Enhanced Antioxidant and Antibacterial Properties of Polybutylene Adipate-Terephthalate/Curcumin Composite Films Using Surface-Modified Cellulose Nanocrystals
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Surface Modification of CNC
2.3. Preparation of DxCNC/PBAT Composite Films
2.4. Preparation of DxCNC/ CUR/PBAT Composite Films
2.5. Characterization
3. Results
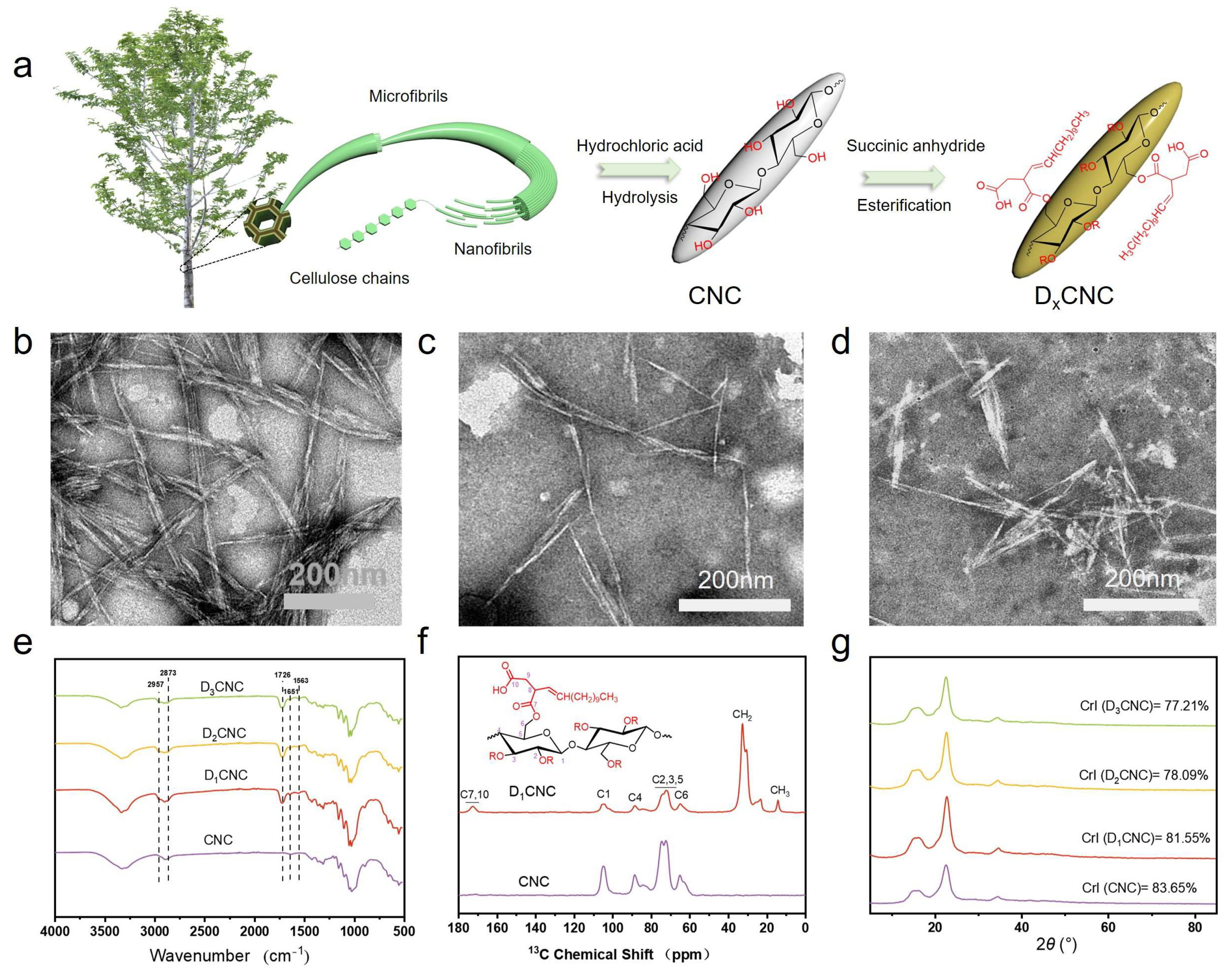
3.1. Morphologies and Structures of DxCNC
3.2. Optical Properties of DxCNC/CUR/PBAT Composite Films
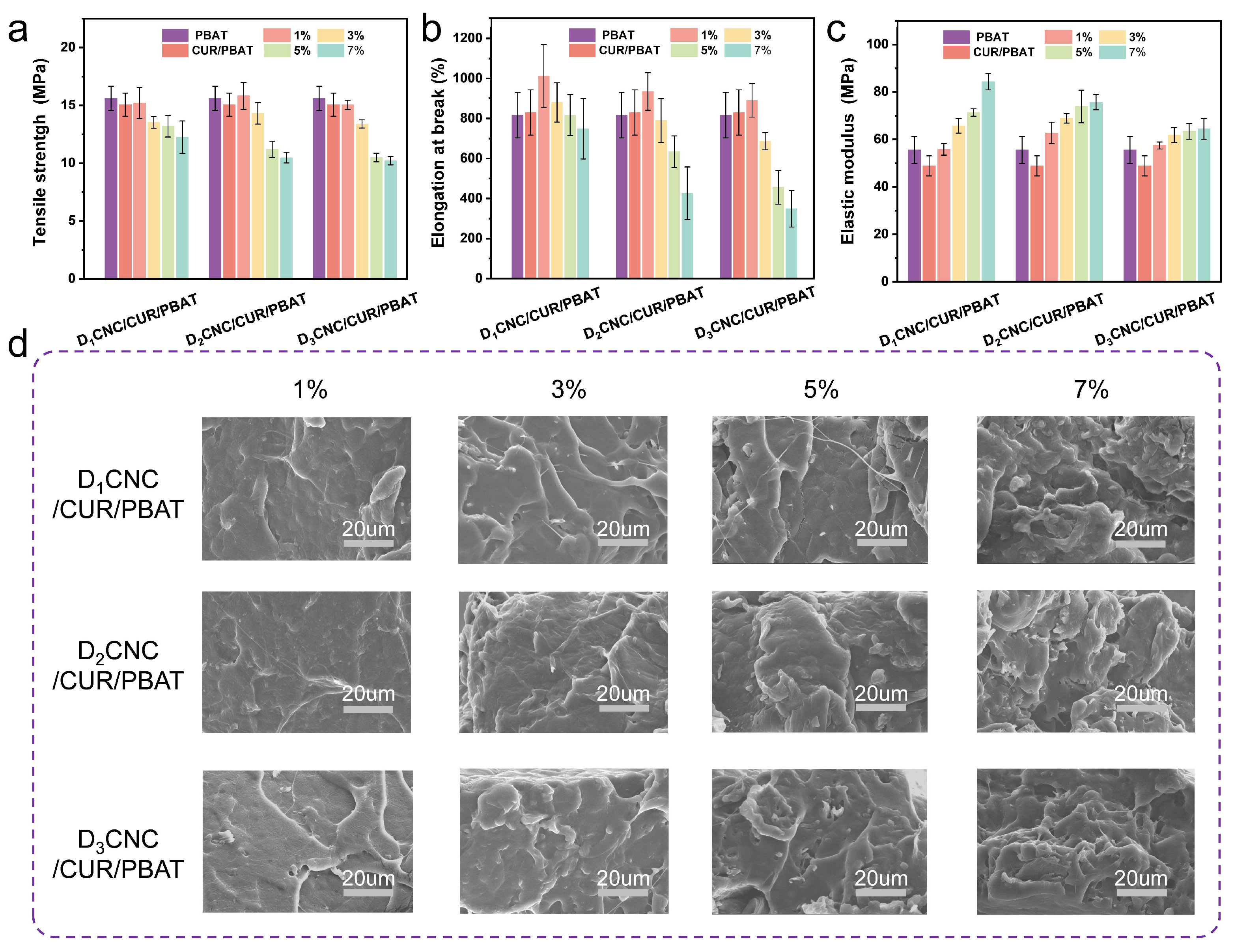
3.3. Mechanical Properties of DxCNC/CUR/PBAT Composite Films
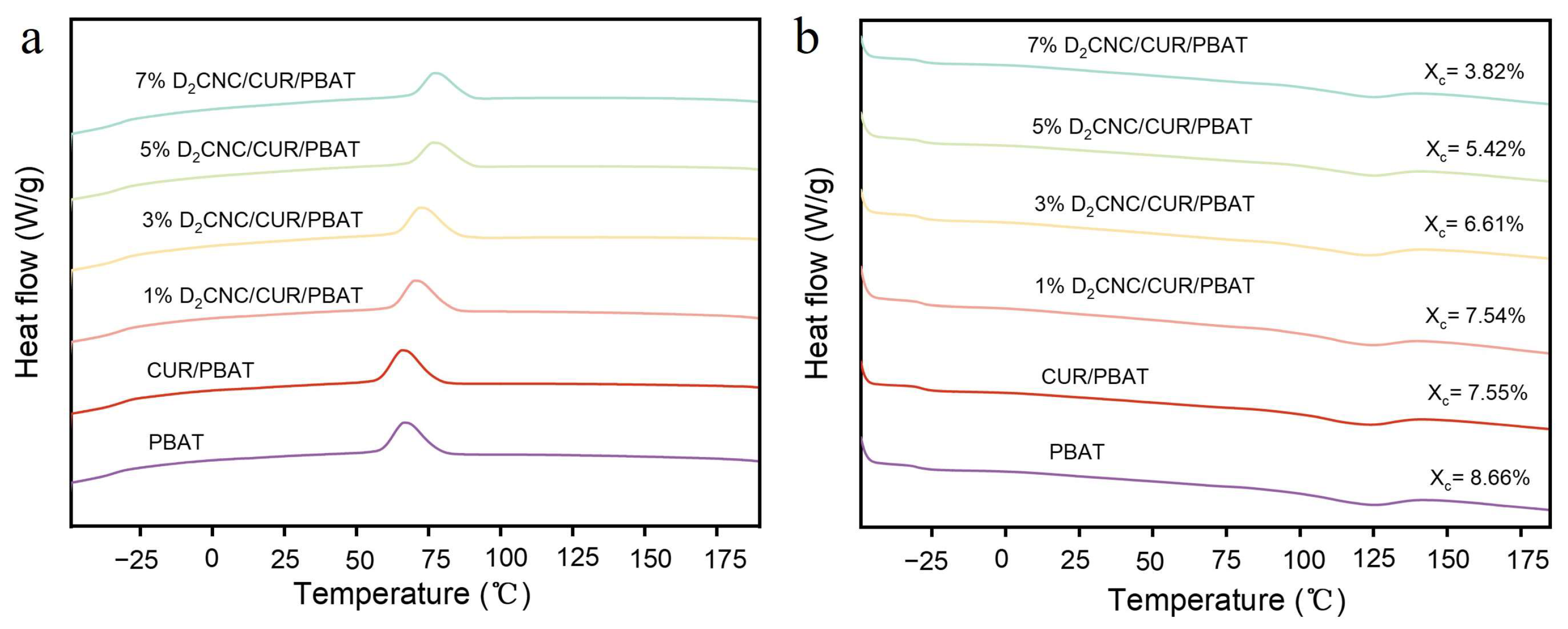
3.4. Thermal Properties of DxCNC/CUR/PBAT Composite Films
3.5. Antioxidant Properties of DxCNC/CUR/PBAT Composite Films
3.6. Antibacterial Properties of DxCNC/CUR/PBAT Composite Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and new opportunities on barrier performance of biodegradable polymers for sustainable packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Sun, B.; Li, B.; Li, K.; Wang, S.; Xu, J.; Liu, Y.; Zhou, Q. Cellulose Microgel Toughening of Starch Nanocomposites: Exploiting the Advantages of Micro-Nanoscale Networks. ACS Sustain. Chem. Eng. 2023, 11, 15721–15731. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Sayed, S.M. Bionanocomposites materials for food packaging applications: Concepts and future outlook. Carbohydr. Polym. 2018, 193, 19–27. [Google Scholar] [CrossRef] [PubMed]
- da Costa, F.A.T.; Parra, D.F.; Cardoso, E.C.L.; Güven, O. PLA, PBAT, Cellulose Nanocrystals (CNCs), and Their Blends: Biodegradation, Compatibilization, and Nanoparticle Interactions. J. Polym. Environ. 2023, 31, 4662–4690. [Google Scholar] [CrossRef]
- Gupta, A.; Chudasama, B.; Chang, B.P.; Mekonnen, T. Robust and sustainable PBAT—Hemp residue biocomposites: Reactive extrusion compatibilization and fabrication. Compos. Sci. Technol. 2021, 215, 109014. [Google Scholar] [CrossRef]
