Molecular Entanglement Facilitated Improvement of Thermal Stability of Cellulose Diacetate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CDA Composites
2.3. Characterization of CDA-Based Materials
2.3.1. Thermogravimetric Analysis
2.3.2. Differential Scanning Calorimetry
2.3.3. Melting Point Measurement
2.3.4. Dynamic Mechanical Analysis
2.3.5. 3D Digital Microscopy
2.3.6. Field Emission Scanning Electron Microscopy
2.3.7. Fourier-Transform Infrared Spectroscopy
3. Results and Discussion
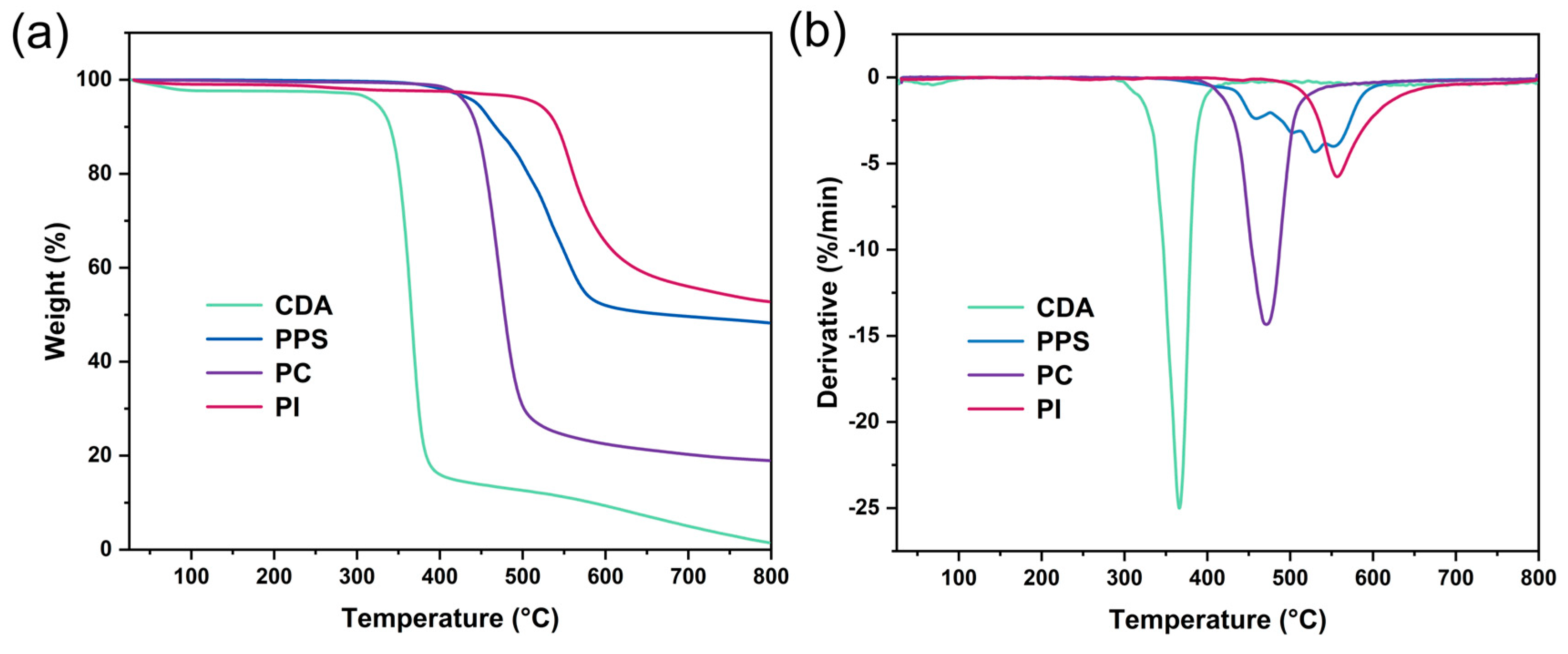
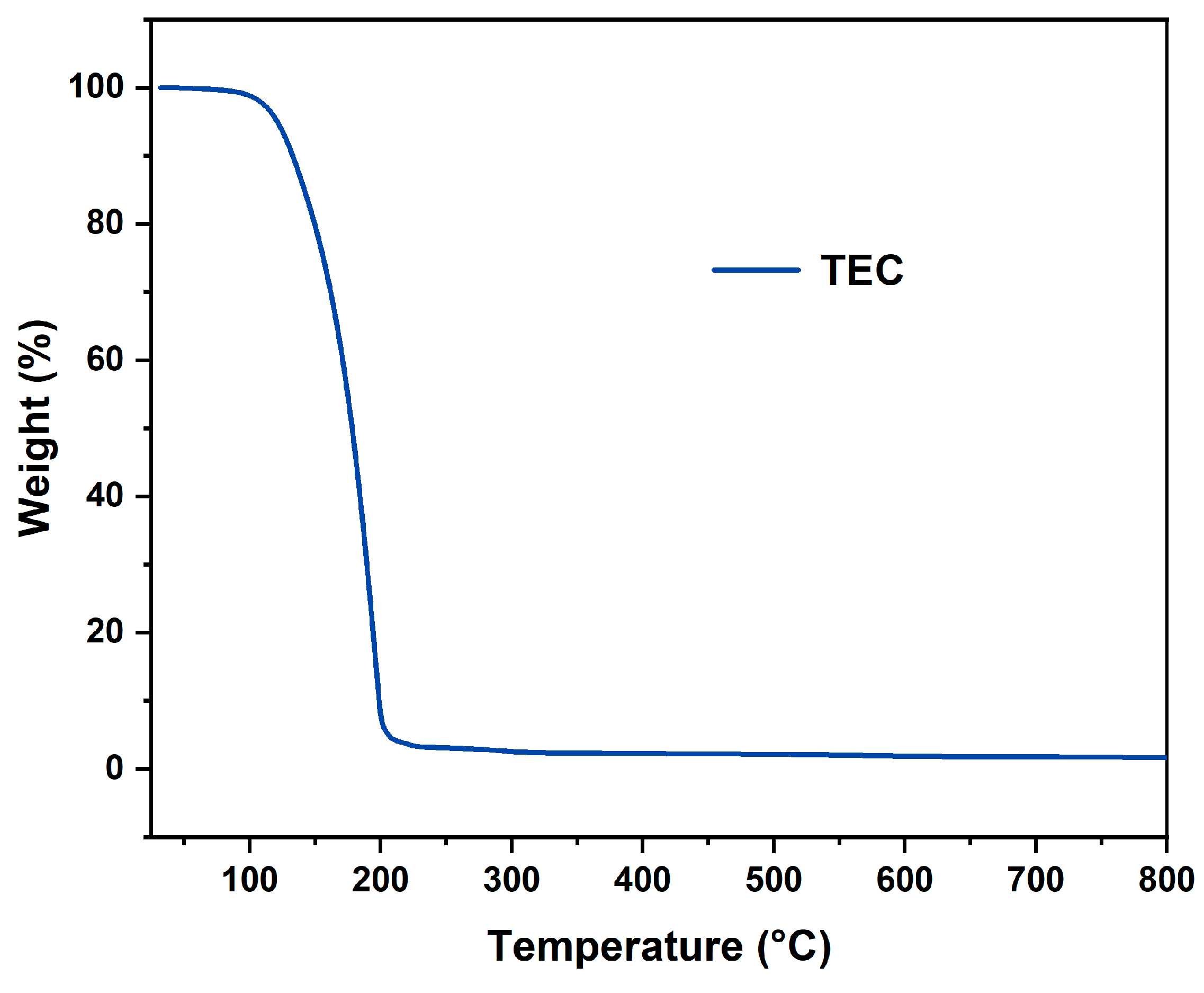
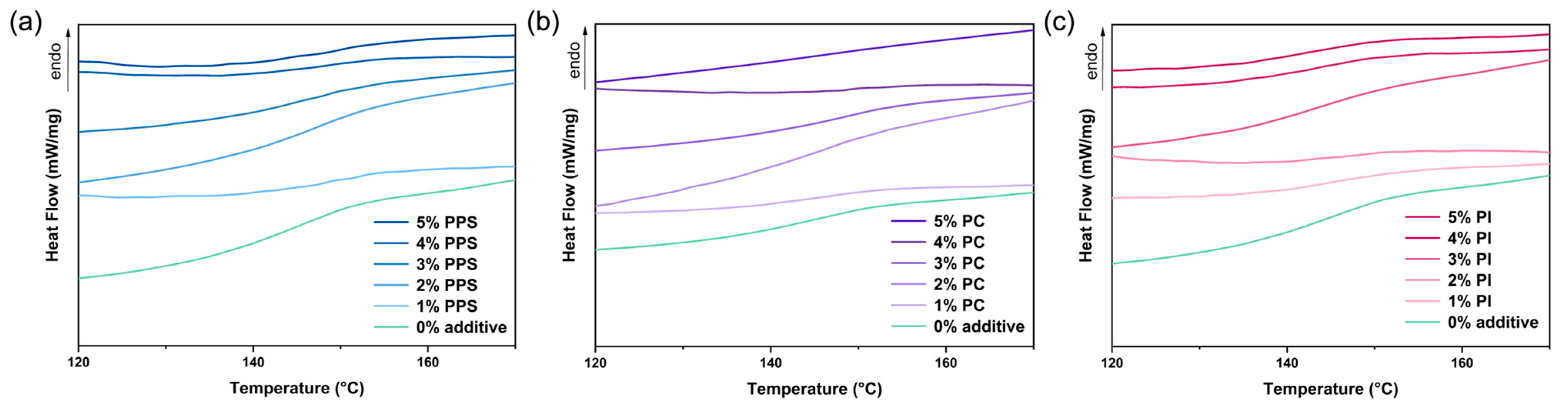
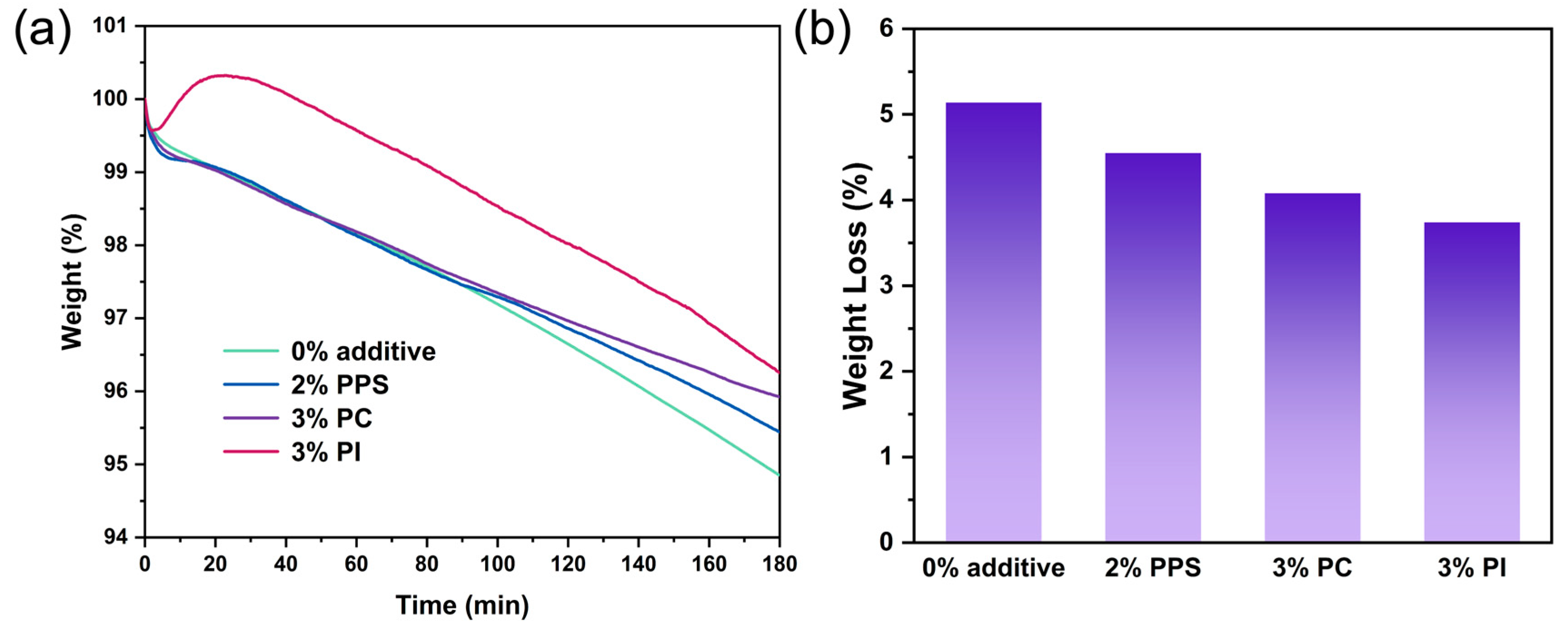
3.1. Thermal Properties
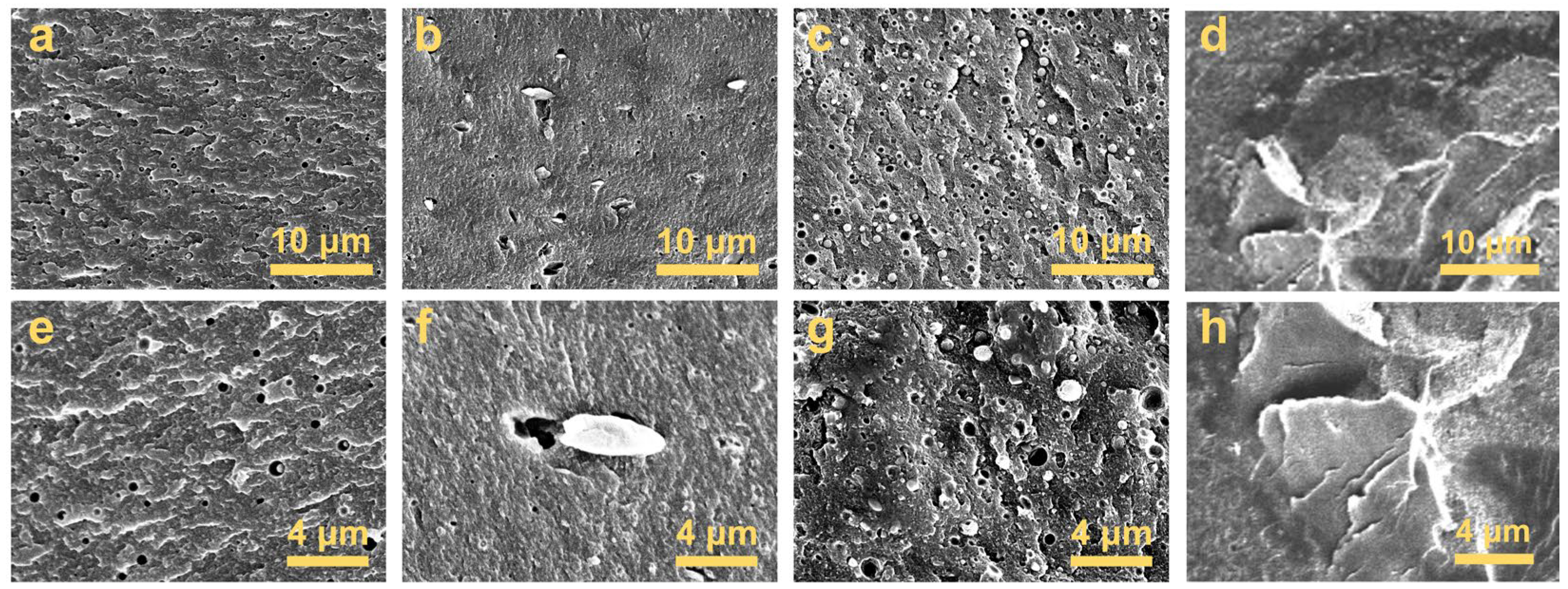
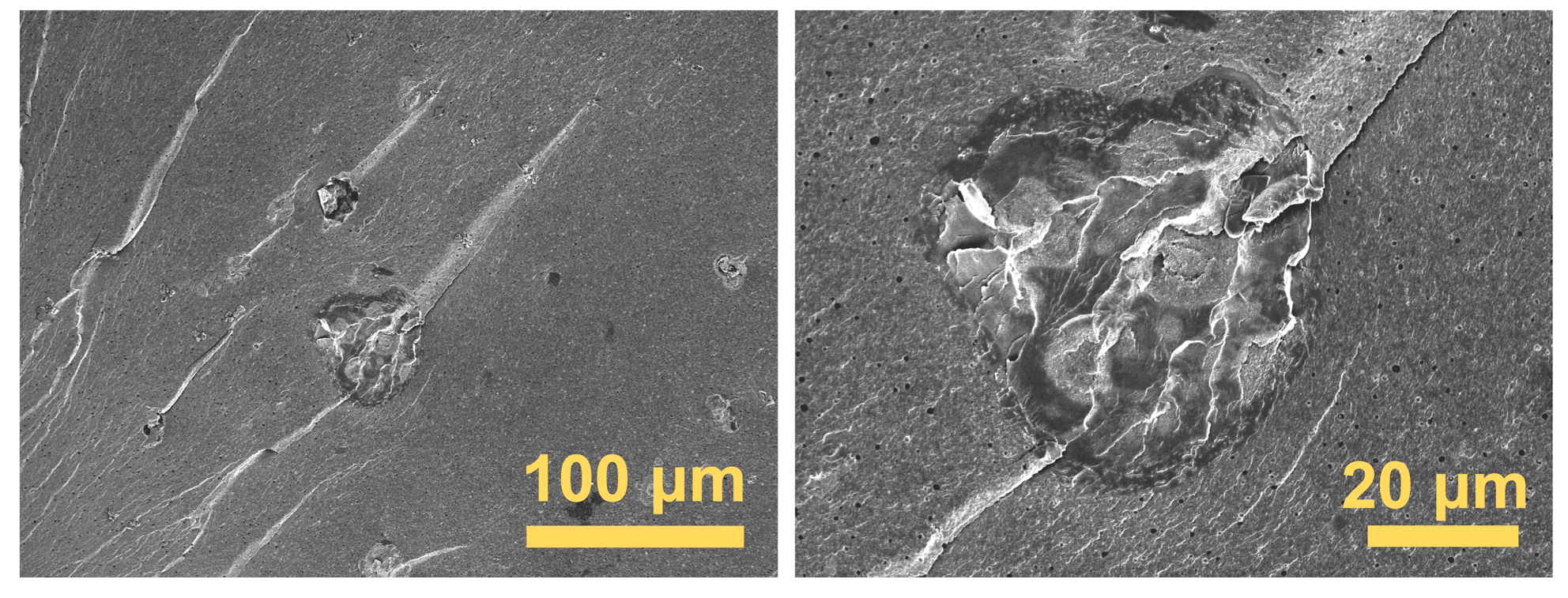
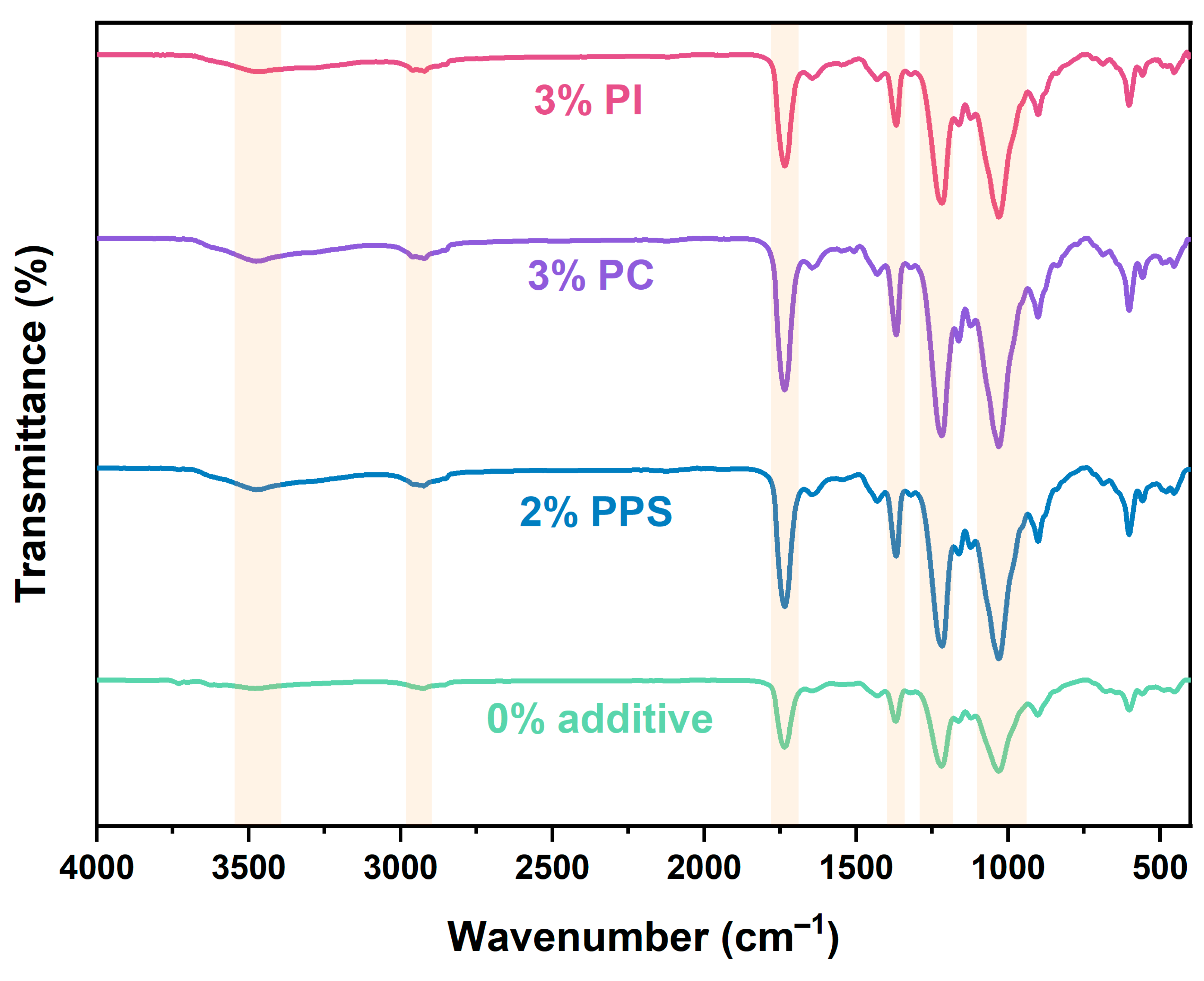
3.2. Morphology and Composition Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perea-Moreno, M.-A.; Samerón-Manzano, E.; Perea-Moreno, A.-J. Biomass as Renewable Energy: Worldwide Research Trends. Sustainability 2019, 11, 863. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Jiang, M.; Zhou, G. Bio-Effects of Bio-Based and Fossil-Based Microplastics: Case Study with Lettuce-Soil System. Environ. Pollut. 2022, 306, 119395. [Google Scholar] [CrossRef]
- Figueira, J.; Peixoto, M.; Gaspar, C.; Loureiro, J.; Martins, R.; Carlos, E.; Pereira, L. Cellulose-Based Encapsulation for All-Printed Flexible Thermoelectric Touch Detectors. J. Mater. Sci. Mater. Electron. 2025, 36, 10. [Google Scholar] [CrossRef]
- Palmieri, E.; Maiolo, L.; Lucarini, I.; Fattorini, A.D.; Tamburri, E.; Orlanducci, S.; Calarco, R.; Maita, F. Toward Sustainable Electronics: Exploiting the Potential of a Biodegradable Cellulose Blend for Photolithographic Processes and Eco-Friendly Devices. Adv. Mater. Technol. 2024, 9, 2301282. [Google Scholar] [CrossRef]
- Palmieri, E.; Cancelliere, R.; Maita, F.; Micheli, L.; Maiolo, L. An Ethyl Cellulose Novel Biodegradable Flexible Substrate Material for Sustainable Screen-Printing. RSC Adv. 2024, 14, 18103–18108. [Google Scholar] [CrossRef]
