Influence of Core Starch and Lignocellulosic Fibers from Plantain (Musa paradisiaca L.) Pseudostem on the Development of Thermoplastic Starches and Biobased Composite Materials
Abstract
:1. Introduction
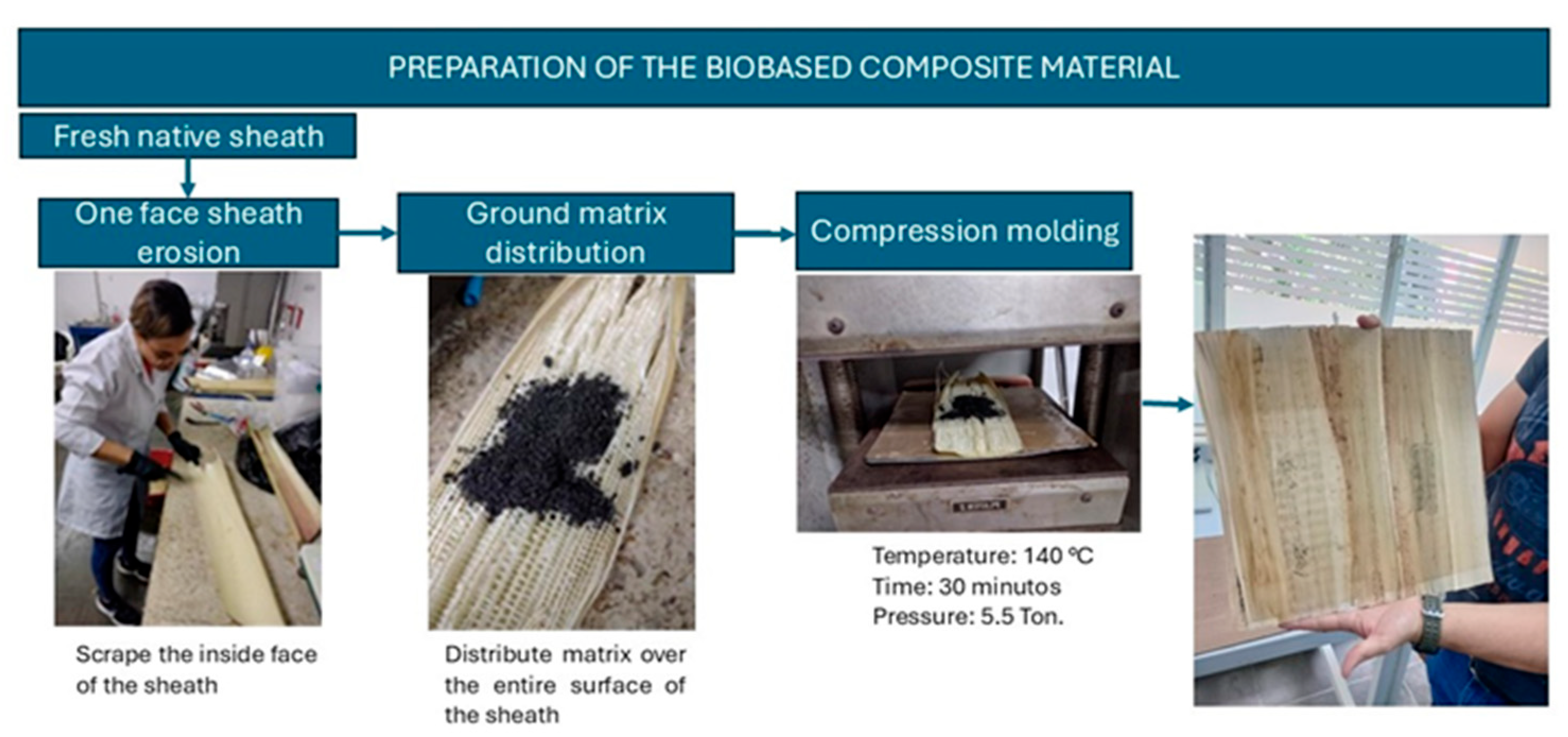
2. Materials and Methods
2.1. Structural Characterization
2.2. Thermal Characterization
2.3. Physicochemical Characterization
2.4. Mechanical Characterization
3. Results and Discussion
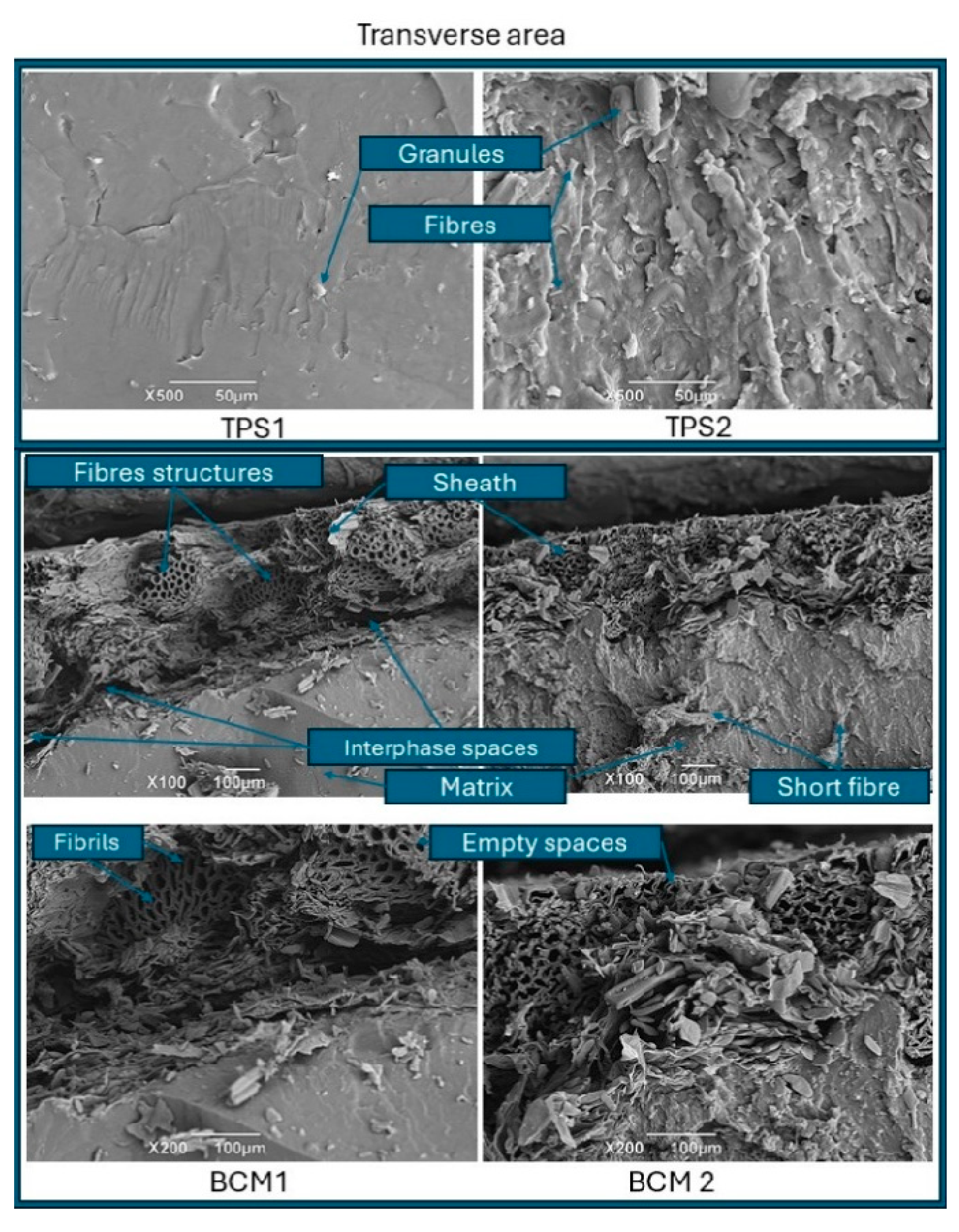
3.1. Structural Characterization
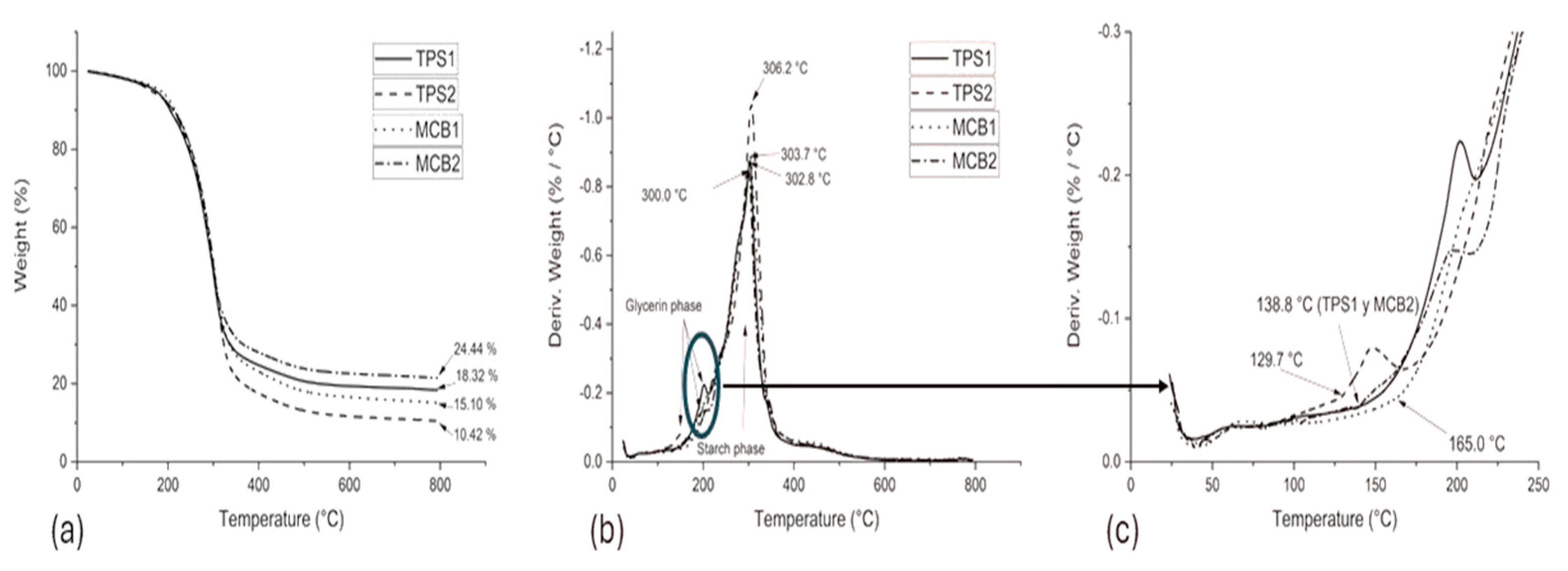
3.2. Thermal Characterization
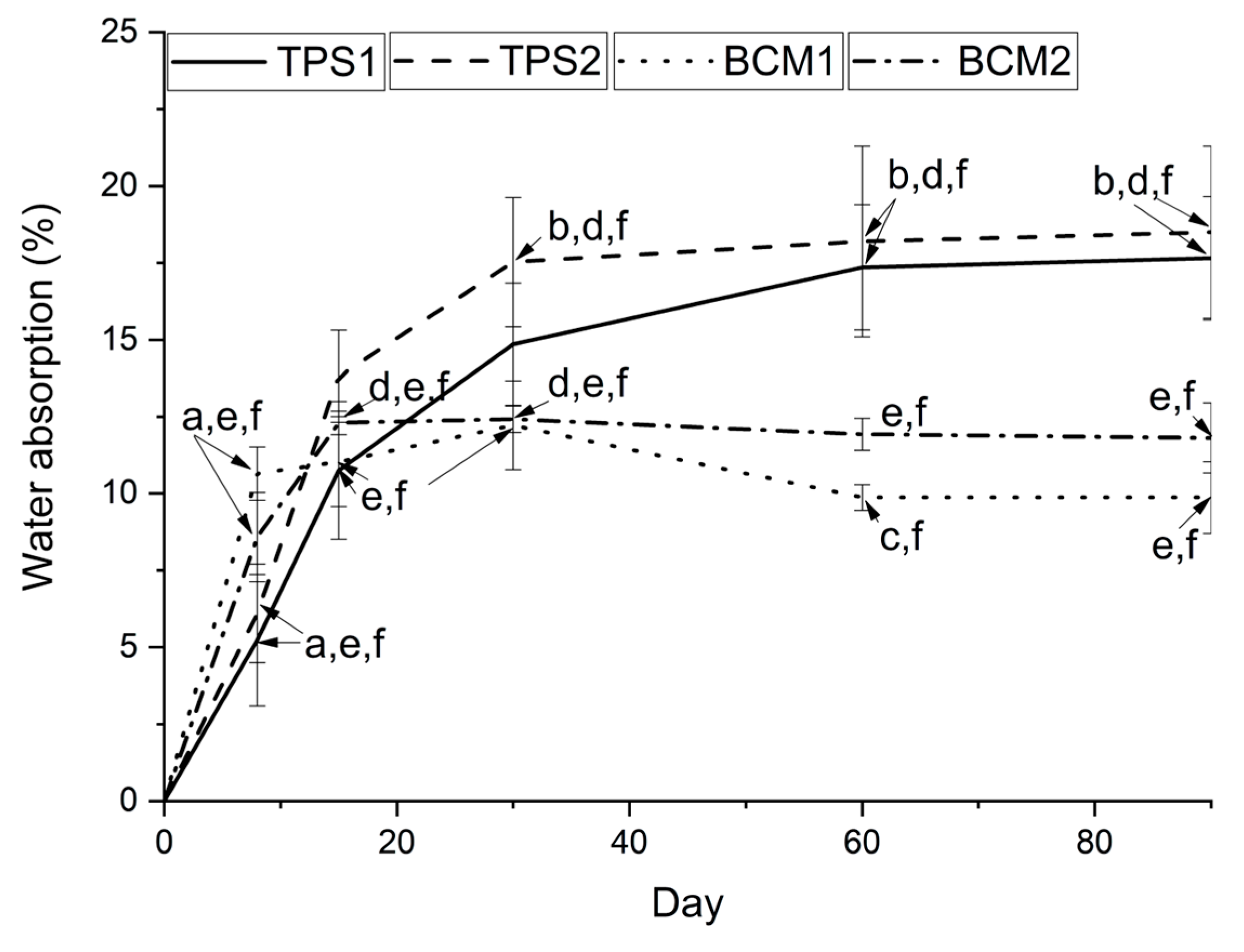
3.3. Physicochemical Characterization
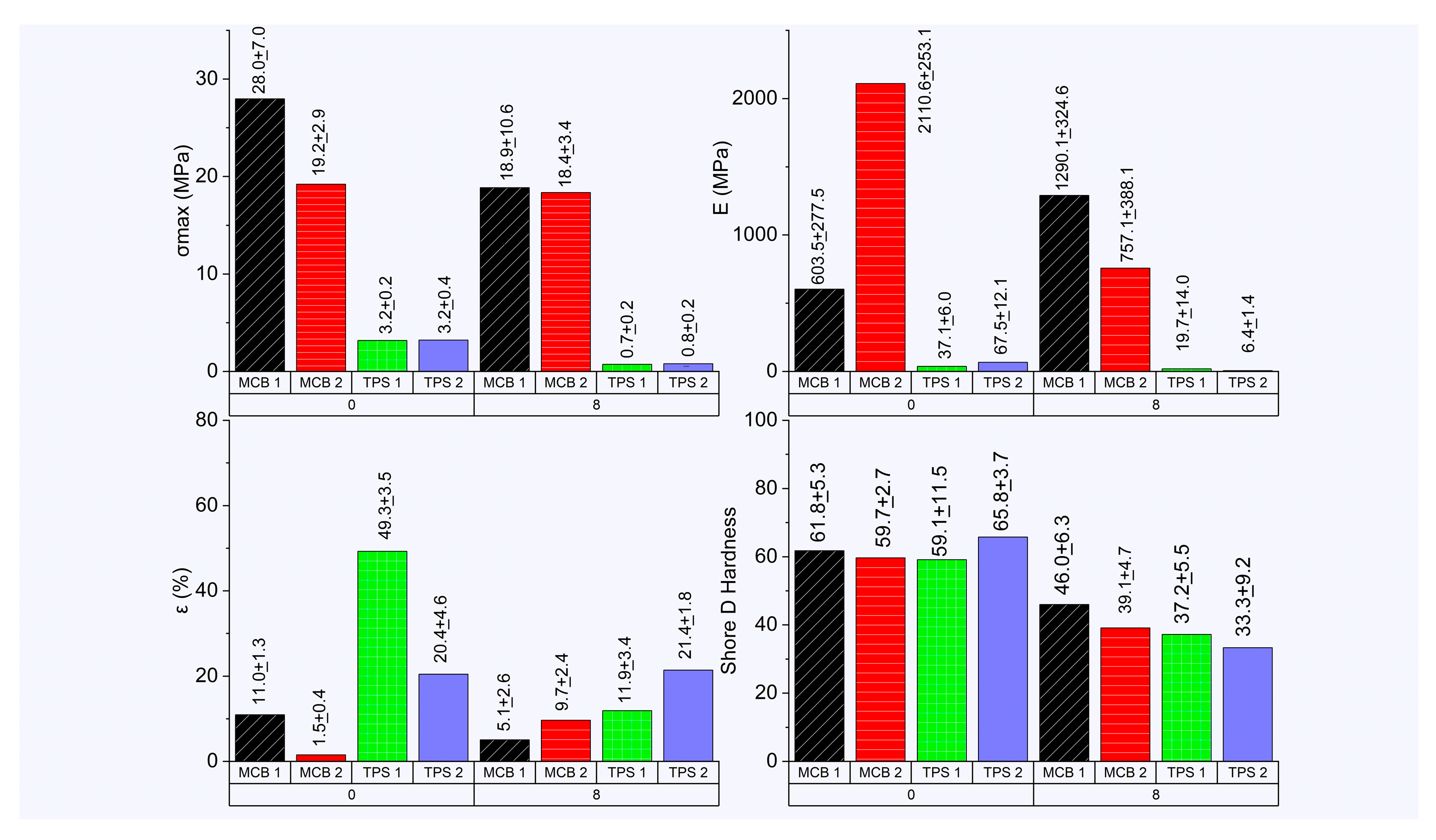
3.4. Mechanical Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kan, M.; Miller, S.A. Environmental Impacts of Plastic Packaging of Food Products. Resour. Conserv. Recycl. 2022, 180, 106156. [Google Scholar] [CrossRef]
- Rispoli, A.L.; Verdone, N.; Vilardi, G. Green Fuel Production by Coupling Plastic Waste Oxy-Combustion and PtG Technologies: Economic, Energy, Exergy and CO2-Cycle Analysis. Fuel Process. Technol. 2021, 221, 106922. [Google Scholar] [CrossRef]
- Wang, J.; Euring, M.; Ostendorf, K.; Zhang, K. Biobased Materials for Food Packaging. J. Bioresour. Bioprod. 2022, 7, 1–13. [Google Scholar] [CrossRef]
- Su, G.; Ong, H.C.; Mofijur, M.; Mahlia, T.M.I.; Ok, Y.S. Pyrolysis of Waste Oils for the Production of Biofuels: A Critical Review. J. Hazard. Mater. 2022, 424, 127396. [Google Scholar] [CrossRef]
- Ibrahim, H.; Mehanny, S.; Darwish, L.; Farag, M. A Comparative Study on the Mechanical and Biodegradation Characteristics of Starch-Based Composites Reinforced with Different Lignocellulosic Fibers. J. Polym. Environ. 2018, 26, 2434–2447. [Google Scholar] [CrossRef]
- Álvarez-Castillo, E.; Felix, M.; Bengoechea, C.; Guerrero, A. Proteins from Agri-Food Industrial Biowastes or Co-Products and Their Applications as Green Materials. Foods 2021, 10, 981. [Google Scholar] [CrossRef]
- Falua, K.J.; Pokharel, A.; Babaei-Ghazvini, A.; Ai, Y.; Acharya, B. Valorization of Starch to Biobased Materials: A Review. Polymers. 2022, 14, 2215. [Google Scholar] [CrossRef]
- López Terán, J.L.; Cabrera, E.V.; Poveda, J.; Araque, J.; Beltrán, M.I. Improving the Behavior of Thermoplastic Starch with the Addition of Gum Arabic: Antibacterial, Mechanical Properties and Biodegradability. Heliyon 2024, 10, e31856. [Google Scholar] [CrossRef]
