Carbon-Supported Hyperbranched Polyethyleneimines: Exploring into Polyamine/Anion Interactions to Design Efficient Polymer-Based Energy and Scavenger Materials
Abstract
:1. Introduction
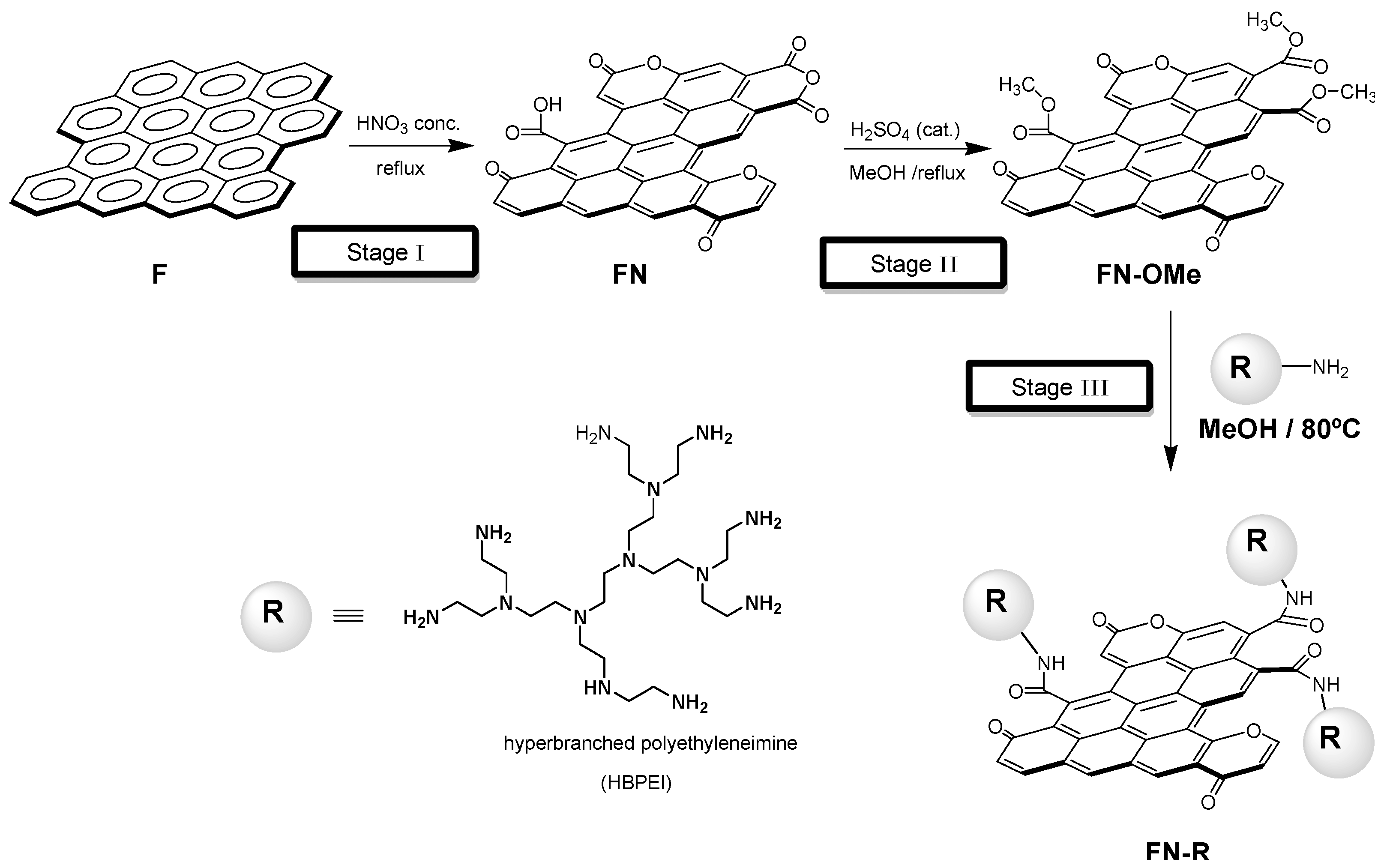
2. Materials and Methods
- (a)
- HBPEI/H+ and HBPEI/anion systems
- (b)
- FN-HBPEI/H+ and FN-HBPEI/anion systems
3. Results and Discussion
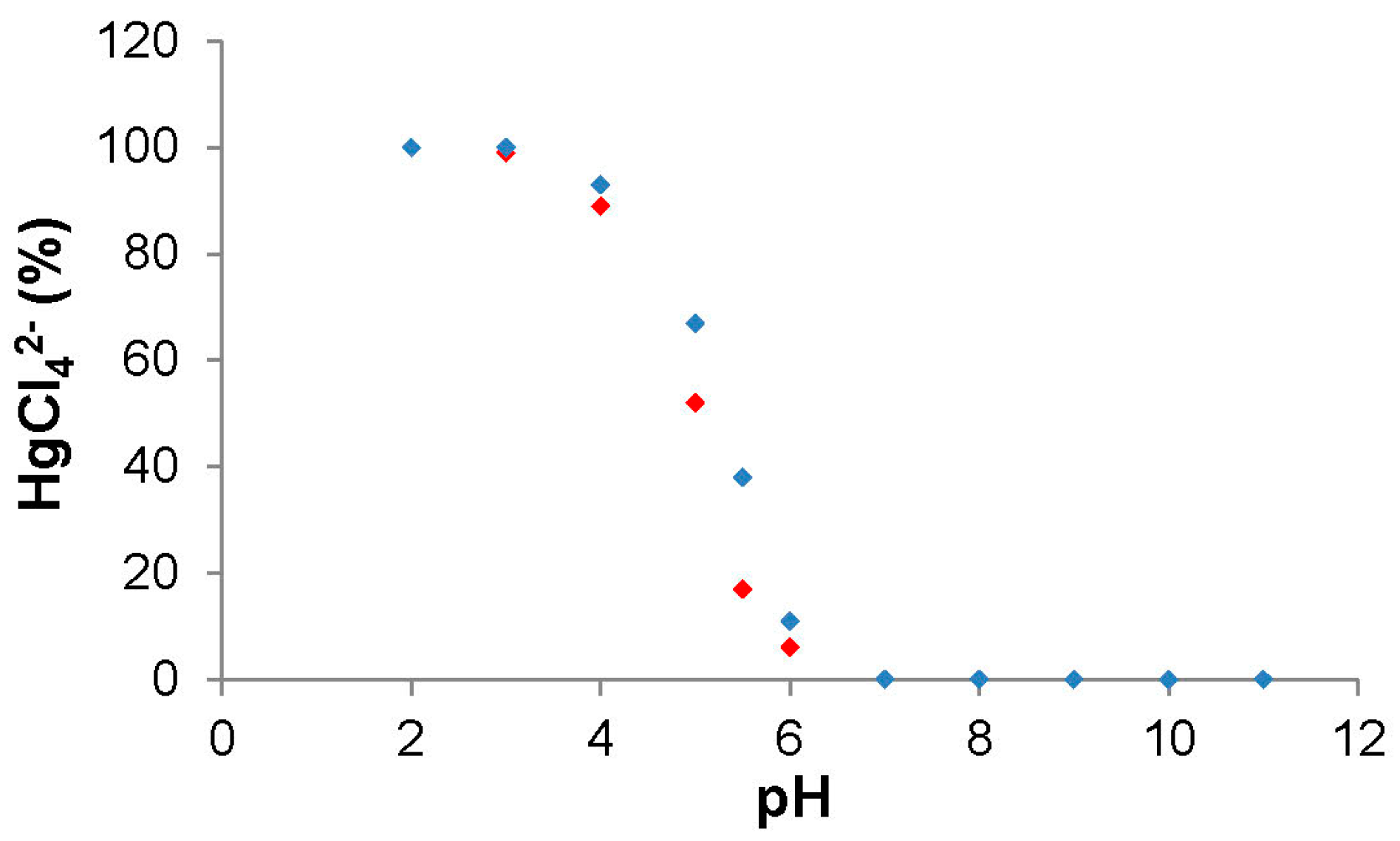
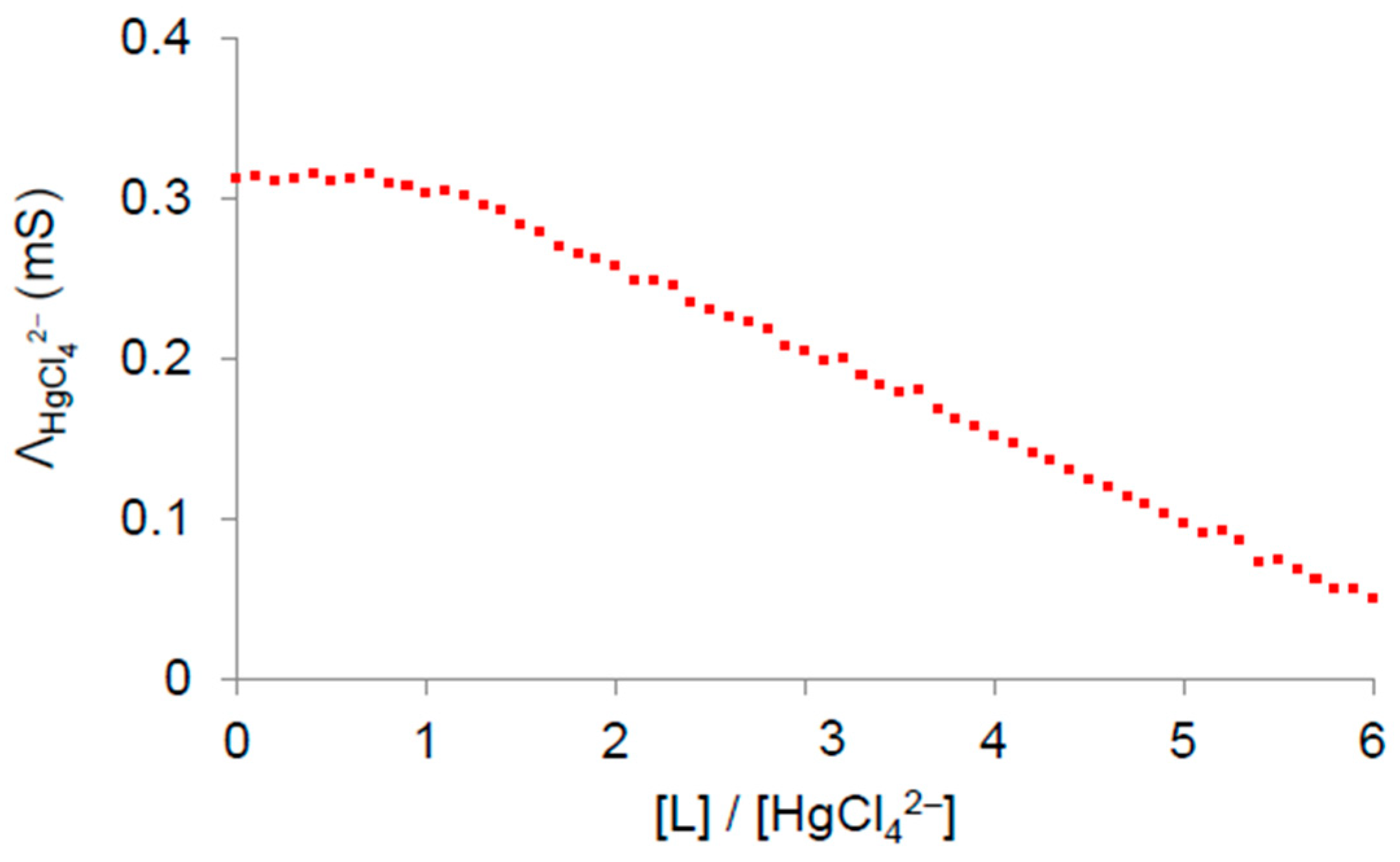
3.1. Complex Formation in HBPEI/Anion Systems
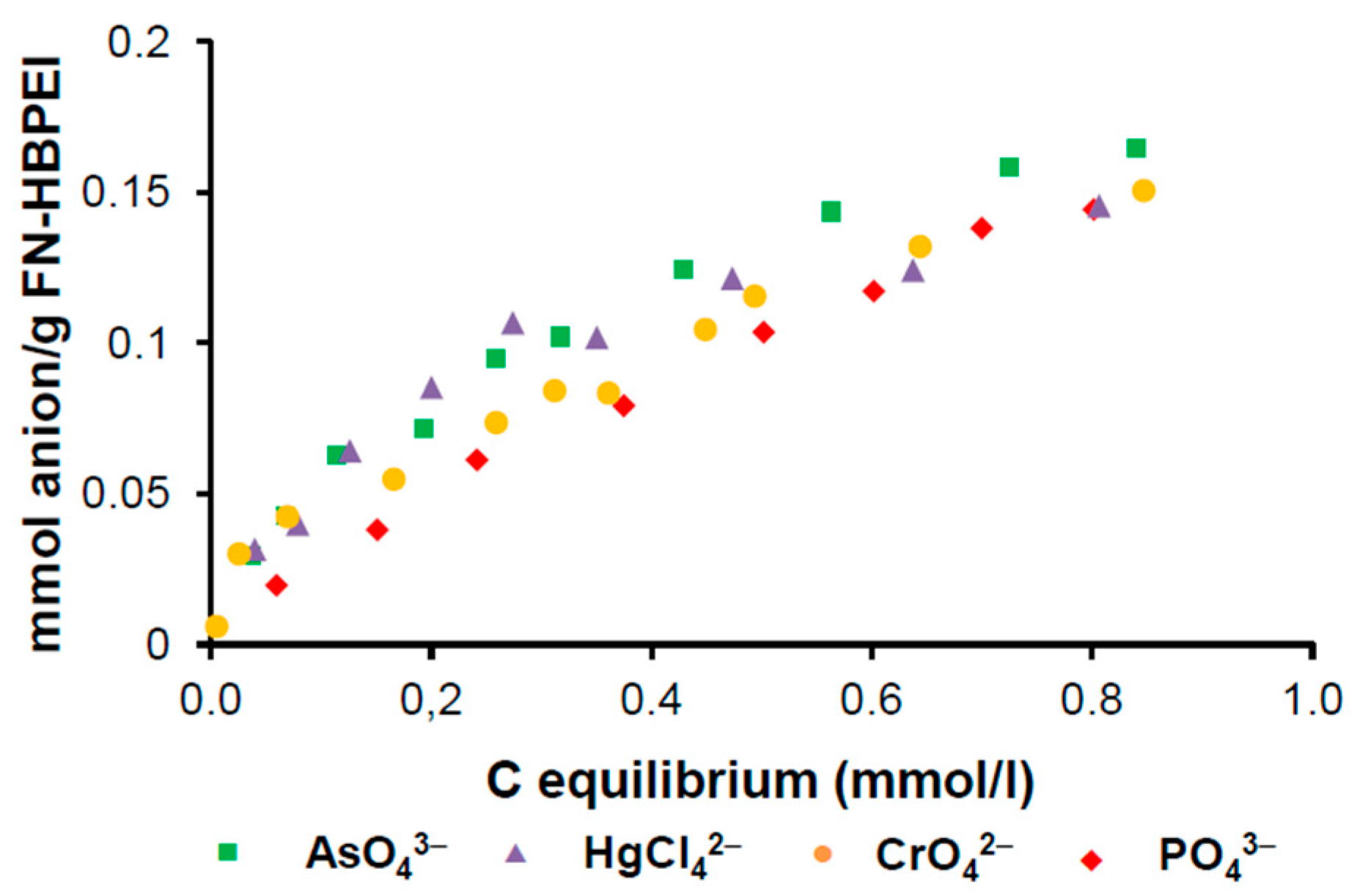
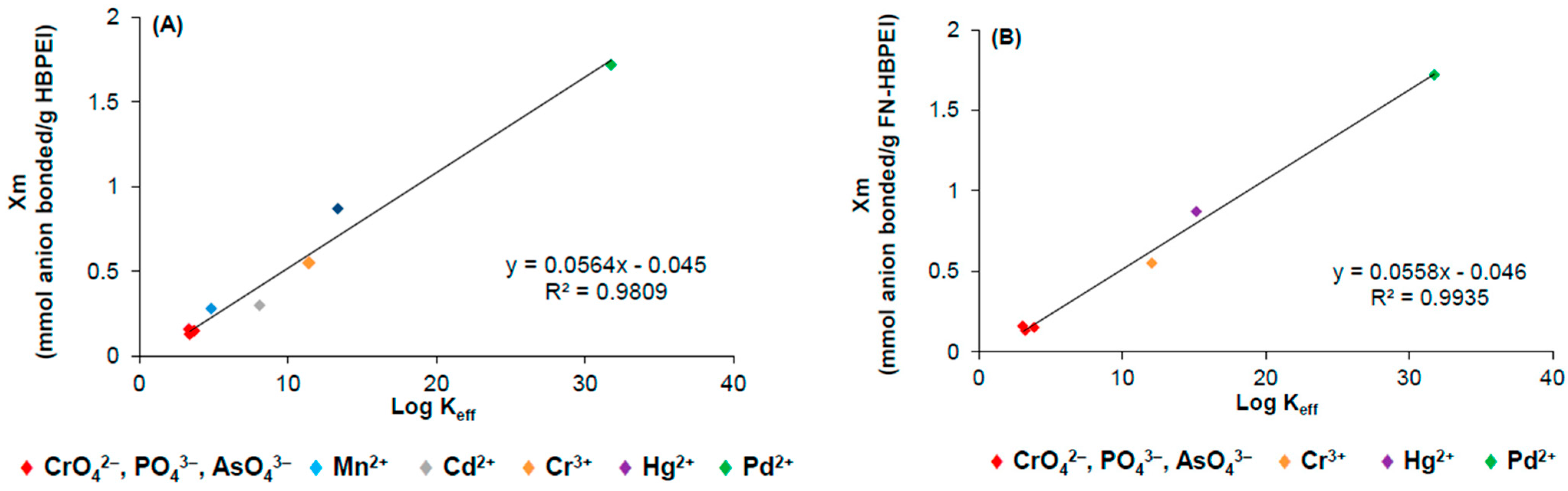
3.2. Adsorption Studies of Anions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HBPEI | Hyperbranched polyethyleneimine |
| AC | Activated carbon |
| F | Commercial activated carbon (Filtracarb) |
| FN | Activated carbon (F) oxidized with HNO3 |
| FN-OME | Activated carbon (FN) esterified |
| FN-HBPEI | Hybrid material based on HBPEI molecules bonded to FN-OMe |
| L | Complexing HBPEI unit based on triethylenetriamine fragments (L= –NH(CH2)2–N[–(CH2)2NH2]–(CH2)2–) |
| HL | Protonated complexing HBPEI unit |
References
- Ruan, D.; Tan, L.; Chen, S.; Fan, J.; Nian, Q.; Chen, L.; Wang, Z.; Ren, X. Solvent versus Anion Chemistry: Unveiling the Structure-Dependent Reactivity in Tailoring Electrochemical Interphases for Lithium-Metal Batteries. JACS Au 2023, 3, 953–963. [Google Scholar] [CrossRef]
- Lee, J.S.M. Anion-cation interactions dictate safe and stable electrolytes for sodium-ion batteries. Commun. Mater. 2024, 5, 119. [Google Scholar] [CrossRef]
- Xiao, H.; Li, X.; Fu, Y. Advances in Anion Chemistry in the Electrolyte Design for Better Lithium Batteries. Nano-Micro Lett. 2025, 17, 149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yan, J.; Pageni, P.; Yan, Y.; Wirth, A.; Chen, Y.P.; Qiao, Y.; Wang, Q.; Decho, A.W.; Tang, C. Anion-Responsive Metallopolymer Hydrogels for Healthcare Applications. Sci. Rep. 2015, 5, 11914. [Google Scholar] [CrossRef] [PubMed]
