Research on the Preparation of Supercapacitor Separators with High Wettability and Excellent Temperature Adaptability Through In Situ Deposition of Nano-Barium Sulfate on Regenerated Cellulose
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Composite Regenerated Cellulose Separator
- In the initial step of the process, Lyocell fibers (CLY) were immersed in a 1 wt% sodium hydroxide solution for a duration of two hours. This step allowed the fibers to fully absorb the solution and undergo swelling, thereby weakening the hydrogen bonds between the fibers. Subsequently, the treated CLY was processed into a 10 wt% fiber stock and fed into a pulper (Shandong Dazhi Papermaking Equipment Co., Ltd., Zhuchengi, China) for fine grinding at an angular velocity of 80,000 rpm at a controlled grinding temperature of 60 °C. The resultant CLY fiber stock exhibited a consistency of 10 wt%. Through this process, the fibers underwent complete fibrillation, ultimately yielding primary fibrillated fibers (MCLY) with a diameter ranging from 1 to 2 μm.
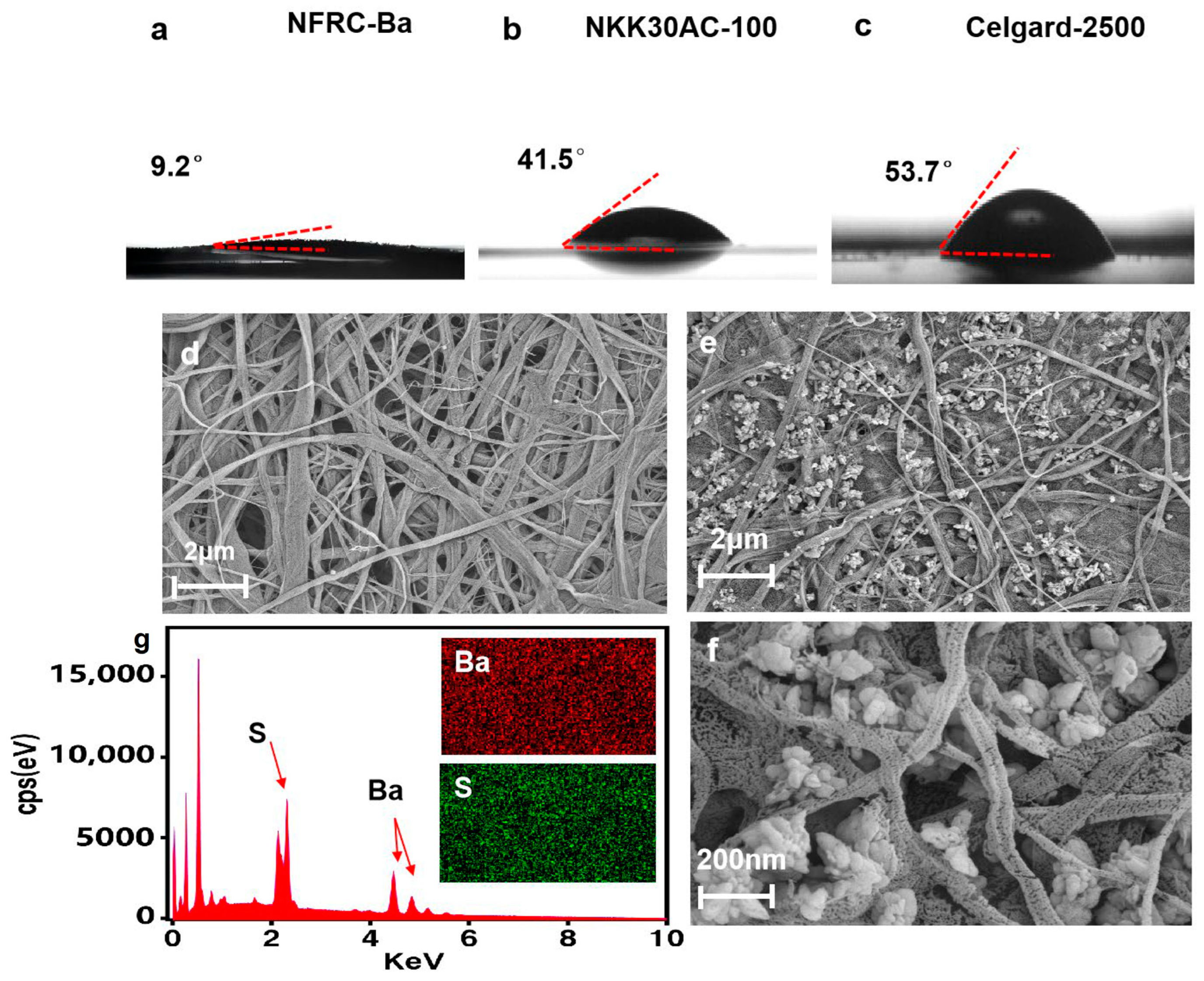
- A 1 M solution of sodium sulfate and a 1 M solution of barium chloride were prepared. The sodium sulfate solution was added to a 3 wt% nanofibrillar cellulose (NFC) mixture in a 5:5 ratio. Then, an ultrasonic disperser (Jining AoChao Electronic Equipment Co., Ltd., Jiningi, China) was used to disperse the NFC system for 30 min at 25 °C to ensure complete dispersion. Subsequently, the barium chloride solution was introduced into the NFC mixture in accordance with the molar ratio of sulfate ions (SO42−) to barium ions (Ba2+) of 1:1, thereby inducing the precipitation of barium sulfate nanoparticles within the NFC system and the effective formulation of the NFC-BA mixture. This process occurs when barium ions in the solution react with sulphate ions in the presence of a regenerated cellulose matrix. Negatively charged functional groups on the cellulose surface attract positively charged barium ions and these adsorbed barium ions subsequently react with the sulphate ions in solution to form barium sulphate nanodeposits on the cellulose fibers.