- Nilsen-Nygaard, J.; Fernández, E.N.; Radusin, T.; Rotabakk, B.T.; Sarfraz, J.; Sharmin, N.; Sivertsvik, M.; Sone, I.; Pettersen, M.K. Current Status of Biobased and Biodegradable Food Packaging Materials: Impact on Food Quality and Effect of Innovative Processing Technologies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1333–1380. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Michelin, M.; Vicente, A.A.; Teixeira, J.A.; Cerqueira, M.Â. Use of Lignocellulosic Materials in Bio-Based Packaging; Springer: Cham, Switzerland, 2018; pp. 65–85. [Google Scholar] [CrossRef]
- Wang, X.; Tian, W.; Ye, Y.; Chen, Y.; Wu, W.; Jiang, S.; Wang, Y.; Han, X. Surface Modifications towards Superhydrophobic Wood-Based Composites: Construction Strategies, Functionalization, and Perspectives. Adv. Colloid Interface Sci. 2024, 326, 103142. [Google Scholar] [CrossRef]
- Bertolini, A. Starches Characterization, Properties and Applications; CRC Press Taylor & Francis Group: Bocaraton, FL, USA, 2010. [Google Scholar]
- Janssen, L.; Moscicki, L. Thermoplastic Starch: A Green Material for Various Industries; Wiley-VCH Verlag: Hoboken, NJ, USA, 2009. [Google Scholar]
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Natural Fibers, Biopolymers, and Biocomposites; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Serna-Jiménez, J.A.; Siles López, J.Á.; de los Ángeles Martín Santos, M.; Chica Pérez, A.F. Exploiting Waste Derived from Musa spp. Processing: Banana and Plantain. Biofuels Bioprod. Biorefining 2023, 17, 1046–1067. [Google Scholar] [CrossRef]
- Nannyonga, S.; Fryer, P.; Robbins, P. Models to Optimise Bioengineering of Banana Wastes to Animal Feeds and Fertilisers. J. Environ. Eng. Sci. 2018, 13, 44–52. [Google Scholar] [CrossRef]
- Ramirez-Cortes, R.; Bello-Pérez, L.A.; Gonzalez-Soto, R.A.; Gutierrez-Meraz, F.; Alvarez-Ramirez, J. Isolation of Plantain Starch on a Large Laboratory Scale. Starch/Staerke 2016, 68, 488–495. [Google Scholar] [CrossRef]
- Hernández-Carmona, F.; Morales-Matos, Y.; Lambis-Miranda, H.; Pasqualino, J. Starch Extraction Potential from Plantain Peel Wastes. J. Environ. Chem. Eng. 2017, 5, 4980–4985. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, Y.; Klomklao, S.; Simpson, B.K. Pectin from Plantain Peels: Green Recovery for Transformation into Reinforced Packaging Films. Waste Manag. 2023, 161, 225–233. [Google Scholar] [CrossRef]
