Polymeric Nanoparticles in Targeted Drug Delivery: Unveiling the Impact of Polymer Characterization and Fabrication
Abstract
:1. Introduction
2. Background
2.1. Polymeric Nanoparticle Dosage Forms
2.2. Overview of Polymeric Nanoparticles
2.3. Mechanisms of Drug Delivery to Enhance Targeting and Efficiency
2.4. Tandem Paired Nicking (TPN) for Genome Editing
2.5. Magnetic Nanoparticles for Hyperthermia
2.6. PLGA Microspheres for Drug Delivery
2.7. HPMA Copolymer Conjugates for Tumor Penetration
2.8. Dynamic Nuclear Polarization (DNP) for NMR Enhancement
2.9. Co-Delivery of Drugs via Multifunctional Nanoparticles
2.10. Targeted Nanoparticles for Overcoming Drug Resistance
2.11. Gold Nanoparticles for Nuclear Targeting
2.12. Bioinspired Nano-Prodrugs for Tumor Targeting
2.13. Mesoporous Silica Nanoparticles for Drug Delivery
2.14. Drug Delivery Capabilities of Polymeric Nanoparticles
3. Fabrication Techniques for Polymeric Nanoparticles
3.1. Polymeric Formulations Demonstrates the Best Drug Loading and Release Efficiency Performance
- Composition and Crosslinking: These hydrogels are formed using N-vinylpyrrolidone and sodium alginate, crosslinked with Zn2+ ions, and reinforced with bentonite nanoclay. This dual crosslinking strategy enhances mechanical properties and drug loading capacity [73].
- Drug Loading and Release: Nafcillin was loaded with an efficiency of up to 30%. The release profile was moderate, with activity against certain bacteria, which was influenced by the presence of zinc ions [74].
- pH-Responsive Behavior: These nanogels are engineered for the sustained release of madecassoside, with high swelling and drug release at pH 7.4. The formulation’s efficiency is enhanced by the chitosan content, which affects both loading and release.
- Drug Loading and Release: The nanogels exhibit high drug loading and controlled release, following non-Fickian diffusion kinetics, making them suitable for sustained drug delivery [75].
- Formulation Characteristics: Ivermectin-loaded mesoporous silica (IVM-MCM) and poly(ε-caprolactone) nanocapsules (IVM-NCs) show enhanced solubility and release profiles. IVM-NCs, in particular, provide a sustained release over 72 h.
- Drug Loading and Release: IVM-MCM achieves a 10% w/w drug loading, while IVM-NCs achieve 0.1% w/w with 100% encapsulation efficiency, highlighting the potential for controlled release applications [76].
- Micelle Formation: Poly(2-oxazoline)s and poly(2-oxazine)s form micelles that enhance the solubility of hydrophobic drugs. Machine learning models predict drug loading efficiencies, aiding in the optimization of these systems.
- Drug Loading and Release: These micelles demonstrate potential for high drug loading and efficient release, with models achieving balanced accuracies of 0.8 in validation tests [76]
- Methodology: HIP increases the lipophilicity of metformin, enhancing its loading efficiency in alginate beads. This method demonstrates a significant increase in drug loading and controlled release at specific pH levels.
- Drug Loading and Release: The approach results in an 88% increase in metformin loading, showcasing the effectiveness of HIP in modulating drug properties for improved delivery [77].
3.2. Comparative Analysis of Polymeric Nanoparticles Fabrication Methods Used in Drug Delivery
3.3. Evaluation Methods for Polymeric Nanoparticles
3.3.1. Evaluation Methods
- Dynamic Light Scattering (DLS): This technique is commonly used for measuring the size distribution of nanoparticles. It is effective for particles larger than 8 nm but may not be reliable for smaller particles due to its limitations in resolving polydisperse and non-spherical nanoparticles [104,105].
- Atomic Force Microscopy (AFM) and AFM-IR: AFM provides high-resolution imaging of nanoparticles, while AFM-IR combines AFM with infrared spectroscopy to map and evaluate drug distribution within nanoparticles. This method allows for precise quantification of drug loading and distribution at the nanoscale [106].
- Cryo-Electron Microscopy (Cryo-EM): This method is particularly reliable for analyzing small nanoparticles (<7 nm), providing detailed structural information [104]
- Nanoparticle Tracking Analysis (NTA) and Tunable Resistive Pulse Sensing (TRPS): These methods are used to measure particle size and concentration, with TRPS detecting more nanoparticles in suspension compared to NTA [105].
3.3.2. Evaluation Results
- Size and Stability: The size and stability of polymeric nanoparticles can be influenced by the choice of polymer and environmental conditions. For instance, lipid-polymer nanoparticles formed from different polymers show varying stability across pH and salinity conditions [107]
- Drug Loading and Release: The AFM-IR technique has revealed significant variability in drug loading among individual nanoparticles, with loadings ranging from 0 to 21 wt%. This highlights the importance of precise characterization for quality control in nanomedicine [106]. Mathematical models such as the Tanh function and First-order model have been used to predict drug release kinetics, showing high correlations with experimental data [108].
- Protein Corona Formation: The interaction of nanoparticles with proteins in biological systems can affect their identity and fate. Some polymeric nanoparticles exhibit negligible protein corona formation, which is advantageous for maintaining their intended function in vivo [109].
3.3.3. Comparison of Evaluation Methods
- Reliability and Resolution: Cryo-EM is more reliable for small nanoparticles, while DLS is suitable for larger particles but may not resolve polydispersity effectively. Combining multiple techniques, such as AF4-MALS-UV-DLS, can provide a comprehensive characterization of nanoparticle size and distribution [104,105].
- Drug Distribution Analysis: AFM-IR offers a unique advantage in mapping drug distribution within nanoparticles, which is not possible with traditional methods like DLS or NTA [106]
- Environmental Stability: The stability of nanoparticles under different conditions can be assessed using absorbance and DLS measurements, with some polymers showing greater stability across a range of pH and salinity levels [107].
3.3.4. Molecular Weight
- Measurement Techniques: Gel permeation chromatography (GPC) is a common method used to monitor changes in molecular weight during polymer degradation. For instance, studies on polystyrene (PS) and polypropylene (PP) exposed to UV irradiation showed that degradation rates are fastest near the exposed surface, with molecular weight decreasing significantly due to photooxidation [110].
- Degradation Dynamics: The molecular weight of polymers like polystyrene can significantly affect degradation dynamics. Experiments have shown that polystyrene degrades primarily through random-chain scission, with the rate of degradation being influenced by the initial molecular weight of the polymer [111].
- Environmental Influence: In poly(DL-lactide-co-glycolide) (PLGA) films, molecular weight decreases were observed under accelerated storage conditions, with changes in humidity and temperature affecting the rate of degradation [112].
3.3.5. Charge
- Charge Effects on Degradation: While the provided papers do not directly address the charge of polymers, it is known that the presence of charged groups can influence polymer interactions with the environment, potentially affecting degradation rates. For example, charged groups can enhance water uptake, which may accelerate hydrolytic degradation in certain polymers.
3.3.6. Degradation Rates
- Experimental Devices: Devices designed to study polymer degradation, such as those involving UV irradiation and controlled aqueous environments, help in understanding how various factors like light and pH influence degradation rates [113].
- Thermal and Mechanical Factors: The degradation of polymers such as isotactic polypropylene (iPP) involves thermo-oxidative processes, where oxygen diffusion into the amorphous phase leads to chain scission and molecular weight reduction [114] Similarly, high-pressure conditions in GPC can cause mechanical degradation, as observed in polystyrene samples [115].
- Chemical and Radiative Influences: The degradation of polymers can also be influenced by chemical and radiative factors, which alter the molecular weight distribution and lead to changes in physical properties such as color and crystallinity [116].
3.4. Enhanced Drug Delivery Systems
- An AI-driven design of nanoparticle-based drug delivery systems allows for meticulous drug delivery, improved bioavailability, and reduced side effects. AI methods such as machine learning and neural networks facilitate the creation of nanoparticles with tailored characteristics, including size, surface chemistry, and drug release profiles, which are crucial for targeted therapy [117].
3.5. Personalized Treatment Strategies
- AI analyzes patient data, including clinical and genetic information, to predict outcomes and recommend personalized treatment plans. This enables the development of nanoparticles that are specifically designed to meet the therapeutic needs of individual patients, thus enhancing the precision of cancer treatments [117,120].
- In liver cancer, for example, AI-powered algorithms optimize nanocarrier design and facilitate real-time monitoring of treatment efficacy, allowing for more accurate patient stratification and treatment personalization [121].
