Adsorption of Bisphenol A onto β-Cyclodextrin–Based Nanosponges and Innovative Supercritical Green Regeneration of the Sustainable Adsorbent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of β-Cyclodextrin Nanosponges
2.3. Batch Adsorption Experiments
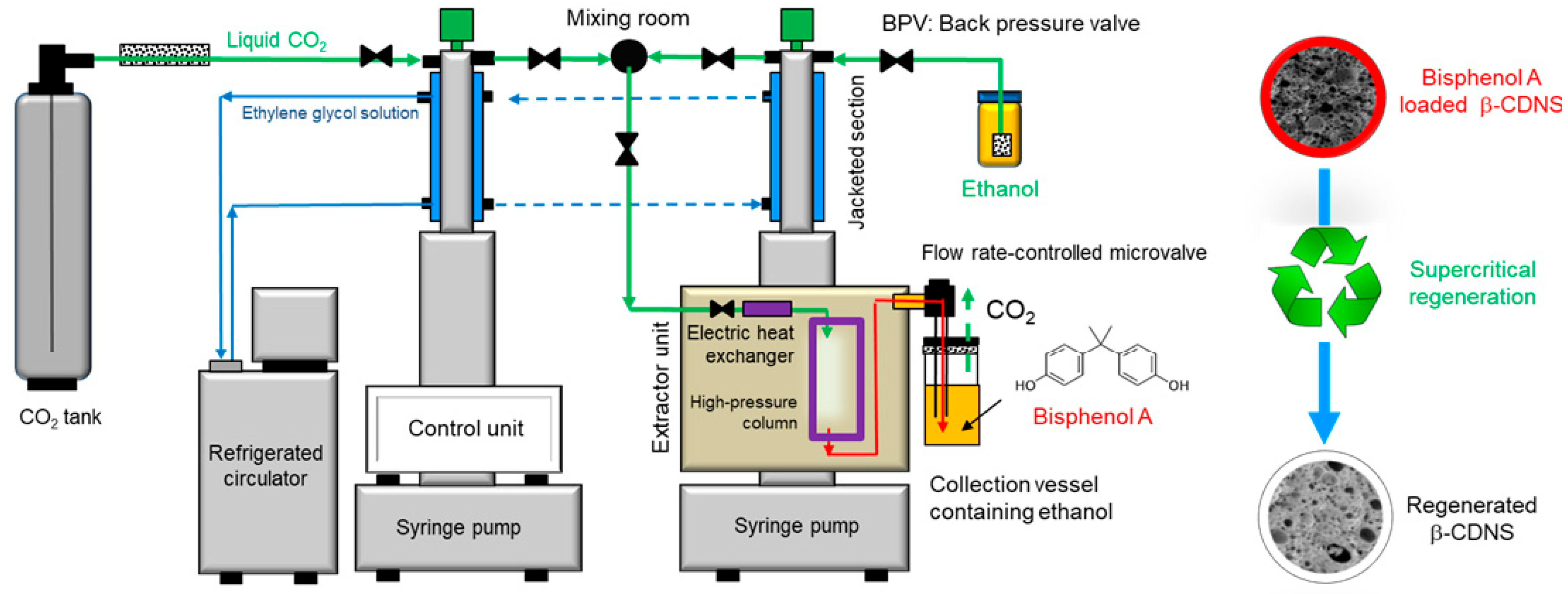
2.4. Regeneration Experiments Using Supercritical CO2-Based Green Solvent
2.5. Characterizations of β-Cyclodextrin Nanosponges
2.6. Computational Approaches for Adsorption Isotherm, Kinetics, and Thermodynamics
3. Results and Discussion
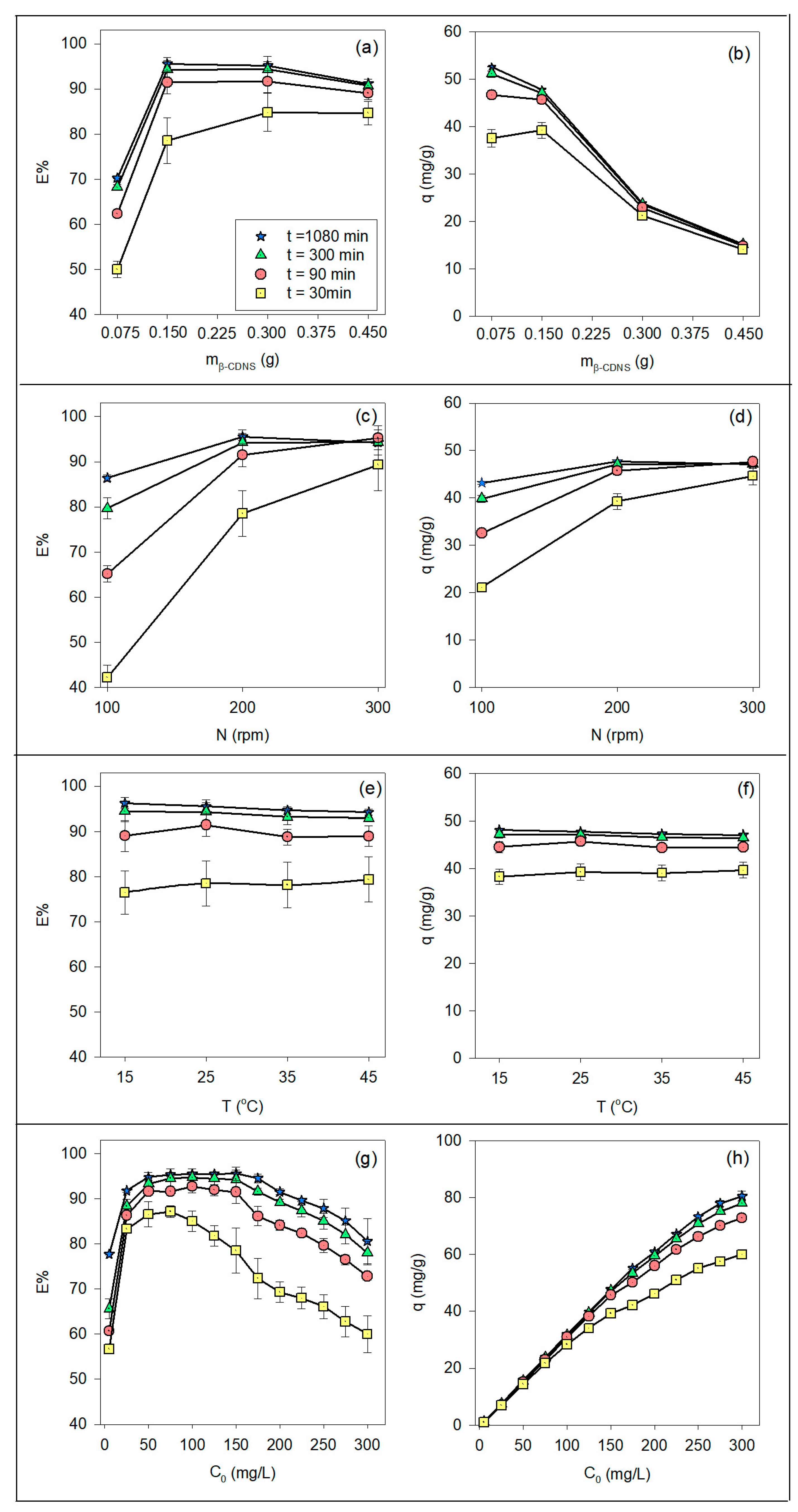
3.1. Adsorption Parameters: Effects on Efficiency and Capacity
3.1.1. Effect of Adsorbent Dosage
3.1.2. Effect of Shaking Speed
3.1.3. Effect of Adsorption Temperature
3.1.4. Effect of Initial Bisphenol A Concentration
3.2. Computational Optimization of Adsorption Models: Isotherm, Kinetics, and Thermodynamics
3.2.1. Adsorption Isotherms
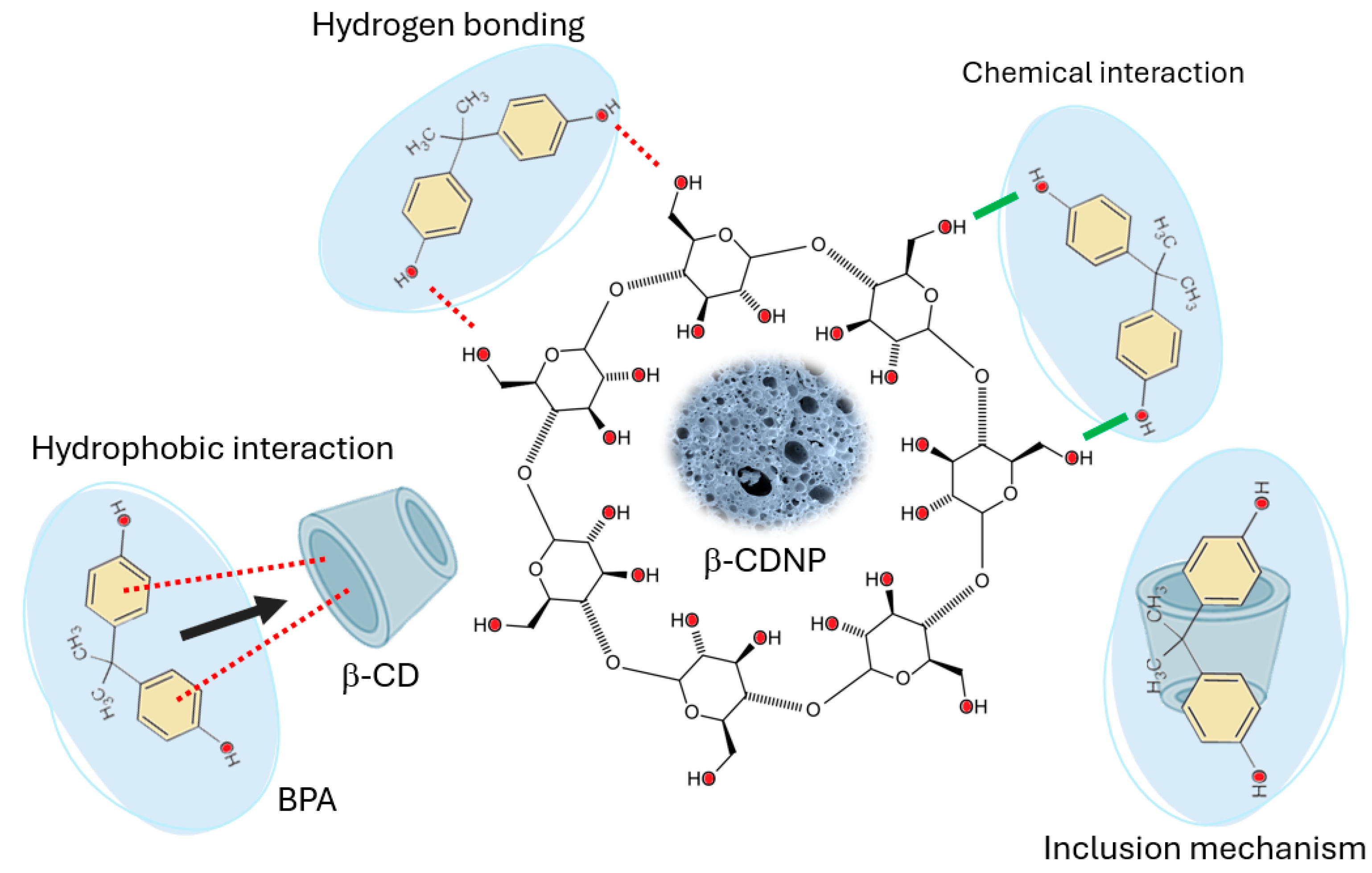
3.2.2. Adsorption Kinetics and Adsorption Mechanism
3.2.3. Adsorption Thermodynamics
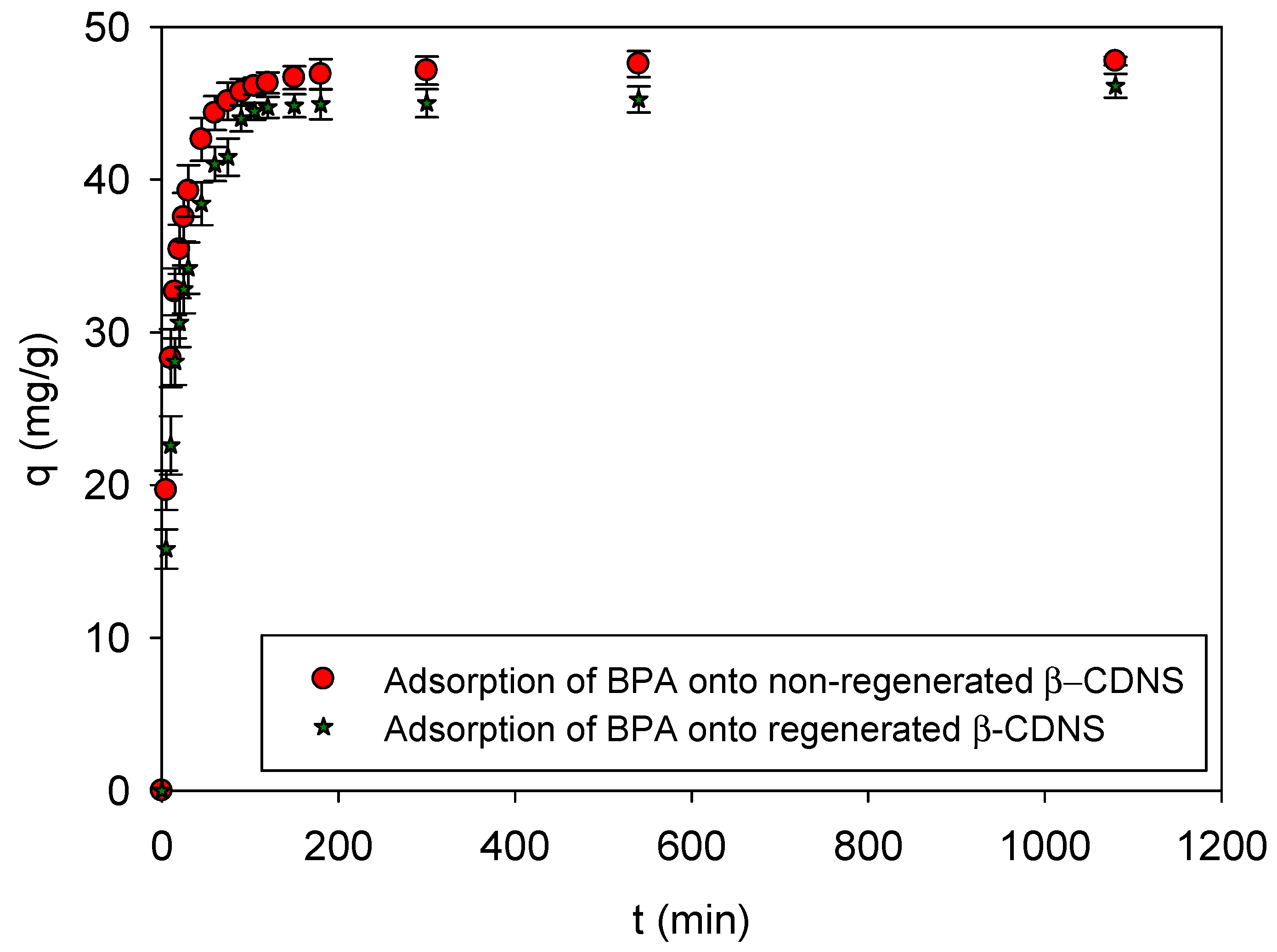
3.3. Supercritical CO2-Based Green Regeneration
3.4. Structural and Functional Characterization of β-CDNSs
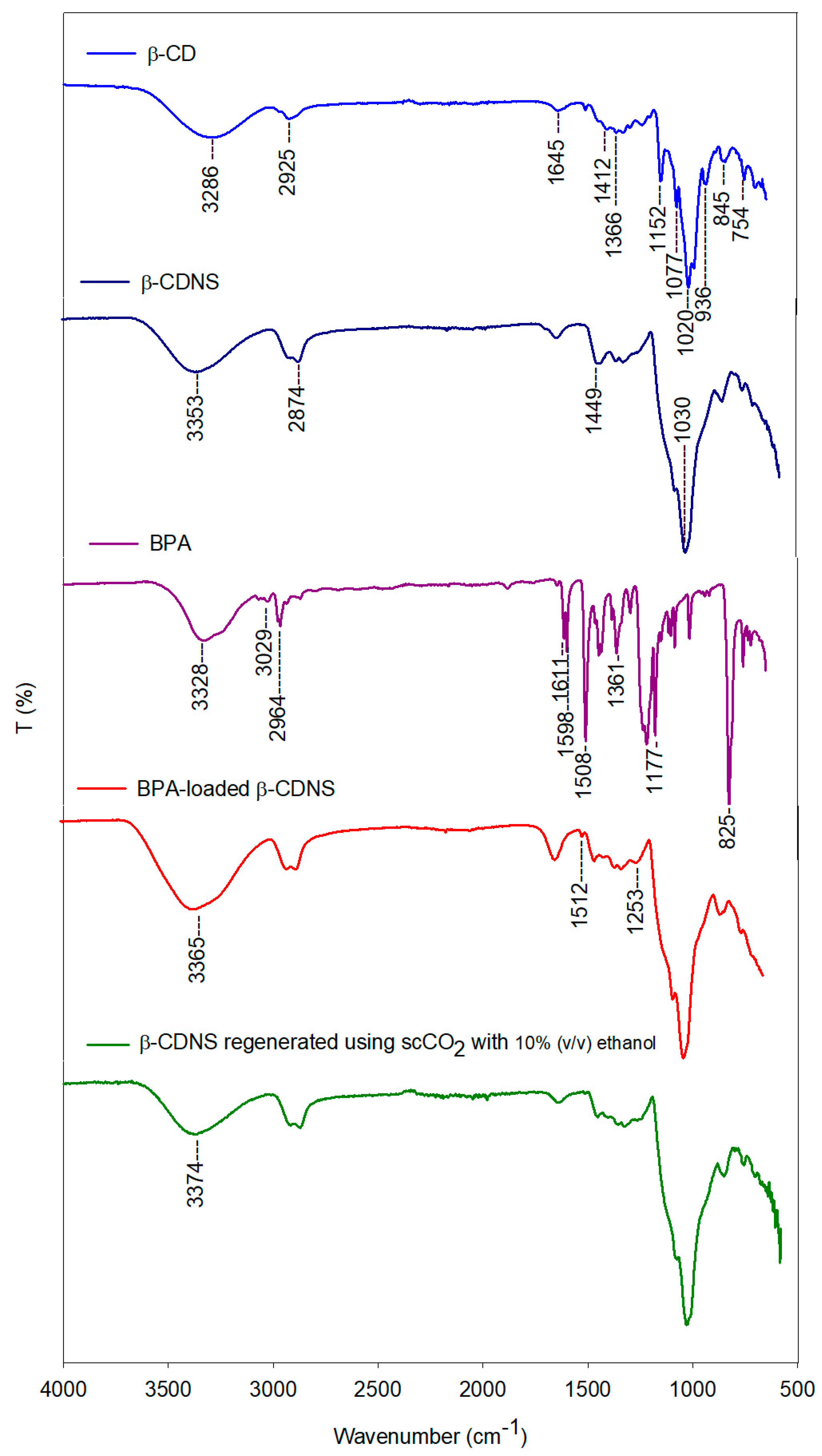
3.4.1. FTIR Characterization and Molecular Interactions of β-CDNSs
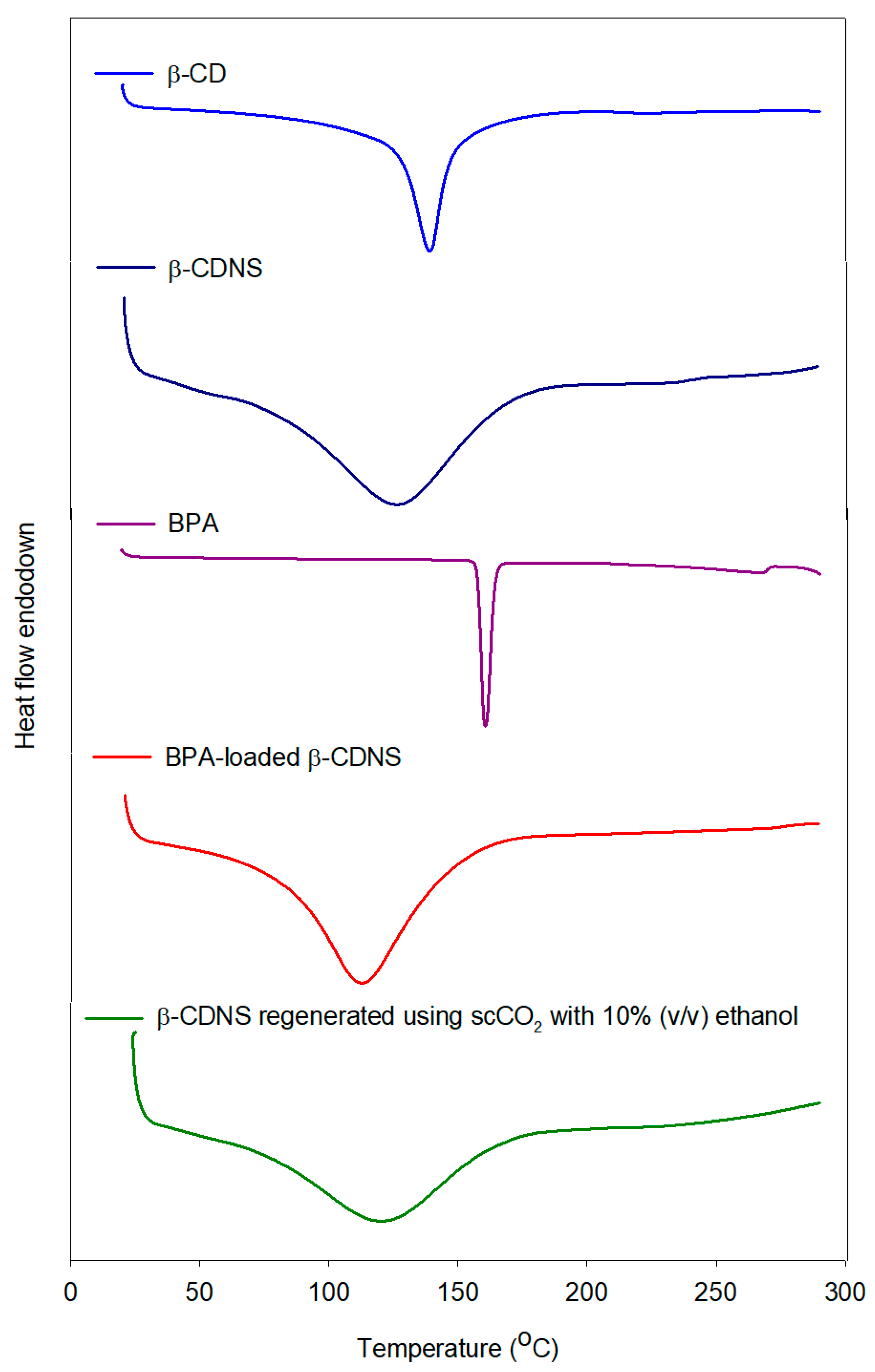
3.4.2. Thermal Characterization of β-Cyclodextrin Nanosponges via DSC
3.4.3. Morphological Characterization of β-Cyclodextrin Nanosponges via SEM