- Decroix, C.; Chalamet, Y.; Sudre, G.; Caroll, V. Thermo-Mechanical Properties and Blend Behaviour of Cellulose Acetate/Lactates and Acid Systems: Natural-Based Plasticizers. Carbohydr. Polym. 2020, 237, 116072. [Google Scholar] [CrossRef]
- Leite, L.S.F.; Battirola, L.C.; Da Silva, L.C.E.; Gonçalves, M.D.C. Morphological Investigation of Cellulose Acetate/Cellulose Nanocrystal Composites Obtained by Melt Extrusion. J Appl. Polym. Sci. 2016, 133, app.44201. [Google Scholar] [CrossRef]
- Martín-Alfonso, M.A. Environmentally Friendly Tailor-Made Oleo-Dispersions of Electrospun Cellulose Acetate Propionate Nanostructures in Castor Oil for Lubricant Applications. Nano Mater. Sci. 2024; in press. [Google Scholar]
- Chen, Z.; Jiang, X.; Boyjoo, Y.; Zhang, L.; Li, W.; Zhao, L.; Liu, Y.; Zhang, Y.; Liu, J.; Li, X. Nanoporous Carbon Materials Derived from Biomass Precursors: Sustainable Materials for Energy Conversion and Storage. Electrochem. Energy Rev. 2024, 7, 26. [Google Scholar] [CrossRef]
- Erdmann, R.; Kabasci, S.; Heim, H.-P. Thermal Properties of Plasticized Cellulose Acetate and Its β-Relaxation Phenomenon. Polymers 2021, 13, 1356. [Google Scholar] [CrossRef]
- Dobos, A.M.; Bargan, A.; Dunca, S.; Rîmbu, C.M.; Filimon, A. Cellulose Acetate/Silica Composites: Physicochemical and Biological Characterization. J. Mech. Behav. Biomed. Mater. 2023, 144, 106002. [Google Scholar] [CrossRef]
- Sunthar, T.P.M.; Boschetto, F.; Doan, H.N.; Honma, T.; Kinashi, K.; Adachi, T.; Marin, E.; Zhu, W.; Pezzotti, G. Antibacterial Property of Cellulose Acetate Composite Materials Reinforced with Aluminum Nitride. Antibiotics 2021, 10, 1292. [Google Scholar] [CrossRef]
- Serbruyns, L.; Van De Perre, D.; Hölter, D. Biodegradability of Cellulose Diacetate in Aqueous Environments. J. Polym. Environ. 2024, 32, 1326–1341. [Google Scholar] [CrossRef]
- Kimura, T.; Kida, T.; Yamaguchi, M. Viscoelastic Properties of Fully Biomass-Based Transparent Plastic Comprising Cellulose Acetate and Citrate Ester. Materials 2022, 15, 3038. [Google Scholar] [CrossRef]