- Xiong, S.-J.; Pang, B.; Zhou, S.-J.; Li, M.-K.; Yang, S.; Wang, Y.-Y.; Shi, Q.; Wang, S.-F.; Yuan, T.-Q.; Sun, R.-C. Economically Competitive Biodegradable PBAT/Lignin Composites: Effect of Lignin Methylation and Compatibilizer. ACS Sustain. Chem. Eng. 2020, 8, 5338–5346. [Google Scholar] [CrossRef]
- Cao, C.; Wang, Y.; Zheng, S.; Zhang, J.; Li, W.; Li, B.; Guo, R.; Yu, J. Poly (butylene adipate-co-terephthalate)/titanium dioxide/silver composite biofilms for food packaging application. LWT 2020, 132, 109874. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.-W. Tocopherol-mediated synthesis of silver nanoparticles and preparation of antimicrobial PBAT/silver nanoparticles composite films. LWT-Food Sci. Technol. 2016, 72, 149–156. [Google Scholar] [CrossRef]
- Venkatesan, R.; Rajeswari, N. Poly (butylene adipate-co-terephthalate) bionanocomposites: Effect of SnO2 NPs on mechanical, thermal, morphological, and antimicrobial activity. Adv. Compos. Hybrid Mater. 2018, 1, 731–740. [Google Scholar] [CrossRef]
- Wang, T.; Shi, Y.; Li, Y.; Liu, L.-Z. The effects of ZnO nanoparticle reinforcement on thermostability, mechanical, and optical properties of the biodegradable PBAT film. J. Polym. Eng. 2021, 41, 835–841. [Google Scholar] [CrossRef]
- Tsikourkitoudi, V.; Henriques-Normark, B.; Sotiriou, G.A. Inorganic nanoparticle engineering against bacterial infections. Curr. Opin. Chem. Eng. 2022, 38, 100872. [Google Scholar] [CrossRef]
- Bhatti, R.; Shakeel, H.; Malik, K.; Qasim, M.; Khan, M.A.; Ahmed, N.; Jabeen, S. Inorganic Nanoparticles: Toxic Effects, Mechanisms of Cytotoxicity and Phytochemical Interactions. Adv. Pharm. Bull. 2022, 12, 757–762. [Google Scholar] [CrossRef]
- Yılmaz, G.E.; Göktürk, I.; Ovezova, M.; Yılmaz, F.; Kılıç, S.; Denizli, A. Antimicrobial Nanomaterials: A Review. Hygiene 2023, 3, 269–290. [Google Scholar] [CrossRef]
