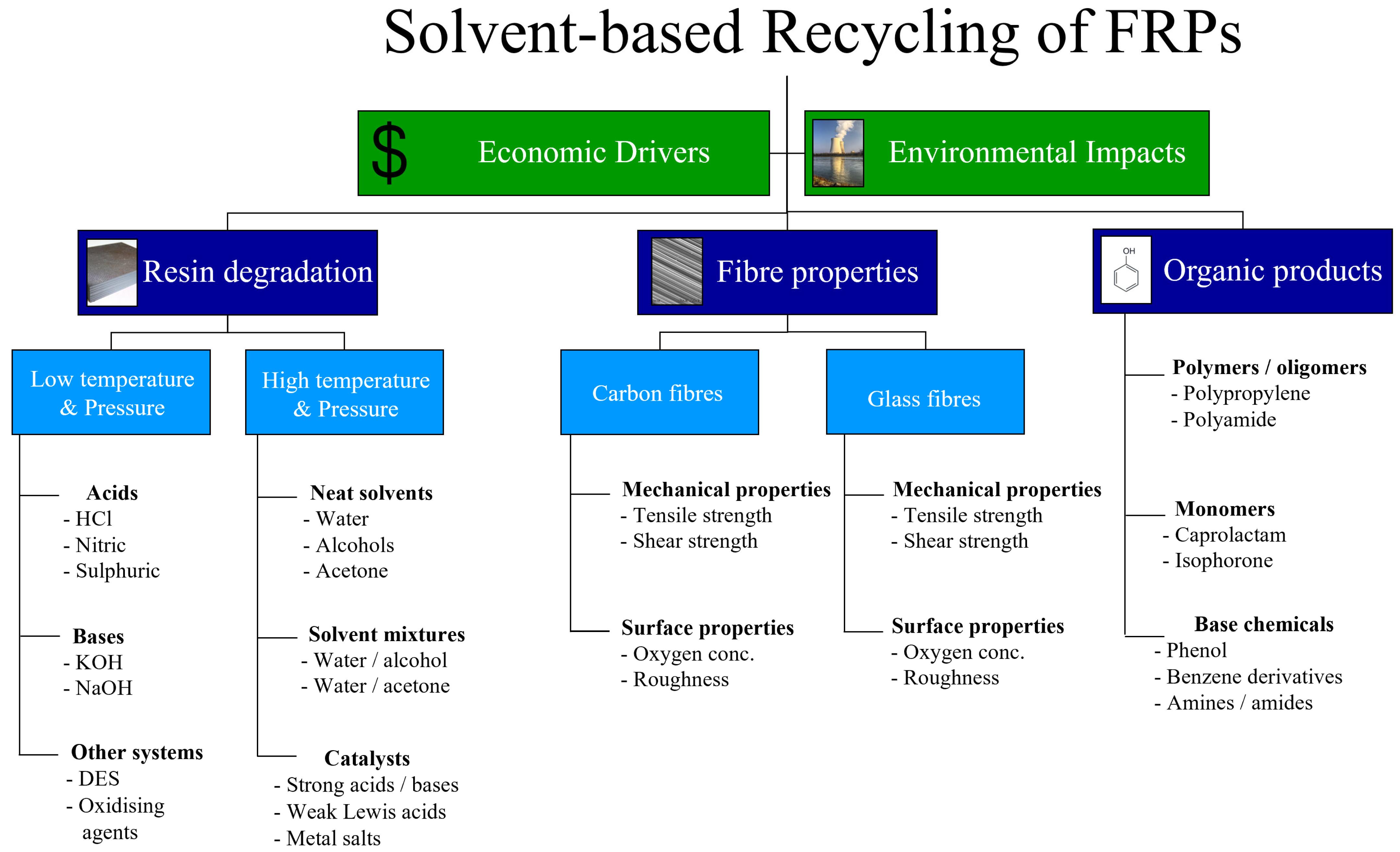
Solvent-Based Recycling as a Waste Management Strategy for Fibre-Reinforced Polymers: Current State of the Art
Abstract
:1. Introduction
2. Why Recycle Fibre-Reinforced Polymers?
2.1. Economic Case
2.2. Environmental Impact
3. Low Temperature and Pressure (LTP) Processes
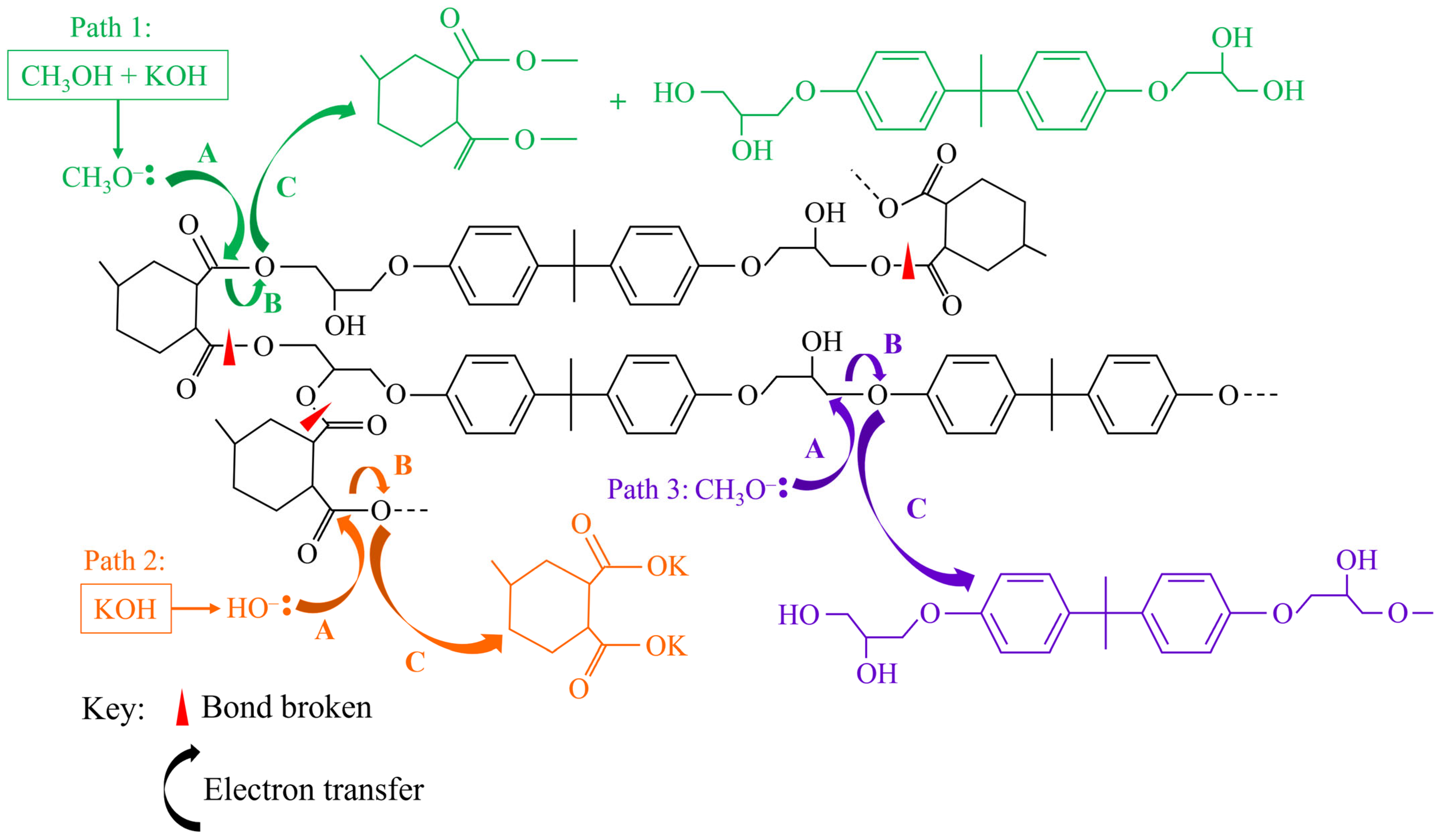
3.1. Acidic Media
3.2. Basic Media
3.3. Other Reaction Systems
4. High Temperature and Pressure (HTP) Processes
4.1. Supercritical Fluids
4.2. Solvents and Solvent Mixtures
4.3. Catalysed Reaction Systems
5. Fibre Properties
5.1. Glass Fibres
5.1.1. LTP Processes
5.1.2. HTP Processes
5.2. Carbon Fibres
5.2.1. LTP Processes
5.2.2. HTP Processes
5.3. Fibre Resizing
6. Organic Products
6.1. Products from Thermosets
6.1.1. LTP Processes
6.1.2. HTP Processes
| Polymer Matrix | Solvent/Reactant | Process Conditions | Products | Reference |
|---|---|---|---|---|
| DGEBA cured with BAC | 4 M nitric acid | 80 °C | Picric acid Low-molecular weight organics | [65] |
| TGDDM cured with DDS | 20 wt.% ZnCl2 in ethanol | 200 °C, several hours | Benzene derivatives with methyl/amine groups | [80] |
| DGEBA cured with DMDC | 10 wt.% AlCl3 in acetic acid | 80 °C, 1.5 h | Benzene derivatives Possible chlorocarbons | [151] |
| Unsaturated polyester | p-TSA and acetic acid | 180 °C, 12 h | Styrene–maleic anhydride (SMA) copolymer Ethylene glycol diacetate (EGDA), Phthalic acid | [69] |
| Vinyl ester cured with DMA and DPO | Propanol with NaOH or KOH | 160 to 240 °C, 60 to 180 min | Isophorone Alklylated aromatics | [153] |
| RTM6 epoxy | Acetone/water (80:20 v/v) | 320 to 360 °C, 15 to 120 min | Alkylated benzene derivatives Short ketones | [124] |
| Anhydride-cured BPA | Methanol | 270 °C, 90 min | BPA-type compounds | [110] |
| Unsaturated polyester | Water | 380 °C, 5 min | Phthalic acid Styrene Styrene derivatives | [156] |
| Unsaturated polyester | Water | 300 °C, 30 min | Benzoic acid Phenyl acetaldehyde Acetone Ethylene glycol Propylene glycol | [117] |
| Unsaturated polyester | Benzyl alcohol/K3PO4 | 300 °C, 240 min | Benzaldehyde Benzoic acid Phenyl ethyl alcohol Styrene derivatives | [158] |
| Unsaturated polyester | Ethanol | 245 °C, 30 min | Diethyl phthalate Diethyl terephthalate Diethyl fumarate Ethyl benzoate | [157] |
| Unsaturated polyester | Propanol | 265 °C, 30 min | Dipropyl phthalate Dipropyl ester of phthalic acid Dipropyl ester of fumaric acid Propyl benzoate | [157] |
6.2. Products from Thermoplastics
6.2.1. LTP Processes
6.2.2. HTP Processes
| Polymer Matrix | Solvent/Reactant | Process Conditions | Products | Reference |
|---|---|---|---|---|
| Polyamide-6 | Water | 280 to 500 °C, 60 to 10 min | ɛ-caprolactam ɛ-aminocaproic acid | [109] |
| Polyamide-6 | Water | 300 to 400 °C, 20 to 35 MPa, 60 to 5 min | ɛ-caprolactam ɛ-aminocaproic acid | [108] |
| Polyamide-6 | Methanol | 370 °C, 39 MPa, time not given | ɛ-caprolactam N-methyl caprolactam | [115] |
| Polyamide-6 | Propanol | 370 °C, 22 MPa, time not given | ɛ-caprolactam | [115] |
| PEEK | Water/ethanol (50:50 v/v), Cs2CO3 catalyst | 350 °C, 30 min | Phenol Dibenzofuran | [125] |
| PEEK | Water/ethanol (50:50 v/v), Cs2CO3 catalyst | 350 °C, 30 min | Phenol Dibenzofuran | [125] |
| PET | Water | 200 to 250 °C, 1.4 to 2.0 MPa, 180 to 300 min | Terephthalic acid Ethylene glycol | [76] |
| PET | Water | 250 to 400 °C, 5.0 to 24 MPa, 1 to 30 min | Terephthalic acid Benzoic acid 1,4-dioxane Acetaldehyde Isophthalic acid | [165] |
| PET | Methanol | 280 to 310 °C, 30 to 70 min | Dimethyl terephthalate Ethylene glycol | [166] |
6.3. Upgrading and Uses of Organic Products
6.3.1. Manufacturing New Resins
6.3.2. Separation and Upgrading Techniques
6.3.3. Energy Recovery
7. Commercial Solvent-Based Recycling Processes
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bunsell, A.R.; Joannes, S.; Thionnet, A. Fundamentals of Fibre Reinforced Composite Materials, 2nd ed.; CRC Press: Oxford, UK, 2021. [Google Scholar]
- Yao, S.-S.; Jin, F.-L.; Rhee, K.Y.; Hui, D.; Park, S.-J. Recent advances in carbon-fiber-reinforced thermoplastic composites: A review. Compos. B Eng. 2018, 142, 241–250. [Google Scholar] [CrossRef]
- Oliveux, G.; Dandy, L.O.; Leeke, G.A. Current status of recycling of fibre reinforced polymers: Review of technologies, reuse and resulting properties. Prog. Mater. Sci. 2015, 72, 61–99. [Google Scholar] [CrossRef]
- Farinha, C.B.; de Brito, J.; Veiga, R. Assessment of glass fibre reinforced polymer waste reuse as filler in mortars. J. Clean. Prod. 2019, 210, 1579–1594. [Google Scholar] [CrossRef]
- Markets and Markets. CF & CFRP Market by Source (Virgin, Recycled), Precursor (PAN, Pitch, Rayon), Resin (Thermosetting, Thermoplastic), Manufacturing Process, End-Use Industry, and Region—Global Forecast to 2028. Available online: https://www.marketsandmarkets.com/Market-Reports/carbon-fiber-composites-market-416.html (accessed on 3 October 2024).