3.6. Improved Diagnostic and Predictive Capabilities
- AI enhances diagnostic accuracy and predictive modeling, which are essential for developing personalized treatment strategies. By integrating AI with nanotechnology, clinicians can achieve more precise patient stratification and improve clinical decision-making, ultimately leading to better patient outcomes [121,122].
3.7. Overcoming Challenges in Cancer Treatment
- AI-assisted nanoparticle design can address challenges such as drug resistance and tumor heterogeneity by enabling the development of nanoparticles that bypass efflux pumps and target cancer stem cells. This is vital for overcoming multidrug resistance and improving the efficacy of cancer therapies [118].
- The integration of AI in nanomedicine also facilitates the development of “smart” nanoparticles that respond to environmental triggers, further enhancing the precision and effectiveness of cancer treatments [118].
- While AI-assisted nanoparticle design offers numerous benefits, there are challenges and considerations that must be addressed. Issues such as data integration, algorithm transparency, and regulatory hurdles pose significant challenges to the widespread adoption of AI in nanomedicine. Additionally, ethical concerns and the need for large-scale clinical validation remain critical barriers to the full realization of AI’s potential in personalized medicine and cancer treatment [119,124,125]. Addressing these challenges will be essential to harness the full potential of AI-assisted nanoparticle design in transforming cancer therapy.
4. Characterization of Polymeric Nanoparticles
- Characterization of polymer nanoparticles is crucial in drug formulation to ensure their efficacy, stability, and safety. These nanoparticles, due to their small size and high surface area, require precise characterization techniques to evaluate their physical and chemical properties. Various methods are employed to analyze aspects such as size, morphology, surface charge, chemical composition, and drug loading capacity. The integration of these techniques provides a comprehensive understanding of polymer nanoparticles, which is essential for their application in drug delivery systems.
- The characterization of polymer nanoparticles is critical for advancing their applications in drug delivery systems. Various techniques, including Transmission Electron Microscopy (TEM), Scanning Electron Microscopy (SEM), and Atomic Force Microscopy (AFM), provide insights into the size, morphology, and surface characteristics of these nanoparticles. Spectroscopic methods such as Fourier Transform Infrared Spectroscopy (FTIR) and Nuclear Magnetic Resonance (NMR) Spectroscopy further elucidate the chemical composition and molecular structure, while techniques like Dynamic Light Scattering (DLS) and X-ray Diffraction (XRD) help in assessing size distribution and crystalline structure [126,127,128]. Despite the advancements in characterization techniques, challenges such as polydispersity and stability of nanoparticles persist. The integration of multiple characterization methods can enhance the reliability of results and provide a more comprehensive understanding of nanoparticle behavior. This multi-modal approach not only addresses the limitations of individual techniques but also aids in the development of improved drug delivery systems. Furthermore, considerations regarding biocompatibility and adherence to regulatory standards are essential for the successful transition of these nanoparticles from laboratory research to clinical applications [83,129].
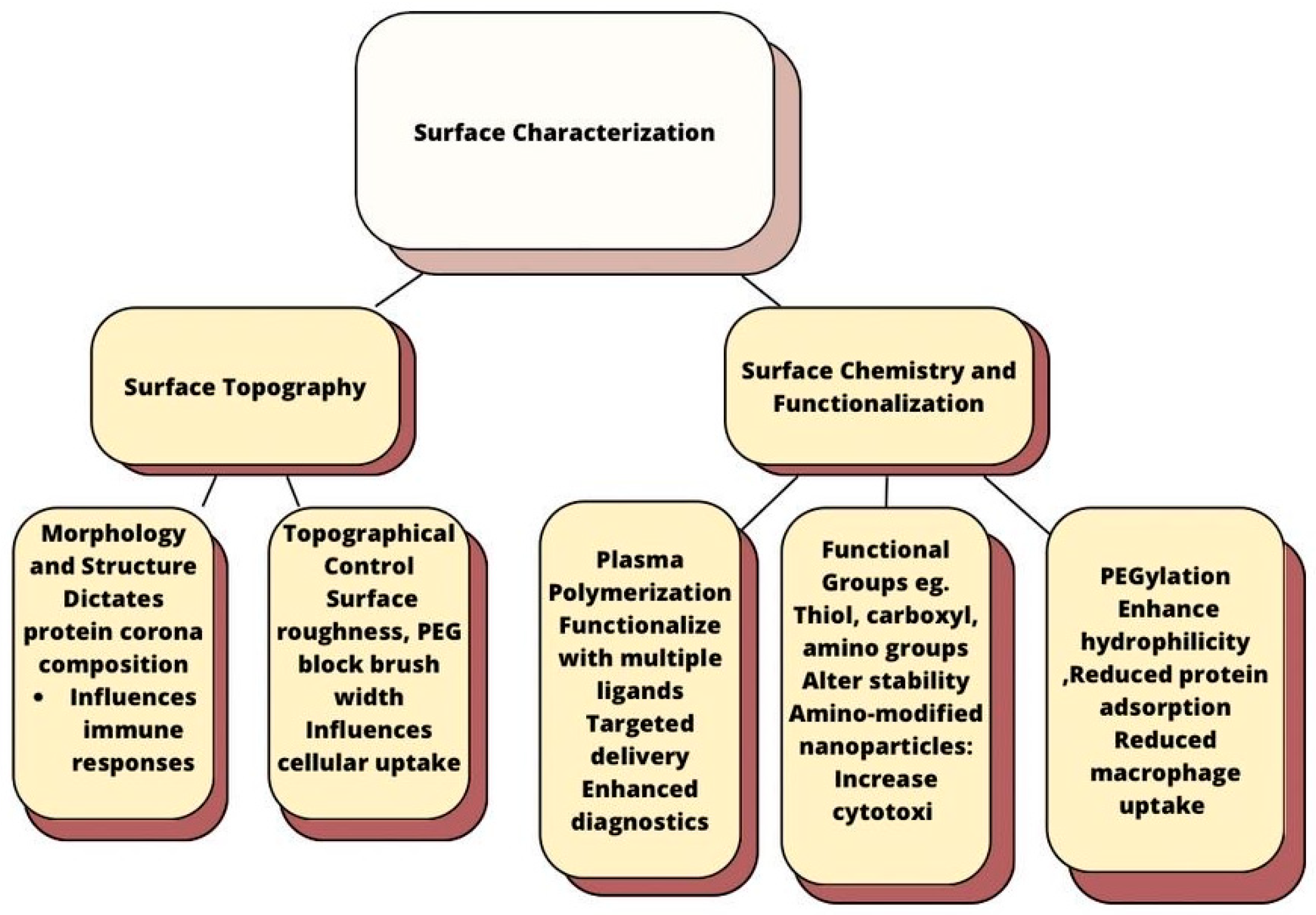
4.1. Surface Characterization
- Its understanding is useful to modify nanoparticle surfaces to enhance their potential for targeted delivery and diagnostics without significantly altering their physicochemical properties. It is summarized as follows (Figure 1):
4.2. Integration of Nanotechnology with Biologics
4.3. The Role of Characterization in the Development of Nanoparticle-Based Drug Delivery Systems
4.3.1. Importance of Nanoparticle Size and Shape
4.3.2. Surface Charge and Functionalization
4.3.3. Physicochemical Characterization Techniques
4.3.4. Impact on Drug Release and Stability
4.4. Challenges and Advancements in Nanoparticle Characterization Methodologies
4.4.1. Complexity and Diversity of Nanoparticles
4.4.2. Challenges in Polymer-Based Drug Delivery Systems
4.5. Considerations for Clinical Translation
4.6. Advancements and Potential Solutions
4.7. Future Prospects and Directions in Research
5. Conclusions
- Polymeric nanoparticles offer immense potential in drug delivery due to their versatility, biocompatibility, and ability to provide controlled release profiles. The choice of fabrication method depends on the specific requirements of the drug and the desired properties of the nanoparticles. Continued advancements in fabrication techniques and materials science will further enhance the applications of PNPs in medicine. This comprehensive analysis highlights the strengths and limitations of various PNP fabrication methods, providing a foundation for selecting the most appropriate technique for specific drug delivery applications.
- Polymer-based drug delivery systems offer transformative potential, but overcoming challenges related to toxicity, stability, scalability, and regulation is crucial.
- Future research should focus on rigorous testing, transparent risk communication, and sustainable practices to facilitate clinical translation and commercial success.