- Jao, W.-C.; Lin, C.-H.; Hsieh, J.-Y.; Yeh, Y.-H.; Liu, C.-Y.; Yang, M.-C. Effect of immobilization of polysaccharides on the biocompatibility of poly (butyleneadipate-co-terephthalate) films. Polym. Adv. Technol. 2010, 21, 543–553. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Kobylińska, A.; Antos-Bielska, M.; Krzyżowska, M.; Gałęski, A. Exploring the potential of lignin nanoparticles in enhancing the mechanical, thermal, and bioactive properties of poly (butylene adipate-co-terephthalate). Int. J. Biol. Macromol. 2024, 262, 129880. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, Z.; Zhang, S.; Hu, C.-Y.; Xu, X. Extrusion-blown oxidized starch/poly (butylene adipate-co-terephthalate) biodegradable active films with adequate material properties and antimicrobial activities for chilled pork preservation. Int. J. Biol. Macromol. 2023, 253, 127408. [Google Scholar] [CrossRef]
- Jain, A.; Duvvuri, L.S.; Farah, S.; Beyth, N.; Domb, A.J.; Khan, W. Antimicrobial Polymers. Adv. Healthc. Mater. 2014, 3, 1969–1985. [Google Scholar] [CrossRef]
- Sharma, R.A.; Gescher, A.J.; Steward, W.P. Curcumin: The story so far. Eur. J. Cancer 2005, 41, 1955–1968. [Google Scholar] [CrossRef]
- Tsekova, P.B.; Spasova, M.G.; Manolova, N.E.; Markova, N.D.; Rashkov, I.B. Electrospun curcumin-loaded cellulose acetate/polyvinylpyrrolidone fibrous materials with complex architecture and antibacterial activity. Mater. Sci. Eng. C 2017, 73, 206–214. [Google Scholar] [CrossRef]
- Zheng, D.; Huang, C.; Huang, H.; Zhao, Y.; Khan, M.R.U.; Zhao, H.; Huang, L. Antibacterial Mechanism of Curcumin: A Review. Chem. Biodivers. 2020, 17, e2000171. [Google Scholar] [CrossRef]
- Kaya, E.; Kahyaoglu, L.N.; Sumnu, G. Development of curcumin incorporated composite films based on chitin and glucan complexes extracted from Agaricus bisporus for active packaging of chicken breast meat. Int. J. Biol. Macromol. 2022, 221, 536–546. [Google Scholar] [CrossRef]
- Krasian, T.; Punyodom, W.; Molloy, R.; Topham, P.D.; Tighe, B.J.; Mahomed, A.; Chaiwarit, T.; Panraksa, P.; Rachtanapun, P.; Jantanasakulwong, K.; et al. Low cytotoxicity, antibacterial property, and curcumin delivery performance of toughness-enhanced electrospun composite membranes based on poly (lactic acid) and MAX phase (Ti3AlC2). Int. J. Biol. Macromol. 