- Loh, W.W.; Lin, Q.; Lim, C.C.; Guo, L.; Tang, Y.K.; Loh, X.J.; Lim, J.Y.C. Hofmeister effects of anions on self-assembled thermogels. Mater. Today Chem. 2022, 23, 100674. [Google Scholar] [CrossRef]
- Heydari, H.; Raissi, H.; Ghahari, A. Engineered crystalline polymers for effective contaminant removal from water. Sci. Rep. 2024, 14, 31869. [Google Scholar] [CrossRef]
- Wang, D.X.; Wang, M.X. Exploring Anion−π Interactions and Their Applications in Supramolecular Chemistry. Acc. Chem. Res. 2020, 53, 1364–1380. [Google Scholar] [CrossRef]
- Nicholas, H.E.; Christopher, J.S.; Kirsten, E.C.; Paul, D.B. Amide and Urea Ferrocene-Containing Macrocycles Capable of the Electrochemical Sensing of Anions. Eur. J. Inorg. Chem. 2012, 2012, 939–944. [Google Scholar]
- Philip, A.G.; Quesada, R. Anion coordination and anion-templated assembly: Highlights from 2002 to 2004. Coord. Chem. Rev. 2006, 250, 3219–3244. [Google Scholar]
- Mateusa, P.; Berniera, N.; Delgado, R. Recognition of anions by polyammonium macrocyclic and cryptand receptors: Influence of the dimensionality on the binding behaviour. Coord. Chem. Rev. 2010, 254, 1726–1747. [Google Scholar] [CrossRef]
- Carvalho, S.; Delgado, R.; Félix, V. Evaluation of the binding ability of a macrobicyclic receptor for anions by potentiometry and molecular dynamics simulations in solution. Tetrahedron 2010, 66, 8714–8721. [Google Scholar] [CrossRef]
- Bianchi, A.; Bowman-James, K.; García-España, E. Supramolecular chemistry of anions, 1. Aufl., 461 S., 175 Abb., 37 Tab., New York, Chichester, Weinheim, Brisbane, Singapore, Toronto, WILEY-VCH, 1997, ISBN 0-471-18622-8. J. Prakt. Chem. 1998, 340, 486–487. [Google Scholar] [CrossRef]