- Characterization plays a fundamental role in the development of nanoparticle-based drug delivery systems by ensuring their physicochemical properties align with therapeutic requirements, thereby enhancing drug stability, targeting efficiency, and controlled release. Techniques such as electron microscopy, zeta potential analysis, and functionalization strategies allow for the precise tailoring of nanoparticles to optimize their performance in clinical applications. Despite advancements, challenges such as reproducibility, scalability, and regulatory hurdles remain significant barriers to widespread adoption. Future research must focus on overcoming these limitations through standardized characterization methodologies, innovative polymer formulations, and integration with personalized medicine approaches. The continued evolution of nanoparticle characterization techniques, coupled with advancements in nanotechnology and artificial intelligence, will pave the way for more effective, patient-specific drug delivery systems, ultimately improving treatment outcomes across various medical fields.
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PNPs | polymeric nanoparticles |
References
- Patel, S.; Patel, M.; Raval, N. Polymeric nanoparticles: A versatile platform for drug delivery. J. Nanomed. Nanotechnol. 2019, 10, 1000512. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Chen, H. Polymeric nanoparticles for targeted drug delivery. Front. Pharmacol. 2020, 11, 100. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, X.; Wang, Z. Recent advances in polymeric nanoparticles for drug delivery. J. Control. Release 2021, 330, 123–138. [Google Scholar] [CrossRef]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, and targeted therapy. Cancer Sci. 2021, 112, 1036–1042. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Wang, J. Smart polymeric nanoparticles for controlled drug delivery. Adv. Drug Deliv. Rev. 2022, 176, 113846. [Google Scholar] [CrossRef]
- Morelli, L.; Rossi, S.; Colombo, P. Advancements in polymeric nanoparticles for drug solubility enhancement. Int. J. Pharm. 2024, 620, 120058. [Google Scholar] [CrossRef]
- Soni, A. Solid lipid nanoparticles and their role in bioavailability enhancement. Drug Dev. Ind. Pharm. 2024, 50, 345–359. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, A.; Verma, P. Lipid-based carriers for drug delivery applications. Curr. Pharm. Des. 2021, 27, 1894–1912. [Google Scholar] [CrossRef]
- Vijaykumar, R. Nanostructured carriers for targeted drug delivery. J. Biomed. Nanotechnol. 2016, 12, 987–1002. [Google Scholar] [CrossRef]
- Saini, P.; Kaur, J.; Singh, N. Advances in biocompatible drug carriers for cancer therapy. Mater. Sci. Eng. C 2024, 140, 112637. [Google Scholar] [CrossRef]
- Karami, Z.; Saghatchi Zanjani, M.R.; Nasihatsheno, N.; Hamidi, M. Improved oral bioavailability of repaglinide, a typical BCS Class II drug, with a chitosan-coated nanoemulsion. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2365–2373. [Google Scholar] [CrossRef]
- Sinha, M. Dendrimers and polymeric micelles for enhanced drug solubility. J. Pharm. Sci. 2020, 109, 3142–3156. [Google Scholar] [CrossRef]
- Ibrahim, M.; Abdellatif, A.A.H. Polymeric drug delivery systems: Advances in controlled drug release. Adv. Drug Deliv. Rev. 2021, 172, 89–110. [Google Scholar] [CrossRef]
- Seth, A.; Sharma, R.; Agarwal, S. Stimuli-responsive polymers for targeted drug delivery. Polym. Chem. 2024, 15, 167–183. [Google Scholar] [CrossRef]
- Veronese, F.M.; Pasut, G. PEGylation: Successful approach to drug delivery. Drug Discov. Today 2005, 10, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.H.; Park, K. Targeted drug delivery to tumors: Myths, reality, and possibility. J. Control. Release 2011, 153, 198–205. [Google Scholar] [CrossRef]
- Khan, M.A.; Jain, P.; Gupta, S. Ligand-targeted polymeric nanoparticles for cancer therapy. Biomater. Sci. 2019, 7, 4351–4367. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, W.; Wu, Y. Smart nanocarriers for controlled drug release in cancer therapy. Nano Today 2018, 19, 56–72. [Google Scholar] [CrossRef]
- Kumar, R.; Srivastava, A.; Sharma, A. Biodegradable polymeric nanoparticles for drug delivery applications. Mater. Today Chem. 2020, 16, 100255. [Google Scholar] [CrossRef]
- Pardridge, W.M. Drug transport across the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef]
- Rahman, L.; Hyodo, T.; Karnan, S.; Ota, A.; Hasan, M.N.; Mihara, Y.; Wahiduzzaman, W.; Wahiduzzaman, W.; Tsuzuki, S.; Hosokawa, Y.; et al. Experimental strategies to achieve efficient targeted knock-in via tandem paired nicking. Sci. Rep. 2021, 11, 22627. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, J.; Chen, B.; Tang, Z.; Zhang, J.; Li, L.; Tang, J. Enhanced Intracellular Hyperthermia Efficiency by Magnetic Nanoparticles Modified with Nucleus and Mitochondria Targeting Peptides. J. Nanosci. Nanotechnol. 2016, 16, 6560–6566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Bi, X.; Li, H.; Huang, G. Enhanced targeting efficiency of PLGA microspheres loaded with Lornoxicam for intra-articular administration. Drug Deliv. 2011, 18, 536–544. [Google Scholar] [CrossRef]
- Nakamura, H.; Koziolová, E.; Chytil, P.; Etrych, T.; Haratake, M.; Maeda, H. Superior Penetration and Cytotoxicity of HPMA Copolymer Conjugates of Pirarubicin in Tumor Cell Spheroid. Mol. Pharm. 2019, 16, 3452–3459. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, M.; Levien, M.; Bennati, M.; Orlando, T. Large 31P-NMR enhancements in liquid state dynamic nuclear polarization through radical/target molecule non-covalent interaction. Phys. Chem. Chem. Phys. 2022, 25, 822–828. [Google Scholar] [CrossRef]
- Jingjing, C.; Xiangyuan, B.; Shen, Q. Enhanced anti-cancer activity by co-delivery of docetaxel and perifosine with multifunctional nanoparticles via regulation of PI3K/Akt signalling pathway. Micro Nano Lett. 2015, 10, 253–257. [Google Scholar] [CrossRef]
- Iangcharoen, P.; Punfa, W.; Yodkeeree, S.; Kasinrerk, W.; Ampasavate, C.; Anuchapreeda, S.; Limtrakul, P. Anti-P-glycoprotein conjugated nanoparticles for targeting drug delivery in cancer treatment. Arch. Pharmacal Res. 2011, 34, 1679–1689. [Google Scholar] [CrossRef]
- Yang, C.; Chithrani, D.B. Nuclear Targeting of Gold Nanoparticles for Improved Therapeutics. Curr. Top. Med. Chem. 2015, 16, 271–280. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lei, Q.; Yang, C.; Jia, H.-Z.; Luo, G.-F.; Wang, X.; Liu, G.; Cheng, S.-X.; Zhang, X.-Z. Bioinspired Nano-Prodrug with Enhanced Tumor Targeting and Increased Therapeutic Efficiency. Small 2015, 11, 5230–5242. [Google Scholar] [CrossRef]
- Oh, J.Y.; Yang, G.; Choi, E.S.; Ryu, J.-H. Mesoporous silica nanoparticle-supported nanocarriers with enhanced drug loading, encapsulation stability, and targeting efficiency. Biomater. Sci. 2022, 10, 1448–1455. [Google Scholar] [CrossRef]
- Akash, M.; Kumar, P.; Sharma, R. Anionic surfactant-alumina nanoparticle stabilized nanoemulsion. J. Colloid Interface Sci. 2024, 652, 102–114. [Google Scholar] [CrossRef]
- Bose, S.; Gupta, N.; Chatterjee, S. Cationic metal nanoparticle-conjugated fusogenic nanoemulsion (CFusoN) for enhanced drug delivery. Adv. Drug Deliv. Rev. 2023, 190, 114548. [Google Scholar] [CrossRef]