2024, 262, 129967. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wei, N.; Wei, J.; Fang, C.; Feng, T.; Liu, F.; Liu, X.; Wu, B. Curcumin and silver nanoparticles loaded antibacterial multifunctional pectin/gelatin films for food packaging applications. Int. J. Biol. Macromol. 2024, 266, 131248. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Yao, Y.; Liu, Y.; Li, Y.; Xu, C.; Fu, L.; Lin, B. Curcumin-loaded HKUST-1@ carboxymethyl starch-based composites with moisture-responsive release properties and synergistic antibacterial effect for perishable fruits. Int. J. Biol. Macromol. 2022, 214, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rhim, J.-W. Curcumin Incorporated Poly (Butylene Adipate-co-Terephthalate) Film with Improved Water Vapor Barrier and Antioxidant Properties. Materials 2020, 13, 4369. [Google Scholar] [CrossRef]
- Oksman, K.; Aitomäki, Y.; Mathew, A.P.; Siqueira, G.; Zhou, Q.; Butylina, S.; Tanpichai, S.; Zhou, X.; Hooshmand, S. Review of the recent developments in cellulose nanocomposite processing. Compos. Part A Appl. Sci. Manuf. 2016, 83, 2–18. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Res. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Lunardi, V.B.; Soetaredjo, F.E.; Putro, J.N.; Santoso, S.P.; Yuliana, M.; Sunarso, J.; Ju, Y.-H.; Ismadji, S. Nanocelluloses: Sources, Pretreatment, Isolations, Modification, and Its Application as the Drug Carriers. Polymers 2021, 13, 2052. [Google Scholar] [CrossRef]
- Pinheiro, I.F.; Ferreira, F.V.; Alves, G.F.; Rodolfo, A.; Morales, A.R.; Mei, L.H.I. Biodegradable PBAT-Based Nanocomposites Reinforced with Functionalized Cellulose Nanocrystals from Pseudobombax munguba: Rheological, Thermal, Mechanical and Biodegradability Properties. J. Polym. Environ. 2019, 27, 757–766. [Google Scholar] [CrossRef]
- Pinheiro, I.F.; Ferreira, F.V.; Souza, D.H.S.; Gouveia, R.F.; Lona, L.M.F.; Morales, A.R.; Mei, L.H.I. Mechanical, rheological and degradation properties of PBAT nanocomposites reinforced by functionalized cellulose nanocrystals. Eur. Polym. J. 2017, 97, 356–365. [Google Scholar] [CrossRef]