- Khan, S.B.; Alamry, K.A.; Bifari, E.N.; Asiri, A.M.; Yasir, M.; Gzara, L.; Ahmad, R.Z. Assessment of Antibacterial Cellulose Nanocomposites for Water Permeability and Salt Rejection. J. Ind. Eng. Chem. 2015, 24, 266–275. [Google Scholar] [CrossRef]
- Kouadri, I.; Layachi, A.; Boubendira, K.; Amor, I.B.; Hemmami, H.; Zeghoud, S.; Seghir, B.B.; Rebiai, A. A Novel Eco-Friendly Source of Cellulose Acetate Extracted from Astragalus Gombo Seeds: Thermal, Structural, and Morphological Characterization. Biomass Conv. Bioref. 2024, 14, 23439–23446. [Google Scholar] [CrossRef]
- Prado, A.G.S.; Faria, E.A.; SouzaDe, J.R.; Torres, J.D. Ammonium Complex of Niobium as a Precursor for the Hydrothermal Preparation of Cellulose Acetate/Nb2O5 Photocatalyst. J. Mol. Catal. A Chem. 2005, 237, 115–119. [Google Scholar] [CrossRef]
- Gontijo De Melo, P.; Fornazier Borges, M.; Afonso Ferreira, J.; Vicente Barbosa Silva, M.; Ruggiero, R. Bio-Based Cellulose Acetate Films Reinforced with Lignin and Glycerol. Int. J. Mol. Sci. 2018, 19, 1143. [Google Scholar] [CrossRef]
- Teixeira, S.C.; Silva, R.R.A.; De Oliveira, T.V.; Stringheta, P.C.; Pinto, M.R.M.R.; Soares, N.D.F.F. Glycerol and Triethyl Citrate Plasticizer Effects on Molecular, Thermal, Mechanical, and Barrier Properties of Cellulose Acetate Films. Food Biosci. 2021, 42, 101202. [Google Scholar] [CrossRef]
- Claro, P.I.C.; Neto, A.R.S.; Bibbo, A.C.C.; Mattoso, L.H.C.; Bastos, M.S.R.; Marconcini, J.M. Biodegradable Blends with Potential Use in Packaging: A Comparison of PLA/Chitosan and PLA/Cellulose Acetate Films. J. Polym. Environ. 2016, 24, 363–371. [Google Scholar] [CrossRef]
- Samios, E.; Dart, R.K.; Dawkins, J.V. Preparation, Characterization and Biodegradation Studies on Cellulose Acetates with Varying Degrees of Substitution. Polymer 1997, 38, 3045–3054. [Google Scholar] [CrossRef]
- Buchanan, C.M.; Gardner, R.M.; Komarek, R.J. Aerobic Biodegradation of Cellulose Acetate. J. Appl. Polym. Sci. 1993, 47, 1709–1719. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Jiang, X.; Zhao, L.; Wang, P.; Zhang, Y. Rational Design of SiOx Based Anode Materials for next Generation Lithium-Ion Batteries. Mater. Adv. 2024, 5, 896–919. [Google Scholar] [CrossRef]