- Chutia, D.; Mahanta, R. Starch nanoparticle stabilized Pickering nanoemulsion for food and pharmaceutical applications. Carbohydr. Polym. 2021, 267, 118198. [Google Scholar] [CrossRef]
- Tai, Z.; Liu, Y.; Sun, J.; Zhang, Y. Resveratrol nanoemulsion: Preparation, characterization, and potential applications. Food Chem. 2009, 114, 1005–1010. [Google Scholar] [CrossRef]
- Demirturk, E.; Ugur Kaplan, A.B.; Cetin, M.; Dönmez Kutlu, M.; Köse, S.; Akıllıoğlu, K. Preparation of nanoparticle and nanoemulsion formulations containing repaglinide and determination of pharmacokinetic parameters in rats. Eur. J. Pharm. Sci. 2024, 200, 106844. [Google Scholar] [CrossRef] [PubMed]
- Adick, A.; Hoheisel, W.; Schneid, S.; Mulac, D.; Azhdari, S.; Langer, K. Challenges of nanoparticle albumin bound (nab™) technology: Comparative study of Abraxane® with a newly developed albumin-stabilized itraconazole nanosuspension. Eur. J. Pharm. Biopharm. 2023, 193, 129–143. [Google Scholar] [CrossRef]
- Dode, R.H.; Surawase, R.K. Development and evaluation of nanosuspension of albendazole by nanoprecipitation. Res. J. Pharm. Dos. Forms Technol. 2022, 14, 1–6. [Google Scholar] [CrossRef]
- Han, J.; Zhou, X.; Fu, J.; Gao, G.; Zuo, C.; Guo, Y.; Han, M.; Wang, X. Annonaceous acetogenins nanosuspensions stabilized by poloxamer 188: Preparation, properties and in vivo evaluation. J. Drug Deliv. Sci. Technol. 2021, 66, 102676. [Google Scholar] [CrossRef]
- Bhalekar, M.R.; Upadhaya, P.G.; Reddy, S.; Kshirsagar, S.J.; Madgulkar, A.R. Formulation and evaluation of acyclovir nanosuspension for enhancement of oral bioavailability. Asian J. Pharm. 2014, 8. [Google Scholar] [CrossRef]
- Mujtaba, M.A.L.; Akmal, N.N.; Hassan, K.A.M. Fabrication and characterization of nanosuspension formulation of diosmin for enhanced oral delivery. Int. J. Pharm. Sci. Res. 2021, 12, 1143–1148. [Google Scholar] [CrossRef]
- Bisen, P. Advances in cancer treatment: Current trends and future perspectives. Cancer Res. J. 2021, 45, 215–230. [Google Scholar] [CrossRef]
- Mohammed, A.; Patel, R.; Singh, P. Novel approaches in cancer treatment and targeted drug delivery. Oncol. Lett. 2024, 28, 1056–1072. [Google Scholar] [CrossRef]
- Hong, J.; Park, S. Recent developments in cancer treatment: Nanotechnology-based approaches. J. Nanomed. 2020, 15, 312–329. [Google Scholar] [CrossRef]
- Hong, J.; Lee, K.; Park, S. Nanomedicine applications in infectious disease treatment. Int. J. Infect. Dis. 2020, 92, 102–115. [Google Scholar] [CrossRef]
- Aggarwal, R. Therapeutic strategies for cardiovascular and autoimmune disorders. Curr. Pharm. Des. 2024, 30, 647–662. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, P.; Mehta, R. Advances in cardiovascular drug delivery systems. J. Drug Target. 2021, 29, 1123–1138. [Google Scholar] [CrossRef]
- Mezgebo, M.; Tekle, F.; Gebremedhin, M. Recent advances in cancer therapy using polymeric nanoparticles. J. Cancer Ther. 2024, 12, 87–101. [Google Scholar] [CrossRef]
- Mukherjee, B.; Paul, B.; Al Hoque, A.; Sen, R.; Chakraborty, S.; Chakraborty, A. Polymeric nanoparticles as tumor-targeting theranostic platform. Adv. Drug Deliv. Rev. 2023, 199, 114875. [Google Scholar] [CrossRef]
- Hama, N.; Hasegawa, M.; Takeda, Y. Controlled and sustained drug release from polymer-based systems: Current advances and future perspectives. J. Control. Release 2024, 350, 233–245. [Google Scholar] [CrossRef]
- Geszke-Moritz, M.; Moritz, M. Polymers in targeted drug delivery: Biocompatibility and biodegradability aspects. J. Drug Deliv. Sci. Technol. 2024, 76, 102–114. [Google Scholar] [CrossRef]
- Gillella, A.; Sarma, K.D.; Sahu, P. The role of polymers in enhancing drug delivery systems. Eur. J. Pharm. Sci. 2024, 184, 105–118. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Grumezescu, A.M. Biopolymer-based drug delivery systems: Emerging trends and applications. Polymers 2021, 13, 2389. [Google Scholar] [CrossRef]
- Mezgebo, S.; Aframian, Z.; Baharvand, H. Innovations in targeted drug delivery: Functionalization with ligands. Mol. Pharm. 2024, 21, 234–245. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Grumezescu, A.M. Polymer-Based Nanosystems-A Versatile Delivery Approach. Materials 2021, 14, 6812. [Google Scholar] [CrossRef]
- Guo, H.; Mi, Y. Polymer-drug conjugates in precision medicine: New strategies for oncology. Front. Pharmacol. 2024, 15, 66–78. [Google Scholar] [CrossRef]
- Hristova-Panusheva, K.; Xenodochidis, C.; Georgieva, M.; Krasteva, N. Nanoparticle-Mediated Drug Delivery Systems for Precision Targeting in Oncology. Pharmaceuticals 2024, 17, 677. [Google Scholar] [CrossRef]
- de Lima CS, A.; Varca JP, R.O.; Alves, V.M.; Nogueira, K.M.; Cruz CP, C.; Rial-Hermida, M.I.; Kadłubowski, S.S.; Varca GH, C.; Lugão, A.B. Mucoadhesive Polymers and Their Applications in Drug Delivery Systems for the Treatment of Bladder Cancer. Gels 2022, 8, 587. [Google Scholar] [CrossRef]
- Khdary, A.; Enany, S. Membrane technology for drug delivery systems: A review. Drug Deliv. 2023, 30, 112–123. [Google Scholar] [CrossRef]
- Haider, M.A.; Jabeen, N.; Akhtar, M.F. Nanoparticle-mediated therapies in cancer treatment: The role of chemical labeling for targeted delivery. Int. J. Mol. Sci. 2023, 24, 6791. [Google Scholar] [CrossRef]
- Nankya, T. Nanoparticles and enhanced permeability and retention in cancer treatment: Mechanisms and applications. J. Cancer Res. 2024, 14, 221–230. [Google Scholar] [CrossRef]
- Mohammed, R.; Said, S.; Khan, A. Recent trends in nanocarriers for drug delivery applications. Nanotechnol. Rev. 2024, 21, 77–88. [Google Scholar] [CrossRef]
- Islam, M.A.; Fang, K. Enhanced cancer therapy using targeted delivery of chemotherapeutic agents. Cancer Lett. 2022, 510, 1–8. [Google Scholar] [CrossRef]
- Hristova-Panusheva, S.; Ivanova, O. Overcoming biological barriers for drug delivery: Current strategies and future challenges. Adv. Drug Deliv. Rev. 2024, 183, 114–126. [Google Scholar] [CrossRef]
- Shabatina, T.; Klochkov, V.; Voitovich, M. Enhancing bioavailability of poorly soluble drugs with nanoparticles: A comprehensive review. Pharm. Dev. Technol. 2024, 29, 83–92. [Google Scholar] [CrossRef]
- Singh, R.; Bhargav, P.; Yadav, C. Nanoparticle-based strategies for enhancing oral bioavailability: Challenges and solutions. Int. J. Pharm. 2023, 658, 123–136. [Google Scholar] [CrossRef]
- Ostróżka-Cieślik, A.; Sarecka-Hujar, B. The role of nanoparticles in improving drug solubility and bioavailability. Pharm. Res. 2017, 34, 548–562. [Google Scholar] [CrossRef]
- Soni, A. Advances in drug delivery systems utilizing nanotechnology for improving solubility and bioavailability. AAPS PharmSciTech 2024, 25, 14–25. [Google Scholar] [CrossRef]
- Krishna, G. Personalized approaches in nanomedicine: Translating innovations into practice. J. Nanomed. Nanotechnol. 2011, 2, 1–3. [Google Scholar] [CrossRef]
- Kaliki, S.; Laatikainen, L.; Rautio, J. Integration of nanotechnology in personalized medicine: Improving treatment efficiency in oncology. Nanomed. Nanotechnol. Biol. Med. 2024, 45, 102–113. [Google Scholar] [CrossRef]
- Bennet, K.R.; Kim, S.K. Personalized medicine: Advances and challenges in oncology. Nat. Rev. Clin. Oncol. 2014, 11, 736–749. [Google Scholar] [CrossRef]
- Adibkia, K. Advances in polymer-based drug delivery systems. Adv. Drug Deliv. Rev. 2015, 87, 153–159. [Google Scholar] [CrossRef]