- Happi Emaga, T.; Robert, C.; Ronkart, S.N.; Wathelet, B.; Paquot, M. Dietary Fibre Components and Pectin Chemical Features of Peels during Ripening in Banana and Plantain Varieties. Bioresour. Technol. 2008, 99, 4346–4354. [Google Scholar] [CrossRef]
- Castañeda-Niño, J.P.; Mina-Hernandez, J.H.; Solanilla-Duque, J.F. Potential of Plantain Pseudostems (Musa AAB Simmonds) for Developing Biobased Composite Materials. Polymers 2024, 16, 1357. [Google Scholar] [CrossRef]
- Chinnathambi, S.; Kumar, P.S.; Shuprajhaa, T.; Shiva, K.N.; Narayanan, S. Elucidation of Techno-Functional, Structural and Rheological Characteristics of Pectin Extracted from the Peel of Different Banana (Musa. spp.) Varieties. Int. J. Biol. Macromol. 2024, 258, 128989. [Google Scholar] [CrossRef]
- Otu, D.A.B.; Owusu, F.W.A.; Acquah, P.G.J.; El Boakye-Gyasi, M.; Edzor-Agbo, Y.; Johnson, R.; Bayor, M.T.; Archer, M.A. Effect of Ripening and Extraction Method on the Physicochemical Properties of Pectin Extracted from Peels of Apem and Apantu Plantain Cultivars in Ghana. J. Chem. 2024, 2024, 6677179. [Google Scholar] [CrossRef]
- Holguín Posso, A.M.; Macías Silva, J.C.; Castañeda Niño, J.P.; Mina Hernandez, J.H.; Fajardo Cabrera de Lima, L.d.P. Characterization and Implementation of Cocoa Pod Husk as a Reinforcing Agent to Obtain Thermoplastic Starches and Bio-Based Composite Materials. Polymers 2024, 16, 1608. [Google Scholar] [CrossRef]
- García-Ramón, J.A.; Carmona-García, R.; Valera-Zaragoza, M.; Aparicio-Saguilán, A.; Bello-Pérez, L.A.; Aguirre-Cruz, A.; Alvarez-Ramirez, J. Morphological, Barrier, and Mechanical Properties of Banana Starch Films Reinforced with Cellulose Nanoparticles from Plantain Rachis. Int. J. Biol. Macromol. 2021, 187, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.S.; Osman, A.F.; Adnan, S.A.; Ibrahim, I.; Salimi, M.N.A.; Alrashdi, A.A. Effective Aging Inhibition of the Thermoplastic Corn Starch Films through the Use of Green Hybrid Filler. Polymers 2022, 14, 2567. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Soto, K.X.; Piñeros-Castro, N.Y.; Ortega-Toro, R. Laminated Composites Reinforced with Chemically Modified Sheets-Stalk of Musa Cavendish. Rev. Mex. Ing. Quim. 2019, 18, 749–758. [Google Scholar] [CrossRef]
- De La Torre-Gutierrez, L.; Torruco-Uco, J.G.; Castellanos-Ruelas, A.; Chel-Guerrero, L.A.; Betancur-Ancona, D. Isolation and Structure Investigations of Square Banana (Musa balbisiana) Starch. Starch/Staerke 2007, 59, 326–333. [Google Scholar] [CrossRef]