- MCLY and NFC-Ba were meticulously amalgamated in a precise proportion of 7:3, yielding a homogeneous liquid with a fiber concentration of 0.3 wt%. Composite regenerated cellulose separator paper (NFNFRC-Ba) with a basis weight of 13 g/m2 was fabricated using a dedicated paper machine. Concurrently, cellulose separator paper (FNFRC-Ba) with an identical basis weight (13 g/m2) to the control sample was fabricated solely utilizing MCLY as the raw material. The comprehensive preparation process for the composite regenerated cellulose separation paper (NFNFRC-Ba) is depicted in Figure 1.
3. Results
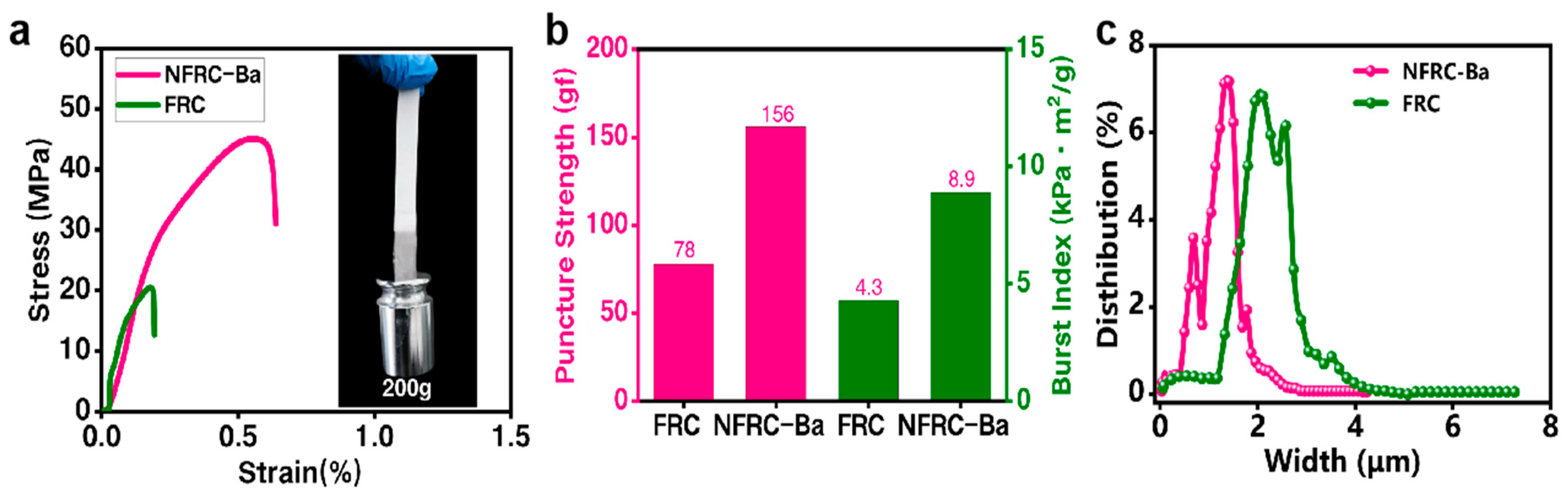
3.1. Physical Properties of the NFRC-Ba
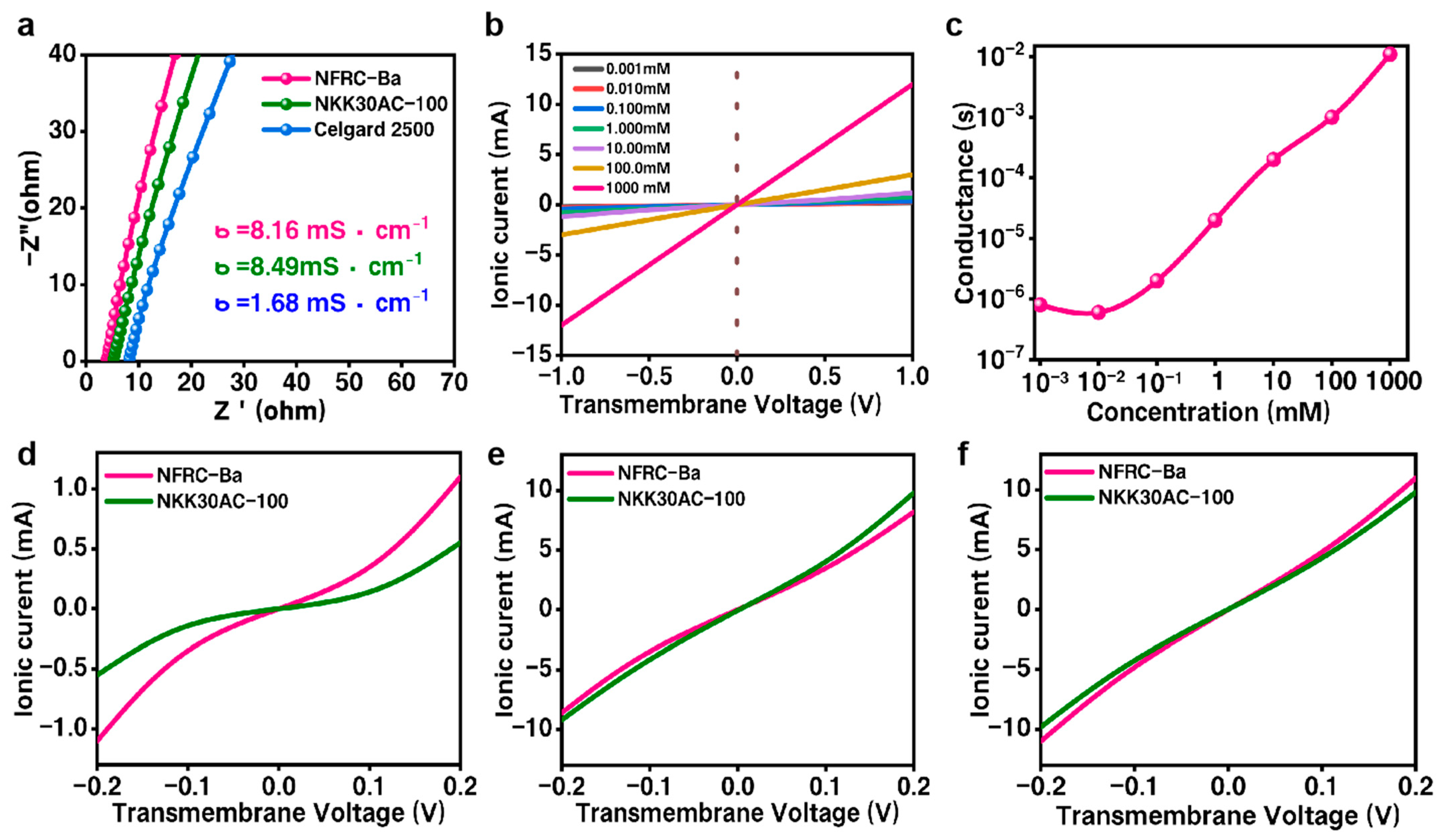
3.2. Ionic Property of the NFRC-Ba Separator
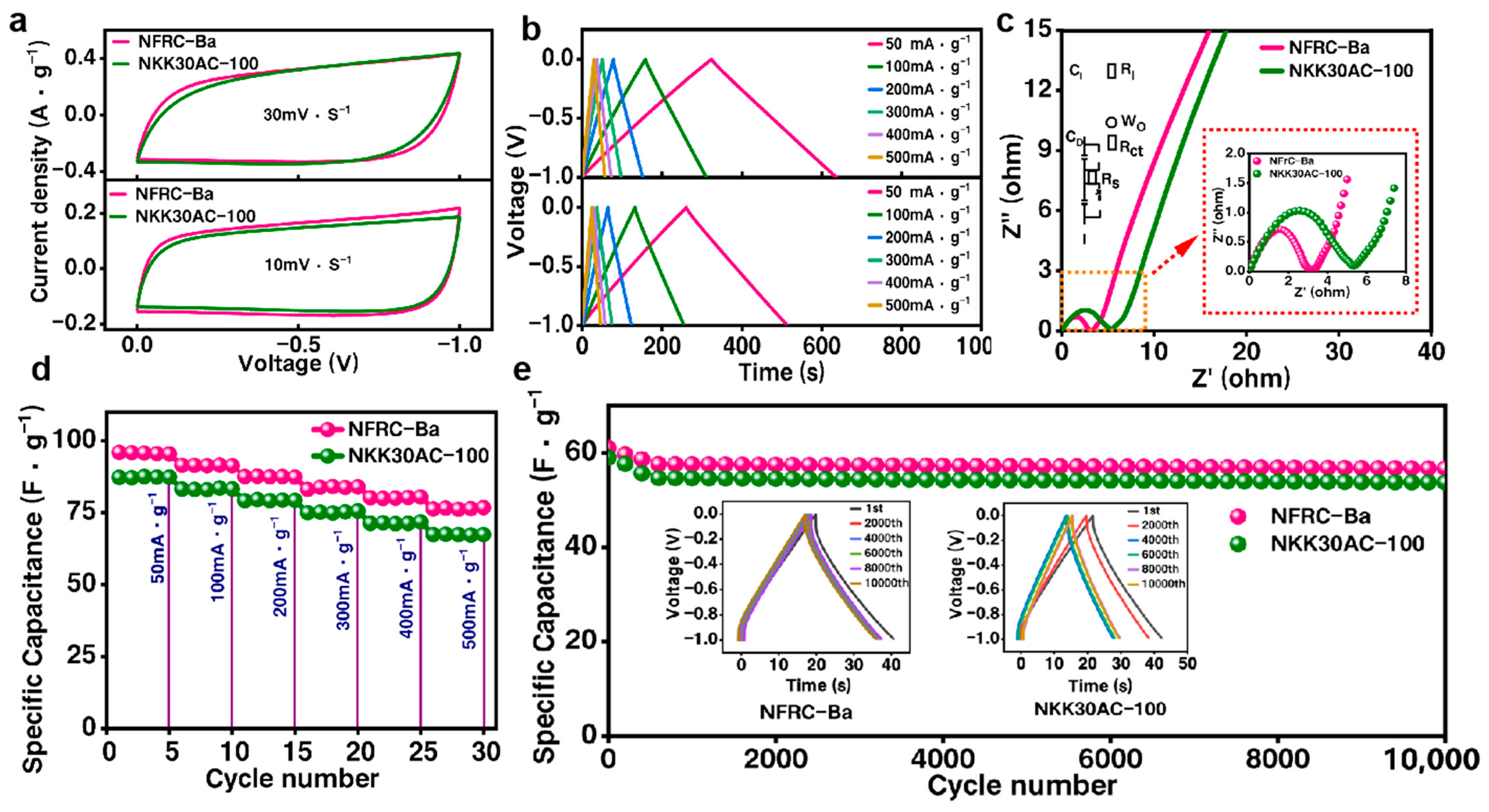
3.3. Charge–Discharge Performance of the NFRC-Ba Separator
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kwade, A.; Haselrieder, W.; Leithoff, R.; Modlinger, A.; Dietrich, F.; Droeder, K. Current status and challenges for automotive battery production technologies. Nat. Energy 2018, 3, 290–300. [Google Scholar] [CrossRef]
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef]
- Huang, X.; He, R.; Li, M.; Chee, M.O.L.; Dong, P.; Lu, J. Functionalized separator for next-generation batteries. Mater. Today 2020, 41, 143–155. [Google Scholar] [CrossRef]
- Lagadec, M.F.; Zahn, R.; Wood, V. Characterization and performance evaluation of lithium-ion battery separators. Nat. Energy 2019, 4, 16–25. [Google Scholar] [CrossRef]
- Xing, J.; Bliznakov, S.; Bonville, L.; Oljaca, M.; Maric, R. A Review of Nonaqueous Electrolytes, Binders, and Separators for Lithium-Ion Batteries. Electrochem. Energy Rev. 2022, 5, 14. [Google Scholar] [CrossRef]
- Hu, W.; Fu, W.; Jhulki, S.; Chen, L.; Narla, A.; Sun, Z.; Wang, F.; Magasinski, A.; Yushin, G. Heat-resistant Al2O3 nanowire-polyetherimide separator for safer and faster lithium-ion batteries. J. Mater. Sci. Technol. 2023, 142, 112–120. [Google Scholar] [CrossRef]
- Zhong, S.; Yuan, B.; Guang, Z.; Chen, D.; Li, Q.; Dong, L.; Ji, Y.; Dong, Y.; Han, J.; He, W. Recent progress in thin separators for upgraded lithium ion batteries. Energy Storage Mater. 2021, 41, 805–841. [Google Scholar] [CrossRef]
- Liu, J.-H.; Wang, P.; Gao, Z.; Li, X.; Cui, W.; Li, R.; Ramakrishna, S.; Zhang, J.; Long, Y.-Z. Review on electrospinning anode and separators for lithium ion batteries. Renew. Sustain. Energy Rev. 2024, 189, 113939. [Google Scholar] [CrossRef]
- Chai, Y.; Ning, D.; Zhou, D.; Gao, J.; Ni, J.; Zhang, G.; Gao, R.; Wu, W.; Wang, J.; Li, Y. Construction of flexible asymmetric composite polymer electrolytes for high-voltage lithium metal batteries with superior performance. Nano Energy 2024, 130, 110160. [Google Scholar] [CrossRef]