- Nicosia, A.; Keppler, T.; Müller, F.A.; Vazquez, B.; Ravegnani, F.; Monticelli, P.; Belosi, F. Cellulose Acetate Nanofiber Electrospun on Nylon Substrate as Novel Composite Matrix for Efficient, Heat-Resistant, Air Filters. Chem. Eng. Sci. 2016, 153, 284–294. [Google Scholar] [CrossRef]
- Abdel-Naby, A.S.; Al-Ghamdi, A.A. Chemical Modification of Cellulose Acetate by N-(Phenyl Amino) Maleimides: Characterization and Properties. Int. J. Biol. Macromol. 2014, 68, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kang, S.W. Cellulose Acetate Containing CaO Coated on Polypropylene for Enhanced Thermal Stability of Separator. Chem. Commun. 2021, 57, 4388–4391. [Google Scholar] [CrossRef]
- Sultana, Q.; Hasan, M.; Iqbal, S.; Shabib, I.; Mitra, A.; Khan, M. Investigation of Mechanical Properties and Morphology of Multi-Walled Carbon Nanotubes Reinforced Cellulose Acetate Fibers. Fibers 2017, 5, 42. [Google Scholar] [CrossRef]
- Jatoi, A.W.; Ogasawara, H.; Kim, I.S.; Ni, Q.-Q. Cellulose Acetate/Multi-Wall Carbon Nanotube/Ag Nanofiber Composite for Antibacterial Applications. Mater. Sci. Eng. C 2020, 110, 110679. [Google Scholar] [CrossRef]
- Nourizadeh Kazerouni, H.; Jafarzadeh, Y.; Yegani, R. Study on the Effect of M-Phenylenediamine as a Cross-Linking Agent on the Mechanical, Chemical and Thermal Properties and Performance of Cellulose Acetate/Nanodiamond Membranes. Polym. Bull. 2020, 77, 405–425. [Google Scholar] [CrossRef]
- Palmieri, E.; Giordanengo, G.; Bonomo, M.; Guglielmotti, V.; Severini, L.; Mazzuca, C.; Barolo, C.; Orlanducci, S. Improving Packaging Sustainability: Cellulose-Based Coatings with Enhanced Barrier Properties and Oxidative Stability. ACS Appl. Polym. Mater. 2024, 6, 11776–11787. [Google Scholar] [CrossRef]
- Baek, W.-I.; Pant, H.R.; Nam, K.-T.; Nirmala, R.; Oh, H.-J.; Kim, I.; Kim, H.-Y. Effect of Adhesive on the Morphology and Mechanical Properties of Electrospun Fibrous Mat of Cellulose Acetate. Carbohydr. Res. 2011, 346, 1956–1961. [Google Scholar] [CrossRef]