3.4.4. Particle Size Distribution and Temporal Stability of β-CDNSs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Yuan, S.; Chi, M.; Wang, Y.; Van Eygen, G.; Zhao, R.; Zhang, X.; Li, G.; Volodine, A.; Hu, S.; et al. Efficient capture of endocrine-disrupting compounds by a high-performance nanofiltration membrane for wastewater treatment. Water Res. 2022, 227, 119322. [Google Scholar] [CrossRef] [PubMed]
- Łukasik, N.; Wikarska, S.; Świątek, H.; Łapiński, M.; Klimczuk, T.; Hemine, K. The influence of magnetic particle incorporation on bisphenol A removal by β-cyclodextrin-derived sorbent. Chemosphere 2023, 338, 139538. [Google Scholar] [CrossRef]
- Gong, Y.; Su, J.; Li, M.; Zhu, A.; Liu, G.; Liu, P. Fabrication and adsorption optimization of novel magnetic core-shell chitosan/graphene oxide/β-cyclodextrin composite materials for bisphenols in aqueous solutions. Materials 2020, 13, 5408. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kwak, S.-Y. Rapid adsorption of bisphenol A from wastewater by β-cyclodextrin-functionalized mesoporous magnetic clusters. Appl. Surf. Sci. 2019, 467–468, 178–184. [Google Scholar] [CrossRef]
- Czarny-Krzymińska, K.; Krawczyk, B.; Szczukocki, D. Bisphenol A and its substitutes in the aquatic environment: Occurrence and toxicity assessment. Chemosphere 2023, 315, 137763. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, X.; Zhou, Y.; Fan, J.; Zeng, G. Microfiltration membranes for the removal of bisphenol A from aqueous solution: Adsorption behavior and mechanism. Water 2022, 14, 2306. [Google Scholar] [CrossRef]