- Tang, L.; Chen, B.; Zhang, Z.; Ma, C.; Chen, J.; Huang, Y.; Zhang, F.; Dong, Q.; Xue, G.; Chen, D.; et al. Polyfluorinated crosslinker-based solid polymer electrolytes for long-cycling 4.5 V lithium metal batteries. Nat. Commun. 2023, 14, 2301. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xu, C.; Wang, J.; Fan, Z.; Xu, L.; He, Y.; Zhang, X.; Ding, B.; Zhang, X. Non-Flammable fluorinated gel polymer electrolyte for safe lithium metal batteries in harsh environments. J. Colloid Interface Sci. 2025, 683, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hu, H.; Yang, J.; He, Z.; Zhu, G.; Wen, C. The Application of Porous Carbon Derived from Furfural Residue as the Electrode Material in Supercapacitors. Polymers 2024, 16, 3421. [Google Scholar] [CrossRef]
- Li, H.; Wu, D.; Wu, J.; Dong, L.; Zhu, Y.; Hu, X. Flexible, High-Wettability and Fire-Resistant Separators Based on Hydroxyapatite Nanowires for Advanced Lithium-Ion Batteries. Adv. Mater. 2017, 29, 1703548. [Google Scholar] [CrossRef]
- Chen, X.; Chu, F.; Li, D.; Si, M.; Liu, M.; Wu, F. In Situ Polymerized Fluorine-Free Ether Gel Polymer Electrolyte with Stable Interface for High-Voltage Lithium Metal Batteries. Adv. Funct. Mater. 2024, 2421965. [Google Scholar] [CrossRef]
- Cheng, X.; Bae, J. Recent Advancements in Fabrication, Separation, and Purification of Hierarchically Porous Polymer separators and Their Applications in Next-Generation Electrochemical Energy Storage Devices. Polymers 2024, 16, 3269. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, J.; Tian, T.; Shen, B.; Peng, Y.; Song, Y.; Jiang, B.; Lu, L.; Yao, H.; Yu, S. Sustainable Separators for High-Performance Lithium Ion Batteries Enabled by Chemical Modifications. Adv. Funct. Mater. 2019, 29, 1902023. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J. Highly Stable Lithium–Sulfur Batteries Based on Laponite Nanosheet-Coated Celgard Separators. Adv. Energy Mater. 2018, 8, 1801778. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, Y.; Han, Q.; Su, M.; Liu, Y.; Wang, S.; Wang, H. Vapor-induced phase inversion of poly (m-phenylene isophthalamide) modified polyethylene separator for high-performance lithium-ion batteries. Chem. Eng. J. 2022, 429, 132429. [Google Scholar] [CrossRef]
- Schadeck, U.; Kyrgyzbaev, K.; Zettl, H.; Gerdes, T.; Moos, R. Flexible, Heat-Resistant, and Flame-Retardant Glass Fiber Nonwoven/Glass Platelet Composite Separator for Lithium-Ion Batteries. Energies 2018, 11, 999. [Google Scholar] [CrossRef]