- Rodriguez, L. Process for Manufacturing of Biocomposite Material Based on Plantain Fibers. Master’s Thesis, Universidad Nacional de Colombia, Manizales, Colombia, 2014. [Google Scholar]
- Mina Hernandez, J.H. Effect of the Incorporation of Polycaprolactone (Pcl) on the Retrogradation of Binary Blends with Cassava Thermoplastic Starch (Tps). Polymers 2021, 13, 38. [Google Scholar] [CrossRef]
- Parra-Campos, A.; Serna-Cock, L.; Solanilla-Duque, J.F. Effect of the Addition of Fique Bagasse Microparticles in Obtaining a Biobased Material Based on Cassava Starch. Int. J. Biol. Macromol. 2022, 207, 289–298. [Google Scholar] [CrossRef]
- Ferreira-Villadiego, J.; García-Echeverri, J.; Vidal, M.V.; Pasqualino, J.; Meza-Castellar, P.; Lambis-Miranda, H.A. Chemical Modification and Characterization of Starch Derived from Plantain (Musa paradisiaca) Peel Waste, as a Source of Biodegradable Material. Chem. Eng. Trans. 2018, 65, 763–768. [Google Scholar] [CrossRef]
- Bodirlau, R.; Teaca, C.A.; Spiridon, I. Influence of Natural Fillers on the Properties of Starch-Based Biocomposite Films. Compos. B Eng. 2013, 44, 575–583. [Google Scholar] [CrossRef]
- Heckadka, S.S.; Kini, M.V.; Ballambat, R.P.; Beloor, S.S.; Udupi, S.R.; Kini, U.A. Flexural Strength Analysis of Starch Based Biodegradable Composite Using Areca Frond Fibre Reinforcement. Int. J. Manuf. Eng. 2014, 2014, 769012. [Google Scholar] [CrossRef]
- Muñoz-Vélez, M.F.; Hidalgo-Salazar, M.A.; Mina-Hernández, J.H. Effect of Content and Surface Modification of Fique Fibers on the Properties of a Low-Density Polyethylene (LDPE)-Al/Fique Composite. Polymers 2018, 10, 1050. [Google Scholar] [CrossRef]
- Tavares, L.; Sousa, L.R.; da Silva, S.M.; Lima, P.S.; Oliveira, J.M. Effect of Incorporation of Graphene Nanoplatelets on Physicochemical, Thermal, Rheological, and Mechanical Properties of Biobased and Biodegradable Blends. Polymers 2023, 15, 3622. [Google Scholar] [CrossRef]
- Singh, A.K.; Chugh, K.; Mhaske, S.T. Improvement in BHETA Yield by Aminolysis and Its Application in Hot Melt Adhesive. Int. J. Adhes. Adhes. 2024, 130, 103610. [Google Scholar] [CrossRef]
- Alanís-López, P.; Pérez-González, J.; Rendón-Villalobos, R.; Jiménez-Pérez, A.; Solorza-Feria, J. Extrusion and Characterization of Thermoplastic Starch Sheets from “Macho” Banana. J. Food Sci. 2011, 76, E465–E471. [Google Scholar] [CrossRef]