- Schhmidtchen, F.P. Reflections on the construction of anion receptors: Is there a sign to resign from design? Coord. Chem. Rev. 2006, 250, 2918–2928. [Google Scholar] [CrossRef]
- Amendola, V.I.; Fabbrizzi, L. Anion receptors that contain metals as structural units. Chem. Commun. 2009, 5, 513–531. [Google Scholar] [CrossRef]
- O’Neil, E.J.; Smith, B.D. Anion recognition using dimetallic coordination complexes. Coord. Chem. Rev. 2006, 250, 3068–3080. [Google Scholar] [CrossRef]
- Rice, C.R. Metal-assembled anion receptors. Coord. Chem. Rev. 2006, 250, 3190–3199. [Google Scholar] [CrossRef]
- Scott, R.J. Cationic inorganic materials for anionic pollutant trapping and catalysis. Chem. Soc. Rev. 2009, 38, 1868–1881. [Google Scholar]
- Vilar, R. Anion Recognition and Templation in Coordination Chemistry. Eur. J. Inorg. Chem. 2008, 2008, 357–367. [Google Scholar] [CrossRef]
- Bazzicalupi, C.; Bencini, A.; Bianchi, A.; Danesi, A.; Giorgi, C.; Valtancoli, B. Anion Binding by Protonated Forms of the Tripodal Ligand Tren. Inorg. Chem. 2009, 48, 2391–2398. [Google Scholar] [CrossRef]
- Bazzicalupi, C.; Bencini, A.; Biagini, S.; Faggi, E.; Meini, S.; Giorgi, C.; Spepi, A.; Valtancoli, B. Exploring the Binding Ability of Phenanthroline-Based Polyammonium Receptors for Anions: Hints for Design of Selective Chemosensors for Nucleotides. J. Org. Chem. 2009, 74, 7349–7363. [Google Scholar] [CrossRef]
- Clifford, T.; Danby, A.; Llinares, J.M.; Mason, S.; Alcock, N.W.; Powell, D.; Aguilar, J.A.; García-España, E.; Bowman-James, K. Anion Binding with Two Polyammonium Macrocycles of Different Dimensionality. Inorg. Chem. 2001, 40, 4710–4720. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.J.; Yatsimirsky, A.K. Selectivity in supramolecular host–guest complexes. Chem. Soc. Rev. 2008, 37, 263–277. [Google Scholar] [CrossRef]