- Hsia, T.-N.; Lu, H.-C.; Hsueh, Y.-C.; Kumar, S.R.; Yen, C.-S.; Yang, C.-C.; Lue, S.J. Superdry poly(vinylidene fluoride-co-hexafluoropropylene) coating on a lithium anode as a protective layer and separator for a high-performance lithium-oxygen battery. J. Colloid Interface Sci. 2022, 626, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhang, D.; Gu, C.; Wang, X.; Wang, S.; Zhang, X.; Qin, J.; Wu, Z. Manipulating Crystallographic Orientation of Zinc Deposition for Dendrite-free Zinc Ion Batteries. Adv. Energy Mater. 2021, 11, 2101299. [Google Scholar] [CrossRef]
- Dong, T.; Arifeen, W.U.; Choi, J.; Yoo, K.; Ko, T. Surface-modified electrospun polyacrylonitrile nano-separator for a lithium-ion battery separator based on phase separation mechanism. Chem. Eng. J. 2020, 398, 125646. [Google Scholar] [CrossRef]
- Zhou, C.; He, Q.; Li, Z.; Meng, J.; Hong, X.; Li, Y.; Zhao, Y.; Xu, X.; Mai, L. A robust electrospun separator modified with in situ grown metal-organic frameworks for lithium-sulfur batteries. Chem. Eng. J. 2020, 395, 124979. [Google Scholar] [CrossRef]
- Raza, N.; Aziz, K.; Javed, M.S.; Tariq, R.; Kanwal, N.; Raza, W.; Sarfraz, M.; Khan, S.; Ismail, M.A.; Akkinepally, B.; et al. Advancements in separator design for supercapacitor technology: A review of characteristics, manufacturing processes, limitations, and emerging trends. J. Energy Storage 2025, 111, 111115328. [Google Scholar] [CrossRef]
- Yu, H.; Tang, Q.; Wu, J.; Lin, Y.; Fan, L.; Huang, M.; Lin, J.; Li, Y.; Yu, F. Using eggshell separator as a separator in supercapacitor. J. Power Sources 2012, 206, 463–468. [Google Scholar] [CrossRef]
- Kalluri, L.; Griggs, J.A.; Janorkar, A.V.; Xu, X.; Chandran, R.; Mei, H.; Nobles, K.P.; Yang, S.; Alberto, L.; Duan, Y. Preparation and optimization of an eggshell separator-based biomaterial for GTR applications. Dent. Mater. 2024, 40, 728–738. [Google Scholar] [CrossRef]
- Zhang, Y.; He, J.; Gao, Z.; Li, X. Converting eggs to flexible, all-solid supercapacitors. Nano Energy 2019, 65, 104045. [Google Scholar] [CrossRef]
- Bai, S.; Kim, B.; Kim, C.; Tamwattana, O.; Park, H.; Kim, J.; Lee, D.; Kang, K. Permselective metal–organic framework gel separator enables long-life cycling of rechargeable organic batteries. Nat. Nanotechnol. 2021, 16, 77–84. [Google Scholar] [CrossRef]
- Sarno, M.; Casa, M. Green and one-step synthesis for Ag/graphene hybrid supercapacitor with remarkable performance. J. Phys. Chem. Solids 2018, 120, 241–249. [Google Scholar] [CrossRef]