- Mendes, J.F.; Paschoalin, R.T.; Carmona, V.B.; Sena Neto, A.R.; Marques, A.C.P.; Marconcini, J.M.; Mattoso, L.H.C.; Medeiros, E.S.; Oliveira, J.E. Biodegradable Polymer Blends Based on Corn Starch and Thermoplastic Chitosan Processed by Extrusion. Carbohydr. Polym. 2016, 137, 452–458. [Google Scholar] [CrossRef]
- Ma, X.; Yu, J.; Kennedy, J.F. Studies on the Properties of Natural Fibers-Reinforced Thermoplastic Starch Composites. Carbohydr. Polym. 2005, 62, 19–24. [Google Scholar] [CrossRef]
- Venegas, R.; Torres, A.; Rueda, A.M.; Morales, M.A.; Arias, M.J.; Porras, A. Development and Characterization of Plantain (Musa paradisiaca) Flour-Based Biopolymer Films Reinforced with Plantain Fibers. Polymers 2022, 14, 748. [Google Scholar] [CrossRef]
- Moriana, R.; Vilaplana, F.; Karlsson, S.; Ribes-Greus, A. Improved Thermo-Mechanical Properties by the Addition of Natural Fibres in Starch-Based Sustainable Biocomposites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 30–40. [Google Scholar] [CrossRef]
- Castañeda-Niño, J.P.; Mina-Hernandez, J.H.; Valdez-González, A. Potential Uses of Musaceae Wastes: Case of Application in the Development of Bio-Based Composites. Polymers 2021, 13, 1844. [Google Scholar] [CrossRef]
- Calambás Pulgarin, H.L.; Caicedo, C.; López, E.F. Effect of Surfactant Content on Rheological, Thermal, Morphological and Surface Properties of Thermoplastic Starch (TPS) and Polylactic Acid (PLA) Blends. Heliyon 2022, 8, e10833. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Long, Z.; Wang, S.; Zhang, J.; Wang, L. Preparation and Performance of Thermoplastic Starch and Microcrystalline Cellulose for Packaging Composites: Extrusion and Hot Pressing. Int. J. Biol. Macromol. 2020, 165, 2295–2302. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, F.; Lin, Y.; Zhou, M.; Zhu, P.; Wu, D. Synergistic Effects of Sodium Adipate/Triethylene Glycol on the Plasticization and Retrogradation of Corn Starch. Carbohydr. Res. 2020, 496, 108112. [Google Scholar] [CrossRef]
- Zainuddin, S.Y.Z.; Ahmad, I.; Kargarzadeh, H.; Abdullah, I.; Dufresne, A. Potential of Using Multiscale Kenaf Fibers as Reinforcing Filler in Cassava Starch-Kenaf Biocomposites. Carbohydr Polym. 2013, 92, 2299–2305. [Google Scholar] [CrossRef]
- Basak, S.; Saxena, S.; Chattopadhyay, S.K.; Narkar, R.; Mahangade, R. Banana Pseudostem Sap: A Waste Plant Resource for Making Thermally Stable Cellulosic Substrate. J. Ind. Text. 2016, 46, 1003–1023. [Google Scholar] [CrossRef]