- Llinares, J.M.; Powell, D.; Bowman-James, K. Ammonium based anion receptors. Coord. Chem. Rev. 2003, 240, 57–75. [Google Scholar] [CrossRef]
- McKee, V.; Nelson, J.; Town, R.M. Caged oxoanions. Chem. Soc. Rev. 2003, 32, 309–325. [Google Scholar] [CrossRef]
- Kang, S.O.; Hossain, M.A.; Bowman-James, K. Influence of dimensionality and charge on anion binding in amide-based macrocyclic receptors. Coord. Chem. Rev. 2006, 250, 3038–3052. [Google Scholar] [CrossRef]
- García-España, E.; Díaz, P.; Llinares, J.M.; Bianchi, A. Anion coordination chemistry in aqueous solution of polyammonium receptors. Coord. Chem. Rev. 2006, 250, 2952–2986. [Google Scholar] [CrossRef]
- Arranz, P.; Bianchi, A.; Cuesta, R.; Giorgi, C.; Godino, M.L.; Gutierrez, M.D.; López, R.; Santiago, A. Correction to Binding and Removal of Sulfate, Phosphate, Arsenate, Tetrachloromercurate, and Chromate in Aqueous Solution by Means of an Activated Carbon Functionalized with a Pyrimidine-Based Anion Receptor (HL). Crystal Structures of [H3L(HgCl4)]·H2O and [H3L(HgBr4)]·H2O Showing Anion−π Interactions. Inorg. Chem. 2012, 51, 4883. [Google Scholar]
- Bagdat, S.; Tokay, F.; Demirci, S.; Yilmaz, S.; Sahiner, N. Removal of Cd(II), Co(II), Cr(III), Ni(II), Pb(II) and Zn(II) ions from wastewater using polyethyleneimine (PEI) cryogels. J. Environ. Manag. 2023, 329, 117002. [Google Scholar] [CrossRef]
- Jarvis, N.V.; Wagener, J.M. Mechanistic studies of metal ion binding to water-soluble polymers using potentiometry. Talanta 1995, 42, 219–226. [Google Scholar] [CrossRef]