- Research and Markets. GFRP Composites—Global Strategic Business Report. Available online: https://www.researchandmarkets.com/reports/4805040/gfrp-composites-global-strategic-business-report (accessed on 3 October 2024).
- Mordor Intelligence. Glass Fiber Reinforced Polymer Market—Growth, Trends, COVID-19 Impact, and Forecasts (2024–2029). Available online: https://www.mordorintelligence.com/industry-reports/glass-fiber-reinforced-polymer-gfrp-market (accessed on 3 October 2024).
- Zhang, J.; Chevali, V.S.; Wang, H.; Wang, C.-H. Current status of carbon fibre and carbon fibre composites recycling. Compos. B Eng. 2020, 193, 108053. [Google Scholar] [CrossRef]
- Global News Wire. Fiber-Reinforced Plastic (FRP) Recycling Market—Growth, Trends, COVID-19 Impact, and Forecasts (2022–2027). ReportLinker. Available online: https://www.globenewswire.com/news-release/2022/04/27/2430016/0/en/Fiber-Reinforced-Plastic-FRP-Recycling-Market-Growth-Trends-COVID-19-Impact-and-Forecasts-2022-2027.html (accessed on 3 October 2024).
- Witik, R.A.; Teuscher, R.; Michaud, V.; Ludwig, C.; Månson, J.-A.E. Carbon fibre reinforced composite waste: An environmental assessment of recycling, energy recovery and landfilling. Compos. Part. A Appl. Sci. Manuf. 2013, 49, 89–99. [Google Scholar] [CrossRef]
- Shen, Y.; Apraku, S.E.; Zhu, Y. Recycling and recovery of fiber-reinforced polymer composites for end-of-life wind turbine blade management. Green Chem. 2023, 25, 9644–9658. [Google Scholar] [CrossRef]
- Pegoretti, A. Towards sustainable structural composites: A review on the recycling of continuous-fiber-reinforced thermoplastics. Adv. Ind. Eng. Polym. Res. 2021, 4, 105–115. [Google Scholar] [CrossRef]
- Gen2Carbon. About Gen2Carbon. Available online: https://www.gen2carbon.com/about/ (accessed on 3 October 2024).
- Mitsubishi Chemical Advanced Materials. Find a Greener Path to Carbon Fiber Benefits. Available online: https://www.mcam.com/en/case-studies/recycled-carbon-fiber#:~:text=Solution,-Add%20recycled%20carbon&text=Thanks%20to%20its%20unique%20offering,fiber%2C%20recycling%20unlocks%20new%20potential.&text=%E2%80%9CAs%20a%20part%20of%20the,portfolio%20for%20their%20specific%20applications.%E2%80%9D (accessed on 3 October 2024).
- Carbon Rivers. Carbon Rivers: G2G+. Available online: https://www.carbonrivers.com/copy-of-composites-recycling (accessed on 26 October 2024).
- ICN Bureau. Toray and Toyota Tsusho to Jointly Promote Carbon Fiber Recycling. Available online: https://www.indianchemicalnews.com/petro-chemical/toray-and-toyota-tsusho-to-jointly-promote-carbon-fiber-recycling-4167 (accessed on 3 October 2024).
- Yousef, S.; Eimontas, J.; Stasiulaitiene, I.; Zakarauskas, K.; Striūgas, N. Recovery of energy and carbon fibre from wind turbine blades waste (carbon fibre/unsaturated polyester resin) using pyrolysis process and its life-cycle assessment. Environ. Res. 2024, 245, 118016. [Google Scholar] [CrossRef]
- Nuccetelli, P.; Maisto, F.; Kraková, L.; Grilli, A.; Takáčová, A.; Šišková, A.O.; Pangallo, D. Evaluation of Microbial Degradation of Thermoplastic and Thermosetting Polymers by Environmental Isolates. Coatings 2024, 14, 982. [Google Scholar] [CrossRef]
- BIZENTE. Applying Enzymes to Resolve End-of-Life Issues of Thermoset Composite Plastics; AITIIP: Zaragoza, Spain, 2024; pp. 4–13. Available online: https://bizente.eu/news/get-now-the-bizente-project-laymans-report/ (accessed on 13 March 2025).
- Moeser, K. Enzymatic Degradation of Epoxy Resins: An Approach for the Recycling of Carbon Fiber Reinforced Polymers. Adv. Mat. Res. 2014, 1018, 131–136. [Google Scholar] [CrossRef]
- Liu, J.; Ikura, R.; Yamaoka, K.; Sugawara, A.; Takahashi, Y.; Kure, B.; Takenaka, N.; Park, J.; Uyama, H.; Takashima, Y. Exploring enzymatic degradation, reinforcement, recycling, and upcycling of poly(ester)-poly(urethane) with movable crosslinks. Chem 2025, 11, 102327. [Google Scholar] [CrossRef]
- Teltschik, J.; Matter, J.; Woebbeking, S.; Jahn, K.; Adasme, Y.B.; Van Paepegem, W.; Drechsler, K.; Tallawi, M. Review on recycling of carbon fibre reinforced thermoplastics with a focus on polyetheretherketone. Compos. Part A Appl. Sci. Manuf. 2024, 184, 108236. [Google Scholar] [CrossRef]
- Karuppannan Gopalraj, S.; Kärki, T. A review on the recycling of waste carbon fibre/glass fibre-reinforced composites: Fibre recovery, properties and life-cycle analysis. SN Appl. Sci. 2020, 2, 433. [Google Scholar] [CrossRef]
- Carbon Fiber Gear. Carbon Fiber vs. Aluminum: A Side-by-Side Comparison. Available online: https://carbonfibergear.com/blogs/carbonfiber/carbon-fiber-vs-aluminum#:~:text=Carbon%20Fiber:%20The%20Premium%20Choice,used%20in%20spacecraft%20and%20airplanes (accessed on 3 October 2024).