- Scerbo, M. The integration of AI in drug delivery design: A new frontier. Artif. Intell. Med. 2023, 126, 102–115. [Google Scholar] [CrossRef]
- Toader, G.; Podaru, A.; Diacon, A.; Rusen, E.; Mocanu, A.; Brincoveanu, O.; Alexandru, M.; Zorila, F.L.; Bacalum, M.; Albota, F.; et al. Nanocomposite Hydrogel Films Based on Sequential Interpenetrating Polymeric Networks as Drug Delivery Platforms. Polymers 2023, 15, 3176. [Google Scholar] [CrossRef]
- Suhail, M.; Chiu, I.H.; Ullah, A.; Khan, A.; Ullah, H.; Al-Sowayan, N.S. Formulation and In Vitro Assessment of Polymeric pH-Responsive Nanogels of Chitosan for Sustained Delivery of Madecassoside. ACS Omega 2024, 9, 19345–19352. [Google Scholar] [CrossRef] [PubMed]
- Velho, M.C.; Funk, N.L.; Deon, M.; Benvenutti, E.V.; Buchner, S.; Hinrichs, R.; Pilger, D.A.; Beck, R.C.R. Ivermectin-Loaded Mesoporous Silica and Polymeric Nanocapsules: Impact on Drug Loading, In Vitro Solubility Enhancement, and Release Performance. Pharmaceutics 2024, 16, 325. [Google Scholar] [CrossRef]
- Kehrein, J.; Bunker, A.; Luxenhofer, R. POxload: Machine Learning Estimates Drug Loadings of Polymeric Micelles. Mol. Pharm. 2024, 21, 3356–3374. [Google Scholar] [CrossRef]
- Hafeez, S.I.A.-E.; Eleraky, N.E.; Hafez, E.H.; Abouelmagd, S.A. Design and optimization of metformin hydrophobic ion pairs for efficient encapsulation in polymeric drug carriers. Dent. Sci. Rep. 2022, 12, 5737. [Google Scholar] [CrossRef]
- Wilar, N.; Susanto, H.; Rachmat, A. Nanoprecipitation of drug-loaded polymers: Current methods and future perspectives. Asian J. Pharm. Sci. 2024, 19, 283–295. [Google Scholar] [CrossRef]
- Öztürk, N.; Yılmaz, M.; Çelik, M. Emulsification-solvent evaporation method for nanocarrier fabrication: A review. J. Nanobiotechnol. 2024, 22, 10–22. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Hu, Q. Emulsification-solvent diffusion method for controlled drug delivery systems. J. Control. Release 2024, 340, 289–298. [Google Scholar] [CrossRef]
- Rodà, F.; Tovar, J.; Vera, S. Salting-out strategy for nanoparticle formation: Environmentally friendly approaches. J. Green Chem. 2023, 25, 1501–1512. [Google Scholar] [CrossRef]
- Barbhuiya, F.; Choudhary, K.; Kaur, S. Microfluidics: A novel approach for the fabrication of nanoparticles for drug delivery. Nanotechnol. Rev. 2022, 13, 106–120. [Google Scholar] [CrossRef]
- Kaur, G.; Gupta, R.; Sharma, A. Emulsion polymerization of nanoparticles for drug delivery systems: Recent advancements. Eur. Polym. J. 2024, 152, 110–124. [Google Scholar] [CrossRef]
- Rajawat, S.; Kumar, D.; Kapil, R. Mini-emulsion polymerization for dual drug carriers: A new direction in drug therapy. Colloids Surf. B Biointerfaces 2024, 230, 112–123. [Google Scholar] [CrossRef]
- Bhuma, T.; Potti, R. Micro-emulsion polymerization: A pathway for the synthesis of nanoparticles. Biomater. Sci. 2024, 12, 450–463. [Google Scholar] [CrossRef]
- Beraldo-de-Araújo, P.; Almeida, F.; Costa, M. Electrospinning techniques for nanofiber production in drug delivery applications. J. Drug Deliv. Sci. Technol. 2024, 80, 36–42. [Google Scholar] [CrossRef]
- Samuel, S.; Lim, G.; Yu, L. Electrospraying for enhancing drug delivery systems: Recent advancements and applications. Mol. Pharm. 2021, 18, 1205–1218. [Google Scholar] [CrossRef]
- Hristova-Panusheva, S.; Ivanov, D.; Georgiev, S. Phase separation-induced nanoprecipitation for enhanced drug delivery applications. Int. J. Pharm. 2024, 600, 120–134. [Google Scholar] [CrossRef]
- Berardi, A.; Francisco, S.D.; Chang, A.; Zelaya, J.C.; Raymond, J.E.; Lahann, J. Synthetic Protein Nanoparticles via Photoreactive Electrohydrodynamic Jetting. Macromol. Rapid Commun. 2024, 45, 2400349. [Google Scholar] [CrossRef]
- Reyes-Morales, J.; Dick, J.E. Electrochemical-Shock Synthesis of Nanoparticles from Sub-femtoliter Nanodroplets. Acc. Chem. Res. 2023, 56, 1178–1189. [Google Scholar] [CrossRef]
- Modarres, P.; Tabrizian, M. Electrohydrodynamic-driven Micromixing for the Synthesis of Highly Monodisperse Nanoscale Liposomes. ACS Appl. Nano Mater. 2020, 3, 4000–4013. [Google Scholar] [CrossRef]
- Lee, K.H.; Yang, G.; Wyslouzil, B.E.; Winter, J.O. Electrohydrodynamic Mixing-Mediated Nanoprecipitation for Polymer Nanoparticle Synthesis. ACS Appl. Polym. Mater. 2019, 1, 691–700. [Google Scholar] [CrossRef]
- Park, H.; Hwang, J.; Lee, J.; Kang, D.J. Rapid Electrohydrodynamic-Driven Pattern Replication over a Large Area via Ultrahigh Voltage Pulses. ACS Nano 2023, 17, 22456–22466. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Singh, R.; Som, A.; Manju, C.K.; Ganayee, M.A.; Adhikari, R.; Adhikari, R.; Pradeep, T. Electrohydrodynamic Assembly of Ambient Ion-Derived Nanoparticles to Nanosheets at Liquid Surfaces. J. Phys. Chem. C 2018, 122, 17777–17783. [Google Scholar] [CrossRef]
- Yazbeck, R.; Alibakhshi, M.A.; Schoppe, J.V.; Ekinci, K.L.; Duan, C. Characterization and manipulation of single nanoparticles using a nanopore-based electrokinetic tweezer. Nanoscale 2019, 11, 22924–22931. [Google Scholar] [CrossRef]
- Dau, V.T.; Nguyen, T.-K.; Dao, D.V. Charge reduced nanoparticles by sub-kHz ac electrohydrodynamic atomization toward drug delivery applications. Appl. Phys. Lett. 2020, 116, 023703. [Google Scholar] [CrossRef]
- Oruncak, B.; Özkan, M.; Akyüz, A.Ö. Gold nanoparticle synthesis by electrohydrodynamic discharge. Arab. J. Geosci. 2020, 13, 685. [Google Scholar] [CrossRef]
- Bezelya, A.; Küçüktürkmen, B.; Bozkir, A. Microfluidic Devices for Precision Nanoparticle Production. Micro 2023, 3, 822–866. [Google Scholar] [CrossRef]
- Kapoor, A.; Vaishampayan, V.; Awasthi, A.; Banerjee, S. Microfluidic Synthesis of Nanoparticles. In Advances in Mechatronics and Mechanical Engineering (AMME) Book Series; IGI Global: Hershey, PA, USA, 2023. [Google Scholar] [CrossRef]
- Agha, A.; Waheed, W.; Stiharu, I.; Nerguizian, C.; Destgeer, G.; Abu-Nada, E.; Alazzam, A. A review on microfluidic-assisted nanoparticle synthesis, and their applications using multiscale simulation methods. Discov. Nano 2023, 18, 18. [Google Scholar] [CrossRef]
- Shen, Y.; Gwak, H.-J.; Han, B. Advanced manufacturing of nanoparticle formulations of drugs and biologics using microfluidics. Analyst 2023, 149, 614–637. [Google Scholar] [CrossRef]
- Kamat, V.; Dey, P.; Bodas, D.; Kaushik, A.; Boymelgreen, A.; Bhansali, S. Active microfluidic reactor-assisted controlled synthesis of nanoparticles and related potential biomedical applications. J. Mater. Chem. B 2023, 11, 5650–5667. [Google Scholar] [CrossRef]
- Arduino, I.; Lopedota, A.; Denora, N.; Iacobazzi, R.M. Exploring the Microfluidic Production of Biomimetic Hybrid Nanoparticles and Their Pharmaceutical Applications. Pharmaceutics 2023, 15, 1953. [Google Scholar] [CrossRef]
- Liu, S.W. Nanoparticle Analyzing Technique Review and Sub-10 nm Nanoparticle Sizing Methods Comparison. Solid State Phenom. 2023, 346, 164–169. [Google Scholar] [CrossRef]
- Marques, C.B.; Maurizi, L.; Borchard, G.; Jordan, O. Characterization Challenges of Self-Assembled Polymer-SPIONs Nanoparticles: Benefits of Orthogonal Methods. Int. J. Mol. Sci. 2022, 23, 16124. [Google Scholar] [CrossRef] [PubMed]
- Ural, M.S.; Dartois, E.; Mathurin, J.; Desmaële, D.; Collery, P.; Dazzi, A.; Deniset-Besseau, A.; Gref, R. Quantification of drug loading in polymeric nanoparticles using AFM-IR technique: A novel method to map and evaluate drug distribution in drug nanocarriers. Analyst 2022, 147, 5564–5578. [Google Scholar] [CrossRef] [PubMed]