- Geckeler, K.; Lange, G.; Eberhardt, H.; Bayer, E. Preparation and application of water-soluble polymer-metal complexes. Pure Appl. Chem. 1980, 52, 1883–1905. [Google Scholar] [CrossRef]
- Kubilay, S.; Demirci, S.; Can, M.; Aktas, N.; Sahiner, N. Dichromate and arsenate anion removal by PEI microgel, cryogel, and bulkgel. J. Environ. Chem. Eng. 2021, 9, 104799. [Google Scholar] [CrossRef]
- Sahiner, N.; Demirci, S.; Sahiner, M.; Yilmaz, S. Application of superporous magnetic cationic cryogels for persistent chromate (toxic chromate and dichromate) uptake from aqueous environments. J. Appl. Polym. Sci. 2016, 133, 43438. [Google Scholar] [CrossRef]
- Sahiner, N.; Demirci, S.; Sahiner, M.; Yilmaz, S.; Al-Lohedan, H. The use of superporous p(3-acrylamidopropyl)trimethyl ammonium chloride cryogels for removal of toxic arsenate anions. J. Environ. Manag. 2015, 152, 66–74. [Google Scholar] [CrossRef]
- Peñas-Sanjuán, A.; López-Garzón, R.; López-Garzón, J.; Pérez-Mendoza, M.; Melguizo, M. Preparation of a poly-alkylamine surface-functionalized carbon with excellent performance as a Pd(II) scavenger. Carbon 2012, 50, 2350–2352. [Google Scholar] [CrossRef]
- Peñas-Sanjuán, A.; Cruz-Sánchez, R.; García-Gallarín, C.; Pérez-Mendoza, M.; López-Garzón, R.; Melguizo, M. A linear free-energy relationship for the prediction of metal ion complexing properties in hybrid carbon-based scavengers. New J. Chem. 2023, 47, 12883. [Google Scholar] [CrossRef]
- Li, L.; Liu, J.; Li, L.; Chen, J.; Liu, J.; Zhou, R.; Zhang, L. Pentafluorophenyl diethoxy phosphate: An electrolyte additive for high-voltage cathodes of lithium-ion batteries. J. Energy Storage 2024, 87, 111364. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, C.; Xie, W.; Liu, X.; Lu, Y.; Hou, Y.; Ma, T.; Yan, Z.; Chen, J. Ether-Modified Nonflammable Phosphate Enabling Anion-Rich Electrolyte for High-Voltage Lithium Metal Batteries. Adv. Mater. 2024, 36, 2312302. [Google Scholar] [CrossRef]
