controlled interphase as a sulfur host. J. Power Sources 2024, 624, 235572. [Google Scholar] [CrossRef]
- Jähnert, T.; Hager, M.D.; Schubert, U.S. Assorted phenoxyl-radical polymers and their application in lithium-organic batteries. macromol. Rapid Commun. 2016, 37, 725–730. [Google Scholar] [CrossRef]
- Facchetti, A. π-conjugated polymers for organic electronics and photovoltaic cell applications. Chem. Mater. 2010, 23, 733–758. [Google Scholar] [CrossRef]
- Heeger, A.J. Semiconducting and metallic polymers: The fourth generation of polymeric materials. J. Phys. Chem. B 2001, 105, 8475–8491. [Google Scholar] [CrossRef]
- Suga, T.; Ohshiro, H.; Sugita, S.; Oyaizu, K.; Nishide, H. Emerging n-type redox-active radical polymer for a totally organic polymer-based Rechargeable Battery. Adv. Mater. 2009, 21, 1627–1630. [Google Scholar] [CrossRef]
- Jahnert, T.; Hager, M.D.; Schubert, U.S. Application of phenolic radicals for antioxidants, as active materials in batteries, magnetic materials and ligands for metal-complexes. J. Mater. Chem. A 2014, 2, 15234–15251. [Google Scholar] [CrossRef]
- Li, L.; Gong, H.X.; Chen, D.Y.; Lin, M.J. Stable bifunctional perylene imide radicals for high-performance organic–lithium redox-flow batteries. Chem. Eur. J. 2018, 24, 13188–13196. [Google Scholar] [CrossRef]
- Gu, S.; Chen, J.; Hussain, I.; Wang, Z.; Chen, X.; Ahmad, M.; Feng, S.P.; Lu, Z.; Zhang, K. Modulation of radical intermediates in rechargeable organic batteries. Adv. Mater. 2023, 36, e2306491. [Google Scholar] [CrossRef] [PubMed]
- Back, J.; Park, J.; Kim, Y.; Kang, H.; Kim, Y.; Park, M.J.; Kim, K.; Lee, E. Triazenyl radicals stabilized by n-heterocyclic carbenes. J. Am. Chem. Soc. 2017, 139, 15300–15303. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.X.; Li, Y.; Huang, F. Persistent and stable organic radicals: Design, synthesis, and applications. Chem 2021, 7, 288–332. [Google Scholar] [CrossRef]
- Bai, W.-L.; Zhang, Z.; Chen, X.; Wei, X.; Zhang, Q.; Xu, Z.-X.; Liu, Y.-S.; Chang, B.; Wang, K.-X.; Chen, J.-S. Boosting the electrochemical performance of Li–O2 batteries with DPPH redox mediator and graphene-luteolin-protected lithium anode. Energy Storage Mater. 2020, 31, 373–381. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Lyu, M.; Meng, N.; Cao, J.; Xiong, C.; Lian, F. Electrically Conductive Functional Polymers and Application Progress in Lithium Batteries. Polymers 2025, 17, 778. https://doi.org/10.3390/polym17060778
Huang Z, Lyu M, Meng N, Cao J, Xiong C, Lian F. Electrically Conductive Functional Polymers and Application Progress in Lithium Batteries. Polymers. 2025; 17(6):778. https://doi.org/10.3390/polym17060778
Chicago/Turabian StyleHuang, Zhe, Mengting Lyu, Nan Meng, Jinxin Cao, Chenyu Xiong, and Fang Lian. 2025. "Electrically Conductive Functional Polymers and Application Progress in Lithium Batteries" Polymers 17, no. 6: 778. https://doi.org/10.3390/polym17060778
APA StyleHuang, Z., Lyu, M., Meng, N., Cao, J., Xiong, C., & Lian, F. (2025). Electrically Conductive Functional Polymers and Application Progress in Lithium Batteries. Polymers, 17(6), 778. https://doi.org/10.3390/polym17060778