- Morelli, C.L.; Belgacem, N.; Bretas, R.E.S.; Bras, J. Melt extruded nanocomposites of polybutylene adipate-co-terephthalate (PBAT) with phenylbutyl isocyanate modified cellulose nanocrystals. J. Appl. Polym. Sci. 2016, 133, 43678. [Google Scholar] [CrossRef]
- Jiang, H.; Zhao, S.; Li, Z.; Chen, L.; Mo, H.; Liu, X. Swan-feathers inspired smart-responsive sustainable carboxymethyl cellulose/polyvinyl alcohol based food packaging film for robustly integrated Intelligent and Active Packaging. Nano Today 2024, 56, 102272. [Google Scholar] [CrossRef]
- ISO 22196:2007; Plastics–Measurement of Antibacterial Activity on Plastics Surfaces. ISO: Geneva, Switzerland, 2007.
- Turalija, M.; Bischof, S.; Budimir, A.; Gaan, S. Antimicrobial PLA films from environment friendly additives. Compos. Part B Eng. 2016, 102, 94. [Google Scholar] [CrossRef]
- Cheng, M.; Qin, Z.; Chen, Y.; Hu, S.; Ren, Z.; Zhu, M. Efficient Extraction of Cellulose Nanocrystals through Hydrochloric Acid Hydrolysis Catalyzed by Inorganic Chlorides under Hydrothermal Conditions. ACS Sustain. Chem. Eng. 2017, 5, 4656. [Google Scholar] [CrossRef]
- Miao, C.; Hamad, W.Y. Alkenylation of cellulose nanocrystals (CNC) and their applications. Polymer 2016, 101, 338. [Google Scholar] [CrossRef]
- Chu, Y.; Sun, Y.; Wu, W.; Xiao, H. Dispersion Properties of Nanocellulose: A Review. Carbohydr. Polym. 2020, 250, 116892. [Google Scholar] [CrossRef]
- Benini, K.C.C.d.C.; Voorwald, H.J.C.; Cioffi, M.O.H.; Rezende, M.C.; Arantes, V. Preparation of nanocellulose from Imperata brasiliensis grass using Taguchi method. Carbohydr. Polym. 2018, 192, 337. [Google Scholar] [CrossRef]
- Niu, W.; Guo, Y.; Huang, W.; Song, L.; Xiao, Z.; Xie, Y.; Wang, Y. Aliphatic chains grafted cellulose nanocrystals with core-corona structures for efficient toughening of PLA composites. Carbohydr. Polym. 2022, 285, 119200. [Google Scholar] [CrossRef]