- Seggem, P.; Chavan, S.N.; Biswas, S.; Jetti, V.R. Phosphate-Based Electrolyte and Pristine Graphite Cathode for a High-Voltage Rechargeable Dual-Ion Magnesium Battery. ACS Appl. Energy Mater. 2021, 4, 5165–5174. [Google Scholar] [CrossRef]
- Liu, D.; Mu, X.; Guo, R.; Xie, J.; Yinm, G.; Zuo, P. Electrochemical performance of CrOx cathode material for high energy density lithium batteries. Int. J. Electrochem. Sci. 2023, 18, 44–48. [Google Scholar] [CrossRef]
- Nejad, M.S.; Sheibani, H. Super-efficient removal of arsenic and mercury ions from wastewater by nanoporous biochar-supported poly 2-aminothiophenol. J. Environ. Chem. Eng. 2022, 10, 107363. [Google Scholar] [CrossRef]
- Ishaq, M.; Jan, F.A.; Khan, M.A.; Ihsanullah, I.; Ahmad, I.; Shakirullah, M.; Roohullah. Effect of mercury and arsenic from industrial effluents on the drinking water and comparison of the water quality of polluted and non-polluted areas: A case study of Peshawar and Lower Dir. Environ Monit Assess 2013, 185, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Sessler, J.L.; Samson, P.I.; Andrievsky, A.; Kral, V. Supramolecular Chemistry of Anions. Application Aspects Involving the Supramolecualr Chemistry of Anions; Wiley: New York, NY, USA, 1997; pp. 355–419. [Google Scholar]
- Gale, P.A. 35 Years of Synthetic Anion Receptor Chemistry 1968–2003. Coord. Chem. Rev. 2003, 240, 1. [Google Scholar] [CrossRef]
- Dudek, M.J.; Ponder, J.W. Accurate modeling of the intramolecular electrostatic energy of proteins. J. Comput. Chem. 1995, 16, 791–816. [Google Scholar] [CrossRef]
- HyperChem, Release 4.5 for Windows; Hypercube, Inc.: Waterloo, ON, Canada, 1995.