- Wang, L.; Cui, L.; Liu, Y.; Riedel, J.; Qian, X.; Liu, Y. Electrospun Polyimide Nanofiber-Coated Polyimide Nonwoven Fabric for Hot Gas Filtration. Adsorpt. Sci. Technol. 2018, 36, 1734–1743. [Google Scholar] [CrossRef]
- Feng, C.; Chen, H.; Yang, M.; Feng, Z.; Wang, Y. Metallization of Polyphenylene Sulfide by Low-Cost Mussel-Inspired Catechol/Polyamine Surface Modification. ACS Appl. Polym. Mater. 2022, 4, 4445–4453. [Google Scholar] [CrossRef]
- Soekoco, A.; Rehman, A.U.; Fauzi, A.; Tasya, H.; Diandra, P.; Tasa, I.; Nugraha; Yuliarto, B. Fabrication of Recycled Polycarbonate Fibre for Thermal Signature Reduction in Camouflage Textiles. Polymers 2022, 14, 1972. [Google Scholar] [CrossRef]
- Celuppi, L.C.M.; Capelezzo, A.P.; Cima, L.B.; Zeferino, R.C.F.; Carniel, T.A.; Zanetti, M.; De Mello, J.M.M.; Fiori, M.A.; Riella, H.G. Microbiological, Thermal and Mechanical Performance of Cellulose Acetate Films with Geranyl Acetate. Int. J. Biol. Macromol. 2023, 228, 517–527. [Google Scholar] [CrossRef]
- Pereira, N.R.L.; Lopes, B.; Fagundes, I.V.; De Moraes, F.M.; Morisso, F.D.P.; Parma, G.O.C.; Zepon, K.M.; Magnago, R.F. Bio-Packaging Based on Cellulose Acetate from Banana Pseudostem and Containing Butia Catarinensis Extracts. Int. J. Biol. Macromol. 2022, 194, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Wnorowska, J.; Ciukaj, S.; Kalisz, S. Thermogravimetric Analysis of Solid Biofuels with Additive under Air Atmosphere. Energies 2021, 14, 2257. [Google Scholar] [CrossRef]
- Tang, H.; Wu, S.; Ding, L.; Fang, N.; Zhang, Q.; Chu, Y. Catalytic Oxidation and Mixed Oxidation of Ethyl Acetate: A Review. Sep. Purif. Technol. 2024, 343, 126980. [Google Scholar] [CrossRef]
- Fraga, L.G.; Silva, J.; Teixeira, S.; Soares, D.; Ferreira, M.; Teixeira, J. Influence of Operating Conditions on the Thermal Behavior and Kinetics of Pine Wood Particles Using Thermogravimetric Analysis. Energies 2020, 13, 2756. [Google Scholar] [CrossRef]
- Wang, B.; Chen, J.; Peng, H.; Gai, J.; Kang, J.; Cao, Y. Investigation on Changes in the Miscibility, Morphology, Rheology and Mechanical Behavior of Melt Processed Cellulose Acetate through Adding Polyethylene Glycol as a Plasticizer. J. Macromol. Sci. Part B 2016, 55, 894–907. [Google Scholar] [CrossRef]