- Hernández-Abreu, A.B.; Álvarez-Torrellas, S.; Rocha, R.P.; Pereira, M.F.R.; Agueda, V.I.; Delgado, J.A.; Larriba, M.; García, J.; Figueiredo, J.L. Effective adsorption of the endocrine disruptor compound bisphenol A from water on surface-modified carbon materials. Appl. Surf. Sci. 2021, 552, 149513. [Google Scholar] [CrossRef]
- Zhou, L.; Huang, Q.; Ji, H.; Tang, X.; Zhang, Y.; Chai, K. Efficient adsorption of bisphenol A from water by a hierarchically porous hyper-crosslinked polymer containing β-cyclodextrin polyurethane. Sep. Purif. Technol. 2023, 319, 124076. [Google Scholar] [CrossRef]
- Sirach, R.; Dave, P.N. Thermal and bisphenol-A adsorption properties of a zinc ferrite/β-cyclodextrin polymer nanocomposite. RSC Adv. 2023, 13, 21991–22006. [Google Scholar] [CrossRef]
- Huang, W.; Hu, Y.; Li, Y.; Zhou, Y.; Niu, D.; Lei, Z.; Zhang, Z. Citric acid-crosslinked β-cyclodextrin for simultaneous removal of bisphenol A, methylene blue and copper: The roles of cavity and surface functional groups. J. Taiwan Inst. Chem. Eng. 2018, 82, 189–197. [Google Scholar] [CrossRef]
- Gupta, V.K.; Agarwal, S.; Sadegh, H.; Ali, G.A.M.; Bharti, A.K.; Makhlouf, A.S.H. Facile route synthesis of novel graphene oxide-β-cyclodextrin nanocomposite and its application as adsorbent for removal of toxic bisphenol A from the aqueous phase. J. Mol. Liq. 2017, 237, 466–472. [Google Scholar] [CrossRef]
- Bucur, S.; Diacon, A.; Mangalagiu, I.; Mocanu, A.; Rizea, F.; Dinescu, A.; Ghebaur, A.; Boscornea, A.C.; Voicu, G.; Rusen, E. Bisphenol A adsorption on silica particles modified with beta-cyclodextrins. Nanomaterials 2022, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Wang, J. Removal of bisphenol A from wastewater by adsorption and membrane separation: Performances and mechanisms. Chem. Eng. J. 2024, 484, 149414. [Google Scholar] [CrossRef]