- Sarno, M.; Scudieri, C.; Ponticorvo, E.; Baldino, L.; Cardea, S.; Reverchon, E. High performance PVDF HFP_RuO2 supercapacitors production by supercritical drying. J. Supercrit. Fluids 2021, 176, 105323. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.; Lee, S.-Y.; Wei, T.; Li, J.; Fan, Z. Nanocellulose: A promising nanomaterial for advanced electrochemical energy storage. Chem. Soc. Rev. 2018, 47, 2837–2872. [Google Scholar] [CrossRef] [PubMed]
- Doobary, S.; Apostolopoulou-Kalkavoura, V.; Mathew, A.P.; Olofsson, B. Nanocellulose: New horizons in organic chemistry and beyond. Chem 2024, 10, 3279–3293. [Google Scholar] [CrossRef]
- El-Shafai, N.M.; Ibrahim, M.M.; Abdelfatah, M.; Ramadan, M.S.; El-Mehasseb, I.M. Synthesis, characterization, and cytotoxicity of self-assembly of hybrid nanocomposite modified separator of carboxymethyl cellulose/graphene oxide for photocatalytic antifouling, energy storage, and supercapacitors application. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 626, 127035. [Google Scholar] [CrossRef]
- Moreira, R.; Rebelo, R.C.; Coelho, J.F.J.; Serra, A.C. Novel thermally regenerated flexible cellulose-based films. Eur. J. Wood Wood Prod. 2024, 82, 1813–1826. [Google Scholar] [CrossRef]
- Chen, L.; Yin, H.; Liu, F.; Abdiryim, T.; Xu, F.; You, J.; Chen, J.; Jing, X.; Li, Y.; Su, M.; et al. Stretchable, self-healing, adhesive and anti-freezing ionic conductive cellulose-based hydrogels for flexible supercapacitors and sensors. Cellulose 2024, 31, 11015–11033. [Google Scholar] [CrossRef]
- Song, H.; Yang, J.; Yang, Y.; Yang, Q.; Hu, J. Characterization of cellulose-nanofiber-modified fibrillated lyocell fiber separator. BioResources 2022, 17, 4689–4704. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, G.; Edgar, K.J.; Zhang, H.; Shao, H. Effect of lyocell fiber cross-sectional shape on structure and properties of lyocell/PLA composites. J. Polym. Eng. 2022, 42, 868–875. [Google Scholar] [CrossRef]
- Xiwen, W.; Jian, H.; Jin, L. Preparation Ultra-fine Fibrillated Lyocell Fiber and Its Application in Battery Separator. Int. J. Electrochem. Sci. 2011, 6, 4999–5004. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, J.; Chen, L.; Long, J.; Hu, J.; Meng, L. Effect of fibrillated fiber morphology on properties of paper-based separators for lithium-ion battery applications. J. Power Sources 2021, 482, 228899. [Google Scholar] [CrossRef]
- Ding, Z.; Yang, X.; Tang, Y. Nanocellulose-based electrodes and separator toward sustainable and flexible all-solid-state supercapacitor. Int. J. Biol. Macromol. 2023, 228, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Prabhakar, M.N.; Zhai, J.-G.; Song, J.-I. High-throughput extraction of cellulose nanofibers from Imperata cylindrica grass for advanced bio composites. Int. J. Biol. Macromol. 2024, 284, 138111. [Google Scholar] [CrossRef] [PubMed]