- Shehab, E.; Meiirbekov, A.; Amantayeva, A.; Suleimen, A.; Tokbolat, S.; Sarfraz, S. A Cost Modelling System for Recycling Carbon Fiber-Reinforced Composites. Polymers 2021, 13, 4208. [Google Scholar] [CrossRef]
- Tapper, R.J.; Longana, M.L.; Norton, A.; Potter, K.D.; Hamerton, I. An evaluation of life cycle assessment and its application to the closed-loop recycling of carbon fibre reinforced polymers. Compos. B Eng. 2020, 184, 107665. [Google Scholar] [CrossRef]
- Ali, B.; Qureshi, L.A.; Khan, S.U. Flexural behavior of glass fiber-reinforced recycled aggregate concrete and its impact on the cost and carbon footprint of concrete pavement. Constr. Build. Mater. 2020, 262, 120820. [Google Scholar] [CrossRef]
- Gardiner, G. The Making of Glass Fiber. Compos. Tech. 2009, 15, 30–35. [Google Scholar]
- European Composites Industry Association. Carbon Fiber LCA Data in EcoCalculator: An Essential Extension; European Composites Industry Association: Stuttgard, Germany, 2018. [Google Scholar]
- Joshi, S.V.; Drzal, L.T.; Mohanty, A.K.; Arora, S. Are natural fiber composites environmentally superior to glass fiber reinforced composites? Compos. Part A Appl. Sci. Manuf. 2004, 35, 371–376. [Google Scholar] [CrossRef]
- Song, Y.S.; Youn, J.R.; Gutowski, T.G. Life cycle energy analysis of fiber-reinforced composites. Compos. Part A Appl. Sci. Manuf. 2009, 40, 1257–1265. [Google Scholar] [CrossRef]
- Trading Economics. Aluminium. Available online: https://tradingeconomics.com/commodity/aluminum (accessed on 3 October 2024).
- Tempelman, E. Multi-parametric study of the effect of materials substitution on life cycle energy use and waste generation of passenger car structures. Transp. Res. D Transp. Environ. 2011, 16, 479–485. [Google Scholar] [CrossRef]
- Ingarao, G.; Deng, Y.; Marino, R.; Di Lorenzo, R.; Franco, A.L. Energy and CO2 life cycle inventory issues for aluminum based components: The case study of a high speed train window panel. J. Clean. Prod. 2016, 126, 493–503. [Google Scholar] [CrossRef]
- Trading Economics. Steel. Available online: https://tradingeconomics.com/commodity/steel (accessed on 3 October 2024).
- Hammond, G.P.; Jones, C.I. Embodied energy and carbon in construction materials. Proc. Inst. Civ. Eng.—Energy 2008, 161, 87–98. [Google Scholar] [CrossRef]
- Trading Economics. Copper. 2024. Available online: https://tradingeconomics.com/commodity/copper (accessed on 3 October 2024).
- Moreno-Leiva, S.; Díaz-Ferrán, G.; Haas, J.; Telsnig, T.; Díaz-Alvarado, F.A.; Palma-Behnke, R.; Kracht, W.; Román, R.; Chudinzow, D.; Eltrop, L. Towards solar power supply for copper production in Chile: Assessment of global warming potential using a life-cycle approach. J. Clean. Prod. 2017, 164, 242–249. [Google Scholar] [CrossRef]
- Röben, F.T.C.; Liu, D.; Reuter, M.A.; Dahmen, M.; Bardow, A. The demand response potential in copper production. J. Clean. Prod. 2022, 362, 132221. [Google Scholar] [CrossRef]
- Grand View Research. Carbon Fiber Reinforced Plastic Market Size, Share & Trends Analysis Report by Raw Material, by Product (Thermosetting, Thermoplastic), by Application, and Segment Forecasts, 2018–2025; Grand View Research: San Francisco, CA, USA, 2022. [Google Scholar]
- Pakdel, E.; Kashi, S.; Varley, R.; Wang, X. Recent progress in recycling carbon fibre reinforced composites and dry carbon fibre wastes. Resour. Conserv. Recycl. 2021, 166, 105340. [Google Scholar] [CrossRef]
- The Senate of the United States. S. 1432: Carbon Fiber Recycling Act of 2015; The Senate of the United States: Washington, DC, USA, 2015. [Google Scholar]
- Confederation of Europe Waste-to-Energy Plants. Landfill Taxes and Restrictions; Confederation of Europe Waste-to-Energy Plants: Dusseldorf, Germany, 2021. [Google Scholar]
- UK Government. Landfill Tax. Environmental Taxes, Reliefs and Schemes for Businesses. Available online: https://www.gov.uk/green-taxes-and-reliefs/landfill-tax (accessed on 4 October 2024).
- Stonell, L. Landfill Tax Raised to £126.15 from 2025/2026. Let’sRecycle. Available online: https://www.letsrecycle.com/news/landfill-tax-to-jump-21-6-to-126-15-from-2025-26/#:~:text=Today’s%20budget%20from%20the%20chancellor,to%20%C2%A34.05%20per%20tonne.&text=The%20move%20was%20confirmed%20in,%C2%A3400m%20over%20three%20years (accessed on 4 October 2024).
- Statista. Average Cost to Landfill Municipal Solid Waste in the United States in 2022 and 2023, by Region. WasteManagement. Available online: https://www.statista.com/statistics/692063/cost-to-landfill-municipal-solid-waste-by-us-region/#:~:text=Price%20of%20landfilling%20municipal%20waste,U.S.%202022%2D2023%2C%20by%20region&text=The%20average%20municipal%20solid%20waste,Environmental%20Protection%20Agency%20(EPA) (accessed on 4 October 2024).
- Vo Dong, P.A.; Azzaro-Pantel, C.; Cadene, A.-L. Economic and environmental assessment of recovery and disposal pathways for CFRP waste management. Resour. Conserv. Recycl. 2018, 133, 63–75. [Google Scholar] [CrossRef]
- Keith, M.J.; Román-Ramírez, L.A.; Leeke, G.; Ingram, A. Recycling a carbon fibre reinforced polymer with a supercritical acetone/water solvent mixture: Comprehensive analysis of reaction kinetics. Polym. Degrad. Stab. 2019, 161, 225–234. [Google Scholar] [CrossRef]
- Merck. 2-Methoxy Furan. Available online: https://www.sigmaaldrich.com/GB/en/product/aldrich/138274 (accessed on 4 October 2024).
- Merck. 1,2,3-Trimethyl Benzene. Available online: https://www.sigmaaldrich.com/GB/en/product/sial/45935 (accessed on 4 October 2024).
- Merck. 2-Methyl 2-Hexanol. Available online: https://www.sigmaaldrich.com/GB/en/product/aldrich/111600 (accessed on 4 October 2024).
- Merck. Phenol. Available online: https://www.sigmaaldrich.com/GB/en/product/sial/33517 (accessed on 4 October 2024).
- Merck. 4-Ethyl Phenol. Available online: https://www.sigmaaldrich.com/GB/en/product/aldrich/e44205 (accessed on 4 October 2024).