- Tuna, D.; Sobolewski, A.L.; Domcke, W. Photochemical Mechanisms of Radiationless Deactivation Processes in Urocanic Acid. J. Phys. Chem. B 2014, 118, 976. [Google Scholar] [CrossRef]
- Zayat, M.; Garcia-Parejo, P.; Levy, D. Preventing UV-light damage of light sensitive materials using a highly protective UV-absorbing coating. Chem. Soc. Rev. 2007, 36, 1270. [Google Scholar] [CrossRef]
- Shamoto, Y.; Yagi, M.; Oguchi-Fujiyama, N.; Miyazawa, K.; Kikuchi, A.J.P.; Sciences, P. Photophysical properties of hexyl diethylaminohydroxybenzoylbenzoate (Uvinul A Plus), a UV-A absorber. Photochem. Photobiol. Sci. 2021, 16, 1449. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Heuzey, M.-C.; Carreau, P.J.; Taguet, A. Interfacial localization of CNCs in PLA/PBAT blends and its effect on rheological, thermal, and mechanical properties. Polymer 2021, 233, 124229. [Google Scholar] [CrossRef]
- Morelli, C.L.; Belgacem, M.N.; Branciforti, M.C.; Bretas, R.E.S.; Crisci, A.; Bras, J. Supramolecular aromatic interactions to enhance biodegradable film properties through incorporation of functionalized cellulose nanocrystals. Compos. Part A Appl. Sci. Manuf. 2016, 83, 80. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Franceschi, W.; Menezes, B.R.C.; Brito, F.S.; Lozano, K.; Coutinho, A.R.; Cividanes, L.S.; Thim, G.P. Dodecylamine functionalization of carbon nanotubes to improve dispersion, thermal and mechanical properties of polyethylene based nanocomposites. Appl. Surf. Sci. 2017, 410, 267. [Google Scholar] [CrossRef]
- Liang, S.; Du, J.; Hong, Y.; Cheng, L.; Gu, Z.; Li, Z.; Li, C. Octenyl succinate anhydride debranched starch-based nanocarriers for curcumin with improved stability and antioxidant activity. Food Hydrocoll. 2017, 135, 108118. [Google Scholar] [CrossRef]
- Liu, Y.; Ying, D.; Cai, Y.; Le, X. Improved antioxidant activity and physicochemical properties of curcumin by adding ovalbumin and its structural characterization. Food Hydrocoll. 2017, 72, 304–311. [Google Scholar] [CrossRef]