- Godino, M.L.; Gutierrez, M.D.; López, R.; Moreno, J.M. Zn(II) complexes with thiopyrimidine derivatives: Solution study, synthesis and crystal structure of a zig-zag chain zinc(II) complex with the ligand 4,6-dimethyl-2-thiopyrimidine. Inorg. Chim. Acta 1994, 221, 177–181. [Google Scholar] [CrossRef]
- Gran, G. Determination of the equivalence point in potentiometric titrations. Part II. Analyst 1952, 77, 661–671. [Google Scholar] [CrossRef]
- Bencini, A.; Bianchi, A.; García-España, E.; Micheloni, M.; Ramirez, J.A. Proton coordination by polyamine compounds in aqueous solution. Coord. Chem. Rev. 1999, 188, 97–156. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Titration and More, Versión 1.2.1; Metrohm LTD: 2007. Available online: https://www.metrohm.com/en_gb/service/software-center/tiamo.html (accessed on 13 March 2025).
- Martell, A.E.; Smith, R.M.; Motekaitis, R.J. Critically Selected Stability Constants of Metal Complexes; NIST Standard Reference Data: 2003. Available online: https://www.nist.gov/system/files/documents/srd/46_8.pdf (accessed on 13 March 2025).
- Radovic, L.; Moreno-Castilla, C.; Rivera-Utrilla, J. Chemistry and Physics of Carbon; Radivic, L., Ed.; Marcel Dekker: New York, NY, USA, 2000; Volume 27. [Google Scholar]
- Radovic, L.R.; Silva, I.F.; Ume, J.I.; Menendez, J.A.; Leon y Leon, C.A.; Scaroni, A.W. An experimental and theoretical study of the adsorption of aromatics possessing electron-withdrawing and electron-donating functional groups by chemically modified activated carbons. Carbon 1997, 35, 1339–1348. [Google Scholar] [CrossRef]
- Gutiérrez-Valero, M.D.; Arranz-Mascarós, P.; Peñas-Sanjuán, A.; Godino-Salido, M.L.; López-Garzón, R.; Santiago-Medina, A.; Melguizo-Guijarro, M.; Pérez-Mendoza, M.; López-Garzón, F.J.; Domingo-García, M. Transferring the properties of molecular receptors to the carbon surface in hybrid materials: The crucial role of porous texture. Mater. Chem. Phys. 2012, 54, 608–615. [Google Scholar] [CrossRef]
- Albelda, M.T.; Bernardo, M.A.; García-España, E.; Godino-Salido, M.; Luis, S.V.; Melo, M.J.; Pina, F.; Soriano, C. Thermodynamics and fluorescence emission studies on potential molecular chemosensors for ATP recognition in aqueous solution. J. Chem. Soc. Perkin Trans. 1999, 2, 2545–2549. [Google Scholar] [CrossRef]

| Equilibrium | Log K * |
|---|---|
| HL2+ + AsO43− ⇆ [HL2(AsO4)]2− | 7.02 (6) |
| HL+ + HAsO42− ⇆ [HL(HAsO42−)]− | 2.98 (8) |
| H2L2+ + HAsO42− ⇆ [H2L(HAsO4)] | 3.70 (4) |
| HL2+ + PO43− ⇆ [HL2(PO4)]2− | 7.85 (6) |
| HL+ + HPO42− ⇆ [HL(HPO42−)]− | 3.12 (6) |
| H2L2+ + HPO42− ⇆ [H2L(HPO4)] | 3.84 (3) |
| HL2+ + CrO42− ⇆ [HL2(CrO4)]− | 4.38 (6) |
| HL+ + CrO42− ⇆ [HL(CrO4)]− | 3.55 (5) |
| H2L2+ + CrO42− ⇆ [H2L(CrO4)] | 3.75 (5) |
| H2L2+ + HCrO4− ⇆ [H2L(HCrO4)]+ | 3.81 (3) |
| Hg2+ + L ⇆ [HgL]2+ | 16.47 (3) |
| Hg2+ + HL+ ⇆ [HgHL]3+ | 13.06 (5) |
| [HgL]2+ + OH− ⇆ [HgL(OH)]+ | 3.87 (4) |