- Li, H.; Xu, S.; Zhao, B.; Yu, Y.; Liu, Y. The Phase Structural Evolution and Gas Separation Performances of Cellulose Acetate/Polyimide Composite Membrane from Polymer to Carbon Stage. Membranes 2021, 11, 618. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Chen, J.; Luo, J.; Min, J.; Fu, Q.; Zhang, J. Efficient Disentanglement of Polycarbonate Melts under Complex Shear Field. Polymer 2020, 201, 122610. [Google Scholar] [CrossRef]
- Oyarzabal, A.; Cristiano-Tassi, A.; Laredo, E.; Newman, D.; Bello, A.; Etxeberría, A.; Eguiazabal, J.I.; Zubitur, M.; Mugica, A.; Müller, A.J. Dielectric, Mechanical and Transport Properties of Bisphenol A Polycarbonate/Graphene Nanocomposites Prepared by Melt Blending. J. Appl. Polym. Sci. 2017, 134, app.44654. [Google Scholar] [CrossRef]
- Hu, S.; Yang, F.; Cao, Y.; Yan, Y.; Wu, T.; Fu, Q. Influence of Chain Entanglements and Melt Memory Effect on the Crystallization Behavior of Polyphenylene Sulfide. Polymer 2023, 285, 126390. [Google Scholar] [CrossRef]
- Nayak, B.; Tanvidkar, P.; Kuncharam, B.V.R. Preparation, Characterization, and CO2 Permeation Testing of Cellulose Acetate and Polyimide Blend Membranes. Polym. Eng. Sci. 2023, pen.26584. [Google Scholar] [CrossRef]
- Olaru, N.; Anghel, N.; Pascariu, P.; Ailiesei, G. Synthesis and Testing of Cellulose Acetate Nicotinate as Adsorbent for Rhodamine B Dye. J. Appl. Polym. Sci. 2019, 136, 47772. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Mallegni, N.; Rizzo, S.; Fiori, S.; Signori, F.; Lazzeri, A. Compatibilization of Poly(Lactic Acid) (PLA)/Plasticized Cellulose Acetate Extruded Blends through the Addition of Reactively Extruded Comb Copolymers. Molecules 2021, 26, 2006. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, X.; Lu, H.; Wu, D.; Du, X.; Li, Z.; Zhang, M. Highly Selective Polyphenylene Sulfide Nanocomposite Membranes Based on Two-Dimensional Metal–Organic Frameworks for Molecular Separation. React. Funct. Polym. 2024, 202, 105971. [Google Scholar] [CrossRef]