- Antunes, B.d.F.; Santana, L.R.; Oliveira, R.M.; Filho, A.V.; Carreno, N.L.V.; Wolke, S.I.; da Silva, R.; Fajardo, A.R.; Dias, A.R.G.; Zavareze, E.d.R. Cellulose, cellulose nanofibers, and cellulose acetate from Butia fruits (Butia odorata): Chemical, morphological, structural, and thermal properties. Int. J. Biol. Macromol. 2024, 281, 136151. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Jiang, C.; Zhao, Y.; Li, Y.; Yu, S.; Huang, L. Cellulose nanofiber-based hybrid hydrogel electrode with superhydrophilicity enabling flexible high energy density supercapacitor and multifunctional sensors. Int. J. Biol. Macromol. 2024, 276, 134003. [Google Scholar] [CrossRef]
- Yuan, T.; Zhang, Z.; Liu, Q.; Liu, X.-T.; Tao, S.-Q.; Yao, C.-L. Cellulose nanofiber/MXene (Ti3C2Tx)/liquid metal film as a highly performance and flexible electrode material for supercapacitors. Int. J. Biol. Macromol. 2024, 262, 130119. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Hu, Y.; Zhuang, Y.; Ji, X.; Yang, G.; He, M. A morphology control engineered strategy of Ti3C2T/sulfated cellulose nanofibril composite film towards high-performance flexible supercapacitor electrode. Int. J. Biol. Macromol. 2023, 243, 124828. [Google Scholar] [CrossRef]
- Chen, Y.; Duan, Y.; Zhao, H.; Liu, K.; Liu, Y.; Wu, M.; Lu, P. Preparation of Bio-Based Foams with a Uniform Pore Structure by Nanocellulose/Nisin/Waterborne-Polyurethane-Stabilized Pickering Emulsion. Polymers 2022, 14, 5159. [Google Scholar] [CrossRef]
- Li, H.; Wang, H.; Xu, Z.; Wang, K.; Ge, M.; Gan, L.; Zhang, Y.; Tang, Y.; Chen, S. Thermal-Responsive and Fire-Resistant Materials for High-Safety Lithium-Ion Batteries. Small 2021, 17, 2103679. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, L.; Xu, D.-M.; Qiao, F.; Yao, X.; Lin, H.; Liu, W.; Pang, L.-X.; Hussain, F.; Darwish, M.A.; et al. Novel Method to Achieve Temperature-Stable Microwave Dielectric Ceramics: A Case in the Fergusonite-Structured NdNbO4 System. ACS Appl. Mater. Interfaces 2023, 15, 19129–19136. [Google Scholar] [CrossRef]
- Jin, S.; Chen, Z.; Bai, S.; Zhang, Y. Highly reversible Zn anode enabled by porous BaSO4 coating with wide band gap and high dielectric constant. J. Power Sources 2024, 591, 228899. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, L.; Zhu, Y.-J.; Wu, J.; Yu, H.-P.; Xie, S.; Li, D.; Wang, Z.; Li, H. Reversible Li plating regulation on graphite anode through a barium sulfate nanofibers-based dielectric separator for fast charging and high-safety lithium-ion battery. J. Energy Chem. 2025, 101, 511–523. [Google Scholar] [CrossRef]