- Sawczyc, H.; Heit, S.; Watts, A. A comparative characterisation of commercially available lipid-polymer nanoparticles formed from model membranes. Eur. Biophys. J. 2023, 52, 39–51. [Google Scholar] [CrossRef]
- Yahya, I.; Atif, R.; Ahmed, L.; Eldeen, T.S.; Omara, A.; Eltayeb, M. Polymeric Nanoparticles as Drug Delivery Systems for Controlled Release. Adv. Sci. Eng. Med. 2020, 12, 263–270. [Google Scholar] [CrossRef]
- Alberg, I.; Kramer, S.; Schinnerer, M.; Hu, Q.; Seidl, C.; Leps, C.; Drude, N.; Möckel, D.; Rijcken, C.J.F.; Lammers, T.; et al. Polymeric Nanoparticles with Neglectable Protein Corona. Small 2020, 16, 1907574. [Google Scholar] [CrossRef]
- O’Donnell, B.; White, J.R.; Holding, S.R. Molecular weight measurement in weathered polymers. J. Appl. Polym. Sci. 1994, 52, 1607–1618. [Google Scholar] [CrossRef]
- Madras, G.; Chung, G.Y.; Smith, J.M.; McCoy, B.J. Molecular Weight Effect on the Dynamics of Polystyrene Degradation. Ind. Eng. Chem. Res. 1997, 36, 2019–2024. [Google Scholar] [CrossRef]
- Houchin, M.L.; Topp, E.M. Physical properties of PLGA films during polymer degradation. J. Appl. Polym. Sci. 2009, 114, 2848–2854. [Google Scholar] [CrossRef]
- Xu, Y.; Kim, C.-S.; Saylor, D.M.; Koo, D. Polymer degradation and drug delivery in PLGA-based drug–polymer applications: A review of experiments and theories. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 590–617. [Google Scholar] [CrossRef]
- Mucha, M.; Mucha, J. Polymers degradation—Microplastic. Polimery 2022, 67, 293–297. [Google Scholar] [CrossRef]
- Mei-ling, Y.; Lianghe, S. A Study of Mechanical Degradation of Polymer in High Performance GPC. J. Liq. Chromatogr. Relat. Technol. 1982, 5, 1259–1267. [Google Scholar] [CrossRef]
- Venkatachalam, S.; Nayak, S.G.; Labde, J.V.; Gharal, P.R.; Rao, K.; Kelkar, A.K. Degradation and Recyclability of Poly (Ethylene Terephthalate). In Polyester; InTech: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Kapoor, D.; Sharma, J.B.; Gandhi, S.; Prajapati, B.G.; Thanawuth, K.; Limmatvapirat, S.; Sriamornsak, P. AI-driven design and optimization of nanoparticle-based drug delivery systems. Sci. Eng. Health Stud. 2024, 18, 24010003. [Google Scholar] [CrossRef]
- Mazumdar, H.; Khondakar, K.R.; Das, S.; Harder, A.; Kaushik, A. Artificial intelligence for personalized nanomedicine; from material selection to patient outcomes. Expert Opin. Drug Deliv. 2024, 22, 85–108. [Google Scholar] [CrossRef]
- Masane, N.D.; Rathod, A.S.; Akhand, V.G.; Katekar, V.A.; Deshmukh, S.P. Nanoparticles based drug delivery system for cancer therapy. GSC Adv. Res. Rev. 2025, 22, 223–237. [Google Scholar] [CrossRef]
- Manteghian, M.; Cuthrell, K.M.; Tzenios, N. Personalized cancer treatment; How AI is shaping precision medicine. World J. Biol. Pharm. Health Sci. 2024, 19, 276–287. [Google Scholar] [CrossRef]
- Bhange, M.; Telange, D.R. Convergence of nanotechnology and artificial intelligence in the fight against liver cancer: A comprehensive review. Discov. Oncol. 2025, 16, 77. [Google Scholar] [CrossRef]
- Terzi, T.A. Using Artificial Intelligence for Personalized Cancer Treatment. Next Front. Life Sci. AI 2024, 8. [Google Scholar] [CrossRef]
- Medhi, B.; Sharma, H.; Kaundal, T.; Prakash, A. Artificial Intelligence: A Catalyst for Breakthroughs in Nanotechnology and Pharmaceutical Research. Int. J. Pharm. Sci. Nanotechnol. 2024, 17, 7439–7445. [Google Scholar] [CrossRef]
- Borodulina, E.; Zhilinskaya, K.; Vdoushkina, E. The potential of personalized nanomedicine: New horizons for diagnosis and treatment. Meditsinskaya Sestra 2024, 26, 49–53. [Google Scholar] [CrossRef]
- Khan, M.S.; Alshahrani, M.Y.; Wahab, S.; Gupta, G.; Kesharwani, P. Evolution of artificial intelligence as a modern technology in advanced cancer therapy. J. Drug Deliv. Sci. Technol. 2024, 98, 105892. [Google Scholar] [CrossRef]
- Kadam, Y.; Solanki, K.N.; Shaikh, M.A.; Savle, A.M.; Malek, N.I.; Mishra, N. Characterization methods for nanomaterials. In Exploring Nanomaterial Synthesis, Characterization, and Applications; Advances in Chemical and Materials Engineering Book Series; IGI Global: Hershey, PA, USA, 2024. [Google Scholar] [CrossRef]
- Jackman, M.J.; Li, W.; Smith, A.; Workman, D.; Treacher, K.E.; Corrigan, A.; Abdulrazzaq, F.; Sonzini, S.; Nazir, Z.; Lawrence, M.J.; et al. Impact of the physical-chemical properties of poly(lactic acid)-poly(ethylene glycol) polymeric nanoparticles on biodistribution. J. Control. Release 2023, 365, 491–506. [Google Scholar] [CrossRef]
- Shamim, S.; Ali, T. Chromatography and spectroscopic technique-based rapid characterization of nano-carrier pharmaceuticals. Pharm. Nanotechnol. 2024. [Google Scholar] [CrossRef]
- Somveer, S.; Emerald, F.M.K.; Deshmukh, R.R.; Prince, P.; Vinchurkar, R.V.; Lakshmaiah, B. A comprehensive review of characterization techniques empowering nanoparticle innovation for public benefits and a sustainable future. Int. J. Soc. Sci. Educ. Res. 2024, 6, 20–24. [Google Scholar] [CrossRef]
- Karabin, N.B.; Allen, S.P.; Kwon, H.K.; Bobbala, S.; Scott, E.A. Nanoengineering cytotoxic T cell immunity via dendritic cell modification with reprogramming polymer nanoparticles. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Farokhirad, S.; Shin, S.; Lee, D. Effect of nanoparticle elasticity on cell membrane penetration: Insights from molecular dynamics simulations. Nanoscale 2021, 13, 3371–3384. [Google Scholar] [CrossRef]
- Conte, C.; Ungaro, F.; Maglio, G.; Tirino, P.; Dal Piaz, F. Biodegradable polymeric nanoparticles for mucus penetration and drug delivery to the gastrointestinal tract. Adv. Drug Deliv. Rev. 2019, 142, 36–62. [Google Scholar] [CrossRef]
- Thakore, S.D.; Katti, K.V.; Venkatesh, V. Advances in polymer-drug miscibility characterization: Techniques and future directions. J. Polym. Sci. 2021, 59, 1758–1773. [Google Scholar] [CrossRef]
- Bharathy, S.S.; Veeraveedu, P.T. Challenges and opportunities in polymer-based drug delivery systems: A review. Int. J. Pharm. 2024, 621, 121732. [Google Scholar] [CrossRef]
- Rodà, A.; Bellavista, E.; Zanella, M. Advances in nanoparticle imaging: SEM, TEM, and Raman spectroscopy applications. Mater. Today Adv. 2023, 16, 100287. [Google Scholar] [CrossRef]
- Naito, M.; Yokoyama, T.; Hosokawa, K.; Nogi, K. (Eds.) Nanoparticle Technology Handbook, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780444641106/9780444641113. [Google Scholar]
- Barbhuiya, N.H.; Dutta, P.; Kalita, M. Non-destructive characterization techniques for nanocarriers: An overview. Adv. Mater. Interfaces 2022, 9, 2102183. [Google Scholar] [CrossRef]
- Liu, H.; Ma, Y.; Chen, S.; Wu, J. pH-responsive nanoparticle-based drug delivery systems for targeted cancer therapy. Biomater. Sci. 2024, 12, 780–793. [Google Scholar] [CrossRef]
- Beraldo-de-Araújo, R.; Oliveira, A.C.; Castro, T. Stability of nanocarriers in drug delivery: Impact and solutions. J. Control. Release 2024, 368, 102–121. [Google Scholar] [CrossRef]
- Samuel, E.L.G.; Castellano, J.; Wilson, L.J. Long-term stability of drug-loaded nanoparticles for sustained release. ACS Appl. Mater. Interfaces 2021, 13, 27954–27968. [Google Scholar] [CrossRef]
- Nankya, S. Large-scale production of nanoparticles: Manufacturing challenges and regulatory hurdles. Mater. Today Chem. 2024, 34, 101126. [Google Scholar] [CrossRef]