- Han, W.; Ren, J.; Xuan, H.; Ge, L. Controllable degradation rates, antibacterial, free-standing and highly transparent films based on polylactic acid and chitosan. Colloids Surf. A Physicochem. Eng. Asp. 2018, 541, 128. [Google Scholar] [CrossRef]
- Mondal, K.; Soundararajan, N.; Goud, V.V.; Katiyar, V. Cellulose Nanocrystals Modulate Curcumin Migration in PLA-Based Active Films and Its Application as Secondary Packaging. ACS Sustain. Chem. Eng. 2024, 12, 9642. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.; Liu, Y.; Hai, Y.; Zhang, M.; Chen, P. Isolation and characterization of cellulose nanofibers from four plant cellulose fibers using a chemical-ultrasonic process. Cellulose 2011, 18, 433–442. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juma, H.; Zhao, C.; Wang, Q.; Guo, Y.; Fan, X.; Fan, W.; Zhao, L.; Sun, J.; Wang, D.; Wang, Y. Enhanced Antioxidant and Antibacterial Properties of Polybutylene Adipate-Terephthalate/Curcumin Composite Films Using Surface-Modified Cellulose Nanocrystals. Polymers 2025, 17, 830. https://doi.org/10.3390/polym17070830
Juma H, Zhao C, Wang Q, Guo Y, Fan X, Fan W, Zhao L, Sun J, Wang D, Wang Y. Enhanced Antioxidant and Antibacterial Properties of Polybutylene Adipate-Terephthalate/Curcumin Composite Films Using Surface-Modified Cellulose Nanocrystals. Polymers. 2025; 17(7):830. https://doi.org/10.3390/polym17070830
Chicago/Turabian StyleJuma, Hashimu, Cunshi Zhao, Qingbo Wang, Yunfeng Guo, Xinyan Fan, Wuming Fan, Linlin Zhao, Jiayi Sun, Dong Wang, and Yonggui Wang. 2025. "Enhanced Antioxidant and Antibacterial Properties of Polybutylene Adipate-Terephthalate/Curcumin Composite Films Using Surface-Modified Cellulose Nanocrystals" Polymers 17, no. 7: 830. https://doi.org/10.3390/polym17070830
APA StyleJuma, H., Zhao, C., Wang, Q., Guo, Y., Fan, X., Fan, W., Zhao, L., Sun, J., Wang, D., & Wang, Y. (2025). Enhanced Antioxidant and Antibacterial Properties of Polybutylene Adipate-Terephthalate/Curcumin Composite Films Using Surface-Modified Cellulose Nanocrystals. Polymers, 17(7), 830. https://doi.org/10.3390/polym17070830