- Kudavalli, J.S.; Rao, S.N.; Bean, D.E.; Sharma, N.D.; Boyd, D.R.; Fowler, P.W.; Gronert, S.; Kamerlin, S.C.L.; Keeffe, J.R.; More O’Ferrall, R.A. Base-Catalyzed Dehydration of 3-Substituted Benzene Cis -1,2-Dihydrodiols: Stabilization of a Cyclohexadienide Anion Intermediate by Negative Aromatic Hyperconjugation. J. Am. Chem. Soc. 2012, 134, 14056–14069. [Google Scholar] [CrossRef]
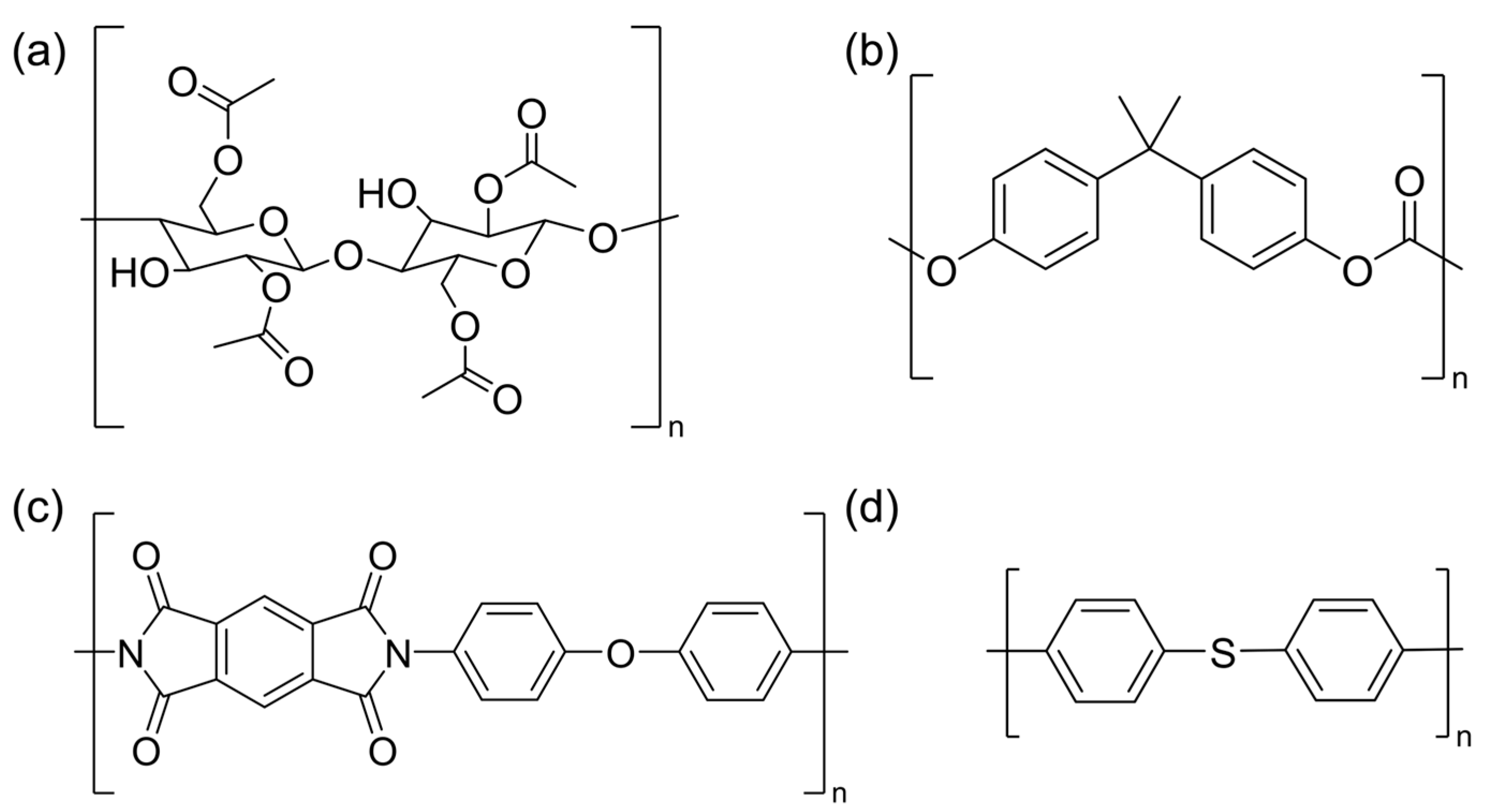




| Material | Mn (kDa) | Mw (kDa) | Mp (kDa) | PDI |
|---|---|---|---|---|
| CDA | 42.7 | 194 | 151 | 4.54 |
| PPS | 18.7 | 44.4 | 43.0 | 2.37 |
| PC | 17.6 | 52.3 | 46.0 | 2.97 |
| PI | 21.0 | 32.1 | 27.0 | 1.53 |
| Materials | CDA (wt, %) | TEC (wt, %) | Irganox 1010 (wt, %) | Irgafos 168 (wt, %) | PPS (wt, %) | PC (wt, %) | PI (wt, %) |
|---|---|---|---|---|---|---|---|
| CDA | 100 | / | / | / | / | / | / |
| PPS | / | / | / | / | 100 | / | / |
| PC | / | / | / | / | / | 100 | / |
| PI | / | / | / | / | / | / | 100 |
| 0% additive | 89 | 10 | 0.5 | 0.5 | / | / | / |
| 1% PPS | 88 | 10 | 0.5 | 0.5 | 1 | / | / |
| 2% PPS | 87 | 10 | 0.5 | 0.5 | 2 | / | / |
| 3% PPS | 86 | 10 | 0.5 | 0.5 | 3 | / | / |
| 4% PPS | 85 | 10 | 0.5 | 0.5 | 4 | / | / |
| 5% PPS | 84 | 10 | 0.5 | 0.5 | 5 | / | / |
| 1% PC | 88 | 10 | 0.5 | 0.5 | / | 1 | / |
| 2% PC | 87 | 10 | 0.5 | 0.5 | / | 2 | / |
| 3% PC | 86 | 10 | 0.5 | 0.5 | / | 3 | / |
| 4% PC | 85 | 10 | 0.5 | 0.5 | / | 4 | / |
| 5% PC | 84 | 10 | 0.5 | 0.5 | / | 5 | / |
| 1% PI | 88 | 10 | 0.5 | 0.5 | / | / | 1 |
| 2% PI | 87 | 10 | 0.5 | 0.5 | / | / | 2 |
| 3% PI | 86 | 10 | 0.5 | 0.5 | / | / | 3 |
| 4% PI | 85 | 10 | 0.5 | 0.5 | / | / | 4 |
| 5% PI | 84 | 10 | 0.5 | 0.5 | / | / | 5 |
| Formulations | Tg (°C) | Tmi (°C) | Tmf (°C) | Mass195°C (%) | Tdmax (°C) |
|---|---|---|---|---|---|
| CDA | 191.5 | 205.7 | 246.4 | 97.6 | 365.9 |
| PPS | 148.7 | 231.1 | 261.2 | 99.9 | 529.4 |
| PC | 140.1 | 185.9 | 234.9 | 99.6 | 471.3 |
| PI | 250.9 | 274.6 | 323.0 | 98.9 | 557.3 |
| 0% additive | 145.2 | 211.6 | 261.7 | 97.6 | 366.1 |
| 1% PPS | 148.3 | 214.5 | 264.2 | 97.7 | 365.4 |
| 2% PPS | 148.2 | 217.9 | 268.7 | 98.0 | 365.1 |