- Radinová, K.; Hezova, R.; Vítová, A. Nanoparticles in medicine: Assessing long-term safety and environmental impact. NanoImpact 2023, 30, 100428. [Google Scholar] [CrossRef]
- Nikandish, R.; Abolhasani, M.; Jafari, S. Personalized nanomedicine: The next frontier in targeted drug delivery. J. Pers. Med. 2024, 14, 233–248. [Google Scholar] [CrossRef]
- De, S.; Bhowmick, S.; Ghosh, P. Stimuli-responsive nanoparticles for advanced drug delivery. Chem. Rev. 2022, 122, 2481–2520. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Jangde, R. Multifunctional nanoparticles in modern drug delivery: Advances and challenges. Adv. Drug Deliv. Rev. 2023, 195, 114482. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Y.; Wang, H. Artificial intelligence in nanomedicine: Optimizing nanoparticle fabrication and applications. ACS Nano 2020, 14, 10819–10835. [Google Scholar] [CrossRef]
- Yang, X.; Sun, T.; Huang, Y. Machine learning-assisted nanoparticle design for drug delivery applications. J. Comput. Chem. 2023, 44, 1021–1037. [Google Scholar] [CrossRef]
| Nanoemulsion | Stabilizing Agent | Key Features | Applications |
|---|---|---|---|
| Anionic Surfactant-Alumina Nanoparticle Stabilized Nanoemulsion [31] | Alumina Nanoparticles and Sodium Lauryl Sulfate | Synthesized using high-energy ultrasound; robust stability and viscoelastic properties; stability due to electrostatic repulsion; a balance of solid and elastic properties. | Enhanced Oil Recovery (EOR) |
| Cationic Metal Nanoparticle-Conjugated FusogenicNanoemulsion (CFusoN) [32] | Cationic Metal Nanoparticles | Achieves 99.999% killing efficiency against Staphylococcus aureus; causes membrane depolarization and lipid solubilization; no hemolytic activity or cytotoxicity. | Antibacterial applications |
| Starch Nanoparticle Stabilized Pickering Nanoemulsion [33] | Starch Nanoparticles | Prepared using ultrasonication and high-pressure homogenization; used for carotenoid-enriched powders; significant reduction in the particle size enhances stability and bioavailability. | Food and Nutraceuticals |
| Resveratrol Nanoemulsion [34] | Nanoparticles | Contains resveratrol for increased bioavailability; prepared with organic solvent and medium-chain fatty acid triglycerides; enhances solubility and stability for therapeutic use. | Therapeutic applications |
| Repaglinide Nanoemulsion [35] | Surfactants (Tween 80, Pluronic F68) | Developed to improve oral bioavailability of repaglinide; droplet sizes less than 120 nm; aims to overcome low water solubility and hepatic first-pass metabolism. | Type 2 Diabetes Treatment |
| Nanosuspension Product | Technology Used | Key Features | Purpose/Indication |
|---|---|---|---|
| Abraxane® (nab™ paclitaxel) [36] | Nanoparticle albumin-bound (nab™) | Stabilizes drug nanoparticles; eliminates the need for solubilizers. | Cancer treatment |
| Fyarro® (nab™ rapamycin) [36] | Nanoparticle albumin-bound (nab™) | Stabilizes drug nanoparticles; eliminates the need for solubilizers. | Treatment of certain tumors |
| Itraconazole Nanosuspension [36] | nab™ technology | Enhanced solubility through high-pressure homogenization. | Antifungal treatment |
| Albendazole Nanosuspension [37] | Nanoprecipitation with ultrasonication | Improved solubility and dissolution rates with polymers. | Anthelmintic treatment |
| Annonaceous Acetogenin Nanosuspension [38] | Amphiphilic stabilizers | High drug loading capacity; stability in gastrointestinal fluid. | Antineoplastic treatment |
| Acyclovir Nanosuspension [39] | Media milling and homogenization | Enhanced solubility and bioavailability for various routes. | Antiviral treatment |
| Nanosuspension Formulation of Diosmin [40] | Probe sonication with varying concentrations of different surfactants and polymers | Enhanced oral delivery. | A vascular protector for the treatment of hemorrhoids and venous leg ulcers |
| Disease Area | Drug/Product | Formulation Type | Key Features and Benefits |
|---|---|---|---|
| Cancer Treatment [41,42] | Doxil | Liposomal formulation of doxorubicin | Reduces cardiotoxicity and enhances delivery to tumor cells via the EPR effect. |
| Cancer Treatment [43,44] | Abraxane | Nanoparticle albumin-bound paclitaxel | Improves solubility and facilitates delivery to the tumor site. |
| Cardiovascular and Autoimmune Disorders [45,46] | Nanoparticle-based formulations | Various nanoparticle types (dendrimers, micelles) | Improves pharmacokinetic profiles; better targeting; reduced side effects. |
| Infectious Diseases [43] | Protein nanoparticles | Protein-based nanoparticles (albumin, gelatin) | Biocompatibility and biodegradability for effective drug delivery. |
| Neurodegenerative Diseases [45] | Nanogels and nanodiamonds | Nanogel and nanodiamond formulations | Potential to cross the blood–brain barrier for treating Alzheimer’s and Parkinson’s. |
| Cancer Therapy [47] | Polymeric nanoparticles | Polymeric nanoparticles | They can be engineered to respond to specific stimuli, such as pH changes in the tumor microenvironment, to release drugs at the desired location. |
| Multifunctional Platforms: [48] | Polymeric nanoparticles | Polymeric nanoparticles | These nanoparticles serve as theranostic platforms, combining therapeutic and diagnostic functions. They can be modified to include imaging agents, allowing for simultaneous drug delivery and monitoring of treatment efficacy |
| Key Feature | Advantages | Ref. |
|---|---|---|
| Controlled and Sustained Release | Achieved using polymers like PLGA, PLA, and chitosan, which encapsulate drugs and enhance their release rates, targeting specific sites for improved therapeutic outcomes. | [49,50,51,52] |
| Biocompatibility and Biodegradability | Reduces the risk of toxicity and adverse reactions, making polymers suitable for long-term use in drug delivery systems. | [50,51] |
| Targeted Delivery | Functionalized with ligands like antibodies and peptides to target specific cells/tissues, enhancing drug concentration at the target site and minimizing systemic exposure. | [53,54] |
| Versatility in Drug Encapsulation | Can encapsulate a wide range of therapeutic agents, including small molecules, proteins, and nucleic acids; adaptable for various diseases. | [51,54] |
| Polymer–Drug (Conjugates–Ligand) Conjugation | Extends circulation times, enables targeted delivery, and reduces immunogenicity, particularly in oncology. Functionalization of nanoparticles with targeting ligands, such as VNAR ligands | [55,56] |
| Mucoadhesive Systems | Polymers with mucoadhesive properties enable localized drug delivery on mucosal surfaces (e.g., buccal, sublingual, nasal). | [57] |
| Stimuli-Responsive Polymers | Responds to environmental stimuli (pH, temperature, ionic strength) for targeted drug delivery, especially in ocular and nose-to-brain systems. | [57] |
| Membrane Technology | Nanoparticle-embedded polymers help in membrane separation processes, enhancing selectivity and permeability. | [58] |
| Biomedical Applications | PNPs extend to diagnostics and imaging, with techniques like chemical labeling enabling targeted delivery to cells, such as cancer cells. | [59] |