- Kundu, S.; Das, B.K.; Wodeyar, A.; Majumder, P.; Jana, S.; Biswas, A.; Das, S.; Besra, R. Clearing the path: Unraveling bisphenol A removal and degradation mechanisms for a cleaner future. J. Environ. Manag. 2025, 373, 123558. [Google Scholar] [CrossRef]
- Mordor Intelligence. Bisphenol-A Market Size & Share Analysis—Growth Trends & Forecasts (2025–2030). Available online: https://www.mordorintelligence.com (accessed on 15 January 2025).
- Wang, J.; Chan, F.K.S.; Johnson, M.F.; Chan, H.K.; Cui, Y.; Chen, J.; Chen, W.-Q. Material Cycles, Environmental Emissions, and Ecological Risks of Bisphenol A (BPA) in China and Implications for Sustainable Plastic Management. Environ. Sci. Technol. 2025, 59, 1631–1646. [Google Scholar] [CrossRef] [PubMed]
- Environment and Climate Change Canada. Federal Environmental Quality Guidelines: Bisphenol A; Government of Canada: Ottawa, ON, Canada, 2018. Available online: https://www.canada.ca/en/environment-climate-change/services/evaluating-existing-substances/federal-environmental-quality-guidelines-bisphenol-a.html (accessed on 8 March 2025).
- SCHEER (Scientific Committee on Health, Environmental and Emerging Risks). Final Opinion on Draft Environmental Quality Standards for Priority Substances under the Water Framework Directive—Bisphenol A; Adopted 12 October 2022; European Commission: Brussels, Belgium, 2022; Available online: https://health.ec.europa.eu/document/download/037b602d-3102-4b6f-8928-92de6fcb7fc7_en?filename=scheer_o_042.pdf (accessed on 8 March 2025).