- Gonzalez, M.S.; Yan, Q.; Holoubek, J.; Wu, Z.; Zhou, H.; Patterson, N.; Petrova, V.; Liu, H.; Liu, P. Draining Over Blocking: Nano-Composite Janus Separators for Mitigating Internal Shorting of Lithium Batteries. Adv. Mater. 2020, 32, e1906836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Z.; Kong, Q.; Zhang, C.; Pang, S.; Yue, L.; Wang, X.; Yao, J.; Cui, G. Renewable and Superior Thermal-Resistant Cellulose-Based Composite Nonwoven as Lithium-Ion Battery Separator. ACS Appl. Mater. Interfaces 2013, 5, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Min, Y. Electrospun polyimide nanofiber-based separators containing carboxyl groups for lithium-ion batteries. J. Appl. Polym. Sci. 2024, 141, e55721. [Google Scholar] [CrossRef]
- Yuan, B.; Wen, K.; Chen, D.; Liu, Y.; Dong, Y.; Feng, C.; Han, Y.; Han, J.; Zhang, Y.; Xia, C.; et al. Composite Separators for Robust High Rate Lithium Ion Batteries. Adv. Funct. Mater. 2021, 31, 2101420. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Cai, Q.; Dong, A.; Yang, D.; Zhao, D. Hierarchically Porous Silica Separator as Separator for High-Performance Lithium-Ion Batteries. Adv. Mater. 2022, 34, 2107957. [Google Scholar] [CrossRef]
- Du, H.; Zhou, X.; Li, T.; Zhao, W.; Zhou, D.; Yang, D.; Wu, T.; Xu, Y. Regulating Built-in Polar States via Atomic Self-Hybridization for Fast Ion Diffusion Kinetics in Potassium Ion Batteries†. Chin. J. Chem. 2024, 42, 2589–2598. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Wu, F.; Chen, H.; Chen, W.W.; Zhao, W.; Kang, K.; Guo, R.; Sun, Y.; Zhai, L.; et al. Hubbard Gap Closure-Induced Dual-Redox Li-Storage Mechanism as the Origin of Anomalously High Capacity and Fast Ion Diffusivity in MOFs-Like Polyoxometalates. Angew. Chem. Int. Ed. Engl. 2025, 64, e202416735. [Google Scholar] [CrossRef]
- Liu, M.; Turcheniuk, K.; Fu, W.; Yang, Y.; Liu, M.; Yushin, G. Scalable, safe, high-rate supercapacitor separators based on the Al2O3 nanowire Polyvinyl butyral nonwoven separators. Nano Energy 2020, 71, 104627. [Google Scholar] [CrossRef]
- Zahn, R.; Lagadec, M.F.; Hess, M.; Wood, V. Improving Ionic Conductivity and Lithium-Ion Transference Number in Lithium-Ion Battery Separators. ACS Appl. Mater. Interfaces 2016, 8, 32637–32642. [Google Scholar] [CrossRef]
- Jiang, S.; Lan, Z.; Zhang, Y.; Kang, X.; Zhao, L.; Wu, X.; Gao, H. Mechanisms by Which Exogenous Substances Enhance Plant Salt Tolerance through the Modulation of Ion Membrane Transport and Reactive Oxygen Species Metabolism. Antioxidants 2024, 13, 1050. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Chen, H.-H.; Chu, C.-W.; Yeh, L.-H. Massively Enhanced Charge Selectivity, Ion Transport, and Osmotic Energy Conversion by Antiswelling Nanoconfined Hydrogels. Nano Lett. 2024, 24, 11756–11762. [Google Scholar] [CrossRef] [PubMed]