- Jayaprabha, J.S.; Brahmakumar, M.; Manilal, V.B. Banana Pseudostem Characterization and Its Fiber Property Evaluation on Physical and Bioextraction. J. Nat. Fibers 2011, 8, 149–160. [Google Scholar] [CrossRef]
- Pooja, N.; Shashank, S.; Singh, B.N.; Mazumder, N. Advancing Sustainable Bioplastics: Chemical and Physical Modification of Starch Films for Enhanced Thermal and Barrier Properties. RSC Adv. 2024, 14, 23943–23951. [Google Scholar] [CrossRef]
- Gáspár, M.; Benko, Z.; Dogossy, G.; Réczey, K.; Czigány, T. Reducing Water Absorption in Compostable Starch-Based Plastics. Polym. Degrad. Stab. 2005, 90, 563–569. [Google Scholar] [CrossRef]
- Gironès, J.; López, J.P.; Mutjé, P.; Carvalho, A.J.F.; Curvelo, A.A.S.; Vilaseca, F. Natural Fiber-Reinforced Thermoplastic Starch Composites Obtained by Melt Processing. Compos. Sci. Technol. 2012, 72, 858–863. [Google Scholar] [CrossRef]
- Mohapatra, D.; Mishra, S.; Meda, V. Plantains and Their Postharvest Uses: An Overview. Stewart Postharvest Rev. 2009, 5, 1–11. [Google Scholar] [CrossRef]
- Prachayawarakorn, J.; Hommanee, L. Effect of Pectin Contents on Properties of Biodegradable Thermoplastic Mung Bean Starch/Low-Density Polyethylene Blends Using Injection Molding Technique. Chiang Mai J. Sci. 2016, 43, 215–225. [Google Scholar]
- Lucas-Aguirre, J.C.; Velásquez-Herrera, J.D.; Quintero-Castaño, V.D. Evaluación de Las Propiedades Térmicas y Composicionales de Almidones Extraidos de 26 Variedades de Musáceas. Vitae 2016, 23 (Suppl. S1), S551–S556. [Google Scholar]
- Islam, S.; Miah, M.A.S.; Islam, M.F.; Tisa, K.J.; Mondol, M.M.H. Enzymatic Extraction of Green Banana Resistant Starch for Future Food Preparation: Structural, Physicochemical and Functional Characterization. Future Foods 2024, 9, 100308. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Ighalo, J.O.; Onifade, D.V. Banana and Plantain Fiber-Reinforced Polymer Composites. J. Polym. Eng. 2019, 39, 597–611. [Google Scholar] [CrossRef]
- Lomelí Ramírez, M.G.; Satyanarayana, K.G.; Iwakiri, S.; De Muniz, G.B.; Tanobe, V.; Flores-Sahagun, T.S. Study of the Properties of Biocomposites. Part I. Cassava Starch-Green Coir Fibers from Brazil. Carbohydr. Polym. 2011, 86, 1712–1722. [Google Scholar] [CrossRef]
- Âverous, L.; Fringant, C.; Moro, L. Plasticized Starch±cellulose Interactions in Polysaccharide Composites. Polymer 2001, 42, 6565–6572. [Google Scholar] [CrossRef]