- Abu Hasan, H.; Muhamad, M.H.; Budi Kurniawan, S.; Buhari, J.; Husain Abuzeyad, O. Managing Bisphenol A Contamination: Advances in Removal Technologies and Future Prospects. Water 2023, 15, 3573. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (EPA). Testing of Bisphenol A. Fed. Regist. 2011, 76, 44535–44544. Available online: https://www.govinfo.gov/content/pkg/FR-2011-07-26/pdf/2011-18842.pdf (accessed on 8 March 2025).
- Hunan Research Institute of Chemical Industry. Polycarbonate Process Wastewater Treatment Method. CN103936216A, 23 July 2014. Available online: https://patents.google.com/patent/CN103936216A/en (accessed on 8 March 2025).
- Yamamoto, T.; Yasuhara, A.; Shiraishi, H.; Nakasugi, O. Bisphenol A in hazardous waste landfill leachates. Chemosphere 2001, 42, 415–418. [Google Scholar] [CrossRef]
- Fischer, B.; Milunov, M.; Floredo, Y.; Hofbauer, P.; Joas, A. Identification of Relevant Emission Pathways to the Environment and Quantification of Environmental Exposure for Bisphenol A, 1st ed.; Federal Environment Agency (Umweltbundesamt): Dessau-Roßlau, Germany, 2014; pp. 95–96. ISSN 1862-4804.
- Godiya, C.B.; Park, B.J. Removal of bisphenol A from wastewater by physical, chemical and biological remediation techniques: A review. Environ. Chem. Lett. 2022, 20, 1801–1837. [Google Scholar] [CrossRef]
- Simić, M.D.; Brdarić, T.P.; Savić Rosić, B.G.; Švorc, Ľ.; Relić, D.J.; Aćimović, D.D. Degradation of bisphenol A via the electro-Fenton process using nanostructured carbon-metal oxide anodes: Intermediates and reaction mechanisms study. J. Environ. Chem. Eng. 2024, 12, 113369. [Google Scholar] [CrossRef]
- Chiang, K.; Lim, T.M.; Tsen, L.; Lee, C.C. Photocatalytic degradation and mineralization of bisphenol A by TiO2 and platinized TiO2. Appl. Catal. A Gen. 2004, 261, 225–237. [Google Scholar] [CrossRef]
- Zhou, Y.; He, J.; Lu, J.; Liu, Y.; Zhou, Y. Enhanced removal of bisphenol A by cyclodextrin in photocatalytic systems: Degradation intermediates and toxicity evaluation. Chin. Chem. Lett. 2020, 31, 2623–2626. [Google Scholar] [CrossRef]
- Dong, B.-z.; Wang, L.; Gao, N.-y. The removal of bisphenol A by ultrafiltration. Desalination 2008, 221, 312–317. [Google Scholar] [CrossRef]
- Dharupaneedi, S.P.; Nataraj, S.K.; Nadagouda, M.; Reddy, K.R.; Shukla, S.S.; Aminabhavi, T.M. Membrane-based separation of potential emerging pollutants. Sep. Purif. Technol. 2019, 210, 850–866. [Google Scholar] [CrossRef] [PubMed]
- Khazaali, F.; Kargari, A.; Rokhsaran, M. Application of low-pressure reverse osmosis for effective recovery of bisphenol A from aqueous wastes. Desalination Water Treat. 2014, 52, 7543–7551. [Google Scholar] [CrossRef]
- Pan, Z.; Yu, F.; Li, L.; Song, C.; Yang, J.; Wang, C.; Pan, Y.; Wang, T. Electrochemical microfiltration treatment of bisphenol A wastewater using coal-based carbon membrane. Sep. Purif. Technol. 2019, 227, 115695. [Google Scholar] [CrossRef]
- Yüksel, S.; Kabay, N.; Yüksel, M. Removal of bisphenol A (BPA) from water by various nanofiltration (NF) and reverse osmosis (RO) membranes. J. Hazard. Mater. 2013, 263, 307–310. [Google Scholar] [CrossRef]
- Moussavi, G.; Abbaszadeh Haddad, F. Bacterial peroxidase-mediated enhanced biodegradation and mineralization of bisphenol A in a batch bioreactor. Chemosphere 2019, 222, 549–555. [Google Scholar] [CrossRef]
- Hong, P.; Zhang, K.; Dai, Y.; Yuen, C.N.T.; Gao, Y.; Gu, Y.; Leung, K.M.Y. Application of aerobic denitrifier for simultaneous removal of nitrogen, zinc, and bisphenol A from wastewater. Bioresour. Technol. 2022, 354, 127192. [Google Scholar] [CrossRef]
- Zielińska, M.; Bułkowska, K.; Cydzik-Kwiatkowska, A.; Bernat, K.; Wojnowska-Baryła, I. Removal of bisphenol A (BPA) from biologically treated wastewater by microfiltration and nanofiltration. Int. J. Environ. Sci. Technol. 2016, 13, 2239–2248. [Google Scholar] [CrossRef]
- Zare, E.N.; Mudhoo, A.; Khan, M.A.; Otero, M.; Bundhoo, Z.M.A.; Navarathna, C.; Patel, M.; Srivastava, A.; Pittman, C.U., Jr.; Mlsna, T.; et al. Water decontamination using bio-based, chemically functionalized, doped, and ionic liquid-enhanced adsorbents: Review. Environ. Chem. Lett. 2021, 19, 3075–3114. [Google Scholar] [CrossRef]
- Katibi, K.K.; Yunos, K.F.; Che Man, H.; Aris, A.Z.; Mohd Nor, M.Z.; Azis, R.S. An insight into a sustainable removal of bisphenol A from aqueous solution by novel palm kernel shell magnetically induced biochar: Synthesis, characterization, kinetic, and thermodynamic studies. Polymers 2021, 13, 3781. [Google Scholar] [CrossRef]
- Rani, S.; Sabharwal, H.; Kumar, P.; Chauhan, A.K.; Khoo, K.S.; Kataria, N. Comparative behavior of carbon-based materials for the removal of emerging bisphenol A from water: Adsorption modelling and mechanism. Groundw. Sustain. Dev. 2024, 25, 101121. [Google Scholar] [CrossRef]
- Li, S.; Gong, Y.; Yang, Y.; He, C.; Hu, L.; Zhu, L.; Sun, L.; Shu, D. Recyclable CNTs/Fe3O4 magnetic nanocomposites as adsorbents to remove bisphenol A from water and their regeneration. Chem. Eng. J. 2015, 260, 231–239. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Min, J.; Li, S.; Deng, X.; Yuan, S.; Zuo, X. Adsorption characteristics of phenolic compounds on graphene oxide and reduced graphene oxide: A batch experiment combined theory calculation. Appl. Sci. 2018, 8, 1950. [Google Scholar] [CrossRef]