- Gojgić, J.; Petrović, M.; Jugović, B.; Jokić, B.; Grgur, B.; Gvozdenović, M. Electrochemical and Electrical Performances of High Energy Storage Polyaniline Electrode with Supercapattery Behavior. Polymers 2022, 14, 5365. [Google Scholar] [CrossRef] [PubMed]
- Park, H.G.; Jeong, J.J.; Kim, J.H.; Lee, J.-S. Ion-Conducting Robust Cross-Linked Organic/Inorganic Polymer Composite as Effective Binder for Electrode of Electrochemical Capacitor. Polymers 2022, 14, 5174. [Google Scholar] [CrossRef]



| Performances | NFRC-Ba Separator | NKK30AC-100 Separator | Celgard-2500 Separator | |
|---|---|---|---|---|
| Mechanical Strength | Tensile Strength | 47.25 MPa | 100 | 117.68 MPa |
| Puncture Strength | 156 gf | 300 gf | 300 gf | |
| Fracture Strength | 8.9 KPa-m2/g | 12 KPa-m2/g | 15 KPa-m2/g | |
| Hole Diameter | 0.6–2 μm | 0.02–0.1 μm | 0.02–0.5 μm | |
| Porosity | 81.3% | 55% | 55% | |
| Contact Angle | 9.2° | 41.5° | 53.7° | |
| Thermal Stability | >180 °C | <140 °C | <110 °C | |
| Electrochemical Properties 1 | Ionic Conductivity | 8.16 mS/cm | 8.49 mS/cm | 1.68 mS/cm |
| Specific Capacitance | 32.45 F/g | 26.72 F/g | 23.54 F/g | |
| Cyclical Durability | >95% | <95% | <95% | |
| Temperature Adaptability | −40–100 °C | RC | RC | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Li, J.; Zhao, C.; Zhao, F. Research on the Preparation of Supercapacitor Separators with High Wettability and Excellent Temperature Adaptability Through In Situ Deposition of Nano-Barium Sulfate on Regenerated Cellulose. Polymers 2025, 17, 842. https://doi.org/10.3390/polym17070842
Li H, Li J, Zhao C, Zhao F. Research on the Preparation of Supercapacitor Separators with High Wettability and Excellent Temperature Adaptability Through In Situ Deposition of Nano-Barium Sulfate on Regenerated Cellulose. Polymers. 2025; 17(7):842. https://doi.org/10.3390/polym17070842
Chicago/Turabian StyleLi, Hui, Jiehua Li, Chuanshan Zhao, and Fenfen Zhao. 2025. "Research on the Preparation of Supercapacitor Separators with High Wettability and Excellent Temperature Adaptability Through In Situ Deposition of Nano-Barium Sulfate on Regenerated Cellulose" Polymers 17, no. 7: 842. https://doi.org/10.3390/polym17070842
APA StyleLi, H., Li, J., Zhao, C., & Zhao, F. (2025). Research on the Preparation of Supercapacitor Separators with High Wettability and Excellent Temperature Adaptability Through In Situ Deposition of Nano-Barium Sulfate on Regenerated Cellulose. Polymers, 17(7), 842. https://doi.org/10.3390/polym17070842