- Howarth, J.; Mareddy, S.S.R.; Mativenga, P.T. Energy intensity and environmental analysis of mechanical recycling of carbon fibre composite. J. Clean. Prod. 2014, 81, 46–50. [Google Scholar] [CrossRef]
- Khalil, Y.F. Comparative environmental and human health evaluations of thermolysis and solvolysis recycling technologies of carbon fiber reinforced polymer waste. Waste Manag. 2018, 76, 767–778. [Google Scholar] [CrossRef]
- Lefeuvre, A.; Yerro, X.; Jean-Marie, A.; Dong, A.V.; Azzaro-Pantel, C. Modelling pyrolysis process for CFRP recycling in a closed-loop supply chain approach. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 2029–2034. [Google Scholar] [CrossRef]
- Meng, F.; McKechnie, J.; Turner, T.A.; Pickering, S.J. Energy and environmental assessment and reuse of fluidised bed recycled carbon fibres. Compos. Part A Appl. Sci. Manuf. 2017, 100, 206–214. [Google Scholar] [CrossRef]
- Dauguet, M.; Mantaux, O.; Perry, N.; Zhao, Y.F. Recycling of CFRP for High Value Applications: Effect of Sizing Removal and Environmental Analysis of the SuperCritical Fluid Solvolysis. Procedia CIRP 2015, 29, 734–739. [Google Scholar] [CrossRef]
- Pillain, B.; Loubet, P.; Pestalozzi, F.; Woidasky, J.; Erriguible, A.; Aymonier, C.; Sonnemann, G. Positioning supercritical solvolysis among innovative recycling and current waste management scenarios for carbon fiber reinforced plastics thanks to comparative life cycle assessment. J. Supercrit. Fluids 2019, 154, 104607. [Google Scholar] [CrossRef]
- Kawajiri, K.; Sakamoto, K. Environmental impact of carbon fibers fabricated by an innovative manufacturing process on life cycle greenhouse gas emissions. Sustain. Mater. Technol. 2022, 31, e00365. [Google Scholar] [CrossRef]
- Meng, F.; Cui, Y.; Pickering, S.; McKechnie, J. From aviation to aviation: Environmental and financial viability of closed-loop recycling of carbon fibre composite. Compos. B Eng. 2020, 200, 108362. [Google Scholar] [CrossRef]
- La Rosa, A.; Blanco, I.; Banatao, D.; Pastine, S.; Björklund, A.; Cicala, G. Innovative Chemical Process for Recycling Thermosets Cured with Recyclamines® by Converting Bio-Epoxy Composites in Reusable Thermoplastic—An LCA Study. Materials 2018, 11, 353. [Google Scholar] [CrossRef]
- Dang, W.; Kubouchi, M.; Yamamoto, S.; Sembokuya, H.; Tsuda, K. An approach to chemical recycling of epoxy resin cured with amine using nitric acid. Polymer 2002, 43, 2953–2958. [Google Scholar] [CrossRef]
- Dang, W.; Kubouchi, M.; Sembokuya, H.; Tsuda, K. Chemical recycling of glass fiber reinforced epoxy resin cured with amine using nitric acid. Polymer 2005, 46, 1905–1912. [Google Scholar] [CrossRef]
- Hanaoka, T.; Arao, Y.; Kayaki, Y.; Kuwata, S.; Kubouchi, M. Analysis of nitric acid decomposition of epoxy resin network structures for chemical recycling. Polym. Degrad. Stab. 2021, 186, 109537. [Google Scholar] [CrossRef]
- Feraboli, P.; Kawakami, H.; Wade, B.; Gasco, F.; DeOto, L.; Masini, A. Recyclability and reutilization of carbon fiber fabric/epoxy composites. J. Compos. Mater. 2012, 46, 1459–1473. [Google Scholar] [CrossRef]
- Xu, P.; Li, J.; Ding, J. Chemical recycling of carbon fibre/epoxy composites in a mixed solution of peroxide hydrogen and N,N-dimethylformamide. Compos. Sci. Technol. 2013, 82, 54–59. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, X.; Ge, H.; Yang, Y.; Wang, Y.; Zhang, C.; Li, J.; Deng, T.; Qin, Z.; Hou, X. Chemical Recycling of Carbon Fiber Reinforced Epoxy Resin Composites via Selective Cleavage of the Carbon–Nitrogen Bond. ACS Sustain. Chem. Eng. 2015, 3, 3332–3337. [Google Scholar] [CrossRef]
- Zhang, N.; Hou, X.; Cui, X.; Chai, L.; Li, H.; Zhang, H.; Wang, Y.; Deng, T. Amphiphilic catalyst for decomposition of unsaturated polyester resins to valuable chemicals with 100% atom utilization efficiency. J. Clean. Prod. 2021, 296, 126492. [Google Scholar] [CrossRef]
- Češarek, U.; Pahovnik, D.; Žagar, E. Chemical Recycling of Aliphatic Polyamides by Microwave-Assisted Hydrolysis for Efficient Monomer Recovery. ACS Sustain. Chem. Eng. 2020, 8, 16274–16282. [Google Scholar] [CrossRef]
- Shukla, S.R.; Harad, A.M.; Mahato, D. Depolymerization of nylon 6 waste fibers. J. Appl. Polym. Sci. 2006, 100, 186–190. [Google Scholar] [CrossRef]
- Hocker, S.; Rhudy, A.K.; Ginsburg, G.; Kranbuehl, D.E. Polyamide hydrolysis accelerated by small weak organic acids. Polymer 2014, 55, 5057–5064. [Google Scholar] [CrossRef]
- Yang, P.; Zhou, Q.; Li, X.-Y.; Yang, K.-K.; Wang, Y.-Z. Chemical recycling of fiber-reinforced epoxy resin using a polyethylene glycol/NaOH system. J. Reinf. Plast. Compos. 2014, 33, 2106–2114. [Google Scholar] [CrossRef]
- Jiang, J.; Deng, G.; Chen, X.; Gao, X.; Guo, Q.; Xu, C.; Zhou, L. On the successful chemical recycling of carbon fiber/epoxy resin composites under the mild condition. Compos. Sci. Technol. 2017, 151, 243–251. [Google Scholar] [CrossRef]
- Zhao, Q.; An, L.; Li, C.; Zhang, L.; Jiang, J.; Li, Y. Environment-friendly recycling of CFRP composites via gentle solvent system at atmospheric pressure. Compos. Sci. Technol. 2022, 224, 109461. [Google Scholar] [CrossRef]
- Sinha, V.; Patel, M.R.; Patel, J.V. PET Waste Management by Chemical Recycling: A Review. J. Polym. Environ. 2010, 18, 8–25. [Google Scholar] [CrossRef]
- Rubio Arias, J.J.; Thielemans, W. Efficient Depolymerization of Glass Fiber Reinforced PET Composites. Polymers 2022, 14, 5171. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X.-L.; Tian, F.; An, W.-L.; Xu, S.; Wang, Y.-Z. A fast and mild closed-loop recycling of anhydride-cured epoxy through microwave-assisted catalytic degradation by trifunctional amine and subsequent reuse without separation. Green Chem. 2019, 21, 2487–2493. [Google Scholar] [CrossRef]
- Deng, T.; Liu, Y.; Cui, X.; Yang, Y.; Jia, S.; Wang, Y.; Lu, C.; Li, D.; Cai, R.; Hou, X. Cleavage of C–N bonds in carbon fiber/epoxy resin composites. Green Chem. 2015, 17, 2141–2145. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, M.; Guo, X.; Liu, C.; Liu, T.; Xin, J.; Zhang, J. Mild chemical recycling of aerospace fiber/epoxy composite wastes and utilization of the decomposed resin. Polym. Degrad. Stab. 2017, 139, 20–27. [Google Scholar] [CrossRef]
- Liu, C.-W.; Hong, W.-J.; Yang, B.-T.; Lin, C.-W.; Wang, L.-C.; Chen, C.-C. Switchable deep eutectic solvents as efficient and sustainable recycling media for carbon fiber reinforced polymer composite waste. J. Clean. Prod. 2022, 378, 134334. [Google Scholar] [CrossRef]
- Ye, L.; Wang, K.; Feng, H.; Wang, Y. Recycling of Carbon Fiber-reinforced Epoxy Resin-based Composites Using a Benzyl Alcohol/Alkaline System. Fibers Polym. 2021, 22, 811–818. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, X.; Feng, K.; An, W.; Tian, F.; Du, R.; Xu, S.; Chen, L.; Wu, G.; Wang, Y. Multicycling of Epoxy Thermoset Through a Two-Step Strategy of Alcoholysis and Hydrolysis using a Self-Separating Catalysis System. ChemSusChem 2022, 15, e202101607. [Google Scholar] [CrossRef]
- Wang, X.-L.; An, W.-L.; Tian, F.; Yang, Y.; Zhao, X.; Xu, P.-P.; Xu, S.; Wang, Y.-Z. Recycling waste thermosetting unsaturated polyester resins into oligomers for preparing amphiphilic aerogels. Waste Manag. 2021, 126, 89–96. [Google Scholar] [CrossRef]
- Tapper, R.J.; Longana, M.L.; Yu, H.; Hamerton, I.; Potter, K.D. Development of a closed-loop recycling process for discontinuous carbon fibre polypropylene composites. Compos. B Eng. 2018, 146, 222–231. [Google Scholar] [CrossRef]
- Poulakis, J.G.; Varelidis, C.; Papaspyrides, C.D. Recycling of polypropylene-based composites. Adv. Polym. Technol. 1997, 16, 313–322. [Google Scholar] [CrossRef]
- Tapper, R.J.; Longana, M.L.; Hamerton, I.; Potter, K.D. A closed-loop recycling process for discontinuous carbon fibre polyamide 6 composites. Compos. B Eng. 2019, 179, 107418. [Google Scholar] [CrossRef]
- Knappich, F.; Klotz, M.; Schlummer, M.; Wölling, J.; Mäurer, A. Recycling process for carbon fiber reinforced plastics with polyamide 6, polyurethane and epoxy matrix by gentle solvent treatment. Waste Manag. 2019, 85, 73–81. [Google Scholar] [CrossRef]
- Cousins, D.S.; Suzuki, Y.; Murray, R.E.; Samaniuk, J.R.; Stebner, A.P. Recycling glass fiber thermoplastic composites from wind turbine blades. J. Clean. Prod. 2019, 209, 1252–1263. [Google Scholar] [CrossRef]
- Tschentscher, C.; Gebhardt, M.; Chakraborty, S.; Meiners, D. Recycling of Elium CFRPs for high temperature dissolution: A study with different solvents. In Proceedings of the Materialtechnik Symposium, Clausthal-Zellerfeld, Germany, 25–26 February 2021; pp. 1–12. [Google Scholar]