| [HgL(OH)]+ + OH− ⇆ [HgL(OH)2] | 3.24 (7) |
| H2L2+ + HgCl42− ⇆ [H2L(HgCl4)] | 8.41 (6) |
| H3L3+ + HgCl42− ⇆ [H3L(HgCl4)]+ | 8.75 (2) |
| Equilibrium | Log K |
|---|---|
| HL2+ + AsO43− ⇆ [HL2(AsO4)]2− | 6.84 (3) |
| HL+ + HAsO42− ⇆ [HL(HAsO42−)]− | 2.13 (6) |
| H2L2+ + HAsO42− ⇆ [H2L(HAsO4)] | 3.99 (4) |
| HL2+ + PO43− ⇆ [HL2(PO4)]2− | 7.15 (4) |
| HL+ + HPO42− ⇆ [HL(HPO42−)]− | 2.92 (7) |
| H2L2+ + HPO42− ⇆ [H2L(HPO4)] | 3.58 (5) |
| HL2+ + CrO42− ⇆ [HL2(CrO4)]− | 4.18 (9) |
| HL+ + CrO42− ⇆ [HL(CrO4)]− | 3.62 (6) |
| H2L2+ + CrO42− ⇆ [H2L(CrO4)] | 3.88 (5) |
| H2L2+ + HCrO4− ⇆ [H2L(HCrO4)]+ | 4.25 (2) |
| Hg2+ + L ⇆ [HgL]2+ | 17.17 (5) |
| Hg2+ + HL+ ⇆ [HgHL]3+ | 14.23 (6) |
| [HgL]2+ + OH− ⇆ [HgL(OH)]+ | 3.57 (2) |
| [HgL(OH)]+ + OH− ⇆ [HgL(OH)2] | 2.01 (8) |
| H2L2+ + HgCl42− ⇆ [H2L(HgCl4)] | --- |
| H3L3+ + HgCl42− ⇆ [H3L(HgCl4)]+ | --- |
| Sample | S (BET) (m2g−1) | V0 (N2) (cm3g−1) | L0 (N2) (nm) | Sext (m2g−1) | V0 (CO2) (cm3g−1) | L0 (CO2) (nm) |
|---|---|---|---|---|---|---|
| FN-HBPEI | 77 | 0.028 | 1.6 | 6.8 | 0.065 | 0.5 |
| F | 1426 | 0.561 | 1.3 | 39.2 | 0.315 | 0.8 |
| Proton Species of HBPEI | Dimensional Sizes (nm) | Min–Max Area Interval (nm2) | Molecular Volume (nm3) | Molecular Deviation (%) | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| L | 2.26 | 1.00 | 2.32 | 2.26–5.23 | 5.25 | 100 |
| HL+ | 2.16 | 0.86 | 2.48 | 1.86–5.36 | 4.62 | 88 |
| HL2+ | 2.05 | 0.87 | 2.22 | 1.78–4.55 | 3.97 | 76 |
| HL3+ | 1.90 | 1.38 | 3.09 | 2.63–5.88 | 8.14 | 155 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peñas-Sanjuán, A.; García-Gallarín, C.; Godino-Salido, M.L.; López-Garzón, R.; Melchionna, M.; Melguizo, M. Carbon-Supported Hyperbranched Polyethyleneimines: Exploring into Polyamine/Anion Interactions to Design Efficient Polymer-Based Energy and Scavenger Materials. Polymers 2025, 17, 786. https://doi.org/10.3390/polym17060786
Peñas-Sanjuán A, García-Gallarín C, Godino-Salido ML, López-Garzón R, Melchionna M, Melguizo M. Carbon-Supported Hyperbranched Polyethyleneimines: Exploring into Polyamine/Anion Interactions to Design Efficient Polymer-Based Energy and Scavenger Materials. Polymers. 2025; 17(6):786. https://doi.org/10.3390/polym17060786
Chicago/Turabian StylePeñas-Sanjuán, Antonio, Celeste García-Gallarín, María L. Godino-Salido, Rafael López-Garzón, Michele Melchionna, and Manuel Melguizo. 2025. "Carbon-Supported Hyperbranched Polyethyleneimines: Exploring into Polyamine/Anion Interactions to Design Efficient Polymer-Based Energy and Scavenger Materials" Polymers 17, no. 6: 786. https://doi.org/10.3390/polym17060786
APA StylePeñas-Sanjuán, A., García-Gallarín, C., Godino-Salido, M. L., López-Garzón, R., Melchionna, M., & Melguizo, M. (2025). Carbon-Supported Hyperbranched Polyethyleneimines: Exploring into Polyamine/Anion Interactions to Design Efficient Polymer-Based Energy and Scavenger Materials. Polymers, 17(6), 786. https://doi.org/10.3390/polym17060786