- Shi, W.; Wang, H.; Yan, J.; Shan, L.; Quan, G.; Pan, X.; Cui, L. Wheat straw derived biochar with hierarchically porous structure for bisphenol A removal: Preparation, characterization, and adsorption properties. Sep. Purif. Technol. 2022, 289, 120796. [Google Scholar] [CrossRef]
- İçten, O.; Özer, D. Magnetite doped metal–organic framework nanocomposites: An efficient adsorbent for removal of bisphenol-A pollutant. New J. Chem. 2021, 45, 2157–2166. [Google Scholar] [CrossRef]
- Xiong, M.; Wang, B.; Wang, H.; Xu, F.; Zeng, Y.; Ren, H.; Zeng, H. Probing the adsorption behavior and mechanism of NO2 and NH2 functionalized covalent organic frameworks (COFs) for removal of bisphenol A. Microporous Mesoporous Mater. 2022, 346, 112299. [Google Scholar] [CrossRef]
- Xie, H.; Wu, J.; Yu, M.; Yan, H.; Masum, S.; Cai, P.; Chen, Y. Bisphenol A adsorption and transport in loess and cationic surfactant/hydrophilic polymer modified bentonite liners. J. Environ. Manag. 2023, 336, 117604. [Google Scholar] [CrossRef]
- Vakili, M.; Mojiri, A.; Kindaichi, T.; Cagnetta, G.; Yuan, J.; Wang, B.; Giwa, A.S. Cross-linked chitosan/zeolite as a fixed-bed column for organic micropollutants removal from aqueous solution, optimization with RSM and artificial neural network. J. Environ. Manag. 2019, 250, 109434. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Ghadermazi, M.; Bhatnagar, A.; Sadighara, P.; Jahed-Khaniki, G.; Heibati, B.; McKay, G. Adsorptive removal of endocrine disrupting bisphenol A from aqueous solution using chitosan. J. Environ. Chem. Eng. 2016, 4, 2647–2655. [Google Scholar] [CrossRef]
- Guo, W.; Hu, W.; Pan, J.; Zhou, H.; Guan, W.; Wang, X.; Dai, J.; Xu, L. Selective adsorption and separation of BPA from aqueous solution using novel molecularly imprinted polymers based on kaolinite/Fe3O4 composites. Chem. Eng. J. 2011, 171, 603–611. [Google Scholar] [CrossRef]
- Li, X.; Zhou, M.; Jia, J.; Ma, J.; Jia, Q. Design of a hyper-crosslinked β-cyclodextrin porous polymer for highly efficient removal toward bisphenol A from water. Sep. Purif. Technol. 2018, 195, 130–137. [Google Scholar] [CrossRef]
- Xiao, G.; Fu, L.; Li, A. Enhanced adsorption of bisphenol A from water by acetylaniline modified hyper-cross-linked polymeric adsorbent: Effect of the cross-linked bridge. Chem. Eng. J. 2012, 191, 171–176. [Google Scholar] [CrossRef]
- Poulsen, B.G.; Alsulami, Q.A.; Sharfalddin, A.; El Agammy, E.F.; Mouffouk, F.; Emwas, A.-H.; Jaremko, L.; Jaremko, M. Cyclodextrins: Structural, chemical, and physical properties, and applications. Polysaccharides 2022, 3, 1–31. [Google Scholar] [CrossRef]
- Narayanan, G.; Shen, J.; Matai, I.; Sachdev, A.; Boy, R.; Tonelli, A.E. Cyclodextrin-based nanostructures. Prog. Mater. Sci. 2022, 124, 100869. [Google Scholar] [CrossRef]
- Özelçağlayan, E.D.; Parker, W.J. β-Cyclodextrin functionalized adsorbents for removal of organic micropollutants from water. Chemosphere 2023, 320, 137964. [Google Scholar] [CrossRef] [PubMed]
- Morin-Crini, N.; Winterton, P.; Fourmentin, S.; Wilson, L.D.; Fenyvesi, É.; Crini, G. Water-insoluble β-cyclodextrin–epichlorohydrin polymers for removal of pollutants from aqueous solutions by sorption processes using batch studies: A review of inclusion mechanisms. Prog. Polym. Sci. 2018, 78, 1–23. [Google Scholar] [CrossRef]
- Sikder, M.T.; Rahman, M.M.; Jakariya, M.; Hosokawa, T.; Kurasaki, M.; Saito, T. Remediation of water pollution with native cyclodextrins and modified cyclodextrins: A comparative overview and perspectives. Chem. Eng. J. 2019, 355, 920–941. [Google Scholar] [CrossRef]
- Salgın, U.; Yıldız, N.; Çalımlı, A. Desorption of salicylic acid from modified bentonite by using supercritical fluids in packed bed column. Sep. Sci. Technol. 2004, 39, 2677–2694. [Google Scholar] [CrossRef]
- Salgın, U.; Yıldız, N.; Çalımlı, A. Regeneration of modified bentonite loaded with phenol using supercritical fluids. Adsorpt. Sci. Technol. 2004, 22, 39–50. [Google Scholar] [CrossRef]
- Brunner, G. Gas Extraction: An Introduction to Fundamentals of Supercritical Fluids and the Application to Separation Processes, 1st ed.; Steinkopff & Springer: Darmstadt, Germany; New York, NY, USA, 1994; pp. 1–387. [Google Scholar]
- Salgın, S.; Salgın, U.; Ayluçtarhan, M. Synthesis of β-cyclodextrin-epichlorohydrin nanospheres: Its application for removal of p-nitrophenol. Chem. Sci. Int. J. 2016, 16, 1–10. [Google Scholar] [CrossRef]
- Du, H.; Piao, M. Facile preparation of microscale hydrogel particles for high efficiency adsorption of bisphenol A from aqueous solution. Environ. Sci. Pollut. Res. 2018, 25, 28562–28571. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M. Adsorption characteristics and mechanism of bisphenol A by magnetic biochar. Int. J. Environ. Res. Public Health 2020, 17, 1075. [Google Scholar] [CrossRef]