- Liu, Y.; Meng, L.; Huang, Y.; Du, J. Recycling of carbon/epoxy composites. J. Appl. Polym. Sci. 2004, 94, 1912–1916. [Google Scholar] [CrossRef]
- Lee, S.-H.; Choi, H.-O.; Kim, J.-S.; Lee, C.-K.; Kim, Y.-K.; Ju, C.-S. Circulating flow reactor for recycling of carbon fiber from carbon fiber reinforced epoxy composite. Korean J. Chem. Eng. 2011, 28, 449–454. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Hashimoto, T.; Kakichi, Y.; Urushisaki, M.; Sakaguchi, T.; Kawabe, K.; Kondo, K.; Iyo, H. Recyclable carbon fiber-reinforced plastics containing degradable acetal linkages: Synthesis, properties, and chemical recycling. J. Polym. Sci. A Polym. Chem. 2015, 53, 1052–1059. [Google Scholar] [CrossRef]
- Li, J.; Xu, P.-L.; Zhu, Y.-K.; Ding, J.-P.; Xue, L.-X.; Wang, Y.-Z. A promising strategy for chemical recycling of carbon fiber/thermoset composites: Self-accelerating decomposition in a mild oxidative system. Green Chem. 2012, 14, 3260. [Google Scholar] [CrossRef]
- Das, M.; Chacko, R.; Varughese, S. An Efficient Method of Recycling of CFRP Waste Using Peracetic Acid. ACS Sustain. Chem. Eng. 2018, 6, 1564–1571. [Google Scholar] [CrossRef]
- Borjan, D.; Knez, Ž.; Knez, M. Recycling of Carbon Fiber-Reinforced Composites—Difficulties and Future Perspectives. Materials 2021, 14, 4191. [Google Scholar] [CrossRef]
- Brunner, G. Applications of Supercritical Fluids. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 321–342. [Google Scholar] [CrossRef]
- Machida, H.; Takesue, M.; Smith, R.L. Green chemical processes with supercritical fluids: Properties, materials, separations and energy. J. Supercrit. Fluids 2011, 60, 2–15. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Z.; Feng, L. Chemical recycling of carbon fibers reinforced epoxy resin composites in oxygen in supercritical water. Mater. Des. 2010, 31, 999–1002. [Google Scholar] [CrossRef]
- Sato, H.; Watanabe, K.; Sengers, J.M.H.L.; Gallagher, J.S.; Hill, P.G.; Straub, J.; Wagner, W. Sixteen Thousand Evaluated Experimental Thermodynamic Property Data for Water and Steam. J. Phys. Chem. Ref. Data 1991, 20, 1023–1044. [Google Scholar] [CrossRef]
- Piñero-Hernanz, R.; Dodds, C.; Hyde, J.; García-Serna, J.; Poliakoff, M.; Lester, E.; Cocero, M.J.; Kingman, S.; Pickering, S.; Wong, K.H. Chemical recycling of carbon fibre reinforced composites in nearcritical and supercritical water. Compos. Part A Appl. Sci. Manuf. 2008, 39, 454–461. [Google Scholar] [CrossRef]
- Kim, Y.N.; Kim, S.Y.; Park, M.; Yang, B.; Kim, J.; Jung, Y.C. Application of supercritical water for green recycling of epoxy-based carbon fiber reinforced plastic. Compos. Sci. Technol. 2019, 173, 66–72. [Google Scholar] [CrossRef]
- Knight, C.C.; Zeng, C.; Zhang, C.; Liang, R. Fabrication and properties of composites utilizing reclaimed woven carbon fiber by sub-critical and supercritical water recycling. Mater. Chem. Phys. 2015, 150, 317–323. [Google Scholar] [CrossRef]
- Prinçaud, M.; Aymonier, C.; Loppinet-Serani, A.; Perry, N.; Sonnemann, G. Environmental Feasibility of the Recycling of Carbon Fibers from CFRPs by Solvolysis Using Supercritical Water. ACS Sustain. Chem. Eng. 2014, 2, 1498–1502. [Google Scholar] [CrossRef]
- Oliveux, G.; Bailleul, J.L.; La Salle, E.L.G.; Lefèvre, N.; Biotteau, G. Recycling of glass fibre reinforced composites using subcritical hydrolysis: Reaction mechanisms and kinetics, influence of the chemical structure of the resin. Polym. Degrad. Stab. 2013, 98, 785–800. [Google Scholar] [CrossRef]
- Henry, L.; Schneller, A.; Doerfler, J.; Mueller, W.M.; Aymonier, C.; Horn, S. Semi-continuous flow recycling method for carbon fibre reinforced thermoset polymers by near- and supercritical solvolysis. Polym. Degrad. Stab. 2016, 133, 264–274. [Google Scholar] [CrossRef]
- Wang, W.; Meng, L.; Huang, Y. Hydrolytic degradation of monomer casting nylon in subcritical water. Polym. Degrad. Stab. 2014, 110, 312–317. [Google Scholar] [CrossRef]
- Iwaya, T.; Sasaki, M.; Goto, M. Kinetic analysis for hydrothermal depolymerization of nylon 6. Polym. Degrad. Stab. 2006, 91, 1989–1995. [Google Scholar] [CrossRef]
- Chaabani, C.; Weiss-Hortala, E.; Soudais, Y. Impact of Solvolysis Process on Both Depolymerization Kinetics of Nylon 6 and Recycling Carbon Fibers from Waste Composite. Waste Biomass Valorization 2017, 8, 2853–2865. [Google Scholar] [CrossRef]
- Okajima, I.; Hiramatsu, M.; Shimamura, Y.; Awaya, T.; Sako, T. Chemical recycling of carbon fiber reinforced plastic using supercritical methanol. J. Supercrit. Fluids 2014, 91, 68–76. [Google Scholar] [CrossRef]
- Piñero-Hernanz, R.; García-Serna, J.; Dodds, C.; Hyde, J.; Poliakoff, M.; Cocero, M.J.; Kingman, S.; Pickering, S.; Lester, E. Chemical recycling of carbon fibre composites using alcohols under subcritical and supercritical conditions. J. Supercrit. Fluids 2008, 46, 83–92. [Google Scholar] [CrossRef]
- Okajima, I.; Sako, T. Recycling fiber-reinforced plastic using supercritical acetone. Polym. Degrad. Stab. 2019, 163, 1–6. [Google Scholar] [CrossRef]
- Sokoli, H.U.; Beauson, J.; Simonsen, M.E.; Fraisse, A.; Brøndsted; Søgaard, E.G. Optimized process for recovery of glass- and carbon fibers with retained mechanical properties by means of near- and supercritical fluids. J. Supercrit. Fluids 2017, 124, 80–89. [Google Scholar] [CrossRef]
- Okajima, I.; Watanabe, K.; Haramiishi, S.; Nakamura, M.; Shimamura, Y.; Sako, T. Recycling of carbon fiber reinforced plastic containing amine-cured epoxy resin using supercritical and subcritical fluids. J. Supercrit. Fluids 2017, 119, 44–51. [Google Scholar] [CrossRef]
- Kaweetirawatt, T.; Yamaguchi, T.; Hayashiyama, S.; Sumimoto, M.; Kamimura, A.; Hori, K. Nylon 6 depolymerization in supercritical alcohols studied by the QM/MC/FEP method. RSC Adv. 2012, 2, 8402. [Google Scholar] [CrossRef]
- Yuyan, L.; Guohua, S.; Linghui, M. Recycling of carbon fibre reinforced composites using water in subcritical conditions. Mater. Sci. Eng. A 2009, 520, 179–183. [Google Scholar] [CrossRef]
- Oliveux, G.; Bailleul, J.-L.; La Salle, E.L.G. Chemical recycling of glass fibre reinforced composites using subcritical water. Compos. Part A Appl. Sci. Manuf. 2012, 43, 1809–1818. [Google Scholar] [CrossRef]
- Liu, T.; Shao, L.; Zhao, B.; Chang, Y.; Zhang, J. Progress in Chemical Recycling of Carbon Fiber Reinforced Epoxy Composites. Macromol. Rapid Commun. 2022, 43, e2200538. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Jiang, Z.; Tang, T. Chemical recycling of carbon fibre reinforced epoxy resin composites in subcritical water: Synergistic effect of phenol and KOH on the decomposition efficiency. Polym. Degrad. Stab. 2012, 97, 214–220. [Google Scholar] [CrossRef]
- Minor, A.-J.; Goldhahn, R.; Rihko-Struckmann, L.; Sundmacher, K. Chemical Recycling Processes of Nylon 6 to Caprolactam: Review and Techno-Economic Assessment. Chem. Eng. J. 2023, 474, 145333. [Google Scholar] [CrossRef]
- Liu, J.; Wang, K.; Ma, L.; Tang, T. Insight into the role of potassium hydroxide for accelerating the degradation of anhydride-cured epoxy resin in subcritical methanol. J. Supercrit. Fluids 2016, 107, 605–611. [Google Scholar] [CrossRef]
- Yan, H.; Lu, C.; Jing, D.; Hou, X. Chemical degradation of amine-cured DGEBA epoxy resin in supercritical 1-propanol for recycling carbon fiber from composites. Chin. J. Polym. Sci. 2014, 32, 1550–1563. [Google Scholar] [CrossRef]
- Keith, M.J.; Leeke, G.A.; Khan; Ingram, A. Catalytic degradation of a carbon fibre reinforced polymer for recycling applications. Polym. Degrad. Stab. 2019, 166, 188–201. [Google Scholar] [CrossRef]
- Keith, M. Recycling High Performance Carbon Fibre Reinforced Polymers Using Sub- and Supercritical Fluids. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2019. Available online: https://etheses.bham.ac.uk//id/eprint/10111/ (accessed on 12 November 2024).
- Dandy, L.O.; Oliveux, G.; Wood, J.; Jenkins, M.J.; Leeke, G.A. Accelerated degradation of Polyetheretherketone (PEEK) composite materials for recycling applications. Polym. Degrad. Stab. 2015, 112, 52–62. [Google Scholar] [CrossRef]
- Dandy, L. Supercritical Fluids and Their Application to the Recycling of High-Performance Carbon Fibre Reinforced Composite Materials. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2015. Available online: http://etheses.bham.ac.uk/5896/ (accessed on 12 November 2024).