| 3% PPS | 149.1 | 214.8 | 266.8 | 98.0 | 365.1 |
| 4% PPS | 149.3 | 213.8 | 277.7 | 98.0 | 365.5 |
| 5% PPS | 150.2 | 213.7 | 260.6 | 98.0 | 364.1 |
| 1% PC | 147.1 | 216.2 | 263.6 | 97.8 | 363.2 |
| 2% PC | 147.7 | 218.9 | 270.7 | 98.1 | 365.2 |
| 3% PC | 149.5 | 224.6 | 274.4 | 98.7 | 363.3 |
| 4% PC | 149.3 | 226.6 | 274.9 | 97.5 | 364.4 |
| 5% PC | 148.9 | 227.2 | 275.1 | 98.3 | 365.2 |
| 1% PI | 150.3 | 214.7 | 263.4 | 96.0 | 364.7 |
| 2% PI | 149.4 | 215.3 | 264.9 | 96.4 | 365.9 |
| 3% PI | 147.1 | 216.2 | 265.6 | 97.4 | 364.1 |
| 4% PI | 147.2 | 214.7 | 265.4 | 96.5 | 365.4 |
| 5% PI | 145.3 | 214.4 | 264.9 | 96.6 | 365.7 |
| Formulations | Wavenumber (cm−1) | |||||
|---|---|---|---|---|---|---|
| O-H Stretching Vibration | C-H Stretching Vibration | C=O Stretching Vibration | CH3 Bending Vibration | C-O-H Stretching Vibration | C-O-C Stretching Vibration | |
| 0% additive | 3478 | 2925 | 1736 | 1370 | 1219 | 1030 |
| 2% PPS | 3475 | 2922 | 1735 | 1368 | 1217 | 1030 |
| 3% PC | 3475 | 2921 | 1735 | 1368 | 1218 | 1030 |
| 3% PI | 3474 | 2921 | 1735 | 1368 | 1217 | 1030 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Hu, Y.; Chen, J.; Yan, Z.; Zhao, L.; Zhan, F.; Wang, J.; Zhang, Y. Molecular Entanglement Facilitated Improvement of Thermal Stability of Cellulose Diacetate. Polymers 2025, 17, 835. https://doi.org/10.3390/polym17070835
Liu Y, Hu Y, Chen J, Yan Z, Zhao L, Zhan F, Wang J, Zhang Y. Molecular Entanglement Facilitated Improvement of Thermal Stability of Cellulose Diacetate. Polymers. 2025; 17(7):835. https://doi.org/10.3390/polym17070835
Chicago/Turabian StyleLiu, Yang, Yin Hu, Jianyu Chen, Zongkai Yan, Lin Zhao, Falu Zhan, Junjie Wang, and Yagang Zhang. 2025. "Molecular Entanglement Facilitated Improvement of Thermal Stability of Cellulose Diacetate" Polymers 17, no. 7: 835. https://doi.org/10.3390/polym17070835
APA StyleLiu, Y., Hu, Y., Chen, J., Yan, Z., Zhao, L., Zhan, F., Wang, J., & Zhang, Y. (2025). Molecular Entanglement Facilitated Improvement of Thermal Stability of Cellulose Diacetate. Polymers, 17(7), 835. https://doi.org/10.3390/polym17070835