- Moghaddam, M.R.A.; Razavi, S.M.A.; Jahani, Y. Effects of Compatibilizer and Thermoplastic Starch (TPS) Concentration on Morphological, Rheological, Tensile, Thermal and Moisture Sorption Properties of Plasticized Polylactic Acid/TPS Blends. J. Polym. Environ. 2018, 26, 3202–3215. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Chandrasekar, M.; Alothman, O.Y.; Fouad, H.; Jawaid, M.; Azeem, M.A. Flexural, Impact and Dynamic Mechanical Analysis of Hybrid Composites: Olive Tree Leaves Powder/ Pineapple Leaf Fibre/Epoxy Matrix. J. Mater. Res. Technol. 2022, 21, 4241–4252. [Google Scholar] [CrossRef]
- Dorigato, A.; Fredi, G.; Pegoretti, A. Thermo-Mechanical Behavior of Novel Wood Laminae-Thermoplastic Starch Biodegradable Composites with Thermal Energy Storage/Release Capability. Front. Mater. 2019, 6, 76. [Google Scholar] [CrossRef]
- de Oliveira Begali, D.; Ferreira, L.F.; de Oliveira, A.C.S.; Borges, S.V.; de Sena Neto, A.R.; de Oliveira, C.R.; Yoshida, M.I.; Sarantopoulos, C.I.G.L. Effect of the Incorporation of Lignin Microparticles on the Properties of the Thermoplastic Starch/Pectin Blend Obtained by Extrusion. Int. J. Biol. Macromol. 2021, 180, 262–271. [Google Scholar] [CrossRef]
- Coffin’, D.R.; Fishman, M.L. Viscoelastic Properties of Pectin/Starch Blends. J. Agric. Food Chem. 1993, 1093, 1192–1193. [Google Scholar] [CrossRef]
- Da Roz, A.L.; Veiga-Santos, P.; Ferreira, A.M.; Antunes, T.C.R.; De Lima Leite, F.; Yamaji, F.M.; De Carvalho, A.J.F. Water Susceptibility and Mechanical Properties of Thermoplastic Starch-Pectin Blends Reactively Extruded with Edible Citric Acid. Mater. Res. 2016, 19, 138–142. [Google Scholar] [CrossRef]
- Yoksan, R.; Boontanimitr, A. Effect of Calcium Carbonate on the Performance of Poly(Butylene Adipate-Co-Terephthalate) Filled with Duckweed Biomass. Ind. Crops Prod. 2023, 205, 117442. [Google Scholar] [CrossRef]
- Li, M.; Jia, Y.; Shen, X.; Shen, T.; Tan, Z.; Zhuang, W.; Zhao, G.; Zhu, C.; Ying, H. Investigation into Lignin Modified PBAT/Thermoplastic Starch Composites: Thermal, Mechanical, Rheological and Water Absorption Properties. Ind. Crops Prod. 2021, 171, 113916. [Google Scholar] [CrossRef]
- Cadena Ch, E.M.; Vélez, R.J.M.; Santa, J.F.; Otálvaro, G.V. Natural Fibers from Plantain Pseudostem (Musa paradisiaca) for Use in Fiber-Reinforced Composites. J. Nat. Fibers 2017, 14, 678–690. [Google Scholar] [CrossRef]
- Gamboni, J.E.; Slavutsky, A.M.; Bertuzzi, M.A. Starch-Pectin Films Obtained by Extrusion and Compression Molding. J. Multidiscip. Eng. Sci. Technol. 2019, 6, 10175–10183. [Google Scholar]