- Ismail, K.I.; Yap, T.C.; Ahmed, R. 3D-Printed Fiber-Reinforced Polymer Composites by Fused Deposition Modelling (FDM): Fiber Length and Fiber Implementation Techniques. Polymers 2022, 14, 4659. [Google Scholar] [CrossRef]
- Longana, M.L.; Ong, N.; Yu, H.; Potter, K.D. Multiple closed loop recycling of carbon fibre composites with the HiPerDiF (High Performance Discontinuous Fibre) method. Compos. Struct. 2016, 153, 271–277. [Google Scholar] [CrossRef]
- Zweben, C.H. Encyclopedia of Condensed Matter Physics. In Encyclopedia of Condensed Matter Physics; Bassani, F., Liedl, G.L., Wyder, P., Eds.; Elsevier: Devon, PA, USA, 2005; Charter Composites: Overview; pp. 192–208. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, L.; Huang, Y.; Liu, L. Method of Recovering the Fibrous Fraction of Glass/Epoxy Composites. J. Reinf. Plast. Compos. 2006, 25, 1525–1533. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, X.; Yang, Q.; Deng, T.; Wang, Y.; Yang, Y.; Jia, S.; Qin, Z.; Hou, X. Chemical recycling of unsaturated polyester resin and its composites via selective cleavage of the ester bond. Green Chem. 2015, 17, 4527–4532. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, P.; Yue, J.; Huan, H.; Bi, G.; Zhang, L. Recycling glass fibers from thermoset epoxy composites by in situ oxonium-type polyionic liquid formation and naphthalene-containing superplasticizer synthesis with the degradation solution of the epoxy resin. Compos. B Eng. 2023, 254, 110435. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Kim, K.-J. MEG Effects on Hydrolysis of Polyamide 66/Glass Fiber Composites and Mechanical Property Changes. Molecules 2019, 24, 755. [Google Scholar] [CrossRef]
- Kamimura, A.; Yamada, K.; Kuratani, T.; Oishi, Y.; Watanabe, T.; Yoshida, T.; Tomonaga, F. DMAP as an Effective Catalyst To Accelerate the Solubilization of Waste Fiber-Reinforced Plastics. ChemSusChem 2008, 1, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.C.; Ghita, O.R.; Hallam, K.R.; Heard, J.; Evans, K.E. Mechanical studies of single glass fibres recycled from hydrolysis process using sub-critical water. Compos. Part A Appl. Sci. Manuf. 2012, 43, 398–406. [Google Scholar] [CrossRef]
- Souza, P.R.; Nunes, C.S.; Freitas, A.R.; Belinato, J.R.; Pilau, E.J.; Fajardo, A.R.; Da Silva Filho, E.A.; Schreiner, W.H.; Muniz, E.C. Sub- and supercritical D-limonene technology as a green process to recover glass fibres from glass fibre-reinforced polyester composites. J. Clean. Prod. 2020, 254, 119984. [Google Scholar] [CrossRef]
- Qiu, Q.; Kumosa, M. Corrosion of E-glass fibers in acidic environments. Compos. Sci. Technol. 1997, 57, 497–507. [Google Scholar] [CrossRef]
- Feng, H.; Xu, X.; Wang, B.; Su, Y.; Liu, Y.; Zhang, C.; Zhu, J.; Ma, S. Facile preparation, closed-loop recycling of multifunctional carbon fiber reinforced polymer composites. Compos. B Eng. 2023, 257, 110677. [Google Scholar] [CrossRef]
- Muhammad Faisal, M.F.; Hassan, A.; Gan, K.W.; Roslan, M.N.; Rashid, A.H.A. Effects of Sulphuric Acid Concentrations during Solvolysis Process of Carbon Fiber Reinforced Epoxy Composite. Sains Malays. 2020, 49, 2073–2081. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, X.; Tian, Z.; Ma, H.; Hou, X.; Wang, Y.; Wang, Y. Controlling degradation and recycling of carbon fiber reinforced bismaleimide resin composites via selective cleavage of imide bonds. Compos. B Eng. 2022, 231, 109595. [Google Scholar] [CrossRef]
- Zabihi, O.; Ahmadi, M.; Liu, C.; Mahmoodi, R.; Li, Q.; Naebe, M. Development of a low cost and green microwave assisted approach towards the circular carbon fibre composites. Compos. B Eng. 2020, 184, 107750. [Google Scholar] [CrossRef]
- Kim, D.H.; Yu, A.; Goh, M. Oxidative chemical depolymerization of thermoset epoxy resin for green recycling. J. Ind. Eng. Chem. 2021, 96, 76–81. [Google Scholar] [CrossRef]
- Lee, M.; Kim, D.H.; Park, J.-J.; You, N.-H.; Goh, M. Fast chemical recycling of carbon fiber reinforced plastic at ambient pressure using an aqueous solvent accelerated by a surfactant. Waste Manag. 2020, 118, 190–196. [Google Scholar] [CrossRef]
- Yan, H.; Lu, C.; Jing, D.; Chang, C.; Liu, N.; Hou, X. Recycling of carbon fibers in epoxy resin composites using supercritical 1-propanol. New Carbon Mater. 2016, 31, 46–54. [Google Scholar] [CrossRef]
- Langston, T.A.; Granata, R.D. Influence of nitric acid treatment time on the mechanical and surface properties of high-strength carbon fibers. J. Compos. Mater. 2014, 48, 259–276. [Google Scholar] [CrossRef]
- Liu, W.; Huang, H.; Cheng, H.; Liu, Z. CFRP Reclamation and Remanufacturing Based on a Closed-loop Recycling Process for Carbon Fibers Using Supercritical N-butanol. Fibers Polym. 2020, 21, 604–618. [Google Scholar] [CrossRef]
- Matrenichev, V.; Belone, M.C.L.; Palola, S.; Laurikainen; Sarlin, E. Resizing Approach to Increase the Viability of Recycled Fibre-Reinforced Composites. Materials 2020, 13, 5773. [Google Scholar] [CrossRef] [PubMed]
- Goethals, F.; Demeyer, E.; De Schrijver, I.; Vanneste, M. Pretreating Recycled Carbon Fiber Nonwoven with a Sizing Formulation to Improve the Performance of Thermoplastic Recycled Fiber-Reinforced Composites. Polymers 2024, 16, 561. [Google Scholar] [CrossRef]
- Laurikainen, P.; Palola, S.; La Calle, A.D.; Elizetxea, C.; García-Arrieta, S.; Sarlin, E. Fiber Resizing, Com-pounding and Validation. In Systemic Circular Economy Solutions for Fiber Reinforced Composites; Springer: Cham, Switzerland, 2022; pp. 125–140. [Google Scholar] [CrossRef]
- Kumar, S.; Krishnan, S. Recycling of carbon fiber with epoxy composites by chemical recycling for future perspective: A review. Chem. Pap. 2020, 74, 3785–3807. [Google Scholar] [CrossRef]
- La Rosa, A.D.; Banatao, D.R.; Pastine, S.J.; Latteri, A.; Cicala, G. Recycling treatment of carbon fibre/epoxy composites: Materials recovery and characterization and environmental impacts through life cycle assessment. Compos. B Eng. 2016, 104, 17–25. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.; Xu, N.; Shen, Y.; Lu, F.; Liu, Y.; Huang, Y.; Hu, Z. Recycling of carbon fibers from unsaturated polyester composites via a hydrolysis-oxidation synergistic catalytic strategy. Compos. Sci. Technol. 2021, 203, 108589. [Google Scholar] [CrossRef]
- Huang, Z.; Deng, Z.; Dong, C.; Fan, J.; Ren, Y. A closed-loop recycling process for carbon fiber reinforced vinyl ester resin composite. Chem. Eng. J. 2022, 446, 137254. [Google Scholar] [CrossRef]
- Oliveux, G.; Dandy, L.O.; Leeke, G.A. Degradation of a model epoxy resin by solvolysis routes. Polym. Degrad. Stab. 2015, 118, 96–103. [Google Scholar] [CrossRef]
- Sokoli, H.U.; Simonsen, M.E.; Nielsen, R.P.; Henriksen, J.; Madsen, M.L.; Pedersen, N.H.; Søgaard, E.G. Characterization of the Liquid Products from Hydrolyzed Epoxy and Polyester Resin Composites Using Solid-Phase Microextraction and Recovery of the Monomer Phthalic Acid. Ind. Eng. Chem. Res. 2016, 55, 9118–9128. [Google Scholar] [CrossRef]
- Sugeta, T.; Nagaoka, S.; Otake, K.; Sako, T. Decomposition of Fiber Reinforced Plastics Using Fluid at High Temperature and Pressure. Kobunshi Ronbunshu 2001, 58, 557–563. [Google Scholar] [CrossRef]
- Sokoli, H.; Søgaard, E.G. Supercritical Degradation of Unsaturated Polyester Resin Composites Using Alcohols. Chem. Eng. Trans. 2015, 43, 967–972. [Google Scholar]
- Iwaya, T.; Tokuno, S.; Sasaki, M.; Goto, M.; Shibata, K. Recycling of fiber reinforced plastics using depolymerization by solvothermal reaction with catalyst. J. Mater. Sci. 2008, 43, 2452–2456. [Google Scholar] [CrossRef]
- Karayannidis, G.P.; Chatziavgoustis, A.P.; Achilias, D.S. Poly(ethylene terephthalate) recycling and recovery of pure terephthalic acid by alkaline hydrolysis. Adv. Polym. Technol. 2002, 21, 250–259. [Google Scholar] [CrossRef]