- Rovani, S.; Santos, J.J.; Guilhen, S.N.; Corio, P.; Fungaro, D.A. Fast, efficient and clean adsorption of bisphenol—A using renewable mesoporous silica nanoparticles from sugarcane waste ash. RSC Adv. 2020, 10, 27706–27712. [Google Scholar] [CrossRef]
- Martín-Lara, M.A.; Calero, M.; Ronda, A.; Iáñez-Rodríguez, I.; Escudero, C. Adsorptive behavior of an activated carbon for bisphenol A removal in single and binary (bisphenol A—Heavy metal) solutions. Water 2020, 12, 2150. [Google Scholar] [CrossRef]
- Liu, S.-H.; Zhu, T.-J.; Lin, C.-W. Biotoxicity monitoring of Bisphenol A using microbial fuel cell sensors: Optimizing the response of sensor’s voltage changes to toxicity and assessing the impact on the application to river water monitoring. Biochem. Eng. J. 2023, 200, 109114. [Google Scholar] [CrossRef]
- Salgın, S.; Salgın, U.; Vatansever, Ö. Synthesis and characterization of β-cyclodextrin nanosponge and its application for the removal of p-nitrophenol from water. Clean Soil Air Water 2017, 45, 1500837. [Google Scholar] [CrossRef]
- Lv, Y.; Ma, J.; Liu, K.; Jiang, Y.; Yang, G.; Liu, Y.; Lin, C.; Ye, X.; Shi, Y.; Liu, M.; et al. Rapid elimination of trace bisphenol pollutants with porous β-cyclodextrin modified cellulose nanofibrous membrane in water: Adsorption behavior and mechanism. J. Hazard. Mater. 2021, 403, 123666. [Google Scholar] [CrossRef]
- Gubta, S.S.; Bhattacharyya, K.G. Kinetics of adsorption of metal ions on inorganic materials: A review. Adv. Colloid Interface Sci. 2011, 162, 39–58. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Favvas, E.P.; Kostoglou, M.; Mitropoulos, A.C. Effect of agitation on batch adsorption process facilitated by using nanobubbles. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125440. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Yuan, X.; Wang, Z.; Sun, S.; Lyu, Q.; Hu, S. Efficient adsorption of BPA and Pb2+ by sulfhydryl-rich β-cyclodextrin polymers. Sep. Purif. Technol. 2023, 309, 122913. [Google Scholar] [CrossRef]
- Hemine, K.; Łukasik, N.; Gazda, M.; Nowak, I. β-Cyclodextrin-containing polymer based on renewable cellulose resources for effective removal of ionic and non-ionic toxic organic pollutants from water. J. Hazard. Mater. 2021, 418, 126286. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhou, L.; Guo, J.; Ye, Q.; Lin, J.-M.; Yuan, J. Adsorption of environmental pollutants using magnetic hybrid nanoparticles modified with β-cyclodextrin. Appl. Surf. Sci. 2014, 305, 267–273. [Google Scholar] [CrossRef]
- Lin, Q.; Wu, Y.; Jiang, X.; Lin, F.; Liu, X.; Lu, B. Removal of bisphenol A from aqueous solution via host-guest interactions based on beta-cyclodextrin grafted cellulose bead. Int. J. Biol. Macromol. 2019, 140, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mamman, S.; Suah, F.B.M.; Raaov, M.; Mehamod, F.S.; Asman, S.; Zain, N.N.M. Removal of bisphenol A from aqueous media using a highly selective adsorbent of hybridization cyclodextrin with magnetic molecularly imprinted polymer. R. Soc. Open Sci. 2021, 8, 201604. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Repo, E.; Yin, D.; Chen, L.; Kalliola, S.; Tang, J.; Iakovleva, E.; Tam, K.C.; Sillanpää, M. One-pot synthesis of trifunctional chitosan-EDTA-β-cyclodextrin polymer for simultaneous removal of metals and organic micropollutants. Sci. Rep. 2017, 7, 15811. [Google Scholar] [CrossRef]
- Ragavan, K.V.; Rastogi, N.K. β-Cyclodextrin capped graphene-magnetite nanocomposite for selective adsorption of Bisphenol-A. Carbohydr. Polym. 2017, 168, 129–137. [Google Scholar] [CrossRef]
- Qu, J.; Li, Z.; Wu, Z.; Bi, F.; Wei, S.; Dong, M.; Hu, Q.; Wang, Y.; Yu, H.; Zhang, Y. Cyclodextrin-functionalized magnetic alginate microspheres for synchronous removal of lead and bisphenol A from contaminated soil. Chem. Eng. J. 2023, 461, 142079. [Google Scholar] [CrossRef]
- Saadi, R.; Saadi, Z.; Fazaeli, R.; Elmi Fard, N. Monolayer and multilayer adsorption isotherm models for sorption from aqueous media. Korean J. Chem. Eng. 2015, 32, 787–799. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, H. Über die Adsorption in Lösungen. Z. Phys. Chem. 1907, 57U, 385–470. [Google Scholar] [CrossRef]
- Temkin, M.I.; Pyzhev, V. Kinetics of ammonia synthesis on promoted iron catalyst. Acta Physicochim. URSS 1940, 12, 327–356. [Google Scholar]
- Dubinin, M.M. The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem. Rev. 1960, 60, 235–241. [Google Scholar] [CrossRef]
- Jovanović, D.S. Physical adsorption of gases I: Isotherms for monolayer and multilayer adsorption. Colloid Polym. Sci. 1969, 235, 1203–1214. [Google Scholar] [CrossRef]
- Elovich, S.Y.; Larionov, O.G. Theory of adsorption from nonelectrolyte solutions on solid adsorbents. Russ. Chem.Bull. 1962, 11, 198–203. [Google Scholar] [CrossRef]
- Redlich, O.; Peterson, D.L. A useful adsorption isotherm. J. Phys. Chem. 1959, 63, 1024. [Google Scholar] [CrossRef]
- Sips, R. On the structure of a catalyst surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Tóth, J. State equations of the solid-gas interface layers. Acta Chim. Acad. Sci. Hung. 1971, 69, 311–328. [Google Scholar]
- Koble, R.A.; Corrigan, T.E. Adsorption isotherms for pure hydrocarbons. Ind. Eng. Chem. 1952, 44, 383–387. [Google Scholar] [CrossRef]