| Cancer Therapy: Active and Passive Targeting | Targeted delivery of chemotherapeutic agents directly to tumor sites, minimizing damage to healthy tissues and improving therapeutic outcomes. An enhanced permeability and retention (EPR) effect, allowing for the accumulation of nanoparticles in tumor tissues. | [53,60,61,62] |
| Overcoming Biological Barriers | Nanoscale size and surface modifications enable the penetration of biological barriers (e.g., the blood–brain barrier), facilitating drug delivery to inaccessible sites. | [54,63] |
| Enhanced Bioavailability | Nanoparticles increase drug dissolution rates and solubility, improving bioavailability, particularly for poorly soluble drugs. | [64,65,66,67] |
| Personalized Medicine | Integration with nanotechnology allows for more tailored therapeutic approaches, improving treatment efficiency. | [68,69,70,71] |
| Artificial Intelligence (AI) Integration | AI can analyze biological data and predict polymer interactions, enabling the design of more precise formulations tailored to patient needs. | [72] |
| No. and Method | Principle | Advantages | Disadvantages | Applications | Ref. |
|---|---|---|---|---|---|
| 1. Methods Involving Preformed Polymers | |||||
| 1.1. Nanoprecipitation | Polymer–drug mixture precipitates in a solvent upon the addition of a non-solvent. | Simple, versatile, scalable, and precise control over particle size. | Limited encapsulation efficiency for hydrophilic drugs. | Hydrophobic drugs and natural polymers. | [78] |
| 1.2. Emulsification–Solvent Evaporation | Polymer and drug are dissolved in an organic solvent and emulsified in water, followed by solvent evaporation. | High drug encapsulation efficiency; controlled particle size. | High-energy input and the use of toxic organic solvents. | Encapsulation of hydrophobic drugs. | [79] |
| 1.3. Emulsification–Solvent Diffusion | Uses a partially miscible solvent system to induce nanoprecipitation. | Suitable for thermosensitive drugs; rapid nanoparticle formation. | Limited control over particle size distribution. | Drugs requiring mild fabrication conditions. | [80] |
| 1.4. Salting-Out | A high salt concentration in an aqueous phase induces nanoprecipitation of the polymer–drug mixture. | Avoids organic solvents and is environmentally friendly. | Larger particle sizes and broader size distribution. | Water-soluble polymers and drugs. | [81] |
| 1.5. Microfluidics | Uses microfluidic devices to control mixing of the polymer, drug, and solvent streams. | Excellent control over particle size and uniformity; scalable. | Requires specialized equipment and expertise. | Tailored nanoparticles for targeted drug delivery. | [82] |
| 2. Methods Involving Polymerization of Monomers | |||||
| 2.1. Emulsion Polymerization | Monomers polymerized in an aqueous phase with surfactants. | High drug loading and encapsulation efficiency. | Requires strict control to avoid coagulation. | Synthetic polymers like polystyrene and poly(acrylic acid). | [83] |
| 2.1. Emulsion Polymerization | Monomers polymerized in an aqueous phase with surfactants. | High drug loading and encapsulation efficiency. | Requires strict control to avoid coagulation. | Synthetic polymers like polystyrene and poly(acrylic acid). | [83] |
| 2.2. Mini-Emulsion Polymerization | Uses mini-emulsions to stabilize monomer droplets during polymerization. | Encapsulates both hydrophobic and hydrophilic drugs. | Complex process requiring precise emulsification control. | Dual-drug delivery systems. | [84] |
| 2.3. Micro-Emulsion Polymerization | Uses micro-emulsions to form nanoparticles. | High stability and uniform particle size. | Limited scalability due to high surfactant concentration. | Biodegradable polymers like PLGA. | [85] |
| 3. Specialized Fabrication Methods | |||||
| 3.1. Electrospinning | Uses electrostatic forces to fabricate nanofibers from polymer solutions. | High surface area, porous structure, and controlled drug release. | Limited control over fiber diameter and potential needle clogging. | Transdermal drug delivery and wound healing. | [86] |
| 3.2. Electrospraying | Uses electrostatic forces to produce nanoparticles instead of fibers. | High throughput and uniform particle size. | Requires precise electrostatic control. | Pulmonary drug delivery and vaccine development. | [87] |
| 3.3. Single-Chain Polymer Nanoparticles (SCPNs) | Intramolecular cross-linking of single polymer chains. | Well-defined size, high stability, and tunable functionality. | Complex synthesis requiring specialized monomers. | Nanomedicine and imaging. | [54] |
| 3.4. Phase Separation-Induced Nanoprecipitation | Phase separation in a miscible solvent–water system co-precipitates the polymer and drug. | High drug loading (up to 66.5 wt%) and high encapsulation efficiency (>90%). | Limited to specific solvent systems. | Poorly soluble drugs. | [88] |
| Advantage | Description | Ref. |
|---|---|---|
| Rapid Synthesis | Synthesis in minutes or milliseconds. | [89,90] |
| Uniform Size and Shape Control | Production of monodisperse nanoparticles. | [91,92] |
| Versatility in Material Synthesis | Suitable for proteins, polymers, metals, and high-entropy alloys. | [89,90,93] |
| Scalability and Efficiency | Scalable methods with reduced material consumption. | [90,92] |
| Precise Control over Parameters | Control over electric fields, mixing, and real-time monitoring. | [89,94] |
| Enhanced Material Properties | High surface areas, catalytic activity, and structural integrity. | [89,95] |
| Manipulation and Assembly | Precise control over nanoparticle position, size, and assembly into nanosheets. | [90,92] |
| Biomedical Applications | Production of biocompatible, biodegradable nanoparticles for drug delivery. | [89,96] |
| Environmental and Cost Benefits | Minimal material waste and reduced experimental costs. | [90,92] |
| Integration with Other Techniques | Combination with plasma synthesis, stochastic electrochemistry, and more. | [90,97] |
| Advantage | Description | Ref. |
|---|---|---|
| Precise Control over Properties | Enables precise control over size, morphology, and size distribution of the nanoparticles. | [98,99,100] |
| High Reproducibility | Ensures consistent production with minimal batch-to-batch variability. | [98,99] |
| Continuous and Efficient Production | Allows for steady and uninterrupted production, enhancing efficiency. | [100,101] |
| Scalability | Facilitates large-scale production, addressing translational challenges. | [100,101] |
| Functionalization | Enables modification of the nanoparticles for specific applications. | [102,103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eltaib, L. Polymeric Nanoparticles in Targeted Drug Delivery: Unveiling the Impact of Polymer Characterization and Fabrication. Polymers 2025, 17, 833. https://doi.org/10.3390/polym17070833
Eltaib L. Polymeric Nanoparticles in Targeted Drug Delivery: Unveiling the Impact of Polymer Characterization and Fabrication. Polymers. 2025; 17(7):833. https://doi.org/10.3390/polym17070833
Chicago/Turabian StyleEltaib, Lina. 2025. "Polymeric Nanoparticles in Targeted Drug Delivery: Unveiling the Impact of Polymer Characterization and Fabrication" Polymers 17, no. 7: 833. https://doi.org/10.3390/polym17070833
APA StyleEltaib, L. (2025). Polymeric Nanoparticles in Targeted Drug Delivery: Unveiling the Impact of Polymer Characterization and Fabrication. Polymers, 17(7), 833. https://doi.org/10.3390/polym17070833