- Montagna, L.S.; Kondo, M.Y.; Callisaya, E.S.; Mello, C.; de Souza, B.R.; Lemes, A.P.; Botelho, E.C.; Costa, M.L.; Alves, M.C.d.S.; Ribeiro, M.V.; et al. A review on research, application, processing, and recycling of PPS based materials. Polímeros 2022, 32, e2022005. [Google Scholar] [CrossRef]
- Kurtz, S.M. Synthesis and Processing of PEEK for Surgical Implants. In PEEK Biomaterials Handbook; Elsevier: Amsterdam, The Netherlands, 2019; pp. 11–25. [Google Scholar] [CrossRef]
- Barnard, E.; Arias, J.J.R.; Thielemans, W. Chemolytic depolymerisation of PET: A review. Green Chem. 2021, 23, 3765–3789. [Google Scholar] [CrossRef]
- Conroy, S.; Zhang, X. Theoretical insights into chemical recycling of polyethylene terephthalate (PET). Polym. Degrad. Stab. 2024, 223, 110729. [Google Scholar] [CrossRef]
- Abedsoltan, H. A focused review on recycling and hydrolysis techniques of polyethylene terephthalate. Polym. Eng. Sci. 2023, 63, 2651–2674. [Google Scholar] [CrossRef]
- Čolnik, M.; Knez, Ž.; Škerget, M. Sub- and supercritical water for chemical recycling of polyethylene terephthalate waste. Chem. Eng. Sci. 2021, 233, 116389. [Google Scholar] [CrossRef]
- Kim, B.-K.; Hwang, G.-C.; Bae, S.-Y.; Yi, S.-C.; Kumazawa, H. Depolymerization of polyethyleneterephthalate in supercritical methanol. J. Appl. Polym. Sci. 2001, 81, 2102–2108. [Google Scholar] [CrossRef]
- Liu, T.; Guo, X.; Liu, W.; Hao, C.; Wang, L.; Hiscox, W.C.; Liu, C.; Jin, C.; Xin, J.; Zhang, J. Selective cleavage of ester linkages of anhydride-cured epoxy using a benign method and reuse of the decomposed polymer in new epoxy preparation. Green Chem. 2017, 19, 4364–4372. [Google Scholar] [CrossRef]
- Nakagawa, T.; Matsugi, S.; Hirota, S.; Miyazaki, T.; Yano, H.; Shibata, K. Enhanced and horizontal recycling of FRP using subcritical water. In Proceedings of the International Symposium on Supercritical Fluids (ISSF), Arcachon, France, 18–20 May 2009. [Google Scholar]
- Manarin, E.; Boumezgane, O.; Giannino, A.; De Fabritiis, V.; Griffini, G.; Turri, S. Towards a zero-waste chemcycling of thermoset polymer composites: Catalyst assisted mild solvolysis for clean carbon fiber liberation and circular coating development. Sustain. Mater. Technol. 2024, 41, e01031. [Google Scholar] [CrossRef]
- Fortunato, G.; Anghileri, L.; Griffini, G.; Turri, S. Simultaneous Recovery of Matrix and Fiber in Carbon Reinforced Composites through a Diels–Alder Solvolysis Process. Polymers 2019, 11, 1007. [Google Scholar] [CrossRef] [PubMed]
- Uwitonze, H.; Goyal, A.; Kim, S.; Kim, S.; Hwang, K.S. Fenske and Kremser Group Methods in the Design of Fully Thermally Coupled Distillation Column. Comput. Aided Chem. Eng. 2014, 33, 1705–1710. [Google Scholar] [CrossRef]
- Maisels, A.; Hiller, A.; Simon, F. Chemical Recycling for Plastic Waste: Status and Perspectives. Chem. Ing. Tech. 2021, 93, 1742–1750. [Google Scholar] [CrossRef]
- Fardhyanti, D.S.; Triwibowo, B.; Istanto, H.; Anajib, M.K.; Larasati, A.; Oktaviani, W. Liquid phase equilibrium of phenol extraction from bio-oil produced by biomass pyrolysis using thermodynamic models. Chin. J. Chem. Eng. 2019, 27, 391–399. [Google Scholar] [CrossRef]
- Sander, A.; Rogošić, M.; Slivar, A.; Žuteg, B. Separation of Hydrocarbons by Means of Liquid-Liquid Extraction with Deep Eutectic Solvents. Solvent Extr. Ion Exch. 2016, 34, 86–98. [Google Scholar] [CrossRef]
- Yildirir, E.; Onwudili, J.A.; Williams, P.T. Recovery of carbon fibres and production of high quality fuel gas from the chemical recycling of carbon fibre reinforced plastic wastes. J. Supercrit. Fluids 2014, 92, 107–114. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Iwuozor, K.O.; Ogunfowora, L.A.; Abdulsalam, A.; Iwuchukwu, F.U.; Itabana, B.; Bright, O.C.; Igwegbe, C.A. Regenerative desulphurisation of pyrolysis oil: A paradigm for the circular economy initiative. J. Environ. Chem. Eng. 2021, 9, 106864. [Google Scholar] [CrossRef]
- Nabgan, W.; Rashidzadeh, M.; Nabgan, B. The catalytic naphtha reforming process: Hydrodesulfurization, catalysts and zeoforming. Environ. Chem. Lett. 2018, 16, 507–522. [Google Scholar] [CrossRef]
- Djandja, O.S.; Wang, Z.; Duan, P.; Wang, F.; Xu, Y. Hydrotreatment of pyrolysis oil from waste tire in tetralin for production of high-quality hydrocarbon rich fuel. Fuel 2021, 285, 119185. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, M.; Jones, I.; Zhang, Z.; Zhang, D. Desulfurization of Spent Tire Pyrolysis Oil and Its Distillate via Combined Catalytic Oxidation using H2O2 with Formic Acid and Selective Adsorption over Al2O3. Energy Fuels 2020, 34, 6209–6219. [Google Scholar] [CrossRef]
- Jovanovic, J.D.; Petkovic, S.D.; Gigov, M.N.; Adnadjevic, B.K. Extractive desulfurization of pyrolysis tire oil with deep eutectic solvent using hydrodynamic cavitation. Environ. Sci. Pollut. Res. 2021, 28, 59268–59276. [Google Scholar] [CrossRef] [PubMed]
- Al-Lal, A.-M.; Bolonio, D.; Llamas, A.; Lapuerta, M.; Canoira, L. Desulfurization of pyrolysis fuels obtained from waste: Lube oils, tires and plastics. Fuel 2015, 150, 208–216. [Google Scholar] [CrossRef]
- Mohebali, G.; Ball, A.S. Biodesulfurization of diesel fuels—Past, present and future perspectives. Int. Biodeterior. Biodegrad. 2016, 110, 163–180. [Google Scholar] [CrossRef]
- Sienkiewicz, A.; Czub, P. Flame Retardancy of Biobased Composites—Research Development. Materials 2020, 13, 5253. [Google Scholar] [CrossRef]
- Fekhar, B.; Gombor, L.; Miskolczi, N. Pyrolysis of chlorine contaminated municipal plastic waste: In-situ upgrading of pyrolysis oils by Ni/ZSM-5, Ni/SAPO-11, red mud and Ca(OH)2 containing catalysts. J. Energy Inst. 2019, 92, 1270–1283. [Google Scholar] [CrossRef]
- Lingaiah, N.; Uddin, M.A.; Muto, A.; Imai, T.; Sakata, Y. Removal of organic chlorine compounds by catalytic dehydrochlorination for the refinement of municipal waste plastic derived oil. Fuel 2001, 80, 1901–1905. [Google Scholar] [CrossRef]
- Torres, D.; Jiang, Y.; Sanchez-Monsalve, D.A.; Leeke, G.A. Hydrochloric acid removal from the thermogravimetric pyrolysis of PVC. J. Anal. Appl. Pyrolysis 2020, 149, 104831. [Google Scholar] [CrossRef]
- Lopez-Urionabarrenechea, A.; de Marco, I.; Caballero, B.M.; Laresgoiti, M.F.; Adrados, A. Catalytic stepwise pyrolysis of packaging plastic waste. J. Anal. Appl. Pyrolysis 2012, 96, 54–62. [Google Scholar] [CrossRef]
- MET Group. Calorific Value of Natural Gas (MJ/m3 and BTU/SCF). Available online: https://group.met.com/en/media/energy-insight/calorific-value-of-natural-gas (accessed on 19 November 2024).
- Branfoot, C.; Folkvord, H.; Keith, M.; Leeke, G.A. Recovery of chemical recyclates from fibre-reinforced composites: A review of progress. Polym. Degrad. Stab. 2023, 215, 110447. [Google Scholar] [CrossRef]
- Adherent Technologies Inc. Recycling Technologies. Available online: https://www.adherent-tech.com/recycling_technologies/ (accessed on 19 November 2024).
- Newswire, P.R. AMT II & Adherent Technologies to Partner in New Carbon Fiber Recycling & Fiber Sizing Facility. Available online: https://www.prnewswire.com/news-releases/amt-ii--adherent-technologies-to-partner-in-new-carbon-fiber-recycling--fiber-sizing-facility-230982821.html (accessed on 19 November 2024).
- Navarro, C.A.; Ma, Y.; Michael, K.H.; Breunig, H.M.; Nutt, S.R.; Williams, T.J. Catalytic, aerobic depolymerization of epoxy thermoset composites. Green Chem. 2021, 23, 6356–6360. [Google Scholar] [CrossRef]
- Shocker Composites LLC. Injection Molding. Available online: https://shockercomposites.com/pages/injection-molding (accessed on 19 November 2024).