- Hill, A.V. The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J. Physiol. 1910, 40, iv–vii. [Google Scholar] [CrossRef]
- Khan, A.R.; Ataullah, R.; Al-Haddad, A. Equilibrium adsorption studies of some aromatic pollutants from dilute aqueous solutions on activated carbon at different temperatures. J. Colloid Interface Sci. 1997, 194, 154–165. [Google Scholar] [CrossRef]
- Khan, A.R.; Al-Waheab, I.R.; Al-Haddad, A. A generalized equation for adsorption isotherms for multi-component organic pollutants in dilute aqueous solution. Environ. Technol. 1996, 17, 13–23. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Huang, X.; Zhang, Q.; Wang, T.; Guo, X. Guideline for modeling solid-liquid adsorption: Kinetics, isotherm, fixed bed, and thermodynamics. Chemosphere 2024, 349, 140736. [Google Scholar] [CrossRef]
- Mphahlele, K.; Onyango, M.S.; Mhlanga, S.D. Kinetics, equilibrium, and thermodynamics of the sorption of bisphenol A onto N-CNTs-β-cyclodextrin and Fe/N-CNTs-β-cyclodextrin nanocomposites. J. Nanomater. 2015, 2015, 214327. [Google Scholar] [CrossRef]
- Martín, Á.; Rodríguez-Rojo, S.; de Pablo, L.; Cocero, M.J. Solubility of bisphenol A in supercritical carbon dioxide. J. Chem. Eng. Data 2011, 56, 3910–3913. [Google Scholar] [CrossRef]
- Sun, J.; Wang, L.; Ding, S.; Sun, X.; Xu, L. Solubility behavior and thermodynamic analysis of bisphenol A in 14 different pure solvents. J. Chem. Eng. Data 2020, 65, 2846–2858. [Google Scholar] [CrossRef]
- Debenedetti, P.G.; Tom, J.W.; Kwauk, X.; Yeo, S.-D. Rapid expansion of supercritical solutions (RESS): Fundamentals and applications. Fluid Phase Equilibria 1993, 82, 311–321. [Google Scholar] [CrossRef]
- Canbolat, M.F.; Celebioglu, A.; Uyar, T. Drug delivery system based on cyclodextrin-naproxen inclusion complex incorporated in electrospun polycaprolactone nanofibers. Colloids Surf. B. Biointerfaces 2014, 115, 15–21. [Google Scholar] [CrossRef]
- Zhang, W.; Gai, H.; Zhang, Q.; Zhang, D.; Zheng, S.; Zhu, G. Preparation, characterization and properties of quercetin cyclodextrin nanosponges. J. Incl. Phenom. Macrocycl. Chem. 2025, 105, 23–34. [Google Scholar] [CrossRef]
- Fei, B.; Chen, C.; Peng, S.; Zhao, X.; Wang, X.; Dong, L. FTIR study of poly(propylene carbonate)/bisphenol A blends. Polym. Int. 2004, 53, 2092–2098. [Google Scholar] [CrossRef]
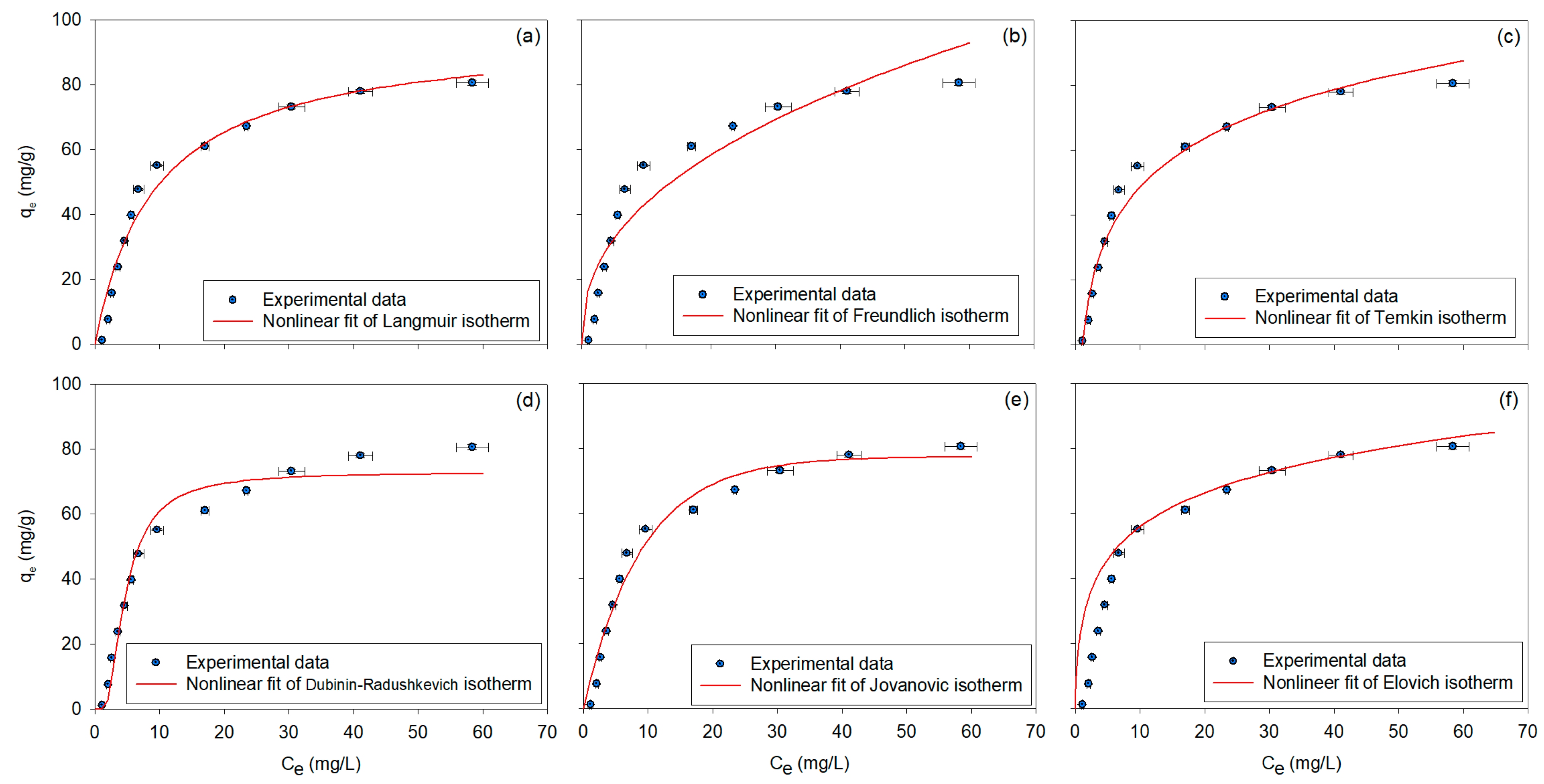





| Isotherm | Equation | Model Parameters | Ref. | |||
|---|---|---|---|---|---|---|
| Two-parameter monolayer adsorption isotherm models | ||||||
| Langmuir | [78] | |||||
| 95.8589 | 0.1068 | 0.9629 | ||||
| Freundlich | [79] | |||||
| 16.3742 | 2.3596 | 0.8782 | ||||
| Temkin | [80] | |||||
| 114.2393 | 0.9391 | 0.9736 | ||||
| Dubinin–Radushkevich (D–R) | [81] | |||||
| 72.7684 | 3.24 × 10−6 | 0.9683 | ||||
| Jovanovic | [82] | |||||
| 77.4110 | 0.1105 | 0.9680 | ||||
| Elovich | [83] | |||||
| 20.1620 | 4.0600 | 0.9770 | ||||
| Three-parameter monolayer adsorption isotherm models | ||||||
| Redlich–Peterson | [84] | |||||
| 8.5450 | 0.0440 | 1.1813 | 0.9629 | |||
| Sips | [85] | |||||
| 77.8437 | 0.0502 | 1.7050 | 0.9861 | |||
| Toth | [86] | |||||
| 78.9506 | 94.1044 | 1.9214 | 0.9746 | |||
| Koble–Corrigan | [87] | |||||
| 3.9049 | 0.0502 | 1.7050 | 0.9861 | |||
| Hill | [88] | |||||
| 77.8436 | 19.9347 | 1.7050 | 0.9861 | |||
| Khan | [89,90] | |||||
| 153.2722 | 0.0603 | 1.2738 | 0.9670 | |||
| Kinetics | Equation | Model Parameters | |||
|---|---|---|---|---|---|
| Pseudo–first order | 0.9739 | ||||
| 46.9502 | 0.0781 | ||||
| Pseudo–second order | 0.9977 | ||||
| 48.3900 | 0.0031 | ||||
| Mixed order | 0.9996 | ||||
| 47.7050 | 0.0119 | 0.0026 | |||
| Pseudo–nth order model | 0.9991 | ||||
| 47.8647 | 0.0074 | 1.7269 | |||
| Elovich | 0.8429 | ||||
| 49.1931 | 3.0549 × 105 | 0.3790 | |||
| Avrami | 0.9997 | ||||
| 47.5724 | 0.2155 | 0.6078 | |||
| T (°C) | ΔGo (J/mol) | ΔHo (kJ/mol) | ΔSo (J/mol K) |
|---|---|---|---|
| 15 | −5130.5 | −11.723 | −23.035 |
| 25 | −4853.8 | ||
| 35 | −4539.9 | ||
| 45 | −4476.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salgın, U.; Alomari, İ.; Soyer, N.; Salgın, S. Adsorption of Bisphenol A onto β-Cyclodextrin–Based Nanosponges and Innovative Supercritical Green Regeneration of the Sustainable Adsorbent. Polymers 2025, 17, 856. https://doi.org/10.3390/polym17070856
Salgın U, Alomari İ, Soyer N, Salgın S. Adsorption of Bisphenol A onto β-Cyclodextrin–Based Nanosponges and Innovative Supercritical Green Regeneration of the Sustainable Adsorbent. Polymers. 2025; 17(7):856. https://doi.org/10.3390/polym17070856
Chicago/Turabian StyleSalgın, Uğur, İsmail Alomari, Nagihan Soyer, and Sema Salgın. 2025. "Adsorption of Bisphenol A onto β-Cyclodextrin–Based Nanosponges and Innovative Supercritical Green Regeneration of the Sustainable Adsorbent" Polymers 17, no. 7: 856. https://doi.org/10.3390/polym17070856
APA StyleSalgın, U., Alomari, İ., Soyer, N., & Salgın, S. (2025). Adsorption of Bisphenol A onto β-Cyclodextrin–Based Nanosponges and Innovative Supercritical Green Regeneration of the Sustainable Adsorbent. Polymers, 17(7), 856. https://doi.org/10.3390/polym17070856