| Treatment | Glycerol (g/100 g) | Pulp and Peel Starch (g/100 g) | Core Starch (g/100 g) | Sheath Short Fiber (g/100 g) | Reinforcement with Treated Sheath (g/100 g) |
|---|---|---|---|---|---|
| TPS1 | 35.0 | 65.0 | 0 | 0 | 0 |
| TPS2 | 35.0 | 60.0 | 5.0 | 0 | 0 |
| BCM1 | 22.1 | 40.3 | 0 | 0.6 | 37 |
| BCM2 | 22.1 | 37.1 | 3.2 | 0.6 | 37 |
| Sample | Day | σmaxF (MPa) | EF (GPa) | ε F (%) |
|---|---|---|---|---|
| BCM1 | 0 | 32.4 ± 7.6 | 3.3 ± 0.9 | 10.4 ± 5.6 |
| 8 | 10.7 ± 3.3 | 0.7 ± 0.2 | 16.5 ± 6.0 | |
| BCM2 | 0 | 53.8 ± 8.7 | 3.5 ± 1.6 | 8.3 ± 5.6 |
| 8 | 14.1 ± 5.1 | 0.7 ± 0.3 | 15.2 ± 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munar, A.M.; Trujillo, D.B.; Méndez, N.M.; Mesa, C.G.; Tenorio, P.A.; Montealegre-Torres, F.; Zapata-Díaz, Y.C.; Ospina-Aguilar, L.G.; Castañeda-Niño, J.P. Influence of Core Starch and Lignocellulosic Fibers from Plantain (Musa paradisiaca L.) Pseudostem on the Development of Thermoplastic Starches and Biobased Composite Materials. Polymers 2025, 17, 859. https://doi.org/10.3390/polym17070859
Munar AM, Trujillo DB, Méndez NM, Mesa CG, Tenorio PA, Montealegre-Torres F, Zapata-Díaz YC, Ospina-Aguilar LG, Castañeda-Niño JP. Influence of Core Starch and Lignocellulosic Fibers from Plantain (Musa paradisiaca L.) Pseudostem on the Development of Thermoplastic Starches and Biobased Composite Materials. Polymers. 2025; 17(7):859. https://doi.org/10.3390/polym17070859
Chicago/Turabian StyleMunar, Andrés Mauricio, Danilo Bonilla Trujillo, Nelly María Méndez, Carlos Guillermo Mesa, Paola Andrea Tenorio, Francisco Montealegre-Torres, Yean Carlos Zapata-Díaz, Lina Gisselth Ospina-Aguilar, and Juan Pablo Castañeda-Niño. 2025. "Influence of Core Starch and Lignocellulosic Fibers from Plantain (Musa paradisiaca L.) Pseudostem on the Development of Thermoplastic Starches and Biobased Composite Materials" Polymers 17, no. 7: 859. https://doi.org/10.3390/polym17070859
APA StyleMunar, A. M., Trujillo, D. B., Méndez, N. M., Mesa, C. G., Tenorio, P. A., Montealegre-Torres, F., Zapata-Díaz, Y. C., Ospina-Aguilar, L. G., & Castañeda-Niño, J. P. (2025). Influence of Core Starch and Lignocellulosic Fibers from Plantain (Musa paradisiaca L.) Pseudostem on the Development of Thermoplastic Starches and Biobased Composite Materials. Polymers, 17(7), 859. https://doi.org/10.3390/polym17070859