- Nakagawa, M.; Kasuga, K.; Aoyagi, K.; Ishihara, K.; Ikeda, Y.; Shibata, K. CFRP recycling technology using depolymerization under ordinary pressure. In Proceedings of the 29th Annual Technical Conference of the American Society for Composites, San Diego, CA, USA, 8–10 September 2014. [Google Scholar]
- Job, S.; Leeke, G.A.; Mativenga, T.; Oliveux, G.; Pickering, S.; Shuaib, N. Composites Recycling: Where Are We Now? Composites: Berkhamsted, UK, 2016. [Google Scholar]
- Mason, H. South Korean Carbon Fiber Recycling Start-Up Scales Up. Composites World, 25 November 2020. [Google Scholar]





| Material | Cost (USD/kg) | Energy Demand (MJ/kg) | GWP (kg CO2 eq/kg) | References |
|---|---|---|---|---|
| Carbon fibre | 30–85 | 171–771 | 24.4–31.0 | [24,26,29] |
| Glass fibre | 0.75–3 | 13.0–48.3 | 2.0 | [27,28,30,31] |
| Epoxy | 4–20 | 76–144 | 4.7–8.1 | [26] |
| Polyester (PE) | 1–2.50 | 63–78 | 2.8–3.1 | [26] |
| Nylon | 2–3 | 139–145 | 6.5–8.3 | [26] |
| Polycarbonate (PC) | 2.50 | 80–112 | 6.0–7.5 | [26] |
| Polypropylene (PP) | 1.50 | 22–112 | 1.9–2.6 | [26] |
| Polyvinyl chloride (PVC) | 1.50 | 53–80 | 2.2 | [26] |
| Low density polyethylene | 1.50 | 65–92 | 1.8 | [26] |
| Aluminium | 2.2–3.5 | 197–298 | 12 | [32,33,34] |
| Steel | 1.6–6.2 | 25.0–44.6 | 2.3–2.5 | [35,36] |
| Copper | 3.5–5 | 30–90 | 1.0–9.0 | [37,38,39] |
| FRP | Reaction System | Process Conditions | Ref. |
|---|---|---|---|
| CF-reinforced thermoset epoxy resin | 8 M nitric acid | 90 °C, 5 h, 40 gcomposite/Lreactant | [91] |
| CF-reinforced thermoset epoxy | 12 M nitric acid | 90 °C, 6 h, 40 gcomposite/Lreactant, flow reactor at 60 mL/min | [92] |
| GF-reinforced anhydride-cured epoxy | 4 M nitric acid | 80 °C, unspecified time | [64] |
| CF-reinforced anhydride-cured epoxy | Sulfuric acid and H2O2, concentration unknown | 110 °C, several hours with agitation | [66] |
| CF-reinforced epoxy cured with vinyl ethers | 0.1 M HCl and THF/water at 9:1 v/v | Room temperature, 24 h | [93] |
| GF-reinforced polyamide | 2.5 HCl/amide mol ratio in water | 200 °C, 10 min, with microwaves | [70] |
| CF-reinforced polyamide | 2.5 HCl/amide mol ratio in water | 200 °C, 10 min, with microwaves | [70] |
| CF-reinforced amine-cured epoxy | Acetic acid pretreatment, 30% H2O2, acetone | Acetic acid refluxed at 120 °C, 30 min, acetone wash 120 °C, 30 min in H2O2 and acetone (1:1 v/v) | [94] |
| Aerospace-grade CFRP | 14 M acetic acid with 14 M H2O2 | 65 °C, 4–5 h, 17 gcomposite/Lreactant | [95] |
| CF-reinforced thermoset epoxy | 1.4 M AlCl3 in acetic acid | 180 °C, 6 h, 200 gcomposite/Lreactant | [68] |
| CF-reinforced thermoset epoxy | 60 wt.% ZnCl2 in water | 210 °C, 9 h | [79] |
| Aerospace-grade CFRP | 20 wt.% ZnCl2 in ethanol | 190 °C, 5 h | [80] |
| CF-reinforced thermoset epoxy | 3.3 wt% ZnCl2 in thymol/decanoic acid | 180 °C, 1.5 h | [81] |
| GF-reinforced epoxy | 0.1 g NaOH/gcomposite in poly(ethylene) glycol | 200 °C, 4 h | [73] |
| CF-reinforced epoxy | 0.1 g NaOH/gcomposite in poly(ethylene) glycol | 200 °C, 4 h | [73] |
| CF-reinforced anhydride-cured epoxy | 0.5 M KOH in mono-ethanolamine (MEA) | 160 °C, 60 min | [75] |
| GF-reinforced PET | 1.25 M KOH in methanol | 120 °C, 5 min, with microwaves | [77] |
| CF-reinforced thermoset epoxy | K3PO4 in benzyl alcohol at 1:10 w/w | 195 °C, 40 min | [82] |
| GF-reinforced thermoplastic Elium® wind turbine | Chloroform dissolution | 72 h | [89] |
| FRP | Solvent | Process Conditions | Ref. |
|---|---|---|---|
| CF-reinforced anhydride-cured epoxy | Water | 440 °C, 35 min, 30 MPa | [99] |
| CF-reinforced RTM6 epoxy | Water | 375 °C, 15 min, 25 MPa, semi-continuous flow reactor | [106] |
| CF-reinforced amine-cured epoxy | Water | 290 °C, 75 min | [116] |
| GF-reinforced unsaturated polyester | Water | 300 °C, 30 min, 0.01 gresin/Lsolvent | [117] |
| CF-reinforced anhydride-cured epoxy | Methanol | 270 to 350 °C, 120 to 10 min, 8 to 10 MPa | [110] |
| CF-reinforced LTM26EL epoxy | Ethanol | 450 °C, 15.5 min, 8.0 MPa, 100 gcomposite/Lsolvent | [111] |
| CF-reinforced LTM26EL epoxy | Propanol | 450 °C, 40 min, 25.4 MPa, 100 gcomposite/Lsolvent | [111] |
| CF-reinforced amine-cured epoxy | Propanol | 320 °C, 25 min, 9.0 MPa | [114] |
| CF-reinforced amine-cured epoxy | Acetone | 320 °C, 20 min, 6.0 MPa | [114] |
| GF-reinforced epoxy (Araldite LY 1564 SP) | Acetone | 260 °C, 30 min, 6.0 MPa, up to 210,100 gcomposite/Lsolvent | [113] |
| CF-reinforced RTM6 epoxy | Ethanol/water (50:50 v/v) | 375 °C, 15 min, semi-continuous flow reactor | [106] |
| CF-reinforced RTM6 epoxy | Acetone/water (80:20 v/v) | 320 °C, 120 min, 30 gcomposite/Lsolvent | [48] |
| Fibre Type | Recycling Process | Strength Change | Other Properties | Reference |
|---|---|---|---|---|
| T-glass fibre | 6 M nitric acid, 70 °C, 250 h | 3.5% reduction | 2.5% reduction in shear strength | [130] |
| T-glass fibre | 4 M nitric acid | Not measured | No mass loss | [64] |
| E-glass fibre | 4 M nitric acid | Not measured | 30% mass loss | [64] |
| T-glass fibres | Sulfuric acid | 70% reduction | - | [132] |
| T-glass fibres | Glycolysis, 130 °C | 45% reduction | 55% reduction in modulus | [133] |
| E-glass fibres | Water, 280 °C | 40% reduction | - | [113] |
| E-glass fibres | Water, 350 °C | 60% reduction | - | [113] |
| T-glass fibres | Acetic acid and AlCl3, 180 °C, 9 h | <4% reduction | - | [131] |
| T-glass fibres | Methanol and DMAP, 275 °C | <7% reduction | - | [135] |
| E-glass fibres | Acetone, 260 to 280 °C | 11 to 15% reduction | - | [113] |
| Fibre Type | Recycling Process | Tensile Properties | Other Properties | Reference |
|---|---|---|---|---|
| Not given | 1.3 M NaOH, 180 °C, 8 h | Strength: 2.4% reduction | No significant changes in surface composition | [140] |
| Toray T700 | Nitric acid, macrogol 400/KOH, 160 °C, 200 min | Strength: 4.4% reduction Stiffness: 3.1% reduction | Increase in surface oxygen and improved wettability | [74] |
| 24k HS | 11 to 18 M sulfuric acid, room temperature | Strength: 0.9 to 5.8% reduction Stiffness: 2.6 to 5.0% reduction | - | [139] |
| Toray T300 3k | 0.1 M HCl in acetone/water (9:1 v/v), room temperature | Strength: 5.1% reduction Stiffness: 2.3% reduction | Increase in surface oxygen. rCFRP had similar shear strength to vCFRP | [138] |
| Not given | 1.4 M AlCl3 in acetic acid, 180 °C, 6 h | Strength: 2.2% reduction Stiffness: 1.9% reduction | ~10% increase in surface oxygen | [131] |
| Not given | 14 M acetic acid, 9 M H2O2, 65 °C, 4 h | Strength: 0 to 26% reduction, dependent on acetic/H2O2 ratio | Additional COH and COOH groups detected | [95] |
| Hexcel CF | H2O2/tartaric acid (2:1 v/w), 1 min microwave irradiation + 30 min soak | Strength: 8% reduction Stiffness: No difference | Increase in surface oxygen concentration | [141] |
| Synthesised in-house | Benzyl alcohol/K3PO4 (1:10), 195 °C, 40 min | Strength: <10% reduction | Surface oxygen comparable to virgin | [82] |
| Toray T700 | Water at 280 to 500 °C, 15 to 20 min | Strength: 7 to 18% reduction | Reduction in purity of graphitic structure | [109] |
| Not given | Water, 1 M phenol, 0.18 M KOH, 315 °C, 9 MPa, 30 min | Strength: Equivalent to virgin | Slight increase in surface oxygen | [119] |
| Not given | Methanol, 270 °C, 8 MPa, 90 min | Strength: 9% reduction | Retention of weave structure | [110] |
| Synthesised in-house | Propanol, 0 to 0.36 M KOH, 320 to 360 °C, 30 to 180 min | Strength: 5 to 15% reduction | KOH caused increase in surface oxygen concentration | [144] |
| Toray T300 3k | Acetone, 320 °C, 1 MPa, 20 min | Strength: Equivalent to virgin | - | [114] |
| Hexcel 48192 | Water/ethanol 50:50 v/v, 350 °C, 25 MPa | Strength: 9 to 19% increase Stiffness: <7% reduction | Slight decrease in surface oxygen. AFM showed similar roughness | [106] |
| Toray T700S | Acetone/water (80:20 v/v), 320 °C, 120 min | Strength: Slight increase Stiffness: Slight decrease | Increase in surface oxygen concentration | [124] |
| Toray T700S | Acetone/water (80:20 v/v), 0.05 M ZnCl2, 290 °C, 90 min | Strength: Up to 22% increase Stiffness: ~3% decrease | Increase in surface oxygen concentration | [124] |
| Toray T700S | Acetone/water (80:20 v/v), 0.005 M AlCl3, 290 °C, 90 min | Strength: 10% reduction Stiffness: 23% decrease | Increase in surface oxygen concentration | [124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keith, M.J.; Al-Duri, B.; McDonald, T.O.; Leeke, G.A. Solvent-Based Recycling as a Waste Management Strategy for Fibre-Reinforced Polymers: Current State of the Art. Polymers 2025, 17, 843. https://doi.org/10.3390/polym17070843
Keith MJ, Al-Duri B, McDonald TO, Leeke GA. Solvent-Based Recycling as a Waste Management Strategy for Fibre-Reinforced Polymers: Current State of the Art. Polymers. 2025; 17(7):843. https://doi.org/10.3390/polym17070843
Chicago/Turabian StyleKeith, Matthew J., Bushra Al-Duri, Tom O. McDonald, and Gary A. Leeke. 2025. "Solvent-Based Recycling as a Waste Management Strategy for Fibre-Reinforced Polymers: Current State of the Art" Polymers 17, no. 7: 843. https://doi.org/10.3390/polym17070843
APA StyleKeith, M. J., Al-Duri, B., McDonald, T. O., & Leeke, G. A. (2025). Solvent-Based Recycling as a Waste Management Strategy for Fibre-Reinforced Polymers: Current State of the Art. Polymers, 17(7), 843. https://doi.org/10.3390/polym17070843